Abstract
Background and Aim
Breath testing has become a commonly used tool in gastroenterology to evaluate changes in the fermentation pattern of the gut microbiome. Currently, hydrogen and methane gas concentrations are measured in breath testing and evaluated against specific cut‐off values for interpretation as normal or abnormal. However, microbial gas kinetics is a complex process that is not currently fully considered when interpreting breath gas results. Gas exchange between hydrogen producers and hydrogen consumers (methanogens and sulfate‐reducing bacteria) is a process whereby hydrogen availability is determined by both its production and removal. Hydrogen sulfide is a crucial gas involved in this process as it is a major hydrogen‐consumptive pathway involved in energy exchange.
Methods
This is a cross‐sectional study evaluating lactulose breath testing with the inclusion of hydrogen sulfide measurements in patients referred for breath testing for gastrointestinal symptoms of bloating, excessive gas, and/or abdominal pain.
Results
A total of 159 patients were analyzed between October 2016 and June 2017. Mean hydrogen concentrations with a positive trend through a 3‐h period (R 2 = 0.97), mean methane concentrations with a positive trend (R 2 = 0.69), and mean hydrogen sulfide concentrations with a negative trend (R 2 = −0.71) were observed.
Conclusion
By incorporating energy exchange in the interpretation of the lactulose breath test, we reevaluated specific breath gas profiles, including those commonly described as “hydrogen nonproducers” and the “double‐peak” phenomenon.
Keywords: breath tests, hydrogen, hydrogen sulfide, methane, microbiome
Introduction
Lactulose breath testing (LBT) has been used as a diagnostic tool for gastrointestinal conditions involving altered microbial fermentation, including small intestinal bacterial overgrowth (SIBO) and maldigestion/malabsorption syndromes.1 Despite breath testing becoming widely used, there is little agreement on the interpretation of results.2 Levitt and colleagues measured hydrogen (H2), carbon dioxide (CO2), methane (CH4), oxygen (O2), nitrogen (N2), hydrogen sulfide (H2S), and ammonia (NH3) in breath and intestinal gas samples collected from healthy subjects.3 The source of breath gases that are exclusively microbial in origin is gut microbial fermentation, with the highly diffusible gases entering the circulation and, subsequently, the pulmonary bed. Of these microbial‐only gases, hydrogen and methane1 are routinely measured in clinical breath testing.
The microbiome of the gastrointestinal tract contains both hydrogen‐producing microbes, mainly Bacteroidetes spp., and hydrogen‐consumptive species, including methanogens (producing methane), sulfate‐reducing bacteria (producing hydrogen sulfide), and acetogens (producing acetate).3, 4, 5 As 40% of the population relies primarily on methanogenesis and only about 5% on acetogenesis, up to 55% of the population relies on sulfate reduction, with H2S production being the main hydrogen‐consumptive pathway.6 However, hydrogen sulfide concentration is not routinely measured in breath tests.
In this study, we will describe the potential relationship between concentration values of hydrogen and that of hydrogen sulfide and/or methane on LBT. We will test the hypothesis that breath testing gas results could be interpreted in terms of a dynamic balance between hydrogen production by fermentation and hydrogen consumption through the competing processes of methanogenesis versus sulfate reduction, sulfate gas elimination, and reaction saturation.
Methods
Patient selection
This study was reviewed and approved by the institutional review board of the New Mexico VA Health Care System. Breath testing data from October 2016 to June 2017 of consecutive patients referred to the GI lab at the Veterans Affairs Medical Center in Albuquerque, NM, USA were reviewed. Data from 159 patients with complete set of data were utilized for the study from a total of 222 patients screened. Patients were referred for a lactulose breath test for symptoms of bloating, excessive gas, and/or abdominal pain. VA providers were aware that breath testing would include hydrogen sulfide measurements, but patients were not specifically referred for hydrogen sulfide testing. Patients were excluded from this study if they had an elevated baseline hydrogen gas concentration of more than 20 parts per million (ppm) prior to lactulose administration, suggestive of inadequate fasting prior to the test; those patients were rescheduled. Patients who did not complete hydrogen sulfide testing were also excluded from the study.
