Abstract
Skeletal muscle regeneration is a finely tuned process involving the activation of various cellular and molecular processes. Satellite cells, the stem cells of skeletal muscle, are indispensable for skeletal muscle regeneration. Their functionality is critically modulated by intrinsic signaling pathways as well as by interactions with the stem cell niche. Here, we discuss the properties of satellite cells, including heterogeneity regarding gene expression and/or their phenotypic traits and the contribution of satellite cells to skeletal muscle regeneration. We also summarize the process of regeneration with a specific emphasis on signaling pathways, cytoskeletal rearrangements, the importance of miRNAs, and the contribution of non-satellite cells such as immune cells, fibro-adipogenic progenitor cells, and PW1-positive/Pax7-negative interstitial cells.
Keywords: Satellite cell, Stem cell, Skeletal muscle, Regeneration, Myogenesis, Heterogeneity
Introduction
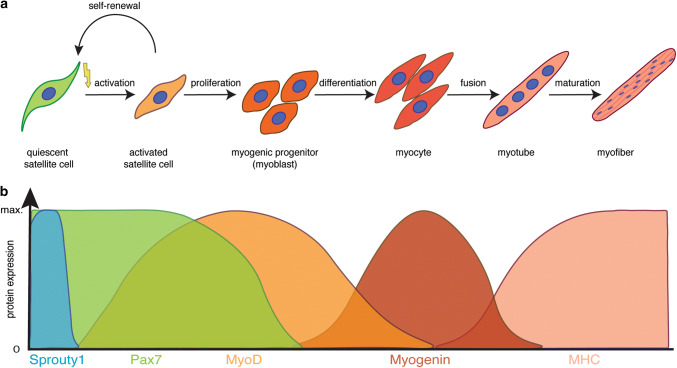
Skeletal muscle fulfils multiple functions in the body including voluntary locomotion, breathing, and postural behaviour. It possesses a remarkable ability to regenerate and to adapt to physiological demands such as growth or training [1]. Muscle stem cells—also termed satellite cells (SCs)—are a prerequisite for regeneration of skeletal muscle, as shown by previous studies using a diphtheria toxin (DTA)-based approach to deplete satellite cells [2–4]. Under resting conditions, satellite cells are quiescent and reside under the basal lamina of the myofiber [5]; this position, between the myofiber and the surrounding extracellular matrix (ECM), was responsible for their naming in 1961 by Alexander Mauro [6]. While quiescent under resting conditions, satellite cells become activated, expand and differentiate during skeletal muscle regeneration, a process controlled by sequential expression of transcription factors, resembling the differentiation program of embryonic myogenesis [1, 7] (Fig. 1). The paired box transcription factor Pax7 is expressed in all satellite cells, and is required for their postnatal maintenance and regeneration of skeletal muscle [8–10]. Upon activation, satellite cells co-express Pax7 and MyoD—an early marker for myogenic commitment— leave the quiescent state and further differentiate into myocytes, before maturing to myofibers. Notably, a subset of activated satellite cells downregulates MyoD and resists the differentiation process, thereby maintaining a mitotically inactive state similar to quiescence, a process depending on Sprouty1 [11–13]. Satellite cells have an enormous myogenic potential, which mostly depends on the expression of Pax genes and subsequent expression of myogenic regulatory factors (MRFs: MyoD, Myf5, Myogenin, and MRF4) [7]. Interestingly, ablation of satellite cells under homeostatic conditions in the adult does not seem to lead to muscle atrophy or myopathies without induced injury, suggesting that their main function in the adult is the regeneration of skeletal muscle [14, 15].
Fig. 1.
Myogenic lineage progression and expression profile of key myogenic regulators. a Schematic illustration of the myogenic lineage progression. Satellite cells are activated, e.g., due to injury, start to proliferate, thereby generating myogenic progenitor cells. Upon differentiation, myogenic progenitor cells differentiate into myocytes, which fuse to form myotubes and mature to become myofibers, the contractile unit of skeletal muscle. b Expression profile of key modulators of myogenic lineage progression
Although skeletal muscle can fully regenerate multiple times in healthy adult mice and men, the functionality of satellite cells declines in the course of various degenerative diseases or states such as aging resulting in impaired regeneration of skeletal muscle [16–21]. Such impaired functionality results from an altered extracellular matrix composition, misbalanced cell–cell interactions with residing cells of the skeletal muscle or changes of systemic factors such as loss of the anti-aging hormone Klotho [22–25]. It was recently demonstrated that soluble Klotho protein counteracts canonical Wnt3a signaling in satellite cells [22]. Importantly, Wnt3a signaling is upregulated in aged skeletal muscle, thereby impairing satellite cell function, resulting in disturbed regeneration [26]. The functional deficits of aged satellite cells can be partially overcome by systemic delivery of Klotho protein [27].
Identification and characterization of satellite cells
Satellite cells were first characterized by their unique localization under the basal lamina of myofibers [6]. Recently, satellite cells have also been termed muscle stem cells (MuSCs), probably due to the fact that the term satellite cell also refers to specific glial cells in the brain. Satellite cells can self-renew, thereby maintaining the satellite cell pool, but also give rise to more differentiated myogenic progenitor cells, which then contribute to regeneration of myofibers after injury. These characteristics demonstrate that satellite cells are bonafide muscle stem cells. In adult skeletal muscle, all satellite cells express the paired box transcription factor Pax7, being essential for satellite cell function [8–10, 28], while subsets also express Pax3 [29] or myogenic regulatory factor 5 (Myf5) [30]. Other markers for satellite cells are located at the plasma membrane making them good candidates for satellite cell isolation via flow cytometry. Amongst others, these markers include α7-Integrin and β1-Integrin, M-Cadherin, Syndecan-4, Calcitonin-Receptor (CALCR), C-X-C Chemokine Receptor type-4 (CXCR4), Vascular Cell Adhesion Molecule 1 (VCAM1), and CD34 [31]. Of the genetic markers listed above, Pax7 is the canonical biomarker for satellite cells, since it is expressed in quiescent and activated satellite cells in most model species including mice, men, zebrafish, and chicken [32].
Adult satellite cells: a heterogeneous cell population
The satellite cell population is heterogeneous. The individual subpopulations can be discriminated by different means, including gene expression or phenotypic traits such as division rate. Pulse-chase experiments using a Tg:Pax7-GFP mouse line demonstrate that satellite cells with a relatively high expression of Pax7 (Pax7High) are less primed for commitment and need a longer time to undergo the first mitosis compared to satellite cells with a relatively low expression of Pax7 (Pax7Low) [33]. Furthermore, those cells segregate their DNA asymmetrically, so that daughter cells receiving the template strand also maintain expression of stem cell markers [34, 35]. Another subset of satellite cells defined by phenotypic traits was identified and termed label-retaining cells (LRCs) using a TetO–H2B–GFP mouse line, in which administration of doxycycline marks rarely dividing or non-cycling cells by retention of the expression of the H2B–GFP reporter [36]. Further evidence for phenotypic heterogeneity within the satellite cell pool arises from a recent study by Tierney et al., who investigated the clonal dynamics of satellite cells following injury and found that, after multiple rounds of injury, the clonal complexity is reduced when using a multicolour lineage tracing approach [37].
