Abstract
Background and Aim
The prohibitively high cost of direct‐acting antivirals (DAA) for hepatitis C virus (HCV) infection remains a barrier to treatment access in Singapore. We aimed to evaluate whether DAA as first‐line therapy would be cost‐effective for genotype 3 (GT3) HCV patients compared with pegylated interferon and ribavirin (PR).
Methods
A decision tree analysis was used to compare the costs and outcomes of DAA and PR as first‐line therapy. Treatment effectiveness, defined as sustained virological response, was assessed using a retrospective cohort of treated GT3 HCV patients. Direct medical costs were estimated from the payer’s perspective using billing information. We obtained health utilities from published literature. We performed extensive one‐way sensitivity analyses and probabilistic sensitivity analyses to account for uncertainties regarding the model parameters.
Results
In base case analysis, first‐line therapy with DAA and PR yielded quality‐adjusted life years (QALYs) of 0.69 and 0.62 at a cost of USD 54 634 and USD 23 857, respectively. The resultant incremental cost‐effectiveness ratio (ICER) (USD 449 232/QALY) exceeded the willingness‐to‐pay threshold (USD 53 302/QALY). The ICER was robust for uncertainties regarding the model parameters. The cost of DAA is the key factor influencing the cost‐effectiveness of HCV treatment. At current price, DAA as first‐line therapy is not cost‐effective compared with PR, with or without consideration of retreatment. Threshold analysis suggested that DAA can be cost‐effective if it costs less than USD 17 002 for a 12‐week treatment course.
Conclusion
At current price, DAA as first‐line therapy is not cost‐effective compared with PR in GT3 HCV patients in Singapore.
Keywords: cost‐effectiveness, direct‐acting antiviral, genotype 3, hepatitis C
Introduction
Chronic hepatitis C virus (HCV) infection is a common cause of decompensated liver cirrhosis, hepatocellular carcinoma (HCC), and death. It is estimated to affect more than 71 million people,1 resulting in a rising trend in HCV‐related morbidity and mortality worldwide.2 In the recent Global Burden of Disease Survey, viral hepatitis accounted for 1.45 million deaths per annum, whereby 48% were due to chronic HCV infection.3 The estimated HCV‐related health‐care cost is expected to rise from $6.5 billion and peak at $9.1 billion in 2024 in the United States.4 In view of this, the World Health Organization (WHO) has set goals to eliminate viral hepatitis by the year 2030. Successful treatment of HCV with sustained virological response (SVR) has been shown to reduce liver‐related morbidity and mortality and HCV‐related extrahepatic complications, reduce transmission of HCV, and improve quality of life.2, 3, 4, 5
Genotype 3 (GT3) HCV infection is highly prevalent in South and Southeast Asia, including Thailand, Malaysia, and India.6 In Singapore, the estimated prevalence of HCV is approximately 0.1% based on estimations by the Ministry of Health, with genotypes 1 (GT1) and 3 (GT3) being the most common.7 Prior to the direct‐acting antiviral (DAA) era, a combination of pegylated interferon and ribavirin (PR) was the mainstay treatment, with SVR ranging from 40% to 70%.8, 9 The high incidence of the CC polymorphism at the interleukin 28B (IL‐28B) gene among Asians is associated with SVR rates between 78% and 92% when treated with interferon‐based regimens. Due to the high baseline prevalence of the CC IL‐28B polymorphism in the Southeast Asian population, there is an inherent advantage of using PR due to its high treatment response rates.10 Coupled with the low cost of PR, there is a strong economic argument for using PR as the first‐line treatment and rationing DAA to those who fail treatment or are intolerant to PR. Nevertheless, the concerns of conventional PR treatment included longer treatment duration, more adverse effects, various contraindications for treatment initiation, and the need of a subcutaneous injection.
The introduction of highly effective oral DAA has revolutionized the treatment of HCV, allowing patients with more advanced disease to be treated over a shorter duration with excellent SVR rates beyond 95%.11, 12, 13 DAA are safe, have minimal side effects, and are easy to administer, making them much easier to deliver on a national scale if the elimination of HCV is to be achieved. However, the prohibitively high cost of DAA remains a barrier for treatment access in many developing countries, including the Asia‐Pacific region. Despite the high cost of DAA, it is widely accepted to be cost‐effective as it takes long‐term health gains into account.14 In addition, PR therapy for GT1 has poor treatment outcomes and longer treatment duration, adding to the cost of therapy, thereby making a compelling argument for the use of DAA as first‐line therapy. However, the same cannot be extrapolated to GT3, in which treatment with PR is associated with shorter treatment durations and higher SVR rates. The data on the cost‐effectiveness of DAA in the treatment of GT3 HCV infection are limited. Current available literature has shown that the use of sofosbuvir (SOF)‐based DAA regimens are not cost‐effective as first‐line treatment at their initial listing prices when compared to PR.15, 16, 17 A Swiss study suggested that DAA with a SOF‐based regimen were indeed cost‐effective, but the comparator was a 12‐week regimen of SOF and PR versus a 24‐week regimen of SOF and ribavirin.18 A more informative comparison would have been between a 12‐week SOF‐based regimen with PR and SOF‐based regimens of equal duration.
