Abstract
Catalytic asymmetric cycloadditions via transition-metal-containing dipolar intermediates are a powerful tool for synthesizing chiral heterocycles. However, within the field of palladium catalysis, compared with the well-developed normal electron-demand cycloadditions with electrophilic dipolarophiles, a general strategy for inverse electron-demand ones with nucleophilic dipolarophiles remains elusive, due to the inherent linear selectivity in the key palladium-catalyzed intermolecular allylations. Herein, based on the switched regioselectivity of iridium-catalyzed allylations, we achieved two asymmetric [4+2] cycloadditions of vinyl aminoalcohols with aldehydes and β,γ-unsaturated ketones through synergetic iridium and amine catalysis. The activation of vinyl aminoalcohols by iridium catalysts and carbonyls by amine catalysts provide a foundation for the subsequent asymmetric [4+2] cycloadditions of the resulting iridium-containing 1,4-dipoles and (di)enamine dipolarophiles. The former provides a straightforward route to a diverse set of enantio-enriched hydroquinolines bearing chiral quaternary stereocenters, and the later represent an enantio- and diastereodivergent synthesis of chiral hydroquinolines.
Subject terms: Asymmetric synthesis, Synthetic chemistry methodology, Stereochemistry
Inverse electron-demand cycloadditions in palladium catalysis are inherently limited by the linear selectivity in the allylic substitution step. To overcome this issue, the authors report a synergetic iridium (branched-selective) and amine catalysis in [4+2] cycloadditions of vinyl aminoalcohols with carbonyls.
Introduction
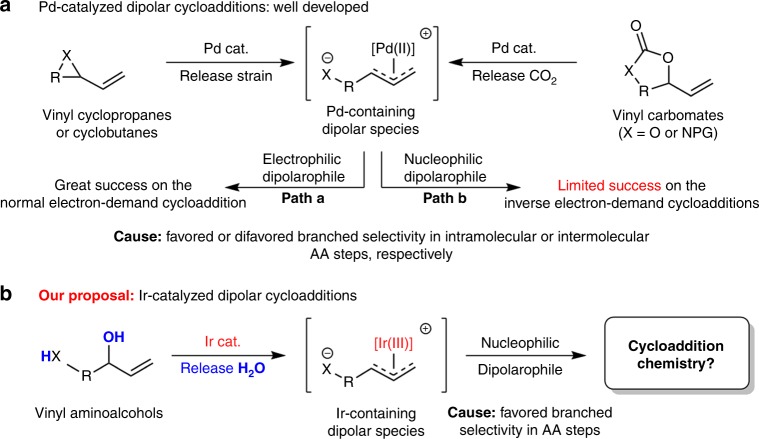
Heterocycles are privileged motifs in fields ranging from pharmaceutical chemistry and agrochemistry to materials chemistry and life sciences. The development of efficient synthetic methods for preparing heterocyclic compounds has been a major focus of the synthetic community1,2. Among the continuing and productive efforts, catalytic asymmetric cycloadditions via transition metal (TM)-containing dipolar intermediates have been determined to be powerful tools for this purpose3–5. Within this realm of palladium catalysis, although many impressive advancements have been made regarding cycloadditions of Pd-containing dipolar intermediates with electrophilic dipolarophiles (Fig. 1a, path a: normal electron-demand cycloadditions)6–12, the inherent linear selectivity of Pd-catalyzed intermolecular allylic alkylation (AA) reactions renders the development of a general strategy for the coupling of such intermediates with nucleophilic dipolarophiles a formidable task (Fig. 1a, path b: inverse electron-demand cycloaddition)13,14. In the few known examples, some additional interaction (i.e., electrostatic interactions or hydrogen bonding interactions) between the Pd-containing dipole and nucleophilic dipolarophile was required to induce branched selectivity in the Pd-catalyzed intermolecular AA processes15–19. For this reason, the exploitation of alternative catalyst systems is required to develop general dipolar cycloadditions and efficiently utilize the synthetic potential of TM catalysis in the synthesis of heterocycles.
Fig. 1.
Research proposal on the Ir-catalyzed cycloadditions of vinyl aminoalcohols. a Well-developed dipolar cycloadditions through asymmetric palladium catalysis and remained problem. b Dipolar cycloadditions through asymmetric iridium catalysis
Based on the pioneering works of Takeuchi20,21 and Helmchen22, as well as the substantial contribution from Helmchen23, Hartwig24, Carreira25, You26 and many other excellent scientists27–29, iridium-catalyzed AA reactions show excellent branched selectivity, which distinguishes them from Pd-catalyzed processes30. Recent studies have indicated that iridium catalysis is compatible with many other catalysis modes (i.e., phase catalysis31, amine catalysis32–34, Lewis base catalysis35, Brønsted acid catalysis36, and Lewis acid catalysis37–40), significantly expanding the scope of Ir-catalyzed asymmetric allylic alkylation (AAA) reactions41. Inspired by these impressive achievements, we question whether a synergetic catalysis strategy involving iridium catalysis can be adopted to resolve the remaining problem associated with Pd-catalyzed inverse electron-demand cycloadditions. More interestingly, readily available vinyl aminoalcohols can be directly utilized as coupling partners in TM-catalyzed cycloadditions using Ir-containing dipoles (Fig. 1b), avoiding the use of vinyl-substituted strained rings and carbonates like those required in Pd-catalyzed cycloaddition (Fig. 1a). Here, through the combination of iridium and amine catalysis, we accomplished the [4+2] cycloadditions of vinyl aminoalcohols with
aldehydes and β,γ-unsaturated ketones. Optically active quinolinones and tetrahydroquinolines were produced in good yields and with high diastereoselectivity and enantioselectivity.
