Abstract
Objective
Food allergy (FA) has become a public health issue of global concern. Short‐chain fatty acids (SCFAs) are one of the most important biomarkers of intestinal metabolites. SCFAs may affect the occurrence and development of FA. Currently, no studies have been reported on the mechanism of FA in response to SCFAs. In this study, the common food allergen ovalbumin (OVA) was used for intestinal sensitization in Balb/c mice to study the effect of FA on intestinal barrier function and regulatory T cells in mice, thus providing a new target for the prevention and treatment of FA.
Methods
Twenty BALB/c mice were randomly divided into the experimental group and control group. The experimental group was given OVA, and the control group was given an equal amount of physiological saline. On the 31st day of modeling, the levels of secretory immunoglobulin A (sIgA) and serum total IgE and diamine oxidase (DAO) were determined using enzyme linked immunosorbent assay (ELISA). At the same time, after in vitro stimulation with different concentrations of SCFAs and histone acetylase inhibitor trichostatin A (TSA), the frequency and function of Treg in OVA‐sensitized mice were detected by flow cytometry.
Results
Different concentrations of SCFAs and TSA selectively proliferate Treg cells in a dose‐dependent manner. SCFAs and TSA‐pretreated PBMCs that were injected intravenously into the OVA‐sensitized mice through the tail vein can significantly reduce the expression of IgE, DAO, and sIgA.
Conclusion
SCFAs and TSA can selectively proliferate Tregs and upregulate the expression of anti‐inflammatory cytokines, thereby suppressing allergic reactions.
Keywords: food allergy, regulatory T cells, short‐chain fatty acids
Introduction
Food allergy (FA) refers to the body's adverse immune response to food allergens. It mainly affects the gastrointestinal tract. Its immunological pathways include food‐specific IgE antibody‐mediated effector cell activation, cell‐mediated subacute or chronic inflammatory responses, and both IgE antibodies and cell‐mediated pathways. In recent years, the prevalence of food allergies has been on the rise. Epidemiological surveys show that their progress demonstrates a phase change with age.1 Previous studies suggest that environmental factors have a role in promoting FAs.2 The intestinal tract is one of the major organs involved in FA. Metabolites of intestinal microflora, short‐chain fatty acids (SCFAs), may directly or indirectly affect the occurrence and development of FAs through multiple pathways. Regulatory T cells (Treg) play a very important role in maintaining immune tolerance. One of the important causes of allergic diseases is the disorder or decline in their number.3 At present, no studies have been reported on the mechanism of FA in response to SCFAs. In this study, the common food allergen ovalbumin (OVA) was used for the first enteral sensitization in Balb/c mice,4 and then, the effect of FA on intestinal barrier function and regulatory T cells in mice was studied. The aim of this study was to investigate the effect of SCFA and its receptor‐signaling pathway intervention on regulatory T cells and its protective effect on FA mice to provide a theoretical basis for better prevention and treatment of FAs.
Materials and methods
Animals, reagents, and materials
Specific pathogen free (SPF) grade 6–8‐week‐old Balb/C mice were purchased from the Experimental Animal Center of Huazhong University of Science and Technology. All mice were fed at the Specific Pathogen Free level animal experiment center. All animal experiments were performed according to the regulations of the Animal Experimental Ethics Committee. Acetic acid (100%) was purchased from Merck, Darmstadt, Germany; propionic acid (100%) was purchased from Acros, Brussels, Belgium; butyric acid (99%), histone acetylase inhibitor trichostatin A (TSA), and OVA) were purchased from Sigma, St. Louis, Missouri, USA; aluminum hydroxide gels were purchased from Thermo, Waltham, MA, USA; diamine oxidase (DAO) assay kits were purchased from Nanjing Jiancheng Company; Tregs flow assay kits were purchased from eBioscience, Los Angeles, CA, USA; OVA‐specific IgE and sIgA ELISA kits were purchased from Chondrex, Redmond, Washington, USA; and the mouse IL‐10 and TNF‐α ELISA kits were purchased from eBioscience.