Economic evaluations of DAA regimens among HCV patients in exclusively Asian populations remain scarce, and outcomes varied between countries because of the diversity of HCV genotype, ethnicity, and cost of DAA.19, 20, 21, 22 In Singapore, a study conducted on GT1 HCV patients showed that boceprevir, a first‐generation DAA, was cost‐effective compared with standard combination treatment using PR alone.23 However, it is unknown whether DAA is cost‐effective compared with PR for the treatment of GT3 HCV infections in Singapore. Hence, the aim of our study, based on the current and the anticipated reduced cost of DAA regimens, is to examine whether it is cost‐effective to use it as first‐line treatment instead of PR. Secondly, we also wish to determine the price threshold at which DAA regimens will be cost‐effective compared with PR to inform value‐based pricing negotiations and policies.
Methods
A decision tree analysis based on a retrospective analysis of 91 consecutively treated patients at Changi General Hospital (CGH) in Singapore was performed. CGH is a large district general hospital that serves the eastern population of Singapore. Prior to January 2018, a universal drug subsidy was only available for PR but not for DAA. As a result, treatment‐naïve, noncirrhotic, and compensated Childs A cirrhotic patients with GT3 HCV were treated with PR; DAA was rationed for patients with decompensated liver cirrhosis or with contraindications to PR, as well as for those who were PR treatment‐experienced.
Ethics review
This study was approved by the SingHealth Centralised Institutional Review Board (Ref: 2017/2539).
Model overview
A decision tree model was constructed to compare costs and quality‐adjusted life years (QALYs) of DAA and PR for the treatment of GT3 HCV infection from the payers’ perspective (Fig. 1). The payers’ perspective was chosen as out‐of‐pocket expenses are substantial for HCV treatment in Singapore. A cohort of treatment‐naïve GT3 HCV patients treated in our institution was simulated. We compared the cost of 12 weeks of SOF, daclastavir (DAC), and ribavirin (SOF + DAC + ribavirin) against 24 weeks of PR at undiscounted prices applicable in 2016 as the base case analysis. To better reflect real‐world practice, patients who had failed PR were retreated with DAA in the simulated decision tree. Patients who failed primary DAA therapy were retreated with a second course of rescue DAA. Patients who failed two treatment courses, including retreatment, were considered to have failed treatment. Costs and outcomes were simulated over a 72‐week time period. This time horizon was chosen because both treatment arms would have completed two courses of treatment in order to assess the attainment of SVR following completion of treatment. We use SVR as our treatment end‐point, which is defined as SVR 24 weeks after completion of PR and SVR 12 weeks after completion of DAA regimens.
Figure 1.
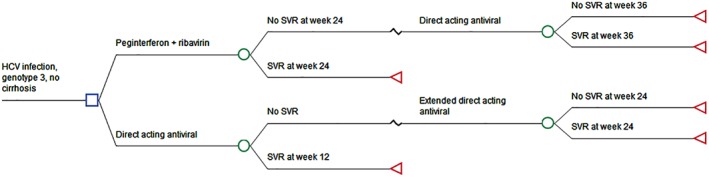
Treatment decision tree on genotype 3 chronic hepatitis C virus (HCV) patients. SVR, sustained virological response.
To account for alternative treatment scenarios with the use of DAA regimens other than SOF + DAC + ribavirin, with and without retreatment, we also modeled for scenarios 2 and 3. The second scenario was modeled on the discount price of DAA and PR using the sofosbuvir and velpastavir (SOF + VEL) regimen and took the retreatment of primary failure into account. The third and final scenario was modeled for the use of SOF + VEL against PR, at a discounted price without retreatment of primary failures. HCV RNA load was measured using an Food and Drug Administration‐approved quantitative real‐time polymerase chain reaction assay for HCV RNA (Roche COBAS Ampliprep/Taqman version 2.0, Roche Molecular System, New Jersey, USA) with a lower detection limit of 12 IU/ml.
Model population
The SVR data obtained from all GT3 HCV patients treated at CGH from 2014 to 2017 were included in the analysis. Patients with human immunodeficiency virus or hepatitis B coinfection were excluded from this analysis. The demographic data and treatment outcomes are summarized in Table 1. A total of 75 and 16 patients received PR and DAA as first‐line treatment, respectively. The overall SVR and SVR in patients treated with DAA and PR was 81.3, 88.2, and 79.8%, respectively. These SVR rates were supplemented with published Asian literature and then applied to the decision tree model. The ranges used in sensitivity analyses were supplemented from published clinical trials of DAA or US populations when no Asian data were available.
