Abstract
Successful pregnancy requires adaptation in maternal physiology. During intrauterine life the mother's circadian timing system supports successful birth and postnatal development. Maternal melatonin is important to transmit circadian timing and day length to the fetus. This study aims to describe the third trimester of pregnancy among day (n = 5) and night (n = 3) workers by assessing their melatonin levels in a natural environment. Additionally, we describe the worker's metabolic profiles and compare the health status of the newborns between groups of day and night working mothers. Our results indicate an occurrence of assisted delivery (cesarean and forceps) among night workers. Moreover, the newborns of night workers showed lower Apgar index and breastfeeding difficulty indicating a worse condition to deal with the immediate outside the womb environment. Additionally, there was lower night-time melatonin production among pregnant night workers compared to day workers. These findings may be related to light-induced suppression of melatonin that occurs during night work. We conclude that night work and consequent exposure to light at unconventional times might compromise the success of pregnancy and the health of the newborn. Further studies need to be carried out to monitor pregnancy and newborn health in pregnant night workers.
Keywords: Pregnancy, Melatonin, Offspring, Metabolic disturbances, Premature birth, Shift work
Graphical abstract
Highlights
-
•
There was lower night-time melatonin production among pregnant night workers compared to day workers.
-
•
Night work might compromise the success of pregnancy.
-
•
Night work during pregnancy might compromise the health of the newborn.
1. Introduction
There is a close relationship between an optimal pregnancy and a healthy transition to postnatal life (Steegers et al., 2016). An increase of non-communicable diseases (Alwan et al., 2010) such as obesity (Proper et al., 2016) and diabetes (Gan et al., 2015) has led to a global disease burden, which among other factors might be a consequence of non-adequate prenatal conditions (Hanson et al., 2011). In this context, pregnancy seems to be an important period to prevent non-communicable diseases in the offspring.
In a study with mice there was evidence that during pregnancy the mother's circadian rhythm gives support to successful birth and postnatal development (Smarr et al., 2017). The circadian timing system of the non-human fetus, however, is not completely developed or is organized differently from that of the adult (Landgraf et al., 2014). This could explain how pregnancy per se represents a crucial time for the prevention of changes that would become chronic and definitely compromise the health of the offspring. Studies by Ferreira et al. (2012) and Varcoe et al. (2011) with non-human subjects suggested circadian misalignment and reduced melatonin synthesis may directly impact energy metabolism. Moreover, during the fetal development, it has been suggested that melatonin has a neuroprotective effect. The melatonin level is increased at the end of pregnancy, contributing to the regulation of sleep patterns, which is important to the neurodevelopment of offspring (Gitto et al., 2013). Studies with rats showed that in addition to this effect, suppression of mother's melatonin over the pregnancy is related to intrauterine growth retardation (Mendez et al., 2012).
Melatonin, distributed ubiquitously in nature, has several functions among which include biological regulation of circadian rhythms, especially in mammals (Arendt, 2006; Epstein and Brzezinski, n.d.), neuroendocrine (Bass and Takahashi, 2010) and neuroimmunological functions (Carrillo-Vico et al., 2005), and reproductive action (Mahoney, 2010). Melatonin, a hormone, is produced primarily by the pineal gland. Pineal melatonin production is controlled by the circadian pacemaker located in the hypothalamic suprachiasmatic nuclei (SCN), restricting melatonin synthesis to the night (dark phase). This endogenous circadian rhythm of melatonin is synchronized to the environmental 24 h light/dark cycle.
In humans, melatonin production peaks around 02:00–03:00 h (Skene and Arendt, 2006). Exposure to light at night acutely suppresses melatonin synthesis resulting in reduced levels in the circulation (Bojkowski et al., 1987; Weitzman et al., 1980). Light exposure at night which occurs during night work, has been hypothesized to contribute to some of the adverse effects of shift work, such as increased breast cancer risk (Stevens et al., 2011). Considering that, in general, a female night worker does not change shift during pregnancy (Palmer et al., 2013), the circadian misalignment caused by night work and the light exposure experienced at night may affect melatonin production (Dumont et al., 2012) and consequently, fetus health.
Night work is a permanent situation around the world. Some countries (e.g. Spain, Germany and other EU countries) do not allow women to do night work during pregnancy, and permit the pregnant woman to transfer to day work as a strategy to promote maternal health and reduce risks of spontaneous abortion/miscarriage (Bonde et al., 2014). By contrast, in Brazil, there are no rules about working night shifts during pregnancy (Campanhole and Campanhole, 1994).
Circadian misalignment, such as occurs during night work, involves disturbances in the temporal organization of many physiological parameters such as hormones, sleep-wake cycle and temperature due to misalignment with external time cues and behaviour. This may affect the pregnant mother and the fetus with negatives consequences for both, such as increased risk of miscarriage (Fernandez et al., 2016) and low birth-weight (Tustin et al., 2004; Zhu et al., 2004). Moreover, circadian misalignment may influence development of the newborn, increasing the risk of developing chronic disease in adult life (Lehnen et al., 2013). A recent study by Strohmaier et al. (2018) has added some controversial points to the discussion. This study investigated possible associations of maternal history of work hours and outcomes in offspring, such as weight from early life to adolescence. The results suggest that rotating night shift prior to pregnancy does not influence the offspring's weight and height at 5 years of age. However, the risk of children being obese or overweight is 11% throughout adolescence and in adulthood (relative risk = 1.11; 95% CI: 1.02–1.21) (Strohmaier et al., 2018).
