Abstract
Working Memory (WM), is an important factor influencing many higher-order cognitive functions that decline with age. Repetitive training appears to increase WM, yet the mechanisms underlying this improvement are not understood. Sleep has been shown to benefit long-term memory formation and may also play a role in WM enhancement in young adults. However, considering age-related decline in sleep, it is uninvestigated whether sleep will facilitate WM in older adults. In the present work, we investigated the impact of a nap, quiet wakefulness (QW) and active wakefulness (AW) on within-day training on the Operation Span (OSPAN) task in older adults. Improvement in WM was found following a nap and QW, but not active wake. Furthermore, better WM was associated with shared electrophysiological features, including slow oscillation (SO, 0.5–1 Hz) power in both the nap and QW, and greater coupling between SO and sigma (12–15 Hz) in the nap. In summary, our data suggest that WM improvement in older adults occurs opportunistically during offline periods that afford enhancement in slow oscillation power, and that further benefits may come with cross-frequency coupling of neural oscillations during sleep.
Keywords: Executive function, Sigma/SO coupling, Napping, Slow oscillation, Quiet wake, Active wake
Highlights
-
•
Working memory improves with repeated, within-day training in older adults, especially across offline periods containing quiet wake and daytime sleep.
-
•
Slow Oscillatory brain activity during quiet wake and sleep was associated with working memory improvement.
-
•
Slow Oscillation/sigma coupling during the nap was also correlated with working memory improvement.
1. Introduction
Working Memory (WM), known to play a critical role in human cognition (Baddeley et al., 1986, Carpenter and Just, 1989), refers to the ability to hold information in mind for a short amount of time. It is hypothesized that WM includes storage mechanisms that maintain its content in an active state, and executive processes that can perform operations on the stored information (Baddeley et al., 1986, Jonides, 1995). WM is a key determinant of several higher-order cognitive functions, such as reasoning, problem solving, intelligence, and language comprehension (Engle, 2002, Borella et al., 2010, Nettelbeck and Burns, 2010). Like many other cognitive domains, WM capacity declines with age (Wingfield et al., 1988). This decline is thought to contribute to a range of cognitive impairments in older adults (Park et al., 2002), such as language learning, decision making, problem solving, and reading comprehension (Engle and Kane, 2004), as well as decreases in daily life functioning, such as medication adherence (Raz, 2000). Thus, WM improvement has been the focus of cognitive training in older adult populations with the expectation that enhanced WM will generalize to a wide range of cognitive functions (see meta analysis Au et al., 2015; Karbach and Verhaeghen, 2014, but see also Melby-Lervåg and Hulme, 2016).
Typical WM training protocols require multiple weeks of daily practice, suggesting that training-induced changes are slow to form, and that the benefits can last up to 18 months (Dahlin et al., 2008). Little is known about the biological factors that may contribute to these long-term changes, but sleep may play a role. To be sure, a sleep deprived brain shows less effective executive functioning, including decreases in WM, attention, and goal-directed behavior (Nilsson et al., 2005). In young adults, studies have shown significant reductions in WM performance after a night of sleep deprivation compared to controls (Chee et al., 2006, Chee and Choo, 2004, Mu et al., 2005). Interestingly, older adults may be somewhat resilient to prolonged wakefulness, with greater maintenance of WM performance after sleep deprivation compared with young adults (Philip et al., 2004, Stenuit and Kerkhofs, 2005, Adam et al., 2006, Duffy et al., 2009). In light of the significant reductions to sleep in older adults (Bliwise, 1993, Ohayon et al., 2004, Yetton et al., 2018), this resilience to sleep deprivation has been viewed as a potentially adaptive response to living with more fragmented and lighter sleep. However, no studies, that we are aware of, have compared whether sleep or wake improves WM among older population. If sleep does confer greater benefit, considering it as a controllable biological factor in cognitive training would likely enhance outcomes and quality of life of older adults (Raz, 2000).
A growing body of studies showed that long-term memory is facilitated by sleep (Diekelmann and Born, 2010). Marr’s theory of memory consolidation (Marr et al., 1991) posits that information is transferred between the hippocampus and neocortex during periods of quiescence, including sleep and quiet rest. Neural activities in non-rapid eye movement (NREM) frequency bands including slow oscillations (SO, <1 Hz, high voltage cortical Up and Down states reflecting periods of neuronal spiking and silencing, respectively) and spindles (bursts of 12–15 Hz thalamo-cortical activity, sigma) have been shown to play critical roles in both formation and consolidation of long-term memory (Mednick et al., 2013). In fact, SOs appear to be the fundamental cellular phenomenon that organize faster electrophysiological frequencies important for memory formation, like sleep spindles (Steriade et al., 1993, Mölle et al., 2011). SO’s originate more frequently in frontal brain areas and are hypothesized to play major role in time-dependent synaptic plasticity during sleep (Massimini et al., 2004). Intriguingly, recent studies suggest that not only are the spindle frequency (sigma power) and SOs independently associated with performance improvement (Clemens et al., 2005, Gais et al., 2002, Mednick et al., 2013, Oyanedel et al., 2014, Schabus et al., 2004), but the coincidence or coupling of SOs and sigma (SO/sigma) may also be a key mechanism of long-term memory formation during sleep (Mölle et al., 2011, Staresina et al., 2015), with several studies suggesting that sigma activity during the SO up-state are optimal (Niknazar et al., 2015, Gais and Born, 2004, Mölle et al., 2009). Older adults typically show decreased amplitude of SOs (0.5–1 Hz) (Carrier et al., 1997, Martin et al., 2000). Additionally, a recent study examining coupling of sigma and SO in relation to fMRI brain activity and memory performance in older adults (Helfrich et al., 2018), reported changes in spindle/SO coupling and decreased coupling over the frontal pole with age. Note that in both age groups spindle/SO coupling predicted post-sleep declarative memory improvement. Together, these findings suggest that long-term memory formation benefits from increased sigma and SO activity independently, as well as from the coordination of these brain rhythms. However, no studies have probed the relationship between these electrophysiological features that usually occur during sleep and working memory in older adults.
It is important to note that memory consolidation may also occur during wake. In particular, post-encoding wake periods free of interference from new learning, such as quiet wake, may engage similar consolidation mechanisms, an idea proposed by the Opportunistic Consolidation Hypothesis (Mednick et al., 2011). This hypothesis posited that replay processes underlying consolidation may initiate under any condition of reduced interference. Consistent with this hypothesis, Rieth and colleagues, demonstrated equivalent levels of learning from sleep and quiet wake on a motor learning task, in which non-sleep factors (i.e., massed practice and circadian confounds) were controlled (Rieth et al., 2010). A similar result was shown for early motor learning (Humiston and Wamsley, 2018). Additionally, Brokaw and colleagues, reported that verbal memory improvement was greater after a brief period with eyes closed, compared with an active wake condition, and increased SO activity during quiet wake was correlated with performance improvement (Brokaw et al., 2016). Given the emerging picture in aging of poor sleep, decreased SO power and sigma/SO coupling, and apparent resilience to the negative cognitive impact of sleep deprivation, it is possible that aging may give rise to plasticity mechanisms that opportunistically activate consolidation processes during non-sleep offline states. Taken together with evidence of marked deterioration in WM and frontal neural structures supporting executive functioning, we propose that understanding offline mechanisms of working memory function in older adults may help slow decline by providing potential routes to interventions.
