Abstract
Perianal fistulas in Crohn's disease (CD) represent a highly debilitating and difficult‐to‐treat condition. Given emerging supportive evidence, we conducted a systematic review and meta‐analysis of all trials/observational studies to establish the safety and efficacy of local injections of mesenchymal stem cells (MSCs). The PRISMA‐P statement was applied for planning and reporting, and MEDLINE, EMBASE, Web of Science, Cochrane, CINAHL, ClinicalTrials.gov database, and ECCO 2017 proceedings were searched for published observational studies and one‐arm and randomized clinical trials (RCTs). Safety was assessed in terms of acute local/systemic events, long‐term events, and relatedness with MSC treatment. Efficacy was evaluated in terms of external and/or radiological closure of fistula tracks. After a review of 211 citations, 23 studies, including 696 participants, were evaluated. Four were RCTs with a total of 483 patients. Overall, fistula closure occurred in 80% of MSC‐treated patients. In RCTs, this rate was 64% in the MSC arm and 37% in the control arm (relative risk (RR) = 1.54). Radiological response occurred in 83% of MSC‐treated patients. Treatment‐related adverse events occurred in 1% of MSC‐treated patients, with severe treatment‐related adverse events reaching 0% over a median follow‐up of 6 months. In RCTs, treatment‐related adverse events occurred in 13% in the MSC arm and 24% in the control arm (RR = 0.65). The relapse rate was 0. These results suggest that a local MSC injection is safe and efficacious. Further clinical trials with standardized end‐points are required to ensure the timely implementation of this new therapy in the management of perianal CD.
Keywords: Crohn's disease fistulas, cryptoglandular fistulas, efficacy, mesenchymal stem cells, safety
Introduction
The development of a fistula track is a relatively common feature of Crohn's disease (CD) and is responsible for a large proportion of its morbidity.1 Perianal fistulas can be particularly challenging due to severe symptoms such as pain, embarrassing discharge, and incontinence, with a significant reduction of quality of life.2 Today, combined medical and surgical therapy is understood to perform better than either treatment alone in achieving fistula healing.3 However, the benefit in terms of sustained fistula closure has proven to be limited, with a relapse rate of 16% at 1 year, 31% at 3 years, and 40% at 5 years.4, 5 In addition, the need to use biological agents, even in association with conventional immunosuppressants, carries an increased risk of opportunistic infections and other complications.6 Unrelated to CD, a second and more common etiology of perianal fistulas is cryptoglandular. These are generally easily managed surgically but sometimes display the same anatomic complexity and difficulty to treat as those related to CD.7
In the recent past, the use of mesenchymal stem cell (MSC) injections in the fistula tract has yielded promising results.8, 9, 10, 11 MSCs are multilineage somatic progenitor cells endowed with unique biological properties, including the lack of substantial immunogenicity that allows use across human leukocyte antigen (HLA) barriers12, homing toward sites of active inflammation13 and regenerative capacity.14 Most importantly, MSCs also exert an extraordinary immunomodulating action on all cells involved in immune response, with the ultimate effect of dampening inflammation while restoring tolerance.15 Taken together, these properties make MSCs particularly suitable for the treatment of conditions characterized by both chronic inflammation and tissue damage, such as fistulas in CD. Following a number of observational studies and case series,8, 9, 10, 11 a phase III double‐blind clinical trial was carried out on 212 patients with nonactive or mildly active CD and complex perianal fistulas refractory to at least one conventional or biological therapy who were randomly assigned to receive one local injection of adipose‐derived MSCs (darvadstrocel, formerly Cx601) or a placebo.16 Those patients who were administered darvadstrocel had a significantly higher rate of combined remission at week 24,16 extending to 52 weeks.17 This has led to a positive opinion from the Committee for Medicinal Products for Human Use of the European Medicines Agency regarding using this product to treat complex perianal fistulas in adults with CD upon an inadequate response to at least one conventional or biologic therapy. Therefore, we aimed to carry out a systematic literature review and meta‐analysis of all the data published to evaluate the safety and efficacy of local injections of MSCs in patients suffering with CD and cryptoglandular fistulas, with the goal of helping to clarify the correct placement of this novel treatment option in the therapeutic algorithm.
Materials and methods
Protocol registration
The study protocol was developed according to the PRISMA‐P guidelines18 and was also registered on the PROSPERO website with the number CRD42017076213, which can be accessed on https://www.crd.york.ac.uk/PROSPERO/. A statistical analysis plan was finalized before data extraction and analysis.
Information sources and literature search
This systematic review was conducted and reported in accordance with the PRISMA guidelines.19 We searched the literature as follows: (i) using electronic databases MEDLINE/PubMed, EMBASE, Web of Science, Cochrane databases, CINAHL, and ClinicalTrials.gov; (ii) hand search of the ECCO 2017 congress proceedings; and (iii) personal knowledge. The search strategy is summarized in Tables S1 A,B, Supporting information. Only original articles and abstracts were selected. Reviews, letters, and meta‐analyses were not considered. All relevant articles published through May 15th, 2017 in English, Italian, French, Spanish, or German were considered.
Study selection and data collection
An initial study selection was performed by the librarian based on the eligibility criteria and the content of the abstract; the selection was supervised and refined by the first author (RC), and full texts were downloaded and stored locally. The data were independently retrieved by two authors (RC and CK), and all discrepancies were resolved through a joint session by reexamining the papers. A third author (GRC) was available to reach an agreement if needed. Data were collected into a database set up in REDCap© (Research Electronic Data Capture),20 a secure web‐based platform, and were subsequently exported into Stata 14 (Stata Corporation, College Station; TX, USA) for analysis. Information on study design, study quality, availability of the chosen end‐points, number of patients, and clinical characteristics of the population was retrieved.
Eligibility inclusion criteria
We included randomized clinical trials (RCTs) and one‐arm clinical trials or cohort studies on patients with CD or cryptoglandular fistulas, treated with local injection(s) of autologous or allogeneic MSCs from any source (alone or versus placebo/comparator or standard of care). Any dose and follow‐up duration was considered.
The following safety end‐points were included:
Number of patients with adverse events (AEs).
Number of patients with treatment‐related AEs.
Number of patients with severe treatment‐related AEs.
Number of patients with local acute AEs.
Number of patients with local late AEs.
Number of patients with systemic acute AEs.
Number of patients with systemic late AEs.
Number of AEs per patient per month.
Number of treatment‐related AEs per patient per month.
Number of severe treatment‐related AEs per patient per month.
Death and hospitalizations.
The following efficacy end‐points were retrieved from the articles (as available):
-
12
External healing (complete or partial) based on surgical inspection. In this regard, a fistula track was considered clinically ‘closed’ when it no longer drained despite gentle finger compression; fistula remission was defined as the absence of any draining fistula opening, and response was defined as a reduction of 50% or more in the number of draining fistulas.
-
13
Clinical assessment: calculation of clinical indexes of activity, that is, Crohn's Disease Activity Index (CDAI21) and Perianal Disease Activity Index (PDAI22).
-
14
Deep fistula healing (radiological healing) based on magnetic resonance imaging (MRI) as evaluated according to the categories and score proposed by van Assche et al.23 if available; otherwise, deep healing was considered based on the description reported in the manuscript.
-
15
Mucosal healing by endoscopic examination if available.
-
16
Clinical composite score [combination of (1) and (3)].
-
17
Fistula recurrence.
Risk of bias
Each individual study was assessed for the risk of bias. For RCTs, the risk of bias was assessed at the study level using the Cochrane risk‐of‐bias assessment instrument.24 Biases were assessed across four domains: random sequence generation, allocation concealment, incomplete outcome data, and selective reporting. The corresponding protocols were examined if published. For one‐arm clinical trials and cohort studies, we considered both the incomplete outcome data and methods for controlling confounding items. Each item was classified as having either a high, low, or unclear risk of bias. A second, subjective, assessment of bias used a 0–100 (with 100 being the best) visual analog scale (VAS) and accounted for study design (RCT ranked highest), complete information provided on efficacy (for instance, number of patients for each end‐point), and complete information provided on safety (for instance, number of patients/events for each safety end‐point). The reported VAS for each study was the mean of two authors’/reviewers’ evaluations (RC and CK).
