Abstract
In the present study, the Cicer arietinum (chickpea) rhizosphere bacterial strains Azotobacter chroococcum (AU-1), Bacillus subtilis (AU-2), Pseudomonas aeruginosa (AU-3) and Bacillus pumilis (AU-4) were isolated and characterized for plant growth-promoting traits with an aim of developing bio-fertilizing agent to improve growth and yield of chickpea plants under normal conditions. The ACC degrading potential of strains AU-1, AU-2, AU-3, and AU-4 was in the range of 600–1700 nmol α-ketobutyrate per mg of cellular protein per hour, respectively. These four rhizobacteria exhibited Indole acetic acid production approximately between 20 and 35.34 µg/ml. The phosphate solubilization potential was in the range of 78–87.64 mg Soluble P/L with maximum solubilization displayed by strains P. aeruginosa and B. pumilis. All the growth-promoting isolates displayed Fe-chelating siderophore and ammonia production while no isolate was able to produce hydrocyanic acid. Besides evaluating the presence of multifaceted in vitro plant growth-promoting traits, these four rhizobacterial isolates were halotolerant as well as water stress (drought) tolerant of up to − 1.2 Mpa of PEG 6000. The optimum pH and temperature for their growth were found to be pH 7 and 30 °C temperature. Under normal conditions, inoculation with formulated bacterial consortia significantly improved the (P ≤ 0.05) germination index, plant height, leaf area index, stem diameter, and chlorophyll content by ~ 50%, 100%, 63%, 185%, and 63%, respectively, as compared to uninoculated chickpea plants. The consortia of halotolerant and drought tolerant bacterial strains were shown to exert a positive impact on the growth of chickpea plants under normal conditions.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1809-2) contains supplementary material, which is available to authorized users.
Keywords: ACC deaminase, Biofertilizer, Indole acetic acid, PGPR, Rhizosphere, ACC deaminase
Introduction
The increasing demand of crop production to feed the growing population with minimum use of synthetic chemical inputs has become a major challenge of today’s agriculture. The intensive use of synthetic agrochemicals has adversely affected the environment and soil fertility, leading to substantial loss in crop production (Mahmood et al. 2015). The agricultural productivity is also degraded by various climatic and biotic stressors such as drought, flooding, salinity, heavy metals contamination and nutrient deficiencies. Plants being sessile, when confronted to diverse forms of abiotic stress, an increase in concentration of phytohormone, ethylene and its precursor 1-aminocyclopropane-1-carboxylic acid (ACC) was observed. This climatic stress-generated ethylene reduced the root and shoot growth and if not monitored properly could even lead to plant death (Kazan 2015; Gamalero and Glick 2015). The rhizosphere is the narrow region of soil that is directly influenced by root secretions (or exudates such as organic acids, amino acids, carbohydrates) and micro-organisms associated with roots (Ali et al. 2017). Bacteria belonging to diverse array of genus such as Bacillus, Azotobacter, Pseudomonas, Azospirillium, Burkholderia, and Enterobacter are reported as root-colonizing PGPRs enhancing plant growth and development. They stimulate the plant growth by employing plethora of growth-promoting mechanism classified into direct and indirect categories. They directly promote nutrient resource procurement (nitrogen, phosphorous, potassium, zinc and other essential nutrients) through nitrogen fixation, insoluble phosphate and zinc solubilization, sequestration of iron from surrounding through siderophore production and modulate phytohormone level through indole acetic acid, gibberellins and cytokinin production as well as reduce stress-generated ethylene levels and its associated growth-reducing adverse effects on plants through ACC deaminase activity, or indirectly reduce inhibitory effects of pathogens as biocontrol agents (Bhattacharyya and Jha 2012; Glick 2012; Egamberdieva and Lugtenberg 2014).
The introduction of root-associated, free-living plant growth-promoting bacterial inoculants under field conditions has garnered significant attention as an environmentally safe and cost-effective approach in ameliorating the negative impact of synthetic agrochemicals manifested on plant growth. Besides this, they also strengthen the plant tolerance against adverse climatic conditions and suppress the phytopathogens. Therefore, their role in sustainable agriculture is highly significant. There exist various published literatures suggesting plant growth rhizobacteria is the better alternative to conventional cultivation practices in enhancing overall yield under stressed and non-stressed conditions and attaining organic sustainable agriculture (Ali and Kim 2018; Bharti and Barnawal 2019; Saleem et al. 2018).Therefore, the objective of the present study was to isolate the rhizospheric bacteria from chickpea (Cicer arietinum) plant and screen them for plant growth-promoting characteristics like production of ACC deaminase, IAA, siderophore, HCN (Hydrocyanic acid) and NH3 (Ammonia), phosphate solubilization and ability to provide tolerance against drought and salinity under in vitro condition. In vivo evaluation of the isolated microbes for their plant growth-promoting abilities was tested on chickpea (C. arietinum) plants under normal field conditions with pot experimental trials.
