Abstract
Persistent human papillomavirus (HPV) infection is causally linked to the development of several human cancers, including cervical, vulvar, vaginal, anal, penile, and oropharyngeal cancers. To address the need for a therapeutic vaccine against HPV-associated diseases, here we test and compare the immunogenicity and therapeutic efficacy of a bacterial exotoxin fusion protein covalently linked to the HPV16 E7 oncoprotein adjuvanted with CpG or GPI-0100 in the C3.43 preclinical HPV16-transformed tumor model. We show that TVGV-1 protein vaccine adjuvanted with either CpG or GPI-0100 adjuvant induces a high frequency of E7-specific CD8+ T cells, and both adjuvants are able to assist the immune response in inducing polyfunctional cytokine-secreting lytic T cells that show therapeutic efficacy against well-established C3.43 tumors. CpG-adjuvanted TVGV-1 resulted in higher frequencies of IFNγ secreting and degranulating E7-specific T cells compared to GPI-0100-adjuvanted TVGV-1, resulting in marginally increased in vivo efficacy. Despite minor differences in immune response outcomes, we consider both CpG ODN and GPI-0100 to be promising vaccine adjuvants to increase the immunogenicity and therapeutic efficacy of the TVGV-1 protein for HPV16-driven cancers.
Keywords: human papillomavirus, therapeutic vaccine, CpG adjuvant, GPI-0100 adjuvant, HPV16-induced tumors
1. Introduction
Persistent infection with high-risk human papillomaviruses (HPVs) are the causative agent in virtually all cervical cancer cases, a significant number of other anogenital cancers, and a subset of oropharyngeal cancers [1]. HPV16 is the most carcinogenic genotype relative to other high risk HPVs, being found in 60% of invasive cervical cancer cases and the predominant genotype also found in vulvar, vaginal, anal, penile lesions, and almost all HPV-associated oropharyngeal head and neck cancers [2–4]. The viral oncoproteins E6 and E7 are the preferred viral protein targets for development of therapeutic vaccines for HPV-induced malignancies due to their role in tumorigenesis and maintenance of transformed cells [5]. Although preventive HPV vaccines have been available for the past decade, prophylactic vaccination to induce protective antibody responses does not address the immediate need for a therapeutic vaccine that targets HPV-associated diseases in people already infected and which requires induction of a strong anti-tumor T cell-mediated immune response. As such, vaccination strategies targeting HPV16 E6 and/or E7 to induce cytotoxic T lymphocytes (CTLs) are the focal point for current therapeutic vaccine development (reviewed in [6]). There have been numerous and extensive studies that have generated promising vaccine candidates tested in clinical trials for HPV-associated diseases. Among them are DNA, RNA, viral vectors, bacterial vectors, dendritic cells, peptides and proteins in various iterations and combinations. These strategies have been supplemented with different adjuvants, heterologous prime-boost regimens, immunostimulants and immunomodulators. Despite this enormous effort, there are currently no approved therapeutic vaccines for HPV-induced cancers or precancerous lesions, though several candidates are currently in clinical trials (reviewed in [7]).
The Pseudomonas Exotoxin-HPV16 E7-KDEL3 fusion protein (PEΔIII-E7-KDEL3; also termed TVGV-1) is a recombinant protein composed of 3 fused subunits: the translocation domains I and II of Pseudomonas aeruginosa exotoxin A at the N-terminus, full length HPV16 E7 protein in the middle and a triple KDEL endoplasmic reticulum (ER) retention signal at the C-terminus [8]. Cell binding and cytoplasmic translocation features of bacterial exotoxins results in retrograde delivery of foreign proteins to cytosolic compartments, enhancing MHC class I presentation of exogenous antigen to T lymphocytes [9].
While PEΔIII-E7-KDEL3 has been designed in an attempt to generate a robust response on its own, the development of weak cellular and humoral immune responses is common following protein-based vaccination in the absence of an adjuvant. Vaccine adjuvants, functionally defined as either vehicles for optimal antigen delivery or direct immunostimulants, are compounds that can enhance the magnitude, breadth, quality and longevity of induced antigen-specific immune responses and are frequently used to augment therapeutic vaccine strategies [10]. Examples of immunostimulatory adjuvants are toll-like receptor (TLR) ligands, cytokines, saponins, and bacterial exotoxins. GPI-0100 is a non-toxic semi-synthetic dodecylamide saponin derivative of Quillaja saponaria Molina soapbark tree extract that functions as an immunostimulatory adjuvant and has been used with investigational vaccines to improve the immunogenicity of both cancer and infectious disease antigens [11–15]. GPI-0100 also facilitates delivery of exogenous protein antigens directly to the cell cytosol for processing by endogenous pathways [12, 16]. This generates a mixed Th1/Th2 immune response (biased toward Th1), and stimulates CTL generation through antigen presenting cell (APC) cross-presentation [11, 17]. Early phase clinical studies show that GPI-0100 is an effective immune adjuvant with a variety of investigational antigens and is well tolerated [15, 18].
CpG synthetic oligodeoxynucleotides (ODNs) containing unmethylated CpG motifs trigger immune cells that express TLR9, which include plasmacytoid dendritic cells (pDCs) and B cells, and stimulates immune responses characterized by the production of Th1 and proinflammatory cytokines [19, 20]. The ODN 1826 used in this study is a class B CpG ODN with a preference for mouse TLR9 and contains a full phosphorothioate backbone with two CpG dinucleotides [21, 22]. CpG ODNs as vaccine adjuvants improve the function of professional APCs and enhance antigen-specific adaptive immunity. Importantly, CpG ODNs have a good safety profile in human clinical trials and show efficacy in increasing immunogenicity of co-administered vaccines [21].
Previous studies have shown that prophylactic vaccination of mice with unadjuvanted PEΔIII-E7-KDEL3 protein results in generation of E7-specific CD8+ and CD4+ T cell responses and protection against challenge with E7-expressing TC-1 tumor cells [8]. Furthermore, therapeutic efficacy was demonstrated against early pulmonary metastasis, reducing the number of lung tumor nodules that develop over time [8]. However, therapeutic activity of this fusion protein against well-established subcutaneous solid tumors has not been investigated nor has this protein been combined with adjuvants that may further enhance cellular immune responses. In this study, we evaluated and compared the immunogenicity and adjuvant activity of GPI-0100 or CpG with TVGV-1 protein therapeutic vaccination in a preclinical mouse model for HPV16-induced cancer.