The present study examined the effect of a nap, quiet wake (QW) or active wake (AW), on repeated within-day training in a WM task in older adults. Participants completed the Operation Span (OSPAN) task (Unsworth et al., 2005) to assess WM performance. Based on the Opportunistic Consolidation hypothesis, we predicted that across a day of training, nap and QW interventions would have similar impacts on WM improvement and would be better for WM than an AW. We additionally predicted that improvement among the nap and QW in within-day WM accuracy would be related to enhanced electrophysiological features, including activity in SO frequency band, and in SO/sigma coupling during sleep.
2. Method
2.1. Subjects
85 (42 females) healthy, non-smoking older adults between the ages of 60 and 85 (M = 69.24, SD = 6.66) gave informed consent to participate in the study. Inclusion criteria included having a regular self-reported sleep schedule, which was defined as getting at least 6 h of total sleep per night on average. Subjects had no personal history of sleep, neurological or psychological disorders, or other chronic illnesses. The personal history of illnesses were measured two times: first, during a pre-screening questionnaire in which a general health condition report was collected over the phone and the eligibility was determined: second, eligible subjects were invited to participate in an orientation in which they were given details about the study. During the orientation, subjects were screened for cognitive impairment and dementia using Digit Span Backwards and Telephone Screening for Dementia questionnaires (referred to as TELE) (Gatz et al., 2002). In the digit span backwards which contains a multi variate length string of digits, subjects were asked to repeat the string of digits backwards after it was read to them. TELE questionnaire consists of variety of questions to identify dementia-like symptoms. All the experimental procedures were approved by the Institutional Review Board of the University of California at Riverside. Subjects with a regular sleep-wake schedule, determined during an in-person interview, were recruited and asked to maintain their schedule for one week prior to their visit, which was monitored with sleep diaries. In addition, subjects were asked to wear an actigraphy (Actiwatch Spectrum, Respironics) for one night prior to their visit. Subjects were rescheduled if they reported poor sleep quality in their sleep diary, such as having more than 2 nights of less than 6 h of sleep during the week prior to their visit, or if subjects’ actigraphy data was showing less than 6 h of sleep the night before the experimental visit. Rescheduled subjects were given another week to fill out a new sleep diary and maintain a regular sleep wake schedule prior to their visit.
2.2. Protocol
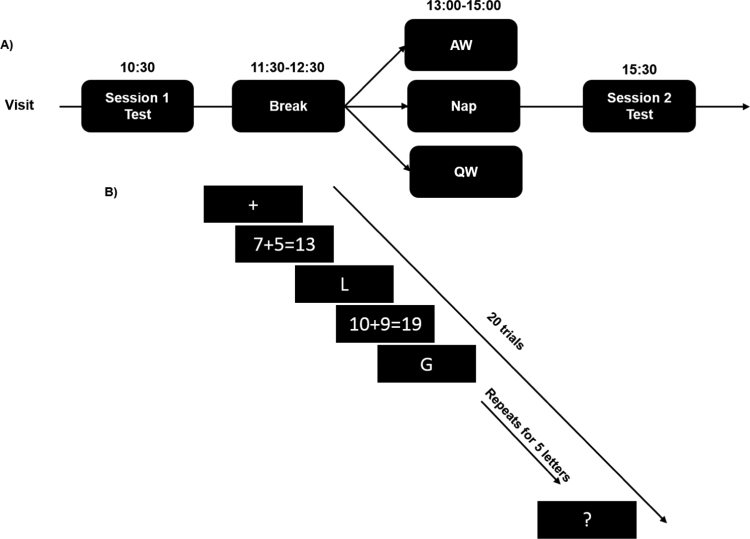
Subjects were asked to refrain from consuming caffeine, alcohol, and all stimulants for 24 h prior to and including the study day. During their visit, Test 1 began at 10:30 (Fig. 1-A). Subjects first were given the Karolinska Sleepiness Scale questionnaire (KSS) (Åkerstedt and Gillberg, 1990) to assess their sleepiness. Next, subjects completed the Operation Span (OSpan) task. The task consisted of a practice phase followed by an immediate test. At 11:30, subjects were given 1hr‐break in the lab and they were instructed to abstain from caffeine, alcohol, exercise and napping. At 12:30, subjects were randomly assigned to either a nap condition (NAP), a quiet wake condition (QW) or an active wake condition (AW). A high-density electroencephalogram (EEG) electrode cap was attached to the scalps of the NAP and QW groups. For the NAP group, subjects were provided a 2‐hour nap opportunity and were allowed to sleep up to 90 min. In the QW conditions, subjects watched a 60-min nature documentary in a dark room lying on the bed while being monitored by lab staff to make sure they did not fall sleep. In the AW group, subjects stayed awake and played a board game with one of the lab research assistants. 30 min after the sleep intervention Test (i.e., Nap, QW or AW), around 15:00, subjects participated in Test 2 during which they were tested on the WM task for approximately 30 min. Subjects completed the KSS and PNQ questionnaire two times throughout the experimental day; at the start of each cognitive Test (i.e., Test 1 and Test 2) to report their sleepiness and mood respectively.
Fig. 1.
A) Experimental visit. At 10:30, subjects completed the Working Memory (WM) task at Session 1, followed by a short break. Next, subjects were randomized to one of three nap interventions (AW, QW, Nap). At 15:30, Session 2 WM testing began. B) The Operation span WM task. The WM task contained nine practice and 20 test trials. The nine practice trials were not counted towards the subjects’ final score. Subjects viewed a serial visual display of letters and math problems. They were asked to hold the letters in memory while simultaneously determining if the simple math problems were correct or not. This was followed by a response period where subjects were asked to type the letters in the previously viewed order.
2.3. WM Task
The WM task contained nine practice and 20 test trials. The nine practice trials were not counted towards the subjects’ final score. Subjects viewed a serial visual display of letters and math problems and then were asked to recall the string of the letters. In fact, they were asked to hold the letters in memory while simultaneously determining if the simple math problems were correct (e.g., 7 + 5 = 13). The letter strings were always 5 letters long (Fig. 1-B). Subjects were tested in the WM task before (Test 1) and after (Test 2) the nap intervention. Each of the simple math equations were presented on the screen for 3000 ms and each letter was presented for 1000 ms. Subjects were instructed to press the “M” key with their right hand if the simple math equation was correct and press the “V” key with their left hand if the equation was not correct. Subjects’ data were only included in the analysis if they performed equal and/or greater than 75 percent correct on the math portion of the task. After each trial, a question mark appeared on the screen to signal the response period. Subjects were required to enter the letters in the same order they were presented. The recall part of the task was not timed and subjects were not able to go to the next trial until the correct number of letters was entered. Then, a “Ready?” prompt appeared on the screen and subjects were instructed to press the space bar to start the next trial when they were ready. Four breaks were inserted throughout the task. Test 2 was identical to Test 1.
2.4. Data Reduction
In this study, if subjects were not comfortable during any part of the experimental day, they were compensated for the portion that was completed and were exempt from finishing the day (2 subjects decided to drop out after Test 1 both from AW group). In addition, three subjects were dropped because they did not pass the math criterion (i.e., 75 percent correct) (1 AW, and 2 QW).