Table 1.
Baseline demographic data and treatment outcome of the hepatitis C virus cohort
| Descriptions | n | % | Total |
|---|---|---|---|
| Age (mean, range) | 50.6 (24–67) | 91 | |
| Gender | |||
| Male | 83 | 91.2 | 91 |
| Female | 8 | 8.8 | — |
| Race | |||
| Chinese | 33 | 36.3 | 91 |
| Malay | 45 | 49.5 | — |
| Indian | 13 | 14.3 | — |
| Baseline Viral load | |||
| <400 000 IU/ml | 36 | 39.6 | 91 |
| ≥400 000 IU/ml | 55 | 60.4 | |
| Fibrosis score | |||
| 0–1 | 17 | 18.7 | 91 |
| 2 | 7 | 7.7 | — |
| 3 | 12 | 13.2 | — |
| 4 | 45 | 49.5 | — |
| Not done | 10 | 11.0 | — |
| Cirrhosis | 45 | 49.5 | 91 |
| Child‐Pugh A | 41 | 91.1 | 45 |
| Child‐Pugh B | 4 | 8.9 | — |
| Child‐Pugh C | 0 | 0.0 | — |
| Ascites | 8 | 17.8 | 45 |
| Esophageal varices | 16 | 35.6 | 45 |
| HE | 2 | 4.4 | 45 |
| DAA Therapy | 17 | 18.7 | 91 |
| MELD score (mean, range) | 8 (6–14) | — | — |
| SVR | |||
| PR | 59† | 79.7 | 74 |
| DAA | 15‡ | 88.2 | 17 |
| Overall | 74 | 81.3 | 91 |
Seven patients who received PR defaulted treatment.
One patient who received DAA passed away during treatment.
DAA, direct‐acting antivirals; MELD, model of end stage liver disease; PR, pegylated interferon and ribavirin; SVR, sustained virological response.
Costs
A payer’s perspective was adopted and included all direct health‐care costs using billing information from the same cohort of GT3 HCV patients treated at CGH. The overall treatment cost, which includes the cost of medications (i.e. DAA, PR, and medications used to manage the associated side effects such as erythropoietin), outpatient visits, emergency department visits, and hospital admissions because of disease or treatment‐related complications, were all included in the analysis.
Health utilities
Health utilities were obtained from published data.24, 25 At the beginning of the analysis, all patients had a baseline utility of 0.93. A disutility was applied to the PR arm to reflect the negative impact on patient’s quality of life due to the use of subcutaneous injections.26 In addition, we applied a disutility for treatment failure to reflect disease progression based on Asian literature.24 Patients who achieved SVR were assumed to return to perfect health for the rest of the modeled duration, up to 72 weeks as per prior published literature.6
Cost‐effectiveness analysis
The incremental cost‐effectiveness ratio (ICER) per QALY was estimated for DAA and compared with PR. The resultant ICER was interpreted against a willingness‐to‐pay (WTP) threshold, equivalent to Singapore’s per capita gross domestic product (GDP) in 2016 (i.e. USD 53 302/ QALY), to determine if DAA is cost‐effective compared with PR. As there is no explicit WTP threshold available in Singapore, we adopted the WTP threshold equivalent to the per capita GDP following a similar cost‐effectiveness study performed on Singaporean GT1 HCV patients.25
Sensitivity analyses
A one‐way sensitivity analysis was performed on all parameters over a set of plausible ranges from our dataset and published literature (Table 2). Probabilistic sensitivity analysis was performed to account for the uncertainty of model parameters concurrently using a Monte Carlo simulation with 10 000 iterations. We used triangular distributions for all parameters. In addition, we performed the following scenario analyses: 1) we used the discounted prices of DAA (USD 32 325 over 12 weeks) and PR (USD 287/week) to re‐estimate the ICER, and 2) no retreatment was performed for those who had failed DAA as first‐line therapy. The model development and analyses were performed using TreeAge Pro Healthcare 2015 (TreeAge Software, Inc., Williamstown, MA, USA).
Table 2.