Another approach for investigating satellite cell heterogeneity is based on genetic analysis using a Myf5-Cre reporter mouse line (R26R-YFP/Myf5-Cre). Here, approximately 10% of satellite cells have never expressed myf5, as demonstrated by the absence of the reporter YFP (yellow fluorescent protein) in Pax7-positive cells [30]. Interestingly, YFP− satellite cells are able to engraft into the satellite cell niche after transplantation, while YFP+ satellite cells give rise to new myofibers and do not home to the satellite cell niche. However, upregulation of myf5 and induction of the YFP reporter gene were demonstrated in YFP− satellite daughter cells, further suggesting that Pax7+/YFP− cells are a rare stem cell sub-population of satellite cells, while Pax7+/YFP+ cells are more committed progenitor cells [30]. Yet, following activation of satellite cells—for instance after injury—both populations of satellite cells (YFP+ and YFP−) are proliferating and undergo planar cell divisions, thereby generating two identical daughter cells (identical in terms of YFP expression). Multiple signaling pathways are driving the symmetric division of YFP− cells, among them Wnt7a in concert with its receptor Fzd7 and the ECM molecule Fibronectin, as well as JAK/STAT signaling [17, 19, 38, 39].
Asymmetric divisions of satellite cells
Asymmetric stem cell divisions are a prerequisite for proper stem cell renewal, concomitant with generation of progenitor cells. The first evidence for asymmetric satellite cell divisions emanated from a study by Shinin and colleagues. With the help of BrdU (bromodeoxyuridine) incorporation experiments, they demonstrated that the cell fate determinant Numb, a Notch signaling inhibitor, was segregated to the same daughter cell as the BrdU label during mitosis, suggesting a role for Numb in self-renewal [35]. Asymmetric satellite cell divisions are controlled by various signaling pathways, including Notch signaling [40]. Of interest is that Notch signaling seems to be responsible for asymmetric divisions of YFP− satellite cells when using the R26R-YFP/Myf5-cre reporter mouse line, since the Notch effectors Notch3 and Delta1 are asymmetrically distributed in the daughter cells [30], the YFP− satellite cells are expressing the Notch ligand Delta1, while the YFP+ satellite cells express the Notch receptor Notch3 [30]. The importance of Notch signaling for asymmetric satellite cell divisions is further supported by the fact that the Notch antagonist Numb is also asymmetrically distributed in different mouse models used to discriminate the different satellite cell subpopulations [34, 41, 42]. Besides asymmetric distribution of signaling molecules and receptors, DNA strands are also asymmetrically segregated during satellite cell division. The daughter cell retaining the template DNA strand shows more stemness characteristics, thereby attenuating the accumulation of replication errors in the parental DNA and the transmission to further daughter cells [33]. In addition, control of cell polarity plays an important role in the regulation of asymmetric satellite cell divisions. For instance, the Par complex, an evolutionary well-conserved complex located at the apical membrane, is a prerequisite for the asymmetric initiation of myogenic differentiation [43]. Activation of the Par complex leads to the selective activation of the p38α/β MAPK pathway, which, in turn, directly regulates MyoD transcription [44]. Dystrophin, a protein mostly known for its function in stabilization of the myofiber, is an essential cofactor for regulating cell polarity during asymmetric satellite cell division [45], suggesting that Duchenne muscular dystrophy is not only affecting the myofiber, but also the satellite cells. This is especially important, since skeletal muscle undergoes constant regeneration under dystrophic conditions, in both mice and humans.
Regeneration of skeletal muscle
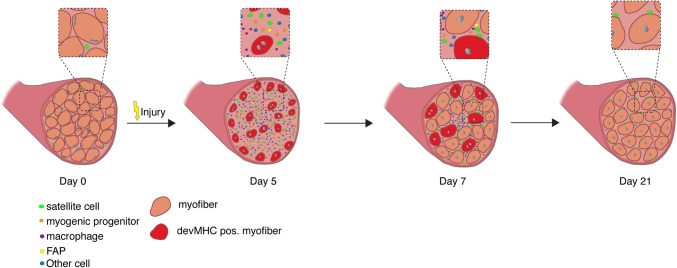
The process of skeletal muscle regeneration can be divided into three phases: the inflammatory phase, the phase of satellite cell activation/differentiation phase, and the maturation phase, when remodeling of the newly formed myofibers occurs (Fig. 2).
Fig. 2.
Regeneration of skeletal muscle. Time course of changes in cellular composition during skeletal muscle regeneration following cardiotoxin (CTX) injury. Satellite cells (in green) are quiescent in resting skeletal muscle. Five days after CTX injury, regenerating muscles are reduced to mostly mono-nuclear cells (satellite cells, immune cells, etc.), but are able to form new myotubes at day 7, which mature to multinucleated myofibers. Of note, the nuclei of intact myofibers are located at the periphery, while newly regenerating myofibers are characterized by centrally located myonuclei. During the maturation process, the myonuclei migrate to the periphery
The muscle degenerates after injury, starting with the necrosis of damaged myofibers. This is accompanied by an increased calcium influx and release of calcium from the sarcoplasmic reticulum of the damaged myofiber, leading to proteolysis and degeneration of the damaged tissue [32]. Inflammatory responses (phase 1 of regeneration) are triggered by necrosis of myofibers, including the recruitment of circulating leucocytes [32, 46]. The first inflammatory cells to be recruited to the damaged muscle are the neutrophils. Recruitment occurs within the first 6 h after muscle damage [47]. Subsequently, macrophages infiltrate the damaged muscle. The macrophage population consists of two distinct populations, the early macrophages infiltrating the muscle are the pro-inflammatory CD68+/CD163− macrophages, followed by the anti-inflammatory CD68−/CD163+ macrophages [48–50]. The early infiltrating macrophages peak around 24 h after injury and are responsible for phagocytosis of damaged tissue parts and secrete pro-inflammatory cytokines such as TNFα and IL-1. The secondary wave of macrophages secretes anti-inflammatory cytokines such as IL-10 and is known to facilitate proliferation and differentiation of satellite cells [51–53]. Their greatest abundance can be observed 2–4 days after injury [25].
The second phase of regeneration of skeletal muscle is characterized by the activation and differentiation of satellite cells, a highly orchestrated process concomitant with morphological changes (Fig. 2). Quiescent under normal resting conditions, satellite cells are characterized by the expression of Pax7, but do not express MyoD [1, 54], although they may enter a G (alert) state priming them for rapid entry into the cell cycle, for instance in response to injury [55]. Upon injury, quiescent satellite cells enter the cell cycle and begin to express MyoD, migrate to the site of injury, and either fuse with damaged myofibers or become myogenic progenitor cells. Migration of satellite cells is controlled by signals from the myofibers, including signaling through Ephrin and Wnt7a [56, 57]. Myogenic progenitor cells are a highly proliferative transient amplifying cell population characterized by the expression of MyoD and Myf5, and are often referred to as myoblasts [5, 58]. The transcription factors Pax7 and Pax3 induce expression of genes responsible for promoting proliferation and commitment to the myogenic lineage and repress genes driving differentiation. The myogenic regulatory factors (MRFs) comprised of Myf5, MyoD, Myogenin, and MRF4 are downstream of Pax7 and Pax3, and promote myogenic differentiation [7, 59, 60]. Myogenin, a direct target of MyoD, initiates the terminal differentiation of myogenic progenitor cells, which is accompanied by downregulation of MyoD expression [1, 7, 60]. Morphologically, myogenic progenitor cells become elongated myocytes, which then fuse to form multinucleated myotubes. Newly formed myofibers are characterized by centrally located nuclei and the expression of devMHC (developmental myosin heavy chain), a myosin heavy chain which is, otherwise, only expressed during embryonic development [1, 32, 54]. This process is followed by maturation into myofibers (phase 3 of regeneration), which are the contractile units of skeletal muscle.