2.5. Electroencephalography (EEG)
EEG data were acquired using a thirty two-channel cap (EASEYCAP GmbH) with Ag/AgCI electrodes placed according to the international 10–20 System (Jasper, 1958). Electrodes included 24 scalp, two electrocardiogram (ECG), two electromyogram (EMG), two electrooculogram (EOG), 1 ground, and 1 on-line common reference channel. EEG signals were recorded at a 1000 Hz sampling rate and referenced on-line to the common reference channel. After data acquisition, EEG signals were re-referenced:the right side of the scalp were re-referenced to the left mastoid and the electrodes on the left side of the scalp were re-referenced to the right mastoid. Sleep architecture variables included minutes and percentage of Stage 1, Stage 2, slow wave sleep (SWS) and rapid eye movement (REM), as well as total sleep time (TST), sleep latency (SL), wake after sleep onset (WASO) and sleep efficiency (SE).
2.6. Electrophysiological data
NAP and QW Raw EEG signals were digitally band-pass filtered between 0.3 and 35 Hz. All epochs of the filtered data with artifacts and arousals were identified by visual inspection and rejected. Next, the EEG data were scored using Hume, a custom MATLAB toolbox. The records were scored in 30 seconds epochs using eight scalp electrodes: Frontal (F3, F4), Central (C3, C4), Parietal (P3, P4), and Occipital (O1, O2). Sleep stages epochs were exported for further analysis in MATLAB 2011b (MathWorks, Natick MA). The MATLAB output files from Hume were first passed to BrainVision Analyzer 2.0 (BrainProducts, Munich Germany) for pre-processing. Next, the data was passed to two other MATLAB based algorithms to measure SO Power and SO/Sigma coupling (developed by Sleep and Cognition Lab (SaC lab) at University of California, Irvine). For both the nap group, the SO power spectral analysis and SO/Sigma coupling analysis were conducted within each sleep stage separately. For EEG power spectrum, we used the Welch method (4 second Hanning windows with 50% overlap) (Campbell, 2009). The SO power was calculated for each sleep stage (i.e., Stage 2, SWS and REM). In addition, given that, SOs show the strongest relative amplitude at frontal electrode cites (Massimini et al., 2004; Murphy et al., 2009), we made the a priori decision to use F3 and F4 electrode cites for our analysis of slow oscillations for both nap and QW data.
SO/Sigma coupling was obtained by means of Modulation Index (MI). First, slow oscillations were detected automatically as described by Massimini and colleagues (2004). Filtered EEG data with negative to positive zero crossings separated by 0.5 to 1.5 second were detected. The detected zero crossings were labeled as SOs if the following criteria were met: a minimum width of 1 second a maximum negative peak width of 10 second and a minimum amplitude of −80 uV. Next, transient coupling between the phase of slow wave oscillations (SO) and sigma power (12–15 Hz) during stage 2 and SWS stages was measured as described by Canolty and colleagues (2006). Assuming an EEG signal (x[n]) sampled at times t_n, n = 1,2,…,N was then bandpass filtered into the two frequency bands of interest, sigma and SO bands. Applying Hilbert Transform to these two filtered signals allowed us to form the composite complex-valued signal (analytic signal) giving us the amplitude of the sigma and the phase of the SO. The absolute value of the mean of Z[n], the so-called MI, provides the coupling between the sigma-amplitude and SO-phase (Canolty et al., 2006). If we assume a uniform distribution of SO-phase, any departure of the distribution of Z[n] from symmetry will show a dependence of the sigma amplitude (sigma-amplitude) on the SO phase. Hence, a non-zero value of MI (here we call it MI_raw) indicates some amount of phase-amplitude coupling (Penny et al., 2008). To take into account the possible over-estimate of MI due to non-white noise in the signal, we normalized MI_raw by comparing it to the possible modulation indexes found in specifically generated surrogate data generated by introducing an arbitrary time lag between sigma-amplitude and SO-phase. Using many different lags, a distribution of surrogate MI values was generated, with mean μ and standard deviation σ and the normalized MI was computed as follows:
M_norm=(M_raw−μ)/σ. Further, given that sigma frequency band shows the most power over central regions (Malerba et al., 2018), for our MI analysis, we made the a priori decision to use C3 and C4 which will likely capture a more accurate representation of SO/sigma activity.
2.6.1. Statistical analysis
Performance was assessed by calculating the following variables for each test of the WM task: Accuracy and Reaction Time (RT). Accuracy represents the total number of correctly remembered letters. RT represents the time spent providing the correct sequence of letters. We used a Univariate ANOVA with Test 1 as the dependent variable and sleep condition as the fixed factor to test for baseline differences in accuracy and RT across the three groups. In addition, to assess performance change related to sleep, we calculated a difference score (Test 2 – Test 1), with positive difference scores indicating an improvement in WM accuracy throughout the day, and positive RT difference scores indicating a deterioration in speed throughout the day. We first used a 2 × 3 repeated measure ANOVA with the dependent variables Test 1 and 2 and the fixed factor sleep condition (i.e., nap, QW and AW). To examine changes in performance throughout the day within each group, we next used paired sample t-test. Finally, we used bivariate correlations to assess the relationships between WM performance and sleep physiology events (i.e., sleep architecture, SO power and normalized MI) for the nap group. In addition, we used bivariate correlations to assess the relationships between WM performance and SO power during QW. Further, to control for multiple comparisons for correlations between WM performance and SO power at different electrodes (i.e., F3 and F4) we used Bonferroni method, meaning that p-values are considered significant if less than/equal 0.025 and trending if greater than 0.025 and less than/equal 0.05. Similarly, we corrected for multiple comparisons for correlations between MI at different electrodes (i.e., C3 and C4) and WM performance.
3. Results
3.1. Working Memory Performance
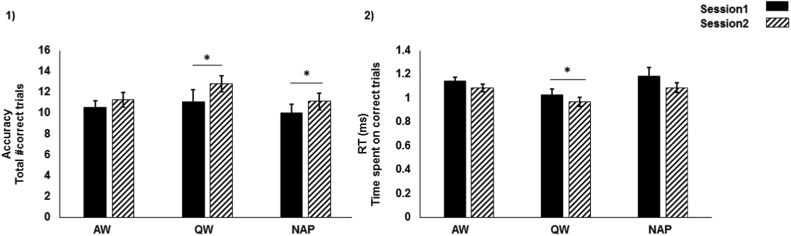
We found no baseline differences between groups in accuracy [Test 1: F(2,77) = 0.34, p = 0.71] or RT [Test 1: F(2,77) = 1.66: p = 0.19], indicating the three groups performed similarly at the start of the training. Our 2 × 3 repeated measure ANOVA, with factors Time (Test 1 and 2) and Group (Nap, QW, AW) revealed no significant interaction effects for accuracy [F(2,77) = 0.60: p = 0.55; RT: F(2,77) = 0.24: p = 0.78] but a significant main effect of Time [accuracy: F(2,77) = 11.7: p = 0.001; RT: F(2,77) = 5.65: p = 0.02]. Thus, regardless of group (i.e. nap, QW or AW), all subjects improved in performance throughout the day. Given our a priori prediction that a nap and QW would confer greater benefit to WM improvement than AW, we statistically tested the magnitude of change in each group, despite the non-significant omnibus interaction. We used student paired t-test to examine the change in performance during the day, from Test 1 to Test 2, separately in each group. WM improvement was not shown in the AW group [accuracy: t(24) = −1.26, p = 0.22; RT: t(24) = 1.35, p = 0.19]. However, QW showed significant improvement in both accuracy and RT throughout the day [accuracy: t(20) = −2.05, p = 0.05; RT: t(20) = 2.29, p = 0.03]. In addition, the nap group showed significant improvement in accuracy [t(33) = −2.56, p = 0.01], but not RT [t(33) = 1.65, p = 0.10] (see Fig. 2). These results indicate that since, all the three groups started at a similar level, any improvement from baseline is more likely related to the sleep intervention. Next, we examined the relation between the WM performance change observed between Test 1 and 2 with sleep features during the nap and quiet wake periods.