Cost and utility variables used in decision tree modeling
| Variables | Base case | References |
|---|---|---|
| SVR | ||
| DAA in TN at week 24 | 0.95 (0.93–0.98) | 23, 27 |
| DAA in TN at week 48 | 0.95 (0.95–0.96) | 28 |
| DAA in TE (PR) at week 72 | 0.9 (0.84–0.91) | 23, 27 |
| PR at week 48 | 0.76 (0.67–0.84) | 8, 9, 10, 29, 30 |
| Utilities, quality of life | ||
| Utility of HCV infection | 0.93 (0.84–1.00) | 24 |
| Utility of SVR at week 72 | 0–1 | 25 |
| Disutility of treatment failure | 0.13 (0.06–0.16) | 25 |
| Disutility of treatment failure at week 12 | 0–0.13 | 24 |
| Disutility of treatment failure at week 24 | 0–0.13 | 24 |
| Disutility of treatment failure at week 48 | 0–0.13 | 24 |
| Disutility associated with using PR | 0.106 (0.091–0.121) | 26 |
| Weekly cost | ||
| PR | 536.18 (429.5–642.8) | CGH HCV cohort |
| DAA | 5592.25 (3847–11 628) | CGH HCV cohort |
| Admission while on DAA | 42.62 (0–396.5) | CGH HCV cohort |
| Outpatient visit while on DAA | 139.43 (61.2–299.2) | CGH HCV cohort |
| ED visit while on DAA | 12.45 (0–243.2) | CGH HCV cohort |
| Admission while on PR | 19.28 (0–281.4) | CGH HCV cohort |
| Outpatient visit while on PR | 100.76 (6.9–345) | CGH HCV cohort |
| ED visit while on PR | 3.93 (0–55) | CGH HCV cohort |
| Erythropoietin while on PR | 0.46 (0–21.3) | CGH HCV cohort |
| Filgrastim while on PR | 0.9 (0–19.6) | CGH HCV cohort |
CGH, Changi General Hospital; DAA, direct‐acting antivirals; ED, emergency department; HCV, hepatitis C virus; PR, pegylated interferon and ribavirin; TE, treatment experienced; TN, treatment naive; SVR, sustained virological response.
Results
In base case analysis, first‐line treatment with DAA using SOF + DAC + ribavirin and PR yielded a QALY of 0.69 and 0.62 at a cost of USD 54 634 and USD 23 857, respectively. The resultant ICER of DAA (SOF + DAC + ribavirin) compared with PR was USD 449 232/QALY. Using a WTP threshold of USD 53 302/QALY, it was found that SOF + DAC + ribavirin is not cost‐effective compared with PR.
One‐way sensitivity analyses showed that the base case ICER was robust for variations in the model parameters (Fig. 2). The cost of DAA regimens was the key driver influencing cost‐effectiveness. Threshold analysis suggested that DAA regimens become cost‐neutral when the weekly cost of DAA is USD 1416.8 (Fig. 3). In order for DAA regimens to be considered cost‐effective as first‐line therapy, a 12‐week regimen of DAA should not exceed USD 17 002.
Figure 2.
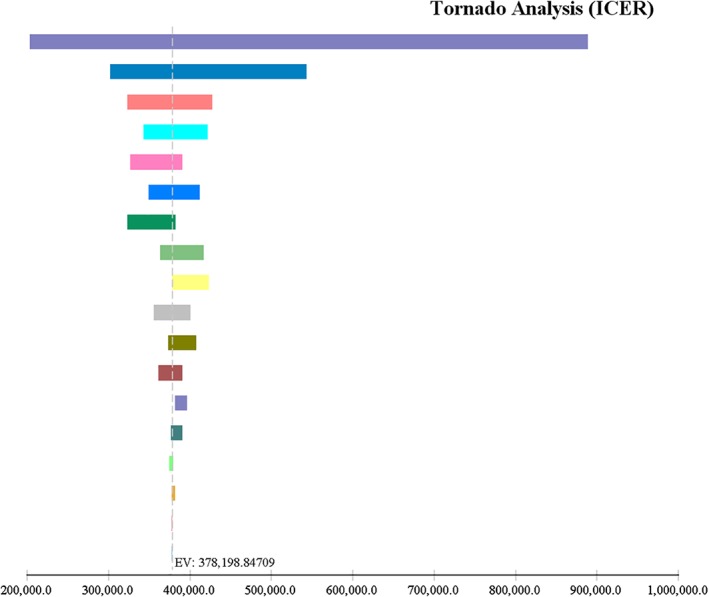
One‐way sensitivity analysis showing all parameters contributing to hepatitis C virus treatment cost. ( ), Weekly cost of direct‐acting antiviral (DAA) (3847.0–11 628.0); (
), Weekly cost of direct‐acting antiviral (DAA) (3847.0–11 628.0); ( ), probability of sustained virological response (SVR) with pegylated interferon and ribavirin (PR) at week 24 (0.67–0.84); (
), probability of sustained virological response (SVR) with pegylated interferon and ribavirin (PR) at week 24 (0.67–0.84); ( ), utility of chronic hepatitis C infection (0.84–1.0); (
), utility of chronic hepatitis C infection (0.84–1.0); ( ), weekly cost of outpatient visits while on PR (6.9–345.0); (