Results
Design plan
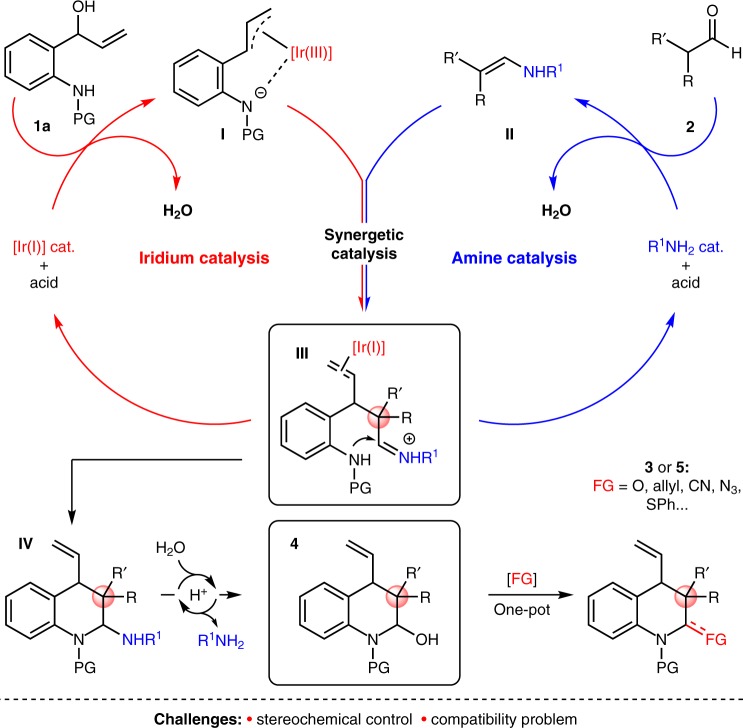
Hydroquinolines are a class of important aza-heterocycles that are ubiquitous in natural alkaloids and functional molecules (i.e., pharmaceuticals, agrochemicals, and chiral ligands)42–44. Among existing methods for synthesizing chiral hydroquinolines, catalytic asymmetric [4+2] cycloadditions stand out as one of the most streamlined approaches45–49. As part of our ongoing studies on the synthesis of heterocycles via TM-catalyzed cycloadditions15,50–55, in this work, we plan to develop reaction methodologies that utilize readily available reagents and feature good selectivity and high synthetic efficiency. A detailed description of a possible mechanism is outlined in Fig. 2. We envision that two catalytic cycles could act in concert to realize this cycloaddition via the following steps: (1) the Ir catalyst reacts with vinyl aminoalcohol 1a to form Ir-containing dipole int. I56,57; (2) the amine catalyst condenses with the aldehyde to generate enamine species II; (3) these two transient species, I and II, react with each other to produce int. III, which can be further converted to hemiacetal 4 through an intramolecular cyclization (III → IV) and acid-promoted hydrolysis process (IV → 4). Based on this mechanism, a diverse set of hydroquinolines can be generated in a facile manner by in situ conversion of the OH group to a variety of other functional groups (4 → 3/5). While attractive in theory, the construction of contiguous stereocenters and chiral all-carbon quaternary stereocenters58–62, as well as the compatibility between the catalysts, substrates, and additives are challenges that must be addressed.
Fig. 2.
Proposed mechanism. Synergetic iridium/amine catalysis for the enantioconvergent syntheses of hydroquinolines
Condition optimization
First, we investigated the Ir/amine-catalyzed cycloaddition/oxidation sequence using vinyl aminoalcohol 1a and aldehyde 2a. The use of 4 mol% of chiral Ir catalyst (2 mol% of [Ir(cod)Cl]2 and 8 mol% of Carreira ligand L1) and 20 mol% of amine catalyst A1, together with 0.5 eq. of CCl3CO2H and 1.0 eq. of H2O as additives, gave hemiaminal 4aa as a white solid in 92% yield; 4aa can be further oxidized to desired hydroquinone 3aa by PCC in 67% yield with 99% ee and >19:1 dr. The absolute configuration of product 3aa was determined by comparing the chiral HPLC spectra with a previous literature55. To circumvent the isolation of intermediate 4aa, we adopted an improved procedure that simply combined these two processes in one pot. To our delight, quinolinone product 3aa was successfully generated with good reaction efficiency and excellent enantiocontrol under the standard conditions (Table 1, entry 1: 64% yield, >99% ee and >19:1 dr). We then found that the protecting group on the nitrogen atom of the vinyl aminoalcohol had a substantial effect on the reaction selectivity. For example, when Boc-protected substrate 1a′ was used instead of 1a, we only identified the byproduct from the allylation of CCl3CO2H (Table 1, entry 2). It is worthy to note that, we have proven that the Ts group on product 3aa could be facilely removed by treatment with Mg powder in methanol (See Supplementary Procedure K). When vinyl aminoalcohol 1a′′ was tested, there was almost no conversion of starting materials (Table 1, entry 3). Furthermore, the effects of the acid and water as additives were investigated (Table 1, entries 4–7). CCl3CO2H provided superior chemo- and enantioselectivities, and the addition of 1 eq. of water increased the reaction efficiency. Considering that the amine catalyst may play a key role in this reaction, various amine catalysts were examined. As shown in Table 1, a bulkier amine, α,α-dimethyl benzylamine (Table 1, entry 8, A2), gave only 22% yield, albeit with >19:1 dr and >99% ee. In addition, when chiral amine catalysts (R)-A3 and (S)-A3 were applied, we did not observe stereodivergent products, but the different amines did result in different reaction efficiencies (Table 1 entries 9 and 10).
Table 1.
Optimization of the reaction conditions for the [4+2] cycloaddition of vinyl aminoalcohols and aldehydesa
|
| |||
|---|---|---|---|
| Entry | Variation from the standard conditions | Yield (%)b | ee (%′)c |
| 1 | none | 64 | 99 |
| 2 | replace 1a with 1a′ | 0 | / |
| 3 | replace 1a with 1a′′ | 0 | / |
| 4 | replace CCl3CO2H with CF3CO2H | 63 | 82 |
| 5 | replace CCl3CO2H with (PhO)2PO2H | 58 | 98 |
| 6 | replace CCl3CO2H with PhCO2H | 0 | / |
| 7 | no H2O | 56 | 99 |
| 8 | replace A1 with A2 | 22 | 95 |
| 9 | replace A1 with (R)-A3 | 62 | 93 |
| 10 | replace A1 with (S)-A3 | 26 | 94 |
| |||
Ts p-toluene sulfonyl, Boc t-butyloxycarbonyl, PCC pyridinium chlorochromate, Am amine catalyst
aStandard conditions: 1a (0.25 mmol), 2a (0.5 mmol), [Ir(cod)Cl]2 (2 mol%), (R)-L1 (8 mol%), amine A1 (20 mol%), CCl3CO2H (0.5 eq.) and H2O (1.0 eq.) in 1,2-dichloroethane (0.5 mL) for 48 h at room temperature; then, PCC (5.0 eq.), silica gel (100 mg) and CH2Cl2 (5 mL) were added, and the mixture was stirred at 40 °C for 10 h
bIsolated yield
cDetermined by chiral HPLC analysis; dr > 19:1
Substrate scope
Having established the optimal conditions, we started to explore the generality of the vinyl aminoalcohol substrate. As summarized in Table 2, a wide range of vinyl aminoalcohols with electron-donating and electron-withdrawing groups, such as Me, F, and CF3, at the 5-position of the phenyl ring can readily react with aldehyde 2a to afford the desired dihydro-quinolinone products in moderate yields over two steps with high diastereo- and enantioselectivities (Table 2, entries 1–4, 3aa–3da: 59–66% yields, 6:1→ 19:1 dr and 94–99% ee). Furthermore, substrates with different substitution patterns, for example, 4-MeO, 4-Cl, and 6-F, were compatible with this asymmetric cycloaddition. The corresponding cycloadducts were achieved in satisfactory reaction efficiencies and stereoselectivities (Table 2, entries 5–7, 3ea–3ga: 61–73% yields, 12:1→19:1 dr and 98–99% ee). An allyl alcohol bearing a naphthyl moiety was also suitable for this reaction, and it was converted to product 3ha in 63% yield, 12:1 dr and 98% ee (Table 2, entry 8).