Summary measures and synthesis of results
Patients’ characteristics were summarized using the median and 25–75th percentiles. Within each study and for each study arm (as applicable), the cumulative incidence of events was calculated as the ratio of the total number of patients with events over the total number of patients overall and in each study arm. The monthly incidence rate was calculated as the total number of events over the total number of patients per month in each arm. The time horizon for acute events (healing and AEs) was set at 2 months, and the median study follow‐up was used for late events. For comparative studies, the relative risk (RR) with its 95% confidence interval (95% CI) for each categorical outcome and the standardized mean difference (SMD, computed from the reported mean difference and standard deviation [SD]) for continuous outcomes were derived from the available data. At least three studies were required to derive RR and SMD. For comparative studies, study RR and SMD were pooled according to the DerSimonian and Laird random effects model.25 To this end, single‐arm study cumulative rates and Poisson‐based rates over time estimates were retrieved/calculated. Statistical heterogeneity among studies was assessed using the Cochran Q test and measured by the I‐squared statistic. The presence of publication bias was investigated by the possible asymmetry in the funnel plot. Data were analyzed with Stata 14.
Results
Study selection
As shown in the flowchart in Figure 1, the bibliographic search identified 345 articles, leaving 211 (inclusive of 41 abstracts) after duplicate removal. Twenty full‐text articles and six abstracts were examined for inclusion. After review, an additional three full‐text articles were removed, and a total of 23 studies (17 studies including three substudies and six abstracts) were retained for the review and meta‐analysis.
Figure 1.

PRISMA flowchart showing study disposition from the bibliographic yield.
Study characteristics
As detailed in Table 1, of the 23 publications describing studies,8, 9, 10, 11, 16, 17, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 6 were RCTs. Of these, one17 was a substudy of a previous RCT16 that compared MSC treatment with placebo, and one study,29 where two dosages of MSCs were randomly used, reported only collapsed results, leaving four RCTs available for comparison. Of these, three studies compared MSCs to placebo, while one study compared MSCs to fibrin glue. Ten studies were one‐arm clinical trials, and seven were observational (with two substudies). Thirteen studies (65%) were multicenter.
Table 1.
Studies included in the review and meta‐analysis
| First author (reference number) | Year | Multicenter | Substudy | Design | Phase/number of arms | Arm combination | Total number patients/months of follow‐up |
|---|---|---|---|---|---|---|---|
| Garcia‐Olmo D 26 | 2010 | No | Case series | —/1 | MSC | 1/1 | |
| Cho YB 27 | 2013 | Yes | One‐arm clinical trial | I/1 | MSC | 10/2 | |
| Lee WY 11 | 2013 | Yes | One‐arm clinical trial | II/1 | MSC | 43/2 | |
| Cho YB 28 | 2015 | Yes | Lee WY, 2013 | Cohort univariable | —/1 | MSC | 41/24 |
| Choi S† , ‡ 29 | 2013 | Yes | RCT | II/2 | Low MSC, high MSC | 15/2 | |
| Molendijk I 30 | 2015 | Yes | RCT | II/2 | Placebo, MSC | 21/3 | |
| Panes J 16 | 2016 | Yes | RCT | III/2 | Placebo, MSC | 212/6 | |
| Garcia‐Olmo D 8 | 2005 | No | One‐arm clinical trial | I/1 | MSC | 5/2 | |
| Garcia‐Olmo D 32 | 2003 | No | Case series | —/1 | MSC | 1/3 | |
| Garcia‐Olmo D 31 | 2009 | Yes | RCT | II/2 | Placebo, MSC | 50/2 | |
| Herreros MD§ 33 | 2012 | Yes | RCT | III/3 | MSC, MSC + fibrin glue, fibrin glue | 200/6 | |
| Ciccocioppo R 9 | 2011 | No | Cohort univariable | —/1 | MSC | 12/12 | |
| de la Portilla F 10 | 2013 | Yes | One‐arm clinical trial | II/2 | MSC | 24/6 | |
| Wainstein C 34 | 2016 | No | Cohort univariable | —/1 | MSC | 9/4 | |
| Garcia ‐Arranz M 35 | 2016 | Yes | One‐arm clinical trial | II/1 | MSC | 10/12 | |
| Lightnert AL‡ 36 | 2016 | No | One‐arm clinical trial | I/1 | MSC | 7/6 | |
| Moniuszko A‡ 37 | 2015 | No | Case series | —/1 | MSC | 1/1 | |
| Panes J‡ 17 | 2018 | Yes | Panes J, 2016 | RCT | —/2 | Placebo, MSC | 131/12 |
| Serrero M‡ 38 | 2017 | Yes | One‐arm clinical trial | II/1 | MSC | 9/3 | |
| Park KJ 39 | 2015 | Yes | One‐arm clinical trial | I/1 | MSC | 6/6 | |
| Ciccocioppo R 40 | 2015 | No | Ciccocioppo R, 2011 | Cohort univariable | —/1 | MSC | 8/72 |
| Baixauli‐Fons J‡ 41 | 2016 | Yes | One‐arm clinical trial | II/1 | MSC | 15/12 | |
| Dietz AB 42 | 2017 | Yes | One‐arm clinical trial | I/1 | MSC | 12/6 |
Two MSC arms; cumulative results reported.
Abstract.
Sixty‐eight patients on MSC and 66 patients on fibrin glue arms included in meta‐analysis.
MSC, mesenchymal stem cells; RCT, randomized clinical trial.
As for the etiology, all studies included patients with CD fistulas, except one where patients with cryptoglandular fistulas were also enrolled31 and one with only the last type included.33 As shown in Table 2, in the vast majority of studies, patients suffering from perianal fistulas were included, and MSCs were mainly autologous and mostly derived from adipose tissue.
Table 2.
Study clinical information
| First author | Year | Fistulas | Type of fistula | Type of MSC | MSC source |
|---|---|---|---|---|---|
| Garcia‐Olmo D | 2010 | Crohn | Rectovaginal | Autologous | Adipose tissue |
| Cho YB | 2013 | Crohn | Perianal | Autologous | Adipose tissue |
| Lee WY | 2013 | Crohn | Perianal | Autologous | Adipose tissue |
| Choi S | 2013 | Crohn | Perianal | Autologous | Adipose tissue |
| Molendijk I | 2015 | Crohn | Perianal | Allogeneic | Bone marrow |
| Panes J | 2016 | Crohn | Perianal | Allogeneic | Adipose tissue |
| Garcia‐Olmo D | 2005 | Crohn | Mixed | Autologous | Adipose tissue |
| Garcia‐Olmo D | 2003 | Crohn | Rectovaginal | Autologous | Adipose tissue |
| Garcia‐Olmo D | 2009 | Cryptoglandular and Crohn | Mixed | Autologous | Adipose tissue |
| Herreros MD | 2012 | Cryptoglandular | Perianal | Autologous | Adipose tissue |
| Ciccocioppo R | 2011 | Crohn | Perianal and enterocutaneous | Autologous | Bone marrow |
| de la Portilla F | 2013 | Crohn | Perianal | Allogeneic | Adipose tissue |
| Wainstein C | 2016 | Crohn | Perianal | Autologous | Adipose tissue |
| Garcia ‐Arranz M | 2016 | Crohn | Rectovaginal | Allogeneic | Adipose tissue |
| Lightnert AL | 2016 | Crohn | Perianal | Autologous | Adipose tissue |
| Moniuszko | 2015 | Crohn | Rectovaginal | Autologous | Adipose tissue |
| Serrero M | 2017 | Crohn | Perianal | Autologous | Adipose tissue |
| Park KJ | 2015 | Crohn | Perianal | Allogeneic | Adipose tissue |
| Baixauli‐Fons J | 2016 | Crohn | Mixed | Autologous | Adipose tissue |
| Dietz AB | 2017 | Crohn | Perianal | Autologous | Adipose tissue |
MSC, mesenchymal stem cells.
