Abstract
Currently, individuals with pre-existing neutralizing antibodies (NABs) against adeno-associated virus (AAV) above titer of 5 are excluded from systemic AAV-based clinical trials. In this study we explored the impact of pre-existing anti-AAV5 NABs on the efficacy of AAV5-based gene therapy. AMT-060 (AAV5-human FIX) was evaluated in 10 adults with hemophilia B who tested negative for pre-existing anti-AAV5 NABs using a GFP-based assay. In this study, using a more sensitive luciferase-based assay, we show that 3 of those 10 patients tested positive for anti-AAV5 NABs. However, no relationship was observed between the presence of pre-treatment anti-AAV5 NABs and the therapeutic efficacy of AMT-060. Further studies in non-human primates (NHPs) showed that AAV5 transduction efficacy was similar following AMT-060 treatment, irrespective of the pre-existing anti-AAV5 NABs titers. We show that therapeutic efficacy of AAV5-mediated gene therapy was achieved in humans with pre-existing anti-AAV5 NABs titers up to 340. Whereas in NHPs circulating human factor IX (hFIX) protein was achieved, at a level therapeutic in humans, with pre-existing anti-AAV5 NABs up to 1030. Based on those results, no patients were excluded from the AMT-061 (AAV5-hFIX-Padua) phase IIb clinical trial (n = 3). All three subjects presented pre-existing anti-AAV5 NABs, yet had therapeutic hFIX activity after AMT-061 administration.
Keywords: AAV, NABs, anti-AAV NABs, hemophilia, gene therapy, hFIX, FIX
Introduction
Pre-clinical and clinical studies have shown that the presence of antibodies directed against the adeno-associated virus (AAV) capsid proteins can negatively affect the efficacy of AAV-based gene therapy.1, 2, 3, 4, 5 Therefore, gene transfer to subjects with pre-existing immunity to AAV is a major concern, especially when systemic AAV vector delivery is considered. The prevalence rate of subjects sero-positive for AAVs can be, depending on the serotype, up to 59% or 50% for AAV2 or AAV1.6 A significant proportion of individuals develop humoral immunity against AAV capsids early in life, starting around 2 years of age. Maternal anti-AAV antibodies can be detected in newborns, and the titers decrease several months after birth before increasing again because of exposure to wild-type AAVs.7, 8 Thus, only during a very limited time frame, humans appear to be naive to AAV. Patients with neutralizing antibodies (NABs) titers above 5 against the AAV serotype to be used as systemic therapeutic delivery vector are currently not entering clinical trials.
Among the AAV serotypes currently considered for clinical use, AAV2 is the most seroprevalent in humans, whereas AAV5 and AAV8 are among the least prevalent.6, 7, 8, 9 The relevance of anti-AAV NABs titers for the initial transgene expression in pre-clinical and clinical studies has been shown for AAV serotypes 2 and 8.1, 2, 3, 4, 5 Indeed, pre-existing anti-AAV NABs levels as low as 1:17 for AAV2 and even 1:1 for bioengineered AAV-Spark10010 capsid3, 10 or 1:5 for AAV8 in non-human primates2 were associated with a lower or even total lack of therapeutic protein expression. Those observations have led to the exclusion of subjects with even low levels of anti-AAV NABs from current AAV-based gene therapy trials.
However, no similar data have been reported for AAV serotype 5. Among the serotypes commonly used as gene therapy vectors, AAV5 has one of the least-conserved capsid sequences,11, 12 and the seroprevalence of anti-AAV5 NABs has been reported to be generally low, with described frequencies ranging from 3% to 40% depending on the methodology used and population studied.6, 9, 13, 14, 15 In seropositive individuals, anti-AAV5 NABs titers have been reported to be low to undetectable.6, 9 To put this in context, up to 59% of the population was reported to be seropositive for AAV2 or 50.5% for AAV1.6 This suggests that gene therapies based on AAV5 vectors might be suitable for a greater proportion of patients than therapies based on AAV1/2 vectors.
AMT-060 is an investigational gene therapy that delivers copies of the human factor IX (hFIX) gene to the liver using an AAV5 transfer vector (AAV5-hFIX). We previously evaluated the safety and efficacy of a single intravenous dose of AMT-060 in 10 adult males with moderate to severe hemophilia B (ClinicalTrials.gov NCT02396342; CT-AMT-060-01).16 Detailed results have been published elsewhere.16 In brief, AMT-060 treatment reduced the clinical severity of hemophilia B from severe to mild (n = 5), severe to moderate (n = 4), or moderate to mild (n = 1), and the phenotype remained stable for the duration of the study. Routine hFIX prophylaxis was discontinued in eight of the nine participants who required regular hFIX infusions prior to gene therapy, resulting in large reductions in annualized hFIX consumption.16 All 10 patients entering the trial were deemed to be negative for anti-AAV5 NABs prior to treatment. However, NABs titers were determined using a standard assay that uses GFP as a reporter gene, whereas emerging evidence suggested that luciferase-based anti-AAV NABs assays might be more sensitive.17
The aim of this study was to investigate the impact of pre-existing anti-AAV5 NABs measured with the newly developed, more sensitive assay on the therapeutic efficacy of AAV5-hFIX (AMT-060) in CT-AMT-060-01 study participants and in non-human primates (NHPs) treated with AAV5-hFIX.