Table 2.
Synthesis of Dihydroquinolinones form Various Vinyl Aminoalcoholsa
|
| |||||
|---|---|---|---|---|---|
| Entry | 1: R1 | 3 | Yield (%)b | drc | ee (%)c |
| 1 | 1a: H | 3aa | 64 | >19:1 | 99 |
| 2 | 1b: 5-Me | 3ba | 59 | 16:1 | 96 |
| 3 | 1c: 5-F | 3ca | 59 | 8:1 | 94 |
| 4 | 1d: 5-CF3 | 3da | 66 | 6:1 | 98 |
| 5 | 1e: 4-MeO | 3ea | 61 | 19:1 | 99 |
| 6 | 1f: 4-Cl | 3fa | 60 | 12:1 | 99 |
| 7 | 1g: 6-F | 3ga | 73 | >19:1 | 99 |
| 8 |

|
63 | 12:1 | 98 | |
aStandard conditions as indicated in entry 1 of Table 1
bIsolated yield
cDetermined by chiral HPLC analysis
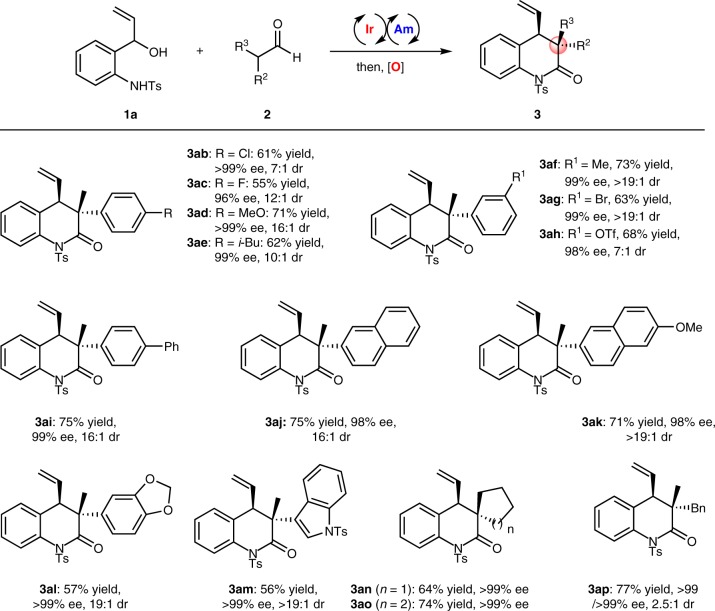
Subsequently, we explored an array of α-disubstituted aldehydes in this Ir- and amine-catalyzed cycloaddition/oxidation procedure. As summarized in Fig. 3, the introduction of various groups at the para- or meta-positions of the 2-phenylpropanal was well tolerated in this reaction, and corresponding products 3ab–3ai were obtained in good yields (55%−75% yields) and with high stereoselectivities (up to >19:1 dr and 96 → 99% ee). Moreover, aldehydes with naphthalene and other polycyclic systems can be converted to corresponding dihydro-quinolinones 3aj–3am in good yields and excellent enantioselectivities (56–75% yields and up to >99% ee). Notably, an indole-derived substrate was also well tolerated in this reaction and provided a good result (3am, 56% yield, >19:1 dr and >99% ee). In addition, cyclic aldehydes with various ring sizes (i.e., five- or six-membered rings) could readily participate in this reaction (3an, 64% yield, >99% ee; 3ao, 74% yield, >99% ee). A dialkyl-substituted acetaldehyde, 2-methyl-3-phenylpropanal, could also be reacted under these conditions to give the desired product 3ap in a good yield and with an excellent enantioselectivity. A linear aldehyde, n-butanal, was also proven suitable for this transformation, affording dihydroquinolinone product 3ar in 31% yield, >99% ee and >20:1 dr (See Supplementary Procedure M).
Fig. 3.