Overall, 696 patients enrolled in the studies were meta‐analyzed: 494 were treated with MSCs, while 202 were in the control arm. The demographic and clinical characteristics of the enrolled patients are summarized in Table 3. Half of the patients were male; the median age across studies was 36 years. The median disease duration was 10 years. Only a few studies provided information on CDAI and PDAI scores, smoking habits, comorbidities, or concomitant therapy. The dosage of MSC injections ranged from 1 to 9 × 107 cells/mL or 20 to 120 × 106 cells suspended in different volumes, thus preventing the statistical analysis.
Table 3.
Population characteristics: Distribution over studies (median [25–75th])
| Variable | Number of studies | Overall | MSC arm | Control arm |
|---|---|---|---|---|
| Studies | 23 (inclusive of three substudies) | 23 | 23 | 4 |
| Patients | 23 | 696 | 494 | 202 |
| Percent male | 17 | 50 (40–60) | 46 (22–60) | 54.5 (51.5–65.5) |
| Age (years) | 16 | 36.5 (33–41.5) | 35 (32.5–39.5) | 41 (37.5–47.5) |
| CDAI | 3 | 92.7 (89–114) | 102.7 (90.2–204) | 85 (76–94) |
| PDAI | 3 | 6.6 (5.2–6.8) | 6.8 (4.4–13) | 5.9 (5.2–6.6) |
| Percent with comorbidities | 1 | 75 (75–75) | 75 | NA |
| Heart | 1 | 0 | 0 | NA |
| Hypertension | 1 | 8 | 8 | NA |
| Diabetes | 1 | 0 | 0 | NA |
| Lung | 1 | 0 | 0 | NA |
| Kidney | 1 | 33 | 33 | NA |
| Liver | 1 | 50 | 50 | NA |
| Percent currently smoking | 1 | 26.5 (20–33) | 20 (20–20) | 33 |
| Percent with concomitant therapy | 6 | 88 (58.5–100) | 97.5 (77–100) | 60.5 (40–81) |
| Steroid | 3 | 11.5 (5.5–25) | 17 (5–33) | 6 (6–6) |
| Immunosuppressants | 4 | 46.5 (28–58) | 50 (33.5–66.5) | 39. 5 (28–51) |
| Biological drugs | 7 | 0 (0–61) | 0 (0–100) | 30.5 (0–61) |
| Antibiotics | 3 | 39 (12–54) | 54 (8–100) | 25.5 (12–39) |
| Disease duration (years) | 13 | 10 (6.5–12) | 10 (6.5–12) | 8.9 (6.8–11) |
CDAI, Crohn's disease activity index; MSC, mesenchymal stem cells; PDAI, perianal disease activity index.
Table 4 details the number of studies that evaluated a given end‐point. As shown, external healing was the efficacy end‐point most frequently evaluated (18 studies and all four comparative RCTs), together with fistula recurrence (10 studies and three of the RCTs). The presence of AEs or severe AEs, including mortality, was reported in the majority of the original studies (17/20), although the relationship with MSC therapy was less frequently assessed. Hospitalization was reported in five articles.
Table 4.
Number of studies with evaluable end‐points
| Number of studies | Overall | MSC arm | Control arm |
|---|---|---|---|
| Safety | |||
| AE (patients) | 17 | 17 | 4 |
| Related AE (patients) | 12 | 12 | 3 |
| Severe related AE (patients) | 17 | 17 | 4 |
| AE (numbers) | 14 | 14 | 2 |
| Related AE (numbers) | 7 | 7 | 1 |
| Severe related AE (numbers) | 8 | 8 | 2 |
| Death acute | 20 | 20 | 4 |
| Death late | 19 | 19 | 4 |
| Hospitalization | 5 | 5 | / |
| Efficacy | |||
| External healing | 18 | 18 | 4 |
| Radiological healing | 7 | 7 | 1 |
| Endoscopic healing | 2 | 2 | 1 |
| Combined end‐point | 3 | 3 | 3 |
| CDAI | 3 | 3 | 2 |
| PDAI | 3 | 3 | 2 |
| Fistula recurrence | 10 | 10 | 3 |
AE, adverse events; CDAI, Crohn's disease activity index; PDAI, perianal disease activity index; MSC, mesenchymal stem cells.
Results of individual studies and synthesis of results
The cumulative incidence of safety end‐points is reported in Tables 5 and 6. AEs were observed in 53% of patients (34% in the observational studies and 61% in the clinical trials, Table 5). In the four RCTs (Table 6), the cumulative incidence of AEs was similar between the MSC arm (71%) and the control arm (66%), with an RR of 1.06 (95% CI: 0.93–1.22). Treatment‐related AEs (e.g. anal abscess and pain) occurred in 13% (95% CI: 5–24) in the MSC arm compared to 24% (95% CI: 14–35) in the control arm, with an RR of 0.65 (95% CI: 0.43–0.97) favoring the MSC arm. Similarly, severe treatment‐related AEs, which were rare, occurred less frequently in the MSC arm (1%, 95% CI: 0–2) than in the control arm (2%, 95% CI: 0–6). There were no fatal events. Individual and meta‐analysis rates of AEs are shown in Figures S2–S5, Supporting information.
Table 5.
Safety end‐points: Meta‐analytical estimates of cumulative incidence (95% confidence interval) (observational longitudinal studies and mesenchymal stem cell arm of trials)
| Safety end‐points | Overall | Observational studies | Clinical trials | |||
|---|---|---|---|---|---|---|
| N | Incidence (95% CI) | N | Incidence (95% CI) | N | Incidence (95% CI) | |
| Proportion with AEs | 17 | 0.53 (0.30 0.75) | 7 | 0.34 (0.00 ‐ 0.90) | 10 | 0.61 (0.36–0.83) |
| Treatment‐related | 12 | 0.01 (0.00–0.07) | 3 | 0.00 (0.00–0.00) | 9 | 0.04 (0.00–0.12) |
| Severe treatment‐related | 17 | 0.00 (0.00–0.00) | 3 | 0.00 (0.00–0.00) | 14 | 0.00 (0.00–0.01) |
| Acute local | 8 | 0.37 (0.00–0.86) | 1 | 0.00 (0.00–0.98) | 7 | 0.41 (0.03–0.88) |
| Acute systemic | 7 | 0.00 (0.00–0.06) | 2 | 0.50 (0.00–1.00) | 5 | 0.01 (0.00–0.07) |
| Rate of late local AE/patient/month | 7 | 0.00 (0.00–0.01) | 2 | 0.01 (0.00–0.01) | 5 | 0.00 (0.00–0.03) |
| Rate of late systemic AE/patient/month | 7 | 0.01 (0.00–0.03) | 1 | 0.00 (0.00–0.01) | 6 | 0.02 (0.00–0.07) |
| Number of AE/patient/month | 13 | 0.13 (0.05–0.24) | 5 | 0.00 (0.00–0.03) | 8 | 0.26 (0.07–0.52) |
| Treatment‐related | 7 | 0.00 (0.00–0.01) | 2 | 0.00 (0.00–0.02) | 5 | 0.01 (0.00–0.01) |
| Severe treatment‐related | 8 | 0.00 (0.00–0.00) | 2 | 0.00 (0.00–0.00) | 6 | 0.00 (0.00–0.00) |
| Proportion acute death | 20 | 0.00 (0.00–0.00) | 5 | 0.00 (0.00–0.01) | 15 | 0.00 (0.00–0.00) |
| Rate of late death per person/month | 19 | 0.00 (0.00–0.00) | 4 | 0.00 (0.00–0.01) | 15 | 0.00 (0.00–0.00) |
| Rate of rehospitalization per person/months | 5 | 0.01 (0.00–0.03) | 2 | 0.00 (0.00–0.00) | 3 | 0.01 (0.01–0.08) |
95% CI, 95% confidence interval; AE, adverse event; N, number of studies.