Lactulose breath testing
Gas samples were tested using gas chromatographs for the concentration of hydrogen sulfide in parts per billion (ppb) (OralChroma, Nissha Company, Osaka, Japan) and for the concentrations of hydrogen and methane in ppm (BreathTracker Analyzer, Quintron, Milwaukee, WI, USA). Patients were instructed to stop proton pump inhibitors for at least 7 days prior to the test date and at least 14 days for any recent antibiotic use, colonoscopy, or barium enema. After a 12‐h fast, a 10 g dose of lactulose was administered and consumed orally. Exhaled breath gas samples were collected in 15‐min intervals from 0 (baseline) to 180 min. At each collection time, the gas samples were immediately measured and then transcribed on a standardized recording sheet.
Data analysis
Hydrogen and methane results were evaluated using published criteria.1 For H2, any rise above 20 ppm prior to 90 min was considered abnormal (positive for H2); for CH4, any rise above 3 ppm was interpreted as abnormal (positive for CH4). For H2S, any detectable value was considered positive. Data were analyzed for a double‐peak profile on the basis of an initial peak rise of breath hydrogen of greater than 20 ppm, with a second peak occurring at a later time point with peak‐to‐trough‐to‐peak changes of ≥10 ppm. Data were analyzed for hydrogen nonproducers7, 8, 9 on the basis of a hydrogen flatline with all values ≤10 ppm and an absence of a hydrogen peak. For each subset of patients, the mean concentrations of each gas (H2, CH4, H2S) were calculated along with the standard error of the mean (SEM). One‐way anova analysis was used to calculate differences between each time point for mean H2S concentration compared to baseline. The correlation coefficient was calculated for each gas (H2, CH4, H2S) to show trends through the measurement time period.
Results
LBT data from 159 patients were analyzed (117 males, 42 females). The subjects were 52.0 ± 1.1 years old. All 159 subjects were evaluated and divided into groups based on their breath gas pattern positive for hydrogen, positive for methane, positive for hydrogen sulfide, or positive for all three (Table 1). A total of 96 subjects tested positive for hydrogen gas, 63 subjects tested positive for methane, 39 subjects tested positive for hydrogen sulfide, and 25 subjects tested positive for all three gases.
Table 1.
Subgroups based on positive breath gas concentrations for hydrogen, methane, and hydrogen sulfide along with mean concentration for each gas
| Number per group | (H2) ± SE | (CH4) ± SE | (H2S) ± SE | |
|---|---|---|---|---|
| H2 | 96 | 51.2 ± 4.9 | 11.6 ± 2.2 | 18.8 ± 8.9 |
| CH4 | 63 | 57.2 ± 6.7 | 14.1 ± .5 | 16.5 ± 5.0 |
| H2S | 39 | 51.1 ± 7.9 | 10.6 ± 2.7 | 44.2 ± 20.8 |
| All 3 positive | 25 | 58.5 ± 11.0 | 14.5 ± 3.9 | 31.1 ± 11.6 |
All study subjects are divided into groups that are positive for hydrogen, methane, and hydrogen sulfide gases. For each group, the number of subjects with positive gases is reported (based on interpretation discussed in Methods section), along with mean concentrations ± standard error (SE) of the mean of all three gases per group.
Mean H2 gas measurements at 15‐min intervals showed a positive trend (R 2 = 0.97), with an overall increasing concentration through the 3‐h time period (Fig. 1). The SEM for each time period increased from start to finish of the experiment, suggesting greater variability of hydrogen gas concentration toward the end of the testing period when compared to the beginning of the testing period.
Figure 1.