Results
The Luciferase-Based Assay Detects Anti-AAV5 NABs More Sensitively Than the GFP-Based Assay
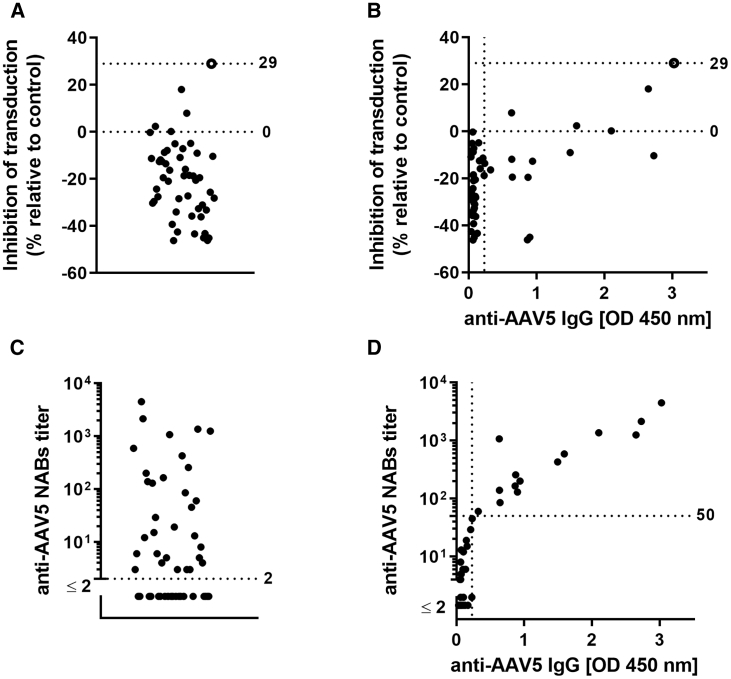
The sensitivities of the luciferase- and GFP-based assays for the detection of anti-AAV5 NABs were evaluated using serum samples from 50 healthy individuals. Overall, 1 in 50 serum samples tested positive for anti-AAV5 NABs using the GFP-based assay (Figure 1A), whereas 32 samples tested positive using the luciferase-based assay (Figure 1C). Results were compared with the levels of anti-AAV5 total immunoglobulin G (IgG) antibody in each serum sample, determined using ELISA (Figures 1B and 1D). Overall, 15 of the 50 samples were positive for anti-AAV5 IgG antibodies; of these, only one was positive for anti-AAV5 NABs using the GFP-based assay, but all 15 were positive using the luciferase-based assay (Figure 1D).
Figure 1.
GFP-Based Anti-AAV5 NABs Assay Was Performed on 50 Healthy Donors’ Serum Samples
Inhibition of transduction of HEK293 cells with AAV5-GFP after pre-incubation with 50 healthy donor serum samples is plotted, and only one donor that is represented as an empty circle was considered to be positive for anti-AAV5 NABs (A). No unequivocal correlation was observed between the anti-AAV5 NABs as measured with GFP-based assay and anti-AAV5 IgG results as measured by ELISA (B). The same 50 healthy donor sera samples were screened for anti-AAV5 NABs with the use of luciferase-based assay (C). A strong correlation was observed between the anti-AAV5 NABs as measured with luciferase-based assay and anti-AAV5 IgG results as measured by ELISA (r = 0.85; p < 0.0001) (D). Limits of quantitation of both methods: for the anti-AAV5 NABs assay, this corresponds to the starting titer of 2; for the ELISA, this corresponds to 0.230 OD. AAV, adeno-associated virus; IgG, immunoglobulin G; NABs, neutralizing antibodies; OD, optical density.
Of the 35 serum samples that were negative for anti-AAV5 IgG, 17 tested marginally positive (titer < 50) for anti-AAV5 NABs using the luciferase-based assay (median titer 5; range 2–29). A strong correlation was observed between anti-AAV5 NABs titers >50 and levels of anti-AAV5 IgG antibodies (Figures 1D and 7B).
Figure 7.
An Additional 100 Healthy Male Donor Serum Samples Were Screened for Presence of Pre-existing Anti-AAV5 NABs
Forty-seven donors who tested above the lower limit of detection (above anti-AAV5 NABs titer of 2) are plotted and median with 95% CI (A). Dotted lines represent current highest pre-existing anti-AAV5 NABs titers that were found not to interfere with the efficacy of AMT-060 (AAV5-hFIX) treatment in humans (titer of 340) or in NHPs (1030). (B) Anti-AAV5 NABs titers versus anti-AAV5 IgG ELISA results are plotted for those 47 donors (each black dot symbol represents paired anti-AAV5 NABs titer and anti-AAV5 IgG ELISA titer). Anti-AAV5 NABs titers and anti-AAV5 IgG ELISA results of hemophilia B clinical trial of patients who tested positive in those assays are shown superimposed (red diamond symbols, patients 3, 4, and 5 as specified). AAV, adeno-associated virus; IgG, immunoglobulin G; NABs, neutralizing antibodies; NHPs, non-human primates.
Re-assessment of Pre-treatment Patients’ Serum Samples from Study CT-AMT-060-01 for the Presence of Anti-AAV5 NABs
The initial screening for the presence of pre-existing anti-AAV5 NABs in the serum samples of the 10 subjects from the AMT-060-01 clinical trial was performed with the GFP-based assay. From this analysis, all 10 patients were determined to be negative for pre-existing anti-AAV5 NABs, and all patients entered the study. However, 2 of those 10 patients (patients 3 and 4) were found low positive for total anti-AAV5 IgG antibodies as measured by ELISA (Table 1).16
Table 1.
Characteristics of Hemophilia B Patients Participating in the CT-AMT-060-01 Clinical Study, Including Levels of hFIX Activity Pre- and Post-treatment with AMT-060 (AAV5-hFIX)
| Patient No | FIX Activity, IU/dL (Basal) | Mean Steady-State FIX Activity, IU/dL (Posttreatment) | Pre-existing Anti-AAV5 NABs Titer (Luciferase-Based Bioassay) | Pre-existing Anti-AAV5 IgG (ELISA) | Pre-existing Anti-AAV5 NABs Titer (GFP-Based Bioassay) |
|---|---|---|---|---|---|
| Low-Dose Cohort | |||||
| 1 | <1 | 6.2 | <4 | negative | negative |
| 2 | <1 | 4.7 | <4 | negative | negative |
| 3 | <1 | 1.3 | 210 | positive (87) | negative |
| 4 | 1.5 | 6.8 | 340 | positive (256) | negative |
| 5 | <1 | 3.0 | 21 | negative | negative |
| High-Dose Cohort | |||||
| 6 | <1 | 12.7 | <4 | negative | negative |
| 7 | <1 | 6.4 | <4 | negative | negative |
| 8 | <1 | 6.8 | <4 | negative | negative |
| 9 | <1 | 3.1 | <4 | negative | negative |
| 10 | <1 | 5.8 | <4 | negative | negative |
Pre-existing anti-AAV5 NABs titers were measured using luciferase- and GFP-based assays, and pre-existing anti-AAV5 IgG titers were measured using ELISA. AAV, adeno-associated virus; hFIX, human factor IX; IgG, immunoglobulin G; NABs, neutralizing antibodies.
