Abstract
Background and Aim
Tacrolimus (TAC) is an important therapeutic option for remission induction in patients with refractory ulcerative colitis (UC). However, there is little evidence available on long‐term outcomes and maintenance treatments after TAC therapy, especially in cases with previous tumor necrosis factor‐α (TNF‐α) inhibitor therapy.
Methods
Long‐term outcomes and remission induction after TAC treatment were retrospectively examined in refractory UC patients with and without previous TNF‐α inhibitor therapy.
Results
The mean disease activity index and the endoscopic activity index scores decreased significantly during the 12‐week treatment after TAC therapy in both groups, showing a significantly greater decrease in the group without TNF‐α inhibitor therapy than in the group with previous TNF‐α inhibitor therapy. One year or more after TAC therapy, TNF‐α inhibitor and/or azathioprine was used as maintenance therapy in most cases in the group without previous TNF‐α inhibitor treatment, while azathioprine was primarily used in the group with previous TNF‐α inhibitor treatment. Colectomy was performed in 45.5% (5/11) and 15.6% (7/45) of the groups with and without previous TNF‐α inhibitor therapy, respectively, and the group without previous TNF‐α inhibitor treatment had a better colectomy‐free rate than the group with previous TNF‐α inhibitor treatment after TAC therapy on Kaplan–Meier analysis.
Conclusions
TAC is effective for remission induction in refractory UC patients with and without previous TNF‐α inhibitor treatment. Maintenance medication after TAC therapy is an issue for the future, especially in UC cases with previous TNF‐α inhibitor treatment failure.
Keywords: long‐term outcomes, maintenance treatments, tacrolimus, tumor necrosis factor‐α inhibitor therapy, ulcerative colitis
Introduction
Tacrolimus (TAC) is effective for patients with ulcerative colitis (UC) refractory to or dependent on corticosteroids (CS) and is usually used as a rescue and bridging therapy before starting azathioprine (AZA) or 6‐mercaptopurine (6‐MP) therapy.1, 2, 3, 4, 5, 6, 7, 8 TAC is one of the calcineurin inhibitors, like cyclosporine (CsA), and it has a 30–100‐fold greater immunosuppressive effect in vitro and a 10–20‐fold greater effect in vivo than CsA, as well as more reliable intestinal absorption, even in the presence of gastrointestinal disease, although TAC and CsA have similar modes of action.1 TAC inhibits transcription of the early activation genes encoding interleukin (IL)‐2, tumor necrosis factor‐α (TNF‐α), and interferon‐γ (IFN‐γ), which are responsible for the development of inflammation.1, 9 In a comparison between calcineurin and TNF‐α inhibitors, CsA was equivalent to infliximab (IFX) for remission induction of refractory UC patients.10 TAC and IFX had similar effects on remission induction in patients with severely active UC.11, 12, 13, 14, 15 TAC is an important therapeutic option for remission induction in refractory UC patients.
Oral TAC was approved for the treatment of steroid‐refractory and steroid‐dependent UC in Japan in 2009, but CsA has not been approved as the standard treatment for UC in Japan.12 The use of higher initial doses of TAC ensured that patients with UC achieved their target levels.16 The treatment duration of oral TAC should be up to 3 months because the long‐term safety and efficacy of TAC have not yet been confirmed.12 Regarding maintenance medication after TAC treatment as remission induction therapy for UC, there have been unsolved problems of long‐term outcomes in refractory UC patients who discontinued oral TAC treatment at up to 3 months and subsequently received other maintenance therapies, such as thiopurine and/or TNF‐α inhibitors, although it has been reported that maintenance treatment with IFX yields better long‐term outcomes than TAC‐thiopurine bridging treatment.13 In addition, it was previously demonstrated that IFX salvage therapy following TAC tended to appear more efficacious in TAC responders (loss of response or no tolerance) than in nonresponders (refractoriness).17 However, there are few reports on TAC salvage therapy following TNF‐α inhibitors in refractory UC cases.
In this study, therefore, long‐term outcomes and remission induction after TAC treatment were retrospectively evaluated in refractory UC patients with and without previous TNF‐α inhibitor therapy using the Mayo score and endoscopic assessment to assess disease activity.
