Abstract
Over the past decades, researchers have reported several mechanisms for doxorubicin (DOX)-induced cardiomyopathy, including oxidative stress, inflammation, and apoptosis. Another mechanism that has been suggested is that DOX interferes with the cell cycle and induces oxidative stress in C-kit+ cells (commonly known as cardiac progenitor cells), reducing their regenerative capacity. Cardiac regeneration through enhancing the regenerative capacity of these cells or administration of other stem cells types has been the axis of several studies over the past 20 years. Several experiments revealed that local or systemic injections with mesenchymal stem cells (MSCs) were associated with significantly improved cardiac function, ameliorated inflammatory response, and reduced myocardial fibrosis. They also showed that several factors can affect the outcome of MSC treatment for DOX cardiomyopathy, including the MSC type, dose, route, and timing of administration. However, there is growing evidence that the C-kit+ cells do not have a cardiac regenerative potential in the adult mammalian heart. Similarly, the protective mechanisms of MSCs against DOX-induced cardiomyopathy are not likely to include direct differentiation into cardiomyocytes and probably occur through paracrine secretion, antioxidant and anti-inflammatory effects. Better understanding of the involved mechanisms and the factors governing the outcomes of MSCs therapy are essential before moving to clinical application in patients with DOX-induced cardiomyopathy.
Keywords: anthracyclines, cardiac progenitor cells, cardiomyopathy, doxorubicin, mesenchymal stem cells
Introduction
Anthracyclines [doxorubicin (DOX), daunorubicin, idarubicin, epirubicin, and anthraquinone] are effective chemotherapeutic agents, used in the management of several tumors. Among them, DOX is the most widely used, especially for the treatment of breast and esophageal cancers, osteosarcoma, Kaposi’s sarcoma, and Hodgkin’s and non-Hodgkin’s lymphomas (Tacar et al., 2013). Despite its efficacy, reports of DOX-induced cardiomyopathy have jeopardized its clinical use due to the high risk of developing heart failure (HF) (Chatterjee et al., 2010; Renu et al., 2018).
DOX cardiotoxicity is categorized (according to its onset) into acute or chronic. Acute cardiotoxicity usually occurs within the first 3 days of administration with an approximate incidence of 11% (Swain et al., 2003; Takemura and Fujiwara, 2007). It has a wide range of non-specific symptoms, including chest pain (due to myopericarditis) and palpitations (secondary to sinus tachycardia, paroxysmal non-sustained supraventricular tachycardia, or premature atrial and ventricular beats). Meanwhile, chronic cardiotoxicity has an incidence of 1.7% and often manifests within 30 days of drug use (Von Hoff et al., 1979). However, it may manifest after a latency period of 6 to 10 years (Ferrans et al., 1997). DOX-induced cardiomyopathy not only is dose-dependent but also is influenced by other factors, including the patient’s age, history of previous cardiovascular diseases, and reduced left ventricular ejection fraction (LVEF) (Von Hoff et al., 1979).
DOX accumulates primarily in the liver, kidneys, and the heart. The heart is highly susceptible to DOX toxicity because the mitochondria-to-cardiomyocyte ratio is elevated, making it more susceptible to oxidative stress. Further, the heart has a low regenerative capacity, compared to other organs (Barry et al., 2007). Cardiac tissues, exposed to DOX, show several structural changes, including myofibrillar loss, eosinophilic fibers, pyknotic nuclei, blood vessel congestion, and widespread infiltration of neutrophils (Abdel-Daim et al., 2017). Functionally, DOX intoxication in humans and animal models is associated with reduced fractional shortening, LVEF, reduced contractility, increased afterload, and increased isovolumetric relaxation time, all of which reflect cardiac dysfunction or even failure (Lipshultz et al., 1991; Stoddard et al., 1992).
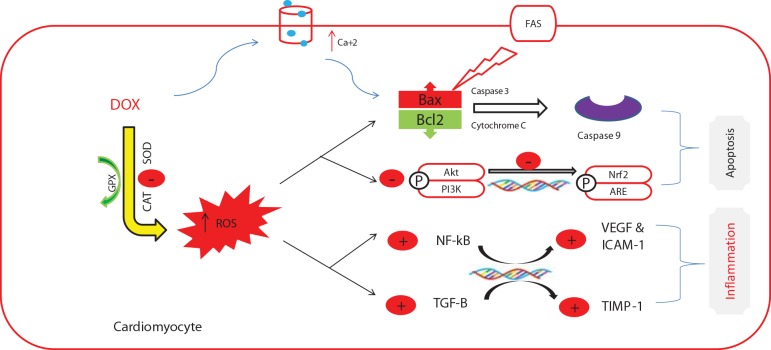
Several studies investigated the pathogenesis of DOX-induced cardiomyopathy and implicated various mechanisms, including oxidative stress {reactive oxygen species (ROS)-rich microenvironment], resulting in endothelial cell injury with subsequent leukocyte infiltration and cytokine secretion [interleukin (IL)-1β, IL-6, and tumor necrosis factor-α] (Pecoraro et al., 2016; Abdel-Daim et al., 2017). Further, DOX increases the expression of nuclear factor kappa-B, cyclooxygenase-2, and inducible nitric oxide synthase (Mantawy et al., 2014). These events, along with suppressed expression of bcl2 (Leung and Wang, 1999), activate the apoptotic cascade. The apoptotic cells can act as a source of damage-associated molecular patterns (DAMPs) that can be identified by the pattern recognition receptors (PRRs), exaggerating the immune response (Krysko et al., 2012). In addition, DOX downregulates the expression of cardiac-muscle-specific and mitochondrial proteins, which are essential for cardiomyocyte function (Ito et al., 1990) ( Figure 1 ).
Figure 1.
The commonly investigated mechanisms of doxorubicin-induced cardiotoxicity. This figure was adapted from a previous publication by the authors (Abushouk et al., 2017) and an adequate permission for reproduction was obtained from the publisher (Elsevier).
Recently, other potential mechanisms are coming into light. For example, DOX inhibits the activity of nuclear topoisomerase IIβ, which is essential for DNA replication and transcription (Lyu et al., 2007). This enzyme is abundantly present in rapidly growing cancer cells (therefore, being a target for DOX), as well as quiescent cardiac myocytes. Inhibition of the enzyme leads to DNA breaks and transcriptional alterations (Zhang et al., 2012). Further, DOX enhances the proteolytic activity of calpain enzyme through the increase of intracellular Ca levels, causing sarcomere disruption and myofibril loss (Lim et al., 2004). This plethora of potential mechanisms suggests that DOX-induced cardiotoxicity probably occurs through a multiple-mechanisms hypothesis (Mele et al., 2016).
Of all the aforementioned mechanisms, oxidative stress has received the most attention (Varricchi et al., 2018). The metabolism of DOX produces DOX–semiquinone, an unstable compound that reacts with O2, generating H2O2 and O2 •− (Cummings et al., 1991). DOX also inhibits mitochondrial iron export, leading to iron accumulation within the mitochondria and production of ROS (Gammella et al., 2014). These radicals cause cardiolipin peroxidation, inducing the release of mitochondrial cytochrome c enzyme, leading to more cardiolipin peroxidation (Goormaghtigh et al., 1990). In addition, DOX augments the activity of extra-mitochondrial oxidative enzymes as xanthine oxidase (Pan and Bachur, 1980) and NADPH oxidase (Zhao et al., 2010). On the other hand, DOX inhibits the activities of endogenous enzymatic and non-enzymatic antioxidants (Abdel-Daim et al., 2017; Abushouk et al., 2017). Therefore, this imbalance between ROS generation and neutralization leads to oxidative stress.
There are no specific guidelines for the amelioration or treatment of DOX-induced cardiotoxicity. The most common approach is decreasing the drug dosage. In practice, the drug is stopped if there is a 10% reduction in the LVEF from baseline (Curigliano et al., 2016). Other approaches include the administration of anthracyclines as infusions rather than as boluses or the use of liposomal DOX (Saidi and Alharethi, 2011). Dexrazoxane, an EDTA chelator, has been shown effective in ameliorating DOX-induced cardiotoxicity. However, its use is limited to patients who receive a cumulative dose of DOX > 300 mg/m2 (Cvetkovic and Scott, 2005). Further, beta-blockers, statins, and angiotensin antagonists might protect against systolic and diastolic dysfunction of DOX toxicity (Kalam and Marwick, 2013), but further clinical trials are required.