A highly orchestrated interplay between the stem cell niche and the satellite cell and other supporting cells is essential for proper regeneration of skeletal muscle. In conditions of perturbed homeostasis—such as aging—functional regeneration is hampered. Examples for changes in the satellite cell niche and changes in interactions with the satellite cell niche during aging include the loss of Fibronectin, altered β1-Integrin activity, or reduced levels of the anti-aging hormone Klotho, all of which result in impaired regeneration of skeletal muscle [22–24, 27]. A finely tuned balance between extrinsic cues and activation of intrinsic signaling pathways is required to accurately control satellite cell function. Multiple signaling pathways coordinate skeletal muscle regeneration. Below, a brief overview of how signaling pathways affect satellite cell function and regeneration of skeletal muscle is given.
Wnt signaling during regeneration of skeletal muscle
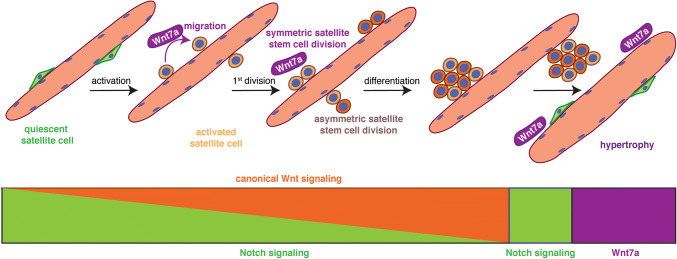
Wnt signaling drives development of skeletal muscle and is one of the key signaling pathways involved in regeneration of skeletal muscle. [58]. Multiple Wnt ligands are expressed during regeneration of skeletal muscle in a temporally controlled manner. During the early phase of regeneration Wnt5a, Wnt5b, and Wnt7a are upregulated, while Wnt4 expression decreases. The later phases of regeneration are characterized by the expression of Wnt3a and Wnt7b [26, 61]. In adult skeletal muscle, canonical Wnt signaling—mainly through the ligand Wnt3a—drives differentiation of satellite cells, while non-canonical Wnt signaling through the ligand Wnt7a is responsible for promoting symmetric satellite cell divisions, migration of satellite cells, and growth of myofibers [26, 38, 39, 56, 62–65] (Fig. 3). In skeletal muscle, Wnt7a always signals through the Frizzled receptor Fzd7. Interestingly, several signaling pathways in satellite cells and myofibers are activated by the interaction of Wnt7a and Fzd7, namely the planar cell polarity pathway (PCP) and the AKT/mTOR pathway [58]. This makes Wnt7a a promising candidate for ameliorative treatment of muscle wasting diseases such as muscular dystrophy [66]. A recent publication describes the importance of R-spondin, a known modulator of canonical Wnt signaling for proper differentiation of myogenic progenitor cells during regeneration, further emphasizing the importance of proper Wnt signaling for regeneration of skeletal muscle [64, 67, 68]. The importance of balanced canonical Wnt signaling for regeneration of skeletal muscle is further highlighted by a recent study from Rudolf et al., who demonstrated that disruption or activation of β-catenin in adult satellite cells impairs the regeneration process [69].
Fig. 3.
A switch from Notch to Wnt signaling is required for proper satellite cell differentiation. Satellite cells express high levels of Notch to retain them in a quiescent state, upon activation canonical Wnt signaling increases. The non-canonical Wnt7a drives the symmetric expansion of satellite stem cells and migration of satellite cells in general. Upon return to quiescence, satellite cells switch to Notch signaling, while Wnt7a drives the growth of already existing myotubes and myofibers, thereby inducing hypertrophy
Notch signaling in satellite cells
A fine balance between signaling pathways and their precise activation is a prerequisite for effective regeneration of skeletal muscle—a good example is the temporal switch from Notch to canonical Wnt signaling required for proper differentiation (Fig. 3). Canonical Wnt signaling antagonizes the effects of Notch signaling, thereby allowing the progression of the myogenic commitment and differentiation [26]. The Notch receptor on the signal-receiving cell is expressed on the cell surface as a heterodimer, while the ligands are located on the opposing signal-sending cell. The Notch ligands are sequestered to the surface of the myofibers, thereby controlling satellite cell proliferation and differentiation [26, 40–42, 70]. The interplay between Notch and the transmembrane receptor Syndecan3 expressed in satellite cells controls the maintenance of the satellite cell pool and myofiber size after regeneration [71]. The importance of proper Notch signaling for satellite cell maintenance is further emphasized by two studies, demonstrating that expression of the downstream effector Recombination Signal Binding Protein for Immunoglobulin Kappa J Region (RBPJ) in satellite cells is a prerequisite for maintaining satellite cell quiescence [70, 72]. In mice lacking Notch1 and Notch2 in satellite cells, an inability to maintain quiescence can be observed, resulting in precocious entry into the cell cycle and in the end to a loss of satellite cells [73]. Cross talk of Notch signaling with the vascular niche is also important for regulating satellite cell quiescence—reminiscent of the role of cell adhesion molecules such as Cadherins, which control the transition from quiescence to activation through interaction with the myofiber niche [74, 75]. The importance of the interaction of Notch with the niche is further highlighted in a recent study by Baghdadi and colleagues who show that Notch-1/RBPJ controls expression of the extracellular matrix (ECM) molecule Collagen-V [76].
Regulation of satellite cell function by miRNAs
miRNAs are controlling multiple signaling pathways in satellite cells. For instance, posttranscriptional regulation by miRNAs has been associated with maintenance of quiescence, activation, and differentiation of satellite cells [77, 78]. The biogenesis of miRNAs involves transcription by Polymerase II and several processing steps of the hairpin containing primary transcript that require the Ribonuclease III enzymes Drosha and Dicer to generate a double-stranded miRNA precursor. Loading of the miRNA precursor to Argonaute (Ago) protein family forms the RNA-induced silencing complex (RISC) that facilitates the final maturation towards a 22 nucleotide short single-stranded miRNA and recruitment to target messenger RNAs [79]. The binding of a miRNA to its target mRNA leads to inhibition of translation followed by mRNA de-capping, de-adenylation, and degradation [80]. The general importance of miRNAs in mouse development was demonstrated by a global Dicer deletion which results in embryonic lethality at embryonic day 7.5 (ED7.5) [81]. In the adult, genetic deletion of Dicer in Pax7-CreER mice revealed a critical role for miRNAs in regulating satellite cell quiescence. Dicer-deficient satellite cells leave quiescence and enter the cell cycle, but are also prone to cell death [82]. Although knowledge of miRNA function in satellite cells and their progeny remains limited, the functions of particular miRNAs have recently been unravelled.
