Abstract
Objective
This study examined the longitudinal effects of co-occurring psychosocial concerns, or syndemics, on HIV-positive sexual minority men’s likelihood of engaging in serodiscordant condomless anal sex (CAS), a health behavior with implications for personal and public health.
Methods
Participants included 390 HIV-positive sexual minority men from two prior secondary prevention trials. Over the course of the one-year data collection period (up to 5 observations per participant), participants completed self-report measures of CAS, as well as six syndemic factors: post-traumatic stress disorder, childhood sexual abuse, depression, anxiety, alcohol abuse, and poly sub stance/stimulant use. We employed multilevel modeling to examine the longitudinal additive effect of syndemics on serodiscordant CAS (binary) over the one-year period.
Results
The number of syndemic conditions was a significant predictor of CAS, with each additional syndemic associated with 1.41 greater odds of CAS (p = 0.0004; 95% CI [1.16, 1.70]). Both the between- (p = 0.0121, 95% CI [1.07, 1.69]) and within-person (p = 0.01, 95% CI [1.11, 2.10]) effects of syndemics were significant predictors, showing that an increase in the number of syndemic conditions across person and time both increased odds of CAS.
Conclusions
Interventions addressing HIV-positive sexual minority men’s sexual health behaviors should address the potential impact of co-occurring psychosocial concerns that affect these behaviors. This will benefit this population’s personal sexual health and reduce transmission of HIV and STIs among sexual minority men.
Keywords: HIV/AIDS, men who have sex with men/MSM, syndemic, sexual behavior, secondary HIV prevention
HIV disproportionately affects sexual minority men (men who identify as gay, bisexual, or another non-heterosexual identity) and other men who have sex with men (MSM) in the United States in terms of both incidence and prevalence. The most recent figures show that at year end of 2015, 59.5% people in the United States living with HIV were MSM and in 2016, 70% of newly diagnosed HIV cases in the United States were MSM (CDC, 2017). Additionally, rates of mental health and associated psychosocial problems among people living with HIV in the U.S. are disproportionately high (Bing et al., 2001; Brandt et al., 2017; Hartzler et al., 2017; O’Cleirigh, Magidson, Skeer, Mayer, & Safren, 2015).
Sexual minority men, regardless of HIV status, experience elevated rates of various mental health problems, including depression, anxiety, psychological distress, self-harm behavior, and alcohol and substance use, as compared to their heterosexual counterparts (Cochran, Greer, & Mays, 2003; King et al., 2008), which is likely due to sexual minority stress (Meyer, 2003). Some of these problems have been shown to be associated with each other and with adverse health behaviors and outcomes (Hirshfield, Remien, Humberstone, Walavalkar, & Chiasson, 2004; Mimiaga et al., 2009; O’Cleirigh et al., 2015; Reisner, Mimiaga, Safren, & Mayer, 2009; Stall et al., 2003; Williams et al., 2015; Wim, Christiana, & Marie, 2014).
A syndemic occurs when a constellation of psychosocial concerns co-occurs in a population and synergistically affects the odds of different health outcomes and health behaviors within that population (Singer & Clair, 2003; Singer et al., 2006). Syndemics theory states that when multiple conditions occur simultaneously, they can worsen the burden of disease on a population and amplify the negative consequences of the individual conditions through their interaction, ultimately heightening health disparities across social groups.
The frequency of syndemics among sexual minority men and their association to HIV acquisition risk behavior has drawn significant research attention. The majority of existing studies are cross-sectional and include samples of primarily HIV-negative individuals or MSM regardless of HIV status (Dyer et al., 2012; Ferlatte, Hottes, Trussler, & Marchand, 2014; Herrick et al., 2013; Mimiaga, Biello, et al., 2015; Mustanski, Garofalo, Herrick, & Donenberg, 2007; Parsons, Grov, & Golub, 2012; Parsons et al., 2017; Stall et al., 2003; Storholm, Satre, Kapadia, & Halkitis, 2016; Tulloch et al., 2015). However, some of these studies had self-reported HIV-positive status as an outcome (Mimiaga, Biello, et al., 2015; Mustanski et al., 2007; Parsons et al., 2012; Stall et al., 2003). More recently, longitudinal studies have examined the association between syndemics and behaviors that could lead to HIV transmission among HIV-negative sexual minority men. Among Thai MSM, a greater quantity of syndemic conditions (forced sex, suicidality, club drug use, alcohol intoxication, selling sex) predicted greater odds of incident HIV infection (Guadamuz et al., 2014). This was the first study to show longitudinal evidence of this syndemic effect. Another study found that the number of syndemic indicators (including depression, alcohol abuse, stimulant use, polysubstance use, and childhood sexual abuse) among MSM was additively associated with CAS and seroconversion over a four-year period (Mimiaga, O’Cleirigh, et al., 2015). Another longitudinal study of young MSM found that greater numbers of syndemics, including substance use, sexual orientation-based violence, and anxiety/depression, were associated with greater likelihood of CAS (Mustanski et al., 2017).
Fewer studies have examined syndemics in HIV-positive individuals. Three of these studies utilized medication adherence as outcomes (Blashill et al., 2015; Friedman et al., 2015; Harkness et al., 2018) and generally found that greater numbers of syndemics were associated with worse adherence. One study examined the association of syndemics and sexual behavior for adolescents living with HIV (not specifically MSM) and found that substance use, emotional distress, and lack of social support formed a syndemic predictive of condomless sex (van den Berg et al., 2017). However, no studies to the authors’ knowledge have examined longitudinally the association between syndemics and behaviors that could lead to HIV transmission, such as serodiscordant CAS, among sexual minority men living with HIV. This is an important area of research due to the disproportionate burden of HIV among sexual minority men, the high levels of syndemics in this population, and the potential for different syndemics to moderate the effects of existing secondary prevention interventions (Safren, Blashill, & O’Cleirigh, 2011). In order to improve and tailor these interventions to improve effectiveness, it is important to better understand and document the relationship between psychosocial syndemics and sexual health behaviors among this population. Therefore, this study assessed the longitudinal additive effects of six syndemic psychosocial concerns on the likelihood of serodiscordant CAS within a group of sexual minority men living with HIV. This study hypothesized that overall, greater numbers of syndemic conditions would contribute to increased likelihood of serodiscordant CAS over time.
Method
Participants
Participants are from two prior secondary prevention trials for sexual minority men (Safren, O’Cleirigh, Skeer, Elsesser, & Mayer, 2013; N = 390; Safren, O’Cleirigh, et al., 2011). The two trials, Project Enhance, an RCT in which the intervention was delivered by medical social workers) and a peer-delivered version of the same intervention, sought to reduce serodiscordant CAS among HIV-positive sexual minority men. Data for these studies were collected from 2004 to 2008. For Project Enhance, baseline condomless sex was part of the eligibility criteria; in contrast, baseline condomless sex was not required for the Peer intervention. All participants completed a baseline visit, at which point they provided informed consent and completed behavioral and psychosocial assessments. Participants in the peer-delivered trial all received the intervention, whereas those in the Project Enhance trial were randomized to either receive treatment or the control condition (treatment as usual in primary care). Participants who received the intervention sessions (Peer and Enhance treatment) covered a variety of topics, such as having sex, using substances, managing stress, exploring triggers for different sexual behaviors, understanding culture and identity, disclosing HIV status, and developing effective relationships (see Knauz et al., 2007 for a description of the interventions and their development). Following study completion, participants completed follow up visits every three months, up to one year, yielding five possible assessment points per participant. All sessions were completed at Fenway Community Health, an organization that provides services to HIV-positive and sexual and gender minority community members.