Additionally, there is strong evidence that melatonin levels differ between day and night shift workers (Daugaard et al., 2017; Liang Zhu et al., 2004) and differ between work days and days off (Daugaard et al., 2017) However, according to Dumont and colleagues these differences could be related to total melatonin production, and not necessarily related to a reduction of melatonin excretion at work (during night work) (Dumont et al., 2012).
On the other hand, offspring whose mothers had a healthy lifestyle before pregnancy, as normal body weight, regular physical activity and no smoking, showed a decreased risk to develop obesity over the lifespan (Dhana et al., 2018). In addition, Devore et al. reported that larger differences (minimum/maximum) in photoperiod exposure throughout gestation may positively influence the risk of depression (Devore et al., 2018).
In short, it has been suggested that during pregnancy, either in human or non-human studies, that maternal melatonin plays an important role transmitting information about day and night fluctuations, day length and its seasonal variation to the fetus (Sáenz de Miera et al., 2017; Serón-Ferré et al., 2012, 1993; Weaver et al., 1987).
Therefore, our aim was, firstly, to describe the third trimester of pregnancy among day and night workers by assessing their melatonin levels in a natural environment. Secondly, we aimed to measure metabolic profiles among day and night workers during pregnancy. Lastly, we compared the health status of the newborns between groups of day and night working mothers.
2. Material and methods
2.1. Study population
Participants were recruited from a large hospital located in São Paulo, Brazil. The research was conducted from August 2016 to April 2017 after participants had signed the informed consent according to the guidelines of the Ethics Committee of University of São Paulo (number 1.581.480) and the Ethics Committee of the Hospital (number 463922147/001000). According to the original study protocol, the pregnant worker could be included in the sample at any gestational trimester. However, we only have melatonin data from pregnant night workers from the third trimester of pregnancy. Thus, we decided not report the first (n = 2 day workers) and second (n = 2 day workers) trimester data since we are not able to compare all workers.
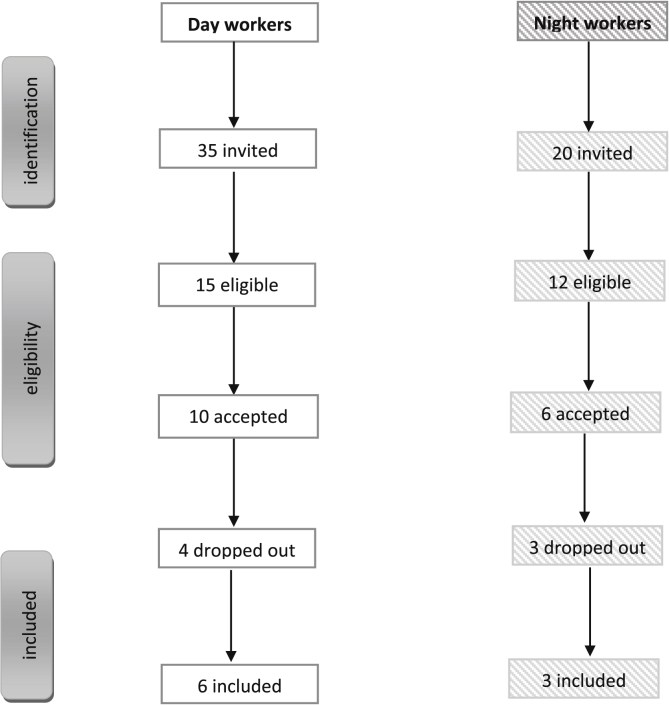
The inclusion criteria were: absence of co-morbidity, and no visual problems. Day workers should not have undertaken night work in the past. All participants were nurses but one day worker was working at the health medical centre. Fig. 1 shows the eligible participants according to the inclusion and exclusion criteria.
Fig. 1.
Diagram of participant recruitment and selection.
Pregnant day (n = 35) and night workers (n = 20) were invited to take part in the study (Fig. 1). Out of 35, 15 pregnant day workers met the inclusion criteria and were eligible to enrol on the field study. Regarding night workers, 12 out of 20 met the inclusion criteria. However, in total only 9 night workers remained in the study (Fig. 1).
2.2. Study protocol
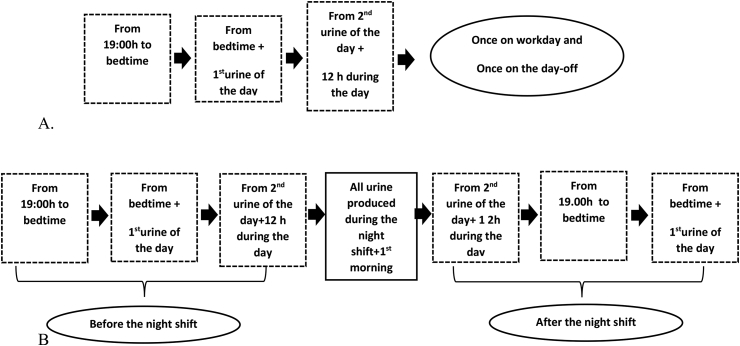
The pregnant workers completed questionnaires giving information on demographics, work, and health status. Melatonin was quantified through measurement of its major metabolite in urine (6-sulfatoxymelatonin - aMT6s). All participants were followed for at least 10 days over the study (Fig. 2A and B). Moreover, the pregnant workers were followed weekly up to 2 months after offspring's birth.
Fig. 2.
Urine collection protocol of day (A) and night (B) pregnant workers.
During the study, no attempt was made to alter the lifestyle, as such as the work schedule of the participants. Urine collection was performed by all participants at home and at work, with no control of environmental light exposure. However, all the participants were asked to avoid caffeinated drinks as well as strenuous exercise during the data collection.