After re-analysis of the patient’s serum samples with the luciferase-based anti-AAV5 NABs assay, 3 out of 10 patients from the AMT-060 clinical trial were found positive for anti-AAV5 NABs pre-treatment (Table 1). The two patients (patients 3 and 4) with the highest levels of anti-AAV5 NABs in the luciferase-based assay (titers of 210 and 340) were found positive for anti-AAV5 IgG antibodies in ELISA-based assay (respectively, titers of 87 and 256). Patient 5, who tested marginally positive (titer < 50) for anti-AAV5 NABs (titer of 21), tested negative for anti-AAV5 IgG antibodies in the ELISA-based assay (Table 1).
Remarkably, no correlation between the presence of anti-AAV5 NABs before therapy and hFIX activity levels obtained after AAV5-hFIX administration (AMT-060 treatment) was observed. Patient 3, with a pre-existing anti-AAV5 NABs titer of 210, was a low responder (mean 1.3 IU/dL hFIX activity), yet patient 4, who presented the highest titer of pre-existing anti-AAV5 NABs at 340, had the highest hFIX activity in the low-dose cohort (mean 6.8 IU/dL) (Table 1; Figure 2).
Figure 2.
Pre-existing Anti-AAV5 NABs Titers in Serum Samples from Patients Participating in the CT-AMT-060-01 Study versus Mean hFIX Activity after Systemic Administration of Low-Dose AMT-060 (AAV5-hFIX)
Anti-AAV5 NABs were measured using the luciferase-based assay. AAV, adeno-associated virus; hFIX, human factor IX; NABs, neutralizing antibodies.
The IgG Depletion of Pre-treatment CT-AMT-060-01 Patient Serum Samples Results in Decrease of Anti-AAV5 NABs Titer
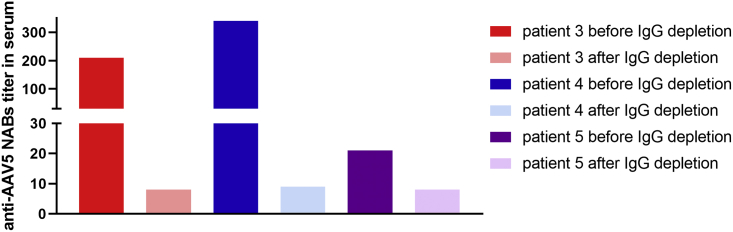
To evaluate whether the inhibition of AAV5-mediated cell transduction in the in vitro anti-AAV5 NABs assay was IgG mediated rather than related to non-antibody-mediated inhibition, we re-assessed serum samples from patients 3, 4, and 5 (all of whom tested positive for anti-AAV5 NABs using the luciferase-based assay) for anti-AAV5 NABs prior to, and after, IgG depletion. IgG depletion was associated with reduced anti-AAV5 NABs titers in all three samples, with titers decreasing from 340 to 9 in patient 4, from 210 to <8 in patient 3, and from 21 to <8 in patient 5 (Figure 3).
Figure 3.
Anti-AAV5 NABs Titers in Serum Samples from Study CT-AMT-060-01 Patients 3, 4, and 5, before and after IgG Depletion
Anti-AAV5 NABs were measured using the luciferase-based assay. AAV, adeno-associated virus; Ig, immunoglobulin; NABs, neutralizing antibodies.
Administration of AMT-060 in Patients with Pre-existing Anti-AAV5 Humoral Immunity Did Not Result in Persistent ALT Elevation or T Cell Activation
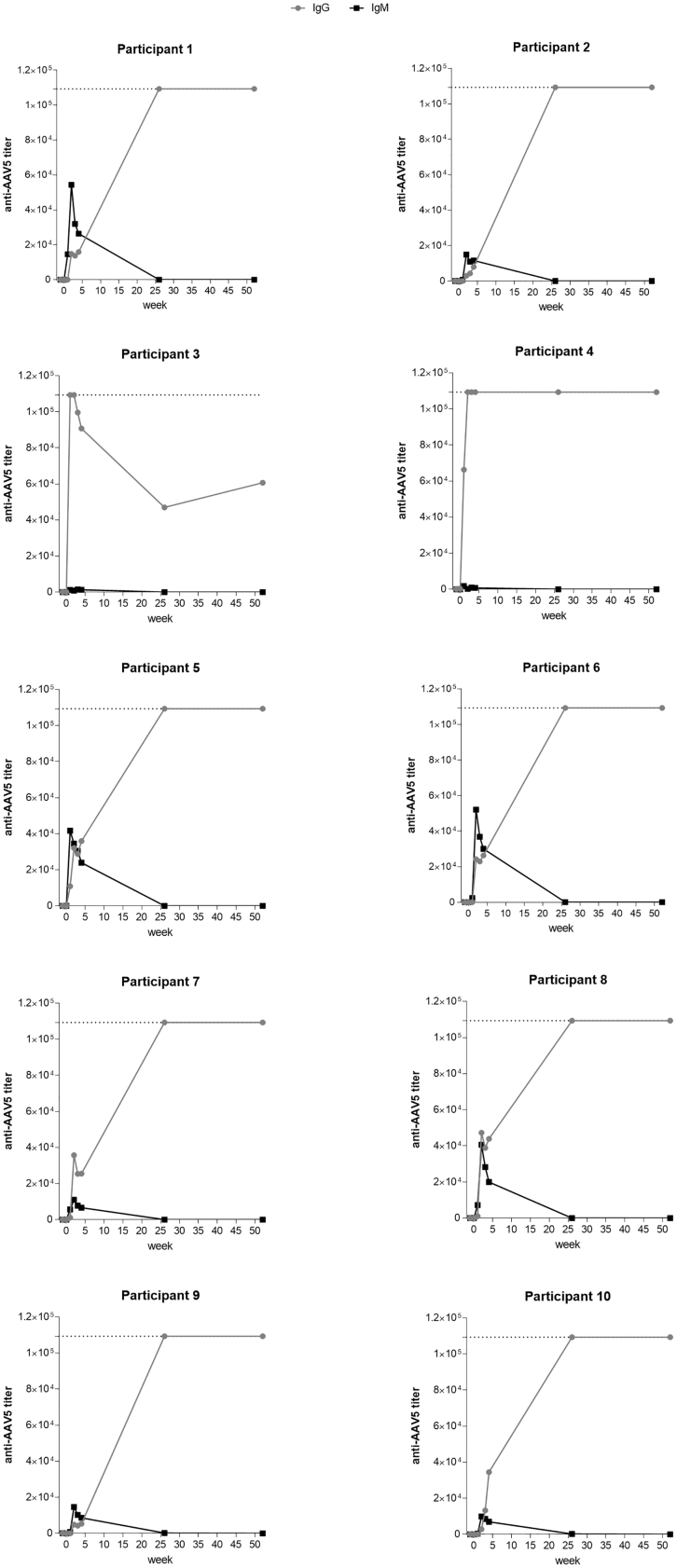
As expected, all subjects injected with AMT-060 developed a humoral immune response to AAV5 within 1 week of treatment. Patients 3 and 4 (who tested positive for pre-existing anti-AAV5 NABs using the luciferase-based assay) showed a rapid increase in AAV5-specific IgG levels after administration of AMT-060, characteristic of an immune boost. In contrast, patient 5 (who tested marginally positive for anti-AAV5 NABs and negative for anti-AAV5 IgG) showed a rapid and transient increase in AAV5-specific IgM followed by increased levels of AAV5-specific IgG, characteristic of primary exposure to an antigen. Similar results were obtained in patients 1, 2, and 6–10, all of whom tested negative for anti-AAV5 NABs and AAV5-specific IgG (Figure 4). Administration of AMT-060 in patients with pre-existing anti-AAV5 NABs did not result in alanine aminotransferase (ALT) elevation, capsid-specific T cell activation, or loss of transgene activity.16
Figure 4.