), weekly cost of outpatient visits while on PR (6.9–345.0); ( ), disutility associated with using PR (0.091–0.121); (
), disutility associated with using PR (0.091–0.121); ( ), weekly cost of hospitalization while on PR (0.0–281.4); (
), weekly cost of hospitalization while on PR (0.0–281.4); ( ), disutility of treatment failure (0.06–0.16); (
), disutility of treatment failure (0.06–0.16); ( ), utility of SVR (0.92–1.0); (
), utility of SVR (0.92–1.0); ( ), weekly cost of Pegylated interferon + ribavirin (429.5–642.8); (
), weekly cost of Pegylated interferon + ribavirin (429.5–642.8); ( ), probability of SVR with DAA at week 12 (0.93–0.98); (
), probability of SVR with DAA at week 12 (0.93–0.98); ( ), weekly cost of hospitalization while on DAA (0.0–396.5); (
), weekly cost of hospitalization while on DAA (0.0–396.5); ( ), weekly cost of emergency department (ED) visit while on DAA (0.0–243.2); (
), weekly cost of emergency department (ED) visit while on DAA (0.0–243.2); ( ), weekly cost of outpatient visits while on DAA (61.2–299.2); (
), weekly cost of outpatient visits while on DAA (61.2–299.2); ( ), weekly cost of erythropoietin while on PR (0.0–21.3); (
), weekly cost of erythropoietin while on PR (0.0–21.3); ( ), weekly cost of filgrastim while on PR (0.0–19.6); (
), weekly cost of filgrastim while on PR (0.0–19.6); ( ), probability of SVR with DAA at week 36 (0.84–0.91); (
), probability of SVR with DAA at week 36 (0.84–0.91); ( ), weekly cost of ED visit while on PR (0.0–55.0); (
), weekly cost of ED visit while on PR (0.0–55.0); ( ), probability of SVR with DAA at week 24 (0.95–0.96).
), probability of SVR with DAA at week 24 (0.95–0.96).
Figure 3.
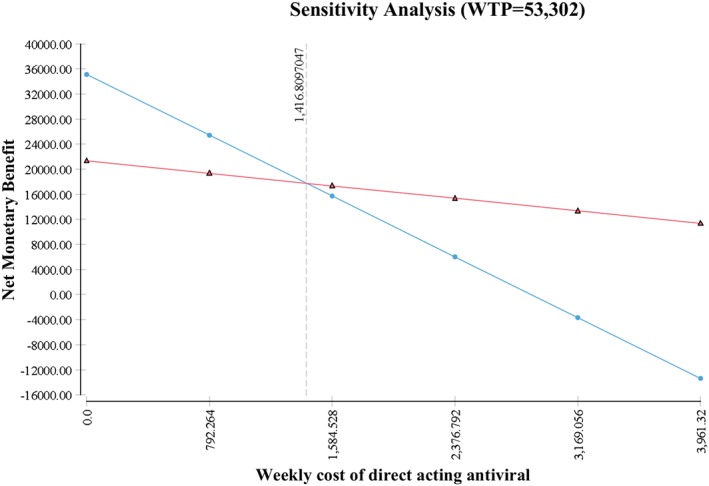
Sensitivity analysis of weekly cost of direct‐acting antiviral (DAA). ( ), DAA; (
), DAA; ( ) peginterferon + ribavirin.
) peginterferon + ribavirin.
The price of both DAA and PR has reduced since 2016. To answer this question at today’s price, we modeled for alternative scenarios and applied the current discounted price of DAA (using sofosbuvir and velpastavir [SOF + VEL] regimen) and PR at the cost of USD 2577/week and USD 287/week, respectively. The resultant cost per therapy to attain an SVR using SOF + VEL as first‐line therapy, with and without consideration of retreatment of primary treatment failures, was USD 35 668 and USD 33 026, respectively (Table 3). Despite the lower ICERs, at the current price, the DAA regimen is not cost‐effective as first‐line therapy in all modeled scenarios as they all exceeded the WTP threshold.
Table 3.
Incremental cost‐effectiveness ratio based on three simulated scenarios
| Strategy | Cost (USD) | Incremental cost (USD) | QALY | ICER |
|---|---|---|---|---|
| Scenario 1 (base case) | ||||
| PR | 23 857.15 | 0 | 0.62 | 0 |
| DAA | 54 634.41 | 30 777.27 | 0.69 | 449 232.57 |
| Scenario 2 (best case) | ||||
| PR | 17 086.19 | 0 | 0.62 | 0 |
| DAA | 35 667.61 | 18 581.42 | 0.69 | 271 218.87 |
| Scenario 3 (no retreatment for DAA arm) | ||||
| PR | 17 086.19 | 0 | 0.62 | 0 |
| DAA | 33 025.56 | 15 939.37 | 0.68 | 259 196.18 |
DAA, direct‐acting antiviral; ICER, incremental cost‐effectiveness ratio; PR, pegylated interferon and ribavirin; QALY, quality‐adjusted life year.