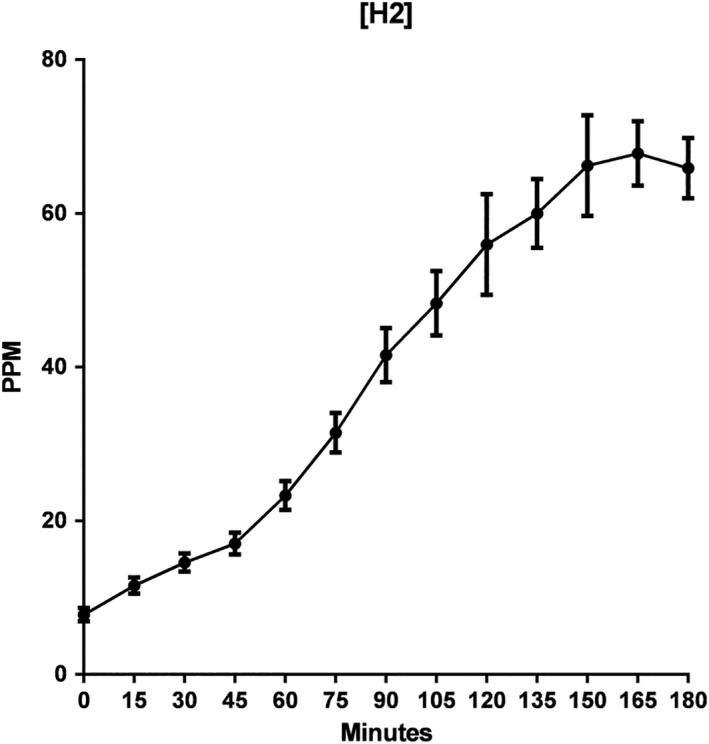
Mean hydrogen (H2) gas concentration in parts per million (ppm) over a 3‐h lactulose breath testing. R 2 of 0.97.
Mean CH4 concentrations also showed a positive trend with an overall increasing concentration (R 2 = 0.69) from start to finish of the 3‐h time period (Fig. 2). Increase in SEM from start to finish of the experiment suggested greater variability of hydrogen gas concentration toward the end of the testing period.
Figure 2.
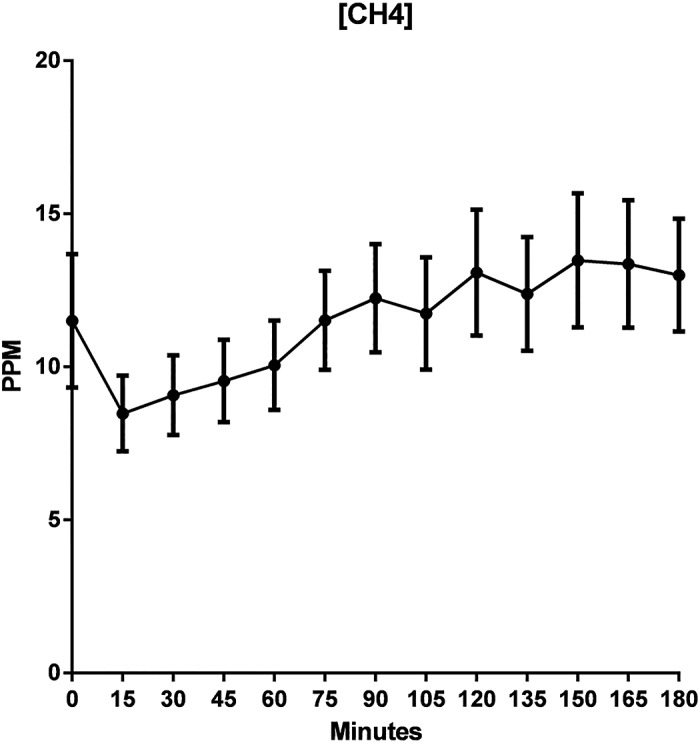
Mean methane (CH4) concentration in parts per million (ppm) over a 3‐h lactulose breath testing. R 2 of 0.71.
In contrast to those of hydrogen or methane, the mean H2S concentrations (Fig. 3) showed a negative (decreasing) trend (R 2 = −0.71) with an overall decreasing concentration through the 3‐h time period; mean H2S concentration along with SEM for each time point are shown in Table 2. Decrease in SEM toward the end of the measuring period suggested less variability of hydrogen sulfide gas concentration when compared to the beginning of the measuring period. Of note, a significant rise in SEM was noted at the 75‐ and 90‐min time points. In addition, the range of concentrations for H2S in ppb is markedly tighter compared to hydrogen and methane in ppm. A high number of individual breath samples measured zero for the concentration of hydrogen sulfide. This contrasted with the consistent detection of hydrogen and methane in every gas sample over the 3‐h time period in those subjects excreting hydrogen or methane.
Figure 3.

Mean values of hydrogen sulfide (H2S) gas in parts per billion (ppb) over a 3‐h lactulose breath testing. R 2 of 0.69.
Table 2.