Fig. 2.
Working Memory (WM) performance across the three experimental groups. 1) WM accuracy was significantly improved from Session 1 (Black bar) to Session 2 (Hatched bar) in a paired sample t-test in the Nap and QW group. 2) WM Reaction Time (RT) was significantly improved from Session 1 to Session 2 only in QW group. (* represent p-values < 0.05).
3.2. Quiet Wake-SO Power and WM Performance
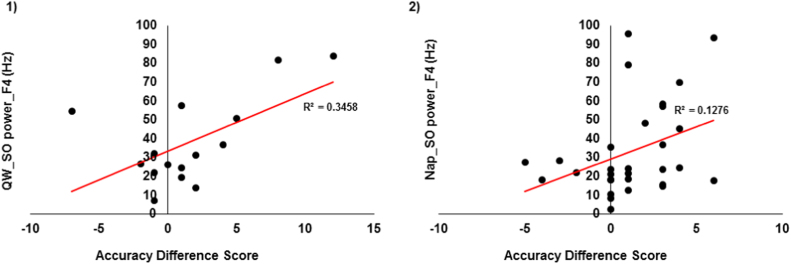
SO power descriptive for QW can be found in Table 2. In the QW group, increase in WM accuracy from Test 1 to Test 2 was positively associated with SO power at F4 (r = 0.58, p = 0.02) (See Fig. 3). This result indicates that higher SO activity during a quiet period relates to greater gain in WM performance among the participants. SO power did not reveal significant association with RT (p> 0.08).
Table 2.
Slow Oscillation power summaries during nap.and quiet wake.
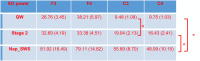 |
SO power during SWS was significantly higher compare to SO power during Stage 2 sleep (all ps<0.001) and SO power during QW (all ps 0.001). Further, SO power in Stage 2 sleep was significantly higher than SO power in QW only at C3 (p = 0.004) and was similar across Stage 2 sleep and QW at C4, F3 and F4 electrode channels (all ps>0.07). * represents p-value<0.001.
Notes: Data are reported as mean (SEM).
Fig. 3.
WM accuracy difference score association with 1) QW_SO power at F4 (significant) and 2) Nap_SO power at F4 (trend).
3.3. Nap Architecture and WM Performance Associations
Sleep architecture can be found in Table 1. No significant association was found between WM accuracy and sleep architecture variables (all ps> 0.09). RT at Test 2 was negatively associated with time spent in SWS (minutes: r = −0.45, p = 0.007; percentage: r = −0.40, p = 0.02) indicating that more time spent in SWS is associated with better RT after the nap.
Table 1.
Sleep summaries during nap.
Notes: Data are reported as mean (SEM). SE; sleep efficiency, SWS: slow wave sleep, WASO; wake after sleep onset, TST; Total Sleep Time.
3.4. Sleep-SO Power and WM Performance Associations
Descriptive analysis of SO power for nap can be found in Table 2. In addition, since only 7 subjects had REM sleep in their nap, the associations between REM sleep and performance were not measured. Further, r effect sizes for all the associations between the WM improvement and brain physiology events can be found in Table 3. In the nap group, increase in WM accuracy from Test 1 to Test 2 had a trending positive association with SO power during stage 2 sleep at F4 (r = 0.36, p = 0.05). In addition, WM accuracy at Test 2 was positively associated with SO power during NREM sleep at F4 (Stage 2 sleep: r = 0.42, p = 0.02; SWS: r = 0.48, p = 0.05 (trend)). Further, faster RT at Test 2 was associated with higher SO power during SWS at F4 (r = −0.51, p = 0.004). Together, these results indicate that WM performance may be increased with greater offline SOs.
Table 3.
QW and nap physiology association with Working Memory performance.
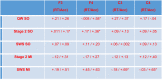 |
Notes: Effect size r for the Working Memory difference score (Reaction-Time/Accuracy) and brain physiology association is reported. Significant associations (p< 0.05) are marked with asterisks.
3.5. Sleep Modulation Index and WM Performance Associations
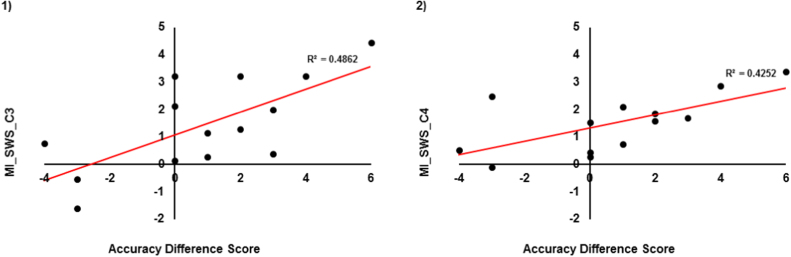
Next, we examined the potential benefit of greater sigma/SO coupling for WM improvement by correlating normalized MI with performance. The accuracy difference score was positively correlated with MI in SWS [C3: r = 0.69, p = 0.006; C4: r = 0.65, p = 02.] (See Fig. 3). In addition, Test 2 WM accuracy was positively correlated with MI in SWS (C4: r = 0.65, p = 0.02). Further, faster RT at Test 2 had a trending association with MI in SWS at C4 (r = −0.59, p = 0.03). Change in RT did not show any association with MI during sleep (all ps> 0.09). Thus, increased phase amplitude coupling between sigma and SO during SWS was related to better WM accuracy. MI in Stage 2 sleep was not associated with WM performance (all ps> 0.38 s). (Fig. 4).
Fig. 4.
WM accuracy difference score association with 1) MI during SWS at C3 (significant) and 2) MI during SWS at C4 (significant).
3.6. Self-reported Sleepiness and WM Performance
We also examined the extent to which sleepiness in each group may have predicted performance differences. Using One Way ANOVA here, we found that the amount of self-reported sleepiness (Karolinska Sleepiness Scale (KSS)) at the beginning of Test 1 (KSS-am) was similar across our three groups (F(2,67) = 1.047, p = 0.36). However, at the beginning of Test 2 (KSS-pm), after the sleep intervention, we found a significant difference in self-reported sleepiness between the groups (F(2,69) = 11.55, p <= 0.001). Post hoc analysis with Bonferroni corrections, revealed that the amount of sleepiness was not different between AW and QW (p = 0.21), but significantly lower in the nappers compared with both AW (p < 0.001) and QW (p = 0.04). In addition, both KSS-am and KSS-pm were was not associated with WM performance at Test 1 and Test 2 respectively in any of the groups [accuracy: all ps> 0.37; RT: all ps> 0.27]. Together, these results suggest that even though the amount of self-reported sleepiness was lower among the nappers compared to both wake groups, considering gain in WM accuracy in both nap and QW and the lack of association between self-reported sleepiness and WM performance among all the three groups, self-reported sleepiness may not have directly contributed to the reported performance changes.