Measures
The same measures were administered in both of the studies from which the participant data was drawn.
Condomless Anal Sex (CAS)
Participants were asked how many times they had condomless anal sex with a negative or unknown status male partner in the past three months. Because responses to this question were highly zero inflated, we elected to treat this as a binary variable, such that participants were coded as either 0 (no CAS) or 1 (CAS) in the past three months.
Syndemics
Participants completed assessments of six possible co-occurring psychosocial syndemic concerns, including childhood sexual abuse (CSA), post-traumatic stress disorder (PTSD), anxiety disorders, depression, alcohol abuse, and polysubstance and/or stimulant use, which are described below. Syndemics scores reflect the total number of syndemic conditions for which participants met criteria at each time point. Because there were six syndemic conditions assessed, participants’ syndemics scores ranged from 0 (no syndemic conditions) to 6 (all syndemic conditions). A prior study using the same syndemic conditions showed that, consistent with syndemics theory, these syndemic conditions were largely co-occurring (Harkness et al., 2018). Logistic regression analyses revealed significant associations (p < 0.05) between seven of the pairs of psychosocial concerns and four associations that trended in the expected direction (p < 0.10). For example, individuals with baseline anxiety had 10.5 (95% CI [5.28,20.88]) greater odds of also having baseline depression. Although co-occurring, they were not redundant (Kappa = 0.338). Some were both depressed and anxious, whereas others were depressed only or anxious only. Following syndemics theory, which holds that intertwined, co-occurring psychosocial concerns would have a worse effect on other health outcomes (e.g. HIV-related health behaviors), we retained all six syndemic conditions in our syndemics variable.
CSA
CSA was assessed using the Juvenile Victimization Questionnaire (Finkelhor, Hamby, Ormrod, & Turner, 2005) at baseline only, as it is a past event that occurred in childhood. Participants who reported having a sexual experience with (1) someone at least five years older, before the age of 13 and/or (2) someone at least ten years older between age 13 and 16 years met criteria for CSA.
PTSD
PTSD was assessed using the SPAN (assesses startle, physiological arousal, anger, and numbness; Meltzer-Brody, Churchill, & Davidson, 1999). The SPAN scoring guidelines determined whether they met criteria for PTSD.
Anxiety disorders
Participants were assessed for social anxiety disorder, panic disorder, and generalized anxiety disorder. The Mini Social Phobia Inventory assessed for social anxiety disorder (Connor, Kobak, Churchill, Katzelnick, & Davidson, 2001) at baseline. The Patient Health Questionnaire (PHQ) assessed for panic disorder and other anxiety disorder (comparable to generalized anxiety disorder; Spitzer, Kroenke, & Williams, 1999) at all time points. Standard scoring procedures and score cutoffs determined whether participants met criteria for social anxiety disorder, panic disorder, and/or generalized anxiety disorder. If they met criteria for any of these three, they met criteria for the composite anxiety disorder variable.
Depression
Participants completed the PHQ (Spitzer et al., 1999), and the standard scoring procedures and score cutoff determined if they met criteria for depression.
Alcohol abuse
Participants reported binge drinking (five or more alcoholic drinks in a day) frequency in the past three months. Those who reported a frequency of one episode or more of binge drinking per week met criteria for alcohol abuse.
Polysubstance and/or stimulant use
Participants reported past three-month sniffing, snorting, smoking, or swallowing substances and/or past 30 day injecting substances. Substances assessed included: (1) marijuana, (2) crack, (3) cocaine, (4) heroin, (5) methamphetamine or amphetamine, (6) ketamine, (7) opiates such as Vicodin, OxyContin, Dilaudid, Percocet, or Darvocet, (8) tranquilizers or barbiturates (e.g. Valium, Xanax, GHB), (9) hallucinogens (e.g. LSD, ecstasy), (10) inhalants (e.g. glue, poppers, nitrous oxide), (11) steroids, or (12) other drugs not listed. The questionnaire did not specifically probe for opiate use as prescribed for pain compared to recreational use, as this study took place prior to the increased national conversation regarding the opioid crisis. Additionally, participants reported past three month crystal methamphetamine use in another questionnaire which we merged into the prior substance use questions. Participants who met criteria for polysubstance use (reported using three or more substances in the past three months) or stimulant use (reported crack, cocaine, and/or methamphetamine/amphetamine use in the past three months) met criteria for this composite variable.
Analytic Plan
Multilevel modeling of the longitudinal effect of syndemics on CAS
Multilevel modeling (MLM) was employed using SAS 9.4 (PROC GLIMMIX) to evaluate the longitudinal effects of syndemics on CAS. Repeated measures were taken every three months over the course of one year, which yielded a dataset of up to five evenly spaced observations nested within each participant. MLM was used because it accounts for repeated measures within individuals and affords the flexibility to include participants with missing data at particular time points (Jackson, 2010).
Predictors of CAS included time (continuous, coded 0 to 4), condition (categorical: Enhance control = reference), a time by condition interaction, and number of syndemics (continuous, coded 0 to 6). Because this was a secondary analysis of participant data from two prior intervention studies, time, condition (which study they were in, and for Enhance whether it was intervention or control group), and condition by time interactions were used as covariates. In each model, statistical significance was determined using a conventional p < .05.
The models were fitted to predict binary CAS in PROC GLIMMIX using Maximum Likelihood estimation (Gauss-Hermite Quardrature) and a logit link function. The unconditional model was first used to compute the ICC, or the variation in CAS, due to between versus within-person effects. Following this, a random effect of time and fixed effect of time, condition, a condition by time interaction term, and number of syndemics were added to the model. The predictor of interest was the number of syndemics, with the remaining variables included as covariates.
Additional variables were considered but not used based on preliminary testing. Although the study was conducted before the findings of “Undetectable = Untransmittable,” and therefore participants in this study were not making sexual decisions using this information, we considered using viral load (binary, detectable vs. undetectable) as a moderator within our model. This, however, was not significant (p = 0.44) and therefore dropped from further analysis. Additionally, lagged effects of syndemics were considered, but ultimately excluded due to statistical issues with multicollinearity, such that the syndemics score and lagged syndemics scores were highly correlated (r = 0.85).
Our analyses evaluated the between-person effect compared to the within-person effect of syndemics on participants’ odds of engaging in serodiscordant CAS. Between person effects were used to examine whether variation in serodiscordant CAS is longitudinally associated with participants’ average syndemic level over time compared to other participants in the study. Within person effects test whether CAS is longitudinally associated with participants’ changes in syndemic levels compared to their average syndemics level across the year-long observation period. To examine the within- and between-person effects of syndemics on serodiscordant CAS, we disaggregated syndemics scores into a person mean (the person’s average level of syndemics over the year they were observed) and a deviation from the mean (how far their syndemics score at a given time point was from their overall average syndemics score). The final model included the disaggregated syndemics scores (person mean and deviation from the mean) replacing the overall syndemics score (Curran & Bauer, 2011). This allowed us to examine within- and between-person effects of syndemics on serodiscordant CAS.