The impairment of cardiac repair after DOX toxicity and the occurrence of late cardiotoxic effects indicate that DOX may influence cardiac adaptation to stress or cardiac regeneration. The latter has been the axis of a major debate recently on whether regenerative stem cells exist or not in the adult mammalian heart. On one hand, some experiments have shown that DOX induces apoptosis of cardiac progenitor cells (CPCs), reducing the pool of available CPCs to regenerate the damaged, senescent, and poorly functioning myocytes (Huang et al., 2010; Spallarossa et al., 2010; Piegari et al., 2013). On the other hand, recent experiments presented strong evidence that the so-called “cardiac progenitor cells” do not have a cardiac regenerative potential in the adult mammalian heart (Van Berlo et al., 2014; Li et al., 2018).
The Heart Stem Cell Debate
The concept of endogenous regenerative cardiac stem cells started in 2001 (Maliken and Molkentin, 2018). Quaini et al. (2002) and Orlic et al. (2001) reported that adult cardiac and bone marrow cells expressing the protein (c-kit) were able to generate new myocytes. Later reports provided further support to this finding (Beltrami et al., 2001; Beltrami et al., 2003). The notion, developed based on these results, was that under normal circumstances, minor cardiac damage causes progenitor cells to migrate to the site of injury and differentiate into cardiac cells (Chimenti et al., 2003; Huang et al., 2010). Therefore, the decline of the progenitor cell pool, cellular senescence, and loss of their functions (as claimed to occur after DOX exposure) lead to impaired cardiac homeostasis and inability of the heart to cope with continuous stress (Chimenti et al., 2003; Spallarossa et al., 2010; Cesselli et al., 2011). Other authors suggested that these cells can be used in cell-based therapy after they are withdrawn from cardiac biopsies and expanded ex vivo (Messina et al., 2004). C-kit+ cells were evaluated for their regenerative capacity in animal models with myocardial infarction (MI) (Barile et al., 2007), as well as in humans (Bolli et al., 2011; Makkar et al., 2012; Dixit and Katare, 2015).
The debate started in 2004 when three investigations by Murry et al. (2004), Balsam et al. (2004), and Nygren et al. (2004) concluded that C-kit+ hematopoietic stem cells could not differentiate into cardiomyocytes, even in the microenvironment of the injured heart. Another paper by Van Berlo et al. (2014) in 2014 confirmed these conclusions that C-kit+ cannot differentiate into cardiac myocytes. However, the cell lineage tracing technique of the latter paper was criticized (Vicinanza et al., 2018). A year later, another research group reported that C-kit+ cells can differentiate into cardiac myocytes in vitro when the optimal conditions are provided. The authors argued that the cells have the potential; however, the microenvironment in the heart after MI does not allow them to perform such differentiation (Hatzistergos et al., 2015). A recent study in 2018 used a dual genetic lineage tracing system and showed that non-myocytes could generate myocytes in the embryonic stage, but not in the adult homeostatic condition or after MI (Li et al., 2018). Later in the same year, several basic science studies were retracted or followed with an editorial expression of concern due to evidence of data falsification or image manipulation. Concurrently, the National Heart, Lung, and Blood Institute (NHLBI) stopped its ongoing CONCERT-HF study, which was testing the regenerative efficacy of the combination of MSCs and C-kit+ cells in patients with HF due to safety concerns.
The Effects of DOX on C-kit+ Cells With Regard to the Recent Debate
The main debate is whether C-kit+ cells can give origin to new myocytes and there is growing evidence that they cannot; however, they may be involved in cardiac repair through other mechanisms. Therefore, impairment of their functions upon DOX exposure might contribute to the observed late toxic effects of DOX. Below is a brief review of the published reports on the effects of DOX on C-kit+ cells that should be revisited in light of the piling evidence, doubting their regenerative capacity.
Huang and colleagues conducted an experiment on a juvenile mouse model to study the mechanism of late-onset DOX cardiomyopathy. They found that treatment with DOX caused a permanent decline in the number of C-kit+ and endothelial progenitor cells (EPCs) in treated mice hearts, as well as telomeric shortening and progressive cell senescence. Moreover, DOX-treated mice became more susceptible to ischemic injuries and MI, and less capable of responding even to minor stresses (Huang et al., 2010). Other studies were conducted on isolated human C-kit+ cells, EPCs, and living rats. DOX-treated cells showed reduced viability and increased apoptosis. After a 6-week period, the myocardium showed almost complete depletion of these cells (Spallarossa et al., 2010; De Angelis et al., 2010). Researchers in another experiment isolated C-kit+ cells from the hearts of DOX-treated patients who died due to cardiomyopathy or other reasons (the primary disease for example) and compared them to C-kit+ cells, isolated from autopsies of patients, not treated with DOX. They found significantly higher cellular senescence in cells obtained from DOX-treated patients. When control cells were treated with DOX, similar effects occurred. To study the persistence of DOX effects on C-kit+ cells, the authors washed the cells from DOX and left them to grow and compared the results with those obtained early after exposure. After a week, the cells showed markedly less apoptosis and higher vitality. However, they still expressed higher senescence, which indicates the long-term toxic effects of DOX (Piegari et al., 2013).
Several mechanisms were suggested to explain the above findings. For example, DOX alters the molecular regulators of the cell cycle, causing cell cycle arrest. The activity of telomerase is also important for the proliferation of progenitor cells. DOX was shown to decrease the activity of telomerase, causing senescence of C-kit+ cells (Huang et al., 2010). Another possible mechanism is the generation of ROS (Spallarossa et al., 2010), which cause damage to myocytes (Doroshow, 1983; Takemura and Fujiwara, 2007). This was proven in vivo and in vitro as anthracyclines were found able to promote oxidative stress in isolated human C-kit+ cells and in living mice (De Angelis et al., 2010; Spallarossa et al., 2010; Piegari et al., 2013). This DNA damage caused by oxidative stress later leads to over- or underexpression of molecular regulators of cell cycle (mainly P53 and Rb genes) (Piegari et al., 2013), which, in turn, causes apoptosis or cellular senescence (Levine, 1997; De Angelis et al., 2010).
In addition, DOX increases the expression of P16INK4A (a marker of cellular senescence that causes cell cycle arrest in the G1 phase) in progenitor cells (Spallarossa et al., 2010; Piegari et al., 2013) through JNK or P38 activation (Wada et al., 2008; Spallarossa et al., 2010). Cyclin D1 and cyclin-dependent kinase 4 (CDK-4) are molecules that work together to phosphorylate the Rb protein into phospho-RbSer798 protein that stops the inhibition of the cell cycle progression (Piegari et al., 2013). Isolated C-kit+ cells after DOX treatment showed a substantial decrease in the levels of cyclin D1, CDK-4, and/or phospho-RbSer798 (De Angelis et al., 2010). Growth factor receptors like insulin-like growth factor-1 (IGF-1) receptor and hepatocyte growth factor receptors (c-Met) are also expressed by C-kit+ cells, and stimulation of these receptors is thought to be crucial for myocyte regeneration in response to injury (D’amario et al., 2011; Piegari et al., 2013). DOX-exposed C-kit+ cells showed reduced expression of IGF-1 receptors and c-Met (Piegari et al., 2013). Therefore, impaired growth factors functions may be another possible mechanism of DOX-induced cardiomyopathy.
The recent findings negating the regenerative capacity of the C-kit+ cells do not necessarily contradict the published observations about DOX effects on these cells. DOX may reduce the ability of these cells to engage in other cardiac repair activities rather than cardiac regeneration. Further research is needed to characterize the structural and functional aspects of C-kit+ cells.
Stem Cell Therapy for DOX-Induced Cardiomyopathy
Stem cell therapy is a promising modality for DOX-induced cardiomyopathy; however, it is still in the preclinical stage. Several studies investigated the role of regenerative medicine as an alternative therapy to heart transplantation (Dimmeler et al., 2005; Pelacho et al., 2007; Mathur et al., 2017). In particular, mesenchymal stem cells (MSCs) have many properties that make them a suitable choice for the prevention and treatment of myocardial diseases, including DOX-induced cardiac damage.