2. Materials and Methods
2.1. Mice, Cell Lines, Antibodies, and Reagents
Pathogen-free 7 week old C57BL/6 female mice were purchased from Taconic Biosciences (Oxnard, CA) and were acclimated to the new housing environment for at least one week prior to use. C3.43, a progressive subclone of the HPV16-transformed B6 mouse embryo cell line C3, was used for tumor challenge studies [23, 24]. C3.43 cells were grown and expanded in vitro with IMDM medium supplemented with 10% fetal bovine serum (FBS; Gemini, Sacramento, CA), 50 uM 2-mercaptoethanol, and 50 μg/mL gentamicin. The following anti-mouse antibodies, isotype controls and reagents were purchased from Biolegend (San Diego, CA): CD3 FITC, CD3 APC-Cy7, CD4 PE, CD4 BV421, CD8a PerCP-Cy5.5, CD8 APC, CD45 APC-Cy7, CD45R/B220 BV510, CD11b BV510, rat IgG2a FITC, rat IgG2b PE, rat IgG1 PE-Cy5, rat IgG2b APC, rat IgG2b PE-Cy7, FluoroFix buffer and Zombie Aqua. CD25 PerCP-Cy5.5, FoxP3 PE-Cy7, and Rat IgG1 PerCP-Cy5.5 were purchased from eBioscience (San Diego, CA). Rat IgG2a FITC, rat IgG2b PE, rat IgG PerCP-Cy5.5, rat IgG1, CD107a FITC, CD107b FITC, IL-2 PE, IFNγ PerCp-Cy5.5, TNFα PE-Cy7, Fc Block, BD GolgiPlug, and BD Cytofix/Cytoperm were purchased from BD Biosciences (San Jose, CA). Phorbol 12-myristate 13-acetate (PMA) and ionomycin were purchased from Sigma-Aldrich (St. Louis, MO). PE labeled H2-Db-HPV16 E7(49–57) tetramer reagent was acquired from the NIH NIAID Tetramer Core Facility at Emory University (Atlanta, GA). CpG oligonucleotide (ODN-1826) was purchased from InvivoGen (San Diego, CA). GPI-0100 was licensed by Hawaii Biotech Inc. (Honolulu, HI) and provided for study investigations by TheVax Genetics Vaccine Co. Ltd. (Taiwan, R.O.C.). The H2-Db-binding peptide, HPV16 E7(49–57) RAHYNIVTF, was synthesized at the University of Chicago (Chicago, IL) and purified by reverse phase high-performance liquid chromatography. Purity was determined to be >95% pure. All in vivo studies were in compliance and approved by University of Southern California Institutional Animal Care and Use Committee (USC IACUC) and comply with the National Institutes of Health guide for the care and use of laboratory animals.
2.2. TVGV-1 vaccine design and protein synthesis
The recombinant TVGV-1 (PE-E7-K3) fusion protein was prepared by slightly modifying a method described by Liao et. al. [8]. In brief, the protein was expressed in E. coli, isolated from inclusion bodies, purified on DEAE-cellulose, then refolded. After refolding, the protein was further purified by size exclusion chromatography. The purified protein was formulated in 1 mM histidine, 4% mannitol, 1% sucrose, and 0.005% Tween 80 (v/v). The protein was then lyophilized and stored at 2°–8°C until use.
2.3. Immunization and Tumor Challenge
To prepare TVGV-1-C vaccine with CpG adjuvant, lyophilized TVGV-1 protein was reconstituted to deliver 100 μg TVGV-1 protein and 50 μg CpG adjuvant in a volume of 100 μL per injection. TVGV-1-G vaccine was reconstituted to deliver 100 μg TVGV-1 protein and 100 μg GPI-0100 adjuvant in a volume of 100 μL per injection. For immunogenicity studies, mice (5/group) were immunized subcutaneously (s.c.) at the base of the neck with 100 μg of TVGV-1-C, TVGV-1-G, or vector control on days 0, 7, and 14. Assays were performed on day 21 or 25. For therapeutic vaccine efficacy studies, mice (10–15/group) were challenged s.c. on the flank with 1×105 C3.43 tumor cells in 100 μl Hanks Balanced Salt Solution on day 0. At the indicated day post challenge, mice were administered 3 doses, one week apart, of 100 μg TVGV-1-C, TVGV-1-G, or vector control. Tumor growth was measured two times per week with manual calipers by measuring tumor length, width, and depth to generate a tumor volume. Tumor volumes exceeding 1500 mm3 or ulcerated tumors resulted in euthanasia as per animal use guidelines. Mice were observed for the duration of each experiment for signs of adjuvant toxicity or pain, including behavioral changes, loss of appetite, dehydration, weight loss, piloerection, and local skin reactions. No toxicities were noted in either the GPI-0100 or CpG vaccinated mice.
2.4. In vivo cytolytic activity
Seven days after the last vaccination, mice (5/group) were injected intravenously under inhalation anesthesia with 10×106 CFSE-labeled naïve splenocytes that were pulsed with 0.5 μg/mL HPV16 E7(49–57) peptide or an irrelevant control peptide. E7-pulsed target cells were labeled with 10 μM CFSE (Vybrant Carboxyfluorescein diacetate succinimidyl ester (CFDA SE) Cell Tracer Kit, Thermo Fisher Scientific, Carlsbad, CA). Control peptide-pulsed cells were labeled with 0.66 μM CFSE. After extensive washing in PBS, cells were mixed in a 1:1 ratio prior to injection. Twenty hours after injection into vaccinated mice, splenocytes were isolated and analyzed by flow cytometry. Percentage of remaining CFSEhi and CFSElow cells were analyzed using FlowJo flow cytometry analysis software (Becton, Dickinson & Company, Ashland, OR). Five thousand CFSE+ events were collected. Peptide specific lysis was calculated with reference to the loss of the CFSEhi target cell population using the following calculation: Percent Specific Lysis = [(%CFSEhi−%CFSElow)]/(%CFSEhi)] × 100.
2.5. CD107 degranulation assay and intracellular cytokine staining
Ten days after the last vaccination, splenocytes were isolated from mice (5/group) and stimulated in vitro with 5 μg/mL HPV16 E7(49–57) peptide or control peptide in the presence of 10 μg/mL GolgiPlug (brefeldin A), 10 μg/mL GolgiStop (monensin) and antibodies to CD107a and CD107b (BD Biosciences) for 6 hours at 37°C, 5% CO2 in a humidified incubator. After incubation, cells were washed and stained with the Zombie Aqua viability dye and antibodies against CD3, CD4, and CD8. CD11b and B220/CD45R staining was used to exclude non-T cells from analysis. Following fixation and permeabilization with BD Cytofix/Cytoperm, cells were stained with IFNγ, TNFα, and IL-2 antibodies or isotype controls. Data acquisition was performed on a BD FACSCanto II flow cytometer (BD Biosciences). Flow data were analyzed utilizing FlowJo software (ver. 10). Gating was set based on isotype control staining.
2.6. Tumor infiltrating lymphocyte (TIL) phenotyping
Tumor tissue was isolated from mice (12–14/group) and processed into single cell suspensions using a mouse tumor dissociation kit with the GentleMACS system (Miltenyi, Auburn, CA) according to manufacturer’s instructions. Isolated TILs were stained with Zombie Aqua to exclude dead cells, then stained for surface antigens. For intracellular FoxP3 staining, cells were permeabilized overnight at 4°C using FoxP3 intracellular stain fixing and permeabilization buffer (ThermoFisher Scientific). The following day, cells were washed in permeabilization buffer and stained with FoxP3 antibody or isotype control antibody. A minimum of 20,000 CD45+ events were acquired on a BD FACSCanto II cytometer. Flow data were analyzed utilizing FlowJo software (ver. 10).
2.7. Statistical Analysis
All statistical analyses were performed using GraphPad Prism version 8.1.0 for Windows (GraphPad Software Inc., San Diego, CA). In vitro immunoassay data were analyzed using a one-way analysis of variance (ANOVA) followed by a Tukey’s multiple comparisons test. Survival data were analyzed by a log rank Mantel-Cox test. Spearman rank-order correlation was used to analyze the association of in vivo lytic activity and CD107 degranulation in antigen-specific T cells. P values <0.05 were considered significant.