Materials and methods
Collection of rhizosphere soil samples
The soil samples were collected randomly during the month of May 2016 from rhizospheric soil of C. arietinum (chickpea), member of leguminous family Fabaceae grown in organic farm of Amity Institute of Organic Agriculture, Noida, Uttar Pradesh, India (28.3239°N 77.1959°E). The field was divided equally into 5 units based on the visual observation. The soil samples were collected by uprooting 5 plants from each unit. The soil adhering to the plants root system was removed and pooled together to form one composite sample of rhizospheric soil. The plant material and coarse roots were removed from the soil and stored at 4 °C for further analysis.
Isolation of rhizospheric bacteria
For isolation of rhizospheric bacteria, 10 gm of rhizospheric soil was suspended in 90 ml of sterilized distilled water. The soil suspension was serially diluted by shaking it for 10 min and dilution was made up to 10−10. 0.1 ml of appropriate dilution was plated on the nutrient agar (NA) media (g/L: Peptone-5.0 g, Beef extract-3.0 g, Agar-15.0 g, pH 7). The plates were incubated at 28 °C for 2–3 days. The morphologically distinct colonies were purified by sub-culturing the isolates and selected for further analysis of plant growth-promoting characteristics.
Molecular identification of bacterial isolates
Based on ACC deaminase activity and other plant growth-promoting (PGP) characteristics, molecular characterization of four bacterial isolates was done based on 16S rRNA gene sequencing. The genomic DNA of four isolates was isolated and amplified in a polymerase chain reaction (PCR) using universal 16S rRNA gene primers pA (5′-AGAGTTTGATCCTGGCTCAG-3′) and pH (5′-AAGGAGGTGATCCAGCCGCA-3′) to obtain approximately 1500 bp product as per standardized protocol (Edwards et al. 1989).
The nucleotide sequences so generated were compared using National Centre of Biotechnology Information (NCBI) BLAST method (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and deposited in NCBI GenBank database. The phylogenetic tree was constructed by Neighbour-joining (NJ) method using software MEGA X with the bootstrap of 1000 replicates and evolutionary distances were computed.
In vitro screening of bacterial isolates for their plant growth promoting (PGP) traits
The rhizobacterial isolates were further assessed for their potential of multifaceted plant growth-promoting characteristics such as ACC deaminase activity, Indole acetic acid production, Tricalcium phosphate solubilization, Fe-chelating siderophore production, Ammonia and HCN production.
The ACC deaminase activity of rhizobacterial isolates was assessedusing sterile minimal DF (Dworkin and Foster 1958) salts media (DF salts per litre: 4.0 g KH2PO4, 6.0 g Na2HPO4, 0.2 g MgSO4.7H2O, 2.0 g Glucose, 2.0 g Gluconic acid and 2.0 g Citric acid with trace elements: 1 mg FeSO4.7H2O, 10 mg H3BO3, 11.19 mg MnSO4.H2O, 124.6 mg ZnSO4.7H2O, 78.22 mg CuSO4.5H2O, 10 mg MoO3, pH 7.2) supplemented with 3 mM ACC as per standardized protocol (Penrose and Glick 2003). The quantitative evaluation of ACC deaminase activity of these four isolates was done in terms of α-ketobutyrate production at 540 nm by comparing with the standard curve of α-ketobutyrate ranging from 0.1 to 1.0 µmol and expressed as the amount of α-ketobutyrate produced in nmol per milligram of cellular protein per hour (Honma and Shimomura 1978). The protein estimation was done as per Bradford methodology (Bradford 1976). The production of IAA of isolates was analyzed spectrophotometrically using Salkowski’s reagent (35% perchloric acid + 0.5 M FeCl3) at 530 nm. The amount of IAA was calculated with the help of standard curve of pure Indole acetic acid (IAA¸ Hi-MEDIA) obtained in the range of 5–50 µg/ml (Ahmad et al. 2008). For phosphate solubilization assay, preliminary screening was done on Pikovaskya’s agar plates supplemented with 2% (w/v) insoluble inorganic Tricalcium phosphate (Ca3(PO4)2, Hi Media) (Nautiyal 2009). Furthermore, the solubilized phosphate (Soluble P mg/L) was quantified in NBRIP medium as per Fiske and Subbarow 1925. All measurements were performed in triplicate and compared with standard curve of KH2PO4 (HI MEDIA). Estimation of ammonia by bacterial isolates was performed using Nesslar’s reagent in accordance with Ahmad et al. (2008). For HCN production, the selected bacterial isolates were streaked on nutrient agar medium supplemented with 0.4% glycine. A Whatman filter paper soaked in picrate solution (2% Na2CO3 + 0.5% picric acid) was placed on the upper lids of Petri plates and monitored for 4 days for the development of orange to red color which indicated cyanogenic activity of isolates (Lorck 1948). The production of siderophores by bacterial isolates was assayed on the Chrome Azurol S (CAS) agar medium as described by Schwyn and Neilands (1987). Development of orange-yellow halo around the growth was considered as positive siderophore producing organisms.