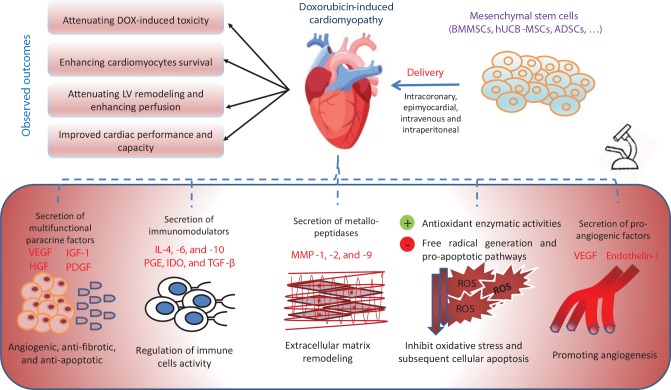
First, MSCs secrete paracrine factors that are involved in the cardiac remodeling process (Garbade et al., 2009). These factors have different functions. For example, IGF-1 increases cell proliferation and inhibits apoptosis by improving the mitochondrial function in different cell types including cardiomyocytes (Duerr et al., 1995; Lai et al., 2003). Some factors have angiogenic functions, e.g., endothelin-1, platelet-derived growth factor, and vascular endothelial growth factor, or participate in the regulation of sympatho-adrenal axis and normalization of noradrenaline and adrenaline plasma levels (Dhein et al., 2006). To confirm the value of paracrine secretion in MSC-observed beneficial effects, Zhang et al. (2015) reported that only the conditioned medium of bone marrow MSCs (BMMSCs) and induced pluripotent stem cells (iPSCs) can alleviate DOX-induced HF, cell apoptosis, and cardiac fibrosis.
Moreover, MSCs ameliorate oxidative stress by controlling redox microenvironment (increasing heme-oxygenase-1 expression and reducing 8-OHdG tissue concentration) and consequently inhibiting apoptosis induced by ROS (Liu et al., 2012; Jin et al., 2013; Calió et al., 2014). Other investigators reported that MSCs have high resistance to oxidative stress, high concentrations of glutathione, and high basal gene expression of glutathione peroxidase, superoxide dismutase, and catalase (Valle-Prieto and Conget, 2010). Xia and Hou (2018a) showed that MSC treatment significantly ameliorated DOX-induced senescence in H9c2 cells as marked by the reduced expression of P53 and P16, probably by inhibiting the miR34aSIRT1 axis.
They also exert anti-inflammatory effects by modulating the functions of different immune cells; they can activate, suppress, and control migration and differentiation of T and B lymphocytes, macrophages, natural killer cells, dendritic cells, and neutrophils by the secretion of different immunomodulators, e.g., IL-4, IL-6, IL-10, prostaglandin E2, transforming growth factor-β, and indoleamine 2,3-dioxygenase. Further, MSCs express Toll-like receptors, e.g., TLR3 and TLR4, which play an important role in the inflammatory process (Liotta et al., 2008). In addition, MSCs have been shown to perform an anti-fibrotic function by secreting matrix metallopeptidase-9 (Ankrum and Karp, 2010; Samper et al., 2013) ( Figure 2 ).
Figure 2.
The mechanisms of action of stem-cell-based treatment in ameliorating doxorubicin-induced cardiotoxicity. ADSCs, adipose-derived stem cells; BMMSCs, bone-marrow mesenchymal stem cells; HGF, hepatocyte growth factor; hUCB-MSCs, human umbilical cord blood mesenchymal stem cells; IDO, indoleamine 2,3-dioxygenase; IGF-1, insulin-like growth factor-1; Il, interleukin; MMP, matrix metallopeptidase; PDGF, platelet-derived growth factor; PGE, prostaglandin E; ROS, reactive oxygen species; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor.
Although MSCs can express some cardiomyocyte markers (Kawada et al., 2004; Gopinath et al., 2010), their settlement in the myocardium was weak even with the local route of administration. Therefore, myocardial regeneration does not involve differentiation of MSCs into cardiomyocytes (Kang et al., 2012). Now, the overall consensus is that C-kit+ cells do not differentiate into cardiomyocytes, and it is not likely that any other stem cell is more cardiogenic (Maliken and Molkentin, 2018).
In addition to the reported beneficial effect of systematic administration of MSCs on DOX-induced cardiomyopathy, they showed significant protection for other organs as well. They were found to decrease DOX-induced nephropathy by decreasing glomerular inflammation and sclerosis (Zoja et al., 2012). Moreover, the anti-inflammatory effect of MSCs makes them protective against DOX-induced inflammation in the brain (Jansen et al., 2008) and the liver (Tulubas et al., 2015).
Factors That Influence the Outcome of MSC Therapy for DOX Cardiomyopathy
The varying results of published experiments on the benefit of MSC use in models of DOX-induced cardiomyopathy indicate that there are several factors that affect the outcome of this treatment. Below is a summary of the most investigated factors in this regard ( Table 1 ).
Table 1.
Summary of the methods and experimental findings on using mesenchymal stem cell therapy for DOX-induced cardiotoxicity.
| Study ID | Animal model | Doxorubicin dose and route | MSCs type and source | MSCs dose | Route and time of MSC administration | Findings |
|---|---|---|---|---|---|---|
| Chen et al. (2006) | Rabbit | 2 mg/kg per week for 8 weeks (i.p.) | BMMSCs or skeletal myoblasts (autologous) | 1 × 107 | Intracoronary, 4 weeks after Dox treatment | The LVEF was not significantly improved in either the BMMSCs or skeletal myoblast-treated groups. |
| Garbade et al. (2009) | Rabbit | 3 mg/kg for 6 weeks (i.p.) | BMMSCs (autologous) | 1.5/2 × 106 | Epimyocardial, 2 weeks after Dox treatment | BMMSCs treatment significantly increased the LVEF. On histological examination, cell-treated hearts exhibited less collagen content and higher capillary density. However, the transplanted cells did not show any cardiac markers. |
| Mohammadi Gorji et al. (2012) | Rat | Three doses of 2.5 mg/kg per week for 2 weeks (i.p.) | BMMSCs (heterologous) | 5 × 106 | Intravenous, 2 weeks after Dox treatment | Both MSCs and its conditioned medium significantly reduced myocardial fibrosis and Bcl-2 expression. Compared to the standard medium, the MSC-conditioned medium had significantly higher levels of HGF and IGF. |
| Yu et al. (2014) | Rat | 2.5 mg/kg per week for 6 weeks (i.p.) | BMMSCs (heterologous) | 5 × 106 | Intravenous, one injection per day (10 times)/10 weeks after Dox treatment | The survival rate and LVEF in rats treated with MSCs, compared to placebo-treated rats. Further, MSC treatment reduced myocardial collagen volume fraction and mRNA expression of TGF-β1, AT1, and CYP11B2. |
| Ammar et al. (2015) | Rat (diabetic) | 2.5 mg/kg 3 times/week for 2 weeks (i.p.) | BMMSCs and ADSCs (from human tissues) | 2 × 106 for either cell type | Intravenous, 4 weeks after the last DOX injection | BM-MSCs and ADSCs were equally effective in alleviating DOX-induced cardiac damage by decreasing immune cell infiltration and collagen deposition and enhancing angiogenesis |
| Dias et al. (2015) | Rat | 3.75 mg/kg/day once a week for 4 weeks (i.p.) | BMMSCs and skeletal myoblasts (autologous) | 5 × 104 | Subepicardial, 4 weeks after the last dose of DOX | The combined stem cell treatment significantly improved the LVEF, compared to the saline-treated group. Histological examination showed proliferation of skeletal muscle cells in the myocardium. |
| Zhang et al. (2015) | Mouse | 3 mg/kg, 3 times per week for 2 weeks (i.p.) | Conditioned medium from BMMSCs (BMMSCs-CdM) or iPSC-derived MSCs | 50 μl of MSCs-CdM | Intramyocardial, after creation of the DOX-induced cardiomyopathy model | Compared to BM-MSCs-CdM, iPSC-MSCs-CdM treatment exhibited better alleviation of heart failure, as well as less cardiomyocyte apoptosis and fibrosis. |
| Deng et al. (2017) | Rat | 2.5 mg/kg for 2 weeks (i.p.) | BMMSCs (cells with or without Nkx2.5 transfection) | 1 × 107 | Intravenous, 3 weeks after Dox treatment | The LVEF was increased by 43.4% and 49.9% in rats treated with BMMSCs and Nkx2.5-transfected BMMSCs, respectively. Further, Nkx2.5 transfection improved MSCs differentiation into cardiomyocyte-like cells and reduced myocardial fibrosis. |
| Zeng et al. (2017) | Rat | 2 mg/kg seven times in 2 days (i.p.) | BMMSCs (heterologous) with and without miR-21 over expression | i.p., injection after cardiotoxicity induction | BMMSCs, overexpressing miR-21, exhibited more proliferation than untransfected cells and significantly enhanced expression of Bcl-2, VEGF and Cx43 and reduced expression of Bax, BNP and troponin T | |