Mean H2S concentrations and standard error of the mean (SEM) for every 15‐min point
| Time (min) | Mean H2S concentration (ppb) | SEM | P‐value |
|---|---|---|---|
| 0 | 33.89 | 4.40 | — |
| 15 | 26.87 | 4.56 | 0.99 |
| 30 | 27.06 | 4.83 | 0.99 |
| 45 | 27.52 | 6.16 | 0.99 |
| 60 | 20.06 | 4.84 | 0.92 |
| 75 | 30.69 | 10.62 | 0.99 |
| 90 | 32.59 | 12.69 | 0.99 |
| 105 | 10.33 | 2.64 | 0.21 |
| 120 | 12.65 | 4.43 | 0.37 |
| 135 | 9.30 | 4.07 | 0.16 |
| 150 | 8.92 | 3.56 | 0.14 |
| 165 | 7.82 | 3.01 | 0.10 |
| 180 | 4.58 | 2.02 | 0.03 |
Mean H2S concentrations in parts per billion (ppb) provided for each 15‐min measurement along with SEM for each time point. Variability at each 15‐min mark is shown. One‐way anova analysis shows a difference between the mean concentration at the starting point at time 0 and the mean concentration at every 15‐min mark.
Data showing the methane and hydrogen sulfide excretion of hydrogen nonproducers demonstrated that, even as none of the mean hydrogen concentrations reached the >20 ppm threshold to be considered abnormal, methane and hydrogen sulfide were positive (Fig. 4), with methane rising in concentration throughout the allotted time and hydrogen sulfide being initially high and then decreasing in concentration over the measuring period, similar to the pattern shown in Figures 2 and 3.
Figure 4.
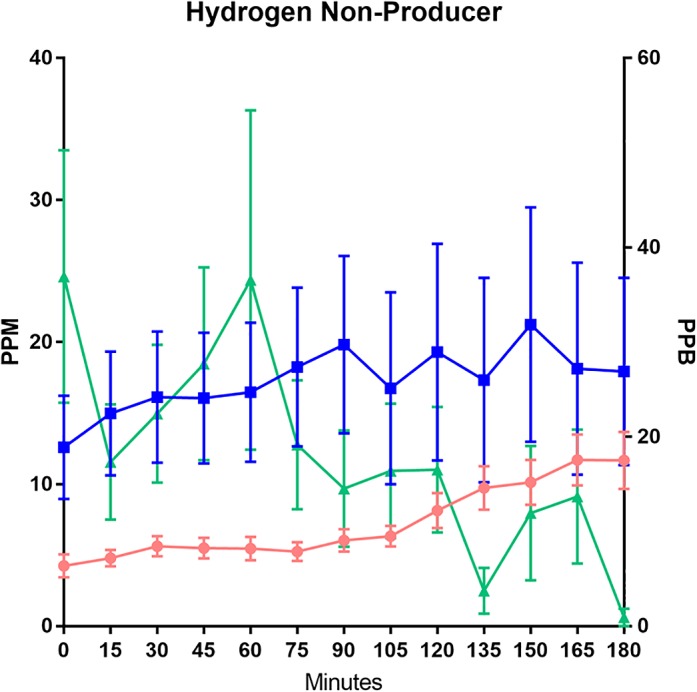
Graphical representation of lactulose breath testing results for patients considered “hydrogen nonproducers”. Hydrogen and methane concentrations are measured in parts per million (ppm) on the left Y‐axis. Hydrogen sulfide gases are measured using parts per billion (ppb) on the right Y‐axis. ( ), Hydrogen; (
), Hydrogen; ( ), methane; (
), methane; ( ), hydrogen sulfide.
), hydrogen sulfide.
Additional analysis was directed at the double‐peak phenomenon of hydrogen gas concentrations (Fig. 5). Hydrogen and methane concentrations were noted to be rising, and hydrogen sulfide concentrations were noted to be falling through the 3‐h time period in patients with a double peak. The mean peak‐to‐trough‐to‐peak change was noted to take place at the 120th min. The first hydrogen peak was noted to have a mean ± SEM of 97.3 ± 16.1 ppm, trough 89.4 ± 6.9 ppm, and the second hydrogen peak was noted at mean ± SEM of 101.3 ± 15.7 ppm. A significantly larger SEM was noted at both hydrogen peaks. This shows that, although the mean values did not reach 10 ppm difference between peak and trough, there is a substantial individual patient variability.
Figure 5.