Discussion
While the introduction of highly effective DAA in recent years has expanded the treatment armamentarium of HCV patients, the prohibitively high cost of DAA remains a barrier for treatment access for HCV patients in Singapore. In this study, we demonstrated that DAA regimens, at their listed prices, are not cost‐effective for GT3 HCV patients as first‐line treatment when compared to PR in Singapore. This is evident from the resultant ICER (USD 449 232/QALY), which exceeded the WTP threshold (USD 53 302/QALY) (Table 3). The prior cost‐effectiveness of DAA has been assessed in Singapore among GT1 HCV patients.25 To the best of our knowledge, this is the first cost‐effectiveness analysis performed on the treatment of GT3 HCV infections in Singapore.
Our findings suggest that DAA regimens, at their current cost, are not cost‐effective for GT3 HCV patients as first‐line treatment when compared with PR. We believe this result was obtained because of the high SVR rate of PR treatment among GT3 HCV patients and the high differential prices of DAA in Singapore. Similar SVR rates have been reported in Taiwan and China, with rates ranging from 75 to 84%.29, 30 Due to the high SVR rates associated with PR treatment, incremental SVR with the use of DAA regimens became marginal. Our findings concur with Moshky and colleagues’ finding that DAA is not cost‐effective as first‐line treatment for GT3 HCV patients due to the cost disparity between SOF + DAC + ribavirin and PR.16 The finding is relevant to many developing countries where the cost of DAA remains a barrier to treatment access, particularly in Southeast Asia, where GT3 is the dominant genotype.
In Singapore, the cost of health care is not fully subsidized by the state or insurer and operates on a copay system. Even though the cost of inpatient health care is partially subsidized by the state, the cost of outpatient care, including the cost of medications, is borne by the patients. Thus, the high cost of DAA can become a barrier to treatment access for HCV patients. We incorporated our real‐world treatment costs and outcomes of our study cohort to simulate the typical treatment practices observed in Singapore based on its unique health‐care system. To put this in Singapore’s perspective, the current cost of achieving an SVR with DAA regimens in Singapore ranges from USD 33 024 to USD 109 274 depending on the regimen choice and duration of treatment. For the purpose of this study, we used SOF + DAC + ribavirin. While the overall treatment cost of a 12‐week regimen of SOF + DAC + ribavirin was USD 54 634, the overall treatment cost of a 24‐week PR regimen was only USD 23 857. The price difference per course of treatment is USD 30 777, which means that, for every patient treated with SOF + DAC + ribavirin, it would be possible to treat two additional patients with PR for a marginal compromise in SVR.
The study also considered alternative scenarios with regard to the treatment algorithm. At the time of this study, the combination of SOF + VEL was introduced as an option for GT3 at a reduced price of USD 2577/week. In the same period, the cost of PR had been discounted to USD 287/week. We took the opportunity to model this change in the price of DAA and PR. The resultant cost per therapy of attaining an SVR was USD 35 667 for SOF + VEL and USD 17 086 for PR, considering the cost of retreatment. The resultant ICER of USD 271 000/QALY still exceeds the predefined WTP threshold. Removing the cost of retreatment did not influence the cost per SVR or the ICER sufficiently (Table 3). This suggests that the reduced cost of SOF + VEL, with and without considering retreatment of SOF + VEL failures, may not be sufficient to support the economic argument of using SOF + VEL regimens for GT3 as first‐line therapy.
To determine a price threshold at which DAAs can be considered cost‐effective, we performed threshold analysis for ICER. At the WTP threshold equivalent to Singapore’s per capita GDP (i.e. USD 53 302/QALY), we found that DAA regimens can be cost‐effective when priced under USD 17 002. In conjunction with WHO’s vision to eliminate viral hepatitis by 2030, reduction in the cost of DAA regimens will improve access to DAA. Cost reduction in DAA has led to success stories of HCV elimination in countries with higher HCV prevalence, such as Egypt (15%) and Georgia (7%)31. As the HCV disease burden in Singapore is much lower, the cost reduction of DAA regimens could make HCV elimination a realistic goal for Singapore.