| Soliman et al. (2017) | Rat | 450 mg/m2 for 3 consecutive days (i.p.) | BMMSCs (heterologous) with and without sodium valproate and electric stimulation (ES) over the shoulder | 5 × 105 | Intravenous, after DOX cardiotoxicity induction | Rats treated by BMMSCs and valproate/ES combination showed similar biochemical parameters to the control group, as well as better histopathological appearance and cardiac homing of MSCs than rats treated by stem cells alone. |
| Oliveira et al. (2013) | Rat | 5 mg/kg weekly for 4 weeks (i.p.) | ADSCs (heterologous) | 3 × 106 | Intravenous, prior to the beginning of the experiment | Unlike C. sinensis extract, treatment by ADSCs significantly improved the LVEF. However, no cell engraftment was detected in the host cardiac tissue. |
| Pınarlı et al. (2013) | Rat | 12 mg/kg as a single dose (i.p.) | ADSCs (alone or with resveratrol) | 2 × 106 | i.p., starting the day after DOX injection, then two times at 5 days interval | The best hemodynamic (left ventricular end diastolic pressure and the rate of pressure development, yet not significant) and histological outcomes were observed in the group, treated by resveratrol and ADSCs. |
| Gopinath et al. (2010) | Mouse and cultured neonatal rat cardiomyocytes | 400 ng/kg per minute (oral) | hUCB-MSCs (human placenta) | 2.5 × 106 | Intravenous, 2 weeks after Dox treatment | hUCB-MSCs exhibited differentiation into cardiomyocyte-like cells, reversed the pathological effects of DOX on cultured myocytes, and induced a shift from pathological hypertrophy towards physiological hypertrophy |
| Di et al. (2012) | Mouse | Three cycles of 3 doses of 2 mg/kg per week (i.p.) | hUCB-MSCs (human placenta) | 1 × 106 | Intravenous, at the end of each Dox cycle | MSC treatment significantly reduced myocardial necrosis and increased LVEF and fractional shortening, probably through reduction of oxidative stress. Further, MSCs treatment had no effect on tumor growth. |
| Mousa et al. (2018) | Rat | 1.25 mg/kg every other day for 1 month (i.p.) | hUCB-MSCs (human placenta) with carvedilol | 1.5 × 106 | Intravenous, single dose, along with carvedilol administration | The combination of hUCB and carvedilol reduced DOX-induced electrocardiographic abnormalities and cardiac concentrations of oxidative stress markers and caspase-3, while increased cardiac concentrations of VEGF and IGF-1 |
| Abd Allah and Hussein (2017) | Rat | 2.5 mg/kg every other day for 2 weeks (i.p.) | hUCB-MSCs (human placenta) | 5 × 106 | Intravenous, 1 week after the last DOX dose | Treatment by hUCB-MSCs resulted in significant amelioration of DOX-induced oxidative stress, ECG abnormalities and histopathological alterations. |
| Mao et al. (2017) | Rat | 2 mg/kg once a week for 8 weeks (i.p.) | hUCB-MSCs (human placenta) | 2.5 × 1 05 (low dose group) and 1 × 106 (high dose group) | Intramuscular, 2 weeks after the last DOX injection | Treatment by hUCB-MSCs significantly increased LVEF, as well as the expression of VEGF, IGF-1, and HGF in the myocardium and attenuated mitochondrial swelling and maintained sarcolemma integrity |
| Haydardedeoglu et al. (2018) | Rat | 2.5 mg/kg on day 1 and 4 mg/kg on day 21 | Fetal-derived MSCs | 2 × 106 | i.p., on days 7, 14, and 21 after the last DOX injection | The intraperitoneal route can be a valid alternative to the intravenous and intra-cardiac routes. |
ADSCs, adipose-derived stem cells; AT-1, angiotensin 1 receptor; BMMSCs, bone-marrow derived mesenchymal stem cells; BNP, brain naturetic peptide; hUCB, human umbilical cord blood; IGF, insulin-like growth factor; LVEF, left-ventricular ejection fraction; TGF-β1, transforming growth factor-β; VEGF, vascular endothelial growth factor.
(A) MSC type: Two main types of stem cells are present: embryonic stem cells (ESCs), present in the inner cell mass of the blastocyst, and adult stem cells, present in different mature tissues to replace dead cells (Swain et al., 2003; Takemura and Fujiwara, 2007). Embryonic stem cells have two considerations that hinder their use; first is the ethical consideration regarding destruction of a developing embryo, and second is the possibility of rejection and the need for lifelong immunosuppressive drugs to maintain the graft (Pfister et al., 2014). Further, the risk of rejection is still present due to potential incompatibility. iPSCs are adult cells, genetically reprogrammed to express genes and factors, required to maintain the properties of ESCs (Choi et al., 2009). Therefore, they overcome the ethical and the rejection problems because they can be withdrawn from the patient (autologous graft). On the other hand, MSCs are able to enhance C-kit+ cell proliferation and differentiation, which may ameliorate the DOX toxicity on the long run (Hatzistergos et al., 2010; Loffredo et al., 2011). The bone marrow, adipose tissue, and umbilical cord blood are the most common sites for MSC extraction. The most commonly used cells in the reviewed studies were BMMSCs (Chen et al., 2006; Garbade et al., 2009; Mohammadi Gorji et al., 2012; Yu et al., 2014; Ammar et al., 2015; Dias et al., 2015; Deng et al., 2017; Soliman et al., 2017; Zeng et al., 2017) and human umbilical cord blood (hUCB)-MSCs (Gopinath et al., 2010; Di et al., 2012; Abd Allah and Hussein, 2017; Mao et al., 2017; Mousa et al., 2018). Fewer studies used adipose-derived stem cells (ADSCs) (Oliveira et al., 2013; Pınarlı et al., 2013) and fetal-derived stem cells (Haydardedeoglu et al., 2018). The majority of these studies showed promising effects; however, to make a conclusion about the most optimal type, head-to-head comparisons are required.
(B) Dose of injected MSCs: In the published studies, the number of injected MSCs in DOX-intoxicated animal models ranged from 5 × 104 to 1 × 107 cells. Chen et al. (2006) used the highest number of BMMSCs (1 × 107 cells), and the results were not satisfactory as regards LVEF. They found further deterioration as regards abnormalities in local ventricular conduction in DOX-treated hearts, as well as positive correlation between the dispersion of the activation time and the number of cells derived from stem cells in the pacing site. Another study used a similar dose and reported beneficial effects, especially when BMMSCs were transfected with Nkx2.5 (Deng et al., 2017). Mao et al. (2017) used two different doses (2.5 × 105 versus 1 × 106) of hUCB-MSCs and reported no dose–effect relationship on LVEF.
(C) Route of administration of MSCs: Two routes of administration are reported in the literature: the local route [either via intracoronary injection by using two balloons introduced through the left common carotid artery (Chen et al., 2006) or via epimyocardial injection (Garbade et al., 2009)] and the systemic route by intravenous injection of MSCs (Di et al., 2012; Yu et al., 2014). The latter was the most commonly used in the reviewed studies, while few studies used the intraperitoneal (Pınarlı et al., 2013; Haydardedeoglu et al., 2018) or the intramuscular routes (Mao et al., 2017), reporting their safety as possible alternatives.
Chen and colleagues have found that the LVEF was not significantly improved by intracoronary administration of BMMSCs after DOX treatment (Chen et al., 2006). However, Garbade et al. (2009) reported that epimyocardial administration of BMMSCs, after DOX treatment, resulted in significant improvement of the LVEF. Similarly, Yu et al. (2014) concluded that systemic administration of BMMSCs resulted in significant reduction of myocardial fibrosis, as well as significant improvement of LVEF. Further, Di et al. (2012) reported significant improvement of the LVEF after systemic administration of hUCB at the end of each DOX cycle.