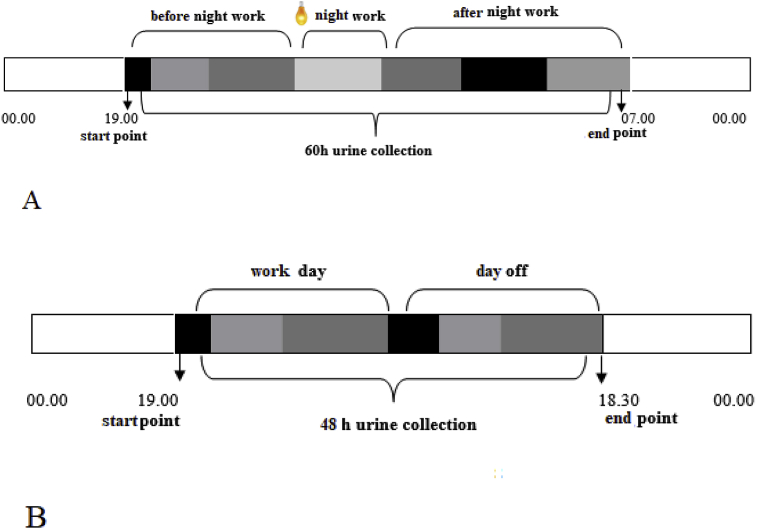
For 10 days of the study, the pregnant night workers were instructed to collect sequential urine samples (before and after the night shift) for measurement of aMT6s (Fig. 3A). The pregnant day workers followed the urine collection protocol on work days and days-off, over a 48 h period (from 19:00 h to time to go to bed; from time to go to bed to the first urine next morning, and 12 h over the day) (Fig. 3B).
Fig. 3.
Urine collection times of a pregnant night worker (A):  19.00 to bed time;
19.00 to bed time;  from bed time to 1st urine of the day;
from bed time to 1st urine of the day;  from 2nd urine of the day +12 h over the day;
from 2nd urine of the day +12 h over the day;  all urine produced during the night shift work;
all urine produced during the night shift work;  from 2nd urine of the day +12 h over the day;
from 2nd urine of the day +12 h over the day;  19.00 to bed time;
19.00 to bed time;  from bed time to 1st urine of the day; and a pregnant day worker (B):
from bed time to 1st urine of the day; and a pregnant day worker (B):  19.00 to bed time;
19.00 to bed time;  from bed time to 1st urine of the day;
from bed time to 1st urine of the day;  from 2nd urine of the day +12 h over the day;
from 2nd urine of the day +12 h over the day;  19.00 to bed time;
19.00 to bed time;  from bed time to 1st urine of the day;
from bed time to 1st urine of the day;  from 2nd urine of the day +12 h over the day.
from 2nd urine of the day +12 h over the day.
Participants were required to void their bladders at the exact times as described in the protocol and were instructed to record the date and time of voiding each sample. Each urine void was collected in a different vial (500 mL or 2 L plastic containers), and kept in a refrigerator. After 24 h, the urine samples were sent to the laboratory in order to measure the total volume of each urine sample and a 10-mL urine aliquot from each container was stored (−20 C) prior to assay of aMT6s. Urinary aMT6s concentrations for each participant was quantified in the same assay run, and determined by ELISA (IBL, Hamburg, Germany), according to the manufacturer's instructions (1.0 ng/mL, mean signal (Zero-Standard) – 2SD). The output for each sequential collection (ng/ml) was converted to ng/h and subjected to cosinor analysis to determine the peak time of production.
2.3. Health evaluation on mothers and offspring
All the pregnant participants had prenatal care with their physician of choice and all had records of their routine examinations. Metabolic measures, as body mass index, blood pressure and blood glucose levels over the trimesters were analysed in the present study. Moreover, we analysed the presence of bleeding/haemorrhage and premature birth of the participants, as well as the type of childbirth, breastfeeding 24 h after birth and the continuity of breastfeeding.
The newborns were evaluated by the Apgar test, on the first- and fifth-minute of life. This procedure was done in order to evaluate five items of the physical examination of the newborn: heart rate, respiratory effort, muscle tone, reflex irritability and skin color. Each of the parameters is classified with a score of 0, 1 or 2, the final score being a maximum of 10. Heart rate was measured in heart beats per minute; breathing was checked by weak or vigorous weeping; muscle tone was analysed by the flexibility and movement of the legs; reflexes were perceived by grimaces, coughs or sneezing before a specific stimulation; and color of the skin is characterized as pale, bluish or pink. The Apgar test is used to evaluate the immediate adjustment of the newborn to life outside the mother's womb. The results of the Apgar test assess this adjustment and determine whether the newborn will need medical care or not (American Academy of Pediatrics Committee on Fetus and Newborn and American College of Obstetricians and Gynecologists Committee on Obstetric Practice, 2015).
2.4. Data analysis
The average and standard deviation of metabolic data for each participant was calculated for both day-offs or workdays. Due to the small sample size comparisons have been kept descriptive.
3. Results
All but one day worker were over 30 years old (32 ± 4.1 years, mean ± SD). All participants were non-smokers and were free from medical illness. Three out of six day workers were nulliparous. All the night workers were multiparas, aged between 33 and 37 years old (35 ± 1.8 years, mean ± SD). All participants reported regular menstrual cycles. The day workers were overweight before the pregnancy (29.6 ± 7.5 kg/m2, mean ± SD) compared to the night workers (23.4 ± 2.0 kg/m2, mean ± SD). None presented anaemia during gestation. None of the participants took medication that could affect sleep and/or melatonin production.