About 351 miRNAs are differentially expressed when comparing quiescent and activated satellite cells, underscoring their importance for activation of satellite cells [82]. For instance, it has been shown that myf5 mRNA is targeted by miR-31, which is highly expressed in quiescent satellite cells. In response to injury, miR-31 is quickly downregulated to allow a rapid translation of the Myf5 protein [83]. Furthermore, the cell cycle-promoting genes Dek, Ccnd2 (Cyclin D2), Cdc25a (Cell division cycle 25A), and Cdc25b1/2 (Cell division cycle 25B) are transcribed in quiescent satellite cells, but their translation is repressed by miR-489, miR-195, and miR-497 [82, 84]. Interestingly, transplantation of cultured satellite cells treated with miR-195/497 contributed more efficiently to regeneration of Dystrophin-deficient mice, suggestive of an increased ability to self-renew and to repopulate the stem cell compartment [84]. Surprisingly, only a few miRNAs have been identified, that are downregulated during differentiation, amongst them miR-125b targeting Insulin-like growth factor II (IGF-II) in myoblasts and miR-221 and miR-222 controlling cell cycle exit [85, 86]. Some microRNAs seem to be largely restricted to the skeletal muscle lineage and are, therefore, called myoMiRs. Of these, miR-1, miR-133 and miR-206 are muscle-specific miRNAs, which are strongly induced during differentiation. Their expression is directly regulated by the MRFs Myf5, MyoD, Myogenin, and Mef2, as well as Serum Response Factor (SRF) [87–90]. Expression of miR-1 is induced by MyoD and directly represses HDAC4 (Histon deacetylase 4), a negative regulator of skeletal muscle gene expression such as Mef2, thereby promoting myogenic differentiation [91]. Although miR-1 and miR-133 are transcribed as a bicistronic transcript, they exert different functions, e.g., miR-133 promotes proliferation of myoblasts by targeting SRF [91]. miRNAs control several signaling pathways important for satellite cell functionality. It has been demonstrated recently that components of the Sonic Hedgehog signaling pathway are controlled by miR-133, guiding the myogenic program during development [92]. Since the miR-206 has a very similar sequence to miR-1, it is assumed that they share the same targets. Consistently miR-206 also promotes differentiation in the myoblast cell line C2C12 cells by targeting HDAC4 [93]. The impact of miRNAs on cell fate decisions is further reflected by the function of miR-133 through the transcriptional regulator Prdm16 (PR domain containing 16), to regulate cell fate choices between the closely related muscle progenitors and brown adipocytes. Antagonism of miR-133 induces active brown adipocytes within regenerating skeletal muscle [94].
Changes in the cytoskeleton of myogenic cells during regeneration
Cytoskeletal rearrangements are essential for proper regeneration of injured skeletal muscle. In resting skeletal muscle, quiescent SCs express high levels of α7- and β1-Integrin, which interact with the extracellular matrix (ECM) and regulate satellite cell fate [95, 96]. Furthermore, the large protein superfamily of Integrins is essential for migration, assembly of the ECM and is mostly associated with focal adhesion sites [97–99]. The importance of proper signaling via Integrins can also be appreciated by the fact that β1-Integrin (Itgb1) signaling is required for activation of satellite cells via the fibroblast growth factor 2 (FGF2) interaction in aged and dystrophic mice [24]. Vice versa, depletion of Itgb1 from satellite cells results in a phenotype resembling aging of satellite cells including deficits in self-renewal and functionality [20, 100, 101]. Integrin-mediated signaling is transmitted by focal adhesion kinases (FAK) at focal adhesion sites, whereas Integrin heterodimers themselves serve as an adaptor for connecting the ECM with the actin cytoskeleton [102]. Binding of FAK to Integrins leads to autophosphorylation of FAK at Tyr397, which, in turn, creates docking sites for other proteins such as Talin [103]. Besides Integrins, work on the Dystroglycan complex revealed important functions of Dystrophin and Dystroglycan in activated satellite cells and asymmetric satellite stem cell divisions in concert with Par1b [45]. In 2002, Cohn et al. demonstrated a functional role for Dystroglycan in satellite cell maintenance and self-renewal [104]. In addition, binding of members of the Dystrophin-associated Glycoprotein Complex (DGC) to Laminin induces the interaction of the small GTPase Rac1 with the DGC, particularly with Syntrophin [105, 106]. Rac activity is important for satellite cell migration by improving motility of satellite cells, thereby enhancing muscle strength after regeneration. Notably, Rac1 activity is also regulated by non-canonical Wnt7/Fzd7 signaling and induces the assembly of the mitotic spindle to drive asymmetric division by interaction with the Par complex [56, 107].
Several studies showed that remodeling of the actin cytoskeleton is pivotal for myoblast fusion and myotube formation, processes crucial for skeletal muscle regeneration [108–110]. FAK has an essential role in myoblast fusion and differentiation through interaction with β1-Integrin in Integrin clusters associated with Vinculin and the actin-binding protein Talin [111–113]. The adaptor protein Paxillin is subsequently recruited to focal adhesion sites [114–117], followed by recruitment of the actin-bundling protein α-Actinin, leading to an increased number of focal adhesion sites [118]. Similar to the actin cytoskeleton, several studies have revealed the importance of microtubule dynamics for the maintenance and formation of skeletal muscle also in human patients [119–121].
Non-myogenic cells involved in regeneration of skeletal muscle
Although skeletal muscle regeneration is mainly driven by satellite cells and their ablation results in a failure to regenerate [2–4], several other cell types support the regeneration process. Those can be divided into two groups, those having myogenic potential such as myoendothelial cells, Pax7-negative Pw1+ interstitial cells (PICs) (PW1+/Pax7− interstitial cells) and Twist2+ cells, contributing directly to the generation of new muscle fibers and those indirectly supporting regeneration. The latter group includes the immune cells and the Fibro-Adipogenic-Progenitors (FAPs).
As described above, immune cells are the first cells attracted to the site of injury in skeletal muscle. They are involved in preparation for regeneration by clearing the skeletal muscle environment from cell debris. To date, several types of immune cells have been shown to be important for proper regeneration of skeletal muscle, e.g., eosinophils, neutrophils, and M1 and M2 macrophages, with the eosinophils being the major source of IL-4 [32, 46]. Knockdown of IL-4 impairs regeneration of skeletal muscle by affecting the fate of FAPs, thus affecting regeneration indirectly [52]. Neutrophils are also recruited within the first hours after injury. Noteworthy, their depletion leads to severely impaired regeneration, since they are important for regulating macrophage function [46, 122–124], which are found in close proximity to blood vessels in resting muscle [125, 126]. Macrophages can be separated into two functional categories, M1 and M2 macrophages. The cytolytic activity of M1 macrophages is promoted by signals from the neutrophils [46, 122–124]. During disease, e.g., chronic infection, the switch from inflammatory M1 towards anti-inflammatory M2 macrophages is prolonged, contributing to impaired muscle regeneration [127]. Depletion of macrophages restrains clearance of necrotic tissue and hampers regeneration by impairing proliferation and differentiation of satellite cells [51, 128].