4. Discussion
We investigated the impact of a nap, quiet wake and active wake on within-day training on a working memory (WM) task in older adults. We found that WM improved within a day of practice among our three groups, with significant levels of improvement shown only after offline periods containing naps and quiet wake, but not active wake. Furthermore, offline slow oscillation power in both the nap and quiet wake was associated with gain in WM performance. The nap provided additional benefit to WM as performance improvement was also correlated with SO/sigma coupling during SWS. Together, these findings suggest that experience-dependent changes in WM in older adults may benefit from specific neural activity shared across quiet wake and deep sleep.
WM, which is critical for a wide variety of cognitive abilities and real-world activities (Baddeley, 2003), declines in late adulthood (Bopp and Verhaeghen, 2005, Payer et al., 2006, Borella et al., 2008). These decreases have been associated with broad cognitive impairments in older adults (Park et al., 2002), including reading comprehension, decision making, problem solving, and vocabulary learning (Engle and Kane,2004). In addition, this decline may impact daily life functioning, such as medication adherence (Raz, 2000). Therefore, investigating the possibilities of improving WM functioning across the lifespan has been a primary focus of many cognitive training studies (for reviews, see Klingberg, 2010; Shipstead et al., 2010; Takeuchi et al., 2010a, Takeuchi et al., 2010b; Morrison and Chein, 2011), with many reporting gains (Mahncke et al., 2006, Buschkuehl et al., 2008, Li et al., 2008, Dahlin et al., 2008, Borella et al., 2010, Schmiedek et al., 2010, Richmond et al., 2011, Kuriyama et al., 2008, Kuriyama et al., 2011). For example, Li and colleagues (2008) examined younger (20–30 years old) and older (70–80 years old) adults in a 45-day practice period on a 2-back WM task and reported substantial performance gains in both age groups compared to the age-matched control. Similarly, young and older adults who practiced a WM task three times a week for five weeks (11 h of training in total) showed significant WM improvement that remained significant at 18-month follow-up testing, compared with controls (Dahlin et al. 2008). Here, we showed the possibility of improvement in WM within a day of practice. Importantly, WM training has been shown to yield broad cognitive benefits as well (reviewed in Morrison and Chein, 2011 cite). For example, training older adults on a complex WM span for 20 tests across four weeks resulted in significant improvement on attention, verbal learning and reading span task (Richmond, 2011). In addition, a sample of 80-year-old adults who underwent WM training twice weekly over 3 months, showed increased visual and episodic memory performance immediately after training completion, compared to the control group (Buschkuehl et al., 2008). In our sample although, WM increased among the participants within a day of practice, the increase was only significant when a period of rest occurred between training sessions. Taken together, these findings indicate that improvement and transfer effects of cognitive training engages neural processes that change slowly over many days of training, and suggests that daily rest that provides the opportunity for slow oscillatory brain rhythms to emerge may be critical (Orban et al., 2006).
An important question to be understood is what mechanisms underlie WM improvement and transfer to other cognitive domains. To date, a handful of neuroimaging studies have attempted to characterize the neural changes associated with WM training (Brehmer et al., 2009; Dahlin et al., 2008; Hempel et al., 2004; McNab et al., 2009; Olesen et al., 2004; Takeuchi et al., 2010a, Takeuchi et al., 2010b; Westerberg and Klingberg, 2007; Wager and Smith, 2003). Results have indicated that cognitive training improves processing efficiency, with changes in recruitment of task-related brain areas including the prefrontal cortex (Heinzel et al., 2016), striatum and hippocampus (Flegal et al., 2018), see Salmi et al., 2018 for a recent meta-analysis. Thus, improvement in working memory may reflect greater efficiency of local neural networks that are associated with both working and long-term memory formation. Interestingly, sleep, in particular SWS, may be critical for restoring prefrontal executive control functions that relate to memory retrieval (Wilckens et al., 2012, Tse et al., 2011). In fact, SOs, which are the fundamental cellular phenomenon underlying EEG activity in SWS may play a role (Steriade et al., 1993; Mölle et al., 2011). While it is well established that improved memory is associated with SOs during sleep (Huber et al., 2004, Marshall et al., 2006), Brokaw and colleagues 2016, showed that, after a period of quiet rest, increased memory for a short story was also associated with increased SO activity in young adults. Similarly, here, we showed that sleep or quiet wake, but not active wake, benefited performance and the gain was associated with SO activity during deep sleep and quiet wake respectively. Taken together with the recent cognitive training literature, greater efficiency in hippocampus-prefrontal cortex dialog may occur during quiet rest and NREM sleep, potentially mediated by SOs, resulting in enhancement of both working and long-term memory. Like sleep, quiet rest is characterized by a dramatic reduction in sensory processing, prevalence of hippocampal “sharp-wave ripples” (Axmacher et al., 2008, Clemens et al., 2011), and decreased levels of the acetylcholine (Marrosu et al., 1995), a neurotransmitter hypothesized to set the appropriate neural dynamics for consolidation by regulating the balance of processing of external and internal signals (Hasselmo et al., 2006). In line with this thinking, the Opportunistic Consolidation Hypothesis posits that the core condition favoring consolidation may not be sleep per se, instead consolidation may be facilitated during any offline state with limited encoding and plasticity, which will reduce retroactive interference and permit SO-driven replay processes to emerge (Mednick et al., 2011). This may be particularly important in aging as individuals are faced with a reduction in sleep across many measures. Therefore, along with SO-rich NREM sleep, quiet wake may also facilitate memory by creating favorable conditions (i.e. reduced interference) for consolidation. Future studies should examine whether these shared neural features of the sleeping and resting brain, as compared to non-resting brain, underlie performance improvements from cognitive training.
Age-related deterioration in sleep quality, efficiency and continuity is well-known (Pace-Schott and Spencer, 2011, Dijk et al., 2010), as well as declines in specific electrophysiological events during sleep (Westerberg et al., 2015, Landolt et al., 1996). In general, older people tend to experience less deep sleep (Bliwise, 1993, Ohayon et al., 2004, Yetton et al., 2018), decreased amplitude of slow waves (0.5–1 Hz) (Carrier et al., 1997), lower total sleep time, higher sleep latency, and a more fragmented sleep. Importantly, these changes in sleep have also been associated with age-related medial prefrontal cortex gray-matter atrophy and abnormal hippocampal-neocortical dialogue (Mander et al., 2013). Compared with youngers, older adults may also experience changes in spindle/SO coupling with lower coupling over the frontal site (Helfrich et al., 2018). Interestingly, Helfrich et al. (2018) reported that post-sleep declarative memory improvement predicted by the spindle/SO coupling in both young and older adults. In the present work, we reported positive associations between WM accuracy improvement and coupling of SO/sigma in SWS in older adults. Taken together, it appears that thalamocortical communication is important for memory improvement, and interventions that boost thalamocortical activity during NREM sleep may be helpful in improving both working and long-term memory in both young and older adults.