Patients and methods
Patients
Between August 2009 and March 2018, 58 patients with UC resistant to or untreatable with conventional therapy were administered oral TAC at Nagoya City University Hospital after informed consent was obtained. This study was approved by the Institutional Review Board (IRB) at Nagoya City University Hospital and was conducted in accordance with the guidelines of the International Conference on Harmonization and ethical principles originating in the Declaration of Helsinki. Of 58 patients with UC, 56 had received more than 1 month of TAC therapy, while TAC therapy was stopped after less than 2 weeks in two patients. Before the start of TAC treatment, bacterial infectious enteritis was ruled out by stool cultures.1, 8, 18 Clostridium difficile infection was ruled out by C. difficile toxin testing and stool cultures.1, 8, 18 Cytomegalovirus infection was ruled out by pathological analysis of lesions.1, 8, 18 The extent of colonic involvement was determined by total colonoscopy.1, 8
Symptoms and endoscopic assessment
Disease activity before and after oral TAC therapy was assessed using the Mayo score (also known as the disease activity index [DAI]) and the endoscopic activity index (EAI).1, 8, 19, 20 Endoscopy was conducted within 1 week before oral TAC administration, and a second endoscopy was performed to evaluate mucosal healing 12 weeks after the patient was started on oral TAC.8 The Mayo score was evaluated at weeks 0 and 12 after the administration of oral TAC.8 The efficacy end‐points analyzed included response per full Mayo score (decrease of ≥3 points and ≥30% from baseline plus a decrease in the rectal bleeding subscore [RBS] ≥1 or an absolute RBS of ≤1), remission (full Mayo score ≤ 2 with no individual subscore >1), and mucosal healing (endoscopy subscore ≤1) at week 12 according to the previous report.18
Treatment
TAC was administered in its oral formulation.5, 8, 9, 21 According to Japanese protocol, the dosage was adjusted to produce TAC whole‐blood trough levels of 10–15 ng/mL to induce remission. After inducing clinical remission, TAC whole‐blood trough concentrations were maintained at a lower level, between 5 and 10 ng/mL.5, 8, 9, 21 TAC is not currently approved in Japan for maintenance therapy; therefore, TAC administration was stopped 3 months after the patient was started on oral TAC.8, 9
Long‐term outcome
One year or more after TAC therapy, the latest maintenance therapies and colectomy were evaluated in this series of UC patients who received more than 1 month of TAC therapy.
Statistical analyses
For the statistical analyses before and after TAC administration, Wilcoxon's t‐test was used to establish the significance of differences in the Mayo and EAI scores in the UC cases with and without previous TNF‐α inhibitor therapy.8 Fisher's exact test and Welch's t test were used to establish the significance of differences in the baseline characteristics of the UC cases with and without previous TNF‐α inhibitor therapy. Colectomy‐free curves were drawn using the Kaplan–Meier method, and statistical comparison between the UC cases with and without previous TNF‐α inhibitor therapy was performed using the log‐rank test. P‐values < 0.05 were considered significant.
Results
Patients’ characteristics
Of 58 patients with UC, 56 had received more than 1 month of TAC therapy, while the TAC therapy was stopped after less than 2 weeks in two patients because of adverse events (nausea and liver function disorder). The baseline characteristics of the 56 patients receiving more than 1 month of TAC therapy are shown in Table 1. The male/female ratio was 34/22, and the median ages at diagnosis and at start of therapy were 41.8 (range 17–85) and 46.9 (range 19–88) years, respectively. Median disease duration was 61 (range 1–312) months. Of the 56 patients, 40 had extensive disease type, and the other 16 patients had left‐sided disease. Regarding previous response to CS, 12 of the 56 patients (21.4%) were refractory to CS, and 44 (78.6%) were dependent on CS. As concomitant medications, 52 patients received prednisolone, 51 received 5‐aminosalicylates, 8 received immunosuppressants (AZA), and 16 received granulocyte and monocyte adsorptive (GMA) therapies (Table 1). Doses of TAC were adjusted to achieve serum trough levels of 10–15 ng/mL for 2 weeks, followed by tapered serum trough levels of 5–10 ng/mL.
Table 1.