Results
Demographics
Complete demographic information for this sample is reported elsewhere (Harkness et al., 2018). Participants ranged in age from 21 to 68 years old (M = 41.95, SD = 8.2). The majority of participants were white (n = 299, 76.7%), followed by Black/African American (n = 41, 10.5%), Hispanic/Latino (n = 30, 7.7%), or another race or ethnicity (n = 20, 5.2%). They were mostly not in a committed relationship (n = 232, 59.5%), whereas about a third were in committed relationships (n = 150, 38.5%). In terms of their HIV health at baseline, about half had an undetectable viral load (n = 218, 55.9%) and their average CD4+T cell count was 536 (SD = 289).
Syndemic Conditions & CAS – Preliminary Analyses
All of the syndemic conditions occurred at relatively high frequencies. The most frequently observed syndemic conditions (30% or more of the sample at any time point) were CSA, PTSD, anxiety, and poly sub stance/stimulant use (see Appendix 1 in the online appendix for description of the frequencies of each syndemic condition over time).
Figure 1 shows that at all time points, the modal syndemics score was 1. About 20% of the sample reported 0 or 2 syndemic conditions at each time point. Approximately 5-15% of the sample reported 3 or 4 syndemic conditions at any time point. Syndemics scores of 5-6 were less common at all time points.
Figure 1.
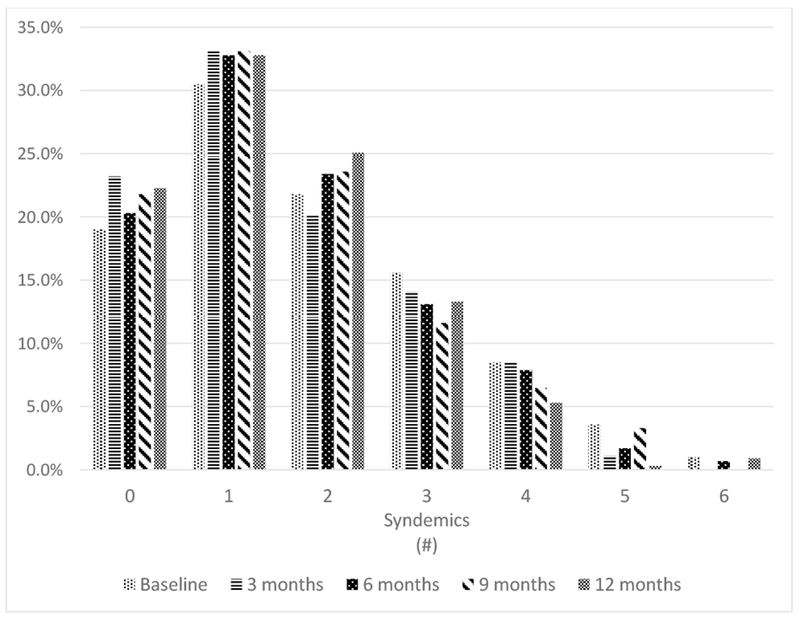
Distribution of participants’ syndemics scores at each visit.
Across the five time points, the number of participants who reported serodiscordant CAS ranged from approximately 27% to approximately 43%. As shown in Figure 2, there was a time effect across the full sample for CAS, such that over time, the frequency of CAS decreased.
Figure 2.
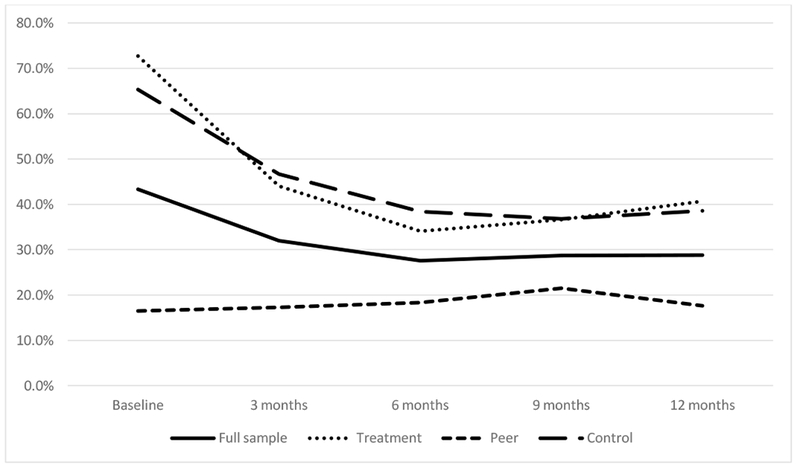
Percentage of participants who reported serodiscordant CAS over time
Longitudinal Effect of Syndemics on CAS
We tested a series of three models, which are summarized in Table 1. The first was the unconditional model, with no predictors entered. The second included all of the predictors and tested our main hypothesis. The third disaggregated syndemics into between- and within-person effects.
Table 1:
Results of multilevel models predicting CAS
| Parameter | Model 1: Unconditional | Model 2: Syndemics total | Model 3: Syndemics disaggregated |
|---|---|---|---|
| Fixed effects | |||
| Intercept Estimate (SE) |
−1.19 (0.15)** | 0.04 (0.35) | 0.12 (0.37) |
| Time Estimate (SE) |
−0.56 (0.13)** | −0.56 (0.13)** | |
| Conditiona | F(2,806) = 35.22** | F(2,806) = 35.42** | |
| Experimental OR (95% CI) |
1.19 (0.53-2.68) | 1.18 (0.53-2.63) | |
| Peer OR (95% CI) |
0.08 (0.04-0.20)** | 0.08 (0.04-0.19)** | |
| Condition × Timea | F(2,806) = 3.52* | F(2,806) = 3.60* | |
| Experimental OR (95% CI) |
0.54 (0.42-0.71) | 0.55 (0.42-0.71) | |
| Peer OR (95% CI) |
0.84 (0.64-1.12)* | 0.85 (0.64-1.13)* | |
| Syndemics OR (95% CI) |
1.41 (1.16-1.70)** | ||
| Between OR (95% CI) |
1.34 (1.07-1.69)* | ||
| Within OR (95% CI) |
1.56 (1.11-2.19)* | ||
| Random effects | |||
| Intercept Estimate (SE) |
4.87 (0.81) | 4.07 (0.91) | 4.06 (0.90) |
| Time Estimate (SE) |
0.43 (0.17) | 0.41 (0.17) | |
Note. Estimates are reported followed by standard errors in parentheses.
Reference = control group.
p ≤ .05,
p ≤ .01
First, we examined the unconditional model in order to partition the observed variance in CAS into between- and within-person components and to compute the ICC. Because error is implied by the response distribution, rather than estimated in hierarchical generalized linear models, the ICC was computed by assuming a variance of 3.29 for the logistic distribution of the binary outcome variable, CAS (Goldstein, Browne, & Rasbash, 2002). With the observed between-person variance (τ = 4.87) and the assumed within-person variance (σ= 3.29), the ICC (0.59) showed that approximately 60% of the variability in CAS was between persons, and the remaining attributable to within-person differences.