Effect of pH and temperature on growth characteristics of plant growth-promoting rhizobacterial isolates
The growth of four rhizobacterial isolates was optimized at various temperatures and pH of the growth medium. The bacterial population was adjusted to 2 × 105 per ml in 50 ml Tryptic soy broth (TSB) medium and incubated at temperature 30 °C, 40 °C, 50 °C, 60 °C and 70 °C for 24 h. The absorbance of aliquots of 1 ml bacterial culture was read at 600 nm in a spectrophotometer.
Further, to assess the effect of pH on the growth of PGP rhizobacterial isolates, the Tryptic soy broth (TSB) medium was amended to different pH from acidic to alkaline range (5, 6, 7, 8, 9). After overnight incubation, the intensity of growth was measured at 600 nm in a spectrophotometer.
In vitro stress tolerance profile of isolates in response to drought and salinity
Salinity stress tolerance
The salt tolerance ability of isolates was evaluated by streaking the bacterial isolates on Luria–Bertani (LB) agar medium supplemented with different salt (NaCl) concentrations (1%, 2%, 5%, 7% and 10%) and incubated at 28 °C for 72 h.
Drought stress tolerance
The drought or water deficit stress tolerance PGP rhizobacterial isolates was observed in TSB media supplemented with 32.6% of polyethylene glycol (PEG-6000) (for inducing osmotic pressure of − 1.2 Mpa) at 28 °C for 24 h (Michel and Kaufmann 1973).
Pot experiment: plant growth promotion assay
A pot experiment was performed to analyze the influence of four selected putative plant growth-promoting rhizobacteria on the growth of C. arietinum (chickpea) plants under normal conditions (Penrose and Glick 2003).
The selected four potential bacterial cultures were inoculated in DF salt minimal medium amended with 3 mM ACC as sole nitrogen source to induce ACC deaminase activity of isolates, incubated at 180 rpm for 48 h and harvested by centrifugation at 12,000 rpm for 15 min. The cell pellet so obtained was suspended in 0.03 M MgSO4 (108 cfu per ml). The consortia were prepared by mixing all the four bacterial suspension of strains AU1, AU-2, AU-3 and AU-4 in the ratio of 1:1. The seeds of C. arietinum (chickpea) procured from Indian Agricultural Research Institute (IARI), New Delhi were first surface sterilized in 70% ethanol followed by 1% sodium hypochlorite and subsequently washed several times with sterile distilled water. For biopriming, the seeds were submerged in the bacterial suspension for 1 h and then air dried in the laminar air flow. The surface-sterilized, uninoculated seeds served as control group. The seeds were then sown in 8″ garden plastic pots at an average of 3 seeds/pot containing 3.2 kg sand–soil mixture in 1:2 ratio. We tested 5 treatments as per: T0—control group with uninoculated seeds: T1—AU-1 inoculated; T2—AU-2 inoculated; T3—AU-3 inoculated; T4—AU-4 inoculated; T5—Consortia (AU-1 + AU-2 + AU-3 + AU-4) inoculated. The pots were arranged randomly with three replicates per treatment in field under natural condition without any application of fertilizer. Water was applied regularly to the plants at 24-h intervals. The different growth parameters like germination percentage, plant height, leaf area index and chlorophyll content were recorded after 30 days of germination.
Statistical analyses
All the data regarding quantitative estimation of PGP traits were subjected to one-way ANOVA followed by Tukey’s test. All the statistical analyses were carried out with help of SPSS software. The experiments were performed in three replicates and the mean as well as standard deviation was calculated using Microsoft Excel 2016.