Patients’ baseline characteristics (n = 56)
| All patients (n = 56) | TAC group without previous anti‐TNF therapy (n = 45) | TAC group with previous anti‐TNF therapy (n = 11) | P‐value | |
|---|---|---|---|---|
| Gender (male/female) | 34/22 | 25/20 | 9/2 | n.s. |
| Age at diagnosis (median [range]) (years) | 41.8 (17–85) | 43.0 (17–85) | 35.7 (21–71) | n.s. |
| Age at start of the therapy (median [range]) (years) | 46.9 (19–88) | 48.6 (19–88) | 38.1 (21–71) | n.s. |
| Disease duration (median [range]) (months) | 61 (1–312) | 67 (1–312) | 29 (1–84) | P = 0.01* |
| Extent of disease | ||||
| Extensive (%) | 40 (71.4%) | 30 (66.7%) | 10 (90.9%) | n.s. |
| Left sided (%) | 16 (28.6%) | 15 (33.3%) | 1 (9.1%) | |
| Response to corticosteroids | ||||
| Corticosteroid refractory (%) | 12 (21.4%) | 8 (17.8%) | 4 (36.4%) | n.s. |
| Corticosteroid dependent (%) | 44 (78.6%) | 37 (82.2%) | 7 (63.6%) | |
| Concomitant medication | ||||
| Predonisolone | 52 | 43 | 9 | n.s. |
| 5‐Aminosalicylates | 51 | 40 | 11 | n.s. |
| Immunosuppresants (AZA) | 8 | 6 | 2 | n.s. |
| GMA | 16 | 13 | 3 | n.s. |
P < 0.05.
AZA, azathioprine; GMA, granulocyte and monocyte adsorptive apheresis; TAC, tacrolimus; TNF, tumor necrosis factor.
In the TAC group without previous anti‐TNF therapy, the male/female ratio was 25/20, and the median ages at diagnosis and at start of therapy were 43.0 (range 17–85) and 48.6 (range 19–88) years, respectively. Median disease duration was 67 (range 1–312) months. Of the 45 patients, 30 had extensive disease type, and the other 15 patients had left‐sided disease. Regarding previous CS, 8 (17.8%) patients were refractory to CS, and 37 (82.2%) were dependent on CS. As concomitant medications, 43 patients received prednisolone, 40 received 5‐aminosalicylates, 6 received immunosuppressants, and 13 received GMA therapies (Table 1).
In the TAC group with previous anti‐TNF therapy, the male/female ratio was 9/2, and the median ages at diagnosis and at start of therapy were 35.7 (range 21–71) and 38.1 (range 21–71) years, respectively. Median disease duration was 29 (range 1–84) months. Of the 11 patients, 10 had extensive disease type, and 1 had left‐sided disease. Regarding previous CS, four (36.4%) patients were refractory to CS, and seven (63.6%) were dependent on CS. As concomitant medications, 9 patients received prednisolone, 11 received 5‐aminosalicylates, 2 received immunosuppressants, and 3 received GMA therapies (Table 1).
There was the significant difference of disease duration between the groups with and without previous anti‐TNF therapy (Table 1).
Mayo scores and EAI scores before and after 12 weeks of TAC therapy
Of the 58 patients with UC treated with TAC, 42 received 3 months of TAC therapy, and endoscopic assessment could be performed at week 12 after the start of TAC therapy. However, TAC therapy was stopped because of disease worsening in 14 cases without the endoscopic assessment at week 12 and was stopped in 2 cases experiencing adverse events. Of the 42 patients who underwent endoscopic assessment at week 12, 33 received TAC therapy without previous TNF‐α inhibitor therapy, and 9 received TAC therapy after TNF‐α inhibitor therapy.
In the TAC group without previous TNF‐α inhibitor therapy (n = 33), the mean Mayo score significantly decreased from 9.0 ± 0.4 (average ± SE) at the start of TAC therapy to 3.3 ± 0.6 at week 12 (P = 0.0000044; Fig. 1a). Response and remission rates were 73 and 45%, respectively. In the TAC group with previous TNF‐α inhibitor therapy (n = 9), the mean Mayo score decreased from 8.6 ± 0.7 at the start of TAC therapy to 2.7 ± 1.0 at week 12 (P = 0.012; Fig. 1b). Response and remission rates were 60 and 47%, respectively.
Figure 1.