3. Results
3.1. TVGV-1 vaccine adjuvanted with either CpG or GPI-0100 induces polyfunctional HPV16 E7-specific CD8+ T cells with in vivo lytic activity
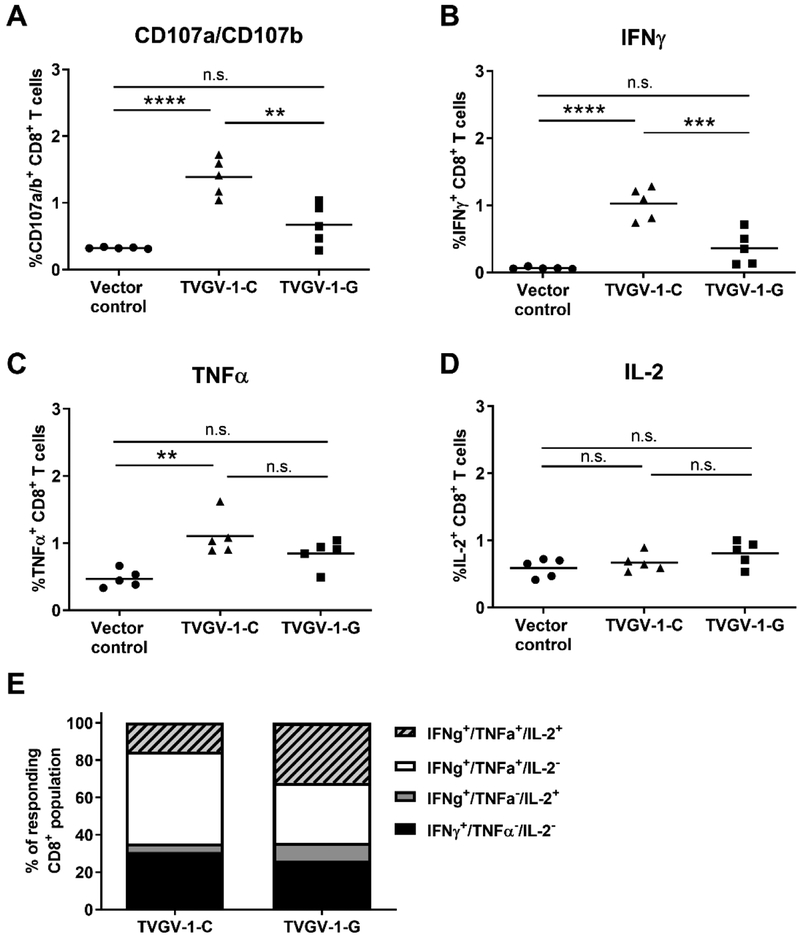
To assess the effect of using either CpG adjuvant or GPI-0100 adjuvant on the immunogenicity of the TVGV-1 (PEΔIII-E7-KDEL3) protein vaccine, we immunized mice three times, one week apart, with 100 μg TVGV-1 formulated with either 50 μg CpG adjuvant (TVGV-1-C) or 100 μg GPI-0100 (TVGV-1-G) or vector control with no adjuvant. The doses of adjuvant were selected based on previous reported literature directly comparing adjuvant properties of CpG ODN 1826 and GPI-0100 [13], as well as the known MTD in mice for GPI-0100, and the manufacturer recommended dose for CpG (www.invivogen.com). These adjuvant doses result in optimal immunogenicity of the TVGV-1 protein vaccine. Immunogenicity was evaluated in vitro 10 days after the last vaccination. Splenocytes were tested for the presence of antigen-specific CD8+ T cells using in vitro stimulation with the immunodominant HPV16 E7(49–57) peptide and flow cytometry to analyze CD107a/CD107b (LAMP-1/LAMP-2) expression as a marker for cytolytic degranulation and the presence of secreted effector cytokines IFNγ TNFα, and IL-2 by intracellular cytokine staining. TVGV-1 vaccine adjuvanted by CpG induced significantly higher frequencies of HPV16E7-specific CD8+ T cells in vaccinated mice compared to TVGV-1 vaccine adjuvanted by GPI-0100. This could be seen for expression of degranulation marker CD107, as well as IFNγ production in response to E7 peptide stimulation (Fig. 1a–b). TVGV-1-C vaccination resulted in a significant increase in E7-specific TNFα-secreting CD8+ T cells compared to the vector control (Fig. 1c). IL-2 expression in vaccinated mice was low and not significantly increased when compared to the vector control (Fig. 1d).
Figure 1. TVGV-1 vaccination induces multi-functional cytolytic and effector cytokine producing HPV-specific CD8+ T cells.
(A-D) Splenocytes from vaccinated mice (5/group) were stimulated with HPV16 E7(49–57) peptide and tested for the ability to express surface CD107a/CD107b, a marker for lytic degranulation (panel A), and produce the effector cytokines IFNγ (panel B), TNFα (panel C), and IL-2 (panel D). Percentage of CD8+ T cells expressing each marker is shown within gated CD8+ T cells. (E) The proportion of polyfunctional cytokine-secreting cells was analyzed through sequential flow cytometry gating and expressed as a percentage of the total HPV16 E7-specific responding CD8+ T cell population. IFNγ secreting cells were assessed for simultaneous secretion of TNFα and IL-2. Percentages from each mouse were averaged to generate proportions. Significance was determined by a one-way ANOVA followed by Tukey’s multiple-comparisons test.**p<0.01, ***p<0.001, ****p<0.0001, n.s., non-significant.
The hallmark cytokine produced by antigen-specific CD8+ T cells is IFNγ; however, it is believed that T cells that secrete more than one effector cytokine are functionally superior in their anti-tumor and anti-viral effects. Therefore, responding T cells from vaccinated mice were analyzed for simultaneous effector cytokine production. As a proportion of the responding CD8+ T cell population, more than 60% of effector T cells produced both IFNγ and TNFα in both TVGV-1 vaccinated groups (Fig. 1e). The GPI-0100 adjuvanted vaccine (TVGV-1-G) induced more IFNγ+/TNFα+/IL-2+-producing cells proportionally compared to the CpG-adjuvanted vaccine (TVGV-1-C). The presence of E7-specific T cells secreting IFNγ and IL-2 together in the absence of TNFα was infrequent and responding cells were more likely to secrete IFNγ alone (IFNγ+/TNFα−/IL-2−) or secrete both IFNγ and TNFα regardless of IL-2 production. These results indicate that although CpG-adjuvanted TVGV-1 induces a higher frequency of E7-specific T cells, both adjuvants are able to assist the immune response in inducing polyfunctional T cells.
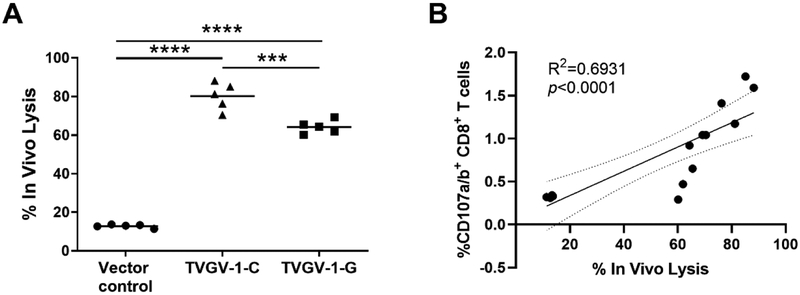
The CD107 mobilization-degranulation assay indicated that TVGV-1 vaccinated mice could be highly lytic. Therefore, we subsequently tested the in vivo cytotoxic activity of T cells induced after vaccination. Differentially-labeled CFSEhi and CFSElow target cells labeled with E7 peptide or an irrelevant peptide, respectively, were injected into vaccinated mice 7 days after the last injection. After 20 hours, loss of the E7-labeled CFSE hi population compared to control was evaluated by flow cytometry and used as a measure of the in vivo lysis capacity of vaccine-induced HPV-specific T cells. Both TVGV-1-C and TVGV-1-G vaccinated groups demonstrated significant in vivo lysis compared to the vector control group which shown minimal in vivo lysis of target cells (p<0.0001) (Fig. 2a). Mice immunized with TVGV-1-C demonstrated significantly higher in vivo lysis compared to mice immunized with TVGV-1-G, which was consistent with the frequency of CD107+ degranulating lytic T cells (Fig. 1a). Strong positive correlation was observed between in vivo lytic activity and the percentage of degranulating CD107 CD8+ T cells (R2=0.6931, Fig. 2b). Vector control immunized mice show very little loss of CFSEhi cell population. Background loss of CFSEhi cells may be related to spontaneous cell death due to the high amount of CFSE label. Overall, TVGV-1-C immunization resulted in the greatest differentiation of phenotypically desirable polyfunctional, cytotoxic T cells.