Results
Isolation and preliminary screening of bacteria for ACC deaminase activity
A total of 10 rhizobacterial isolates were successfully isolated from the rhizospheric soil of Chickpea using nutrient agar medium as pure colonies based on differences in morphology. Out of ten, four bacterial isolates, AU-1, AU-2, AU-3 and AU-4, were selected for further PGP characteristics and plant growth-promotion experiments based on their growth on selective media, i.e. DF media supplemented with 3 mM ACC as nitrogen source. Sub-culturing of isolates on DF-ACC agar medium confirmed them as ACC degraders or ACC deaminase producers.
Molecular identification and phylogenetic analysis
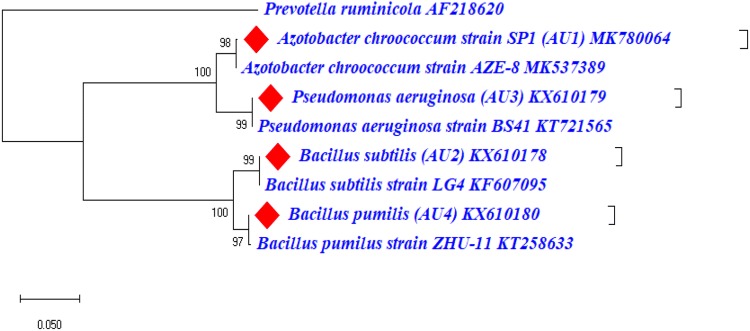
The identity of four PGPR isolates AU-1, AU-2, AU-3 and AU-4 was identified using 16S rRNA gene sequencing as Azotobacter chroococcum, Bacillus subtilis, Pseudomonas aeruginosa and Bacillus pumilis showed sequence similarity with gene sequences of Azotobacter, Bacillus and Pseudomonas sp. The 16S rRNA gene sequence of AU-1, AU-2, AU-3 and AU-4 has been submitted to NCBI GenBank database under the accession numbers MK780064, KX610178 KX610179 and KX610180, respectively. The Phylogenetic analysis of these 4 PGPR strains using MEGA X software revealed their relatedness with other strains of respective species (Fig. 1).
Fig. 1.
Phylogenetic tree based on partial 16S rRNA nucleotide sequences showing relationship between four PGPR strains AU-1, AU-2, AU-3, AU-4 and related strains of genus Azotobacter, Bacillus and Pseudomonas. The 16S rRNA gene sequences of closely related species were retrieved from NCBI GenBank databases. The Neighbor joining phylogenetic tree was inferred using MEGA-X software; evolutionary distance was computed using Maximum Composite Likelihood method at bootstrap value of 1000. Numbers at nodes indicate bootstrap percentages and only values above 95% are shown
Screening for plant growth-promoting (PGP) traits
As per quantitative assay, the ACC deaminase activity of strains AU-1, AU-2, AU-3 and AU-4 was determined as 941.496 ± 8.50, 1710 ± 6.89, 1607 ± 9.19 and 933.539 ± 4.64 nmol α-ketobutyrate mg/protein h, respectively (Fig. 2). All the isolates were able to solubilize inorganic complex of phosphorous-forming clear yellow zone of solubilization around spot-inoculated colonies on Pikovaskya’s agar amended with 2% tri-calcium phosphate ((Ca3(PO4)2, TCP). The amount of phosphate solubilized by isolates was quantified in NBRIP medium, revealing that strains AU3 and AU4 solubilized significantly (P < 0.05) higher amount as 87.34 ± 1.69 and 87.67 ± 1.68, respectively, in comparison to that of other strains AU2 (82 ± 1.63) and AU1 (78 ± 2.16). The isolates were able to produce indole acetic acid as phytohormone in the range between 20 and 35 µg/ml with AU-2 being maximum IAA producer among the four strains (Fig. 2). In addition to this, all the isolates were able to chelate iron from surrounding environment by producing iron-chelating siderophores on Chrome Azurol S agar medium, significantly changing its blue color to greenish color as shown in Fig. 3. Moreover, the isolates were positive for other indirect growth-promoting traits such as ammonia production and producing dark brown color on addition of Nessler reagent. However, the isolates were not able to turn yellow picrate filter paper into red brown, negative for Hydrocyanic acid production (Table 1).
Fig. 2.