Table 6.
Safety end‐points: Meta‐analytical estimates of cumulative incidence (95% confidence interval) and relative risk in randomized clinical trials
| Variable | N | Incidence MSC arm (95% CI) | Incidence CTRL arm (95% CI) | RR (95% CI) |
|---|---|---|---|---|
| Proportion with AEs | 4 | 0.71 (0.35–0.96) | 0.66 (0.38–0.8) | 1.06 (0.93–1.22) |
| Treatment‐related | 3 | 0.13 (0.05–0.24) | 0.24 (0.14–0.35) | 0.65 (0.43–0.97) |
| Severe treatment‐related | 5† | 0.01 (0.00–0.02) | 0.02 (0.00–0.06) | / |
| Acute local | 2 | 0.08 (0.03–0.16) | 0.05 (0.00–0.13) | / |
| Acute systemic | 2 | 0.00 (0.00–0.03) | 0.00 (0.00–0.01) | / |
| Rate of late local AE/patient/month | 2 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | / |
| Rate of late systemic AE/patient/month | 2 | 0.00 (0.00–0.03) | 0.00 (0.00–0.00) | / |
| Number of AE/patient/month | 2 | 0.52 (0.42–0.62) | 0.46 (0.34–0.54) | / |
| Treatment‐related | 1 | 0.00 (0.00–0.00) | 0.00 (0.00–0.19) | / |
| Severe treatment‐related | 2 | 0.00 (0.00–0.02) | 0.02 (0.00–0.06) | / |
| Rate of rehospitalization per person month | 3 | 0.04 (0.00–0.08) | / | / |
One substudy.
95% CI, 95% confidence interval; AE, adverse event; CTRL, control; MSC, mesenchymal stem cells; N, number of studies; RR, relative risk.
The cumulative incidences of meeting efficacy end‐points are listed in Tables 7 and 8. External healing (18 studies) occurred in 80% of patients (84% in the observational studies and 77% in the clinical trials, Table 7 and Fig. 2a). In the four RCTs, (Table 8, Fig. 2b) external healing was observed in 64% of the MSC treated arm and 37% of the control arm, with an RR of 1.57 (95% CI: 1.07–2.31, Fig. 2c) favoring the MSC arm. Similarly, in those studies assessing the combined external and radiological healing end‐point, the RR favored the MSC arm; RR 1.57 (95% CI: 1.07–2.31, Table 6). The incidence of radiological healing (seven studies) was comparable (83% of patients) to that of external healing (Table 7, Fig. S1a). The meta‐analytical incidence rate of fistula recurrence was 0, both in observational studies (95% CI: 0–1) and in clinical trials (95% CI: 0–4) (Fig. S1b,c), over a median follow‐up of 6 months (25–75th: 2.5–9 months).
Table 7.
Efficacy end‐points: Meta‐analytical estimates of cumulative incidence (95% confidence interval) (observational longitudinal studies and mesenchymal stem cell arm of trials)
| Efficacy end‐point | Overall | Observational studies | Clinical trials | |||
|---|---|---|---|---|---|---|
| N | Incidence (95% CI) | N | Incidence (95% CI) | N | Incidence (95% CI) | |
| Proportion with external healing | 18 | 0.80 (0.70 to 0.89) | 5 | 0.84 (0.58–1.00) | 13 | 0.77 (0.67–0.89) |
| Partial | 14 | 0.22 (0.10 to 0.36) | 4 | 0.14 (0.00 to 0.43) | 10 | 0.25 (0.12 to 0.40) |
| Total | 16 | 0.51 (00.40 to 0.62) | 5 | 0.56 (0.28 to 0.83) | 11 | 0.50 (0.38 to 0.62) |
| Proportion with radiological healing | 7 | 0.83 (0.65 to 0.96) | 2 | 0.72 (0.50 to 0.90) | 5 | 0.88 (0.65 to 1.00) |
| Proportion with endoscopic healing | 2 | 0.17 (0.04 to 0.35) | 1 | 0.58 (0.28 to 0.85) | 1 | 0.00 (0.00 to 0.22) |
| Proportion with combined response | 3 | 0.48 (0.40 to 0.57) | 0 | / | 3 | 0.48 (0.40 to 0.57) |
| Rate of recurrence (per person months) | 10 | 0.00 (0.00 to 0.01) | 4 | 0.00 (0.00 to 0.03) | 6 | 0.00 (0.00 to 0.04) |
| Median (25 to –75th) | Median (25 to –75th) | Median (25 to –75th) | ||||
| Change in CDAI | 3 | 12 (−5.7 to 195) | 1 | 195 (195 to 195) | 2 | 3.15 (−5.7 to 12.00) |
| Change in PDAI | 3 | 2.3 (1.8 to 8.5) | 1 | 8.5 (8.5 to 8.5) | 2 | 2.05 (1.80 to 2.30) |
95% CI, 95% confidence interval; CDAI, Crohn's disease activity index; N, number of studies; PDAI, perianal disease activity index.
Table 8.
Efficacy end‐points: Meta‐analytical estimates of cumulative incidence (95% confidence interval) and relative risk (RR) in randomized clinical trials
| Variable | Number of RCTs | Incidence MSC arm (95% CI) | Incidence CTRL arm (95% CI) | RR (95% CI) |
|---|---|---|---|---|
| Proportion with external healing | 4 | 0.64 (0.57 to 0.70) | 0.37 (0.21 to 0.55) | 1.54 (1.03 to 2.29) |
| Partial | 3 | 0.22 (0.06 to 0.44) | 0.09 (0.04 to 0.15) | 2.06 (0.60 to 7.04) |
| Total | 3 | 0.42 (0.24 to 0.62) | 0.25 (0.04 to 0.55) | 1.34 (1.02 to 1.77) |
| Proportion with radiological healing | 1 | 0.53 (0.27 to 0.79) | 0.27 (0.00 to 0.64) | / |
| Proportion with endoscopic healing | 1 | 0.00 (0.00 to 0.22) | 0.00 (0.00 to 0.48) | / |
| Proportion with combined response | 3 | 0.48 (0.40 to 0.57) | 0.35 (0.29 to 0.41) | 1.57 (1.07 to 2.31) |
| Rate of recurrence (per person months) | 3 | 0.00 (0.00 to 0.02) | 0.00 (0.00 to 0.01) | / |
| Median (25 to –75th) | Median (25 to –75th) | SMD | ||
| Change in CDAI | 2 | 3.15 (−5.70 to 12.00) | −28.10 (−54.00 to −2.20) | / |
| Change in PDAI | 2 | 2.05 (1.80 to 2.30) | 0.50 (−0.30 to 1.30) | / |
95% CI, 95% confidence interval; CDAI, Crohn's disease activity index; CTRL, control; MSC, mesenchymal stem cells; N, number of studies; PDAI, perianal disease activity index; RCT, randomized clinical trial; RR, relative risk: SMD, standardized mean difference.
Figure 2.