Synthesis of dihydroquinolinones from various aldehydes. Reaction conditions: as indicated in entry 1 in Table 1; isolated yield; the ee and dr values were determined by chiral HPLC analysis
Demonstrations of synthetic utility
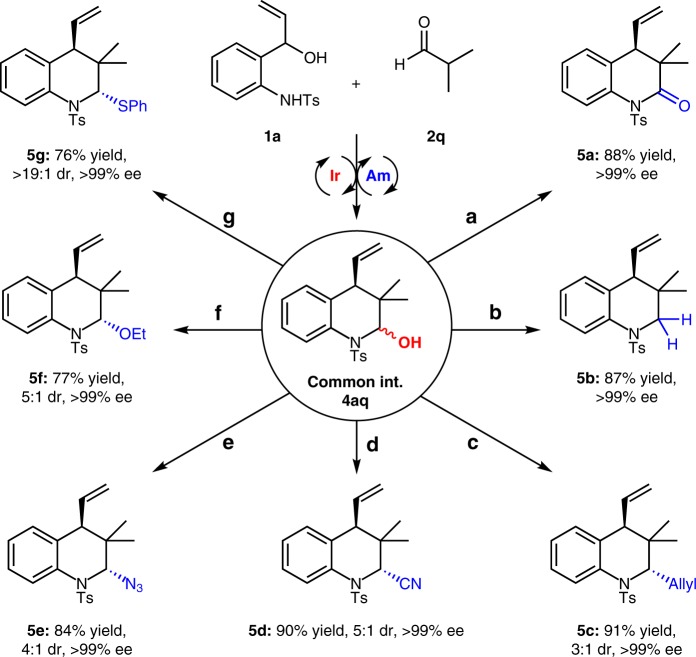
To illustrate the versatility of this cycloaddition, we explored various derivatizations of the hemiaminal intermediate. As highlighted in Fig. 4, taking key hemiaminal 4aq (>99% ee and 5:1 dr), which was generated from the asymmetric cycloaddition of vinyl aminoalcohol 1a and isobutyraldehyde 2q, as an example, treatment with various organic reagents in one-pot reactions indeed provided a diverse set of functionalized hydroquinolines in generally high yields and excellent enantioselectivities. Except in the oxidation with PCC, the OH group of hemiaminal 4aq could be removed by treating the compound with Et3SiH and Et2O·BF3 in DCM, giving tetrahydroquinoline 5b in 87% yield and >99% ee. Similarly, replacement of the hydroxyl group with other organosilicon reagents could deliver 2-allyl-, 2-CN- and 2-N3-substituted tetrahydroquinolines 5c–5e in 84–91% yields and >99% ee, albeit with modest dr values. In addition, the OH group can be successfully converted to ether or sulfide moieties in good yields and excellent enantioselectivities (5f, 77% yield, 5:1 dr and >99% ee; 5g, 76% yield, >19:1 dr and >99% ee. The high diastereoselectivity of product 5g may be result of the steric effect (See Supplementary Procedures I and J), and its absolute configuration was established through the X-ray diffraction analysis (CCDC 1821791)63.
Fig. 4.
Divergent synthetic transformations of chiral hemiaminal 4aq. Reaction conditions: a) PCC (5.0 eq.), silica (100 mg), CH2Cl2 (5 mL), 40 °C, 10 h; b) Et3SiH (3.0 eq.), Et2O·BF3 (3.0 eq.), CH2Cl2 (3 mL), 0 °C, 5 min; c) allyltrimethylsilane (3.0 eq.), Et2O·BF3 (3.0 eq.), CH2Cl2 (3 mL), 0 °C, 5 min; d) TMSCN (2.0 eq.), Et2O·BF3 (3.0 eq.), CH2Cl2 (3 mL), 0 °C, 5 min; e) TMSN3 (2.0 eq.), Et2O·BF3 (3.0 eq.), CH2Cl2 (3 mL), 0 °C, 5 min; f) p-TSA (20 mol%), EtOH (2.5 mL), 30 °C, 10 h. g) PhSH (1.5 eq.), Et2O·BF3 (3.0 eq.), CH2Cl2 (3 mL), 0 °C, 5 min; isolated yield; the ee and dr values were determined by chiral HPLC analysis of purified products and 1H NMR analysis of reaction mixtures, respectively. p-TSA: p-toluenesulfonic acid
Stereodivergent [4+2] cycloadditions
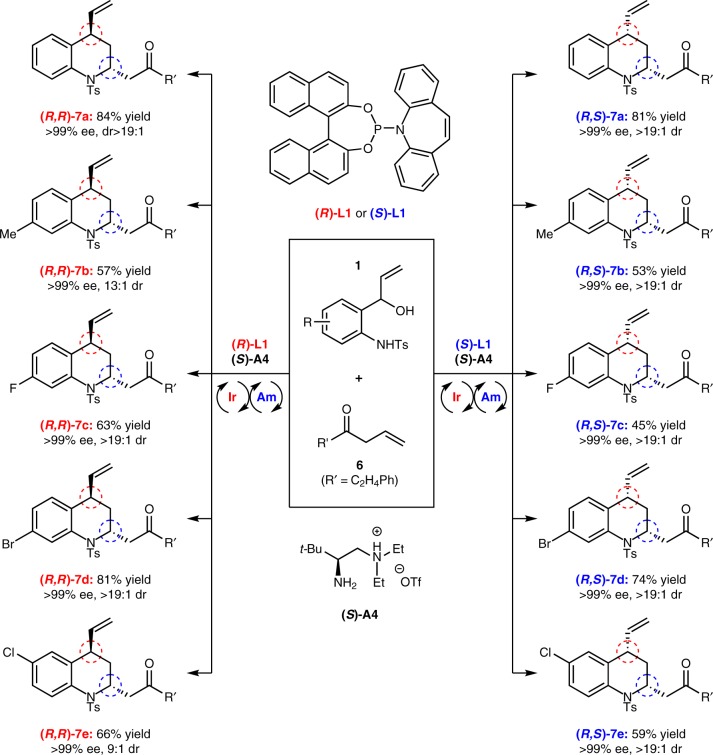
In addition to activating the α-position of the aldehyde via an enamine mechanism64, amine catalysis can also be used to efficiently activate the γ-position of the β,γ-unsaturated ketone through the formation of an dienamine species65–69. Based on this information and our above impressive results, we further probed the asymmetric [4 + 2] cycloadditions of vinyl aminoalcohols with β,γ-unsaturated ketones by merging iridium catalysis with dienamine catalysis. With the cycloaddition of vinyl aminoalcohol 1a and ketone 6 as model substrates, a simple optimization of the reaction parameters including chiral amine catalyst, solvent, and acid defined the optimal reaction conditions (See Supplementary Table 3 for the details of condition optimization). To our delight, unlike the cycloaddition of vinyl aminoalcohols 1 with aldehyde 2, which provides a pair of diastereomers, by reasonably using Carreira’s chiral phosphoramidite ligands, (R)-L1 or (S)-L1, and Luo’s primary amine catalyst (S)-A470, this reaction can provide both members of either of two pairs of diastereomers in good yields and with excellent enantio- and diastereoselectivities (Fig. 5, (R,R)-7a, 84% yield, >99% ee and >19:1 dr; (R,S)-7a, 81% yield, >99% ee and >19:1 dr). Worthy to note that, other two stereoisomers of 7a have been synthesized by using the enantiomer of Luo’s catalyst (S)-A4, too (See Supplementary Table 3 for details). The absolute configuration of product (R, S)-7a were established by analyzing its derivative through the X-ray diffraction analysis (CCDC 1869868, See Supplementary Note 1 for details)25. Encouraged by these results, experiments to preliminarily examine the scope of vinyl aminoalcohols for this stereodivergent cycloaddition were performed. As highlighted in Fig. 4, moderate to good yields together with high levels of enantio- and diastereocontrol were generally observed for both pairs of diastereomers. In a word, this is an interesting but challenging work that high diastereo- and enantioselectivities have been simultaneously accomplished in a stereodivergent manner, demonstrating the power and potential of synergetic catalysis strategies in the field of TM-catalyzed dipolar cycloaddition.