(a) Mayo scores before and 12 weeks after the start of tacrolimus therapy in the group without previous tumor necrosis factor‐α (TNF‐α) inhibitor therapy. (b) Mayo scores before and 12 weeks after the start of tacrolimus therapy in the group with previous TNF‐α inhibitor therapy.
In the TAC group without previous TNF‐α inhibitor therapy (n = 33), the mean EAI score decreased significantly from 2.5 ± 0.1 (average ± SE) at the start of TAC therapy to 1.1 ± 0.2 at week 12 (P = 0.0000098, Fig. 2a). The mucosal healing rate was 64%, and the rate of endoscopy subscore 0 was 27%. In the TAC group with previous TNF‐α inhibitor therapy (n = 9), the mean EAI score also decreased significantly from 3.0 ± 0.0 at the start of TAC therapy to 0.9 ± 0.4 at week 12 (P=0.012, Fig. 2b). The mucosal healing rate was 53%, and the rate of endoscopy subscore 0 was 26%.
Figure 2.
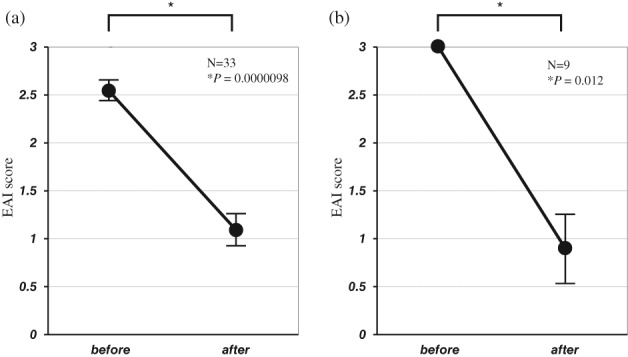
(a) Endoscopic activity index (EAI) scores before and 12 weeks after the start of tacrolimus therapy in the group without previous tumor necrosis factor‐α (TNF‐α) inhibitor therapy. (b) EAI scores before and 12 weeks after the start of tacrolimus therapy in the group with previous TNF‐α inhibitor therapy.
There were no statistical differences in response, remission, mucosal healing, and endoscopy subscore 0 between the groups with and without previous anti‐TNF therapy.
Maintenance therapy or colectomy after TAC therapy
The latest statuses of the TAC group without previous TNF‐α inhibitor therapy (n = 46) and the TAC group with previous TNF‐α inhibitor therapy (n = 12) are summarized in Figures 3 and 4, respectively. In the TAC group without previous TNF‐α inhibitor therapy, seven patients (15.6%) required colectomy. The remaining 38 patients were treated basically with oral 5‐aminosalicylic acid (5‐ASA) if they had no adverse events to 5‐ASA. Of the 38 cases, 10 (22.2%), 15 (33.3%), 5 (11.1%), 1 (2.2%), and 7 (15.6%) patients were treated with the combination of TNF‐α inhibitor and AZA, TNF‐α inhibitor, AZA, continuing TAC, and other therapy, respectively. Regarding other therapy, three entered clinical trials, three had only 5‐ASA therapy, and one had combination 5‐ASA and CS (Fig. 3). In the TAC group with previous TNF‐α inhibitor therapy, five patients (45.5%) required colectomy. The remaining six patients were basically treated with oral 5‐ASA. Of the six cases, one (9.0%), four (36.4), and one (9.0%) patients were treated with TNF‐α inhibitor, AZA, and only oral 5‐ASA therapy, respectively (Fig. 4).
Figure 3.
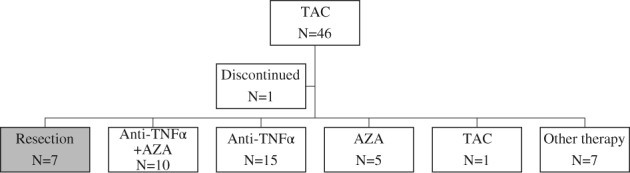
Disposition and flow of patients after tacrolimus therapy in the group without previous TNF‐α inhibitor therapy. (TAC, tacrolimus; TNF, tumor necrosis factor; AZA, azathioprine).
Figure 4.