Most of the participants were accustomed to exercise before pregnancy, however, this stopped during pregnancy (Table 1). All participants completed high school and some (33% of day and night workers) graduated from University. Although some of them dropped out the university, they have concluded high school, which is above the average education level in Brazil.
Table 1.
Participants’ age, Body Mass Index, glucose and blood pressure over the three trimesters, according to their shift pattern.
| Age at start of study (years) | Pregestationala | Body Mass Index (kg/m2) |
Glucose (mg/dl) |
Blood Pressure (mmHg) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trimester | |||||||||||
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | 1st | 2nd | 3rd | |||
| Day workers | |||||||||||
| A | 34.2 | 35.8 | 34.8 | 36.5 | 38.2 | 92 | 150 | 178 | 120 × 60 | 130 × 110 | 120 × 80 |
| B | 24.1 | 22.7 | 23.8 | 25.0 | 27.3 | 89 | 88 | 64 | 100 × 70 | 90 × 50 | 100 × 60 |
| C | 37.7 | 40.6 | 37.2 | 35.5 | 35.0 | 121 | 100 | 82 | 140 × 100 | 120 × 80 | 110 × 60 |
| D | 30.9 | 21.3 | 21.1 | 25.4 | 27.4 | 85 | 88 | 71 | 100 × 70 | 90 × 60 | 100 × 60 |
| E | 32.8 | 29.1 | 30.0 | 30.4 | 37.5 | 90 | 95 | 99 | 110 × 90 | 124 × 72 | 110 × 80 |
| F | 32.7 | 27.9 | 29.0 | 30.1 | 31.2 | 99 | 90 | 88 | 110 × 90 | 110 × 70 | 110 × 70 |
| mean | 31.6 | 29.6 | 29.3 | 30.5 | 32.8 | 96 | 102 | 97 | 113x80 | 111x74 | 108x68 |
| SD | 4.9 | 7.5 | 6 | 4.8 | 4.8 | 13 | 24 | 42 | 15x15 | 18x20 | 8x10 |
| Night workers | |||||||||||
| G | 34.0 | 21.1 | 22.3 | 25.2 | 25.9 | 89 | 94 | 83 | 110 × 60 | 110 × 60 | 110 × 60 |
| H | 33.2 | 24.4 | 260 | 26.4 | 26.8 | 121 | 98 | 90 | 120 × 70 | 110 × 60 | 120 × 70 |
| I | 37.3 | 24.6 | 24.6 | 27.3 | 28.5 | 80 | 82 | 95 | 100 × 60 | 90 × 50 | 110 × 60 |
| mean | 34.8 | 23.4 | 24.3 | 26.3 | 27.1 | 97 | 91 | 89 | 110x63 | 103x57 | 113x63 |
| SD | 2.2 | 1.9 | 1 | 1.1 | 1.3 | 22 | 8 | 6 | 10x05 | 11x5 | 6x6 |
Pregestational = before pregnancy.
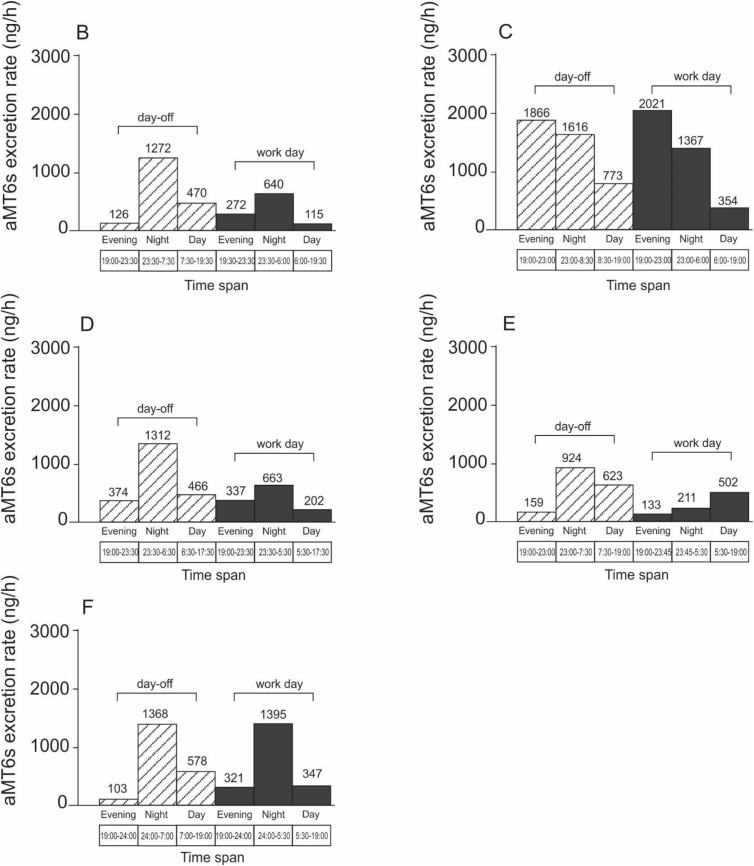
Urinary aMT6s profiles in pregnant day workers during the third trimester are shown in Fig. 4.
Fig. 4.
aMT6s profiles (excretion rate, μg/h) across days-off and work days in pregnant day workers during the third trimester. Each graph (B, C, D, E, F) represents a different participant. day-off and
day-off and  workday.
workday.