Changes in Anti-AAV5 IgG and IgM Antibody Titers over Time in CT-AMT-060-01 Study Participants
Patients received a single systemic administration of AMT-060 (AAV5-hFIX) on day 0. IgG and IgM levels were determined using ELISAs. The lower limit of detection for IgG/IgM was 50. Dotted lines represent the upper limit of detection for the anti-AAV5 IgG ELISA (109350). AAV, adeno-associated virus; hFIX, human factor IX; Ig, immunoglobulin.
Pre-existing Anti-AAV5 NABs Serum Titers up to 1030 Have No Effect on Liver Transduction following AMT-060 Administration in NHPs
Pre-treatment serum samples of 14 NHPs were re-analyzed for the levels of pre-existing anti-AAV5 NABs with a luciferase-based assay.
NHPs were injected intravenously with an AAV5 vector (AAV5-hFIX) at a dose of 5e11 genome copies (gc)/kg (n = 3), 5e12 gc/kg (n = 5), 2.5e13 gc/kg (n = 3), or 9.3e13 gc/kg (n = 3). Transduction efficiency was assessed by measuring transgene protein levels in plasma over time from the day of administration until sacrifice, and levels of AAV5 vector DNA and hFIX mRNA in the liver 6 months after vector injection (post mortem).
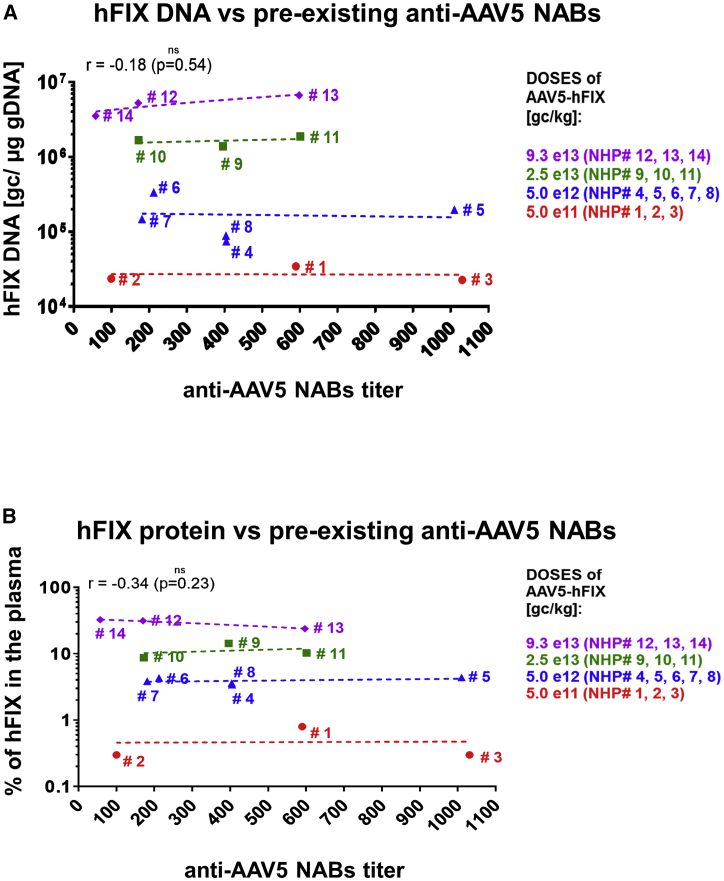
Using the anti-AAV5 NABs luciferase-based assay, all animals tested positive for pre-existing anti-AAV5 NABs, at titers ranging from 57 to 1030. At sacrifice, the amounts of AAV5 vector DNA, transgene mRNA in the liver, and hFIX protein concentration in the plasma over time were directly proportional to the injected dose of AAV5 and found to be similar within animal groups tested, regardless of the level of anti-AAV5 NABs measured in the sera pre-administration (Figures 5A and 5B).
Figure 5.
Lack of Correlation between Pre-Existing Anti-AAV5 NABs Titers in Serum Samples from NHPs and Efficacy of AMT-060 (AAV5-hFIX) Treatment
Pre-existing anti-AAV5 NABs titers in serum samples from NHPs versus (A) levels of hFIX DNA in the liver 6 months after a single intravenous administration of AMT-060 (AAV5-hFIX) and (B) percentage of hFIX protein in plasma 1 week after a single intravenous administration of AMT-060 (AAV5-hFIX). Anti-AAV5 NABs titers were measured using the luciferase-based assay, hFIX DNA was measured using qPCR, and percentage of hFIX protein was measured in an ELISA-based assay. AAV, adeno-associated virus; hFIX, human factor IX; NABs, neutralizing antibodies; NHPs, non-human primates.