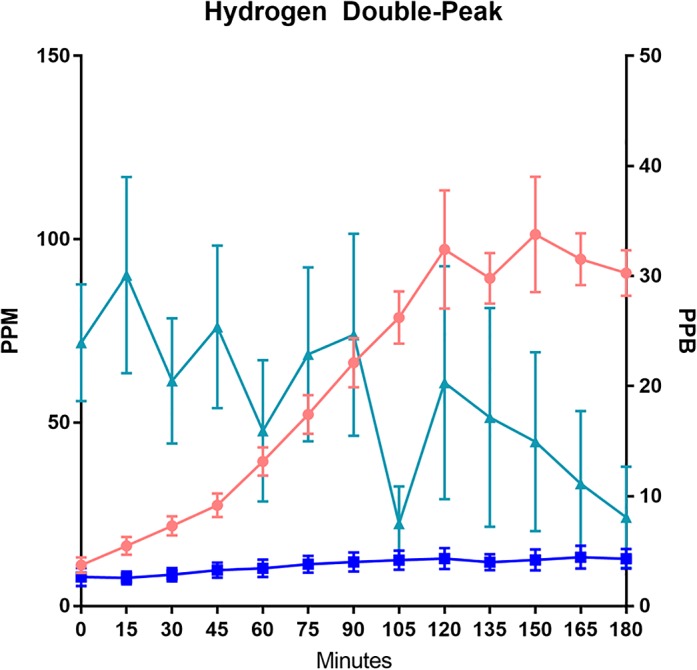
Graphical representation of lactulose breath testing results for patients with hydrogen double peak. Hydrogen and methane concentrations are measured in parts per million (ppm) on the left Y‐axis. Hydrogen sulfide gases are measured using parts per billion (ppb) on the right Y‐axis. ( ), Hydrogen; (
), Hydrogen; ( ), methane; (
), methane; ( ), hydrogen sulfide.
), hydrogen sulfide.
Discussion
The impact of hydrogen producers and hydrogen consumers (methanogens and sulfate‐reducing bacteria) changing the availability of hydrogen in the exhaled breath has not been adequately considered or evaluated. In turn, the interpretation of LBT results has been based on an incomplete picture. As hydrogen consumers convert hydrogen to methane and hydrogen sulfide, the amount of H2 remaining and entering the circulation and appearing in the exhaled breath decreases. As commercially available gas chromatographs measure only H2 and CH4, could the current approach in interpreting breath gas results be flawed when only a part of the gas exchange is seen?
Hydrogen concentration rose throughout the 3‐h period (Fig. 1), while H2S decreased continuously from baseline (Fig. 3) in the same period. As hydrogen sulfide is converted from hydrogen, one might have expected to see a proportional rise in hydrogen sulfide as hydrogen rises.3, 10 Considering the highly toxic nature of hydrogen sulfide, such a proportional rise could be harmful to the human host. Thus, the absence of a direct, proportional relationship between hydrogen and hydrogen sulfide is consistent with the known existence of an effective host mechanism for removing and preventing build‐up of this gas through detoxification.11 H2S is known to be detoxified by colonic mucosa via oxidation,11, 12 by hepatocytes via oxidative metabolism,13 and by blood via enzyme thiol methyltransferase.14, 15 Additional mechanisms for detoxification include expulsion of intestinal gas by the passing of flatus and excretion of gases via pulmonary and nonpulmonary routes, such as the skin.12, 15 As the hydrogen concentration continues to climb across the 3‐h period, with the hydrogen sulfide concentration dropping, a possible interpretation is that sulfate reduction for converting hydrogen to hydrogen sulfide is saturable, but the detoxification for hydrogen sulfide is not. This explanation would account for a continuous decline in the concentration of hydrogen sulfide while hydrogen concentration rises and could explain the precipitous drop in the mean H2S concentration seen at the 90‐min mark (Fig. 3). The difference noted in starting and ending mean H2S concentrations (Table 2) also suggests that the detoxification mechanism is not saturated throughout the 3 h.