In a second model, we tested the fixed and random effects for our full set of predictors. Regarding the control variables, the model showed that the fixed effect of time (γ = −0.56, SE = 0.13, t(343) = −4.25, p < 0.0001), intervention condition (F(2,806) = 35.22, p < 0.0001) and the time by intervention condition interaction (F(2,806) = 3.52, p = 0.03) were significant. As predicted via the primary study hypothesis, syndemics was a significant predictor of CAS over and above the control variables, such that for each additional syndemic condition, participants were 41% more likely to report CAS (OR = 1.41; 95% CI [1.16, 1.70]; p = 0.0004).
Finally, the third model disaggregated participants’ syndemics scores at each time point into an overall person mean (their average syndemics score across the five time points; between-person effects) and the deviation from their mean at each time point (the difference between their observed syndemics score and their average syndemics score; within-person effects). In this model, we replaced the syndemics score from model 2 with these disaggregated variables for syndemics. All other covariates remained the same. This model showed that both the within- and between-person effects of syndemics were significantly predictive of serodiscordant CAS. Accordingly, the between person effect of syndemics revealed that participants’ average number of syndemics across the five time points was a significant predictor of CAS, t(806) = 2.51, p = 0.0121. A mean syndemics score increase of 1 was associated with 1.34 (95% CI [1.07, 1.69]) greater odds of CAS. The within person effect of syndemics over time, t(806) = 2.58, p = 0.01 revealed that a one unit increase in the syndemics score at a given time point (compared to their average score) was associated with 1.56 (95% CI [1.11, 2.10]) greater odds of CAS over time. In other words, participants’ syndemics scores significantly predicted serodiscordant CAS (Model 2), and this effect was attributable to within person changes in syndemics scores and between person differences in average syndemics scores (Model 3). Figure 3 illustrates the odds ratios associated with a one unit increase in each of the three syndemics scores we evaluated through models 2 and 3.
Figure 3.
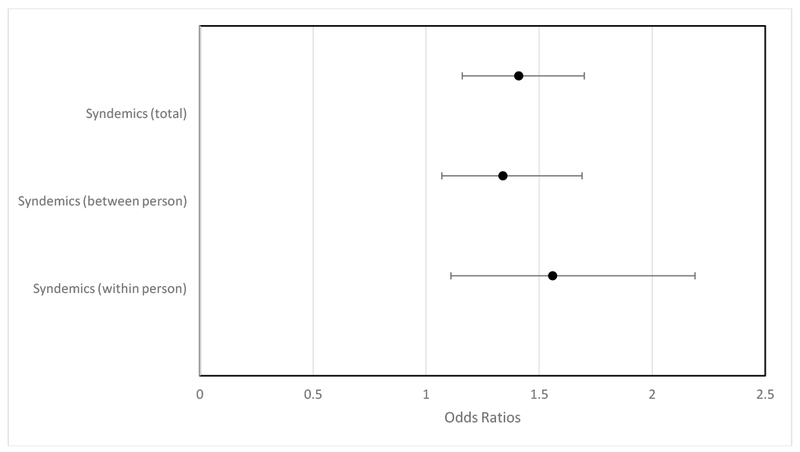
Odds of CAS across predictors from Models 2 and 3
Note. The odds ratios refer to the increase in odds of serodiscordant CAS for a 1 unit increase in the continuous predictor. For syndemics, this refers to (1) Total: a one unit increase on the 0-6 total syndemics scores, (2) Between: a one unit increase in the person’s average syndemics score across the 5 time points, and (3) Within: a one unit increase in the participant’s deviation from their average score at a particular time point.
Discussion
Our study shows that co-occurring syndemic conditions are associated with greater likelihood of engaging in serodiscordant CAS among sexual minority men living with HIV. The findings were significant when comparing participants to each other, as well as when looking at individuals: those with higher average syndemics levels were more likely to engage in serodiscordant CAS, and if a participant had more syndemics than their average at a particular visit, they were also more likely to engage in serodiscordant CAS. Together, the syndemic conditions observed in this study (childhood sexual abuse, PTSD, depression, anxiety, poly sub stance/stimulant use, and alcohol abuse) longitudinally predicted serodiscordant CAS. This study builds on prior findings that syndemics are associated longitudinally with another health behavior, ART adherence, among sexual minority men living with HIV (Friedman et al., 2015; Harkness et al., 2018) and cross-sectional research showing the relationship between syndemics and condomless sex among a general population of adolescents living with HIV (van den Berg et al., 2017). This is the first study to the authors’ knowledge to show this longitudinal relationship between syndemics and serodiscordant CAS among HIV-positive sexual minority men.
In addition to our main finding, we found both a between- and within-person effect of syndemics on CAS. In our sample, participants’ average syndemics level over the one-year period was associated with their odds of engaging in CAS. Additionally, the change in their syndemics level from one time point to the next was associated with CAS. Different factors could explain these unique relationships, which may be useful to explore in future research. For those who had a higher overall level of syndemics over time, it could be that managing chronic, high levels of stress due to multiple psychosocial problems over time requires substantial use of one’s cognitive coping resources, leaving few resources to plan or engage in additional self-care behaviors when these coping resources are already depleted. For instance, chronic stress from these different psychosocial conditions over time could affect one’s self-efficacy for asserting or negotiating condom use or for HIV status disclosure as well as the perceived benefits of these behaviors (i.e. Klein, 2011). There may be unique mediators, such as distress intolerance or emotion dysregulation, for those who had a relative elevation in syndemics level from one time point to the next. Puckett et al. (2017) showed that negative urgency, or a pattern of acting impulsively in response to distress, moderates the link between internalized stigma and CAS in young MSM, suggesting the potential utility of distress tolerance training for individuals with higher levels of negative urgency. Related, Rendina et al. (2017) found that among HIV-positive sexual minority men, difficulties with emotion regulation mediated observed relationships between internalized stigma and serodiscordant CAS. Future studies might examine these and other potential mediators and moderators of the relationship between syndemics and sexual behavior in order to tailor individual interventions and develop structural interventions.
Additionally, this study of sexual minority men living with HIV showed high rates of syndemic psychosocial conditions. We compared the rates of syndemic conditions observed in this study to two prior samples of sexual minority men: a cross-sectional sample of mostly HIV-negative sexual minority men collected in New York City from 2003-4 (Parsons et al., 2012) and a longitudinal sample of HIV-negative MSM collected in multiple U.S. cities from 1999-2001 (Mimiaga, O’Cleirigh, et al., 2015). Participants in this study had similar rates of childhood sexual abuse (44.9%) to Mimiaga, O’Cleirigh et al. (2015; 37.5% - 41.6%) but lower than Parsons et al. (2012; 10.2%). This could be associated with differences in measurement. Sexual minority men in this sample had lower rates of depression (11.6% - 13.8%) compared to the prior studies which used the Center for Epidemiological Studies Depression Scale (Parsons et al. = 47.4%; Mimiaga et al. = 35.4% - 47.3%). Alcohol abuse was somewhat higher in this sample (13.7% - 20.3%) than Mimiaga et al. (4+ drinks daily or 6+ drinks on a typical drinking day; 4.9% - 10.5%). This sample also had higher polysubstance/stimulant use (22.0% - 37.7%) compared to the other studies (Parsons et al., polysubstance = 8.4%; Mimiaga et al., polysubstance = 5.9% - 13.7% and stimulant = 16.9% - 25.1%). Consistent with other studies, this group of participants was quite burdened with syndemic psychosocial concerns.