The plant growth promoting features of four isolates AU-1, AU-2, AU-3 and AU-4 from Cicer arietinum rhizospheric soil. Panel A showing quantative determination of ACC deaminase activity, IAA production and phosphate solubilization whereas Panel B represent qualitative estimation of phosphate solubilization on Pikovaskya’s agar with 2% Ca3(PO4)2 plates and siderophore production on CAS agar plate. Columns represent Mean values while bars represent Standard deviation (n = 3); Different letters shows statistically significant different values (P < 0.05) from each other as evaluated from Turkey’s test
Fig. 3.
Effect of pH and temperature on the growth of four plant growth-promoting rhizobacterial isolates from Cicer arietinum rhizospheric soil. Columns represent Mean values while bars represent Standard deviation (n = 3)
Table 1.
The molecular and functional characterization of selected plant growth-promoting rhizobacterial isolates from chickpea (Cicer arietinum) plants
| Strains | Closest species | Accession number | IAA production (µg/ml) | Phosphate solubilization (mg Soluble P/L) | ACC deaminase activity (nmol α-ketobutyrate mg protein−1 h−1) |
|---|---|---|---|---|---|
| AU-1 | Azotobacter chroococcum group | MK780064 | 23 | 78 ± 2.16 | 941.496 ± 8.50 |
| AU-2 | Bacillus subtilis group | KX610178 | 35.34 | 82 ± 1.63 | 1710 ± 6.89 |
| AU-3 | Pseudomonas aeruginosa group | KX610179 | 25.34 | 87.34 ± 1.69 | 1607 ± 9.19 |
| AU-4 | Bacillus pumilus group | KX610180 | 30.67 | 87.67 ± 1.68 | 933.539 ± 4.64 |
All strains were producing siderophore and ammonia but not able to produce HCN
Temperature and pH optimization studies of plant growth promoting rhizobacteria
The effect of pH ranged between 5 and 9 and temperatures over the range 30–70 °C on the growth characteristics of four putative ACC deaminase-producing PGP rhizobacterial isolates were monitored as shown in Fig. 3. According to the results obtained, the optimum pH and temperature were found to be pH 7 and 30 °C, respectively, for the maximum growth of these four ACC deaminase-producing isolates.
In vitro stress tolerance profile of isolates in response to drought and salinity
In case of salinity, the isolates AU-2 and AU-4 were able to withstand the NaCl concentration of 7% (w/v) in the growth medium while other two isolates exhibited growth with 2% NaCl-supplemented media. No isolates could tolerate high concentration of 10% (w/v) NaCl in growth media. The AU-3 isolate was able to tolerate the osmotic stress of − 1.2 Mpa PEG 6000 while others were not able to tolerate drought conditions artificially imposed by PEG 6000.
Pot experiment: plant growth promotion assay in normal field conditions
These four potent ACC deaminase-producing plant growth-promoting rhizobacteria were evaluated for growth-promoting attributes by pot experiments. The experiment was done with surface-sterilized chickpea (C. arietinum) seeds (Fig. S1). It was found that consortia (AU-1 + AU-2 + AU-3 + AU-4) treated seeds showed considerable increment in germination percentage, Plant height, Leaf area index, Stem diameter as well as in chlorophyll content of chickpea seedlings as compared to uninoculated (control) seeds as well as individually bioprimed seeds (Table 2).
Table 2.
Effect of ACC deaminase-producing rhizobacterial isolates on physio-morphological and biochemical parameters of chickpea (Cicer arietinum) plants
| Treatment details | Germination percentage (%) | Plant height (cm) | Leaf Area Index | Stem diameter (mm) | Chlorophyll content (mg g/FW) |
|---|---|---|---|---|---|
| Control (unprimed seeds) | 61.00 ± 0.81a | 19.33 ± 0.94a | 1.63 ± 0.18a | 0.34 ± 0.02a | 0.77 ± 0.07a |
| AU-1 inoculated | 88.91 ± 0.04b | 23.87 ± 1.14b | 1.89 ± 0.07b | 0.39 ± 0.08a | 0.86 ± 0.01b |
| AU-2 inoculated | 88.88 ± 9.07c | 33.97 ± 1.21c | 1.97 ± 0.02b | 0.69 ± 0.06b | 0.93 ± 0.08c |
| AU-3 inoculated | 77.78 ± 9.06b | 29.05 ± 1.22d | 1.72 ± 0.04a | 0.61 ± 0.03c | 0.82 ± 0.02d |
| AU-4 inoculated | 85.15 ± 5.27d | 28.95 ± 1.25d | 1.64 ± 0.008a | 0.73 ± 0.02b | 0.81 ± 0.02a |
| Consortia (AU-1 + AU-2 + AU-3 + AU-4) inoculated | 92.59 ± 5.24e | 38.29 ± 0.75e | 2.67 ± 0.12c | 0.97 ± 0.01d | 1.26 ± 0.04e |
Values are mean of 3 replicates; different letters show statistically significant different values (P < 0.05) from each other as evaluated from Turkey’s test
Discussion
The microbial communities present in rhizospheric soil play a significant role in crop production, soil structure and soil health. Pulses are known to improve soil health as they harbour beneficial microbes in their rhizosphere. Chickpea is an important pulse of India and its production is highest among all pulses (Pérez-Montaño et al. 2014).