Methods
Grouping and modeling
Twenty Balb/c mice were fed with no test protein, and their body weight was 18–22 g. Males and females were divided into the experimental group (OVA group) and control group (NS group). The sensitization was performed according to the literature method as follows4: (i) Basic sensitization—the animals in the experimental group were injected intraperitoneally with a 0.5 mL solution of sterile physiological saline containing OVA 10 mg and Al(OH)3 1 mg on the first day; (ii) Enhanced sensitization—on the 15th day, 0.5 mL of sterile normal saline containing OVA 10 mg was used for intraperitoneal injection again; (iii) Intragastric administration—on the 20th, 24th, and 28th days, 0.5 mL of sterile normal saline containing OVA 0.5 mg was intragastrically administered. The control group received intraperitoneal injection and intragastric administration of equal volume of normal saline. Diarrhea is a sign of success.
Determination of serum total IgE and DAO
On the 31st day after model establishment, mice were bled by eyeballs and sacrificed. After whole blood was allowed to stand at 37°C for 1 h, serum was separated by centrifugation at 4°C. According to the DAO assay kit instructions, the final microplate reader detects DAO. Serum OVA‐specific IgE levels were determined by ELISA.
Determination of small intestinal mucus sIgA
The ileum of 4 cm at the end of the small intestine of all mice was flattened on the filter paper and then cut longitudinally, and the contents of the intestine and mucus were scraped into the EP tube, fully homogenized by adding 1 mL of PBS, centrifuged at 10 000 g for 15 min, and the supernatant was taken. The mucus slgA was determined by ELISA.
Effect of SCFAs on the frequency and function of Tregs in peripheral blood mononuclear lymphocytes (PBMC) of mice
Peripheral blood PBMCs of OVA‐sensitized mice were isolated, and the cell concentration was adjusted to (1, 2) × 106/L. Then 5, 30 mM acetic acid; 2, 5, 30 mM propionic acid; 0.5, 1, 2, 5, 10 mM butyrate; and 5, 20, 100 ng/mL TSA were added in vitro and incubated at 37°C for 30 min. CD4‐FITC, CD25‐APC, and 7AAD were surface‐stained, light‐shielded at 4° for 30 min, and finally fixed and perforated. FoxP3 was added and placed at 4°C for 30 min. Flow cytometry was used to detect the frequency of CD4+CD25+Foxp3+ Treg cells. Cell supernatants were collected for the detection of cytokines IL‐10 and TNF‐α.
Effect of SCFAs on OVA‐sensitized mice
A total of 2 × 106/L PBMCs were pretreated with 30 mmol/L acetic acid, 30 mmol/L propionic acid, 10 mmol/L butyric acid, and 100 ng/mL TSA for adoptive reinfusion experiments. These cells were injected intravenously into the OVA‐sensitized mice through the tail vein. After 72 h, the serum IgE and DAO levels and the expression of sIgA in the mucus of the small intestine were detected again.
Statistical analysis
The experimental results were analyzed using the SPSS 22.0 statistical software package (IBM Corp., Armonk, NY, USA). An independent sample t‐test was used to compare the mean of the two groups, and an analysis of variance was used to compare the three groups. P < 0.05 was considered statistically significant. The experimental data were plotted using GraphPad Prism 5.0 software (GraphPad Company, San Diego, California, USA).
Results
Serum total IgE and DAO
The serum total IgE content of mice in the experimental group was significantly higher than that of the control group (P = 0.001) (Fig. 1a). The OVA FA mouse model in this study showed a high IgE response, suggesting a successful model establishment. DAO is mostly distributed in the mucosal or villous upper layers of mammals. If the intestinal mucosal mechanical barrier is damaged, then the permeability increases, and DAO will be released into the blood, resulting in increased DAO levels in peripheral blood. The serum DAO content in FA mice was significantly higher than that in the control group (P = 0.005) (Fig. 1b). This result suggests that the intestinal permeability of OVA‐sensitized mice is increased.