Figure 2. CpG and GPI-0100 adjuvanted TVGV-1 vaccine induce HPV16 E7-specific T cells with efficient in vivo cytolytic activity.
(A) Mice (5/group) were vaccinated three times, one week apart, with 100 μg TVGV-1-C, TVGV-1-G, or TVGV-vector control. CFSE-labeled peptide-loaded target cells (equal numbers of E7(49–57) peptide-loaded CFSE-hi and control peptide loaded CFSE-low) were injected into vaccinated or control mice 7 days after the last vaccination. Twenty hours later, splenocytes were harvested and analyzed by flow cytometry for the proportion of target cells remaining. In vivo cytotoxic activity (% in vivo lysis) is based on calculated percentage loss of the CFSE-hi target cell population. Each point represents an individual mouse. Horizontal line indicates group mean. Significance was determined by a one-way ANOVA followed by Tukey’s multiple-comparisons test. ***p<0.001, ****p<0.0001. (B) Spearman correlation analysis of E7-specific in vivo lytic activity with CD107a/b degranulation for all vaccine cohorts. High in vivo lytic activity is significantly associated with increased in vitro T cell degranulation (R2=0.6931, p<0.0001).
3.2. Therapeutic vaccination of tumor-bearing mice with TVGV-1 vaccine adjuvanted with either CpG or GPI-0100 induces tumor regression and increases overall survival
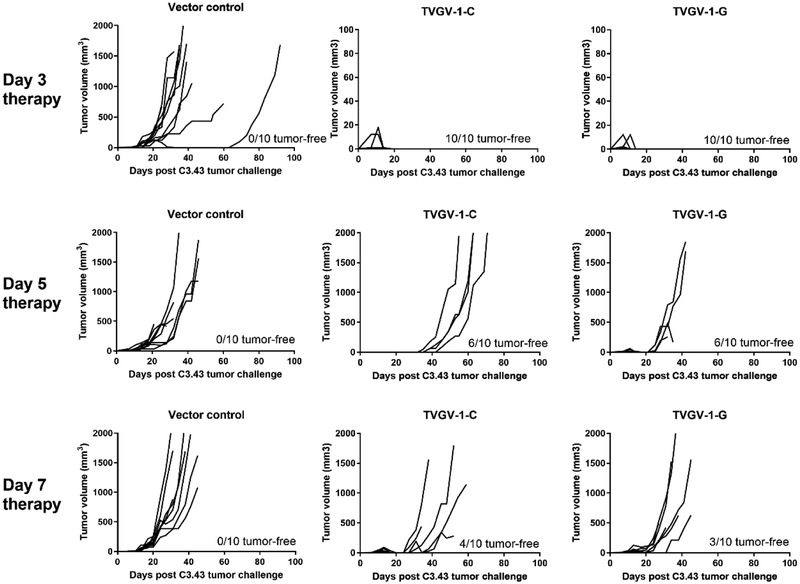
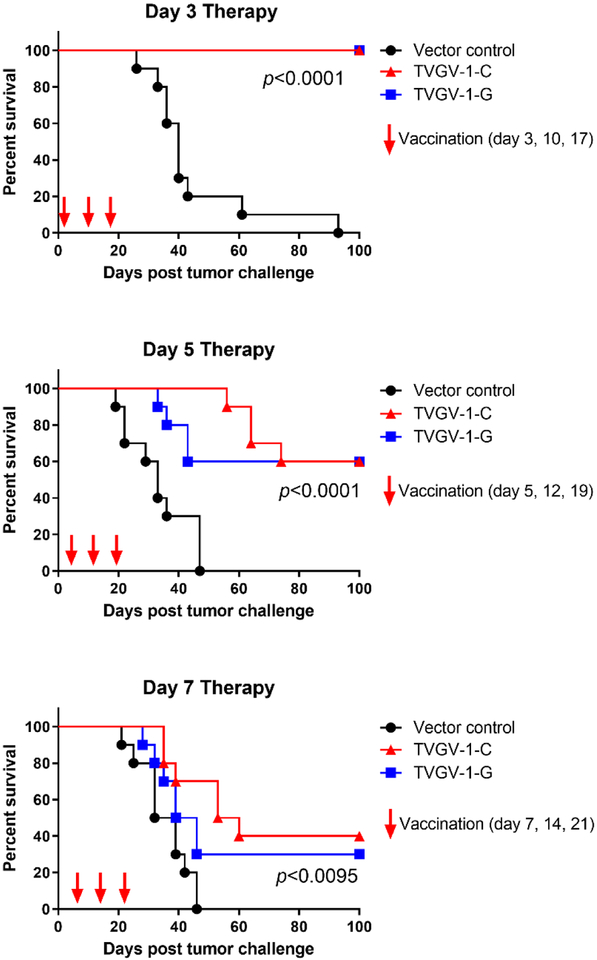
In order to investigate the therapeutic efficacy of TVGV-1 vaccination on established tumors, we used the HPV16-induced C3.43 tumor model. Mice were implanted with 1×105 C3.43 tumor cells subcutaneously in the rear flank and immunized either 3, 5, or 7 days post-tumor engraftment to test vaccine efficacy with the two different adjuvants with the expectation that more advanced day 7 established tumors might be more difficult to treat than earlier engrafted tumors. Mice were boosted two additional times one week apart for a total of 3 doses of vaccine per regimen. For mice immunized on days 3, 10 and 17, both CpG-adjuvanted and GPI-0100-adjuvanted TVGV-1 vaccine resulting in 100% tumor regression within 18 days, whereas all control treated mice developed progressive tumors over time (p<0.0001) (Fig. 3, top panel). Several mice developed small but measurable tumors in the vaccine groups, which subsequently regressed over the course of the vaccine regimen, resulting in 100% survival of all mice treated with TVGV-1 with either adjuvant (Fig. 4, top). For mice immunized on days 5, 12, and 19, the two groups immunized with either TVGV-1-C and TVGV-1-G showed 60% tumor regression while 4/10 mice developed progressing tumors (Fig. 3, middle panel). Although tumors in TVGV-1-C treated mice grew at a slower rate compared to TVGV-1-G vaccinated mice, overall long term survival and tumor-free percentage of both groups was 60% (p<0.0001) (Fig. 4, middle). For mice immunized on days 7, 14 and 21, sixty-percent (6/10) of mice treated with TVGV-1-C vaccine developed progressive tumors. Seventy-percent (7/10) of mice treated with TVGV-1-G vaccine developed progressive tumors (Fig. 3, bottom panel). The remaining tumors regressed for overall survival percentages between 30–40% (p<0.0095) (Fig. 4, bottom). As was expected, earlier vaccination results in more significant tumor therapy and prolonged survival compared to treatment at advanced growth stages. Importantly, all TVGV-1 vaccinated groups, regardless of adjuvant used or the start day of treatment, showed induction of significant anti-tumor effects resulting in a survival advantage over vector control treated mice. Although there were no significant differences in survival between the CpG adjuvant group and the GPI-0100 adjuvant group for all treatment cohorts, the day 5 and day 7 therapy cohort results suggest a trend towards the TVGV-1 vaccine adjuvanted with CpG having slightly more therapeutic efficacy than the vaccine adjuvanted with GPI-0100, which is likely related to the higher frequency of antigen-specific T cells that are generated with in vivo lytic capabilities.