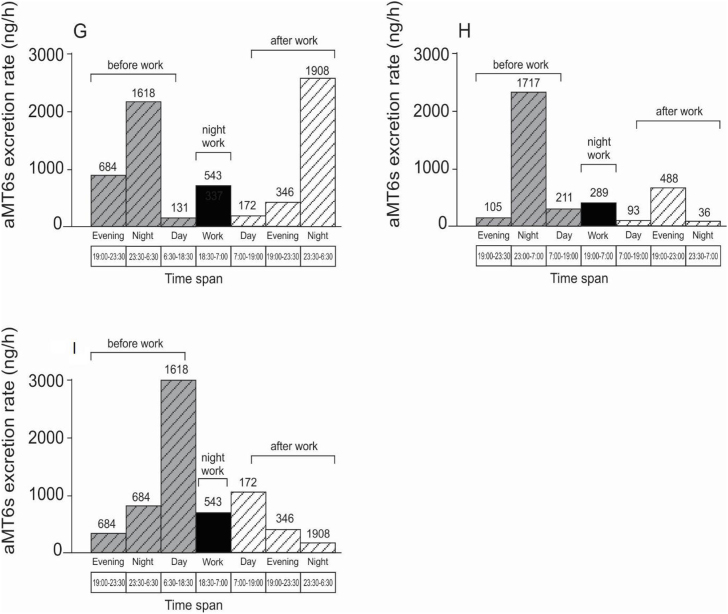
Only data from the third trimester were analysed. Their aMT6s profiles for nights-off and during night work are presented in Fig. 5. For these participants, melatonin suppression was observed during the night shift, as shown by lower melatonin production during night work and higher levels at night before and/or after night shift (Fig. 5). There was lower night-time melatonin production among pregnant night workers (night work plus the next evening) compared to day workers (evening plus night after the day work).
Fig. 5.
aMT6s profiles (excretion rate, μg/h) across work nights and nights-off in pregnant night workers during the third trimester. Each graph (G,H,I) represents a different participant.  night-off before night work;
night-off before night work;  night work and
night work and  night-off after night work.
night-off after night work.
Metabolic characteristics of the nine pregnant workers across the 3 trimesters of gestation are presented in Table 2. Day workers exhibited higher pregestational BMI than night workers. Glucose and blood pressure levels were also higher among day workers than night workers.
Table 2.
Type of birth, birth weight, breastfeeding and Apgar information according the mother's shift.
| Variable | Mother's shift | |
| Day | Night | |
| n | n | |
| Type of childbirth | ||
| Natural | 4 | 1 |
| Surgery (cesarean) | 2 | 1 |
| Forceps | 0 | 1 |
| Breast feeding 24 h after birth | ||
| Yes | 6 | 1 |
| No | 0 | 2 |
| Continue Breastfeeding after 2 monthsa | ||
| Yes | 5 | 2 |
| No | 1 | 1 |
| Apgar | ||
| 1stminute | 8.8 | 7.6 |
| 5thminute | 9.8 | 8.6 |
Apgar score 0 = minimum value (worst) 10 = maximum value (excellent).
One newborn of a night working mother was breastfeed after 2 months but not on the first 24 h.
During the pregnancy two out of three night workers were affected by premature birth and haemorrhage, as well as reported previous miscarriage, even though they had no disorders at the beginning of followed pregnancy in this study. The offspring did not present health problems immediately after birth. The mean Apgar test score was, however, lower for the newborns of the night working mothers compared to the day working mothers (Table 2).
The predominant type of delivery among day workers was the natural one, whereas night workers presented different types of delivery (natural, cesarean and forceps). During the first 24 h, two of the three newborns of night working mothers were not breastfeed. Thus, it is not surprising that 1/3 of the newborns after two months were already in the stage of weaning.
4. Discussion
The aim of this study was firstly to describe melatonin rhythms in the third trimester in both pregnant day and night workers and secondly to assess the health of the newborn in day and night workers. Moreover, our results may provide insights about the melatonin production and work hours on 3rd trimester of gestation. Since evidence from animal models (Jud and Albrecht, 2006; Serón-Ferré et al., 2013) propose that maternal circadian misalignment would have negative effects on offspring health, studies looking at the effect of shift work on newborn health are needed. In some species, for example rats and sheep, the amount of melatonin during pregnancy was higher compared to the levels postpartum (Deguchi, 1975; Yellon and Longo, 1988). In humans, variation of serum melatonin levels over the pregnancy was observed by Tamura et al. (2008) with higher values near the end possibly preparing the fetus for birth. Experimental and clinical reports have suggested that reduction of maternal melatonin during pregnancy is a potential damage to the offspring, that could be explained by an epigenetic function of melatonin (Tain et al., 2014).
Our data have revealed some adverse effects on pregnant women who work at night. We observed bleeding and miscarriage in the night workers whereas the day workers did not show these symptoms. These results provide some evidence to support a link between maternal circadian misalignment such as occurs in night workers and gestational health. Shift work has been associated with risk of miscarriage and pre-term labor (Mcdonald et al., 1988; Zhu et al., 2004). Despite the small sample numbers in the present study differences between day and night workers were observed. Moreover, our results link night work, pregnancy and melatonin production and rhythmicity for the first time as far as we know. We observed lower night-time melatonin production among pregnant night workers, and lower Apgar test score for the babies of night workers’ mothers.
The reduction in the urinary 6-sulfatoxymelatonin levels on the night shift suggests that night work may disturb the rhythmicity of melatonin production in this population. Night work increases a person's exposure to artificial light at night, which in turn suppresses melatonin synthesis (Bojkowski et al., 1987). It was not possible to observe how many days-off would be necessary to re-establish the night-time melatonin level, due to the data collection protocol.