There are some limitations in the current work. In the current study, we did not examine EEG frequency patterns during the active wake state preventing examination of what brain activity in quiet vs active wake led to large WM increases in the former and only small increases in the latter. We hypothesize that though the majority of active wake is spent engaged in processing sensory input and executing behavioral responses, there are short bouts of inward mind-wandering during which the brain may experience increases in slower frequency activity (Braboszcz and Delorme, 2011, Aeschbach et al., 1997, Smallwood and Schooler, 2006, Mason et al., 2007). Prior work has reported that delta activity is present and increases across prolonged periods of wakefulness (Aeschbach et al., 1997, Braboszcz and Delorme, 2011). Aeschbach and colleagues (1997) recorded EEG at half-hourly intervals during ~40 h of sustained wakefulness where EEG power density in the 0.75–9.0 Hz range exhibited a global increasing trend, as well as local increases in the delta (0.75–4.0 Hz), theta (4.25–7.0 Hz) and lower alpha band (7.25–9.0 Hz). In addition, in mind-wandering episodes; periods of task-unrelated thoughts that result in attention drifting away from the task at hand (Smallwood and Schooler, 2006, Mason et al., 2007), slower frequencies such as delta (2–3.5 Hz) and theta (4–7 Hz) EEG activity increase, compared with alpha (9–11 Hz) and beta (15–30 Hz) frequencies (Braboszcz and Delorme, 2011). Together, these results support the hypothesis that the actively waking brain is speckled with episodes of low frequency brain activity that indicate inward reflection and mind wandering. However, the impact of this jostling between active and quiet wake on cognitive improvement needs further study. Second, since spindles are not present during QW, we only measured MI during sleep. As a result, the extent to which QW is mirroring sleep mechanisms in memory formation remains unclear.
In conclusion, we show that improvements in WM across a day of training may be more facilitated by a mid-day nap or a quiet period compared to wake. In addition, we hypothesize that, similarities between sleep and resting brain’s electrophysiology events such as slow oscillations may play a role in this process. Additionally, our data suggest that WM improvement may benefit from similar sleep-dependent mechanisms as long-term memory consolidation (e.g., coupling of sigma and SOs). Therefore, promoting more and better sleep quality in older adults could improve their WM, especially in the context of brain training.
Acknowledgment
This research was supported by the National Institutes of Health, United States, R01AG046646.
The EEG data were scored using Hume, a custom MATLAB toolbox designed by Jared Saletin.
Acknowledgments
Conflicts of interest statement
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- Adam M., Rétey J.V., Khatami R., Landolt H.P. Age-related changes in the time course of vigilant attention during 40 h without sleep in men. Sleep. 2006;29(1):55–57. doi: 10.1093/sleep/29.1.55. [DOI] [PubMed] [Google Scholar]
- Aeschbach D., Matthews J.R., Postolache T.T., Jackson M.A., Giesen H.A., Wehr T.A. Dynamics of the human EEG during prolonged wakefulness: evidence for frequency-specific circadian and homeostatic influences. Neurosci. Lett. 1997;239(2–3):121–124. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T., Gillberg M. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 1990;52(1–2):29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- Au J., Sheehan E., Tsai N., Duncan G.J., Buschkuehl M., Jaeggi S.M. Improving fluid intelligence with training on working memory: a meta-analysis. Psychon. Bull. Rev. 2015;22(2):366–377. doi: 10.3758/s13423-014-0699-x. [DOI] [PubMed] [Google Scholar]
- Axmacher N., Elger C.E., Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131(7):1806–1817. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat. Rev. Neurosci. 2003;4(10):829. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley A., Logie R., Bressi S., Sala S.D., Spinnler H. Dementia and working memory. Q. J. Exp. Psychol. 1986;38(4):603–618. doi: 10.1080/14640748608401616. [DOI] [PubMed] [Google Scholar]
- Bliwise D.L. Sleep in normal aging and dementia. Sleep: J. Sleep Res. Sleep Med. 1993 doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Bopp K.L., Verhaeghen P. Aging and verbal memory span: a meta-analysis. J. Gerontol. Ser. B: Psychol. Sci. Social. Sci. 2005;60(5):P223–P233. doi: 10.1093/geronb/60.5.p223. [DOI] [PubMed] [Google Scholar]
- Borella E., Carretti B., De Beni R. Working memory and inhibition across the adult life-span. Acta Psychol. 2008;128(1):33–44. doi: 10.1016/j.actpsy.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Borella E., Carretti B., Riboldi F., De Beni R. Working memory training in older adults: evidence of transfer and maintenance effects. Psychol. Aging. 2010;25(4):767. doi: 10.1037/a0020683. [DOI] [PubMed] [Google Scholar]
- Braboszcz C., Delorme A. Lost in thoughts: neural markers of low alertness during mind wandering. Neuroimage. 2011;54(4):3040–3047. doi: 10.1016/j.neuroimage.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Brehmer Y., Westerberg H., Bellander M., Fürth D., Karlsson S., Bäckman L. Working memory plasticity modulated by dopamine transporter genotype. Neurosci. Lett. 2009;467(2):117–120. doi: 10.1016/j.neulet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Brokaw K., Tishler W., Manceor S., Hamilton K., Gaulden A., Parr E., Wamsley E.J. Resting state EEG correlates of memory consolidation. Neurobiol. Learn. Mem. 2016;130:17–25. doi: 10.1016/j.nlm.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Buschkuehl M., Jaeggi S.M., Hutchison S., Perrig-Chiello P., Däpp C., Müller M., Perrig W.J. Impact of working memory training on memory performance in old-old adults. Psychol. Aging. 2008;23(4):743. doi: 10.1037/a0014342. [DOI] [PubMed] [Google Scholar]
- Campbell I.G. EEG recording and analysis for sleep research. Curr. Protoc. Neurosci. 2009:10–12. doi: 10.1002/0471142301.ns1002s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty R.T., Edwards E., Dalal S.S., Soltani M., Nagarajan S.S., Kirsch H.E., Knight R.T. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313(5793):1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter P.A., Just M.A. Complex Information Processing: The Impact of Herbert A. Simon. 1989. The role of working memory in language comprehension; pp. 31–68. [Google Scholar]
- Carrier J., Monk T.H., Buysse D.J., Kupfer D.J. Sleep and morningness‐eveningness in the ‘middle’years of life (20–59y) J. Sleep Res. 1997;6(4):230–237. doi: 10.1111/j.1365-2869.1997.00230.x. [DOI] [PubMed] [Google Scholar]
- Chee M.W., Choo W.C. Functional imaging of working memory after 24 h of total sleep deprivation. J. Neurosci. 2004;24(19):4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M.W., Chuah L.Y., Venkatraman V., Chan W.Y., Philip P., Dinges D.F. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: correlations of fronto-parietal activation with performance. Neuroimage. 2006;31(1):419–428. doi: 10.1016/j.neuroimage.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Clemens Z., Fabo D., Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132(2):529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Clemens Z., Mölle M., Erőss L., Jakus R., Rásonyi G., Halász P., Born J. Fine‐tuned coupling between human parahippocampal ripples and sleep spindles. Eur. J. Neurosci. 2011;33(3):511–520. doi: 10.1111/j.1460-9568.2010.07505.x. [DOI] [PubMed] [Google Scholar]