The root-colonizing plant growth-promoting rhizobacteria (PGPR) play a prominent role in today’s agricultural system majorly dominated by synthetic agrochemicals, pesticides, herbicides, etc. In the present study, isolation of plant growth-promoting bacteria was done from rhizospheric soil of chickpea (C. arietinum). These bacteria hydrolyze immediate precursor of stressed ethylene, ACC into α-ketobutyrate and ammonia through the possession of ACC deaminase activity and reduces the growth inhibition triggered by high concentration of ethylene under unfavourable conditions (Dimkpa et al. 2009; Maxton et al. 2018). Four bacterial isolates AU-1, AU-2, AU-3 and AU-4 (40%) with high ACC deaminase activity were further evaluated for other attributes for considering them as plant growth-promoting rhizobacteria (PGPR).
The in vitro screening of bacterial isolates for production of IAA revealed that all four isolates are significant producers of IAA suggesting that they could be used as PGPR (Etesami et al. 2015). Indole acetic acid is one of the most important plant growth hormones and it has been reported that it is synthesized in 80% of rhizospheric microflora of crops which in turn promote root elongation and lateral root formation and improve nutrient uptake efficiency of plants (Patten and Glick 2002). The microorganisms stimulate plant growth by plant hormones which in turn enhance production of plant metabolites which can be beneficial for the growth of the microorganisms (Egamberdieva et al. 2017).
Phosphorous is the second most growth-limiting plant nutrient after nitrogen and it is widely available in both organic as well as inorganic forms in the soil. The plentiful amount of phosphorous present is of no use to plants since they absorb only monobasic ( ) and dibasic ( ) ionic forms of phosphorous (Bhattacharyya and Jha 2012). Phosphate-solubilizing bacteria (PSB) are considered as the potential agent for converting unavailable inorganic and organic form of phosphorous into plant accessible form. Therefore, phosphate solubilization is another important attribute of PGPR and the isolates of current study revealed significant release of PO43− from inorganic phosphate complex, Tricalcium phosphate ((Ca3(PO4)2). The findings of current study are in agreement with numerous published literature reporting solubilization of phosphate by Bacillus, Pseudomonas and Azotobacter (Ahemad and Khan 2010; Panhwar et al. 2014).
The production of siderophore, ammonia and HCN is another attribute that comprises indirect traits of plant growth promoting bacteria to promote growth and stress tolerance to plants. These traits are found among all four isolates of the present study except HCN production. Siderophores are the low molecular weight compounds produced by certain soil microbes that improve iron nutrient accessibility to plants. Iron occurs principally in Fe3+ form which forms hydro oxides and oxy hydroxides in aerobic environment and renders itself inaccessible to both plants and microbes (Khan et al. 2006). There are several reports describing production of siderophores by the rhizospheric microflora enhancing the iron uptake of plants (Kotasthane et al. 2017). The production of ammonia by the microbes helps the plants both directly and indirectly. The ammonia excreted by diazotrophic bacteria is one of the most important characters of the PGPRs which benefits the crop. This accumulation of ammonia in soil may increase the soil pH becoming unfavorable for growth of certain pathogenic fungi and bacteria. It also disturbs the equilibrium of microbial community and inhibits germination of spores of many fungi (Arumugam et al.2017). Likewise, the plant growth-promoting Pseudomonas and Bacillus species from maize rhizopshere were reported to produce ammonia metabolite and indirectly increase maize production (Agbodjato et al. 2015).
The stress tolerance of four putative plant growth-promoting strains was evaluated and it was observed that these strains could be used as stress tolerating agent in soils confronting salinity and water stress conditions.