A limitation of this study and area for future research is the time at which the data was collected and accounting for viral load detectability when examining the relationship between syndemics and serodiscordant CAS. We chose to examine serodiscordant CAS as the outcome for this longitudinal model, using all of the HIV-positive sexual minority men in the sample because data was collected prior to the dissemination of the “Undetectable = Untransmittable” public health messaging (Cohen et al., 2011; Rodger et al., 2016). We were interested in examining the association of psychosocial variables to what was considered transmission risk behavior during the time of data collection. Since at that time, individuals were not making sexual decisions based on this knowledge, serodiscordant CAS was considered a behavior that could lead to HIV transmission. Consistent with this, a meta-analysis conducted during the same time period showed there was no difference in HIV acquisition risk behavior by viral load or ART status (Crepaz et al., 2009). We did not hypothesize that the psychosocial syndemics in this study would be differentially related to serodiscordant CAS based on viral load status, which was consistent with our test of possible moderating effects of viral load. Given that “Undetectable = Untransmittable” is in the process of being disseminated it will be important for future studies to re-evaluate the relationship between syndemics and what is now considered sexual behavior that could lead to HIV transmission. The current study’s findings can be used as a point of comparison for future studies conducted in the context of updated public health messaging. Additionally, this analysis did not distinguish risk based on sexual positioning; therefore, future studies would benefit from further analyses with consideration for both viral load and seropositioning to develop a more nuanced and current understanding of the relationship between these factors. Finally, as this was an analysis of the impact of syndemics on sexual behavior, we did not examine the associations between individual psychosocial concerns and CAS.
As a secondary analysis, this study’s conceptualization of syndemics was limited to the initial set of measures that were collected for the larger study from which the data was drawn. CSA, a fixed past event, was only assessed at baseline as was PTSD. Social anxiety disorder was only assessed at baseline, however generalized anxiety and panic disorder were assessed at all time points, therefore anxiety scores varied over time. In future longitudinal research, it will be useful to continue expanding the syndemic factors that are observed longitudinally, including PTSD and distress related to CSA, which does vary over time. This will also facilitate developing and implementing interventions that can intervene upon all of the psychosocial syndemic conditions assessed, as distress related to CSA can be intervened upon, whereas developmental occurrences of CSA cannot be changed.
An ongoing area of discussion within the HIV-related syndemics literature is how to best operationalize and test syndemics theory. Some have suggested testing interaction terms between syndemic conditions to examine synergistic effects (Singer, Bulled, Ostrach, & Mendenhall, 2017; Tsai & Burns, 2015). Stall et al. (2015) suggest that although interaction effects may be tested, syndemics theory as it has been applied remains an essential part of syndemics theory, which has been widely supported by the literature. Testing numerous interaction terms could lead to detection of spurious effects without an a priori hypothesis about particular interactions and larger (up to 6-way interactions in this study) interactions may not lend themselves toward clinical utility. There have been mixed findings regarding interaction effects. Ferlatte et al. (2018) showed evidence of statistical interaction among syndemic conditions in predicting syphilis diagnosis among Canadian sexual minority men. Additionally, Storholm et al. (2016) found an interaction effect between depression and sexual compulsivity, such that sexual compulsivity’s effects on sexual behavior was moderated by depression. In contrast, Morrison et al. (2018) did not detect interaction effects, which they interpreted to support the additive syndemics model. Tomori et al. (2018) found only one significant two-way interaction when testing 2- and 3-way interactions among five syndemic conditions associated with CAS among MSM in India. Accordingly, they suggest that the operationalization of syndemics be expanded to include cumulative models, which consistently show effects. Consistent with recent HIV-related syndemics studies with MSM (Friedman et al., 2015; Guadamuz et al., 2014; Mimiaga, Biello, et al., 2015; Mimiaga, O’Cleirigh, et al., 2015; Parsons et al., 2017; Tulloch et al., 2015), the present analysis shows evidence of disease concentration, as well as mutual causality between the syndemic conditions. We believe this approach provides clear public health and clinical implications, however, future research will continue exploring different approaches to conceptualizing and testing syndemics theory.
These findings point to further areas of research and practice. Engaging in serodiscordant CAS is a health behavior that is modifiable through behavioral intervention. A recent meta-analysis identified evidence-based interventions for reducing behaviors associated with risk of HIV transmission and highlighted that those that addressed mental health were more likely to be effective (Crepaz et al., 2014). Safren, Blashill, and O’Cleirigh (2011) pointed out that secondary prevention interventions need to be integrated with treatment of the co-occurring syndemic conditions that make it difficult to uptake safer sexual behaviors, as this will improve effect sizes of secondary prevention interventions. The present study’s findings support this claim and suggest that tailoring behavioral interventions to meet the needs of those with higher levels of syndemic conditions may result in greater personal and public health benefit. Although provision of more intensive treatment to those who require it may be more costly in the short term, the long term benefits in terms of new infections averted would likely be cost effective (Safren et al., 2016; Safren, Perry, Blashill, O’Cleirigh, & Mayer, 2015). Further, this would improve the mental health of sexual minority men living with HIV, as well as their sexual health and that of their sexual partners.
Another potential implication for practice is to continue expanding the focus of secondary prevention programming beyond preventing transmission of HIV to new partners. Taking up safer sexual behaviors also benefits HIV-positive sexual minority men’s own sexual health. For instance, condom use and disclosure of status and testing history can help this population to avoid reinfection or superinfection with a medication resistant strain of HIV as well as avoid infection with other STIs (World Health Organization, 2011). Focusing on the benefits of condom use and other sexual health behaviors for one’s own health may be a useful strategy for reducing stigma and focusing on self-care in general. This broader approach to secondary prevention is consistent with what HIV-positive sexual minority men describe wanting in these types of interventions (Vanable et al., 2012). Additionally, Pachankis (2015) recommends that mental health providers conceptualize sexual health as including sex that is pleasurable, assertive, and positive. Clinicians are encouraged to take a clearly affirming stance regarding sexual minority men’s expression of sexuality to address some of the minority stress pathways through which syndemic conditions develop. Taken together, this suggests the utility of multidisciplinary providers and institutions, including public health, medical providers, and mental health counselors affirming HIV-positive sexual minority men’s sexuality broadly in order to optimize sexual health.