(D) Time of administration: The published studies used two different timings for administering MSCs. The first is a preventive strategy that entails administering stem cells before, during, or just after DOX administration (Di et al., 2012). The second involves administering stem cells after cardiomyopathy has been established (regenerative strategy) (Gopinath et al., 2010). Di et al. (2012) have adopted the preventive strategy (by systemic administration of hUCB at the end of each DOX cycle) that resulted in both histological (reduced apoptosis) and functional improvements (significant increase in LVEF), while Gopinath et al. (2010) have adopted the regenerative strategy (by systemic administration of hUCB-MSCs, 2 weeks after DOX treatment) that resulted in histological improvement in the form of decreased fibrosis (2.9% compared to 7.2% when mice were treated by DOX alone); however, the functional status was not investigated.
Further, studies that adopted the regenerative strategy used different intervals between DOX administration and MSC administration. Early epimyocardial administration of BMMSCs after 2 weeks of DOX treatment has resulted in significant improvement of LVEF (Garbade et al., 2009), while insignificant improvement was noted following intracoronary administration of BMMSCs, 4 weeks after DOX injection (Chen et al., 2006).
(E) Degree of DOX-induced cardiac injury: The improvement in cardiac function after MSC treatment can be related to the degree of cardiac affection by DOX and the pretreatment functional status. Administration of a total dose of 16 mg/kg of DOX in a rabbit model has resulted in a pretreatment EF of 77.6 ± 1.6% in the MSC group. Comparing this EF to the control group 81.5 ± 1.5% shows that there was no marked reduction in EF after DOX treatment, and this led to nonsignificant improvement in LVEF (Chen et al., 2006), while administration of a total dose of 18 mg/kg of DOX in a rabbit model resulted in a pretreatment EF of 33 ± 1.3% in the MSC group. Comparing this EF to the control group (around 41%) shows a significant reduction in EF after DOX treatment, which was improved later by stem cell treatment (Garbade et al., 2009). Another study (Yu et al., 2014) may support this conclusion; in a rat model, DOX treatment has resulted in a pretreatment EF of 66 ± 1.4% in the MSC group, compared to 88.9 ± 0.8% in the control group. These data indicate that minimal functional impairment of the heart after DOX administration results in nonsignificant improvement after MSC treatment. However, more studies are needed to verify this conclusion.
(F) Time of assessment: The time of assessment and follow-up duration may impact the results of MSC treatment of DOX cardiotoxicity, depending on the time given to the stem cells to exert their effects. Chen et al. (2006) have related the nonsignificant improvement in LVEF partly to the number of animals and the short follow-up time, which may have underestimated the improvement of LVEF after stem cell infusion.
(G) Addition of supporting elements: Some studies investigated whether editing the genetic properties of MSCs or supplementing them with pharmacological compounds can enhance their therapeutic efficacy. For example, Deng et al. (2017) reported that Nkx2.5 transfection improved MSC differentiation into cardiomyocyte-like cells (as claimed by the authors) and reduced myocardial fibrosis. Nkx2.5 is a gene that encodes a homeobox-containing transcription factor that is essential for proper heart formation and development (Jamali et al., 2001). Another study by Zeng et al. evaluated the effect of miR-21 overexpression in BMMSCs. They reported that BMMSCs, overexpressing miR-21, exhibited more proliferation than untransfected cells and significantly enhanced expression of Bcl-2, VEGF, and Cx43 and reduced expression of Bax and troponin T (Zeng et al., 2017).
Other studies explored the value of pharmacological support for MSCs function. Mousa et al. reported that the combination of hUCB-MSCs and carvedilol reduced DOX-induced electrocardiographic abnormalities and cardiac concentrations of oxidative stress markers and caspase-3. This may be due to its antioxidant and anti-inflammatory effects (Mousa et al., 2018). DOX has been reported to induce oxidative stress and senescence in MSCs (Xia and Hou, 2018b; Kozhukharova et al., 2018). Therefore, supplementation with antioxidants like resveratrol may be a promising strategy for future studies (Pınarlı et al., 2013; Shaban et al., 2017). In another study, the cardiac tissues of rats treated by BMMSCs and sodium valproate combination showed better histopathological appearance and cardiac homing of MSCs than rats treated by stem cells alone. To enhance homing of BMMSCs, Soliman et al. investigated whether supplementing BMMSCs with valproate along with an electric current over the shoulder would enhance cardiac homing. They concluded that this pharmaco-electrical method can be beneficial in enhancing MSCs homing in the injured myocardium (Soliman et al., 2017).
All the previous factors may affect the outcomes of stem cell treatment. More studies are needed to investigate and fulfill all these factors to know the optimal cell type, dose, and route of administration, as well as the proper time interval between their use and DOX injection.
Discussion and Implications for Future Research
The experiments reviewed here demonstrated favorable effects for administering MSCs in DOX-induced cardiomyopathy through diverse mechanisms. They also showed that the efficacy of MSC therapy for this condition depends on several factors that must be further studied and accommodated for. Some lessons for further preclinical experiments and future trials can be learnt from discussing the previous trials on MSC therapy for other cardiovascular conditions as HF and ischemic cardiomyopathy.
Stem cell-based therapies have been tested in numerous HF and MI trials and were proven to be clinically feasible (Sanganalmath and Bolli, 2013). However, it has recently become evident that the improvements observed in these trials are not because of direct differentiation of stem cells into cardiomyocytes, but because of the immune regulation, angiogenic abilities, and paracrine factors produced by these cells (Eschenhagen et al., 2017). The extent of cardiac performance improvement in these trials varies as well. Some trials in ischemic cardiomyopathy reported significant satisfactory improvements, while others reported significant yet modest improvement (Fisher et al., 2015). The heterogeneity of their results can be attributed to the differences in the used stem cell type, the routes of administration, and timing of the interventions. Despite these limitations, clinical trials are continuing, while a more comprehensive understanding of the basic science is still required.
Although several studies have been conducted to test the effectiveness of stem cell therapy in human subjects, the optimal conditions for the therapy are still debated. Regarding the timing of administration, delivery of the stem cells within the first week of insult seems to be more beneficial. Further, we would expect that higher doses of cells would be associated with more significant results. However, as indicated before, this is not necessarily the case (Chen et al., 2006; Mao et al., 2017). With regard to the cell type, various stem cell types have demonstrated the ability to improve cardiac functions; however, certain types are generally less recommended. For example, authors using skeletal myoblasts have reported ventricular arrhythmias in their subjects, suggesting lack of synchrony of these cells (Sanganalmath and Bolli, 2013). Further, it has been shown that MSCs may increase the resistance of tumor cells to DOX (Chen et al., 2014). However, other studies have revealed contradictory findings; that is, MSCs increase tumor chemosensitivity to DOX (Klopp et al., 2011; Kucerova et al., 2013). Another study on mice and humans with metastasis hinted that some MSC populations may aggravate tumor growth, suggesting a tumorigenic potential for MSCs (Kudo-Saito, 2015). However, a meta-analysis of 36 clinical studies found no association between MSC treatment and tumor development (Lalu et al., 2012). Further research and long-term follow-up are needed to establish the safety of MSC therapy.
Despite these limitations and uncertainties, cell-based therapy remains a viable option for the treatment of DOX-induced cardiotoxicity because the subjects in these trials were patients who suffered from severe cardiac injury (from ischemia) and have markedly decreased LVEF. On the contrary, patients treated with DOX are less likely to be as severely injured. Additionally, the mechanisms of cardiac insult in the two conditions are different. Whether cell therapy is going to be a clinical solution for DOX cardiomyopathy and other cardiac conditions is a question that will be answered with time and further investigations. For now, this potential therapeutic approach needs more time in the bench before moving to the clinic.
Overall, the reviewed studies reported favorable effects for administering MSCs in DOX-induced cardiomyopathy through diverse mechanisms; however, these mechanisms are unlikely to involve cardiogenic differentiation. These effects may depend on several factors, including the cell type, dose, and route of administration. Better understanding of the involved mechanisms and the factors governing the outcomes of MSC therapy is essential before moving to clinical application in patients with DOX-induced cardiomyopathy.
Author Contributions
AIA contributed to idea conception and designing the manuscript structure. AAS, AS, and AMA contributed to searching the literature and summarizing the published reports on the topic. All authors contributed to writing and revising the manuscript draft and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BMMSCs, bone-marrow mesenchymal stem cells; DOX, doxorubicin; EPCs, endothelial progenitor cells; hUCB, human umbilical cord blood; iPSCs, induced pluripotent stem cells; MSCs, mesenchymal stem cells.