Figure 3. TVGV-1 vaccination induces tumor regression in mice vaccinated therapeutically at day 3, 5, or 7 with TVGV-1-C, TVGV-1-G vaccines in the C3.43 HPV16-induced mouse tumor model.
Mice (10/group) were challenged with 1 × 105 C3.43 tumor cells s.c. in the flank. At the indicated time after challenge, mice were vaccinated three times, one week apart, with 100 μg TVGV-vector control, TVGV-1-C, or TVGV-1-G. Individual tumor volumes are shown for each group. Number of tumor free mice at the end of the study is indicated in each graph.
Figure 4. TVGV-1 vaccination increases overall survival in mice vaccinated therapeutically at day 3, 5, or 7 with TVGV-1-C, TVGV-1-G vaccines in the C3.43 HPV16-induced mouse tumor model.
Mice (10/group) were vaccinated at the indicated days post tumor challenge as described in Figure 3 and followed for up to 100 days. Mice euthanized per protocol were marked as dead the following day. Significance was calculated using the log rank (Mantel-Cox) test for survival. Overall P-values (all significant) are indicated in each graph. Differences in survival between TVGV-1-C and TVGV-1-G vaccinated groups were non-significant for all therapy cohorts.
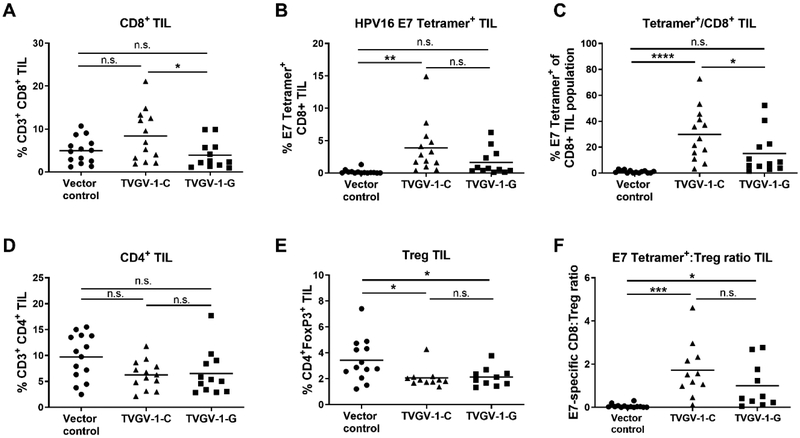
3.3. TVGV-1 vaccination results in increased lymphocyte effector cells within in the tumor microenvironment
In addition to induction of systemic cellular immune responses, an effective therapeutic vaccine should trigger migration of antigen-specific CD8+ T cells to the tumor site. Since tumor regression of more advanced tumors was less frequent than smaller tumors, we next aimed to characterize the tumor TIL populations from mice whose tumors were not cleared in order to potentially gain a mechanistic understanding of why day 7 therapy was suboptimal. Mice (15/group) were challenged with 1×105 C3.43 tumor cells and vaccinated on days 7, 14, and 21. Progressively growing tumors were harvested between days 30–60 post challenge, depending on their growth rate. The presence of CD8+ T cells, CD4+ T cells, regulatory T cells (Tregs), and HPV16 E7-tetramer specific T cells was evaluated by flow cytometry analysis of all CD45+ hematopoietic TIL after tumor tissue dissociation. In tumor-bearing mice that received the TVGV-1-C vaccine, we observed a significantly greater increase in the overall percentage of CD8+ TIL and in the HPV16 E7-specific CD8+ TIL population compared to mice that received the TVGV-1-G vaccine (Fig. 5a, 5b). Notably, of the infiltrating CD8+ T cells, a significant percentage were E7(49–57)-tetramer positive (Fig. 5c), whereas no significant tetramer positive cells were observed in the vector control mice. There were no significant changes in the overall frequency of CD4+ TIL (Fig. 5d), however, both TVGV-1-C and TVGV-1-G vaccinated groups demonstrated a significant decrease in the frequency of CD4+FoxP3+ Tregs compared to vector control (Fig. 5e). Both TVGV-1-C and TVGV-1-G vaccinated mice demonstrated statistically significant increases in the ratio of E7-specific CD8+ T cells to Tregs in the tumor microenvironment (Fig. 5f), which is often observed to be prognostically favorable for tumor clearance both in animal tumor models and in human cancers. These results suggest that even though TVGV-1 therapeutic vaccination is suboptimal in a subset of mice bearing advanced tumors, vaccination with TVGV-1 induces HPV-specific T cells that are capable of migrating to and infiltrating the tumor site, although it is unclear whether they are still functional or have been suppressed by other cells and mechanisms in the local tumor microenvironment.
Figure 5. TVGV-1 therapeutic vaccination induces changes in the immune phenotype of tumor-infiltrating lymphocytes (TIL) in mice vaccinated one week post C3.43 tumor challenge.
Mice (15/group) were challenged with C3.43 tumor cells s.c. in the flank. Starting at day 7, mice were vaccinated three times, one week apart, with 100 μg TVGV-vector control, TVGV-1-C, or TVGV-1-G. Tumors from tumor-bearing mice were harvested between days 30–60 when mice were euthanized due to increasing tumor size or tumor ulceration. TIL were evaluated by flow cytometry for indicated cell populations. (A) Shown is the percentage of CD8+ T cell infiltration of CD45+ cells into tumors in treated mice. (B) Shown is the percentage of HPV16 E7(49–57) MHC tetramer positive CD8 T cell infiltration of CD45+ cells in tumors of treated mice. (C) Shown is the proportion of HPV16 E7(49–57)-tetramer positive cells within the CD8+ T cell subset. (D) Shown is the percentage of CD4+ T cell infiltration of CD45+ cells into tumors in treated mice. (E) Shown is the percentage of Treg (CD4+FoxP3+) cell infiltration of CD45+ cells into tumors of treated mice. (F) Shown is the ratio of E7-specific CD8+ T cells to Tregs in treated mice. Each point represents a single tumor. Horizontal line indicates group mean. *p<0.05, **p<0.01, ****p<0.0001 determined by one-way ANOVA followed by Tukey’s multiple comparisons test. n.s., non-significant.