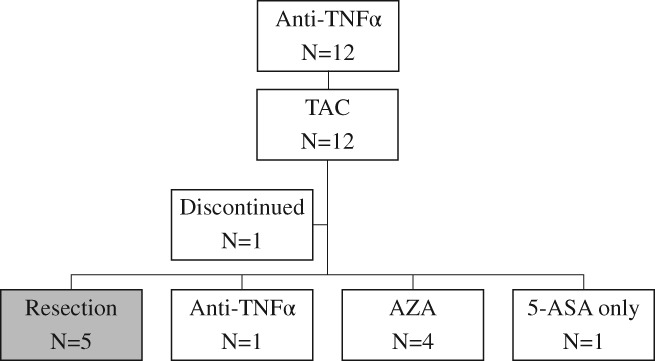
Disposition and flow of patients after tacrolimus therapy in the group with previous TNF‐α inhibitor therapy. (5‐ASA, 5‐aminosalicylic acid; AZA, azathioprine; TAC, tacrolimus; TNF, tumor necrosis factor).
In the TAC group without previous TNF‐α inhibitor therapy (n = 46), 44 cases (95.6%) received oral CS before TAC therapy. One year or more after TAC therapy, 36 cases (78.2%) were steroid‐free, while the remaining 8 (17.4%) were exposed to CS. Of the eight cases, three (6.5%), three (6.5%), and two (4.3%) cases were treated with combination TNF‐α inhibitor and AZA, TNF‐α inhibitor, and other therapy (clinical trials and combination 5‐ASA and CS), respectively. In the TAC group with previous TNF‐α inhibitor therapy (n = 12), 10 cases (83.3%) received oral CS before TAC therapy. One year or more after TAC therapy, all cases were steroid‐free.
The colectomy rates were 15.6% (7/45) in the TAC group without previous TNF‐α inhibitor therapy and 45.5% (5/11) in the TAC group with previous TNF‐α inhibitor therapy.
Colectomy‐free rate after TAC therapy
The median follow‐up period of the 56 patients with more than 1 month of TAC therapy (TAC group without previous TNF‐α inhibitor therapy, n = 45; TAC group with previous TNF‐α inhibitor therapy: n = 11) was 49 (range 1–108) months. On Kaplan–Meier analysis, the overall cumulative colectomy‐free rate was 74.9% at 108 months. The cumulative colectomy‐free rates were 80.9% at 108 (range 1–108) months in the TAC group without previous TNF‐α inhibitor therapy and 42.4% at 84 (range 1–84) months in the TAC group with previous TNF‐α inhibitor therapy (log‐rank: P = 0.02, Fig. 5).
Figure 5.
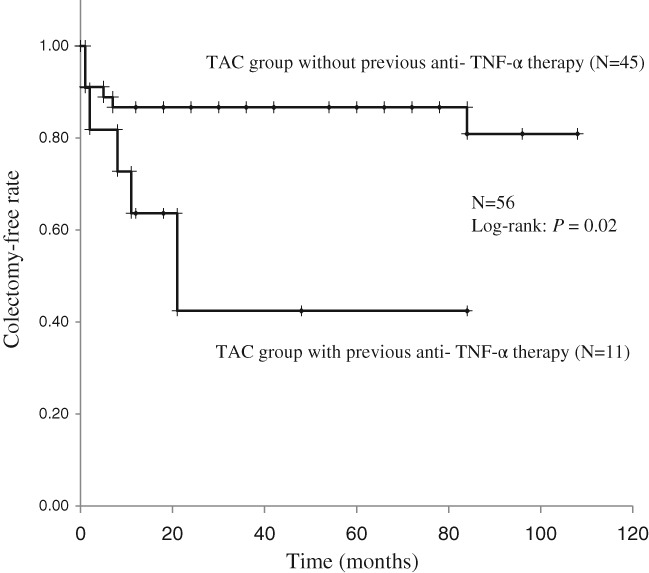
Kaplan–Meier analysis of cumulative colectomy‐free rates. (TAC, tacrolimus; TNF, tumor necrosis factor).