Levitt reported that most microbial gases produced in healthy individuals are colonic in source.16 It has long been expected that time‐to‐rise of breath hydrogen during LBT should coincide with the arrival of lactulose in the colon (cecum). This has led to the use of LBT for the measurement of orocecal transit time based on the idea that the rise of breath hydrogen concentration would be timed to the arrival of lactulose in the cecum where fermentation would begin as the fermentable substrate encounters the colon microbial community. However, there are many findings that would argue against this traditional interpretation. For example, there is often an inexplicable discordance between the assumed orocecal transit time as measured by the “time‐to‐rise of breath hydrogen concentration” and the cecal arrival of a radioactive tracer.4 These observations could have an alternative interpretation based on the dynamic balance between hydrogen production and consumption by methanogenesis or sulfate reduction. Scintigraphic cecal arrival could indicate the arrival of the head of lactulose bolus into the cecum. However, breath hydrogen would only rise when hydrogen production has exceeded the hydrogen consumptive processes. Thus, the time to rise for breath hydrogen would always be later in time than scintigraphic cecal entry.
In a study by Yu et al., orocecal scintigraphy was compared to LBT results; these authors found that, in a majority of cases, time to rise of breath hydrogen occurred after cecal arrival by scintigraphy.17 This study concluded that, given the temporal relationship between scintigraphy and breath testing, LBT was not reliable for the diagnosis of SIBO.17 This discrepancy could be explained by the work of hydrogen‐consuming microbes. As hydrogen gas is rapidly used up by hydrogen consumers in methanogenesis or sulfate reduction, the “delayed” time to rise of breath hydrogen, when compared to scintigraphic cecal arrival, could be explained as follows: upon entry into the cecum, lactulose is fermented, and hydrogen is produced, but hydrogen does not appear in the exhaled breath until hydrogen‐consuming pathways are saturated. This dynamic process of energy exchange is further masked from interpretation when hydrogen sulfide is not measured in patients dependent on sulfate reduction as their hydrogen‐consumptive pathway. During scintigraphy, the end‐point of orocecal transit time is measured as the arrival of radionuclide markers to the cecum.7, 9, 18, 19 In contrast, time to rise of breath hydrogen depends on both contact of the fermentable substrate with hydrogen‐producing microbes and their interaction with hydrogen‐consuming microbes along with the entire intestinal tract. As such, there should be a delay between breath hydrogen measurements and the arrival of radioactive tracer in the cecum and rise of breath hydrogen.17, 20, 21, 22, 23 There is no reason for scintigraphic transit and LBT to match perfectly in timing. Additional studies are necessary to evaluate the effects of H2S in relation to orocecal transit time given the significant changes noted in H2S concentration at the 90‐min mark (Fig. 3).
Often, in interpreting LBT, the term “hydrogen nonproducers” is used for a hydrogen concentration profile that is a “flatline”.7, 18, 22, 24, 25, 26, 27, 28 This idea that some individuals may be hydrogen nonproducers conflicts with published studies describing the universal inclusion in the human gut microbiota of Bacteroidetes, a phylum of hydrogen‐producing species.3, 5 When evaluating subjects with a “flatlined” hydrogen time course, we found the concurrent presence of methane and hydrogen sulfide in the exhaled breath, suggesting that hydrogen had been produced as an initial part of fermentation by microbes but that it was converted fully to these other microbial gases (Fig. 4). Our results support that hydrogen consumers have relatively low saturation points and reach their limits quickly. It is only then that hydrogen gas will start to accumulate and enter the circulation, leading to its appearance in the exhaled breath and its recording as a rise in breath H2 concentration on LBT. Based on our interpretation, if the production of hydrogen does not exceed the saturation threshold for the use of hydrogen by hydrogen consumers during a 3‐h LBT, there may not be any measurable hydrogen reaching the exhaled breath, leading to a “flatline” hydrogen profile. Breath testing alone, however, cannot provide a detailed look at saturation points involved in gas kinetics but rather provides measureable final gas concentrations.
Currently, a peak hydrogen concentration exceeding 20 ppm is often used as a threshold criterion for determining an abnormal LBT. Under that usage, a flatlined hydrogen profile would not be considered abnormal. However, based on energy exchange involving hydrogen consumers, a high concentration of hydrogen need not always be present even in the setting of abnormally excessive microbial fermentation and abnormally high hydrogen production if hydrogen‐consumptive capacity were to exceed that of hydrogen production. Measuring hydrogen simultaneously with methane and hydrogen sulfide on LBT may be required to observe the energy exchange involved in the interaction of hydrogen producers and hydrogen consumers. A lack of hydrogen gas in the exhaled breath should not be interpreted as the absence of hydrogen production.