The other non-myogenic cells important for regeneration of skeletal muscle are the FAPs, which are located in the interstitium and can be identified by expression of PDGFRα (platelet-derived growth factor receptor-α). These are quiescent in healthy muscle, but proliferate upon injury. FAPs are a bipotent cell population, capable of differentiation into adipocytes and fibroblasts. Differentiation into adipocytes is controlled by different factors such as nitric oxide (NO) [129]. However, undifferentiated FAPs can have positive effects on activated myoblasts. In vitro and in vivo studies show that undifferentiated FAPs can induce differentiation of activated myoblasts by secreting molecules like IL-6, IGF-1, Wnt1, Wnt3a, and Wnt5a [130, 131]. In addition, FAPs also control satellite cell activation and proliferation in vitro in fiber culture assays [132]. The positive effect of undifferentiated FAPs is controlled by signals from intact muscle fibers under homeostatic conditions preventing differentiation of FAPs into adipocytes [131, 133]; while differentiation of FAPs into adipocytes is inhibited during regeneration by secretion of IL-4 by the eosinophils [52]. Besides differentiating into adipocytes, FAPs contribute to disturbed regeneration in disease or during aging by differentiating into fibroblasts, leading to increased fibrosis through secretion of collagen type I [131]. Interestingly, under disease or chronic injury conditions, more PDGFRα+ cells are present in skeletal muscle, with some of them already differentiated into myofibroblasts [134]. The increased number of myofibroblasts might be due to changes in the expression profile of macrophages. This is supported by the finding that macrophages express TGFβ (transforming growth factor beta), thereby inducing differentiation of FAPs into fibroblasts under disease conditions, while, in healthy muscle, apoptosis is initiated through expression of TNFα (tumor necrosis factor alpha) in macrophages [135, 136].
The group of non-satellite cells with myogenic potential includes myoendothelial cells, PICs and Twist2+ cells. PW1+/Pax7− interstitial cells (PICs) were identified as a new population of cells having myogenic capacity [137]. In vitro studies showed that they start expressing Pax7/MyoD prior to formation of MHC-positive myotubes through fusion with other PICs or through fusion to satellite cell-derived myotubes [137, 138]. When transplanted into a regenerating muscle, PICs contribute to the formation of new muscle fibers at a level comparable to transplanted satellite cells [137, 139]. Not only do they contribute to muscle fiber formation during regeneration of skeletal muscle, but also secrete factors such as FGF-2 and IGF-1, known to promote satellite cell functionality [140]. Hence, PICs contribute to regeneration both directly and indirectly.
The Twist2+/Pax7− cells are located in the interstitium of the skeletal muscle. In vitro analyses showed that Twist2+ cells lose Twist2 expression and start to express Pax7 when differentiation is induced. These cells are able to fuse with each other and with satellite cells in vitro. In vivo experiments further demonstrated that they contribute to regeneration of skeletal muscle by fusing to existing myofibers, as well as initiating myofiber formation. However, during embryogenesis, Twist2+ cells do not contribute to development of skeletal muscle [141].
Concluding remarks and future perspectives
Satellite cells are the main drivers of skeletal muscle regeneration. The finely tuned balance between the states of quiescence, activation, and differentiation is a prerequisite for proper regeneration. Alongside cell intrinsic signaling, interactions with other cell types and the extracellular matrix play an important role in controlling these processes. In the future, the biggest challenge will be to gain a comprehensive understanding of how those processes interact and how they are altered in age and disease.
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft to J.v.M. (MA-3975/2-1) and from the Else-Kröner-Fresenius Stiftung to B.v.E. (2016_A58).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuel Schmidt and Svenja C. Schüler have contributed equally.
References
- 1.von Maltzahn J, Bentzinger CF, Rudnicki MA. Characteristics of satellite cells and multipotent adult stem cells in the skeletal muscle. Springer. 2013;12:63–73. [Google Scholar]
- 2.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 5.Chang NC, Rudnicki MA. Satellite cells: the architects of skeletal muscle. Curr Top Dev Biol. 2014;107:161–181. doi: 10.1016/B978-0-12-416022-4.00006-8. [DOI] [PubMed] [Google Scholar]
- 6.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez-Hernandez JM, Garcia-Gonzalez EG, Brun CE, Rudnicki MA. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin Cell Dev Biol. 2017;72:10–18. doi: 10.1016/j.semcdb.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/S0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 9.Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci USA. 2013;110:16474–16479. doi: 10.1073/pnas.1307680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shea KL, Xiang W, LaPorta VS, Licht JD, Keller C, Basson MA, Brack AS. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell. 2010;6:117–129. doi: 10.1016/j.stem.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2015;21:76–80. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakkalakal J, Brack A. Extrinsic regulation of satellite cell function and muscle regeneration capacity during aging. J Stem Cell Res Ther. 2012;Suppl 11:001. doi: 10.4172/2157-7633.S11-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J, Rudnicki MA. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat Med. 2014;20:1174–1181. doi: 10.1038/nm.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardi M, Ballestar E, Gonzalez S, Serrano AL, Perdiguero E, Munoz-Canoves P. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 19.Tierney MT, Aydogdu T, Sala D, Malecova B, Gatto S, Puri PL, Latella L, Sacco A. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med. 2014;20:1182–1186. doi: 10.1038/nm.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schworer S, Becker F, Feller C, Baig AH, Kober U, Henze H, Kraus JM, Xin B, Lechel A, Lipka DB, Varghese CS, Schmidt M, Rohs R, Aebersold R, Medina KL, Kestler HA, Neri F, von Maltzahn J, Tumpel S, Rudolph KL. Epigenetic stress responses induce muscle stem-cell ageing by Hoxa9 developmental signals. Nature. 2016;540:428–432. doi: 10.1038/nature20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahrens HE, Huettemeister J, Schmidt M, Kaether C, von Maltzahn J. Klotho expression is a prerequisite for proper muscle stem cell function and regeneration of skeletal muscle. Skelet Muscle. 2018;8:20. doi: 10.1186/s13395-018-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukjanenko L, Jung MJ, Hegde N, Perruisseau-Carrier C, Migliavacca E, Rozo M, Karaz S, Jacot G, Schmidt M, Li L, Metairon S, Raymond F, Lee U, Sizzano F, Wilson DH, Dumont NA, Palini A, Fassler R, Steiner P, Descombes P, Rudnicki MA, Fan CM, von Maltzahn J, Feige JN, Bentzinger CF. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat Med. 2016;22:897–905. doi: 10.1038/nm.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozo M, Li L, Fan CM. Targeting beta1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice. Nat Med. 2016;22:889–896. doi: 10.1038/nm.