Figure 1.
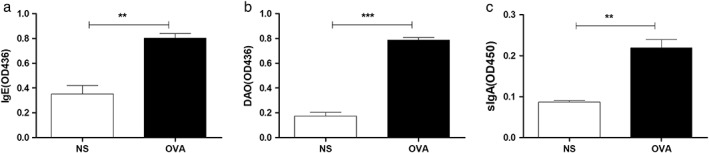
IgE, diamine oxidase (DAO), and intestinal mucosal sIgA expression in ovalbumin (OVA)‐sensitized mice: On the 31st day after model establishment, mice were bled by eyeballs, and small intestinal mucus was collected; ELISA detected serum IgE (a), DAO (b), and small intestinal mucus sIgA (c) expression. **P < 0.01; ***P < 0.001.
Small intestinal mucus sIgA
In the experimental group, the content of slgA in intestinal mucus was significantly higher than that in the control group (P = 0.002) (Fig. 1c). The slgA is an important part of the intestinal barrier, which binds to food antigens and enhances the capture of antigens by the pooled lymph nodules, thereby strengthening the intestinal immune barrier function. This result suggests that, in the mouse model of OVA FA, the sIgA response system of the mouse is hyperactive.
Effects of SCFAs (acetic acid, propionic acid, butyric acid) and TSA treatment on Treg in mouse PBMC
Different concentrations of SCFAs and TSA selectively proliferate Treg cells, with the increase of SCFA concentration. The frequency of Treg cells increased in a dose‐dependent manner (Fig. 2).
Figure 2.
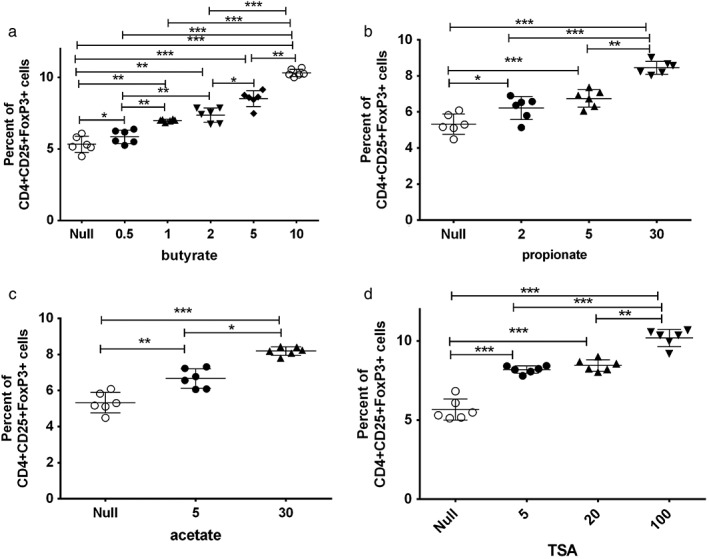
Effects of short‐chain fatty acids (acetic acid, propionic acid, butyric acid) and trichostatin A (TSA) on mouse peripheral blood mononuclear lymphocyte (PBMC) Tregs in vitro: after isolation of PBMCs from ovalbumin (OVA)‐sensitized mice, different concentrations of short‐chain fatty acids (acetic acid, propionic acid, butyric acid) and TSA were stimulated for 30 min, and FACS was used to detect Treg frequency. **P < 0.01; ***P < 0.001.
Effects of SCFAs (acetic acid, propionic acid, butyric acid) and TSA on the secretion of cytokines (IL‐10, TNF‐α) in mouse PBMCs
Different concentrations of SCFAs and TSA promote the expression of anti‐inflammatory cytokines 10 while inhibiting the expression of pro‐inflammatory cytokines TNF‐α. Their expression was also dose‐dependent (Fig. 3).
Figure 3.