References
- Abd Allah S. H., Hussein S. (2017). Functional and structural assessment of the effect of human umbilical cord blood mesenchymal stem cells in doxorubicin-induced cardiotoxicity. J. Cell Biochem. 118 (10), 3119–3129. 10.1002/jcb.26168 [DOI] [PubMed] [Google Scholar]
- Abdel-Daim M. M., Khalifa H. A., Ahmed A. A. (2017). Allicin ameliorates doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Cancer Chemother. Pharmacol. 80 (4), 745–753. 10.1007/s00280-017-3413-7 [DOI] [PubMed] [Google Scholar]
- Abushouk A. I., Ismail A., Salem A. M. A., Afifi A. M., Abdel-Daim M. M. (2017). Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 90, 935–946. 10.1016/j.biopha.2017.04.033 [DOI] [PubMed] [Google Scholar]
- Ammar H. I., Sequiera G. L., Nashed M. B., Ammar R. I., Gabr H. M., Elsayed H. E., et al. (2015). Comparison of adipose tissue- and bone marrow-derived mesenchymal stem cells for alleviating doxorubicin-induced cardiac dysfunction in diabetic rats. Stem Cell Res. Ther. 6 (1), 148. 10.1186/s13287-015-0142-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankrum J., Karp J. M. (2010). Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol. Med. 16 (5), 203–209. 10.1016/j.molmed.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam L. B., Wagers A. J., Christensen J. L., Kofidis T., Weissman I. L., Robbins R. C. (2004). Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 428 (6983), 668. 10.1038/nature02460 [DOI] [PubMed] [Google Scholar]
- Barile L., Chimenti I., Gaetani R., Forte E., Miraldi F., Frati G., et al. (2007). Cardiac stem cells: isolation, expansion and experimental use for myocardial regeneration. Nat. Rev. Cardiol. 4 (S1), S9. 10.1038/ncpcardio0738 [DOI] [PubMed] [Google Scholar]
- Barry E., Alvarez J. A., Scully R. E., Miller T. L., Lipshultz S. E. (2007). Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin. Pharmacother. 8 (8), 1039–1058. 10.1517/14656566.8.8.1039 [DOI] [PubMed] [Google Scholar]
- Beltrami A. P., Urbanek K., Kajstura J., Yan S.-M., Finato N., Bussani R., et al. (2003). Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114 (6), 763–776. 10.1016/S0092-8674(03)00687-1 [DOI] [PubMed] [Google Scholar]
- Beltrami A. P., Urbanek K., Kajstura J., Yan S.-M., Finato N., Bussani R., et al. (2001). Evidence that human cardiac myocytes divide after myocardial infarction. N. Engl. J. Med. 344 (23), 1750–1757. 10.1056/NEJM200106073442303 [DOI] [PubMed] [Google Scholar]
- Bolli R., Chugh A. R., D'Amario D., Loughran J. H., Stoddard M. F., Ikram S., et al. (2011). Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378 (9806), 1847–1857. 10.1016/S0140-6736(11)61590-0 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Calió M. L., Marinho D. S., Ko G. M., Ribeiro R. R., Carbonel A. F., Oyama L. M., et al. (2014). Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radic. Biol. Med. 70, 141–154. 10.1016/j.freeradbiomed.2014.01.024 [DOI] [PubMed] [Google Scholar]
- Cesselli D., Beltrami A. P., D'Aurizio F., Marcon P., Bergamin N., Toffoletto B., et al. (2011). Effects of age and heart failure on human cardiac stem cell function. Am. J. Pathol. 179 (1), 349–366. 10.1016/j.ajpath.2011.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee K., Zhang J., Honbo N., Karliner J. S. (2010). Doxorubicin cardiomyopathy. Cardiology 115 (2), 155–162. 10.1159/000265166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.-R., Lu D.-Y., Lin H.-Y., Yeh W.-L. (2014). Mesenchymal stem cell-induced doxorubicin resistance in triple negative breast cancer. Biomed. Res. Int. 2014, 532161. 10.1155/2014/532161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Fan Z. C., Liu X. J., Deng J. L., Zhang L., Rao L., et al. (2006). Effects of autologous stem cell transplantation on ventricular electrophysiology in doxorubicin-induced heart failure. Cell Biol. Int. 30 (7), 576–582. 10.1016/j.cellbi.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Chimenti C., Kajstura J., Torella D., Urbanek K., Heleniak H., Colussi C., et al. (2003). Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ. Res. 93 (7), 604–613. 10.1161/01.RES.0000093985.76901.AF [DOI] [PubMed] [Google Scholar]
- Choi K. D., Yu J., Smuga-Otto K., Salvagiotto G., Rehrauer W., Vodyanik M., et al. (2009). Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 27 (3), 559–567. 10.1002/stem.20080922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J., Anderson L., Willmott N., Smyth J. F. (1991). The molecular pharmacology of doxorubicin in vivo. Eur. J. Cancer Clin. Oncol. 27 (5), 532–535. 10.1016/0277-5379(91)90209-V [DOI] [PubMed] [Google Scholar]
- Curigliano G., Cardinale D., Dent S., Criscitiello C., Aseyev O., Lenihan D., Cipolla C. M. (2016). Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J. Clin. 66 (4), 309–325. 10.3322/caac.21341 [DOI] [PubMed] [Google Scholar]
- Cvetkovic R. S., Scott L. J. (2005). Dexrazoxane: a review of its use for cardioprotection during anthracycline chemotherapy. Drugs 65 (7), 1005–1024. 10.2165/00003495-200565070-00008 [DOI] [PubMed] [Google Scholar]
- D’amario D., Cabral-Da-Silva M. C., Zheng H., Fiorini C., Goichberg P., Steadman E., et al. (2011). Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circ. Res. 108 (12), 1467–1481. 10.1161/CIRCRESAHA.111.240648 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- De Angelis A., Piegari E., Cappetta D., Marino L., Filippelli A., Berrino L., et al. (2010). Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation 121 (2), 276–292. 10.1161/CIRCULATIONAHA.109.895771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng B., Wang J. X., Hu X. X., Duan P., Wang L., Li Y., Zhu Q. L. (2017). Nkx2.5 enhances the efficacy of mesenchymal stem cells transplantation in treatment heart failure in rats. Life Sci. 182, 65–72. 10.1016/j.lfs.2017.06.014 [DOI] [PubMed] [Google Scholar]
- Dhein S., Garbade J., Rouabah D., Abraham G., Ungemach F.-R., Schneider K., et al. (2006). Effects of autologous bone marrow stem cell transplantation on beta-adrenoceptor density and electrical activation pattern in a rabbit model of non-ischemic heart failure. J. Cardiothorac. Surg. 1 (1), 17. 10.1186/1749-8090-1-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di G.-H., Jiang S., Li F.-Q., Sun J.-Z., Wu C.-T., Hu X., Duan H.-F. (2012). Human umbilical cord mesenchymal stromal cells mitigate chemotherapy-associated tissue injury in a pre-clinical mouse model. Cytotherapy 14 (4), 412–422. 10.3109/14653249.2011.646044 [DOI] [PubMed] [Google Scholar]
- Dias C., Francisco J., Cardoso M., Cunha R., Simeoni R. (2015). Cardiac analysis of autologous transplantation of cocultured skeletal myoblasts and mesenchymal cells in a rat model doxorubicin-induced cardiotoxicity: histopathological and functional studies. J. Clin. Exp. Cardiolog. 6 (407), 2. 10.4172/2155-9880.1000407 [DOI] [Google Scholar]
- Dimmeler S., Zeiher A. M., Schneider M. D. (2005). Unchain my heart: the scientific foundations of cardiac repair. J. Clin. Invest. 115 (3), 572–583. 10.1172/JCI200524283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit P., Katare R. (2015). Challenges in identifying the best source of stem cells for cardiac regeneration therapy. Stem Cell Res. Ther. 6 (1), 26. 10.1186/s13287-015-0010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshow J. H. (1983). Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res. 43 (2), 460–472. [PubMed] [Google Scholar]