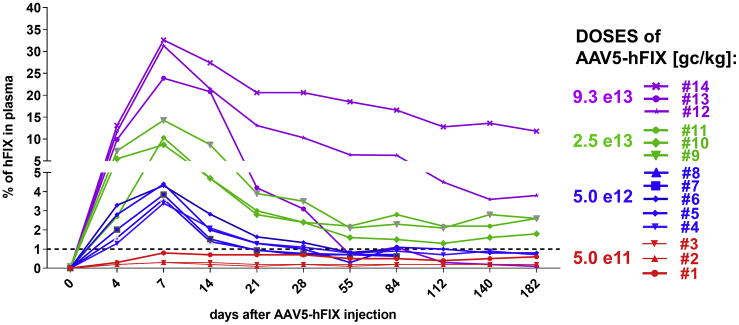
As previously observed in NHPs injected with AAV-hFIX, the levels of hFIX peaked 1 week after injection before decreasing and stabilizing.2, 18, 19 Furthermore, as reported in other studies, an immune response against the human FIX transgene protein occurred. One of the NHPs (#13) had a significant decrease of circulating hFIX protein by day 55 (Figure 6), which was associated with a raise in anti-hFIX antibodies18, 19, 20, 21, 22, 23 (data not shown).
Figure 6.
Changes in Percentage of hFIX in NHPs Plasma over Time, after a Single Intravenous Administration of AAV5-hFIX
Different colors represent different doses of AAV5-hFIX. Percentage of hFIX protein was measured using ELISA-based assay. AAV, adeno-associated virus; hFIX, human factor IX; NHPs, non-human primates.
Overall, the results obtained demonstrate that a successful and comparable AAV5-hFIX transduction was achieved, independent of the level of circulating pre-existing anti-AAV5 NABs. Remarkably, even anti-AAV5 NABs titers as high as 1030 did not negatively impact transduction of the liver by AAV5 in NHPs.
Prevalence of Anti-AAV5 NABs in the Second Healthy Donor Cohort (n = 100) as Measured with the Luciferase-Based Assay
In order to gather more information on the prevalence of anti-AAV5 NABs in the general population, serum samples from 100 healthy male donors (none of which were included in the previous analysis comparing GFP- and luciferase-based NABs assays) were screened using the highly sensitive luciferase-based assay. No anti-AAV5 NABs were detected (titer < 2) in 53 of the 100 samples, and the remaining 47 samples had titers ranging from 3 to 10077 (median 27) (Figure 7A). As expected, a strong correlation was observed between levels of anti-AAV5 NABs and anti-AAV5 IgG, especially in samples with anti-AAV5 NABs titers >50 (Figure 7B). Of note, the levels of anti-AAV5 NABs and anti-AAV5 IgG observed in the three seropositive subjects taking part in study CT-AMT-060-01 were within the ranges observed in the general population (Figure 7B).
Discussion
The present study demonstrates limited significance of pre-existing anti-AAV5 NABs as predictive indicator of the efficacy of AAV5-based gene transfer. Using a sensitive assay for anti-AAV5 NABs, we showed that 3 out of the 10 patients included in the hemophilia B clinical trial (CT-AMT-060-01) had pre-existing anti-AAV5 NABs prior to treatment, and pre-exposure to AAV5 was confirmed in 2 patients who showed a typical immune boost response following treatment with AAV5-hFIX. However, the presence of these neutralizing antibodies in the serum at measured titers up to 340 did not negatively affect expression of the therapeutic transgene. This observation was supported by NHPs data showing successful liver transduction in the presence of pre-existing anti-AAV5 NABs titers up to 1030.
Low levels of anti-AAV2 or anti-AAV8 NABs have been related to a decrease or even total impairment of liver transduction in both NHPs or humans,1, 2, 3, 5 and patients with detectable anti-AAV NABs titers, as low as 1:5, are currently commonly excluded from clinical trials.17, 24 It should be noted that assays designed to detect pre-existing humoral immunity against AAV capsids are not standardized between different clinical or research centers, and consequently NABs titers can be compared only relatively. Nonetheless, our results suggest that differences in the neutralization ability of antibodies might exist between AAV serotypes. Even though anti-AAV5 NABs were detected in human serum samples in vitro, those antibodies did not impair the efficacy of in vivo transduction of AAV5-based vector at the doses of AAV5-hFIX used in our clinical trial. Additionally, the data obtained in the NHPs study indicate that the efficacy of AAV5-hFIX transduction was preserved at all vector doses (high and low), irrespective of the level of pre-existing anti-AAV5 NABs. Hence, the efficacy of transduction of NHPs livers in the presence of neutralizing antibodies was not simply a consequence of a high dose of AAV5 vector escaping antibody binding. It is currently unknown why neutralizing antibodies to distinct AAV serotypes have different effects on liver transduction. Previous studies show that NABs against AAV serotypes with highly conserved capsid sequences (e.g., AAV1 and AAV2) can recognize and cross-neutralize serotypes with a similar capsid sequence, and that most humans test positive for anti-AAV NABs across a panel of serotypes.13 Among the serotypes commonly used as gene therapy vectors, AAV5 has one of the least-conserved capsid sequences.11, 12 This suggests that it is less likely to be recognized and cross-neutralized by non-AAV5 NABs. However, it is also conceivable that NABs that inhibit AAV vector transduction in vitro do not interfere with transduction in vivo, due to low binding affinities or high “off” rates. Further studies are required to fully understand the differences between AAV serotypes in terms of the neutralizing potential of anti-AAV NABs.
This study addresses the utility of anti-AAV NABs assays in general and their significance for therapeutic success of AAV-based treatments. In the hemophilia B clinical trial with AAV5-hFIX (AMT-060), patients were initially screened for anti-AAV5 NABs with a validated GFP-based bio-assay. All patients tested negative for anti-AAV5 NABs and therefore were included in the study. During the clinical study, a more sensitive assay for anti-AAV5 NABs was developed. In contrast with the GFP-based assay that was used for eligibility testing, the novel anti-AAV5 NABs assay was based on luciferase because luminescence results in a more sensitive detection of NABs than fluorescence.17 Titers measured with our luciferase-based anti-AAV5 NABs bio-assay closely correlated to total anti-AAV5 IgG levels detected in the ELISA. Importantly, patients 3 and 4, with anti-AAV5 NABs as detected by the NABs luciferase-based assay, demonstrated a rapid increase in AAV5-specific IgG after administration of AAV5-hFIX (AMT-060), which is a characteristic of an immune boost, confirming that those patients were previously exposed to AAV5 antigen. In contrast, patient 5, who tested marginally positive for anti-AAV5 NABs (titer of 21) and negative for anti-AAV5 IgG, had a transient increase in anti-AAV5-specific IgM followed by a rise in AAV5-specific IgG typical of humoral immune response after primary exposure to an antigen. The IgG depletion from the serum samples of AMT-060 patients 3 and 4, but also patient 5, confirmed that the transduction neutralization observed in the in vitro anti-AAV5 NABs assay was predominantly IgG antibody dependent. No correlation between the presence of anti-AAV5 NABs before therapy and hFIX activity levels obtained after AAV5-hFIX administration (AMT-060 treatment) was observed. Patients 3 and 5, with pre-existing anti-AAV5 NABs titers of 210 and 21, were lower responders in their dose cohort (mean 1.3 IU/dL and mean 3 IU/dL hFIX activity). Interestingly, they are also siblings, which suggests genetic and/or environmental factors that were likely to contribute to lower susceptibility to AAV transduction. Yet patient 4, who presented the highest titer of pre-existing anti-AAV5 NABs at 340, had the highest hFIX activity in the low-dose cohort (mean 6.8 IU/dL) (Table 1; Figure 2).