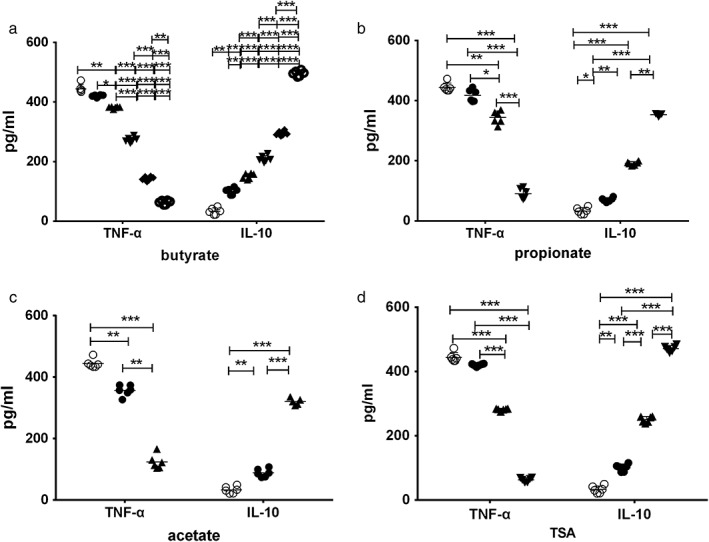
Effect of short‐chain fatty acids (acetic acid, propionic acid, butyric acid) and trichostatin A (TSA) treatment on the secretion of cytokines (IL‐10, TNF‐α) in mouse peripheral blood mononuclear lymphocytes (PBMCs): after isolation of PBMCs from ovalbumin (OVA)‐sensitized mice, different concentrations of short‐chain fatty acids (acetic acid, propionic acid, butyric acid) and TSA were stimulated for 30 min; cell supernatants were collected for the detection of cytokines IL‐10 and TNF‐α. **P < 0.01; ***P < 0.001.(a): ( ), null; (
), null; ( ), 0.5; (
), 0.5; ( ), 1; (
), 1; ( ), 2; (
), 2; ( ), 5; (
), 5; ( ), 10. (b): (
), 10. (b): ( ), null; (
), null; ( ), 2; (
), 2; ( ), 5; (
), 5; ( ), 30. (c): (
), 30. (c): ( ), null; (
), null; ( ), 5; (
), 5; ( ), 30. (d): (
), 30. (d): ( ), null; (
), null; ( ), 5; (
), 5; ( ), 20; (
), 20; ( ), 100.
), 100.
Effects of SCFAs (acetic acid, propionic acid, butyric acid) and TSA‐treated Tregs on expression of IgE, DAO, and sIgA in FA mice
SCFAs and TSA‐pretreated Tregs were transferred to OVA‐sensitized mice and significantly reduced the expression of IgE, DAO, and sIgA (Fig. 4). This result suggests that SCFAs (butyrate, propionate, and acetate) and TSA enhance the function of the intestinal barrier, thus playing an antiallergic role.
Figure 4.
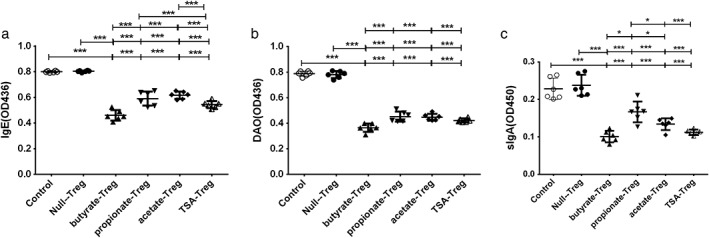
Effect of short‐chain fatty acids (acetic acid, propionic acid, butyric acid) and trichostatin A (TSA) on IgE, diamine oxidase (DAO), and sIgA expression in ovalbumin (OVA)‐sensitized mice: short‐chain fatty acids (SCFAs) and TSA pretreated PBMCs were injected intravenously into the OVA‐sensitized mice through the tail vein. After 72 h, eyelid bleeds and intestinal mucus were collected. ELISA was used to detect serum IgE, DAO, and small intestinal mucus sIgA. *P < 0.05; **P < 0.01; ***P < 0.001. ( ), Control; (
), Control; ( ), null‐Treg; (
), null‐Treg; ( ), butyrate‐Treg; (
), butyrate‐Treg; ( ), propionate‐Treg; (
), propionate‐Treg; ( ), acetate‐Treg; (
), acetate‐Treg; ( ), TSA‐Treg.