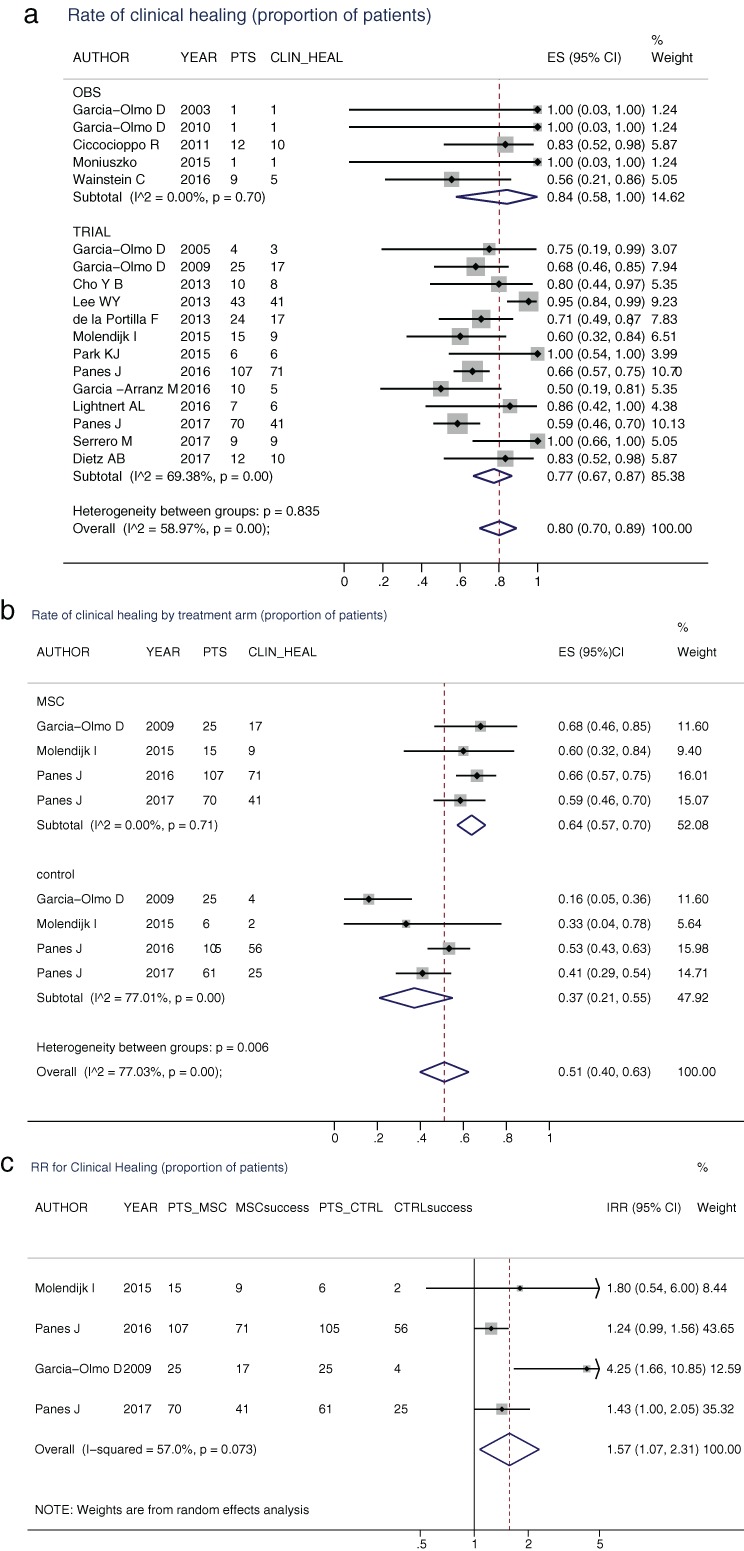
(a) Cumulative incidence of clinical healing by study design. Diamonds represent the meta‐analytical estimate of the cumulative incidence (95% CI) of clinical healing for observational studies (OBS), for clinical trials (TRIAL), and overall. Dots and whiskers represent the incidences and 95% CIs derived from the single studies. The dotted vertical line corresponds to the overall incidence. (b) Cumulative incidence of clinical healing by treatment arm in the four randomized clinical trials (RCTs). (c) Relative risk (RR) of healing in the four RCTs. The diamond represents the meta‐analytical estimate of the RR (95% CI) of clinical healing. Dots and whiskers represent the RRs and 95% CIs derived from the single studies. The continuous vertical line corresponds to null effect, the dotted vertical line to the meta‐analytical RR.
Risk of bias
None of the observational studies and single‐arm trials was controlled for confounding, mostly due to the low number of patients included in each study. Randomization and concealment was adequate in three RCTs but was unclear or not described in the other cases. The assessment of missing data was unclear or not considered in 10 studies, and reporting was lacking in 16 studies. The subjective assessment of the risk of bias across all studies yielded a median VAS of 22 (25–75th: 11–60). No evidence of publication bias was elicited from the funnel plots of the RCTs (Fig. 3) when considering the efficacy end‐points, external healing and the clinical composite, or the safety end‐points, AEs and treatment‐related AEs.
Figure 3.

Funnel plots for the identification of publication bias for the efficacy end‐points in terms of clinical healing (a) and combined healing (b) and for the safety end‐points in terms of adverse events (c) and treatment‐related adverse events (d). (LN_IRR, log‐transformed incidence rate ratio; s.e., standard error).
Discussion
MSC therapy is an emerging potential treatment for a number of medical conditions triggered and sustained by a dysregulated immune response resulting in tissue damage.15 This systematic literature review with meta‐analysis was conducted to assess the safety and efficacy of MSC local injections in fistulas (most perianal) of both CD and cryptoglandular origin, thus providing information to clinicians and patients considering this treatment option. Our analysis clearly shows that the use of MSCs results in a high rate of external and radiological healing in patients with perianal CD, up to 80% in observational studies and 64% in RCTs, with treatment‐related AEs seen in approximately 1% of patients. Remarkably, fistula healing appears durable, with isolated recurrences over a 6‐month time period. Among RCTs, MSC treatment results in an estimated 50% higher rate of fistula healing compared to the control arm. Conversely, the proportion of MSC‐treated patients with AEs is similar to controls. This evidence supports the concept that MSC local injections represent an important step forward in ameliorating patient outcomes, avoiding current invasive surgical procedures, which result in postoperative complications in a substantial number of cases.43, 44 In addition, the surgical technique applied (seton placement, obturation with fibrin glue or plug, mucosal advancement flap, muscle transposition, ligation of the inter‐sphincteric tract, sphincteroplasty) differs among centers depending on personal experience and preference, resulting in disparate outcomes.45 For medical therapy, the efficacy of biological agents in terms of complete fistula closure was 64% for infliximab at week 1446 and approximately 30% for adalimumab at week 2647 and for vedolizumab at week 14,48 while no definitive data are available for ustekinumab.49, 50 With longer follow‐up (around 1 year), these values fell to 23, 33, and 16%, respectively. For thiopurines, a meta‐analysis showed that the efficacy rate after a mean follow‐up of 26 weeks was 54%, with a pooled odds ratio of 4.44 (95% CI: 1.50–13.20) favoring fistula healing.51 Previously, antibiotics were used as first‐line therapy but did not result in fistula closure.52
A contributing factor to the difficulty in fistula treatment is the uncertainty of its pathogenesis.1 It is clear that immune mechanisms are only one component, and additional factors, such as the supportive stroma and the microvascular bed, favor the development and maintenance of tissue damage. In this regard, MSCs are known to exert a multifaceted action not only on those cells involved in immune response but also on epithelial cells, capillaries, and stroma (for review, see Ciccocioppo et al.53). Currently, the precise mechanism of action of MSCs in fistula healing is not well understood. In the few studies addressing the mechanism of action of MSCs in fistula,9, 30, 35 an increase of T‐cells with regulatory function at both rectal mucosa and peripheral blood level was evident.9 However, no modification of cytokine profile was found at the rectal level30 or in peripheral blood,35 although neither interleukin‐13 nor transforming growth factor‐β, both considered key molecules in fistula pathogenesis,1 were assessed.