The first screening on healthy donor serum samples (n = 50) was performed to compare the GFP-based assay with the luciferase-based assay. Thirty-two samples returned positive for NABs with the luciferase-based assay, but only 15 samples returned positive for the presence of anti-AAV5 IgG antibodies as measured with ELISA. Remarkably, all of the 17 samples negative for anti-AAV5 IgG, yet marginally positive for anti-AAV5 NABs (titer < 50), presented very low NABs titers (median = 5, with minimum titer of 2 and maximum of 29). Overall, these results confirm the anti-AAV5 NABs luciferase assay is more sensitive than the anti-AAV5 IgG ELISA. However, it cannot be excluded that the very low NABs titers measured in the NABs bio-assay might also be caused by uncharacterized non-IgG serum components able to impair AAV transduction efficacy in vitro. Importantly, whether IgG mediated or not, it has to be noted that the low level of transduction inhibition observed in vitro does not negatively affect AAV5-mediated liver transduction in vivo.
Results obtained from our hemophilia B clinical trial demonstrated that an anti-AAV5 NABs titer as high as 340 did not impair the clinically efficacy of the AAV5-hFIX therapy. To place the levels of pre-existing anti-AAV5 NABs and anti-AAV5 ELISA IgG measured in the patients’ serum samples in a broader context, a healthy male donor cohort population (n = 100) was screened. The results obtained showed that 92% of the donors had titers below 340 and therefore could be considered for treatment with AAV5 vectors (Figure 4). Importantly, pre-treatment levels of anti-AAV5 NABs and anti-AAV5 IgG in the three seropositive subjects from study CT-AMT-060-01 are within the range observed in the general population (Figure 7B). Even though very high titers of AAV5 NABs are expected to interfere with transduction efficacy in humans,25 our data generated in NHPs indicate that anti-AAV5 titers up to 1030 are compatible with efficient AAV5-based gene delivery. Assuming these results could be extrapolated to humans, at least 97% of our second healthy donors’ cohort screening for anti-AAV5 NABs (n = 100) would be eligible for treatment with AAV5-based vectors (Figure 7).
In summary, our study shows that patients can be successfully treated by systemic administration of AAV5-based vectors in the presence of detectable pre-existing anti-AAV5 NABs. Additional studies are needed to determine at what titer anti-AAV5 NABs become relevant to impact the outcomes of clinical gene transfer using AAV5 vectors. Consequent to this study, patients are currently not excluded on the basis of anti-AAV5 NABs levels from the AMT-061 (AAV5-hFIX-Padua) trial (NCT03569891), full results of which will be reported elsewhere. The levels of pre-existing anti-AAV5 NABs are, however, measured and registered. Three patients participating in phase IIb of the trial presented low levels of anti-AAV5 NABs (48, 44, and 25, respectively), while two of them were excluded from another gene therapy trial because of pre-existing NABs to a different AAV vector. Remarkably, all three patients have demonstrated increasing and sustained hFIX levels after the one-time administration of AMT-061. Mean hFIX activity for the three patients at 12 weeks increased to 38% of normal.26
Materials and Methods
Serum Samples from CT-AMT-060-01 Study Participants
Pre-treatment serum samples were obtained from 10 adults with hemophilia B participating in study CT-AMT-060-01 (ClinicalTrials.gov: NCT02396342; EudraCT: 2013-005579-42). The study design and results have been previously described by Miesbach et al.16
Serum Samples from Healthy Donors
Serum samples from 125 healthy American male donors and 25 healthy American female donors were purchased from BioreclamationIVT (formerly Seralab, West Sussex, UK).
Serum and Plasma Samples from NHPs
Pre-treatment sera samples were obtained from NHPs from non-clinical studies conducted in Charles River Test Facilities in accordance with the Organization for Economic Cooperation and Development (OECD) Principles of Good Laboratory Practice. Fourteen male Macaca fascicularis of Mauritius origin, age ranging between 2 years 6 months and 3 years 9 months, were injected intravenously with different doses of AMT-060 (AAV5-hFIX): 5e11 gc/kg (n = 3), 5e12 gc/kg (n = 5), 2e13 gc/kg (n = 3), and 9.3e13 gc/kg (n = 3). Plasma samples for hFIX analysis were collected prior to AAV5-hFIX injections and at different time points after the injections.
GFP-Based Anti-AAV5 NABs Assay
The assay entails incubation of the 1:50 dilution of test sera with an AAV5-based reporter vector that carries the GFP gene. This incubation allows any neutralizing antibodies, or other interfering factors present in the test serum, to bind to the reporter vector particles. These mixtures were subsequently transferred to wells seeded with HEK293 cells in a 96-well plate format, allowing non-neutralized reporter vector particles to transduce cells and express GFP. The cells were analyzed by flow cytometry for the percentage of GFP-expressing (and hence fluorescent) cells. Each analytical run included negative controls (control sample without AAV5-GFP reporter vector addition and pooled human serum control negative for anti-AAV5 NABs as determined during the assay development). Additional technical controls include a negative and positive assay control, consisting of monkey serum obtained pre- and post-immunization with AAV5-hFIX, respectively. The readout of the assay was the percent of inhibition of transduction, relative to normalized negative control serum. This percentage inhibition of transduction is then held against the pre-defined cut-point of 29%, which means that test sera that inhibit transduction by 29% or more are considered positive. Cut-point (cut-point = mean % inhibition + 2.33 × SD) was calculated at the 99% confidence level from the percent inhibition data obtained from the initial four test runs of 48 human sera samples screened in the development of GFP-based anti-AAV5 NABs assay.