Discussion
The present study results provide clear evidence that the group without previous TNF‐α inhibitor treatment had a better colectomy‐free rate than the group with previous TNF‐α inhibitor treatment after remission induction therapy with TAC in refractory UC patients (Fig. 5). Regarding colectomy after TAC therapy in refractory UC patients, 12 (24.0%) refractory UC patients without previous TNF‐α inhibitor therapy underwent colectomy at week 52.12 The cumulative colectomy‐free survival was 77.3% at 118 (range 2–118) months in refractory UC patients without previous TNF‐α inhibitor therapy.11 The cumulative colectomy rates at 1, 6, and 12 months after treatment initiation were 24.5, 28.2, and 37.8% in the TAC group of refractory UC patients without previous TNF‐α inhibitor therapies.14 In refractory UC cases including both with and without previous TNF‐α inhibitor therapies, the colectomy‐free survival rates at 6, 12, and 18 months were 96, 92, and 82%, respectively.13 To the best of our knowledge, this report appears to be the first involving refractory UC cases to compare colectomy‐free survival after TAC therapy between patients with and without previous TNF‐α inhibitor therapy.
It is well known that TAC appears to be effective for remission induction treatment of moderate/severe UC patients, being equivalent to the remission induction efficacy of IFX.11, 12, 14, 15 However, maintenance treatment after remission induction with TAC therapy is an issue for the future as the treatment duration of oral TAC administration approved in Japan is up to 3 months, suggesting that maintenance treatment with IFX shows better long‐term outcomes than TAC‐thiopurine bridging treatment in refractory UC patients.13 In fact, there is little evidence regarding long‐term outcomes and maintenance treatment after TAC remission induction therapy in refractory active UC patients with or without previous TNF‐α inhibitor therapies. Regarding the long‐term outcomes after TAC therapy in the group without previous TNF‐α inhibitor therapies, of 50 moderate‐to‐severe active UC patients, 35 (70.0%) and 7 (14.0%) patients had AZA and IFX, respectively, as maintenance therapy, while 12 (24.0%) underwent colectomy.12 Of 29 refractory UC patients, 17 (58.6%) and 6 (20.7%) patients had AZA and TNF‐α inhibitor therapies, respectively, as maintenance therapy, while 11 (37.9%) underwent colectomy.14 Of 22 severe UC patients, 11 (50.0%) and 6 (27.3%) had continuing TAC and IFX, respectively, as maintenance therapy, while 5 (22.7%) underwent colectomy.11 In the present study, of 45 refractory UC patients without previous TNF‐α inhibitor treatment, 10 (22.2%), 15 (33.3%), 5 (11.1%), 1 (2.2%), and 7 (15.6%) had TNF‐α inhibitor + AZA, TNF‐α inhibitor, AZA, continuing TAC, and other therapies, respectively, as maintenance therapy, while 7 (15.6%) underwent colectomy (Fig. 3). At least half (25/45, 55.6%) of the patients without a previous TNF‐α inhibitor received it after TAC therapy, suggesting the efficacy of TNF‐α inhibitor as maintenance therapy. We consider that TNF‐α inhibitor is one of the important optional treatments of remission maintenance after the induction of remission with TAC in the UC patients without previous TNF‐α inhibitor therapy. Regarding the long‐term outcomes after TAC therapy in the group with previous TNF‐α inhibitor therapies, of 50 moderate‐to‐severe active UC patients, 3 (6.0%) with TNF‐α inhibitor treatment failure had oral TAC.12 Of these three cases treated with TAC after TNF‐α inhibitor treatment failure, one and two had AZA maintenance therapy and colectomy as surgical resection, respectively.12 Of seven severe UC patients treated with IFX, three (42.9%) with IFX treatment failure had oral TAC; all three had continuing TAC, with clinical remission.11 None of the cases of TNF‐α inhibitor treatment failure had oral TAC therapy.14 In the present study, of 11 refractory UC patients with previous TNF‐α inhibitor treatment, 1 (9.0%), 4 (36.4), and 1 (9.0%) had TNF‐α inhibitor, AZA, and only oral 5‐ASA, respectively, as maintenance therapy, while 5 (45.5%) underwent colectomy (Fig. 4). Regarding other biological therapies in refractory UC patients treated with TAC, there have been two reports of the combination of TAC and vedolizumab,22, 23 although vedolizumab has only recently been approved for UC in July, 2018 in Japan. Further large‐scale studies may also be needed to evaluate the long‐term outcomes and maintenance medication after TAC remission induction therapy in refractory active UC patients with and without previous TNF‐α inhibitor therapy.