Nonetheless, our evidence was obtained from a sizable number of patients (494 with MSCs and 202 with comparator), with 412 of them recruited in two RCTs.16, 33 We found a striking effect of MSCs in inducing external healing of the fistula tracks, with a cumulative rate of 80% in the short term. When assessing data from observational studies in comparison to RCTs, the healing rate was similar (84% versus 77%). It is conceivable that the lack of imaging evaluation in several early studies may have led to an overestimation of benefit; however, when performed, radiological assessment was generally consistent with the clinical evaluation. Finally, an improvement of the clinical indexes of activity, CDAI and PDAI, in the studies where they were assessed is demonstrated, whereas only a few studies evaluated mucosal healing.9, 10 In our opinion, this is an important end‐point as rectal inflammation sustains fistula formation.54 Accordingly, we found that mucosal healing paralleled fistula closure,9 whereas in the darvadstrocel phase III trial, the presence of active inflammation of the rectal mucosa was an exclusion criterion.16
A further interesting point is that the results were invariably favorable despite differences in the anatomy of fistulas (anal, rectovaginal, entercutaneous), etiology (CD and/or cryptoglandular), assessment time point, MSC source, HLA setting, dose, and schedule of injections. This is critical as allogeneic MSCs have the advantage of being widely available without the infrastructure and lag time needed for the production of autologous clinical‐grade MSCs.55 Overall, a wide heterogeneity in dosage was observed in the reported articles; thus, the impact of MSC dosage on efficacy has not yet been established. However, no evidence of a clear dose‐dependent efficacy was observed in the studies where dose escalation was performed.10, 11, 27, 29, 30, 31, 35, 39 Therefore, a definitive conclusion about optimal dosing cannot be drawn, and standardization of both cellular concentration and total volume to be injected are important issues that need to be addressed.55 In this regard, an adaptation of both the MSC dosage and number of injections was performed in two studies9, 11 in an attempt to address this specific issue.
Long‐standing disease does not seem to hamper MSC efficacy as the mean duration in the studies evaluated was 10 years (range 6.5–12). Whether treating with MSC earlier on in the disease prevents organ dysfunction and if using serial injections instead of single administration reduces the recurrence rate are important issues that remain to be determined. In this regard, we found an irrelevant rate of fistula recurrence on a median follow‐up of 6 months even though, in all studies, the patients enrolled had had an inadequate response to conventional or biological therapy. In contrast, the probability of fistula relapse‐free survival, defined as the percentage of patients who do not need to restart medical therapy, decreases progressively over time,56 reaching at 88% at 1 year, 50% at 2 years, and 37% at 5 years.40 However, these rates are more favorable than those observed after biological46, 47, 48, 49, 50 or surgical57 therapy.
In contrast with CD fistulas, only scant information is available on the safety and efficacy of MSC local treatment in cryptoglandular complex perianal fistulas. Indeed, this condition was explored in only three studies29, 31, 33 with contrasting results: in two studies, the MSC injection proved to be successful in achieving fistula healing at week 8 [69.2%29 and 71%31], and in one study, no apparent benefit was shown.33 However, when dissecting these last results among the participating centers, the analysis carried out on the subpopulation treated at the leading center showed healing rates of 54.55 and 83.33% when using MSC treatment alone or in combination with fibrin glue, respectively, compared to 18.18% when using fibrin glue alone,33 thus highlighting the need for standardization techniques and training.
Considering safety, it is widely known that conventional immunosuppressive and immunomodulant therapies are associated with severe AEs, including opportunistic infections and the potential for malignancy.58 In contrast, the safety profile of MSCs appears favorable. The most frequently reported treatment‐related AEs were anal abscess and proctalgia that developed in 17.5 and 29.4% of the MSC and placebo arm, respectively, in the pivotal phase III trial and were attributed to the surgical procedure.16 Moreover, although 53 of 107 MSC‐treated patients developed anti‐HLA class I antibodies, there was no association with positivity for donor‐specific antibodies and AEs or therapeutic response.16 The most important issue when evaluating the long‐term safety of cellular therapies is malignancy. In this regard, a potential carcinogenic risk had been postulated based on the in vitro demonstration of MSC malignant transformation.59, 60 However, this finding was subsequently refuted by the same authors and explained by cross‐culture contamination.61, 62 When moving to in vivo results, our data confirmed previous evidence of absence of cancer development among patients who underwent intravascular MSC treatment,63 and neither ectopic growth of tissues nor opportunistic infections have been recorded. Possibly, the absence of long‐term engraftment might protect against this risk, while the anti‐inflammatory effect might contribute to reducing the risk of tumor development. Indeed, patients with perianal fistulas in CD have an increased risk of malignancy,64, 65, 66 with the inflammatory milieu being the main driving force.67 Finally, neither fertility nor pregnancy has been shown to be affected by MSC local therapy.68
In conclusion, despite the lack of standardization in the collection of end‐points and information derived mostly from uncontrolled observational studies and one‐arm clinical trials, the possibility of achieving sustained fistula closure with favorable safety makes MSCs an attractive therapeutic strategy for patients with perianal fistulas in CD. Therefore, local MSC therapy should appropriately be placed in the treatment algorithm of this condition. Further studies aimed at assessing dosage and schedule of MSC injections, functional potency of the cellular suspension, donor heterogeneity, implementation of the manufacturing process, and efficacy of this treatment in comparison with biologics using standardized safety and efficacy end‐points are required. Finally, although the cost‐effectiveness was beyond the scope of this work, this represents a crucial point that needs to be appropriately investigated.
Supporting information
Figure S1 (a) Cumulative incidence of radiological healing by study design, (b) of fistula recurrence by study design, and (c) of fistula recurrence by treatment arm in the four randomized clinical trials. Diamonds represent the meta‐analytical estimate of the cumulative incidence (95% confidence interval [95% CI]). Dots and whiskers represent the incidences and 95% CI derived from the single studies. The dotted vertical line corresponds to the overall incidence.
Figure S2 (a) Cumulative incidence of adverse events by study design, (b) of treatment related adverse events by study design, and (c) of severe treatment related adverse events by study design.
Figure S3 (a) Cumulative incidence of local acute adverse events by study design, (b) of systemic acute adverse events by study design, (c) incidence of local late adverse events by study design, and (d) of systemic late adverse events by study design.
Figure S4 (a) Incidence of hospitalization by study design; (b) acute mortality by study design; and (c) late mortality by study design.
Figures S5 (a) Incidence of adverse events by treatment arm in randomized clinical trials, (b) of treatment‐related adverse events by treatment arm in randomized clinical trials, and (c) of severe treatment‐related adverse events by treatment arm in randomized clinical trials.
Table S1A Search strategy 1 (performed on 2017‐05‐13).
Table S1B Search strategy 2 (performed on 2017‐05‐13).
Acknowledgments
The authors are grateful to the librarian Ms Chiara Rebuffi for her contribution to the bibliographic search and screening. This study was funded by Takeda Pharmaceutical Company Limited.
Declaration of conflict of interest: RC, CK, and GRC received a consulting (honorary) fee from Takeda Pharmaceuticals; DL, RR, and DB are employees of Takeda Pharmaceutical Company Limited (USA).
Author contribution: RC and CK planned the study design, prepared the protocol, interpreted the data, reviewed the literature, and wrote the first draft of manuscript. CK performed the statistical analysis. DL, RR, and DB participated in the study design and protocol development, data interpretation, and drafting of manuscript and critically revised the article for important intellectual content. GRC critically revised the article for important intellectual content. All authors read and approved the final manuscript.