Luciferase-Based Anti-AAV5 NABs Assay
The assay entails incubation of the test sera dilution series with an AAV5-based reporter vector that carries the luciferase gene. As in the GFP-based assay, this incubation allows neutralizing antibodies in the test serum to bind to the reporter vector particles. These mixtures are subsequently transferred to wells seeded with HEK293T cells in a 96-well plate format, where reporter vector particles can transduce cells and mediate expression of luciferase. After 2 h, the supernatants of each well are replaced by cell culture medium to maintain maximum cell viability. On the next day, all wells are analyzed for luciferase expression by luciferin substrate conversion-based chemiluminescent readout. The anti-AAV5 neutralizing antibody titer is determined with the use of LabKey software analysis that calculates the percent of neutralization for each serum dilution after subtraction of background activity and fits a curve to the neutralization profile. This curve is used to calculate neutralizing antibody titers, area under the curve (AUC), and error estimates. The four-parameter method is currently used to calculate curve fits. LabKey calculates IC50, the dilution at which the antibodies inhibit transduction by 50%. LabKey also calculates “point-based” titers. This is done by linearly interpolating between the two replicates on either side of the target neutralization percentage. Each analytical run includes positive controls (wells without sample sera but with AAV5-luciferase), negative controls (wells that have only medium, without sample sera and without AAV5-luciferase), and negative control sample serum (heat-inactivated fetal bovine serum [FBS]) to assess the specificity of AAV5-luciferase neutralization. MOI in the luciferase-based anti-AAV5 NABs assay was 378.4, whereas the target relative light units (RLUs) that were to be read in the luminometer after AAV5-luciferase transduction of HEK293T cells in the positive control wells were to be approximately 1000 RLUs, and negative control wells that consisted of only HEK293T cells would have reads of approximately 50 RLUs.
Total Anti-AAV5 IgG and IgM ELISAs
Screening Assay
Diluted test serum samples are incubated for 1 h at room temperature, on immunosorbent plates coated with AAV5. After washing, anti-human IgM or IgG conjugated with horseradish peroxidase (HRP) is added, which binds to the Fc region of any bound IgM or IgG. After a final wash, tetramethylbenzidine (TMB) is added as a substrate to react with bound HRP, enabling readout by colorimetric reaction. Antibody levels are deduced from the returned optical density (OD). Specificity for AAV5 was confirmed by pre-incubating the test sera with excess AAV5 before ELISA. An initial evaluation of 50 test human sera samples was performed. These sera had been analyzed by ELISA at a 1/50 dilution shortly before validation of the assay, in order to assess seroprevalence and to identify positive control sera. As shown in Figures 1B and 1C, the sera returned across a wide range of optical densities (ODs). In the validated assay, samples are scored for antibodies by comparing the returned OD with a floating cut-point. During the actual measurements, no cut-point had yet been defined. For the current study, the average cut-point determined during the validation runs was used, which was OD 0.230 (validation returned floating cut-points ranging from 0.146 to 0.330).
For Patients’ Sera Samples that Tested Positive in the Screening Assay, Titration Assay Was Performed as Described
Serum samples are diluted in diluent buffer to obtain a dilution curve. These dilution curves are obtained by diluting an aliquot of the sample to have a total of eight dilutions. The dilutions are added onto the AAV5-coated immunosorbent plates in duplicates. For each curve the titer value (inverted dilution factor) is determined by extrapolation of the titer curve down the assessed cut-point.
IgG Depletion of Human Serum Samples
Depletion procedure of IgG from human serum samples was performed with the use of PureProteome Protein G Magnetic Beads System (Millipore) according to the manufacturer’s instructions. In brief, four times diluted serum samples were incubated with Protein G magnetic beads for 30 min at room temperature with continuous mixing. After incubation, tubes with serum samples were placed on the magnetic stand. After allowing the beads to migrate to the magnet, supernatant that represents the depleted serum sample is removed. Due to pre-dilution of serum samples prior to IgG depletion, IgG-depleted serum samples were subjected to anti-AAV5 NABs assay with the starting serum sample dilution equal to 8.
hFIX Protein Activity in Human Plasma
Plasma samples for the measurement of hFIX activity were centrifuged within 90 min of collection, frozen at −20°C and transferred on dry ice to the central laboratory. hFIX activity was measured using a one-stage activated partial thromboplastin time (aPTT) assay, as described previously.16 Endogenous hFIX activity was analyzed using samples obtained at least 10 days after exogenous hFIX infusion in order to exclude the possibility of residual activity from exogenous treatment. The lower limit of quantification was 0.6 IU/dL.
hFIX Transgene Expression in NHPs Plasma
hFIX protein levels were measured in plasma taken from NHPs at different time points. This was performed using a sandwich ELISA developed in-house, as previously described.25
Vector DNA Quantification in NHPs Liver
Liver samples were collected from NHPs at necropsy (6 months after AMT-060 administration), and total genomic DNA (gDNA) was extracted from frozen pulverized tissue. qPCR was conducted on 250-ng samples (three reactions per sample) using a primer-probe set specific for AAV5-hFIX (AMT-060) vector genome. For quantification, serial dilutions of a plasmid containing the qPCR target sequence were subjected to qPCR in parallel to the samples. From the results of the plasmid dilutions, a calibrator curve was established by means of linear regression. Vector DNA copies in the samples were calculated by means of interpolation from the calibrator curve. To control for the absence of inhibitors in the samples, 250-ng duplicates of each gDNA were subjected to qPCR using a primer-probe set specific for a sequence unique to the monkey porphobilinogen deaminase (PBGD) locus, in parallel to the hFIX samples.
ELISpot Assay
T cell responses were measured using an enzyme-linked immunospot (ELISpot) assay for interferon-γ production. Participant peripheral blood mononuclear cells (PBMCs) were collected using standard tubes containing sodium citrate anticoagulant. Tubes were stored at room temperature and centrifuged at 1,590 × g for 20 min, following which the tube sample was mixed. PBMCs were transferred to the central laboratory at ambient temperature. PBMCs were sampled up to week 26 following AMT-060 treatment and were cultured without any stimulus (negative control), phytohemagglutinin (PHA; positive control), or AAV5 antigen to identify individual interferon-γ-positive spot-forming colonies (SFCs) per 1 × 106 PBMCs.