Day workers showed urinary 6-sulfatoxymelatonin rhythms consistent with the normal profile for this hormone, peaking at night (Arendt, 2005, 1998; Montagnese et al., 2015). The similarity in aMT6s profiles on both days-off and workdays was expected, considering they were day workers sleeping at night and thus had no/little light exposure during the night. However, there were individual differences, and two day workers seem to be affected by the early start times of work schedules, which might explain the lower levels of nocturnal melatonin in these participants.
In summary, we present for the first time, in a case series conducted in the field, melatonin profiles during the third trimester of gestation and the impact of night work on the production of this hormone, since our study assessed melatonin levels during both day and night work as well as on days- and nights-off. The birth data from the offspring of the night workers may point to putative repercussions of night work for newborn health. Night workers presented proportionally more problems during pregnancy than day workers and, at birth, the offspring of the night workers showed lower Apgar scores and difficulty in immediate breastfeeding. These practices are recognized as key to long-term health maintenance, such as exclusive breastfeeding up to the sixth month of life.
This study has some limitations such as low sample size, which was lower than initially planned. Inclusion criteria such as having been working during conception and remain working at night were the main reasons for the reduced sample size. Due to this limitation, interpretation of the results should be taken with caution. Another issue was the level of light exposure, known to be an acute suppressor of melatonin, that was not measured during sample collection at home or at the workplace.
It is relevant to highlight that labor activity has a central role in society, considering that other activities inherent to human living are developed around the work schedule (Costa et al., 2004; Eurofound and the International Labour Office, 2017). In this context, economy plays an important contribution to the changes in the structure of the work environment, which includes new work schedules, and consequently effects on lifestyle. At the same time, the world has observed expansion of the participation of women in the workforce, for instance in the European Union (EU), where from 2005 to 2015 female employment increased 4%. Although the trend on weekly working hours is declining, there is evidence that working night shifts will remain. Around 19% of workers in the EU reported working at night. Males mainly work at night, with 24% of men working in this shift, while women who work night shifts are under 15% (Eurofound and the International Labour Office, 2017).
5. Conclusions and remarks
There is currently a wide-ranging discussion about the deleterious effects of circadian misalignment such as occurs during night work, which may extend beyond the individual and be transmitted to the offspring. Studies using animal models have made significant progress in clarifying the extent to which shift work, especially night work and consequent exposure to light, is the triggering factor for circadian misalignment. In addition, melatonin production is suppressed by night shift light exposure, which was corroborated by our results. It seems that very early start times of work schedules among day workers might also reduce melatonin production due to the early wake up times. Changes in the pattern of melatonin production might contribute to the development of gestational problems as we have observed among night workers. In addition, the offspring of the night workers showed a worse ability to deal with the immediate environment and to engage in breastfeeding nourishment. Further research on the putative correlation between melatonin reduction in pregnancy and mother and offspring health in a larger sample, and in other work or environmental situations is warranted.
Declarations of interest
None.
Funding
This work was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo – FAPESP [grant numbers 2014/50457-0; 2016/11155-3]; Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior – CAPES– Finance Code 001; CAPES/Stint [grant numbers 021/14]; and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq [grant numbers 142140/2015-5].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbscr.2019.04.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alwan A., MacLean D.R., Riley L.M., D'Espaignet E.T., Mathers C.D., Stevens G.A., Bettcher D. Monitoring and surveillance of chronic non-communicable diseases: progress and capacity in high-burden countries. Lancet. 2010;376(9755):1861–1868. doi: 10.1016/S0140-6736(10)61853-3. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Fetus and Newborn and American College of Obstetricians and Gynecologists Committee on Obstetric Practice, A.C.O.O.A.G.C.O.O. The apgar score. Pediatrics. 2015 doi: 10.1542/peds.2015-2651. [DOI] [Google Scholar]
- Arendt J. Chronobiology International. 2006. Melatonin and human rhythms. [DOI] [Google Scholar]
- Arendt J. Melatonin: characteristics, concerns, and prospects. J. Biol. Rhythm. 2005;20:291–303. doi: 10.1177/0748730405277492. [DOI] [PubMed] [Google Scholar]
- Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev. Reprod. 1998;3:13–22. doi: 10.1530/ror.0.0030013. [DOI] [PubMed] [Google Scholar]
- Bass J., Takahashi J.S. Circadian integration of metabolism and energetics. Science (80-. ) 2010 doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojkowski C.J., Aldhous M.E., English J., Franey C., Poulton A.L., Skene D.J., Arendt J. Suppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in man. Horm. Metab. Res. 1987;19:437–440. doi: 10.1055/s-2007-1011846. [DOI] [PubMed] [Google Scholar]