HIV-related syndemics research should also continue to expand and account for other co-occurring factors that may affect sexual health behaviors. Much of the syndemics research within MSM has focused on the intertwined psychosocial factors that heighten risk of HIV acquisition (Dyer et al., 2012; Mimiaga, Biello, et al., 2015; Mimiaga, O’Cleirigh, et al., 2015; Mustanski et al., 2007; Parsons et al., 2012, 2017; Ron Stall et al., 2003). However, structural and environmental factors are also facets of syndemics theory as originally articulated (Singer & Clair, 2003). For example, anti-gay discrimination and stigma, sexual orientation based violence, racism, and financial hardship are also part of the syndemic associated with CAS among MSM (Ayala, Bingham, Kim, Wheeler, & Millett, 2012; Duncan et al., 2017; Ferlatte et al., 2014; Mustanski et al., 2017). Similarly, life course events associated with stress and stigma are associated with the psychosocial syndemic conditions that have been shown to be associated with greater HIV-related behaviors among MSM (Herrick et al., 2013). Continuing to incorporate these broader social determinants of health (i.e. stigma, heterosexism, marginalization, poverty, structural violence) in future studies examining syndemics among MSM will further bolster our knowledge of the individual, structural, and environmental syndemic conditions that perpetuate HIV-related health outcomes among MSM. Relatedly, using an intersectional lens instead of conceptualizing all sexual minority men living with HIV as one homogenous group can inform research on HIV-related syndemics, as multiple layers of identity and experience intersect to produce the social conditions that create these syndemics (Carnes, 2016). Future syndemics research should consider the unique syndemics that may occur and contribute to sexual health behaviors for subpopulations of sexual minority men across geographic region, race/ethnicity, age, and/or socioeconomic class. An intersectionality approach can inform tailored intervention to meet the needs of community members experiencing different networks of syndemic conditions that contribute to HIV-related health.
Although this study highlights the prevalence of these syndemic conditions and their impact on sexual health behavior, many sexual minority men living with HIV demonstrate resilience in the face of these stressors and take steps to protect their own and their partners’ sexual health (Crepaz et al., 2009; McConnell, Bragg, Shiboski, & Grant, 2010). Capitalizing on the strengths and resilience of sexual minority men will be important in continuing efforts to move from a deficit based model of intervention and toward strengths based models (Herrick et al., 2011; Kurtz, Buttram, Surratt, & Stall, 2012). Identifying the protective factors that enable sexual minority men living with HIV to cope effectively with syndemic conditions in order to protect their own and their partners’ sexual health can inform strengths-based interventions.
In conclusion, we found that multiple, co-occurring syndemic conditions are additively associated with greater likelihood of engaging in serodiscordant CAS among HIV-positive sexual minority men over a one-year period both comparing participants to each other, and within participants over time. In order to promote HIV-positive sexual minority men’s sexual health and reduce onward transmission of HIV to uninfected partners, it will be important for researchers and clinicians to appreciate the contribution of these stressors to this population’s sexual health behaviors. New interventions may be more effective by specifically addressing the multiple, overlapping factors that contribute to sexual health behaviors in this population.
Supplementary Material
Acknowledgments
This research was supported in part by HRSA (H97HA01293) and NIMH (5R01MH068746) awarded to Drs. Kenneth H. Mayer and Steven A. Safren. Some of the author time was supported by NIDA (9K24DA040489) awarded to Dr. Steven A. Safren. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Funding: This research was supported in part by HRSA (H97HA01293) and NIMH (5R01MH068746) awarded to Drs. Kenneth H. Mayer and Steven A. Safren. Some of the author time was supported by NIDA (9K24DA040489) awarded to Dr. Steven A. Safren.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained for all participants included in the study.
References
- Ayala G, Bingham T, Kim J, Wheeler DP, & Millett GA (2012). Modeling the impact of social discrimination and financial hardship on the sexual risk of HIV among Latino and Black men who have sex with men. American Journal of Public Health, 102(Suppl 2), S242–S249. 10.2105/AJPH.2011.300641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, … Shapiro M (2001). Psychiatric disorders and drug use among Human Immunodeficiency Virus-infected adults in the United States. Archives of General Psychiatry, 58, 721–728. [DOI] [PubMed] [Google Scholar]
- Blashill AJ, Bedoya CA, Mayer KH, O’Cleirigh C, Pinkston MM, Remmert JE, … Safren SA (2015). Psychosocial syndemics are additively associated with worse ART adherence in HIV-infected individuals. AIDS and Behavior, 19(6), 981–986. 10.1007/s10461-014-0925-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C, Zvolensky MJ, Woods SP, Gonzalez A, Safren SA, & O’Cleirigh CM (2017). Anxiety symptoms and disorders among adults living with HIV and AIDS: A critical review and integrative synthesis of the empirical literature. Clinical Psychology Review, 51, 164–184. 10.1016/j.cpr.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnes N (2016). Gay men and men who have sex with men: Intersectionality and syndemics In Wright ER & Carnes N (Eds.), Understanding the HIV/AIDS Epidemic in the United States (pp. 43–69). Springer International Publishing; 10.1007/978-3-319-34004-3_3 [DOI] [Google Scholar]
- CDC. (2017). HIV Surveillance Report, 2016 (No. 28). Centers for Disease Control and Prevention; Retrieved from https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2016-vol-28.pdf [Google Scholar]
- Cochran SD, Greer J, & Mays VM (2003). Prevalence of mental disorders, psychological distress, and mental health services use among lesbian, gay, and bisexual adults in the United States. Journal of Consulting and Clinical Psychology, 71(1), 53–61. 10.1037/0022-006X.71.1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, … Fleming TR (2011). Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine, 365(6), 493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, Kobak KA, Churchill LE, Katzelnick D, & Davidson JR (2001). Mini-SPIN: A brief screening assessment for generalized social anxiety disorder. Depression and Anxiety, 14(2), 137–140. 10.1002/da.1055 [DOI] [PubMed] [Google Scholar]
- Crepaz N, Marks G, Liau A, Mullins MM, Aupont LW, Marshall KJ, … others. (2009). Prevalence of unprotected anal intercourse among HIV-diagnosed MSM in the United States: A meta-analysis. AIDS, 23(13), 1617–1629. 10.1097/QAD.0b013e32832effae [DOI] [PubMed] [Google Scholar]
- Crepaz N, Tungol MLV, Higa DH, Vosburgh HW, Mullins MM, Barham T, … Lyles CM (2014). A systematic review of interventions for reducing HIV risk behaviors among people living with HIV in the United States, 1988–2012. AIDS, 28(5), 633–656. 10.1097/QAD.0000000000000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, & Bauer DJ (2011). The disaggregation of within-person and between-person effects in longitudinal models of change. Annual Review of Psychology, 62, 583–619. 10.1146/annurev.psych.093008.100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan DT, Park SH, Schneider JA, Al-Ajlouni YA, Goedel WC, Elbel B, … Mayer KH (2017). Financial hardship, condomless anal intercourse and HIV risk among men who have sex with men. AIDS and Behavior, 21(12), 3478–3485. 10.1007/s10461-017-1930-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer TP, Shoptaw S, Guadamuz TE, Plankey M, Kao U, Ostrow D, … Stall R (2012). Application of syndemic theory to Black men who have sex with men in the Multicenter AIDS Cohort Study. Journal of Urban Health, 89(4), 697–708. 10.1007/s11524-012-9674-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlatte O, Hottes TS, Trussler T, & Marchand R (2014). Evidence of a syndemic among young Canadian gay and bisexual men: Uncovering the associations between anti-gay experiences, psychosocial issues, and HIV risk. AIDS and Behavior, 18(7), 1256–1263. 10.1007/s10461-013-0639-1 [DOI] [PubMed] [Google Scholar]