4. Discussion
Antigenic proteins linked to bacterial exotoxins combined with strong immune adjuvants provides a potential platform for increasing immunogenicity and therapeutic efficacy of proteins that alone do not generate a strong enough immune response to show significant anti-tumor activity. The P. aeruginosa exotoxin A domains I and II have unique cell binding and translocation functions that allow them to transport covalently linked proteins into the cytosol of professional APCs when injected in vivo [25]. Deletion of the ADP-ribosylating catalytic domain III renders the exotoxin non-cytopathic to cells and greatly reduces associated toxicity [9, 26]. The KDEL ER retention domain of the TVGV-1 fusion protein drives the E7 antigen into the cross presentation pathway for MHC class I, improving antigen-specific immune responses [8]. Prior studies have shown the importance of directing antigen into the cytosol and ER for generation of anti-HPV16 E6 and/or E7 tumor immunity using various strategies, including the use of heat shock protein fusions, calreticulin, and Bordatella pertussis adenylate cyclase, for example [27–29]. The goal of this study was to investigate the effect of combining TVGV-1 (PE ΔIII-E7-KDEL3) with two different immunostimulatory adjuvants and to compare immunogenicity and anti-tumor activity of the resulting immune response in the established HPV16-induced C3.43 tumor model. Our results demonstrate that TVGV-1 protein vaccine adjuvanted with either CpG or the GPI-0100 adjuvant induces a high frequency of tumor-infiltrating E7-specific CD8+ T cells. Furthermore, both adjuvants are able to assist the immune response in inducing polyfunctional cytokine-secreting lytic T cells that show substantial therapeutic efficacy against well-established C3.43 tumors as a proof of concept. The E6 antigen in this mouse model and in others (TC-1) is less important for tumor rejection, since E7-derived peptides are more immunogenic in C57BL/6 mice based on H2-Kb and H2-Db MHC peptide binding [23]. Indeed, Chen et. al. reported that vaccination with a PEΔIII-E7-KDEL3 monovalent fusion protein could elicit better immune and anti-tumor immune responses compared to a PE ΔIII-E6-KDEL3 monovalent fusion protein in the TC-1 tumor model [30]. However, the authors also demonstrated that combining the two vaccines (E6 + E7) could produce synergistic effects leading to enhanced survival in tumor-bearing mice. Because both E6 and E7 play important roles in HPV-associated carcinogenesis in humans, a therapeutic vaccine strategy based on this reported platform would benefit from inclusion of both viral oncogenes as target tumor antigens if being developed clinically.
Many adjuvants have been tested in human clinical trials, however the only ones approved for human use are aluminum salts (Alum), oil-in-water emulsions (MF59, AS03), liposome-based virosomes, and various combinations of immunostimulants [31]. The AS01 adjuvant system is a combination of saponin QS-21 and TLR4 agonist monophosphoryl lipid A (MPLA) with liposomes currently FDA approved for use in the Herpes zoster vaccine (SHINGRIX, GlaxoSmithKline) and approved for use in Europe for the world’s first malaria vaccine (Mosquirix™) [32]. AS04 combines MPLA and Alum to induce a Th1-biased immune response and is currently used in a HBV vaccine and HPV vaccine developed by GlaxoSmithKline [33]. Other adjuvants in clinical development but not yet FDA-approved include flagellin (TLR5 agonist), Poly I:C (TLR3 agonist), imidazoquinolines (TLR7/8 agonist), saponins (Quil A, GPI-0100), bacterial toxins and cytokine mixtures [10]. Although imiquimod (TLR7/8 agonist, Aldara™) is not approved as a vaccine adjuvant, it is approved by the FDA as a stand-alone topical treatment for actinic keratosis, superficial basal cell carcinoma and external genital warts. Saponin-based adjuvants, such as Quil A, its derivative QS-21, and now the semi-synthetic GPI-0100, are unique in their capacity to induce and enhance Th1 immune responses and generate CTLs against exogenous antigens, an essential characteristic with respect to vaccination against intracellular pathogens or to enhance therapeutic cancer vaccines [34]. Because the undesirable toxic and hemolytic properties of saponin-based adjuvants have been removed, it has opened the door for their safe use for human and veterinary applications [35–38]. In practice, Evans et. al. showed that use of the QS-21 adjuvant allows for reduced antigen doses to elicit similar titers of an anti-HIV gp120 subunit vaccine compared to use of no adjuvant, providing a means for dose reduction of immunogen for vaccination [39]. Consistent with other studies using GPI-0100 as an adjuvant, we show that the GPI-0100-adjuvanted TVGV-1 vaccine stimulated an anti-tumor CD8+ T cell response characterized by IFNγ and TNFα secretion supporting a Th1-biased immune response, and furthermore we did not observe any toxicity associated with its use. In several clinical trials, GPI-0100 adjuvant has been administered to humans without significant toxicity, primarily eliciting mild to moderate injection site reactions [18, 40, 41].
Interestingly, our data showed that CpG-adjuvanted TVGV-1 vaccine stimulated an anti-tumor CD8+ T cell response that was similar, but slightly more effectively, than GPI-0100-adjuvanted TVGV-1. Differences in the quantity and quality of observed anti-E7 immune responses in this study between the two vaccine formulations are presumed to be related to the different mechanism of action of each adjuvant since the immunogen was the same in each group. Adjuvants can have very profound effects on the nature of immune responses with some adjuvants favoring humoral and Th2-biased responses, and others favoring Th1 and CTL production. While both GPI-0100 and CpG trigger immune responses characterized by strong Th1, CTL, antibody responses, and activation of NK cells, they do so through different cellular interactions [10]. GPI-0100, like other saponins, targets antigen processing through enhanced antigen uptake by APC via endocytosis, as well as providing direct stimulation of T cells through a lipophilic acyl group moiety, but which has been rendered non-toxic in GPI-0100 compared to its natural counterpart [12]. In contrast, CpG ODNs target TLR9, a receptor for conserved pathogen-associated molecular patterns (PAMPS), which when engaged causes downstream signaling events that lead to activation of NFκB and IRF3, followed by transcriptional activation and expression of proinflammatory cytokines, including type I interferons [21]. In humans, the primary cell targets that express TLR9 are B cells and plasmacytoid dendritic cells (pDCs), while in mice, TLR9-expressing cells also include monocytes, macrophages, and conventional DCs [21]. CpG 1018 is currently FDA approved as an adjuvant in the HEPLISAV-B hepatitis B vaccine (Dynavax) [42]. Importantly, a number of phase I-III clinical trials support the therapeutic potential of CpG ODNs for cancer vaccines [10, 43]. In a phase I study in 41 patients with chronic lymphocytic leukemia, a single intravenous dose of CpG 7909 was well tolerated with no significant toxicity up to 1.05 mg/kg, and a maximum tolerated dose of 0.45 mg/kg was found when delivered subcutaneously [44]. In a phase III study in non-small-cell lung cancer, CpG 7909 administration (0.2 mg/kg subcutaneous) in combination with chemotherapy, common CpG-related reported adverse events were mild to moderate injection site reactions and flu-like symptoms, which would be expected from CpG’s immune stimulating activity [45].
Importantly, our data showed that GPI-0100 and CpG-adjuvanted TVGV-1 were both able to induce polyfunctional E7-specific CD8+ T cells characterized by cytokine secretion of IFNγ, TNFα and IL-2 and degranulation of the lysosomal marker CD107, a marker for lytic activity. While CpG-immunized mice showed twice the level of CD107 degranulation as GPI-0100-immunized mice by intracellular cytokine staining, in vivo CTL lysis differences, although significant, were not as prominent. These data do indicate, however, that the two adjuvants are not functionally equivalent in providing adjuvant activity to TVGV-1. The differences in the induced immune responses might indeed be related to stronger activation of the proinflammatory response through CpG-induced TLR9 activation, as we observed higher numbers of E7-specific T cells, greater anti-tumor efficacy, and even within mice whose tumors were not controlled, there were greater frequencies of E7-specific TIL within the CD8+ TIL population. Understandably, combining adjuvants has been proposed as a strategy to improve desired immune responses compared to single-agent adjuvant in order to derive benefit from the differential immunostimulatory properties. For example, the saponin-based adjuvant QS-21 was tested in twelve different adjuvant combinations by Kim et. al. to determine which combination induced a more potent immune response to two cancer antigens GD3 and MUC1 [13]. Their results revealed five adjuvant combinations that showed superiority in augmenting antibody responses to these antigens with a strong correlation to a Th1 response, one combination in particular being QS-21 + CpG ODN 1826. Similarly, the AS15 adjuvant system (MPLA, QS-21, and CpG 7909; GlaxoSmithKline) was chosen for its immunostimulatory activity to support clinical development of melanoma immunotherapeutic vaccines with human safety results being within acceptable limits [33, 46–48]. While we did not test adjuvant combinations in this study, it remains an intriguing proposition to drive optimal immunogenicity with poorly immunogenic protein vaccines.