A common pattern seen in LBT is the double‐peak phenomenon where there are two distinct rise and fall patterns of breath hydrogen concentration, with the first rise representing small bowel fermentation and the second rise representing colonic fermentation.29 This pattern has been used as a criterion for diagnosing SIBO.4 Our mean hydrogen profile does show a pattern consistent with double‐peak, with a higher SEM noted at the two peaks (Fig. 5). We found a continuous rise of breath hydrogen throughout the entire testing period, suggesting that the double‐peak phenomenon could be better explained on the basis of a dynamic process whereby the amount of hydrogen produced by fermentation intermittently exceeds the hydrogen‐consumptive capacity to drive a spike in hydrogen concentration. Thus, breath hydrogen concentration rises when the amount of hydrogen exceeds the hydrogen‐consumptive capacity but falls when the hydrogen produced is consumed as the amount of available hydrogen drops below the saturation point for its conversion to either methane or hydrogen sulfide. In addition, it is not surprising that it has been reported that the “small bowel” and “large bowel” breath hydrogen peaks did not match scintigraphic radionuclide locations.4
This study analyzed an alternative interpretation based on LBT with concurrent hydrogen, methane, and hydrogen sulfide concentration results, all available from the same patient. The idea that breath hydrogen concentration may depend on the interaction of hydrogen producers and consumers provides a novel conceptual framework for understanding some of the puzzling findings observed during a lactulose breath test and in several published studies involving LBT and simultaneous scintigraphy. The addition of hydrogen sulfide in the breath gas measurements is affected not only by sulfate reduction by sulfate‐reducing bacteria but also by multiple host detoxification mechanisms. Recording methane gas as the sole route of hydrogen consumption on LBT leads to an incomplete interpretation of the complex interactions involved. We hope that an appreciation and a better understanding of this dynamic system, considering hydrogen production as well as multiple pathways of hydrogen consumptions, will provide researchers with a more complete approach to reviewing lactulose breath tests and should offer the necessary tools to correctly interpret lactulose breath tests in the setting of diseases such as SIBO and irritable bowel syndrome.
Acknowledgment
This study was supported, in part, by the Winkler Bacterial Overgrowth Research Fund.
Declaration of conflict of interest: Dr Lin has patent rights in a related area.
References
- 1. Rezaie A, Buresi M, Lembo A et al Hydrogen and methane‐based breath testing in gastrointestinal disorders: the North American Consensus. Am. J. Gastroenterol. 2017; 112: 775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Stefano M, Mengoli C, Bergonzi M et al Breath methane excretion is not an accurate marker of colonic methane production in irritable bowel syndrome. Am. J. Gastroenterol. 2015; 110: 891–8. [DOI] [PubMed] [Google Scholar]
- 3. Nakamura N, Lin HC, McSweeney CS, Mackie RI, Gaskins HR. Mechanisms of microbial hydrogen disposal in the human colon and implications for health and disease. Annu. Rev. Food Sci. Technol. 2010; 1: 363–95. [DOI] [PubMed] [Google Scholar]
- 4. Zhao J, Zheng X, Chu H et al A study of the methodological and clinical validity of the combined lactulose hydrogen breath test with scintigraphic oro‐cecal transit test for diagnosing small intestinal bacterial overgrowth in IBS patients. Neurogastroenterol. Motil. 2014; 26: 794–802. [DOI] [PubMed] [Google Scholar]
- 5. Levitt MD. Volume and composition of human intestinal gas determined by means of an intestinal washout technic. N. Engl. J. Med. 1971; 284: 1394–8. [DOI] [PubMed] [Google Scholar]
- 6. Triantafyllou K, Chang C, Pimentel M. Methanogens, methane and gastrointestinal motility. J. Neurogastroenterol. Motil. 2014; 20: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghoshal UC. How to interpret hydrogen breath tests. J. Neurogastroenterol. Motil. 2011; 17: 312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pimentel M. Breath testing for small intestinal bacterial overgrowth: should we bother? Am. J. Gastroenterol. 2016; 111: 307–8. [DOI] [PubMed] [Google Scholar]