References
- 1. Panés J, Rimola J. Perianal fistulizing Crohn's disease: pathogenesis, diagnosis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2017; 14: 652–64. [DOI] [PubMed] [Google Scholar]
- 2. Mahadev S, Young JM, Selby W, Solomon MJ. Quality of life in perianal Crohn's disease: what do patients consider important? Dis. Colon Rectum. 2011; 54: 579–85. [DOI] [PubMed] [Google Scholar]
- 3. Yassin NA, Askari A, Warusavitarne J et al Systematic review: the combined surgical and medical treatment of fistulising perianal Crohn's disease. Aliment. Pharmacol. Ther. 2014; 40: 741–9. [DOI] [PubMed] [Google Scholar]
- 4. Bouguen G, Siproudhis L, Gizard E et al Long‐term outcome of perianal fistulising Crohn's disease treated with infliximab. Clin. Gastroenterol. Hepatol. 2013; 11: 975–81. [DOI] [PubMed] [Google Scholar]
- 5. Molendijk I, Nuij VJ, van der Meulen‐de Jong AE. Disappointing durable remission rates in complex Crohn's disease fistula. Inflamm. Bowel Dis. 2014; 20: 2022–8. [DOI] [PubMed] [Google Scholar]
- 6. Ford AC, Peyrin‐Biroulet L. Opportunistic infections with anti‐tumor necrosis factor‐α therapy in inflammatory bowel disease: meta‐analysis of randomized controlled trials. Am. J. Gastroenterol. 2013; 108: 1268–76. [DOI] [PubMed] [Google Scholar]
- 7. Whiteford MH, Kilkenny J III, Hyman N et al Practice parameters for the treatment of perianal abscess and fistula‐in‐ano (revised). Dis. Colon Rectum. 2005; 48: 1337–42. [DOI] [PubMed] [Google Scholar]
- 8. Garcia‐Olmo D, Garcia‐Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez‐Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis. Colon Rectum. 2005; 48: 1416–23. [DOI] [PubMed] [Google Scholar]
- 9. Ciccocioppo R, Bernardo ME, Sgarella A et al Autologous bone marrow‐derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011; 60: 788–98. [DOI] [PubMed] [Google Scholar]
- 10. de la Portilla F, Alba F, García‐Olmo D, Herrerías JM, González FX, Galindo A. Expanded allogeneic adipose‐derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn's disease: results from a multicenter phase I/IIa clinical trial. Int. J. Colorectal Dis. 2013; 28: 313–23. [DOI] [PubMed] [Google Scholar]
- 11. Lee WY, Park KJ, Cho YB et al Autologous adipose tissue‐derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn's fistula. Stem Cells. 2013; 31: 2575–81. [DOI] [PubMed] [Google Scholar]
- 12. Sundin M, Ringdén O, Sundberg B, Nava S, Gotherstrom C, Le Blanc K.. No alloantibodies against mesenchymal stromal cells, but presence of anti‐fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007; 92: 1208–15. [DOI] [PubMed] [Google Scholar]
- 13. Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009; 87: S42–5. [DOI] [PubMed] [Google Scholar]
- 14. Jiang Y, Jahagirdar BN, Reinhardt RL et al Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002; 418: 41–9. [DOI] [PubMed] [Google Scholar]
- 15. Ben‐Ami E, Berrih‐Aknin S, Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun. Rev. 2011; 10: 410–5. [DOI] [PubMed] [Google Scholar]
- 16. Panés J, García‐Olmo D, Van Assche G et al Expanded allogeneic adipose‐derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double‐blind controlled trial. Lancet. 2016; 388: 1281–90. [DOI] [PubMed] [Google Scholar]
- 17. Panés J, García‐Olmo D, Van Assche G et al ADMIRE CD Study Group Collaborators. Long‐term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn's disease. Gastroenterology. 2018; 154: 1334–42. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Shamseer L, Clarke M et al Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst. Rev. 2015; 4: 1 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann. Intern. Med. 2009; 151: 264–9. [DOI] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Best WR, Beckel JM, Singleton JW, Kern F. Development of a Crohn's disease activity index: National Cooperative Crohn's disease Study. Gastroenterology. 1976; 70: 439–44. [PubMed] [Google Scholar]
- 22. Gecse KB, Bemelman W, Kamm MA et al A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn's disease. Gut. 2014; 63: 1381–92. [DOI] [PubMed] [Google Scholar]
- 23. van Assche G, Vanbeckevoort D, Bielen D et al Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn's disease. Am. J. Gastroenterol. 2003; 98: 332–9. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. The Cochrane Collaboration, 2011. Available from URL: http://handbook.cochrane.org
- 25. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control. Clin. Trials. 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 26. García‐Olmo D, Herreros D, De‐La‐Quintana P et al Adipose‐derived stem cells in Crohn's rectovaginal fistula. Case Rep. Med. 2010; 2010: 1–3. 10.1155/2010/961758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cho YB, Lee WY, Park KJ, Kim M, Yoo HW, Yu CS. Autologous adipose tissue‐derived stem cells for the treatment of Crohn's fistula: a phase I clinical study. Cell Transplant. 2013; 22: 279–85. [DOI] [PubMed] [Google Scholar]
- 28. Cho YB, Park KJ, Yoon SN et al Long‐term results of adipose‐derived stem cell therapy for the treatment of Crohn's fistula. Stem Cells Transl. Med. 2015; 4: 532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi S, Park K, Kim D et al Autologous adipose tissue‐derived mesenchymal stem cells for the treatment of complex perianal fistulas. Dis. Colon Rectum. 2013; 56: 4.23222273 [Google Scholar]
- 30. Molendijk I, Bonsing BA, Roelofs H et al Allogeneic bone marrow‐derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn's disease. Gastroenterology. 2015; 149: 918–27. [DOI] [PubMed] [Google Scholar]
- 31. García‐Olmo D, Herreros D, Pascual I et al Expanded adipose‐derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis. Colon Rectum. 2009; 52: 79–86. [DOI] [PubMed] [Google Scholar]
- 32. Garcia‐Olmo D, Garcia‐Arranz M, Gomez Garcia L et al Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn's disease: a new cell‐based therapy. Int. J. Colorectal Dis. 2003; 18: 451–4. [DOI] [PubMed] [Google Scholar]
- 33. Herreros MD, Garcia Arranz M, Guadalajara H et al Autologous expanded adipose‐derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase II randomized clinical trial (FATT 1: Fistula Advanced Therapy Trial 1) and long‐term evaluation. Dis. Colon Rectum. 2012; 55: 762–72. [DOI] [PubMed] [Google Scholar]
- 34. Wainstein C, Quera R, Kromberg U et al Mesenchymal stem cells and platelet‐rich plasma in the treatment of patients with perianal Crohn's disease. Int. J. Colorectal Dis. 2016; 31: 725–6. [DOI] [PubMed] [Google Scholar]
- 35. Garcia‐Arranz M, Herreros MD, Gonzalez‐Gomez C et al Treatment of Crohn's‐related rectovaginal fistula with allogeneic expanded‐adipose derived stem cells: a phase I‐IIa clinical trial. Stem Cells Translational Med. 2016; 5: 1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lightnert AL, Dozois E, Fletcher JG, Dietz A, Friton J, Faubion W. Early results using an adipose derived mesenchymal stem cells coated fistula plug for the treatment of refractory perianal fistulizing Crohns Disease. Gastroenterology. 2016; 150: S483. [Google Scholar]
- 37. Moniuszko A, Sarnowska A, Rogowski W, Durlik M, Rydzewska G. Adipose derived regenerative cells injection as a novel method of enterovesical fistula treatment in Crohn's disease: a case report. J. Crohns Colitis. 2015; 9: S282. [Google Scholar]
- 38. Serrero M, Philandrianos C, Visee C et al An innovative treatment for refractory perianal fistulas in Crohn's disease: local micro reinjection of autologous fat and adipose derived stromal vascular fraction. J Crohns Colitis. 2017; 11: OP008. [Google Scholar]