), TSA‐Treg.
Discussion
Since 2010, the National Institute of Allergy and Infectious Diseases of the United States formally proposed that FA refers to a reproducible adverse health effect caused by a specific immune response when exposed to a specific food.5 Currently, FA has become a public health problem of global concern. In recent years, the incidence rate has continued to rise. About 5% of adults and up to 8% of children and infants are allergic to one or more foods.6 According to data from Europe and the United States, the incidence of milk protein allergy among infants and young children within 1 year of age is between 2 and 7.5%.7 As children's age continues to increase, allergic diseases will undergo periodic changes.8 The clinical manifestations are different, such as nausea, vomiting, diarrhea, abdominal pain, gastrointestinal disorders, and other gastrointestinal allergy symptoms, and severe cases can cause asthma attacks, shocks, and even death. Prolonged FAs, especially in children with multiple FAs, may affect growth and development. However, the mechanism of FA is still unclear. In addition to the combination of food proteins and impaired oral tolerance,6, 7current etiological studies have focused on Thl/Th2 cell imbalances, hygienic hypotheses, and bacterial toxins.
Tregs is a group of cells with immunosuppressive functions that can inhibit the activation of effector T cells and play an important role in maintaining immune tolerance and immune homeostasis. Its dysfunction or number decline is one of the important causes of autoimmune and allergic diseases.3, 9 Hygiene hypothesis points out the use of antibiotics and improved hygiene conditions. The increase in the prevalence of allergic diseases is due to a decrease in early exposure of microorganisms.10, 11 The establishment of newborn microorganisms starts from birth. The microbial composition of a vaginal‐delivered infant is similar to the mother's vaginal microbiota. However, infants born through caesarean section receive microbiota from the maternal skin, and it has been shown that the incidence of asthma and allergies is high in caesarean.12, 13 Recent research demonstrated that the development of FA is correlated with dysbiosis in a mouse model of allergic dysregulation.14 Food‐allergic mice exhibited reduced abundance in members of the Firmicutes phylum and an increase in Proteobacteria phylum compared with WT mice. The transfer of allergen‐specific Treg cells blocked the development of allergic responses in mice while also preventing the food‐allergic dysbiosis observed in control mice.14 Our study also confirmed that acetic acid, propionic acid, and butyric acid can all selectively proliferate Tregs and upregulate the expression of anti‐inflammatory cytokines, thereby suppressing allergic reactions.
SCFAs are the most important metabolites of the intestinal flora. In vivo, SCFA is mainly composed of acetic acid, propionic acid, and butyric acid, accounting for about 90–95% of the total SCFA. The types and numbers of SCFAs produced by bacteria of different species in the intestine are different, and the intestinal flora in children with allergic diseases is significantly different from those in normal children.7 Studies have demonstrated the ability of SCFAs to restore the frequency and function of intestinal Treg in sterile mice.15 In the gastrointestinal tract, two major factors affecting immune tolerance are the composition and function of diet and microbiota.16 Kim demonstrated that, under normal physiological conditions, macromolecules in the diet induce the development of a large number of Tregs, which is essential for suppressing FAs in the immune response. Observational studies have shown that the early introduction of peanut,17 egg,18 or milk19 may prevent allergic reactions to these foods. There is increasing evidence that the pattern of altered microbial exposure in early life can lead to the development of FA by adversely affecting the development of the immune system.20 Therefore, the gut microbiota can be considered a potential target for preventing and treating FA intervention. This study also confirmed that SCFAs, the main metabolite of the intestinal flora, can reduce allergic reactions in OVA‐sensitized mice.