- Dahlin E., Neely A.S., Larsson A., Bäckman L., Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320(5882):1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Dahlin E., Nyberg L., Bäckman L., Neely A.S. Plasticity of executive functioning in young and older adults: immediate training gains, transfer, and long-term maintenance. Psychol. aging. 2008;23(4):720. doi: 10.1037/a0014296. [DOI] [PubMed] [Google Scholar]
- Diekelmann S., Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11(2):114. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dijk D.J., Groeger J.A., Stanley N., Deacon S. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep. 2010;33(2):211–223. doi: 10.1093/sleep/33.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J.F., Willson H.J., Wang W., Czeisler C.A. Healthy older adults better tolerate sleep deprivation than young adults. J. Am. Geriatr. Soc. 2009;57(7):1245–1251. doi: 10.1111/j.1532-5415.2009.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle R.W. Working memory capacity as executive attention. Curr. Dir. Psychol. Sci. 2002;11(1):19–23. [Google Scholar]
- Engle R.W., Kane M.J. Executive attention, working memory capacity, and a two-factor theory of cognitive control. Psychol. Learn. Motiv. 2004;44:145–200. [Google Scholar]
- Flegal K.E., Ragland J.D., Ranganath C. Adaptive task difficulty influences neural plasticity and transfer of training. NeuroImage. 2018 doi: 10.1016/j.neuroimage.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S., Born J. Declarative memory consolidation: mechanisms acting during human sleep. Learn. Mem. 2004;11(6):679–685. doi: 10.1101/lm.80504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S., Mölle M., Helms K., Born J. Learning-dependent increases in sleep spindle density. J. Neurosci. 2002;22(15):6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz M., Reynolds C.A., John R., Johansson B., Mortimer J.A., Pedersen N.L. Telephone screening to identify potential dementia cases in a population-based sample of older adults. Int. Psychogeriatrics. 2002;14(3):273–289. doi: 10.1017/s1041610202008475. [DOI] [PubMed] [Google Scholar]
- Heinzel S., Lorenz R.C., Pelz P., Heinz A., Walter H., Kathmann N., Stelzel C. Neural correlates of training and transfer effects in working memory in older adults. Neuroimage. 2016;134:236–249. doi: 10.1016/j.neuroimage.2016.03.068. [DOI] [PubMed] [Google Scholar]
- Helfrich R.F., Mander B.A., Jagust W.J., Knight R.T., Walker M.P. Old brains come uncoupled in sleep: slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron. 2018;97(1):221–230. doi: 10.1016/j.neuron.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel A., Giesel F.L., Garcia Caraballo N.M., Amann M., Meyer H., Wüstenberg T., Schröder J. Plasticity of cortical activation related to working memory during training. Am. J. Psychiatry. 2004;161(4):745–747. doi: 10.1176/appi.ajp.161.4.745. [DOI] [PubMed] [Google Scholar]
- Huber R., Ghilardi M.F., Massimini M., Tononi G. Local sleep and learning. Nature. 2004;430(6995):78. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Humiston, G.B., Wamsley, E.J., 2018. A brief period of eyes-closed rest enhances motor skill consolidation. Neurobiology of Learning and Memory. [DOI] [PMC free article] [PubMed]
- Jonides J. Working memory and thinking. Invit. Cogn. Sci.: Think. 1995;3:215–265. [Google Scholar]
- Karbach J., Verhaeghen P. Making working memory work: a meta-analysis of executive-control and working memory training in older adults. Psychol. Sci. 2014;25(11):2027–2037. doi: 10.1177/0956797614548725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends Cogn. Sci. 2010;14(7):317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Kuriyama K., Honma M., Shimazaki M., Horie M., Yoshiike T., Koyama S., Kim Y. An N-methyl-d-aspartate receptor agonist facilitates sleep-independent synaptic plasticity associated with working memory capacity enhancement. Sci. Rep. 2011;1:127. doi: 10.1038/srep00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama K., Mishima K., Suzuki H., Aritake S., Uchiyama M. Sleep accelerates the improvement in working memory performance. J. Neurosci. 2008;28(40):10145–10150. doi: 10.1523/JNEUROSCI.2039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt H.P., Dijk D.J., Achermann P., Borbely A.A. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738(2):205–212. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- Li S.C., Schmiedek F., Huxhold O., Röcke C., Smith J., Lindenberger U. Working memory plasticity in old age: practice gain, transfer, and maintenance. Psychol. Aging. 2008;23(4):731. doi: 10.1037/a0014343. [DOI] [PubMed] [Google Scholar]
- Mahncke H.W., Connor B.B., Appelman J., Ahsanuddin O.N., Hardy J.L., Wood R.A., Merzenich M.M. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc. Natl. Acad. Sci. USA. 2006;103(33):12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malerba P., Whitehurst L.N., Simons S.B., Mednick S.C. Spatio-temporal structure of sleep slow oscillations on the electrode manifold and its relation to spindles. Sleep. 2018 doi: 10.1093/sleep/zsy197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander B.A., Rao V., Lu B., Saletin J.M., Lindquist J.R., Ancoli-Israel S., Walker M.P. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat. Neurosci. 2013;16(3):357. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D., Willshaw D., McNaughton B. From the Retina to the Neocortex. Birkhäuser Boston; 1991. Simple memory: a theory for archicortex; pp. 59–128. [Google Scholar]
- Marshall L., Helgadóttir H., Mölle M., Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Martin J., Shochat T., Ancoli-Israel S. Assessment and treatment of sleep disturbances in older adults. Clin. Psychol. Rev. 2000;20(6):783–805. doi: 10.1016/s0272-7358(99)00063-x. [DOI] [PubMed] [Google Scholar]
- Marrosu F., Portas C., Mascia M.S., Casu M.A., Fà M., Giagheddu M., Gessa G.L. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671(2):329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- Mason M.F., Norton M.I., Van Horn J.D., Wegner D.M., Grafton S.T., Macrae C.N. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M., Huber R., Ferrarelli F., Hill S., Tononi G. The sleep slow oscillation as a traveling wave. J. Neurosci. 2004 doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F., Varrone A., Farde L., Jucaite A., Bystritsky P., Forssberg H., Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323(5915):800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Mednick S.C., Cai D.J., Shuman T., Anagnostaras S., Wixted J.T. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011;34(10):504–514. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S.C., McDevitt E.A., Walsh J.K., Wamsley E., Paulus M., Kanady J.C., Drummond S.P. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J. Neurosci. 2013;33(10):4494–4504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby-Lervåg M., Hulme C. There is no convincing evidence that working memory training is effective: a reply to Au et al. (2014) and Karbach and Verhaeghen (2014) Psychon. Bull. Rev. 2016;23(1):324–330. doi: 10.3758/s13423-015-0862-z. [DOI] [PubMed] [Google Scholar]