- 39. Park KJ, Ryoo S‐B, Kim JS et al Allogeneic adipose‐derived stem cells for the treatment of perianal fistula in Crohn's disease: a pilot clinical trial. Colorectal Dis. 2015; 18: 468–76. [DOI] [PubMed] [Google Scholar]
- 40. Ciccocioppo R, Gallia A, Sgarella A, Kruzliak P, Gobbi PG, Corazza GR. Long‐term follow‐up of Crohn disease fistulas after local injections of bone marrow‐derived mesenchymal stem cells. Mayo Clin. Proc. 2015; 90: 747–55. [DOI] [PubMed] [Google Scholar]
- 41. Baixauli‐Fons J, Nunez‐Cordoba JM, Garcia‐Olmo D et al Cell therapy in patients with fistulous Crohn's disease is safe and effective: a phase I‐II clinical trial. Colorectal Dis. 2016; 18: 24. [Google Scholar]
- 42. Dietz AB, Dozois EJ, Fletcher JG et al Autologous mesenchymal stem cells, applied in bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn's disease. Gastroenterology. 2017; 153: 59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Koperen PJ, Safiruddin F, Bemelman WA, Slors JFM. Outcome of surgical treatment for fistula in ano in Crohn's disease. Br. J. Surg. 2009; 96: 675–9. [DOI] [PubMed] [Google Scholar]
- 44. Ritchie RD, Sackier JM, Hodde JP. Incontinence rates after cutting seton treatment for anal fistula. Colorectal Dis. 2009; 11: 564–71. [DOI] [PubMed] [Google Scholar]
- 45. Akiba RT, Rodrigues FG, da Silva G. Management of complex perineal fistula disease. Clin. Colon Rectal Surg. 2016; 29: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sands BE, Anderson FH, Bernstein CN et al Infliximab maintenance therapy for fistulizing Crohn's disease. N. Engl. J. Med. 2004; 350: 876–85. [DOI] [PubMed] [Google Scholar]
- 47. Colombel JF, Sandborn WJ, Rutgeerts P et al Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007; 132: 52–65. [DOI] [PubMed] [Google Scholar]
- 48. Feagan BG, Schwartz D, Danese S et al Efficacy of Vedolizumab in fistulising Crohn's disease: exploratory analyses of data from GEMINI 2. J. Crohns Colitis. 2018; 12: 621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sands BE, Gasink C, Jacobestein D et al Fistula healing in pivotal studies of ustekinumab in Crohn's disease. Presented at DDW 2017. Gastroenterology. 2017; 152: S185. [Google Scholar]
- 50. Battat R, Bessissow T, Strohl M et al Ustekinumab for the treatment of perianal fistulas in patients with Crohn's disease. J. Crohns Colitis. 2017; 11: S400–1. [Google Scholar]
- 51. Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6‐mercaptopurine in Crohn disease. A meta‐analysis. Ann. Int. Med. 1995; 123: 132–42. [DOI] [PubMed] [Google Scholar]
- 52. Present DH. Crohn's fistula: current concepts in management. Gastroenterology. 2003; 124: 1629–35. [DOI] [PubMed] [Google Scholar]
- 53. Ciccocioppo R, Cangemi GC, Kruzliak P, Corazza GR. Cellular therapies: the potential to regenerate and restore tolerance in immune‐mediated intestinal diseases. Stem Cells. 2016; 34: 1474–86. [DOI] [PubMed] [Google Scholar]
- 54. Schwartz DA, Ghazi LJ, Regueiro M et al Guidelines for the multidisciplinary management of Crohn's perianal fistulas: summary statement. Inflamm. Bowel Dis. 2015; 21: 723–30. [DOI] [PubMed] [Google Scholar]
- 55. Ciccocioppo R, Corazza G. Mesenchymal stem cells for fistulising Crohn's disease. Lancet. 2016; 388: 1251–2. [DOI] [PubMed] [Google Scholar]
- 56. Guadalajara H, Herreros D, De‐La‐Quintana P, Trebol J, Garcia‐Arranz M, Garcia‐Olmo D. Long‐term follow‐up of patients undergoing adipose‐derived adult stem cell administration to treat complex perianal fistulas. Int. J. Colorectal Dis. 2012; 27: 595–600. [DOI] [PubMed] [Google Scholar]
- 57. van der Hagen SJ, Baeten CG, Soeters PB, van Gemert WG. Long‐term outcome following mucosal advancement flap for high perianal fistulas and fistulotomy for low perianal fistulas: recurrent perianal fistulas: failure of treatment or recurrent patient disease? Int. J. Colorectal Dis. 2006; 21: 784–90. [DOI] [PubMed] [Google Scholar]
- 58. Levy C, Tremaine WJ. Management of internal fistulas in Crohn's disease. Inflamm. Bowel Dis. 2002; 8: 106–11. [DOI] [PubMed] [Google Scholar]
- 59. Rubio D, Garcia‐Castro J, Martin MC et al Spontaneous human adult stem cell transformation. Cancer Res. 2005; 65: 3035–9. [DOI] [PubMed] [Google Scholar]
- 60. Rosland GV, Svendsen A, Torsvik A et al Long‐term cultures of bone marrow‐derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009; 69: 5331–9. [DOI] [PubMed] [Google Scholar]
- 61. Torsvik A, Røsland GV, Svendsen A et al Spontaneous malignant transformation of human mesenchymal stem cells reflects cross‐contamination: putting the research field on track—letter. Cancer Res. 2010; 70: 6393–6. [DOI] [PubMed] [Google Scholar]
- 62. Garcia S, Bernad A, Martin MC, Cigudosa JC, Garcia‐Castro J, de la Fuente R. Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp. Cell Res. 2010; 316: 1648–50. [DOI] [PubMed] [Google Scholar]
- 63. Lalu MM, McIntyre L, Pugliese C et al Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta‐analysis of clinical trials. PLoS One. 2012; 7: e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Iesalnieks I, Gaertner WB, Glass H et al Fistula‐associated anal adenocarcinoma in Crohn's disease. Inflamm. Bowel Dis. 2010; 16: 1643–8. [DOI] [PubMed] [Google Scholar]
- 65. Baars JE, Kuipers EJ, Djkstra G et al Malignant transformation of perianal and enterocutaneous fistulas is rare: results of 17 years of follow‐up from The Netherlands. Scand. J. Gastroenterol. 2011; 46: 319–25. [DOI] [PubMed] [Google Scholar]
- 66. Papaconstantineou I, Mantzos DS, Kondi‐Pafiti A, Koutroubakis IE. Anal adenocarcinoma complicating chronic Crohn's disease. Int. J. Surg. Case Rep. 2015; 10: 201–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature. 2008; 454: 436–44. [DOI] [PubMed] [Google Scholar]
- 68. Sanz‐Baro R, Garcia‐Arranz M, Guadalajara H, de la Quintana P, Herreros MD, Garcia‐Olmo D. First‐in‐human case study: pregnancy in women with Crohn's perianal fistula treated with adipose‐derived stem cells: a safety study. Stem Cells Transl. Med. 2015; 4: 598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (a) Cumulative incidence of radiological healing by study design, (b) of fistula recurrence by study design, and (c) of fistula recurrence by treatment arm in the four randomized clinical trials. Diamonds represent the meta‐analytical estimate of the cumulative incidence (95% confidence interval [95% CI]). Dots and whiskers represent the incidences and 95% CI derived from the single studies. The dotted vertical line corresponds to the overall incidence.
Figure S2 (a) Cumulative incidence of adverse events by study design, (b) of treatment related adverse events by study design, and (c) of severe treatment related adverse events by study design.
Figure S3 (a) Cumulative incidence of local acute adverse events by study design, (b) of systemic acute adverse events by study design, (c) incidence of local late adverse events by study design, and (d) of systemic late adverse events by study design.
Figure S4 (a) Incidence of hospitalization by study design; (b) acute mortality by study design; and (c) late mortality by study design.
Figures S5 (a) Incidence of adverse events by treatment arm in randomized clinical trials, (b) of treatment‐related adverse events by treatment arm in randomized clinical trials, and (c) of severe treatment‐related adverse events by treatment arm in randomized clinical trials.
Table S1A Search strategy 1 (performed on 2017‐05‐13).
Table S1B Search strategy 2 (performed on 2017‐05‐13).