- Duerr R. L., Huang S., Miraliakbar H. R., Clark R., Chien K. R., Ross J. (1995). Insulin-like growth factor-1 enhances ventricular hypertrophy and function during the onset of experimental cardiac failure. J. Clin. Invest. 95 (2), 619–627. 10.1172/JCI117706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenhagen T., Bolli R., Braun T., Field L. J., Fleischmann B. K., Frisen J., et al. (2017). Cardiomyocyte regeneration: a consensus statement. Circulation 136 (7), 680–686. 10.1161/CIRCULATIONAHA.117.029343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrans V., Clark J., Zhang J., Yu Z., Herman E. (1997). Pathogenesis and prevention of doxorubicin cardiomyopathy. Tsitologiia 39 (10), 928–937. [PubMed] [Google Scholar]
- Fisher S. A., Doree C., Mathur A., Martin-Rendon E. (2015). Meta-analysis of cell therapy trials for patients with heart failure. Circ. Res. 116 (8), 1361–1377. 10.1161/CIRCRESAHA.116.304386 [DOI] [PubMed] [Google Scholar]
- Gammella E., Maccarinelli F., Buratti P., Recalcati S., Cairo G. (2014). The role of iron in anthracycline cardiotoxicity. Front. Pharmacol. 5, 25. 10.3389/fphar.2014.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbade J., Dhein S., Lipinski C., Aupperle H., Arsalan M., Borger M. A., et al. (2009). Bone marrow-derived stem cells attenuate impaired contractility and enhance capillary density in a rabbit model of doxorubicin-induced failing hearts. J. Card. Surg. 24 (5), 591–599. 10.1111/j.1540-8191.2009.00844.x [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., Huart P., Praet M., Brasseur R., Ruysschaert J.-M. (1990). Structure of the adriamycin–cardiolipin complex: role in mitochondrial toxicity. Biophys. Chem. 35 (2–3), 247–257. 10.1016/0301-4622(90)80012-V [DOI] [PubMed] [Google Scholar]
- Gopinath S., Vanamala S. K., Gondi C. S., Rao J. S. (2010). Human umbilical cord blood derived stem cells repair doxorubicin-induced pathological cardiac hypertrophy in mice. Biochem. Biophys. Res. Commun. 395 (3), 367–372. 10.1016/j.bbrc.2010.04.021 [DOI] [PubMed] [Google Scholar]
- Hatzistergos K. E., Quevedo H., Oskouei B. N., Hu Q., Feigenbaum G. S., Margitich I. S., et al. (2010). Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ. Res. 107 (7), 913–922. 10.1161/CIRCRESAHA.110.222703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzistergos K. E., Takeuchi L. M., Saur D., Seidler B., Dymecki S. M., Mai J. J., et al. (2015). cKit+ cardiac progenitors of neural crest origin. Proc. Natl. Acad. Sci. U.S.A. 112 (42), 13051–13056. 10.1073/pnas.1517201112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydardedeoglu A. E., Özgermen D. B. B., Yavuz O. (2018). Mesenchymal stem cells reduce left ventricular mass in rats with doxorubicin-induced cardiomyopathy. Int. J. Morphol. 36 (1), 48–53. 10.4067/S0717-95022018000100048 [DOI] [Google Scholar]
- Huang C., Zhang X., Ramil J. M., Rikka S., Kim L., Lee Y, et al. (2010). Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation 121 (5), 675–683. 10.1161/CIRCULATIONAHA.109.902221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Miller S. C., Billingham M. E., Akimoto H., Torti S. V., Wade R., et al. (1990). Doxorubicin selectively inhibits muscle gene expression in cardiac muscle cells in vivo and in vitro. Proc. Natl. Acad. Sci. U.S.A. 87 (11), 4275–4279. 10.1073/pnas.87.11.4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali M., Rogerson P. J., Wilton S., Skerjanc I. S. (2001). Nkx2–5 activity is essential for cardiomyogenesis. J. Biol. Chem. 276 (45), 42252–42258. 10.1074/jbc.M107814200 [DOI] [PubMed] [Google Scholar]
- Jansen C. E., Dodd M. J., Miaskowski C. A., Dowling G. A., Kramer J. (2008). Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psychooncology 17 (12), 1189–1195. 10.1002/pon.1342 [DOI] [PubMed] [Google Scholar]
- Jin G., Qiu G., Wu D., Hu Y., Qiao P., Fan C., Gao F. (2013). Allogeneic bone marrow-derived mesenchymal stem cells attenuate hepatic ischemia–reperfusion injury by suppressing oxidative stress and inhibiting apoptosis in rats. Int. J. Mol. Med. 31 (6), 1395–1401. 10.3892/ijmm.2013.1340 [DOI] [PubMed] [Google Scholar]
- Kalam K., Marwick T. H. (2013). Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur. J. Cancer 49 (13), 2900–2909. 10.1016/j.ejca.2013.04.030 [DOI] [PubMed] [Google Scholar]
- Kang S. K., Shin I. S., Ko M. S., Jo J. Y., Ra J. C. (2012). Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int. 2012, 342968. 10.1155/2012/342968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H., Fujita J., Kinjo K., Matsuzaki Y., Tsuma M., Miyatake H, et al. (2004). Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 104 (12), 3581–3587. 10.1182/blood-2004-04-1488 [DOI] [PubMed] [Google Scholar]
- Klopp A. H., Gupta A., Spaeth E., Andreeff M., Marini F., III (2011). Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells 29 (1), 11–19. 10.1002/stem.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhukharova I., Zemelko V., Kovaleva Z., Alekseenko L., Lyublinskaya O., Nikolsky N. (2018). Therapeutic doses of doxorubicin induce premature senescence of human mesenchymal stem cells derived from menstrual blood, bone marrow and adipose tissue. Int. J. Hematol. 107 (3), 286–296. 10.1007/s12185-017-2346-6 [DOI] [PubMed] [Google Scholar]
- Krysko D. V., Garg A. D., Kaczmarek A., Krysko O., Agostinis P., Vandenabeele P. (2012). Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12 (12), 860. 10.1038/nrc3380 [DOI] [PubMed] [Google Scholar]
- Kucerova L., Skolekova S., Matuskova M., Bohac M., Kozovska Z. (2013). Altered features and increased chemosensitivity of human breast cancer cells mediated by adipose tissue-derived mesenchymal stromal cells. BMC Cancer 13 (1), 535. 10.1186/1471-2407-13-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo-Saito C. (2015). Cancer-associated mesenchymal stem cells aggravate tumor progression. Front. Cell Dev. Biol. 3, 23. 10.3389/fcell.2015.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H.-C., Liu T.-J., Ting C.-T., Sharma P. M., Wang P. H. (2003). Insulin-like growth factor-1 prevents loss of electrochemical gradient in cardiac muscle mitochondria via activation of PI 3 kinase/Akt pathway. Mol. Cell. Endocrinol. 205 (1–2), 99–106. 10.1016/S0303-7207(03)00200-4 [DOI] [PubMed] [Google Scholar]
- Lalu M. M., McIntyre L., Pugliese C., Fergusson D., Winston B. W., Marshall J. C., et al. (2012). Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One 7 (10), e47559. 10.1371/journal.pone.0047559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. K., Wang T. T. (1999). Differential effects of chemotherapeutic agents on the Bcl-2/Bax apoptosis pathway in human breast cancer cell line MCF-7. Breast Cancer Res. Treat. 55 (1), 73–83. 10.1023/A:1006190802590 [DOI] [PubMed] [Google Scholar]
- Levine A. J. (1997). p53, the cellular gatekeeper for growth and division. Cell 88 (3), 323–331. 10.1016/S0092-8674(00)81871-1 [DOI] [PubMed] [Google Scholar]
- Li Y., He L., Huang X., Bhaloo S. I., Zhao H., Zhang S., et al. (2018). Genetic lineage tracing of nonmyocyte population by dual recombinases. Circulation 138 (8), 793–805. 10.1161/CIRCULATIONAHA.118.034250 [DOI] [PubMed] [Google Scholar]
- Lim C. C., Zuppinger C., Guo X., Kuster G. M., Helmes M., Eppenberger H. M., et al. (2004). Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J. Biol. Chem. 279 (9), 8290–8299. 10.1074/jbc.M308033200 [DOI] [PubMed] [Google Scholar]
- Liotta F., Angeli R., Cosmi L., Fili L., Manuelli C., Frosali F., et al. (2008). Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells 26 (1), 279–289. 10.1634/stemcells.2007-0454 [DOI] [PubMed] [Google Scholar]