Over the past decade, environmental factors related to changes in lifestyle may have contributed to our loss of allergen tolerance and may require better improvement. Better understanding on how symbiotic microorganisms or their metabolites affect the production of Treg cells and how ecological disorders affect allergic reactions may provide new strategies for the treatment of allergic diseases.
Acknowledgments
This study is supported by the Health and Family Planning Commission United Fund of Hubei Province (WJ2018H0153).
Declaration of conflict of interest: None.
Funding support: Health and Family Planning Commission United Fund of Hubei Province WJ2018H0153
References
- 1. Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014; 133: 291–307 308. [DOI] [PubMed] [Google Scholar]
- 2. Gilbert JA, Quinn RA, Debelius J et al Microbiome‐wide association studies link dynamic microbial consortia to disease. Nature. 2016; 535: 94–103. [DOI] [PubMed] [Google Scholar]
- 3. Onuora S. Immunology: metabolic changes modify Treg cell function. Nat. Rev. Rheumatol. 2016; 12: 621. [DOI] [PubMed] [Google Scholar]
- 4. Knippels LM, Penninks AH, Spanhaak S, Houben GF. Oral sensitization to food proteins: a Brown Norway rat model. Clin. Exp. Allergy. 1998; 28: 368–75. [DOI] [PubMed] [Google Scholar]
- 5. Boyce JA, Assa'Ad A, Burks AW et al Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID‐sponsored expert panel report. J. Allergy Clin. Immunol. 2010; 126: 1105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nwaru BI, Hickstein L, Panesar SS et al The epidemiology of food allergy in Europe: a systematic review and meta‐analysis. Allergy. 2014; 69: 62–75. [DOI] [PubMed] [Google Scholar]
- 7. Chin S, Vickery BP. Pathogenesis of food allergy in the pediatric patient. Curr. Allergy Asthma Rep. 2012; 12: 621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wickman M. When allergies complicate allergies. Allergy. 2005; 60(Suppl. 79): 14–18. [DOI] [PubMed] [Google Scholar]
- 9. Togashi Y, Nishikawa H. Regulatory T cells: molecular and cellular basis for immunoregulation. Curr. Top. Microbiol. Immunol. 2017; 410: 3–27. [DOI] [PubMed] [Google Scholar]
- 10. Okada H, Kuhn C, Feillet H, Bach JF. The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin. Exp. Immunol. 2010; 160: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N. Engl. J. Med. 2003; 348: 977–85. [DOI] [PubMed] [Google Scholar]
- 12. Jakobsson HE, Abrahamsson TR, Jenmalm MC et al Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014; 63: 559–66. [DOI] [PubMed] [Google Scholar]
- 13. Legatzki A, Rosler B, von Mutius E. Microbiome diversity and asthma and allergy risk. Curr. Allergy Asthma Rep. 2014; 14: 466. [DOI] [PubMed] [Google Scholar]
- 14. Noval RM, Burton OT, Wise P et al A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J. Allergy Clin. Immunol. 2013; 131: 201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith PM, Howitt MR, Panikov N et al The microbial metabolites, short‐chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013; 341: 569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim KS, Hong SW, Han D et al Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016; 351: 858–63. [DOI] [PubMed] [Google Scholar]
- 17. Du Toit G, Katz Y, Sasieni P et al Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J. Allergy Clin. Immunol. 2008; 122: 984–91. [DOI] [PubMed] [Google Scholar]
- 18. Koplin JJ, Osborne NJ, Wake M et al Can early introduction of egg prevent egg allergy in infants? A population‐based study. J. Allergy Clin. Immunol. 2010; 126: 807–13. [DOI] [PubMed] [Google Scholar]
- 19. Katz Y, Rajuan N, Goldberg MR et al Early exposure to cow's milk protein is protective against IgE‐mediated cow's milk protein allergy. J. Allergy Clin. Immunol. 2010; 126: 77–82. [DOI] [PubMed] [Google Scholar]
- 20. Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat. Med. 2016; 22: 713–22. [DOI] [PubMed] [Google Scholar]