The four plant growth-enhancing strains AU-1, AU-2, AU-3 and AU-4 isolated from rhizospheric soil of chickpea were identified as Azotobacter, B. subtilis, P. aeruginosa, and B. pumilis through 16 s rRNA sequencing. The result of the present study is comparable to several studies that have reported the most important genera of PGPR are Pseudomonas, Enterobacter, Clostridium, Arthrobacter, Achromobacter, Micrococcus, Flavobacterium, Azospirillum, Azotobacter and Bacillus with Azotobacter and Bacillus being the most common group of bacteria isolated from soil and other environments (Vessey 2003; Sreevidya and Gopalakrishnan 2017).
The present study deals with the inoculation of chickpea (C. arietinum) seeds with all the four potential plant growth-promoting rhizobacterial strains and a significant enhancement in physio-morphological parameters such as seed germination percentage, plant height, leaf area index and biochemical attributes such as chlorophyll content was observed in consortia-inoculated plants over the un-inoculated (control) and individually primed ones. This represents the synergistic and cumulative effect of all the strains when applied together in the agricultural production. The findings of the present study were in agreement with the study done by Pérez-Fernández and Valentine (2017), Liu et al. (2015), Samaddar et al. (2019) suggesting beneficial effects of consortia of PGPR is more pronounced than application of individual PGPR. Co-inoculation of microbes increases growth and yield, provides the plants with balanced nutrition and increases availability of minerals and nutrients (Manjunath et al. 2011; Felici et al. 2008). The presence of multifarious plant growth-promoting traits such as ACC deaminase activity, IAA production, phosphate solubilization and production of siderophore and ammonia could be the added advantage of these isolates which could be utilized to develop these strains as bio-fertilizing agent in wide range of plants under normal conditions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Department of Science and Technology-Science and Engineering Research Board (DST-SERB) for providing financial support with research Grant ECR/2017/000080 to carry out this research work. The authors are also thankful to the Amity University Uttar Pradesh for providing infrastructural support. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Agbodjato N, Noumavo PA, Baba-Moussa F, et al. Characterization of potential plant growth promoting rhizobacteria isolated from maize (Zea mays L.) in Central and Northern Benin (West Africa) Appl Environ Soil Sci. 2015;2015:9. doi: 10.1155/2015/901656. [DOI] [Google Scholar]
- Ahemad M, Khan MS. Phosphate-solubilizing and plant-growth-promoting Pseudomonas aeruginosa PS1 improves greengram performance in quizalafop-p-ethyl and Clodinafop amended soil. Arch Environ ContamToxicol. 2010;58:361–372. doi: 10.1007/s00244-009-9382-z. [DOI] [PubMed] [Google Scholar]
- Ahmad F, Ahmad I, Khan MS. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res. 2008;163:173–181. doi: 10.1016/j.micres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Ali S, Kim W-C. Plant growth promotion under water: decrease of waterlogging-induced ACC and ethylene levels by ACC deaminase-producing bacteria. Front Microbiol. 2018;9:1096. doi: 10.3389/fmicb.2018.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MA, Naveed M, Mustafa A, Abbas A. The good, the bad, and the ugly of rhizosphere microbiome. In: Kumar V, Kumar M, Sharma S, Prasad R, editors. Probiotics and plant health. Berlin: Springer; 2017. pp. 253–290. [Google Scholar]
- Arumugam S, Vijayabharathi R, Gopalakrishnan S. Plant growth-promoting actinobacteria: a new strategy for enhancing sustainable production and protection of grain legumes. Biotech. 2017;7:102. doi: 10.1007/s13205-017-0736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti N, Barnawal D. Chapter five—amelioration of salinity stress by PGPR: ACC deaminase and ROS scavenging enzymes activity. In: Singh AK, Kumar A, Singh PK, editors. PGPR amelioration in sustainable agriculture. Cambridge: Woodhead Publishing; 2019. pp. 85–106. [Google Scholar]
- Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dimkpa C, Weinand T, Asch F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009;32:1682–1694. doi: 10.1111/j.1365-3040.2009.02028.x. [DOI] [PubMed] [Google Scholar]
- Dworkin M, Foster JW. Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol. 1958;75:592–603. doi: 10.1128/jb.75.5.592-603.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards U, Rogall T, Emde M, Böttger E. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989;17:7803–7853. doi: 10.1093/nar/17.19.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egamberdieva D, Lugtenberg B. Use of plant growth-promoting rhizobacteria to alleviate salinity stress in plants. Use Microbes Allev Soil Stress. 2014;1:73–96. [Google Scholar]