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA. Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 2013;14:1062–1072. doi: 10.1038/embor.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Sahu A, Mamiya H, Shinde SN, Cheikhi A, Winter LL, Vo NV, Stolz D, Roginskaya V, Tang WY, St Croix C, Sanders LH, Franti M, Van Houten B, Rando TA, Barchowsky A, Ambrosio F. Age-related declines in alpha-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat Commun. 2018;9:4859. doi: 10.1038/s41467-018-07253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunther S, Kim J, Kostin S, Lepper C, Fan CM, Braun T. Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell. 2013;13:590–601. doi: 10.1016/j.stem.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 30.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YX, Dumont NA, Rudnicki MA. Muscle stem cells at a glance. J Cell Sci. 2014;127:4543–4548. doi: 10.1242/jcs.151209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148:112–125. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 34.Shinin V, Gayraud-Morel B, Tajbakhsh S. Template DNA-strand co-segregation and asymmetric cell division in skeletal muscle stem cells. Methods Mol Biol. 2009;482:295–317. doi: 10.1007/978-1-59745-060-7_19. [DOI] [PubMed] [Google Scholar]
- 35.Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8:677–687. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- 36.Chakkalakal JV, Christensen J, Xiang W, Tierney MT, Boscolo FS, Sacco A, Brack AS. Early forming label-retaining muscle stem cells require p27kip1 for maintenance of the primitive state. Development. 2014;141:1649–1659. doi: 10.1242/dev.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tierney MT, Stec MJ, Rulands S, Simons BD, Sacco A. Muscle stem cells exhibit distinct clonal dynamics in response to tissue repair and homeostatic aging. Cell Stem Cell. 2018;22(119–127):e3. doi: 10.1016/j.stem.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12:75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen Y, Bi P, Liu W, Asakura A, Keller C, Kuang S. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol Cell Biol. 2012;32:2300–2311. doi: 10.1128/MCB.06753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conboy L, Seymour CM, Monopoli MP, O’Sullivan NC, Murphy KJ, Regan CM. Notch signalling becomes transiently attenuated during long-term memory consolidation in adult Wistar rats. Neurobiol Learn Mem. 2007;88:342–351. doi: 10.1016/j.nlm.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/S1534-5807(02)00254-X. [DOI] [PubMed] [Google Scholar]
- 43.Troy A, Cadwallader AB, Fedorov Y, Tyner K, Tanaka KK, Olwin BB. Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38alpha/beta MAPK. Cell Stem Cell. 2012;11:541–553. doi: 10.1016/j.stem.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palacios D, Mozzetta C, Consalvi S, Caretti G, Saccone V, Proserpio V, Marquez VE, Valente S, Mai A, Forcales SV, Sartorelli V, Puri PL. TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7:455–469. doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dumont NA, Wang YX, von Maltzahn J, Pasut A, Bentzinger CF, Brun CE, Rudnicki MA. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med. 2015;21:1455–1463. doi: 10.1038/nm.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc. 1995;27:1022–1032. doi: 10.1249/00005768-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am J Physiol. 1993;265:R166–R172. doi: 10.1152/ajpcell.1993.265.1.C166. [DOI] [PubMed] [Google Scholar]
- 48.Cantini M, Giurisato E, Radu C, Tiozzo S, Pampinella F, Senigaglia D, Zaniolo G, Mazzoleni F, Vitiello L. Macrophage-secreted myogenic factors: a promising tool for greatly enhancing the proliferative capacity of myoblasts in vitro and in vivo. Neurol Sci. 2002;23:189–194. doi: 10.1007/s100720200060. [DOI] [PubMed] [Google Scholar]
- 49.Chazaud B, Brigitte M, Yacoub-Youssef H, Arnold L, Gherardi R, Sonnet C, Lafuste P, Chretien F. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev. 2009;37:18–22. doi: 10.1097/JES.0b013e318190ebdb. [DOI] [PubMed] [Google Scholar]
- 50.Merly F, Lescaudron L, Rouaud T, Crossin F, Gardahaut MF. Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve. 1999;22:724–732. doi: 10.1002/(SICI)1097-4598(199906)22:6<724::AID-MUS9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 51.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31:384–396. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- 54.Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol. 2012;4:a008342. doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai CR, Goodell MA, Rando TA. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert) Nature. 2014;510:393–396. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bentzinger CF, von Maltzahn J, Dumont NA, Stark DA, Wang YX, Nhan K, Frenette J, Cornelison DD, Rudnicki MA. Wnt7a stimulates myogenic stem cell motility and engraftment resulting in improved muscle strength. J Cell Biol. 2014;205:97–111. doi: 10.1083/jcb.201310035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stark DA, Karvas RM, Siegel AL, Cornelison DD. Eph/ephrin interactions modulate muscle satellite cell motility and patterning. Development. 2011;138:5279–5289. doi: 10.1242/dev.068411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA. Wnt signaling in myogenesis. Trends Cell Biol. 2012;22:602–609. doi: 10.1016/j.tcb.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soleimani VD, Punch VG, Kawabe Y, Jones AE, Palidwor GA, Porter CJ, Cross JW, Carvajal JJ, Kockx CE, van Ijcken WF, Perkins TJ, Rigby PW, Grosveld F, Rudnicki MA. Transcriptional dominance of Pax7 in adult myogenesis is due to high-affinity recognition of homeodomain motifs. Dev Cell. 2012;22:1208–1220. doi: 10.1016/j.devcel.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh K, Dilworth FJ. Differential modulation of cell cycle progression distinguishes members of the myogenic regulatory factor family of transcription factors. FEBS J. 2013;280:3991–4003. doi: 10.1111/febs.12188. [DOI] [PubMed] [Google Scholar]
- 61.Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/S0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 62.Otto A, Schmidt C, Luke G, Allen S, Valasek P, Muntoni F, Lawrence-Watt D, Patel K. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci. 2008;121:2939–2950. doi: 10.1242/jcs.026534. [DOI] [PubMed] [Google Scholar]