The results of the present study provide clear evidence that TAC treatment is effective for remission induction in refractory active UC patients with or without previous TNF‐α inhibitor therapy. Regarding TAC therapy in refractory active UC without previous TNF‐α inhibitor therapies, mucosal healing was achieved in 78.9% (15/19) of patients in the high trough concentration (10–15 ng/mL) group, compared with 12.5% (2/16) in the placebo group, at week 2.1 The clinical remission rate at 12 weeks was 55% in the TAC group of steroid‐refractory active UC patients.14 The clinical remission rate at 14 weeks after treatment was 50% (32/64) in the TAC group in patients with moderate to severe UC.15 The mean DAI score decreased significantly during the 12‐week treatment in the TAC group (P < 0.0001).12 Regarding TAC therapy in the group including refractory active UC patients both with and without previous TNF‐α inhibitor therapy, it was previously shown that both mean DAI and EAI scores were significantly reduced in 26 UC patients receiving more than 1 month of TAC therapy at week 12 after starting oral TAC (P < 0.0001).8 The clinical remission rate at 2 months was 55.3% in the TAC group, including 10.6% with previous IFX treatment, in steroid‐refractory UC patients.13 However, there is little evidence for TAC remission induction therapy in refractory active UC patients with previous TNF‐α inhibitor therapy. In the present study, the mean DAI and EAI scores decreased significantly during the 12‐week treatment after administration of oral TAC in refractory UC patients with previous TNF‐α inhibitor therapy, showing greater decreases in the mean DAI and EAI scores at week 12 after TAC therapy in the group without previous TNF‐α inhibitor therapy than in the group with previous TNF‐α inhibitor therapy. We consider TAC treatment effective for remission induction therapy in refractory UC patients, especially in cases without previous TNF‐α inhibitor therapy.
It is very important to analyze the relevant factors for possible long‐term maintenance after TAC therapy for patients who previously received anti‐TNF‐α agents. We analyzed the factors of the likelihood, including gender, age at diagnosis, age at start of the therapy, disease duration, extent of disease, response to CS, and concomitant medication, but we could not find the candidate factors. Further studies may be needed to evaluate the relevant factors for possible long‐term maintenance after TAC therapy in refractory UC patients with previous TNF‐α inhibitor therapy.
In conclusion, TAC is effective for remission induction in refractory UC patients with and without previous TNF‐α inhibitor treatment. Maintenance medication after TAC therapy is an issue for the future, especially in UC cases with previous TNF‐α inhibitor treatment failure.
Acknowledgments
This study was supported by a Grant‐in‐Aid (Kiban C, 17K09355) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Declaration of Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1. Ogata H, Matsui T, Nakamura M et al A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut. 2006; 55: 1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baumgart DC, Pintoffl JP, Sturm A, Wiedenmann B, Dignass AU. Tacrolimus is safe and effective in patients with severe steroid‐refractory or steroid‐dependent inflammatory bowel disease—a long‐term follow‐up. Am. J. Gastroenterol. 2006; 101: 1048–56. [DOI] [PubMed] [Google Scholar]
- 3. Fellermann K, Tanko Z, Herrlinger KR et al Response of refractory colitis to intravenous or oral tacrolimus (FK506). Inflamm. Bowel Dis. 2002; 8: 317–24. [DOI] [PubMed] [Google Scholar]
- 4. Ng SC, Arebi N, Kamm MA. Medium‐term results of oral tacrolimus treatment in refractory inflammatory bowel disease. Inflamm. Bowel Dis. 2007; 13: 129–34. [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto S, Nakase H, Mikami S et al Long‐term effect of tacrolimus therapy in patients with refractory ulcerative colitis. Aliment. Pharmacol. Ther. 2008; 28: 589–97. [DOI] [PubMed] [Google Scholar]
- 6. Hogenauer C, Wenzl HH, Hinterleitner TA, Petritsch W. Effect of oral tacrolimus (FK 506) on steroid‐refractory moderate/severe ulcerative colitis. Aliment. Pharmacol. Ther. 2003; 18: 415–23. [DOI] [PubMed] [Google Scholar]