Previous studies testing the PE ΔIII-E7-KDEL3 vaccine in the TC-1 HPV16-transformed tumor model has shown vaccine efficacy in the preventative setting in the absence of adjuvant [8]. Liao et. al. demonstrated that delivery of the E7 protein alone does not induce any anti-tumor respon se. Similarly, fusion of only the PE ΔIII domain to E7 was suboptimal for inducing a T cell response. Only after adding the KDEL ER targeting signal was substantial anti-E7 immunity induced, and only after three doses were administered. These responses were sufficient in quantity to reduce the number of pulmonary metastasis of TC-1 cells when mice were vaccinated two days after tumor challenge [8]. For this study we tested vaccine efficacy in the C3.43 tumor model in which the entire native HPV16 genome was used to generate the tumor cell line [23]. Importantly, expression of E7 protein is under physiologic regulation and is likely similar to the low levels of expression found in HPV16-infected human tissue [49]. Non-optimized codon usage in the papillomavirus genome keeps viral protein expression low in mammalian cells to avoid triggering immune detection [50, 51]. Indeed, E7-specific CD8+ T cells isolated from TriVax-HPV vaccinated mice were 50% less activated by C3.43 tumor cell stimulation compared to TC-1 tumor cells, some of which was partially rescued by IFNγ-mediated upregulation of MHC class I expression on the surface of C3.43 cells [52]. These data indirectly suggest that C3.43 cells express less E7 peptide MHC complexes on the surface compared to TC-1 cells, and therefore may require a more robust immune response to see similar tumor regression efficacy in vivo with any given therapeutic vaccine.
Tumor burden also plays a role in determining vaccine and adjuvant efficacy, since more established tumors are generally more difficult to treat. This is reflected in our study when we compare the overall tumor burden and survival of day 3 therapeutically treated mice to day 7 treated mice. Despite the observation that a few mice are not able to clear tumors completely, we do see that the majority of vaccinated mice in both TVGV-1-C and TVGV-1-G vaccinated groups develop very robust anti-tumor immunity capable of clearing pre-established tumors or significantly delaying their growth, resulting in a significant survival advantage.
In summary, targeting the MHC class I cross-presentation pathway using the translocating properties of bacterial exotoxin in an HPV16 E7 KDEL fusion protein adjuvanted with either CpG or GPI-0100 results in a strongly immunogenic antigen-specific T cell profile that demonstrates therapeutic efficacy in a very aggressive pre-clinical HPV-driven tumor model in which expression of the E7 antigen is physiologically expressed under the natural viral promoter, similar to that which would be found in virally-induced human cancers. Therefore, vaccination with the TVGV-1 construct in combination with adjuvant represents a viable therapeutic vaccine for the treatment of HPV-associated tumors.
Acknowledgements
This study was funded by TheVax Genetics Vaccine Co., Ltd. The C3.43 tumor model was developed with support from National Institutes of Health (NIH) grant R01 CA074397 (to W.M.K). W.M.K. holds the Walter A. Richter Cancer Chair. We thank the Norris Comprehensive Cancer Center Beckman Center for Immune Monitoring (supported in part by the NIH Cancer Center Support Grant P30CA014089) for technical assistance. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
DMD, JGS, ECJ, KPL, and WMK have no competing interests to declare. JMW, CMW and KH are employees of TheVax Genetics Vaccine Co., Ltd. The funder, TheVax Genetics Vaccine Co., Ltd, played a role in study design and decision to submit article for publication.
References
- [1].Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30 Suppl 5:F12–23. [DOI] [PubMed] [Google Scholar]
- [2].de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. [DOI] [PubMed] [Google Scholar]
- [3].Serrano B, de Sanjose S, Tous S, Quiros B, Munoz N, Bosch X, et al. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur J Cancer. 2015;51:1732–41. [DOI] [PubMed] [Google Scholar]
- [4].Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–75. [DOI] [PubMed] [Google Scholar]
- [5].Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30 Suppl 5:F55–70. [DOI] [PubMed] [Google Scholar]
- [6].Skeate JG, Woodham AW, Einstein MH, Da Silva DM, Kast WM. Current therapeutic vaccination and immunotherapy strategies for HPV-related diseases. Hum Vaccin Immunother. 2016;12:1418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chabeda A, Yanez RJR, Lamprecht R, Meyers AE, Rybicki EP, Hitzeroth II. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018;5:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liao CW, Chen CA, Lee CN, Su YN, Chang MC, Syu MH, et al. Fusion protein vaccine by domains of bacterial exotoxin linked with a tumor antigen generates potent immunologic responses and antitumor effects. Cancer Res. 2005;65:9089–98. [DOI] [PubMed] [Google Scholar]
- [9].Goletz TJ, Klimpel KR, Leppla SH, Keith JM, Berzofsky JA. Delivery of antigens to the MHC class I pathway using bacterial toxins. Human Immunol. 1997;54:129–36. [DOI] [PubMed] [Google Scholar]
- [10].Dubensky TW Jr., Reed SG. Adjuvants for cancer vaccines. Semin Immunol. 2010;22:155–61. [DOI] [PubMed] [Google Scholar]
- [11].Marciani DJ, Press JB, Reynolds RC, Pathak AK, Pathak V, Gundy LE, et al. Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine. 2000;18:3141–51. [DOI] [PubMed] [Google Scholar]
- [12].Marciani DJ, Reynolds RC, Pathak AK, Finley-Woodman K, May RD. Fractionation, structural studies, and immunological characterization of the semi-synthetic Quillaja saponins derivative GPI-0100. Vaccine. 2003;21:3961–71. [DOI] [PubMed] [Google Scholar]
- [13].Kim SK, Ragupathi G, Cappello S, Kagan E, Livingston PO. Effect of immunological adjuvant combinations on the antibody and T-cell response to vaccination with MUC1-KLH and GD3-KLH conjugates. Vaccine. 2000;19:530–7. [DOI] [PubMed] [Google Scholar]
- [14].Ragupathi G, Coltart DM, Williams LJ, Koide F, Kagan E, Allen J, et al. On the power of chemical synthesis: immunological evaluation of models for multiantigenic carbohydrate-based cancer vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang P, Yang QB, Marciani DJ, Martin M, Clements JD, Michalek SM, et al. Effectiveness of the quillaja saponin semi-synthetic analog GPI-0100 in potentiating mucosal and systemic responses to recombinant HagB from Porphyromonas gingivalis. Vaccine. 2003;21:4459–71. [DOI] [PubMed] [Google Scholar]
- [16].Kensil CR, Wu JY, Soltysik S. Structural and immunological characterization of the vaccine adjuvant QS-21. Pharm Biotechnol. 1995;6:525–41. [DOI] [PubMed] [Google Scholar]
- [17].Wu JY, Gardner BH, Murphy CI, Seals JR, Kensil CR, Recchia J, et al. Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J Immunol. 1992;148:1519–25. [PubMed] [Google Scholar]