- 63.von Maltzahn J, Zinoviev R, Chang NC, Bentzinger CF, Rudnicki MA. A truncated Wnt7a retains full biological activity in skeletal muscle. Nat Commun. 2013;4:2869. doi: 10.1038/ncomms3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Girardi F, Le Grand F. Wnt signaling in skeletal muscle development and regeneration. Prog Mol Biol Transl Sci. 2018;153:157–179. doi: 10.1016/bs.pmbts.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 65.von Maltzahn J, Bentzinger CF, Rudnicki MA. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat Cell Biol. 2011;14:186–191. doi: 10.1038/ncb2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Maltzahn J, Renaud JM, Parise G, Rudnicki MA. Wnt7a treatment ameliorates muscular dystrophy. Proc Natl Acad Sci USA. 2012;109:20614–20619. doi: 10.1073/pnas.1215765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lacour F, Vezin E, Bentzinger CF, Sincennes MC, Giordani L, Ferry A, Mitchell R, Patel K, Rudnicki MA, Chaboissier MC, Chassot AA, Le Grand F. R-spondin1 controls muscle cell fusion through dual regulation of antagonistic Wnt signaling pathways. Cell Rep. 2017;18:2320–2330. doi: 10.1016/j.celrep.2017.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huels DJ, Sansom OJ. R-spondin is more than just Wnt’s sidekick. Dev Cell. 2017;41:456–458. doi: 10.1016/j.devcel.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 69.Rudolf A, Schirwis E, Giordani L, Parisi A, Lepper C, Taketo MM, Le Grand F. Beta-catenin activation in muscle progenitor cells regulates tissue repair. Cell Rep. 2016;15:1277–1290. doi: 10.1016/j.celrep.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 70.Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012;30:243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- 71.Pisconti A, Cornelison DD, Olguin HC, Antwine TL, Olwin BB. Syndecan-3 and Notch cooperate in regulating adult myogenesis. J Cell Biol. 2010;190:427–441. doi: 10.1083/jcb.201003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30:232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujimaki S, Seko D, Kitajima Y, Yoshioka K, Tsuchiya Y, Masuda S, Ono Y. Notch1 and Notch2 coordinately regulate stem cell function in the quiescent and activated states of muscle satellite cells. Stem Cells. 2018;36:278–285. doi: 10.1002/stem.2743. [DOI] [PubMed] [Google Scholar]
- 74.Verma M, Asakura Y, Murakonda BSR, Pengo T, Latroche C, Chazaud B, McLoon LK, Asakura A. Muscle satellite cell cross-talk with a vascular niche maintains quiescence via VEGF and notch signaling. Cell Stem Cell. 2018;23(530–543):e9. doi: 10.1016/j.stem.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goel AJ, Rieder MK, Arnold HH, Radice GL, Krauss RS. Niche cadherins control the quiescence-to-activation transition in muscle stem cells. Cell Rep. 2017;21:2236–2250. doi: 10.1016/j.celrep.2017.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baghdadi MB, Castel D, Machado L, Fukada SI, Birk DE, Relaix F, Tajbakhsh S, Mourikis P. Reciprocal signalling by Notch-Collagen V-CALCR retains muscle stem cells in their niche. Nature. 2018;557:714–718. doi: 10.1038/s41586-018-0144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mok GF, Lozano-Velasco E, Munsterberg A. microRNAs in skeletal muscle development. Semin Cell Dev Biol. 2017;72:67–76. doi: 10.1016/j.semcdb.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Yang LZ, Zhang JS, Gong JX, Wang YH, Zhang CL, Chen H, Fang XT. Effects of microRNAs on skeletal muscle development. Gene. 2018;668:107–113. doi: 10.1016/j.gene.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 79.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 80.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 81.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 82.Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, Yoo B, Hoang P, Rando TA. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482:524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crist CG, Montarras D, Buckingham M. Muscle satellite cells are primed for myogenesis but maintain quiescence with sequestration of Myf5 mRNA targeted by microRNA-31 in mRNP granules. Cell Stem Cell. 2012;11:118–126. doi: 10.1016/j.stem.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 84.Sato T, Yamamoto T, Sehara-Fujisawa A. miR-195/497 induce postnatal quiescence of skeletal muscle stem cells. Nat Commun. 2014;5:4597. doi: 10.1038/ncomms5597. [DOI] [PubMed] [Google Scholar]
- 85.Ge Y, Sun Y, Chen J. IGF-II is regulated by microRNA-125b in skeletal myogenesis. J Cell Biol. 2011;192:69–81. doi: 10.1083/jcb.201007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cardinali B, Castellani L, Fasanaro P, Basso A, Alema S, Martelli F, Falcone G. Microrna-221 and microrna-222 modulate differentiation and maturation of skeletal muscle cells. PLoS One. 2009;4:e7607. doi: 10.1371/journal.pone.0007607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu N, Williams AH, Kim Y, McAnally J, Bezprozvannaya S, Sutherland LB, Richardson JA, Bassel-Duby R, Olson EN. An intragenic MEF2-dependent enhancer directs muscle-specific expression of microRNAs 1 and 133. Proc Natl Acad Sci USA. 2007;104:20844–20849. doi: 10.1073/pnas.0710558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rao PK, Kumar RM, Farkhondeh M, Baskerville S, Lodish HF. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci USA. 2006;103:8721–8726. doi: 10.1073/pnas.0602831103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sweetman D, Goljanek K, Rathjen T, Oustanina S, Braun T, Dalmay T, Munsterberg A. Specific requirements of MRFs for the expression of muscle specific microRNAs, miR-1, miR-206 and miR-133. Dev Biol. 2008;321:491–499. doi: 10.1016/j.ydbio.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 90.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 91.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mok GF, Lozano-Velasco E, Maniou E, Viaut C, Moxon S, Wheeler G, Munsterberg A. miR-133-mediated regulation of the Hedgehog pathway orchestrates embryo myogenesis. Development. 2018;145:dev159657. doi: 10.1242/dev.159657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Winbanks CE, Wang B, Beyer C, Koh P, White L, Kantharidis P, Gregorevic P. TGF-beta regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J Biol Chem. 2011;286:13805–13814. doi: 10.1074/jbc.M110.192625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, Thorn S, Seale P, Fernando P, van Ijcken W, Grosveld F, Dekemp RA, Boushel R, Harper ME, Rudnicki MA. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 2013;17:210–224. doi: 10.1016/j.cmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feige P, Brun CE, Ritso M, Rudnicki MA. Orienting muscle stem cells for regeneration in homeostasis. Aging Dis Cell Stem Cell. 2018;23:653–664. doi: 10.1016/j.stem.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mashinchian O, Pisconti A, Le Moal E, Bentzinger CF. The muscle stem cell Niche in health and disease. Curr Top Dev Biol. 2018;126:23–65. doi: 10.1016/bs.ctdb.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 97.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Ann Rev Cell Dev Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 98.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 99.Wu C, Keivenst VM, O’Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 100.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 101.Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, Olwin BB. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med. 2014;20:265–271. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta (BBA) Mol Cell Res. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 103.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophysca Acta (BBA) Mol Cell Res. 2001;1540:1–21. doi: 10.1016/S0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 104.Cohn RD, Henry MD, Michele DE, Barresi R, Saito F, Moore SA, Flanagan JD, Skwarchuk MW, Robbins ME, Mendell JR, Williamson RA, Campbell KP. Disruption of Dag1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell. 2002;110:639–648. doi: 10.1016/S0092-8674(02)00907-8. [DOI] [PubMed] [Google Scholar]
- 105.Oak SA, Zhou YW, Jarrett HW. Skeletal muscle signaling pathway through the dystrophin glycoprotein complex and Rac1. J Biol Chem. 2003;278:39287–39295. doi: 10.1074/jbc.M305551200. [DOI] [PubMed] [Google Scholar]
- 106.Charrasse S, Causeret M, Comunale F, Bonet-Kerrache A, Gauthier-Rouviere C. Rho GTPases and cadherin-based cell adhesion in skeletal muscle development. J Muscle Res Cell Motil. 2003;24:309–313. doi: 10.1023/A:1025429924231. [DOI] [PubMed] [Google Scholar]