This study should be interpreted within its context of limitations of retrospective design. First, as the patient selection for HCV treatment is physician‐driven, selection bias leading to overestimation the treatment response in the PR group is a concern. The SVR for the DAA cohort is 88.2% because a majority of these patients were cirrhotic. However, it is worth noting that the subjects were comparable between both treatment arms, with most subjects having compensated cirrhosis and none being treatment‐experienced (Table 1). Furthermore, the range of SVRs of the PR group incorporated into the decision tree model was supported by published Asian literature and tested in a robust sensitivity analysis with over 10 000 iterations based on the given SVR range. Second, this study does not take the long‐term gains from an SVR into account but, rather, seeks to determine the immediate cost–benefit of a treatment algorithm. This may have resulted in an underestimation of the long‐term benefits of DAA to health care. However, several studies support that DAA is not cost‐effective as first‐line treatment for GT3 HCV patients, even when simulated over a lifetime period.15, 16 Future economic evaluations that include long‐term outcomes are required to verify this finding, particularly among Asian GT3 HCV patients where SVR is generally higher with interferon‐based treatment. Third, we did not consider reinfection of HCV in our model. Finally, only direct medical costs were included in our study. Direct nonmedical costs, such as transport and paid caregiving, and indirect costs, such as reduced productivity, were not included in this study. Unlike analysis performed from a societal perspective, whereby there is an obligation to care for patients and society by mitigating the economic impact on a larger scale, this analysis was performed from a payer’s perspective, which generally does not include indirect health‐care costs.32
It is important to clarify that this study and its findings do not seek to undermine the value of DAA in treating GT3 HCV. Rather, it seeks to determine the price at which DAA therapy becomes cost‐effective, or at the very least cost‐neutral, for widespread use. This finding is relevant to many developing countries where the treatment cost of DAA remains a barrier to treatment access for HCV infection. In conclusion, our study demonstrated that, at the present prices outlined above, DAA is not cost‐effective as first‐line treatment for GT3 HCV patients compared with PR. In order for DAA to be cost‐effective as first‐line therapy, a 12‐week course of DAA regimens should cost less than USD 17 002 based on a WTP threshold of USD 53 302/QALY. Revision of current DAA prices will help policymakers implement the widespread nationwide elimination of HCV with a reasonable budget impact.
Declaration of conflict of interest: None.
References
- 1. Blach S, Zeuzem S, Manns M et al Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol. Hepatol. 2017; 2: 161–76. [DOI] [PubMed] [Google Scholar]
- 2. Chan HLY, Chen CJ, Omede O et al The present and future disease burden of hepatitis C virus infections with today’s treatment paradigm: volume 4. J. Viral Hepat. 2017; 24(Suppl 2): 25–43. [DOI] [PubMed] [Google Scholar]
- 3. Stanaway JD, Flaxman AD, Naghavi M et al The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016; 388: 1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Razavi H, ElKhoury AC, Elbasha E et al Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013; 57: 2164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long‐term treat‐ Q21 ment outcomes of patients infected with hepatitis C Virus: a systematic review and meta‐analysis of the survival benefit of achieving a sustained virological response. Clin. Infect. Dis. 2015; 61: 730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thrift AP, El‐Serag HB, Kanwal F. Global epidemiology and burden of HCV infection and HCV‐related disease. Nat. Rev. Gastroenterol. Hepatol. 2017; 14: 122–32. [DOI] [PubMed] [Google Scholar]
- 7. Lim SG, Aghemo A, Chen P‐J et al Management of hepatitis C virus infection in the Asia‐Pacific region: an update. Lancet Gastroenterol. Hepatol. 2017; 2: 52–62. [DOI] [PubMed] [Google Scholar]
- 8. Hadziyannis SJ, Sette HJ, Morgan TR et al Peginterferon‐alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 2004; 140: 346–55. [DOI] [PubMed] [Google Scholar]
- 9. Tan SS, Abu Hassan MR, Abdullah A, Ooi BP, Korompis T, Merican MI. Safety and efficacy of an escalating dose regimen of pegylated interferon alpha‐2b in the treatment of haemodialysis patients with chronic hepatitis C. J. Viral Hepat. 2010; 17: 410–18. [DOI] [PubMed] [Google Scholar]
- 10. Rangnekar AS, Fontana RJ. IL‐28B polymorphisms and the response to antiviral therapy in HCV genotype 2 and 3 varies by ethnicity: a meta‐analysis. J. Viral Hepat. 2013; 20: 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacobson IM, Lawitz E, Gane EJ et al Efficacy of 8 weeks of sofosbuvir, velpatasvir, and voxilaprevir in patients with chronic HCV infection: 2 phase 3 randomized trials. Gastroenterology. 2017; 153: 113–22. [DOI] [PubMed] [Google Scholar]