- 9. Koetse HA, Vonk RJ, Pasterkamp S, Pal J, de Bruijn S, Stellaard F. Variations in colonic H2 and CO2 production as a cause of inadequate diagnosis of carbohydrate maldigestion in breath tests. Scand. J. Gastroenterol. 2000; 35: 607–11. [DOI] [PubMed] [Google Scholar]
- 10. Barton LL, Fardeau M‐L, Fauque GD. Hydrogen sulfide: a toxic gas produced by dissimilatory sulfate and sulfur reduction and consumed by microbial oxidation. Met. Ions Life Sci. 2014; 14: 237–77. [DOI] [PubMed] [Google Scholar]
- 11. Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J. Clin. Invest. 1999; 104: 1107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Furne J, Springfield J, Koenig T, DeMaster E, Levitt MD. Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa. Biochem. Pharmacol. 2001; 62: 255–9. [DOI] [PubMed] [Google Scholar]
- 13. Norris EJ, Culberson CR, Narasimhan S, Clemens MG. The liver as a central regulator of hydrogen sulfide. Shock. 2011; 36: 242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olson KR. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim. Biophys. Acta. 2009; 1787: 856–63. [DOI] [PubMed] [Google Scholar]
- 15. Pitcher MCL, Beatty ER, Harris RM, Waring RH, Cummings JH. Sulfur metabolism in ulcerative colitis: investigation of detoxification enzymes in peripheral blood. Dig. Dis. Sci. 1998; 43: 2080–5. [DOI] [PubMed] [Google Scholar]
- 16. Levitt MD. Production and excretion of hydrogen gas in man. N. Engl. J. Med. 1969; 281: 122–7. [DOI] [PubMed] [Google Scholar]
- 17. Yu D, Cheeseman F, Vanner S. Combined oro‐caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro‐caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011; 60: 334–40. [DOI] [PubMed] [Google Scholar]
- 18. Kajs TM, Fitzgerald JA, Buckner RY et al Influence of a methanogenic flora on the breath H2 and symptom response to ingestion of sorbitol or oat fiber. Am. J. Gastroenterol. 1997; 92: 89–94. [PubMed] [Google Scholar]
- 19. Ning Y, Lou C, Huang Z et al Clinical value of radionuclide small intestine transit time measurement combined with lactulose hydrogen breath test for the diagnosis of bacterial overgrowth in irritable bowel syndrome. Hell. J. Nucl. Med. 2016; 19: 124–9. [DOI] [PubMed] [Google Scholar]
- 20. Kokubo T, Matsui S, Ishiguro M. Meta‐analysis of oro‐cecal transit time in fasting subjects. Pharm. Res. 2013; 30: 402–11. [DOI] [PubMed] [Google Scholar]
- 21. Lin HC, Prather C, Fisher RS et al Measurement of gastrointestinal transit. Dig. Dis. Sci. 2005; 50: 989–1004. [DOI] [PubMed] [Google Scholar]
- 22. Scarpellini E, Abenavoli L, Balsano C, Gabrielli M, Luzza F, Tack J. Breath tests for the assessment of the orocecal transit time. Eur. Rev. Med. Pharmacol. Sci. 2013; 17 (Suppl. 2): 39–44. [PubMed] [Google Scholar]
- 23. Lin EC, Massey BT. Scintigraphy demonstrates high rate of false‐positive results from glucose breath tests for small bowel bacterial overgrowth. Clin. Gastroenterol. Hepatol. 2016; 14: 203–8. [DOI] [PubMed] [Google Scholar]
- 24. Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J. Gastroenterol. 2015; 21: 8787–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. K Sunny J, Garcia CJ, McCallum RW. Interpreting the lactulose breath test for the diagnosis of small intestinal bacterial overgrowth. Am. J. Med. Sci. 2016; 351: 229–32. [DOI] [PubMed] [Google Scholar]
- 26. Moran C, Sheehan D, Shanahan F. The small bowel microbiota. Curr. Opin. Gastroenterol. 2015; 31: 130–6. [DOI] [PubMed] [Google Scholar]
- 27. Yao CK, Tuck CJ. The clinical value of breath hydrogen testing. J. Gastroenterol. Hepatol. 2017; 32: 20–2. [DOI] [PubMed] [Google Scholar]
- 28. Newman A. Breath‐analysis tests in gastroenterology. Gut. 1974; 15: 308–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diggory RT, Cuschieri A. The effect of dose and osmolality of lactulose on the oral‐caecal transit time determined by the hydrogen breath test and the reproducibility of the test in normal subjects. Ann. Clin. Res. 1985; 17: 331–3. [PubMed] [Google Scholar]