- Mölle M., Bergmann T.O., Marshall L., Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34(10):1411–1421. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M., Eschenko O., Gais S., Sara S.J., Born J. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur. J. Neurosci. 2009;29(5):1071–1081. doi: 10.1111/j.1460-9568.2009.06654.x. [DOI] [PubMed] [Google Scholar]
- Morrison A.B., Chein J.M. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychon. Bull. Rev. 2011;18(1):46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- Mu Q., Nahas Z., Johnson K.A., Yamanaka K., Mishory A., Koola J., George M.S. Decreased cortical response to verbal working memory following sleep deprivation. Sleep. 2005;28(1):55–67. doi: 10.1093/sleep/28.1.55. [DOI] [PubMed] [Google Scholar]
- Nettelbeck T., Burns N.R. Processing speed, working memory and reasoning ability from childhood to old age. Pers. Individ. Differ. 2010;48(4):379–384. [Google Scholar]
- Niknazar M., Krishnan G.P., Bazhenov M., Mednick S.C. Coupling of thalamocortical sleep oscillations are important for memory consolidation in humans. PLoS One. 2015;10(12):e0144720. doi: 10.1371/journal.pone.0144720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J.P., Söderström M., Karlsson A.U., Lekander M., Åkerstedt T., Lindroth N.E., Axelsson J. Less effective executive functioning after one night’s sleep deprivation. J. Sleep Res. 2005;14(1):1–6. doi: 10.1111/j.1365-2869.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- Ohayon M.M., Carskadon M.A., Guilleminault C., Vitiello M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Olesen P.J., Westerberg H., Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat. Neurosci. 2004;7(1):75. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Orban P., Rauchs G., Balteau E., Degueldre C., Luxen A., Maquet P., Peigneux P. Sleep after spatial learning promotes covert reorganization of brain activity. Proc. Natl. Acad. Sci. USA. 2006;103(18):7124–7129. doi: 10.1073/pnas.0510198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyanedel C.N., Binder S., Kelemen E., Petersen K., Born J., Inostroza M. Role of slow oscillatory activity and slow wave sleep in consolidation of episodic-like memory in rats. Behav. Brain Res. 2014;275:126–130. doi: 10.1016/j.bbr.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Pace-Schott E.F., Spencer R.M. Progress in Brain Research. Vol. 191. Elsevier; 2011. Age-related changes in the cognitive function of sleep; pp. 75–89. [DOI] [PubMed] [Google Scholar]
- Park D.C., Lautenschlager G., Hedden T., Davidson N.S., Smith A.D., Smith P.K. Models of visuospatial and verbal memory across the adult life span. Psychol. Aging. 2002;17(2):299. [PubMed] [Google Scholar]
- Payer D., Marshuetz C., Sutton B., Hebrank A., Welsh R.C., Park D.C. Decreased neural specialization in old adults on a working memory task. Neuroreport. 2006;17(5):487–491. doi: 10.1097/01.wnr.0000209005.40481.31. [DOI] [PubMed] [Google Scholar]
- Penny W.D., Duzel E., Miller K.J., Ojemann J.G. Testing for nested oscillation. J. Neurosci. Methods. 2008;174(1):50–61. doi: 10.1016/j.jneumeth.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip P., Taillard J., Sagaspe P., Valtat C., Sanchez‐Ortuno M., Moore N., Bioulac B. Age, performance and sleep deprivation. J. Sleep Res. 2004;13(2):105–110. doi: 10.1111/j.1365-2869.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- Raz N.B. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik F.I.M., Salthouse T.A., editors. The handbook of Aging and Cognition. Erlbaum; Mahwah, NJ: 2000. pp. 1–90. [Google Scholar]
- Rieth C.A., Cai D.J., McDevitt E.A., Mednick S.C. The role of sleep and practice in implicit and explicit motor learning. Behav. Brain Res. 2010;214(2):470–474. doi: 10.1016/j.bbr.2010.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond L.L., Morrison A.B., Chein J.M., Olson I.R. Working memory training and transfer in older adults. Psychol. Aging. 2011;26(4):813. doi: 10.1037/a0023631. [DOI] [PubMed] [Google Scholar]
- Salmi J., Nyberg L., Laine M. Working memory training mostly engages general-purpose large-scale networks for learning. Neurosci. Biobehav. Rev. 2018 doi: 10.1016/j.neubiorev.2018.03.019. [DOI] [PubMed] [Google Scholar]
- Schabus M., Gruber G., Parapatics S., Sauter C., Klösch G., Anderer P., Zeitlhofer J. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479–1485. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- Schmiedek F., Lövdén M., Lindenberger U. Hundred days of cognitive training enhance broad cognitive abilities in adulthood: findings from the COGITO study. Front. Aging Neurosci. 2010;2:27. doi: 10.3389/fnagi.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipstead Z., Redick T.S., Engle R.W. Does working memory training generalize? Psychol. Belg. 2010;50(3):245–276. [Google Scholar]
- Smallwood J., Schooler J.W. The restless mind. Psychol. Bull. 2006;132(6):946. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Staresina B.P., Bergmann T.O., Bonnefond M., Van Der Meij R., Jensen O., Deuker L., Fell J. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 2015;18(11):1679. doi: 10.1038/nn.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenuit P., Kerkhofs M. Age modulates the effects of sleep restriction in women. Sleep. 2005;28(10):1283–1288. doi: 10.1093/sleep/28.10.1283. [DOI] [PubMed] [Google Scholar]
- Steriade M., Nunez A., Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 1993;13(8):3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Sekiguchi A., Taki Y., Yokoyama S., Yomogida Y., Komuro N., Kawashima R. Training of working memory impacts structural connectivity. J. Neurosci. 2010;30(9):3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Sekiguchi A., Taki Y., Yokoyama S., Yomogida Y., Komuro N., Kawashima R. Training of working memory impacts structural connectivity. J. Neurosci. 2010;30(9):3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D., Takeuchi T., Kakeyama M., Kajii Y., Okuno H., Tohyama C., Morris R.G. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333(6044):891–895. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- Unsworth N., Heitz R.P., Schrock J.C., Engle R.W. An automated version of the operation span task. Behav. Res. methods. 2005;37(3):498–505. doi: 10.3758/bf03192720. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Smith E.E. Neuroimaging studies of working memory. Cogn. Affect. Behav. Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Westerberg C.E., Florczak S.M., Weintraub S., Mesulam M.M., Marshall L., Zee P.C., Paller K.A. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiol. Aging. 2015;36(9):2577–2586. doi: 10.1016/j.neurobiolaging.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg H., Klingberg T. Changes in cortical activity after training of working memory—a single-subject analysis. Physiol. Behav. 2007;92(1–2):186–192. doi: 10.1016/j.physbeh.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Wilckens K.A., Erickson K.I., Wheeler M.E. Age-related decline in controlled retrieval: the role of the PFC and sleep. Neural Plast. 2012:2012. doi: 10.1155/2012/624795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield A., Stine E.A., Lahar C.J., Aberdeen J.S. Does the capacity of working memory change with age? Exp. Aging Res. 1988;14(2):103–107. doi: 10.1080/03610738808259731. [DOI] [PubMed] [Google Scholar]
- Yetton B.D., McDevitt E.A., Cellini N., Shelton C., Mednick S.C. Quantifying sleep architecture dynamics and individual differences using big data and Bayesian networks. PLoS One. 2018;13(4):e0194604. doi: 10.1371/journal.pone.0194604. [DOI] [PMC free article] [PubMed] [Google Scholar]