- Egamberdieva D, Wirth SJ, Alqarawi AA, et al. Phytohormones and beneficial microbes: essential components for plants to balance stress and fitness. Front Microbiol. 2017;8:2104. doi: 10.3389/fmicb.2017.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etesami H, Alikhani HA, Hosseini HM. Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX. 2015;2:72–78. doi: 10.1016/j.mex.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felici C, Vettori L, Giraldi E, et al. Single and co-inoculation of Bacillus subtilis and Azospirillum brasilense on Lycopersicon esculentum: effects on plant growth and rhizosphere microbial community. Appl Soil Ecol. 2008;40:260–270. doi: 10.1016/j.apsoil.2008.05.002. [DOI] [Google Scholar]
- Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- Gamalero E, Glick BR. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015;169:13–22. doi: 10.1104/pp.15.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. Plant growth-promoting bacteria: mechanisms and applications. Scientifica (Cairo) 2012;2012:963401. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M, Shimomura T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem. 1978;42:1825–1831. doi: 10.1271/bbb1961.42.1825. [DOI] [Google Scholar]
- Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015;20:219–229. doi: 10.1016/j.tplants.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Khan A, Geetha R, Akolkar A, et al. Differential cross-utilization of heterologous siderophores by nodule bacteria of Cajanuscajan and its possible role in growth under iron-limited conditions. Appl Soil Ecol. 2006;34:19–26. doi: 10.1016/j.apsoil.2005.12.001. [DOI] [Google Scholar]
- Kotasthane AS, Agrawal T, Zaidi NW, Singh US. Identification of siderophore producing and cynogenic fluorescent pseudomonas and a simple confrontation assay to identify potential bio-control agent for collar rot of chickpea. 3 Biotech. 2017;7(2):137. doi: 10.1007/s13205-017-0761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Xie BM, Shi XH, et al. Effects of two plant growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase on oat growth in petroleum-contaminated soil. Int J Environ Sci Technol. 2015;12:3887–3894. doi: 10.1007/s13762-015-0798-x. [DOI] [Google Scholar]
- Lorck H. Production of hydrocyanic acid by bacteria. Physiol Plant. 1948;1:142–146. doi: 10.1111/j.1399-3054.1948.tb07118.x. [DOI] [Google Scholar]
- Mahmood I, Imadi S, Shazadi K, et al. et al. Effects of pesticides on environment. In: Hakeem KR, et al.et al., editors. Plant, soil and microbes. Berlin: Springer; 2015. [Google Scholar]
- Manjunath M, Prasanna R, Sharma P, et al. Developing PGPR consortia using novel genera Providencia and Alcaligenes along with cyanobacteria for wheat. Arch Agron Soil Sci. 2011;57:873–887. doi: 10.1080/03650340.2010.499902. [DOI] [Google Scholar]
- Maxton A, Singh P, Masih SA. ACC deaminase-producing bacteria mediated drought and salt tolerance in Capsicum annuum. J Plant Nutr. 2018;41:574–583. doi: 10.1080/01904167.2017.1392574. [DOI] [Google Scholar]
- Michel BE, Kaufmann MR. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973;51:914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 2009;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- Panhwar QA, Naher UA, Jusop S, et al. Biochemical and molecular characterization of potential phosphate-solubilizing bacteria in acid sulfate soils and their beneficial effects on rice growth. PLoS ONE. 2014;9:1–14. doi: 10.1371/journal.pone.0097241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol. 2002;68:3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Fernández M, Valentine A. Enhanced plant performance in Cicer arietinum L. due to the addition of a combination of plant growth-promoting bacteria. Agriculture. 2017 doi: 10.3390/agriculture7050040. [DOI] [Google Scholar]
- Pérez-Montaño F, Alías-Villegas C, Bellogín RA, et al. Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res. 2014;169:325–336. doi: 10.1016/j.micres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Saleem AR, Brunetti C, Khalid A, et al. Drought response of Mucuna pruriens (L.) DC. inoculated with ACC deaminase and IAA producing rhizobacteria. PLoS ONE. 2018;13:1–18. doi: 10.1371/journal.pone.0191218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaddar S, Chatterjee P, Choudhury AR, et al. Interactions between Pseudomonas spp. and their role in improving the red pepper plant growth under salinity stress. Microbiol Res. 2019;219:66–73. doi: 10.1016/j.micres.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Schwyn B, Neilands JB. Universal CAS assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Sreevidya M, Gopalakrishnan S. Direct and indirect plant growth-promoting abilities of Bacillus species on chickpea, isolated from compost and rhizosphere soils. Org Agric. 2017;7:31–40. doi: 10.1007/s13165-015-0141-3. [DOI] [Google Scholar]
- Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.