- Ferlatte O, Salway T, Samji H, Dove N, Gesink D, Gilbert M, … Wong J (2018). An application of syndemic theory to identify drivers of the syphilis epidemic among gay, bisexual, and other men who have sex with men. Sexually Transmitted Diseases, 45(3), 163–168. 10.1097/OLQ.0000000000000713 [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Hamby SL, Ormrod R, & Turner H (2005). The Juvenile Victimization Questionnaire: Reliability, validity, and national norms. Child Abuse & Neglect, 29(4), 383–412. 10.1016/j.chiabu.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Friedman MR, Stall R, Silvestre AJ, Wei C, Shoptaw S, Herrick A, … Plankey MW (2015). Effects of syndemics on HIV viral load and medication adherence in the multicentre AIDS cohort study. AIDS, 29(9), 1087–1096. 10.1097/QAD.0000000000000657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein H, Browne W, & Rasbash J (2002). Partitioning variation in multilevel models. Understanding Statistics, 1(4), 223–231. 10.1207/S15328031US0104_02 [DOI] [Google Scholar]
- Guadamuz TE, McCarthy K, Wimonsate W, Thienkrua W, Varangrat A, Chaikummao S, … Griensven F van. (2014). Psychosocial health conditions and HIV prevalence and incidence in a cohort of men who have sex with men in Bangkok, Thailand: Evidence of a syndemic effect. AIDS and Behavior, 18(11), 2089–2096. 10.1007/s10461-014-0826-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness A, Bainter SA, O’Cleirigh C, Mendez NA, Mayer KH, & Safren SA (2018). Longitudinal effects of syndemics on ART non-adherence among sexual minority men. AIDS and Behavior, 22(8), 2564–2574. 10.1007/s10461-018-2180-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Dombrowski JC, Crane HM, Eron JJ, Geng EH, Mathews WC, … Donovan DM (2017). Prevalence and predictors of substance use disorders among HIV care enrollees in the United States. AIDS and Behavior, 21(4), 1138–1148. 10.1007/s10461-016-1584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick AL, Lim SH, Plankey MW, Chmiel JS, Guadamuz TT, Kao U, … Stall R (2013). Adversity and syndemic production among men participating in the Multicenter AIDS Cohort Study: A life-course approach. American Journal of Public Health, 103(1), 79–85. 10.2105/AJPH.2012.300810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick AL, Lim SH, Wei C, Smith H, Guadamuz T, Friedman MS, & Stall R (2011). Resilience as an untapped resource in behavioral intervention design for gay men. AIDS and Behavior, 15(S1), 25–29. 10.1007/s10461-011-9895-0 [DOI] [PubMed] [Google Scholar]
- Hirshfield S, Remien RH, Humberstone M, Walavalkar I, & Chiasson MA (2004). Substance use and high-risk sex among men who have sex with men: a national online study in the USA. AIDS Care, 16(8), 1036–1047. 10.1080/09540120412331292525 [DOI] [PubMed] [Google Scholar]
- Jackson DL (2010). Reporting results of latent growth modeling and multilevel modeling analyses: Some recommendations for rehabilitation psychology. Rehabilitation Psychology, 55(3), 272. [DOI] [PubMed] [Google Scholar]
- King M, Semlyen J, Tai SS, Killaspy H, Osborn D, Popelyuk D, & Nazareth I (2008). A systematic review of mental disorder, suicide, and deliberate self harm in lesbian, gay and bisexual people. BMC Psychiatry, 8, 70 10.1186/1471-244X-8-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H (2011). Using a syndemics theory approach to study HIV risk taking in a population of men who use the internet to find partners for unprotected sex. American Journal of Men’s Health, 5(6), 466–476. 10.1177/1557988311398472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauz RO, Safren SA, O’Cleirigh C, Capistrant BD, Driskell JR, Aguilar D, … Mayer KH (2007). Developing an HIV-prevention intervention for HIV-infected men who have sex with men in HIV care: Project Enhance. AIDS and Behavior, 11(1), S117–26. 10.1007/s10461-007-9257-0 [DOI] [PubMed] [Google Scholar]
- Kurtz SP, Buttram ME, Surratt HL, & Stall RD (2012). Resilience, syndemic factors, and serosorting behaviors among HIV-positive and HIV-negative substance-using MSM. AIDS Education and Prevention, 24(3), 193–205. 10.1521/aeap.2012.24.3.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell JJ, Bragg L, Shiboski S, & Grant RM (2010). Sexual seroadaptation: Lessons for prevention and sex research from a cohort of HIV-positive men who have sex with men. PLOS ONE, 5(1), e8831 10.1371/journal.pone.0008831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S, Churchill E, & Davidson JR (1999). Derivation of the SPAN, a brief diagnostic screening test for post-traumatic stress disorder. Psychiatry Research, 88(1), 63–70. 10.1016/S0165-1781(99)00070-0 [DOI] [PubMed] [Google Scholar]
- Meyer IH (2003). Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: Conceptual issues and research evidence. Psychological Bulletin, 129(5), 674–697. 10.1037/0033-2909.129.5.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga MJ, Biello KB, Robertson AM, Oldenburg CE, Rosenberger JG, O’Cleirigh C, … Safren SA (2015). High Prevalence of Multiple Syndemic Conditions Associated with Sexual Risk Behavior and HIV Infection Among a Large Sample of Spanish- and Portuguese-Speaking Men Who Have Sex with Men in Latin America. Archives of Sexual Behavior, 44(7), 1869–1878. 10.1007/s10508-015-0488-2 [DOI] [PubMed] [Google Scholar]
- Mimiaga MJ, Noonan E, Donnell D, Safren SA, Koenen KC, Gortmaker S, … Mayer KH (2009). Childhood sexual abuse is highly associated with HIV risk-taking behavior and infection among MSM in the EXPLORE Study. Journal of Acquired Immune Deficiency Syndromes (1999), 51(3), 340–348. 10.1097/QAI.0b013e3181a24b38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga MJ, OʼCleirigh C, Biello KB, Robertson AM, Safren SA, Coates TJ, … Mayer KH (2015). The effect of psychosocial syndemic production on 4-year HIV incidence and risk behavior in a large cohort of sexually active men who have sex with men. Journal of Acquired Immune Deficiency Syndromes, 68(3), 329–336. 10.1097/QAI.0000000000000475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SA, Yoong D, Hart TA, MacPherson P, Bogoch I, Sivarajah V, … Tan DHS (2018). High prevalence of syndemic health problems in patients seeking post-exposure prophylaxis for sexual exposures to HIV. PLOS ONE, 13(5), e0197998 10.1371/journal.pone.0197998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski B, Garofalo R, Herrick A, & Donenberg G (2007). Psychosocial health problems increase risk for HIV among urban young men who have sex with men: preliminary evidence of a syndemic in need of attention. Annals of Behavioral Medicine, 34(1), 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski B, Phillips G, Ryan DT, Swann G, Kuhns L, & Garofalo R (2017). Prospective effects of a syndemic on HIV and STI incidence and risk behaviors in a cohort of young men who have sex with men. AIDS and Behavior, 21(3), 845–857. 10.1007/s10461-016-1607-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Cleirigh C, Magidson JF, Skeer MR, Mayer KH, & Safren SA (2015). Prevalence of psychiatric and substance abuse symptomatology among HIV-infected gay and bisexual men in HIV primary care. Psychosomatics, 56(5), 470–478. 10.1016/j.psym.