Fig. 5.
Enantioselective and diastereodivergent [4+2] cycloadditions of vinyl aminoalcohols and β,γ-unsaturated ketones. Reaction conditions: 1 (0.25 mmol), 6 (1.25 mmol), [Ir(cod)Cl]2 (2 mol%), (R)-L1 or (S)-L1 (8 mol%), amine (S)-A4 (20 mol%), in 1,2-dichloroethane (0.3 mL) for 48 h at 50 oC; isolated yield; the values of ee and dr were determined by chiral HPLC analysis
Discussion
Overall, we have successfully developed two enantioselective [4+2] cycloadditions of vinyl aminoalcohols with carbonyls, namely, aldehydes and β,γ-unsaturated ketones, through synergetic iridium and amine catalysis. The cycloaddition with aldehydes provides a diverse set of hydroquinolines bearing chiral quaternary stereocenters with a high level of enantiocontrol. The reaction with β,γ-unsaturated ketones allows the enantio- and diastereodivergent synthesis of hydroquinoline products using reasonable chiral iridium catalysts and chiral amine catalysts. Obviously, the formation of Ir-containing dipoles from vinyl aminoalcohols and chiral iridium catalysts and (di)enamine dipolarophiles from carbonyls and amine catalysts provides a foundation for subsequent asymmetric [4+2] dipolar cycloadditions. As demonstrated in this work, the application of synergetic catalysis makes transition metal-catalyzed cycloadditions more practical and powerful for the synthesis of chiral heterocycles.
Methods
Preparation of product 3
In a dried Schlenk tube under N2, [Ir(cod)Cl]2 (0.005 mmol, 3.35 mg) and chiral ligand (R)-L1 (0.02 mmol, 10.15 mg) were mixed in 0.5 mL DCE and stirred at ambient temperature for 20 min under argon atmosphere. Then allylic alcohol 1 (0.25 mmol, 1.0 eq), aldehyde 2 (0.5 mmol, 2.0 eq), Cl3CCOOH (0.125 mmol, 20.4 mg), amine A1 (0.05 mmol, 9.2 mg, 8.7 μL), 4.5 μL H2O (1.0 eq.) were added to the mixture successively. The reaction mixture was stirred at ambient temperature until substrate 1 disappeared on TLC. Then the mixture was diluted with 5 mL DCM, PCC (275 mg, 5.0 eq.) and 100 mg silica gel were added to the mixture. The mixture was stirred at 40 oC for 12 h and the final product was achieved by flash column chromatography.
Preparation of product 7
In a dried Schlenk tube under N2, [Ir(cod)Cl]2 (0.005 mmol, 3.35 mg) and chiral ligand (R)-L or (S)-L1 (0.02 mmol, 10.15 mg) were mixed in 0.3 mL DCE and stirred at ambient temperature for 20 min under argon atmosphere. Then allylic alcohol 1 (0.25 mmol, 1.0 eq), ketone 2 (0.5 mmol, 2.0 eq) and chiral amine catalyst (S)-A4 (0.05 mmol, 16.1 mg) were added to the mixture successively. After 8 h, the other 3.0 eq. ketone 2 was added in portions. Stirring at 50 oC for 48 h. The residue was directly purified by flash silica gel chromatography to afford the title compound.
Supplementary information
Acknowledgements
We are grateful to the National Science Foundation of China (No. 21822103, 21820102003, 21772052, 21772053 and 21572074), the Program of Introducing Talents of Discipline to Universities of China (111 Program, B17019), the Natural Science Foundation of Hubei Province (2017AHB047) and International Joint Research Center for Intelligent Biosensing Technology and Health for support of this research. Prof. Dr. Sanzhong Luo in Institute of Chemistry (CAS, Beijing) is sincerely acknowledged for helpful discussion about the enantio- and diastereodivergent cycloaddition of aminoalcohols with β,γ-unsaturated ketones and for providing their chiral primary amine catalysts.
Author contributions
L.-Q.L. and W.-J.X. supervised the project. M.-M.Z., Y.-N.W., B.-C.W., and X.-W.C. performed the experiments. L.-Q.L. and W.-J.X. wrote the manuscript, and all the authors contributed to the discussion.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information file, or from the corresponding author upon reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 1821791 and CCDC 1869868. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liang-Qiu Lu, Email: luliangqiu@mail.ccnu.edu.cn.
Wen-Jing Xiao, Email: wxiao@mail.ccnu.edu.cn.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-10674-3.
References
- 1.Pozharskii, A. R., Soldatenkov, A. & Katritzky, A. R. Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry and Applications (Willey, 2010).
- 2.Katritzky, A. R., Ramsden, C. A., Joule, J. A. & Zhdankin, V. V. Handbook of Heterocyclic Chemistry, Third Edition (Elsevier, 2010).