- Bonde J.P.E., Jørgensen K.T., Bonzini M., Palmer K.T., Keith T. Europe PMC Funders Group Risk of miscarriage and occupational activity : a systematic review and meta-analysis regarding shift work , working hours , lifting , standing and physical workload. Scand. J. Work. Environ. Health. 2014;39:325–334. doi: 10.5271/sjweh.3337.Risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanhole A., Campanhole H.L. 90 edição. 1994. Consolidação das leis do trabalho, legislação complementar. São Paulo. [Google Scholar]
- Carrillo-Vico A., Guerrero J.M., Lardone P.J., Reiter R.J. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27(2):189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- Costa G., Akerstedt T., Nachreiner F., Baltieri F., Carvalhais J., Folkard S., Dresen M.F., Gadbois C., Gartner J., Sukalo H.G., Harma M., Kandolin I., Sartori S., Silverio J. Flexible working hours, health, and well-being in Europe: some considerations from a SALSTA project. Chronobiol. Int. 2004;21:831–844. doi: 10.1081/LCBI-200035935. [DOI] [PubMed] [Google Scholar]
- Daugaard S., Garde A.H., Bonde J.P.E., Christoffersen J., Hansen Ä.M., Markvart J., Schlünssen V., Skene D.J., Vistisen H.T., Kolstad H.A. Night work, light exposure and melatonin on work days and days off. Chronobiol. Int. 2017;34(7):942–955. doi: 10.1080/07420528.2017.1327867. [DOI] [PubMed] [Google Scholar]
- Deguchi T. Ontogenesis of a biological clock for serotonin:acetyl coenzyme A N-acetyltransferase in pineal gland of rat (circadian/neural regulation/melatonin) Physiology. 1975;72:2814–2818. doi: 10.1073/pnas.72.7.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore E.E., Chang S.C., Okereke O.I., McMahon D.G., Schernhammer E.S. Photoperiod during maternal pregnancy and lifetime depression in offspring. J. Psychiatr. Res. 2018;104:169–175. doi: 10.1016/j.jpsychires.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhana K., Zong G., Yuan C., Schernhammer E., Zhang C., Wang X., Hu F.B., Chavarro J.E., Field A.E., Sun Q. Lifestyle of women before pregnancy and the risk of offspring obesity during childhood through early adulthood. Int. J. Obes. 2018;42:1275–1284. doi: 10.1038/s41366-018-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M., Lanctt V., Cadieux-Viau R., Paquet J. Melatonin production and light exposure of rotating night workers. Chronobiol. Int. 2012;29:203–210. doi: 10.3109/07420528.2011.647177. [DOI] [PubMed] [Google Scholar]
- Epstein, F.H., Brzezinski, A., n.d. Review Articles Mechanisms of Disease the New England Journal of Medicine MELATONIN IN HUMANS.
- Eurofound and the International Labour Office . Eurofound; 2017. Working Anytime, Anywhere: the Effects on the World of Work. [DOI] [Google Scholar]
- Fernandez R.C., Marino J.L., Varcoe T.J., Davis S., Moran L.J., Rumbold A.R., Brown H.M., Whitrow M.J., Davies M.J., Moore V.M. Fixed or rotating night shift work undertaken by women: implications for fertility and miscarriage. Semin. Reprod. Med. 2016;34(02):074–082. doi: 10.1055/s-0036-1571354. [DOI] [PubMed] [Google Scholar]
- Ferreira D.S., Amaral F.G., Mesquita C.C., Barbosa A.P.L., Lellis-Santos C., Turati A.O., Santos L.R., Sollon C.S., Gomes P.R., Faria J.A., Cipolla-Neto J., Bordin S., Anhê G.F. Maternal melatonin programs the daily pattern of energy metabolism in adult offspring. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y., Yang C., Tong X., Sun H., Cong Y., Yin X., Li L., Cao S., Dong X., Gong Y., Shi O., Deng J., Bi H., Lu Z. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup. Environ. Med. 2015;72:72–78. doi: 10.1136/oemed-2014-102150. [DOI] [PubMed] [Google Scholar]
- Gitto E., Marseglia L., Manti S., D'Angelo G., Barberi I., Salpietro C., Reiter R.J. Protective role of melatonin in neonatal diseases. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/980374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M., Godfrey K.M., Lillycrop K.A., Burdge G.C., Gluckman P.D. Developmental plasticity and developmental origins of non-communicable disease: theoretical considerations and epigenetic mechanisms. Prog. Biophys. Mol. Biol. 2011;106(1):272–280. doi: 10.1016/j.pbiomolbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Jud C., Albrecht U. Circadian rhythms in murine pups develop in absence of a functional maternal circadian clock. J. Biol. Rhythm. 2006;21(2) doi: 10.1177/0748730406286264. [DOI] [PubMed] [Google Scholar]
- Landgraf D., Koch C.E., Oster H. Embryonic development of circadian clocks in the mammalian suprachiasmatic nuclei. Front. Neuroanat. 2014;8:143. doi: 10.3389/fnana.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnen H., Zechner U., Haaf T. Epigenetics of gestational diabetes mellitus and offspring health: the time for action is in early stages of life. Mol. Hum. Reprod. 2013;19(7):415–422. doi: 10.1093/molehr/gat020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Zhu J., Henrik Hjollund N., Nybo Andersen A.-M., Olsen J. Vol. 46. 2004. pp. 1144–1149. (Shift work, job stress, and late fetal loss: The National Birth Cohort in Denmark). [DOI] [PubMed] [Google Scholar]