- 107.Kim SK. Cell polarity: new PARtners for Cdc42 and Rac. Nat Cell Biol. 2000;2:E143. doi: 10.1038/35019620. [DOI] [PubMed] [Google Scholar]
- 108.Kim S-J, Kim S, Shin H, Uhm C-S. Intercellular interaction observed by atomic force microscopy. Ultramicroscopy. 2008;108:1148–1151. doi: 10.1016/j.ultramic.2008.04.081. [DOI] [PubMed] [Google Scholar]
- 109.Nowak SJ, Nahirney PC, Hadjantonakis A-K, Baylies MK. Nap1-mediated actin remodeling is essential for mammalian myoblast fusion. J Cell Sci. 2009;122:3282. doi: 10.1242/jcs.047597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pajcini KV, Pomerantz JH, Alkan O, Doyonnas R, Blau HM. Myoblasts and macrophages share molecular components that contribute to cell–cell fusion. J Cell Biol. 2008;180:1005. doi: 10.1083/jcb.200707191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Quach NL, Biressi S, Reichardt LF, Keller C, Rando TA. Focal adhesion kinase signaling regulates the expression of caveolin 3 and β1 integrin, genes essential for normal myoblast fusion. Mol Biol Cell. 2009;20:3422–3435. doi: 10.1091/mbc.e09-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee H-S, Bellin RM, Walker DL, Patel B, Powers P, Liu H, Garcia-Alvarez B, de Pereda JM, Liddington RC, Volkmann N, Hanein D, Critchley DR, Robson RM. Characterization of an actin-binding site within the Talin FERM domain. J Mol Biol. 2004;343:771–784. doi: 10.1016/j.jmb.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 113.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin β tails: a final common step in integrin activation. Science. 2003;302:103. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 114.Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of α5 integrin, paxillin, and α-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wiseman PW, Brown CM, Webb DJ, Hebert B, Johnson NL, Squier JA, Ellisman MH, Horwitz AF. Spatial mapping of integrin interactions and dynamics during cell migration by image correlation microscopy. J Cell Sci. 2004;117:5521. doi: 10.1242/jcs.01416. [DOI] [PubMed] [Google Scholar]
- 116.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK–Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 117.Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179:1043–1057. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:1039. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kellie HB. Colchicine-induced rhabdomyolysis. Ann Pharmacother. 2002;36:824–826. doi: 10.1345/aph.1A288. [DOI] [PubMed] [Google Scholar]
- 120.Caglar K, Odabasi Z, Safali M, Yenicesu M, Vural A. Colchicine-induced myopathy with myotonia in a patient with chronic renal failure. Clin Neurol Neurosurg. 2003;105:274–276. doi: 10.1016/S0303-8467(03)00030-1. [DOI] [PubMed] [Google Scholar]
- 121.Wilbur K, Makowsky M. Colchicine myotoxicity: case reports and literature review. Pharmacotherapy. 2004;24:1784–1792. doi: 10.1592/phco.24.17.1784.52334. [DOI] [PubMed] [Google Scholar]
- 122.Nguyen HX, Tidball JG. Interactions between neutrophils and macrophages promote macrophage killing of rat muscle cells in vitro. J Physiol. 2003;547:125–132. doi: 10.1113/jphysiol.2002.031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schwab N, Waschbisch A, Wrobel B, Lochmuller H, Sommer C, Wiendl H. Human myoblasts modulate the function of antigen-presenting cells. J Neuroimmunol. 2008;200:62–70. doi: 10.1016/j.jneuroim.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 124.Teixeira CF, Zamuner SR, Zuliani JP, Fernandes CM, Cruz-Hofling MA, Fernandes I, Chaves F, Gutierrez JM. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle Nerve. 2003;28:449–459. doi: 10.1002/mus.10453. [DOI] [PubMed] [Google Scholar]
- 125.Honda H, Kimura H, Rostami A. Demonstration and phenotypic characterization of resident macrophages in rat skeletal muscle. Immunology. 1990;70:272–277. [PMC free article] [PubMed] [Google Scholar]
- 126.Krippendorf BB, Riley DA. Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle Nerve. 1993;16:99–108. doi: 10.1002/mus.880160116. [DOI] [PubMed] [Google Scholar]
- 127.Jin RM, Warunek J, Wohlfert EA. Chronic infection stunts macrophage heterogeneity and disrupts immune-mediated myogenesis. JCI Insight. 2018;3:121549. doi: 10.1172/jci.insight.121549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Segawa M, Fukada S, Yamamoto Y, Yahagi H, Kanematsu M, Sato M, Ito T, Uezumi A, Hayashi S, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp Cell Res. 2008;314:3232–3244. doi: 10.1016/j.yexcr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 129.Cordani N, Pisa V, Pozzi L, Sciorati C, Clementi E. Nitric oxide controls fat deposition in dystrophic skeletal muscle by regulating fibro-adipogenic precursor differentiation. Stem Cells. 2014;32:874–885. doi: 10.1002/stem.1587. [DOI] [PubMed] [Google Scholar]
- 130.Dammone G, Karaz S, Lukjanenko L, Winkler C, Sizzano F, Jacot G, Migliavacca E, Palini A, Desvergne B, Gilardi F, Feige JN. PPARgamma controls ectopic adipogenesis and cross-talks with myogenesis during skeletal muscle regeneration. Int J Mol Sci. 2018;19:E2044. doi: 10.3390/ijms19072044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fiore D, Judson RN, Low M, Lee S, Zhang E, Hopkins C, Xu P, Lenzi A, Rossi FM, Lemos DR. Pharmacological blockage of fibro/adipogenic progenitor expansion and suppression of regenerative fibrogenesis is associated with impaired skeletal muscle regeneration. Stem Cell Res. 2016;17:161–169. doi: 10.1016/j.scr.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 133.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 134.Contreras O, Rebolledo DL, Oyarzun JE, Olguin HC, Brandan E. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res. 2016;364:647–660. doi: 10.1007/s00441-015-2343-0. [DOI] [PubMed] [Google Scholar]
- 135.Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21:786–794. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 136.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 137.Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 138.Lewis FC, Henning BJ, Marazzi G, Sassoon D, Ellison GM, Nadal-Ginard B. Porcine skeletal muscle-derived multipotent PW1pos/Pax7neg interstitial cells: isolation, characterization, and long-term culture. Stem Cells Transl Med. 2014;3:702–712. doi: 10.5966/sctm.2013-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cottle BJ, Lewis FC, Shone V, Ellison-Hughes GM. Skeletal muscle-derived interstitial progenitor cells (PICs) display stem cell properties, being clonogenic, self-renewing, and multi-potent in vitro and in vivo. Stem Cell Res Ther. 2017;8:158. doi: 10.1186/s13287-017-0612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lewis FC, Cottle BJ, Shone V, Marazzi G, Sassoon D, Tseng CCS, Dankers PYW, Chamuleau SAJ, Nadal-Ginard B, Ellison-Hughes GM. Transplantation of allogeneic PW1(pos)/Pax7(neg) interstitial cells enhance endogenous repair of injured porcine skeletal muscle. JACC Basic Transl Sci. 2017;2:717–736. doi: 10.1016/j.jacbts.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu N, Garry GA, Li S, Bezprozvannaya S, Sanchez-Ortiz E, Chen B, Shelton JM, Jaichander P, Bassel-Duby R, Olson EN. A Twist2-dependent progenitor cell contributes to adult skeletal muscle. Nat Cell Biol. 2017;19:202–213. doi: 10.1038/ncb3477. [DOI] [PMC free article] [PubMed] [Google Scholar]