- 3.Kusama H, Iwasawa N. Cycloaddition reactions of transition metal-containing benzopyrylium and related zwitterionic intermediates. Chem. Lett. 2006;35:1082–1087. doi: 10.1246/cl.2006.1082. [DOI] [Google Scholar]
- 4.Xu X, Doyle MP. The [3+3]-cycloaddition alternative for heterocycle syntheses: catalytically generated metalloenolcarbenes as dipolar adducts. Acc. Chem. Res. 2014;47:1396–1405. doi: 10.1021/ar5000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De N, Yoo EJ. Recent advances in the catalytic cycloaddition of 1,n‑dipoles. ACS Catal. 2017;8:48–58. doi: 10.1021/acscatal.7b03346. [DOI] [Google Scholar]
- 6.Weaver JD, Recio A, Grenning AJ, Tunge JA. Transition metal-catalyzed decarboxylative allylation and benzylation reactions. Chem. Rev. 2011;111:1846–1913. doi: 10.1021/cr1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen BDW, Lakeland CP, Harrity JPA. Utilizing palladium-stabilized zwitterions for the construction of N-heterocycles. Chem. Eur. J. 2017;23:13830–13857. doi: 10.1002/chem.201702486. [DOI] [PubMed] [Google Scholar]
- 8.Lowe MA, et al. Palladium-mediated annulation of vinyl aziridines with michael acceptors: stereocontrolled synthesis of substituted pyrrolidines and its application in a formal synthesis of (-)-α-kainic acid. Angew. Chem. Int. Ed. 2011;50:6370–6374. doi: 10.1002/anie.201101389. [DOI] [PubMed] [Google Scholar]
- 9.Shintani R, Moriya K, Hayashi T. Guiding the nitrogen nucleophile to the middle: palladium-catalyzed decarboxylative cyclopropanation of 2-alkylidenetrimethylene carbonates with isocyanates. Chem. Commun. 2011;47:3057–3059. doi: 10.1039/c0cc05308b. [DOI] [PubMed] [Google Scholar]
- 10.Ohmatsu K, Imagawa N, Ooi T. Ligand-enabled multiple absolute stereocontrol in metal-catalysed cycloaddition for construction of contiguous all-carbon quaternary stereocentres. Nat. Chem. 2013;6:47–51. doi: 10.1038/nchem.1796. [DOI] [PubMed] [Google Scholar]
- 11.Khan A, et al. Palladium-catalyzed decarboxylative cycloaddition of vinylethylene carbonates with formaldehyde: enantioselective construction of tertiary vinylglycols. Angew. Chem. Int. Ed. 2014;53:6439–6442. doi: 10.1002/anie.201403754. [DOI] [PubMed] [Google Scholar]
- 12.Xu C-F, Zheng B-H, Suo J-J, Ding C-H, Hou X-L. Highly diastereo- and enantioselective palladium-catalyzed [3+2] cycloaddition of vinyl aziridines and α, β-unsaturated ketones. Angew. Chem. Int. Ed. 2014;54:1624–1627. doi: 10.1002/anie.201409467. [DOI] [PubMed] [Google Scholar]
- 13.Poli G., Prestat G., Liron F. & Kammerer-Pentier C. Selectivity in Palladium-Catalyzed Allylic Substitution. In Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis. Topics in Organometallic Chemistry, (eds Kazmaier, U.) vol. 38. (Springer, Berlin, Heidelbe 2011).
- 14.Wang Y-N, Lu L-Q, Xiao W-J. Non-bonding interactions enable the selective formation of branched products in palladium-catalyzed allylic substitution reactions. Chem. Asian J. 2018;13:2174–2183. doi: 10.1002/asia.201800496. [DOI] [PubMed] [Google Scholar]
- 15.Li T-R, et al. Asymmetric trapping of zwitterionic intermediates by sulphur ylides in a palladium-catalysed decarboxylation-cycloaddition sequence. Nat. Commun. 2014;5:5500–5509. doi: 10.1038/ncomms6500. [DOI] [PubMed] [Google Scholar]
- 16.Guo C, Fleige M, Janssen-Mgller D, Daniliuc CG, Glorius F. Cooperative N-heterocyclic carbene/palladium-catalyzed enantioselective umpolung annulations. J. Am. Chem. Soc. 2016;138:7840–7843. doi: 10.1021/jacs.6b04364. [DOI] [PubMed] [Google Scholar]
- 17.Guo C, et al. Mechanistic studies on a cooperative NHC organocatalysis/palladium catalysis system: uncovering significant lessons for mixed chiral Pd(NHC)(PR3) catalyst design. J. Am. Chem. Soc. 2017;139:4443–4451. doi: 10.1021/jacs.7b00462. [DOI] [PubMed] [Google Scholar]
- 18.Mei G-J, et al. Catalytic asymmetric construction of the tryptanthrin skeleton via an enantioselective decarboxylative [4+2] cyclization. Org. Lett. 2017;19:3219–3222. doi: 10.1021/acs.orglett.7b01336. [DOI] [PubMed] [Google Scholar]
- 19.Singha S, Patra T, Daniliuc CG, Glorius F. Highly enantioselective [5+2] annulations through cooperative N‑heterocyclic carbene (NHC) organocatalysis and palladium catalysis. J. Am. Chem. Soc. 2018;140:3551–3554. doi: 10.1021/jacs.8b00868. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi R, Kashio M. Highly selective allylic alkylation with a carbon nucleophile at the more substituted allylic terminus catalyzed by an iridium complex: an efficient method for constructing quaternary carbon centers. Angew. Chem. Int. Ed. 1997;36:263–264. doi: 10.1002/anie.199702631. [DOI] [Google Scholar]
- 21.Janssen JP, Helmchen G. First enantioselective alkylations of monosubstituted allylic acetates catalyzed by chiral iridium complexes. Tetrahedron Lett. 1997;38:8025–8026. doi: 10.1016/S0040-4039(97)10220-9. [DOI] [Google Scholar]
- 22.Takeuchi R, Kashio M. Iridium complex-catalyzed allylic alkylation of allylic esters and allylic alcohols: unique regio- and stereoselectivity. J. Am. Chem. Soc. 1998;120:8647–8655. doi: 10.1021/ja981560p. [DOI] [Google Scholar]
- 23.Qu J, Helmchen G. Applications of iridium-catalyzed asymmetric allylic substitution reactions in target-oriented synthesis. Acc. Chem. Res. 2017;50:2539–2555. doi: 10.1021/acs.accounts.7b00300. [DOI] [PubMed] [Google Scholar]
- 24.Hartwig JF, Levi M. Stanley mechanistically driven development of iridium catalysts for asymmetric allylic substitution. Acc. Chem. Res. 2010;43:1461–1475. doi: 10.1021/ar100047x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krautwald S, Carreira EM. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 2017;139:5627–5639. doi: 10.1021/jacs.6b13340. [DOI] [PubMed] [Google Scholar]
- 26.Zhuo C-X, Zheng C, You S-L. Transition-metal-catalyzed asymmetric allylic dearomatization reactions. Acc. Chem. Res. 2014;47:2558–2573. doi: 10.1021/ar500167f. [DOI] [PubMed] [Google Scholar]
- 27.Lu Z, Ma S. Metal-catalyzed enantioselective allylation in asymmetric synthesis. Angew. Chem. Int. Ed. 2008;47:258–297. doi: 10.1002/anie.200605113. [DOI] [PubMed] [Google Scholar]
- 28.Liu W-B, Xia J-B, You S-L. Iridium-catalyzed asymmetric allylic substitutions. Top. Organomet. Chem. 2012;38:155–208. doi: 10.1007/3418_2011_10. [DOI] [Google Scholar]
- 29.Hartwig JF, Pouy MJ. Iridium-catalyzed allylic substitution. Top. Organomet. Chem. 2011;34:169–208. doi: 10.1007/978-3-642-15334-1_7. [DOI] [Google Scholar]
- 30.Helmchen, G. Iridium-Catalyzed Allylic Substitutions, Iridium Complexes in Organic Synthesis. (eds Oro, L. A. & Claver, G.) pp 211–250 (Willey, 2009).