- Mahoney M.M. Shift work, jet lag, and female reproduction. Internet J. Endocrinol. 2010;813764:2010. doi: 10.1155/2010/813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald A.D., Mcdonald J.C., Armstrong B., Cherry N.M., Cote R., Lavoie J., Nolin A.D., Robert D. 1988. Fetal Death and Work in Pregnancy; pp. 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez N., Abarzua-Catalan L., Vilches N., Galdames H.A., Spichiger C., Richter H.G., Valenzuela G.J., Seron-Ferre M., Torres-Farfan C. Timed maternal melatonin treatment reverses circadian disruption of the fetal adrenal clock imposed by exposure to constant light. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnese S., Middleton B., Corrias M., Mani A.R., Skene D.J., Morgan M.Y. Assessment of 6-sulfatoxymelatonin rhythms and melatonin response to light in disease states: lessons from cirrhosis. Chronobiol. Int. 2015;32:187–194. doi: 10.3109/07420528.2014.961607. [DOI] [PubMed] [Google Scholar]
- Palmer K.T., Bonzini M., Harris E.C., Linaker C., Bonde J.P. vol. 70. 2013. pp. 213–222. (Europe PMC Funders Group Work Activities and Risk of Prematurity , Low Birthweight and Pre- Eclampsia : an Updated Review with Meta-Analysis). (Work) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proper K.I., Van De Langenberg D., Rodenburg W., Vermeulen R.C.H., Van Der Beek A.J., Van Steeg H., Van Kerkhof L.W.M. The relationship between shift work and metabolic risk factors: a systematic review of longitudinal studies. Am. J. Prev. Med. 2016;50:e147–e157. doi: 10.1016/j.amepre.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Sáenz de Miera C., Bothorel B., Jaeger C., Simonneaux V., Hazlerigg D. Maternal photoperiod programs hypothalamic thyroid status via the fetal pituitary gland. Proc. Natl. Acad. Sci. Unit. States Am. 2017;114(31):8408–8413. doi: 10.1073/pnas.1702943114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serón-Ferré M., Ducsay C.A., Valenzuela G.J. Circadian rhythms during pregnancy. Endocr. Rev. 1993;14:594–609. doi: 10.1210/edrv-14-5-594. [DOI] [PubMed] [Google Scholar]
- Serón-Ferré M., Forcelledo M.L., Torres-Farfan C., Valenzuela F.J., Rojas A., Vergara M., Rojas-Garcia P.P., Recabarren M.P., Valenzuela G.J. Impact of chronodisruption during primate pregnancy on the maternal and newborn temperature rhythms. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0057710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serón-Ferré M., Mendez N., Abarzua-Catalan L., Vilches N., Valenzuela F.J., Reynolds H.E., Llanos A.J., Rojas A., Valenzuela G.J., Torres-Farfan C. Circadian rhythms in the fetus. Mol. Cell. Endocrinol. 2012;349:68–75. doi: 10.1016/j.mce.2011.07.039. [DOI] [PubMed] [Google Scholar]
- Skene D.J., Arendt J. Human circadian rhythms: physiological and therapeutic relevance of light and melatonin. Ann. Clin. Biochem. 2006;43(5) doi: 10.1258/000456306778520142. [DOI] [PubMed] [Google Scholar]
- Smarr B.L., Grant A.D., Perez L., Zucker I., Kriegsfeld L.J. Maternal and early-life circadian disruption have long-lasting negative consequences on offspring development and adult behavior in mice. Sci. Rep. 2017;7:3326. doi: 10.1038/s41598-017-03406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers E.A.P., Barker M.E., Steegers-Theunissen R.P.M., Williams M.A. Societal valorisation of new knowledge to improve perinatal health: time to act. Paediatr. Perinat. Epidemiol. 2016;30(2) doi: 10.1111/ppe.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R.G., Hansen J., Costa G., Haus E., Kauppinen T., Aronson K.J., Castaño-Vinyals G., Davis S., Frings-Dresen M.H.W., Fritschi L., Kogevinas M., Kogi K., Lie J.A., Lowden A., Peplonska B., Pesch B., Pukkala E., Schernhammer E., Travis R.C., Vermeulen R., Zheng T., Cogliano V., Straif K. Considerations of circadian impact for defining “shift work” in cancer studies: IARC Working Group Report. Occup. Environ. Med. 2011;68:154–162. doi: 10.1136/oem.2009.053512. [DOI] [PubMed] [Google Scholar]
- Strohmaier S., Devore E.E., Vetter C., Missmer S., Eliassen A.H., Rosner B., Rich- Edwards J., Field A.E., Schernhammer E.S. 2018. Night shift work before and during pregnancy and offspring weight outcomes through adolescence; p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tain Y.L., Huang L.T., Chan J.Y.H. Transcriptional regulation of programmed hypertension by melatonin: an epigenetic perspective. Int. J. Mol. Sci. 2014;15(10):18484–18495. doi: 10.3390/ijms151018484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H., Nakamura Y., Terron M.P., Flores L.J., Manchester L.C., Tan D.X., Sugino N., Reiter R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008;25(3):291–303. doi: 10.1016/j.reprotox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Tustin K., Gross J., Hayne H. Maternal exposure to first-trimester sunshine is associated with increased birth weight in human infants. Dev. Psychobiol. 2004;45:221–230. doi: 10.1002/dev.20030. [DOI] [PubMed] [Google Scholar]
- Varcoe T.J., Wight N., Voultsios A., Salkeld M.D., Kennaway D.J. Chronic phase shifts of the photoperiod throughout pregnancy programs glucose intolerance and insulin resistance in the rat. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0018504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D.R., Keohan J.T., Reppert S.M. Definition of a prenatal sensitive period for maternal-fetal communication of day length. Am. J. Physiol. Metab. 1987;253(6):E701–E704. doi: 10.1152/ajpendo.1987.253.6.E701. [DOI] [PubMed] [Google Scholar]
- Weitzman E.D., York N., Moore-ede M.C., Knauer R.S. Light suppresses melatonm secretion in humans. Science (80-. ) 1980;210:1267–1268. [Google Scholar]
- Yellon2 S.M., Longo L.D. Effect of maternal pinealectomy and reverse photoperiod on the circadian melatonin rhythm in the sheep and fetus during the last trimester of pregnancy’. Biol. Reprod. 1988;39:1093–1099. doi: 10.1095/biolreprod39.5.1093. [DOI] [PubMed] [Google Scholar]
- Zhu J.L., Hjollund N.H., Olsen J. Shift work, duration of pregnancy, and birth weight. Am. J. Obstet. Gynecol. 2004;191:285–291. doi: 10.1016/j.ajog.2003.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.