Author Contributions
A.M. developed the luciferase-based anti-AAV5 NABs assay, planned and performed most of the experiments, and wrote the manuscript. B.N. was involved in planning the study and helped arrange analysis of the samples. M.H.L. developed the anti-AAV5 IgG ELISA and reviewed the manuscript. L.S. sourced the NHPs serum samples and reviewed the manuscript. M.d.H. was responsible for the non-clinical studies and reviewed the manuscript. H.P. was involved in planning the study and reviewed the manuscript. S.J.v.D. gave advice and reviewed and edited the manuscript. C.M. helped source the patient sera samples. M.T. was involved in study planning, helped source patient serum samples, and reviewed and edited the manuscript. V.F. planned the study and wrote the manuscript.
Acknowledgments
This work was supported by uniQure. The authors would also like to thank the following individuals for their assistance: Karin L. Kwikkers for assistance with patient sample analysis; Ilse Tuinhof for arranging patient serum samples; Vector Production Unit at uniQure for AAV5 batches production and analysis (Erich Ehlert, Tamar Grevelink, Lisanne Schulte); Charles River Laboratories for sera samples analysis with GFP-based anti-AAV5 NABs; and Eileen Sawyer for review of the manuscript.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2019.05.009.
Supplemental Information
References
- 1.Hurlbut G.D., Ziegler R.J., Nietupski J.B., Foley J.W., Woodworth L.A., Meyers E., Bercury S.D., Pande N.N., Souza D.W., Bree M.P. Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol. Ther. 2010;18:1983–1994. doi: 10.1038/mt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang H., Couto L.B., Patarroyo-White S., Liu T., Nagy D., Vargas J.A., Zhou S., Scallan C.D., Sommer J., Vijay S. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 4.Scallan C.D., Jiang H., Liu T., Patarroyo-White S., Sommer J.M., Zhou S., Couto L.B., Pierce G.F. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 5.Wang L., Calcedo R., Bell P., Lin J., Grant R.L., Siegel D.L., Wilson J.M. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum. Gene Ther. 2011;22:1389–1401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 7.Calcedo R., Morizono H., Wang L., McCarter R., He J., Jones D., Batshaw M.L., Wilson J.M. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calcedo R., Vandenberghe L.H., Gao G., Lin J., Wilson J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C., Narkbunnam N., Samulski R.J., Asokan A., Hu G., Jacobson L.J., Manco-Johnson M.J., Monahan P.E., Joint Outcome Study Investigators Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 2012;19:288–294. doi: 10.1038/gt.2011.90. [DOI] [PubMed] [Google Scholar]
- 10.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiorini J.A., Afione S., Kotin R.M. Adeno-associated virus (AAV) type 5 Rep protein cleaves a unique terminal resolution site compared with other AAV serotypes. J. Virol. 1999;73:4293–4298. doi: 10.1128/jvi.73.5.4293-4298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao G., Vandenberghe L.H., Wilson J.M. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- 13.Halbert C.L., Miller A.D., McNamara S., Emerson J., Gibson R.L., Ramsey B., Aitken M.L. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2006;17:440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q., Huang W., Zhang H., Wang Y., Zhao J., Song A., Xie H., Zhao C., Gao D., Wang Y. Neutralizing antibodies against AAV2, AAV5 and AAV8 in healthy and HIV-1-infected subjects in China: implications for gene therapy using AAV vectors. Gene Ther. 2014;21:732–738. doi: 10.1038/gt.2014.47. [DOI] [PubMed] [Google Scholar]
- 15.Mimuro J., Mizukami H., Shima M., Matsushita T., Taki M., Muto S., Higasa S., Sakai M., Ohmori T., Madoiwa S. The prevalence of neutralizing antibodies against adeno-associated virus capsids is reduced in young Japanese individuals. J. Med. Virol. 2014;86:1990–1997. doi: 10.1002/jmv.23818. [DOI] [PubMed] [Google Scholar]
- 16.Miesbach W., Meijer K., Coppens M., Kampmann P., Klamroth R., Schutgens R., Tangelder M., Castaman G., Schwäble J., Bonig H. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2018;131:1022–1031. doi: 10.1182/blood-2017-09-804419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M., Crosby A., Hastie E., Samulski J.J., McPhee S., Joshua G., Samulski R.J., Li C. Prediction of adeno-associated virus neutralizing antibody activity for clinical application. Gene Ther. 2015;22:984–992. doi: 10.1038/gt.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathwani A.C., Gray J.T., McIntosh J., Ng C.Y., Zhou J., Spence Y., Cochrane M., Gray E., Tuddenham E.G., Davidoff A.M. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mingozzi F., Hasbrouck N.C., Basner-Tschakarjan E., Edmonson S.A., Hui D.J., Sabatino D.E., Zhou S., Wright J.F., Jiang H., Pierce G.F. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathwani A.C., Rosales C., McIntosh J., Rastegarlari G., Nathwani D., Raj D., Nawathe S., Waddington S.N., Bronson R., Jackson S. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol. Ther. 2011;19:876–885. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathwani A.C., Gray J.T., Ng C.Y., Zhou J., Spence Y., Waddington S.N., Tuddenham E.G., Kemball-Cook G., McIntosh J., Boon-Spijker M. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathwani A.C., Davidoff A.M., Hanawa H., Hu Y., Hoffer F.A., Nikanorov A., Slaughter C., Ng C.Y., Zhou J., Lozier J.N. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100:1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- 23.Davidoff A.M., Gray J.T., Ng C.Y., Zhang Y., Zhou J., Spence Y., Bakar Y., Nathwani A.C. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol. Ther. 2005;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Falese L., Sandza K., Yates B., Triffault S., Gangar S., Long B., Tsuruda L., Carter B., Vettermann C., Zoog S.J., Fong S. Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther. 2017;24:768–778. doi: 10.1038/gt.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majowicz A., Salas D., Zabaleta N., Rodríguez-Garcia E., González-Aseguinolaza G., Petry H., Ferreira V. Successful Repeated Hepatic Gene Delivery in Mice and Non-human Primates Achieved by Sequential Administration of AAV5ch and AAV1. Mol. Ther. 2017;25:1831–1842. doi: 10.1016/j.ymthe.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Von Drygalski A., Giermasz A., Castaman G., Key N.S., Lattimore S., Leebeek F.W.G., Miesbach W., Gut R., Recht M., Pipe S.W. Oral Presentation OR10: Phase 2b trial of AMT-061 (AAV5-Padua hFIX): translation into humans of an enhanced gene transfer vector for adults with severe or moderate-severe hemophilia B. Haemophilia. 2019;25(Suppl 1):25–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.