- 31.Kanayama T, Yoshida K, Miyabe H, Kimachi T, Takemoto Y. Synthesis of β-substituted α-amino acids with use of iridium-catalyzed asymmetric allylic substitution. J. Org. Chem. 2003;68:6197–6201. doi: 10.1021/jo034638f. [DOI] [PubMed] [Google Scholar]
- 32.Krautwald S, Sarlah D, Schafroth MA, Carreira EM. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science. 2013;340:1065–1068. doi: 10.1126/science.1237068. [DOI] [PubMed] [Google Scholar]
- 33.Krautwald S, Schafroth MA, Sarlah D, Carreira EM. Stereodivergent α‑allylation of linear aldehydes with dual iridium and amine catalysis. J. Am. Chem. Soc. 2014;136:3020–3023. doi: 10.1021/ja5003247. [DOI] [PubMed] [Google Scholar]
- 34.Næsborg L, Halskov KS, Tur F, Mønsted SMN, Jørgensen KA. Asymmetric γ-allylation of α, β-unsaturated aldehydes by combined organocatalysis and transition-metal catalysis. Angew. Chem. Int. Ed. 2015;54:10193–10197. doi: 10.1002/anie.201504749. [DOI] [PubMed] [Google Scholar]
- 35.Jiang X, Beiger JJ, Hartwig JF. Stereodivergent allylic substitutions with aryl acetic acid esters by synergistic iridium and lewis base catalysis. J. Am. Chem. Soc. 2017;139:87–90. doi: 10.1021/jacs.6b11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen D, Chen Q, Yan P, Zeng X, Zhong G. Enantioselective dearomatization of naphthol derivatives with allylic alcohols by cooperative iridium and Brønsted acid catalysis. Angew. Chem. Int. Ed. 2017;56:3242–3246. doi: 10.1002/anie.201609693. [DOI] [PubMed] [Google Scholar]
- 37.Huo X, He R, Zhang X, Zhang W. An Ir/Zn dual catalysis for enantio- and diastereodivergent α‑allylation of α‑hydroxyketones. J. Am. Chem. Soc. 2016;138:11093–11096. doi: 10.1021/jacs.6b06156. [DOI] [PubMed] [Google Scholar]
- 38.Jiang X, Boehm P, Hartwig JF. Stereodivergent allylation of azaaryl acetamides and acetates by synergistic iridium and copper catalysis. J. Am. Chem. Soc. 2018;140:1239–1242. doi: 10.1021/jacs.7b12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei L, Zhu Q, Xu S-M, Chang X, Wang C-J. Stereodivergent synthesis of α,α-disubstituted α‑amino acids via synergistic Cu/Ir catalysis. J. Am. Chem. Soc. 2018;140:1508. doi: 10.1021/jacs.7b12174. [DOI] [PubMed] [Google Scholar]
- 40.Huo X, Zhang J, Fu J, He R, Zhang W. Ir/Cu dual catalysis: enantio- and diastereodivergent access to α,α-disubstituted α-amino acids bearing vicinal stereocenters. J. Am. Chem. Soc. 2018;140:2080–2084. doi: 10.1021/jacs.8b00187. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M-M, Luo Y-Y, Lu L-Q, Xiao W-J. Advances on asymmetric allylic substitutions under synergetic catalysis system with transition metals and organocatalysts. Acta Chim. Sin. 2018;76:838–849. doi: 10.6023/A18060237. [DOI] [Google Scholar]
- 42.Sridharan V, Suryavanshi PA, Mendez JC. Advances in the chemistry of tetrahydroquinolines. Chem. Rev. 2011;111:7157–7259. doi: 10.1021/cr100307m. [DOI] [PubMed] [Google Scholar]
- 43.Gómez, C. M. M. & Kouznetsov, V. Recent Development on Antimicrobial Quinoline Chemistry, in Microbial Pathogens and Strategies for Combating Them: Science Technology and Educations vol. 1A. (eds Mendez-Vilas), pp 666–677 (Formatex, Badajoz, 2013).