- 12. Foster GR, Pianko S, Brown A et al Efficacy of sofosbuvir plus ribavirin with or without peginterferon‐alfa in patients with hepatitis C virus genotype 3 infection and treatment‐experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology. 2015; 149: 1462–70. [DOI] [PubMed] [Google Scholar]
- 13. Bourliere M, Gordon SC, Flamm SL et al Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N. Engl. J. Med. 2017; 376: 2134–46. [DOI] [PubMed] [Google Scholar]
- 14. Chhatwal J, He T, Hur C, Lopez‐Olivo MA. Direct‐acting antiviral agents for patients with hepatitis C virus genotype 1 infection are cost‐saving. Clin. Gastroenterol. Hepatol. 2017; 15: 827–837.e8. [DOI] [PubMed] [Google Scholar]
- 15. Zhang S, Bastian ND, Griffin PM. Cost‐effectiveness of sofosbuvir‐based treatments for chronic hepatitis C in the US. BMC Gastroenterol. 2015; 15: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moshyk A, Martel M‐J, Tahami Monfared AA, Goeree R. Cost‐effectiveness of daclatasvir plus sofosbuvir‐based regimen for treatment of hepatitis C virus genotype 3 infection in Canada. J. Med. Econ. 2016; 19: 181–92. [DOI] [PubMed] [Google Scholar]
- 17. Najafzadeh M, Andersson K, Shrank WH et al Cost‐effectiveness of novel regimens for the treatment of hepatitis C virus. Ann. Intern. Med. 2015; 162: 407–19. [DOI] [PubMed] [Google Scholar]
- 18. Pfeil AM, Reich O, Guerra IM et al Cost‐effectiveness analysis of sofosbuvir compared to current standard treatment in Swiss patients with chronic hepatitis C. PLoS One. 2015; 10: e0126984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen G‐F, Wei L, Chen J et al Will sofosbuvir/ledipasvir (harvoni) Be cost‐effective and affordable for Chinese patients infected with hepatitis C virus? An economic analysis using real‐world data. PLoS One. 2016; 11: e0155934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Igarashi A, Tang W, Guerra I, Marie L, Cure S, Lopresti M. Cost‐utility analysis of ledipasvir/sofosbuvir for the treatment of genotype 1 chronic hepatitis C in Japan. Curr. Med. Res. Opin. 2017; 33: 11–21. [DOI] [PubMed] [Google Scholar]
- 21. Aggarwal R, Chen Q, Goel A et al Cost‐effectiveness of hepatitis C treatment using generic direct‐acting antivirals available in India. PLoS One. 2017; 12: e0176503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lo AO‐S, Chan HL‐Y, Wong VW‐S, Wong GL‐H. Cost‐effectiveness of the highly effective direct‐acting antivirals in the treatment of chronic hepatitis C in Hong Kong. J. Gastroenterol. Hepatol. 2017; 32: 1071–8. [DOI] [PubMed] [Google Scholar]
- 23. Dan YY, Ferrante SA, Elbasha EH, Hsu T‐Y. Cost‐effectiveness of boceprevir co‐administration versus pegylated interferon‐alpha2b and ribavirin only for patients with hepatitis C genotype 1 in Singapore. Antivir. Ther. 2015; 20: 209–16. [DOI] [PubMed] [Google Scholar]
- 24. Crawford B, Yeung C‐K, Tanaka E, Kraemer M, Leteneux C. Hepatitis C virus in Asia: utility values based on the Short Form‐36 questionnaire. Expert Rev. Pharmacoecon. Outcomes Res. 2012; 12: 765–73. [DOI] [PubMed] [Google Scholar]
- 25. Zhao YJ, Khoo AL, Lin L et al Cost‐effectiveness of strategy‐based approach to the treatment of genotype 1 chronic hepatitis C. J. Gastroenterol. Hepatol. 2016; 31: 1628–37. [DOI] [PubMed] [Google Scholar]
- 26. Westerhout KY, Treur M, Mehnert A, Pascoe K, Ladha I, Belsey J. Cost‐utility analysis of simeprevir with peginterferon + ribavirin (SMV/PR) in the management of genotype 1 (G1) and 4 (G4) hepatitis C virus (HCV) infection; from the perspective of the UK National Health Service (NHS). Value Health. 2014; 17: A679. [DOI] [PubMed] [Google Scholar]
- 27. Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin. Infect. Dis. 2011; 52: 889–900. [DOI] [PubMed] [Google Scholar]
- 28. Mahale P, Engels EA, Li R et al The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut. 2018; 67: 553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu C‐J, Chuang W‐L, Lee C‐M et al Peginterferon alfa‐2a plus ribavirin for the treatment of dual chronic infection with hepatitis B and C viruses. Gastroenterology. 2009; 136: 496–504.e3. [DOI] [PubMed] [Google Scholar]
- 30. Yu J‐W, Wang G‐Q, Sun L‐J, Li X‐G, Li S‐C. Predictive value of rapid virological response and early virological response on the sustained virological response in HCV patients treated with pegylated interferon alpha‐2a and ribavirin. J. Gastroenterol. Hepatol. 2007; 22: 832–6. [DOI] [PubMed] [Google Scholar]
- 31. Amer F. Large‐scale hepatitis C combating campaigns in Egypt and Georgia; past, current and future challanges. J Infect Dev Ctries. 2018; 12: 404–414. [DOI] [PubMed] [Google Scholar]
- 32. Ernst R. Indirect costs and cost‐effectiveness analysis. Value Health. 2006; 9: 253–61. [DOI] [PubMed] [Google Scholar]