- 7. Ziring DA, Wu SS, Mow WS, Martin MG, Mehra M, Ament ME. Oral tacrolimus for steroid‐dependent and steroid‐resistant ulcerative colitis in children. J. Pediatr. Gastroenterol. Nutr. 2007; 45: 306–11. [DOI] [PubMed] [Google Scholar]
- 8. Mizoshita T, Tanida S, Tsukamoto H et al Colon mucosa exhibits loss of ectopic MUC5AC expression in patients with ulcerative colitis treated with oral tacrolimus. ISRN Gastroenterol. 2013; 2013: 304894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mizushima T, Tanida S, Mizoshita T et al A complicated case of tacrolimus‐induced rapid remission after cesarean section in the early third trimester for refractory severe ulcerative colitis flaring in the initial period of gestation. Case Rep. Gastroenterol. 2011; 5: 144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laharie D, Bourreille A, Branche J et al Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open‐label randomised controlled trial. Lancet. 2012; 380: 1909–15. [DOI] [PubMed] [Google Scholar]
- 11. Minami N, Yoshino T, Matsuura M et al Tacrolimus or infliximab for severe ulcerative colitis: short‐term and long‐term data from a retrospective observational study. BMJ Open Gastroenterol. 2015; 2: e000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamamoto T, Shimoyama T, Umegae S, Matsumoto K. Tacrolimus vs. anti‐tumour necrosis factor agents for moderately to severely active ulcerative colitis: a retrospective observational study. Aliment. Pharmacol. Ther. 2016; 43: 705–16. [DOI] [PubMed] [Google Scholar]
- 13. Endo K, Onodera M, Shiga H et al A comparison of short‐ and long‐term therapeutic outcomes of infliximab‐ versus tacrolimus‐based strategies for steroid‐refractory ulcerative colitis. Gastroenterol. Res. Pract. 2016; 2016: 3162595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsumoto S, Kawamura H, Nishikawa T, Sagihara N, Miyatani H, Mashima H. Tacrolimus versus anti‐tumor necrosis factor agents for steroid‐refractory active ulcerative colitis based on the severity of endoscopic findings: a single‐center, open‐label cohort study. Clin. Exp. Gastroenterol. 2017; 10: 249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamagami H, Nishida Y, Nagami Y et al A comparison of short‐term therapeutic efficacy between infliximab and tacrolimus for moderate to severe ulcerative colitis. Rom. J. Intern. Med. 2017; 55: 151–7. [DOI] [PubMed] [Google Scholar]
- 16. Naganuma M, Fujii T, Watanabe M. The use of traditional and newer calcineurin inhibitors in inflammatory bowel disease. J. Gastroenterol. 2011; 46: 129–37. [DOI] [PubMed] [Google Scholar]
- 17. Tsukamoto H, Tanida S, Mizoshita T et al Infliximab salvage therapy for patients with ulcerative colitis who failed to respond to tacrolimus. Eur. J. Gastroenterol. Hepatol. 2013; 25: 714–8. [DOI] [PubMed] [Google Scholar]
- 18. Mizoshita T, Katano T, Tanida S et al Prospective comparison of preference and efficacy of adalimumab and infliximab for treating ulcerative colitis naive to antitumor necrosis factor therapy. Medicine (Baltimore). 2017; 96: e7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987; 317: 1625–9. [DOI] [PubMed] [Google Scholar]
- 20. Sutherland LR, Martin F, Greer S et al 5‐Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987; 92: 1894–8. [DOI] [PubMed] [Google Scholar]
- 21. Yamamoto S, Nakase H, Matsuura M, Masuda S, Inui K, Chiba T. Tacrolimus therapy as an alternative to thiopurines for maintaining remission in patients with refractory ulcerative colitis. J. Clin. Gastroenterol. 2011; 45: 526–30. [DOI] [PubMed] [Google Scholar]
- 22. Hamel B, Wu M, Hamel EO, Bass DM, Park KT. Outcome of tacrolimus and vedolizumab after corticosteroid and anti‐TNF failure in paediatric severe colitis. BMJ Open Gastroenterol. 2018; 5: e000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Christensen B, Gibson P, Micic D et al Safety and efficacy of combination treatment with calcineurin inhibitors and vedolizumab in patients with refractory inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2018. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]