- 44.Liu W-B, Zheng C, Zhuo C-X, Dai L-X, You S-L. Iridium-catalyzed allylic alkylation reaction with N-aryl phosphoramidite ligands: scope and mechanistic studies. J. Am. Chem. Soc. 2012;134:4812–4821. doi: 10.1021/ja210923k. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Tunge JA. Asymmetric cycloadditions of palladium-polarized aza-o-xylylenes. J. Am. Chem. Soc. 2008;130:8118–8119. doi: 10.1021/ja801742h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, et al. An organocatalytic cascade approach toward polysubstituted quinolines and chiral 1,4-dihydroquinolines-unanticipated effect of N-protecting groups. Angew. Chem. Int. Ed. 2012;51:7282–7286. doi: 10.1002/anie.201202161. [DOI] [PubMed] [Google Scholar]
- 47.Leth LA, et al. Decarboxylative [4 + 2] Cycloaddition by synergistic palladium and organocatalysis. Angew. Chem. Int. Ed. 2016;55:15272–15276. doi: 10.1002/anie.201607788. [DOI] [PubMed] [Google Scholar]
- 48.Fu Z, Jiang K, Zhu T, Torres J, Chi Y-R. Access to oxoquinoline heterocycles by N-heterocyclic carbene catalyzed ester activation for selective reaction with an enone. Angew. Chem. Int. Ed. 2014;53:6506–6510. doi: 10.1002/anie.201402620. [DOI] [PubMed] [Google Scholar]
- 49.Song J, Zhang Z-J, Gong L-Z. Asymmetric [4+2] annulation of c1 ammonium enolates with copper-allenylidenes. Angew. Chem. Int. Ed. 2017;56:5212–5216. doi: 10.1002/anie.201700105. [DOI] [PubMed] [Google Scholar]
- 50.Lu L-Q, Li T-R, Wang Q, Xiao W-J. Beyond sulfide-centric catalysis: recent advances in the catalytic cyclization reactions of sulfur ylides. Chem. Soc. Rev. 2017;46:4135–4149. doi: 10.1039/C6CS00276E. [DOI] [PubMed] [Google Scholar]
- 51.Li T-R, Wang Y-N, Xiao W-J, Lu L-Q. Transition-metal-catalyzed cyclization reactions using vinyl and ethynyl benzoxazinones as dipole precursors. Tetrahedron Lett. 2018;59:1521–1530. doi: 10.1016/j.tetlet.2018.02.081. [DOI] [Google Scholar]
- 52.Wang Q, et al. Catalytic asymmetric [4+1] annulation of sulfur ylides with copper-allenylidene intermediates. J. Am. Chem. Soc. 2016;138:8360–8363. doi: 10.1021/jacs.6b04414. [DOI] [PubMed] [Google Scholar]
- 53.Wei Y, et al. P,S ligands for the asymmetric construction of quaternary stereo-centers in palladium-catalyzed decarboxylative [4+2] cycloadditions. Angew. Chem. Int. Ed. 2016;55:2200–2204. doi: 10.1002/anie.201509731. [DOI] [PubMed] [Google Scholar]
- 54.Wang Q, et al. Iron-catalyzed decarboxylative (4+1) cycloadditions: exploiting the reactivity of ambident iron-stabilized intermediates. Angew. Chem. Int. Ed. 2016;55:2840–2844. doi: 10.1002/anie.201510413. [DOI] [PubMed] [Google Scholar]
- 55.Li M-M, et al. Sequential visible-light photoactivation and palladium catalysis enabling enantioselective [4+2] cycloadditions. J. Am. Chem. Soc. 2017;139:14707. doi: 10.1021/jacs.7b08310. [DOI] [PubMed] [Google Scholar]
- 56.Liang X, et al. Ir-catalyzed asymmetric total synthesis of (-)-communesin F. J. Am. Chem. Soc. 2018;139:3364–3367. doi: 10.1021/jacs.7b00854. [DOI] [PubMed] [Google Scholar]
- 57.Sandmeier T, Krautwald S, Carreira EM. Stereoselective synthesis of piperidines by iridium-catalyzed cyclocondensation. Angew. Chem. Int. Ed. 2017;56:11515–11519. doi: 10.1002/anie.201706374. [DOI] [PubMed] [Google Scholar]
- 58.Quasdorf KW, Overman LE. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature. 2014;516:181–191. doi: 10.1038/nature14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Han SJ, Liu W-B, Stoltz BM. Catalytic enantioselective construction of quaternary stereocenters: assembly of key building blocks for the synthesis of biologically active molecules. Acc. Chem. Res. 2015;48:740–751. doi: 10.1021/ar5004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukherjee S, List B. Chiral counteranions in asymmetric transition-metal catalysis: highly enantioselective Pd/Brønsted acid-catalyzed direct α-allylation of aldehydes. J. Am. Chem. Soc. 2007;129:11336–11337. doi: 10.1021/ja074678r. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida M, Terumine T, Masaki E, Hara S. Direct asymmetric α‑allylation of α‑branched aldehydes by two catalytic systems with an achiral Pd complex and a chiral primary α‑amino acid. J. Org. Chem. 2013;78:10853–10859. doi: 10.1021/jo4018414. [DOI] [PubMed] [Google Scholar]
- 62.Wang P-S, Lin H-C, Zhai Y-J, Han Z-Y, Gong L-Z. Chiral counteranion strategy for asymmetric oxidative C(sp3)-H/C(sp3)-H coupling: enantioselective α-allylation of aldehydes with terminal alkenes. Angew. Chem. Int. Ed. 2014;53:12218–12221. doi: 10.1002/anie.201408199. [DOI] [PubMed] [Google Scholar]
- 63.Song W, et al. Iridium-catalyzed highly regioselective and diastereoselective allylic etherification to access cis-2,6-disubstituted dihydropyridinones. J. Org. Chem. 2018;83:12822–12830. doi: 10.1021/acs.joc.8b01598. [DOI] [PubMed] [Google Scholar]
- 64.Mukherjee S, Yang J-W, Hoffmann S, List B. Asymmetric enamine catalysis. Chem. Rev. 2007;107:5471–5569. doi: 10.1021/cr0684016. [DOI] [PubMed] [Google Scholar]
- 65.Bertelsen S, Marigo M, Brandes S, Dinér P, Jørgensen KA. Dienamine catalysis: organocatalytic asymmetric γ-amination of α,β-unsaturated aldehydes. J. Am. Chem. Soc. 2006;128:12973–1298. doi: 10.1021/ja064637f. [DOI] [PubMed] [Google Scholar]
- 66.Li J-L, Liu T-Y, Chen Y-C. Aminocatalytic asymmetric Diels-Alder reactions via homo activation. Acc. Chem. Res. 2012;45:1491–1500. doi: 10.1021/ar3000822. [DOI] [PubMed] [Google Scholar]
- 67.Zhu B, et al. Direct asymmetric vinylogous aldol reaction of allyl ketones with isatins: divergent synthesis of 3-hydroxy-2-oxindole derivatives. Angew. Chem. Int. Ed. 2013;52:6666–6670. doi: 10.1002/anie.201302274. [DOI] [PubMed] [Google Scholar]
- 68.Zhan G, He Q, Yuan X, Chen Y-C. Asymmetric direct vinylogous Michael additions of allyl alkyl ketones to maleimides through dienamine catalysis. Org. Lett. 2014;16:6000–6003. doi: 10.1021/ol503017h. [DOI] [PubMed] [Google Scholar]
- 69.Shi M-L, Zhan G, Zhou S-L, Du W, Chen Y-C. Asymmetric inverse-electron-demand oxa-Diels-Alder reaction of allylic ketones through dienamine catalysis. Org. Lett. 2016;18:6480–6483. doi: 10.1021/acs.orglett.6b03384. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Fu N, Luo S. Pushing the limits of aminocatalysis: enantioselective transformations of α-branched β-ketocarbonyls and vinyl ketones by chiral primary amines. Acc. Chem. Res. 2015;48:986–997. doi: 10.1021/acs.accounts.5b00028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information file, or from the corresponding author upon reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 1821791 and CCDC 1869868. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.