- [18].Slovin SF, Ragupathi G, Fernandez C, Jefferson MP, Diani M, Wilton AS, et al. A bivalent conjugate vaccine in the treatment of biochemically relapsed prostate cancer: a study of glycosylated MUC-2-KLH and Globo H-KLH conjugate vaccines given with the new semi-synthetic saponin immunological adjuvant GPI-0100 OR QS-21. Vaccine. 2005;23:3114–22. [DOI] [PubMed] [Google Scholar]
- [19].Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. [DOI] [PubMed] [Google Scholar]
- [20].Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–63. [DOI] [PubMed] [Google Scholar]
- [23].Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–9. [DOI] [PubMed] [Google Scholar]
- [24].Smith KA, Meisenburg BL, Tam VL, Pagarigan RR, Wong R, Joea DK, et al. Lymph node-targeted immunotherapy mediates potent immunity resulting in regression of isolated or metastatic human papillomavirus-transformed tumors. Clin Cancer Res. 2009;15:6167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jinno Y, Ogata M, Chaudhary VK, Willingham MC, Adhya S, FitzGerald D, et al. Domain II mutants of Pseudomonas exotoxin deficient in translocation. J Biol Chem. 1989;264:15953–9. [PubMed] [Google Scholar]
- [26].Chaudhary VK, Jinno Y, FitzGerald D, Pastan I. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huang CY, Chen CA, Lee CN, Chang MC, Su YN, Lin YC, et al. DNA vaccine encoding heat shock protein 60 co-linked to HPV16 E6 and E7 tumor antigens generates more potent immunotherapeutic effects than respective E6 or E7 tumor antigens. Gynecologic Oncol. 2007;107:404–12. [DOI] [PubMed] [Google Scholar]
- [28].Peng S, Tomson TT, Trimble C, He L, Hung CF, Wu TC. A combination of DNA vaccines targeting human papillomavirus type 16 E6 and E7 generates potent antitumor effects. Gene Ther. 2006;13:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Preville X, Ladant D, Timmerman B, Leclerc C. Eradication of established tumors by vaccination with recombinant Bordetella pertussis adenylate cyclase carrying the human papillomavirus 16 E7 oncoprotein. Cancer Res. 2005;65:641–9. [PubMed] [Google Scholar]
- [30].Chen S, Liao C, Lai Y, Fan Y, Lu G, Wang H, et al. De-oncogenic HPV E6/E7 vaccine gets enhanced antigenicity and promotes tumoricidal synergy with cisplatin. Acta Biochim Biophys Sin (Shanghai). 2014;46:6–14. [DOI] [PubMed] [Google Scholar]
- [31].Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw. 2015;15:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. New Engl J Med. 2015;372:2087–96. [DOI] [PubMed] [Google Scholar]
- [33].Garcon N, Di Pasquale A. From discovery to licensure, the Adjuvant System story. Hum Vaccin Immunother. 2017;13:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sun HX, Xie Y, Ye YP. Advances in saponin-based adjuvants. Vaccine. 2009;27:1787–96. [DOI] [PubMed] [Google Scholar]
- [35].Marciani DJ, Kensil CR, Beltz GA, Hung CH, Cronier J, Aubert A. Genetically-engineered subunit vaccine against feline leukaemia virus: protective immune response in cats. Vaccine. 1991;9:89–96. [DOI] [PubMed] [Google Scholar]
- [36].Newman MJ, Wu JY, Gardner BH, Anderson CA, Kensil CR, Recchia J, et al. Induction of cross-reactive cytotoxic T-lymphocyte responses specific for HIV-1 gp120 using saponin adjuvant (QS-21) supplemented subunit vaccine formulations. Vaccine. 1997;15:1001–7. [DOI] [PubMed] [Google Scholar]
- [37].Vandepapeliere P, Horsmans Y, Moris P, Van Mechelen M, Janssens M, Koutsoukos M, et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine. 2008;26:1375–86. [DOI] [PubMed] [Google Scholar]
- [38].Santos FN, Borja-Cabrera GP, Miyashiro LM, Grechi J, Reis AB, Moreira MA, et al. Immunotherapy against experimental canine visceral leishmaniasis with the saponin enriched-Leishmune vaccine. Vaccine. 2007;25:6176–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Evans TG, McElrath MJ, Matthews T, Montefiori D, Weinhold K, Wolff M, et al. QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immunization in humans. Vaccine. 2001;19:2080–91. [DOI] [PubMed] [Google Scholar]
- [40].Amato RJ, Shetty A, Lu Y, Ellis R, Low PS. A phase I study of folate immune therapy (EC90 vaccine administered with GPI-0100 adjuvant followed by EC17) in patients with renal cell carcinoma. J Immunother. 2013;36:268–75. [DOI] [PubMed] [Google Scholar]
- [41].Amato RJ, Shetty A, Lu Y, Ellis PR, Mohlere V, Carnahan N, et al. A Phase I/Ib study of folate immune (EC90 vaccine administered with GPI-0100 adjuvant followed by EC17) with interferon-alpha and interleukin-2 in patients with renal cell carcinoma. J Immunother. 2014;37:237–44. [DOI] [PubMed] [Google Scholar]
- [42].Jackson S, Lentino J, Kopp J, Murray L, Ellison W, Rhee M, et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine. 2018;36:668–74. [DOI] [PubMed] [Google Scholar]
- [43].Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Advanced Drug Delivery Rev. 2009;61:195–204. [DOI] [PubMed] [Google Scholar]
- [44].Zent CS, Smith BJ, Ballas ZK, Wooldridge JE, Link BK, Call TG, et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hirsh V, Paz-Ares L, Boyer M, Rosell R, Middleton G, Eberhardt WE, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:2667–74. [DOI] [PubMed] [Google Scholar]
- [46].Kruit WH, Suciu S, Dreno B, Mortier L, Robert C, Chiarion-Sileni V, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol. 2013;31:2413–20. [DOI] [PubMed] [Google Scholar]
- [47].Slingluff CL Jr., Petroni GR, Olson WC, Smolkin ME, Chianese-Bullock KA, Mauldin IS, et al. A randomized pilot trial testing the safety and immunologic effects of a MAGE-A3 protein plus AS15 immunostimulant administered into muscle or into dermal/subcutaneous sites. Cancer Immunol Immunother. 2016;65:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dreno B, Thompson JF, Smithers BM, Santinami M, Jouary T, Gutzmer R, et al. MAGE-A3 immunotherapeutic as adjuvant therapy for patients with resected, MAGE-A3-positive, stage III melanoma (DERMA): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:916–29. [DOI] [PubMed] [Google Scholar]
- [49].Isaacson Wechsler E, Wang Q, Roberts I, Pagliarulo E, Jackson D, Untersperger C, et al. Reconstruction of human papillomavirus type 16-mediated early-stage neoplasia implicates E6/E7 deregulation and the loss of contact inhibition in neoplastic progression. J Virol. 2012;86:6358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kanodia S, Fahey LM, Kast WM. Mechanisms used by human papillomaviruses to escape the host immune response. Curr Cancer Drug Targets. 2007;7:79–89. [DOI] [PubMed] [Google Scholar]
- [51].Steinbach A, Riemer AB. Immune evasion mechanisms of human papillomavirus: An update. International J Cancer. 2018;142:224–9. [DOI] [PubMed] [Google Scholar]
- [52].Barrios K, Celis E. TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer Immunol Immunother. 2012;61:1307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]