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachankis JE (2015). A transdiagnostic minority stress treatment approach for gay and bisexual men’s syndemic health conditions. Archives of Sexual Behavior, 44(7), 1843–1860. 10.1007/s10508-015-0480-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Grov C, & Golub SA (2012). Sexual compulsivity, co-occurring psychosocial health problems, and HIV risk among gay and bisexual men: further evidence of a syndemic. American Journal of Public Health, 102(1), 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Millar BM, Moody RL, Starks TJ, Rendina HJ, & Grov C (2017). Syndemic conditions and HIV transmission risk behavior among HIV-negative gay and bisexual men in a U.S. national sample. Health Psychology, 36(7), 695–703. 10.1037/hea0000509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett JA, Newcomb ME, Garofalo R, & Mustanski B (2017). Examining the conditions under which internalized homophobia is associated with substance use and condomless sex in young MSM: The moderating role of impulsivity. Annals of Behavioral Medicine, 51(4), 567–577. 10.1007/s12160-017-9878-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner SL, Mimiaga MJ, Safren SA, & Mayer KH (2009). Stressful or traumatic life events, post-traumatic stress disorder (PTSD) symptoms, and HIV sexual risk taking among men who have sex with men. AIDS Care, 21(12), 1481–1489. 10.1080/09540120902893258 [DOI] [PubMed] [Google Scholar]
- Rendina HJ, Gamarel KE, Pachankis JE, Ventuneac A, Grov C, & Parsons JT (2017). Extending the minority stress model to incorporate HIV-positive gay and bisexual men’s experiences: A longitudinal examination of mental health and sexual risk behavior. Annals of Behavioral Medicine. 10.1007/s12160-016-9822-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, … Lundgren J (2016). Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA, 316(2), 171–181. 10.1001/jama.2016.5148 [DOI] [PubMed] [Google Scholar]
- Safren SA, Blashill AJ, & O’Cleirigh CM (2011). Promoting the sexual health of MSM in the context of comorbid mental health problems. AIDS and Behavior, 15(Suppl 1), S30–S34. 10.1007/s10461-011-9898-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Hughes JP, Mimiaga MJ, Moore AT, Friedman RK, Srithanaviboonchai K, … HPTN063 Study Team. (2016). Frequency and predictors of estimated HIV transmissions and bacterial STI acquisition among HIV-positive patients in HIV care across three continents. Journal of the International AIDS Society, 19(1), 21096 10.7448/IAS.19.1.21096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh CM, Skeer M, Elsesser SA, & Mayer KH (2013). Project Enhance: A randomized controlled trial of an individualized HIV prevention intervention for HIV-infected men who have sex with men conducted in a primary care setting. Health Psychology, 32(2), 171–179. 10.1037/a0028581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh C, Skeer MR, Driskell J, Goshe BM, Covahey C, & Mayer KH (2011). Demonstration and evaluation of a peer-delivered, individually-tailored, HIV prevention intervention for HIV-infected MSM in their primary care setting. AIDS and Behavior, 15(5), 949–958. 10.1007/s10461-010-9807-8 [DOI] [PubMed] [Google Scholar]
- Safren SA, Perry NS, Blashill AJ, O’Cleirigh C, & Mayer KH (2015). The cost and intensity of behavioral interventions to promote HIV treatment for prevention among article-title>HIV-positive men who have sex with men. Archives of Sexual Behavior, 44(7), 1833–1841. 10.1007/s10508-014-0455-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer MC, Bulled N, Ostrach B, & Mendenhall E (2017). Syndemics and the biosocial conception of health. The Lancet, 389(10072), 941–950. 10.1016/S0140-6736(17)30003-X [DOI] [PubMed] [Google Scholar]
- Singer MC, & Clair S (2003). Syndemics and public health: Reconceptualizing disease in bio-social context. Medical Anthropology Quarterly, 17(4), 423–441. 10.1525/maq.2003.17.4.423 [DOI] [PubMed] [Google Scholar]
- Singer MC, Erickson PI, Badiane L, Diaz R, Ortiz D, Abraham T, & Nicolaysen AM (2006). Syndemics, sex and the city: Understanding sexually transmitted diseases in social and cultural context. Social Science & Medicine, 63(8), 2010–2021. 10.1016/j.socscimed.2006.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JBW, & Group, and the P. H. Q. P. C. S. (1999). Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. JAMA, 282(18), 1737–1744. 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- Stall R, Coulter RWS, Friedman MR, & Plankey MW (2015). Commentary on “Syndemics of psychosocial problems and HIV risk: A systematic review of empirical tests of the disease interaction concept” by A. Tsai and B. Burns. Social Science & Medicine (1982), 145, 129–131. 10.1016/j.socscimed.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Stall R, Mills TC, Williamson J, Hart T, Greenwood G, Paul J, … Catania JA (2003). Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. American Journal of Public Health, 93(6), 939–942. 10.2105/AJPH.93.6.939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storholm ED, Satre DD, Kapadia F, & Halkitis PN (2016). Depression, compulsive sexual behavior, and sexual risk-taking among urban young gay and bisexual men: The P18 cohort study. Archives of Sexual Behavior, 45(6), 1431–1441. 10.1007/s10508-015-0566-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomori C, McFall AM, Solomon SS, Srikrishnan AK, Anand S, Balakrishnan P, … Celentano DD (2018). Is there synergy in syndemics? Psychosocial conditions and sexual risk among men who have sex with men in India. Social Science & Medicine, 206, 110–116. 10.1016/j.socscimed.2018.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, & Burns BFO (2015). Syndemics of psychosocial problems and HIV risk: A systematic review of empirical tests of the disease interaction concept. Social Science & Medicine, 139, 26–35. 10.1016/j.socscimed.2015.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch TG, Rotondi NK, Ing S, Myers T, Calzavara LM, Loutfy MR, & Hart TA (2015). Retrospective reports of developmental stressors, syndemics, and their association with sexual risk outcomes among gay men. Archives of Sexual Behavior, 44(7), 1879–1889. 10.1007/s10508-015-0479-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg JJ, Isabel Fernández M, Fava JL, Operario D, Rudy BJ, Wilson PA, & ATN 086/106 Protocol Teams for the Adolescent Medicine Trials Network for HIV/ADS Interventions. (2017). Using syndemics theory to investigate risk and protective factors associated with condomless sex among youth living with HIV in 17 U.S. cities. AIDS and Behavior, 21(3), 833–844. 10.1007/s10461-016-1550-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanable PA, Carey MP, Brown JL, Littlewood RA, Bostwick R, & Blair D (2012). What HIV-positive MSM want from sexual risk reduction interventions: Findings from a qualitative study. AIDS and Behavior, 16(3), 554–563. 10.1007/s10461-011-0047-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JK, Wilton L, Magnus M, Wang L, Wang J, Dyer TP, … Cummings V (2015). Relation of childhood sexual abuse, intimate partner violence, and depression to risk factors for HIV among black men who have sex with men in 6 U.S. cities. American Journal of Public Health, 105(12), 2473–2481. 10.2105/AJPH.2015.302878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wim VB, Christiana N, & Marie L (2014). Syndemic and other risk factors for unprotected anal intercourse among an online sample of Belgian HIV negative men who have sex with men. AIDS and Behavior, 18(1), 50–58. 10.1007/s10461-013-0516-y [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2011). WHO | Prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender people. Retrieved from http://www.who.int/hiv/pub/guidelines/msm_guidelines2011/en/ [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