- Lipshultz S. E., Colan S. D., Gelber R. D., Perez-Atayde A. R., Sallan S. E., Sanders S. P. (1991). Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N. Engl. J. Med. 324 (12), 808–815. 10.1056/NEJM199103213241205 [DOI] [PubMed] [Google Scholar]
- Liu H., McTaggart S. J., Johnson D. W., Gobe G. C. (2012). Original article anti-oxidant pathways are stimulated by mesenchymal stromal cells in renal repair after ischemic injury. Cytotherapy 14 (2), 162–172. 10.3109/14653249.2011.613927 [DOI] [PubMed] [Google Scholar]
- Loffredo F. S., Steinhauser M. L., Gannon J., Lee R. T. (2011). Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell 8 (4), 389–398. 10.1016/j.stem.2011.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y. L., Kerrigan J. E., Lin C.-P., Azarova A. M., Tsai Y.-C., Ban Y., Liu L. F. (2007). Topoisomerase IIβ-mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 67 (18), 8839–8846. 10.1158/0008-5472.CAN-07-1649 [DOI] [PubMed] [Google Scholar]
- Makkar R. R., Smith R. R., Cheng K., Malliaras K., Thomson L. E., Berman D., et al. (2012). Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379 (9819), 895–904. 10.1016/S0140-6736(12)60195-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliken B. D., Molkentin J. D. (2018). Undeniable evidence that the adult mammalian heart lacks an endogenous regenerative stem cell. Circulation 138 (8), 806–808. 10.1161/CIRCULATIONAHA.118.035186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantawy E. M., El-Bakly W. M., Esmat A., Badr A. M., El-Demerdash E. (2014). Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur. J. Pharmacol. 728, 107–118. 10.1016/j.ejphar.2014.01.065 [DOI] [PubMed] [Google Scholar]
- Mao C., Hou X., Wang B., Chi J., Jiang Y., Zhang C., Li Z. (2017). Intramuscular injection of human umbilical cord-derived mesenchymal stem cells improves cardiac function in dilated cardiomyopathy rats. Stem Cell Res. Ther. 8 (1), 18. 10.1186/s13287-017-0472-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A., Fernández-Avilés F., Dimmeler S., Hauskeller C., Janssens S., Menasche P., et al. (2017). The consensus of the Task Force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for the treatment of acute myocardial infarction and heart failure: update 2016. Eur. Heart J. 38 (39), 2930–2935. 10.1093/eurheartj/ehw640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele D., Nardozza M., Spallarossa P., Frassoldati A., Tocchetti C. G., Cadeddu C., et al. (2016). Current views on anthracycline cardiotoxicity. Heart Fail. Rev. 21 (5), 621–634. 10.1007/s10741-016-9564-5 [DOI] [PubMed] [Google Scholar]
- Messina E., De Angelis L., Frati G., Morrone S., Chimenti S., Fiordaliso F., et al. (2004). Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 95 (9), 911–921. 10.1161/01.RES.0000147315.71699.51 [DOI] [PubMed] [Google Scholar]
- Mohammadi Gorji S., Karimpor Malekshah A. A., Hashemi-Soteh M. B., Rafiei A., Parivar K., Aghdami N. (2012). Effect of mesenchymal stem cells on Doxorubicin-induced fibrosis. Cell J. 14 (2), 142–151. 10.22074/cellj.2012.989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa H. S. E., Abdel Aal S. M., Abbas N. A. T. (2018). Umbilical cord blood–mesenchymal stem cells and carvedilol reduce doxorubicin-induced cardiotoxicity: possible role of insulin-like growth factor-1. Biomed. Pharmacother. 105, 1192–1204. 10.1016/j.biopha.2018.06.051 [DOI] [PubMed] [Google Scholar]
- Murry C. E., Soonpaa M. H., Reinecke H., Nakajima H., Nakajima H. O., Rubart M., et al. (2004). Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 428 (6983), 664. 10.1038/nature02446 [DOI] [PubMed] [Google Scholar]
- Nygren J. M., Jovinge S., Breitbach M., Säwén P., Röll W., Hescheler J., et al. (2004). Bone marrow–derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nature Medicine 10 (5), 494. 10.1038/nm1040 [DOI] [PubMed] [Google Scholar]
- Oliveira M. S., Melo M. B., Carvalho J. L., Melo I. M., Lavor M. S., Gomes D. A., et al. (2013). Doxorubicin cardiotoxicity and cardiac function improvement after stem cell therapy diagnosed by strain echocardiography. J. Cancer Sci. Ther. 5 (2), 52–57. 10.4172/1948-5956.1000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S. M., Li B., et al. (2001). Bone marrow cells regenerate infarcted myocardium. Nature 410 (6829), 701. 10.1038/35070587 [DOI] [PubMed] [Google Scholar]
- Pan S.-S., Bachur N. R. (1980). Xanthine oxidase catalyzed reductive cleavage of anthracycline antibiotics and free radical formation. Mol. Pharmacol. 17 (1), 95–99. [PubMed] [Google Scholar]
- Pecoraro M., Del Pizzo M., Marzocco S., Sorrentino R., Ciccarelli M., Iaccarino G., et al. (2016). Inflammatory mediators in a short-time mouse model of doxorubicin-induced cardiotoxicity. Toxicol. Appl. Pharmacol. 293, 44–52. 10.1016/j.taap.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Pelacho B., Aranguren X. L., Mazo M., Abizanda G., Gavira J. J., Clavel C., et al. (2007). Plasticity and cardiovascular applications of multipotent adult progenitor cells. Nat. Rev. Cardiol. 4 (S1), S15. 10.1038/ncpcardio0735 [DOI] [PubMed] [Google Scholar]
- Pfister O., Della Verde G., Liao R., Kuster G. M. (2014). Regenerative therapy for cardiovascular disease. Transl. Res. 163 (4), 307–320. 10.1016/j.trsl.2013.12.005 [DOI] [PubMed] [Google Scholar]
- Piegari E., De Angelis A., Cappetta D., Russo R., Esposito G., Costantino S., et al. (2013). Doxorubicin induces senescence and impairs function of human cardiac progenitor cells. Basic Res. Cardiol. 108 (2), 334. 10.1007/s00395-013-0334-4 [DOI] [PubMed] [Google Scholar]
- Pınarlı F. A., Turan N. N., Güçlü Pınarlı F., Okur A., Sönmez D., Ulus T., et al. (2013). Resveratrol and adipose-derived mesenchymal stem cells are effective in the prevention and treatment of doxorubicin cardiotoxicity in rats. Pediatr. Hematol. Oncol. 30 (3), 226–238. 10.3109/08880018.2012.762962 [DOI] [PubMed] [Google Scholar]
- Quaini F., Urbanek K., Beltrami A. P., Finato N., Beltrami C. A., Nadal-Ginard B., et al. (2002). Chimerism of the transplanted heart. N. Engl. J. Med. 346 (1), 5–15. 10.1056/NEJMoa012081 [DOI] [PubMed] [Google Scholar]
- Renu K., Abilash V., Tirupathi Pichiah P. B, Arunachalam S. (2018). Molecular mechanism of doxorubicin-induced cardiomyopathy—an update. Eur. J. Pharmacol. 818, 241–253. 10.1016/j.ejphar.2017.10.043 [DOI] [PubMed] [Google Scholar]
- Saidi A., Alharethi R. (2011). Management of chemotherapy induced cardiomyopathy. Curr. Cardiol. Rev. 7 (4), 245–249. 10.2174/157340311799960681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samper E., Diez-Juan A., Montero J., Sepulveda P. (2013). Cardiac cell therapy: boosting mesenchymal stem cells effects. Stem Cell Rev. 9 (3), 266–280. 10.1007/s12015-012-9353-z [DOI] [PubMed] [Google Scholar]
- Sanganalmath S. K., Bolli R. (2013). Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 113 (6), 810–834. 10.1161/CIRCRESAHA.113.300219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaban S., El-Husseny M. W. A., Abushouk A. I., Salem A. M. A., Mamdouh M., Abdel-Daim M. M. (2017). Effects of antioxidant supplements on the survival and differentiation of stem cells. Oxid. Med. Cell. Longev. 2017, 5032102. 10.1155/2017/5032102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman N., Abd-Allah S. H., Hussein S., Alaa Eldeen M. (2017). Factors enhancing the migration and the homing of mesenchymal stem cells in experimentally induced cardiotoxicity in rats. IUBMB Life 69 (3), 162–169. 10.1002/iub.1600 [DOI] [PubMed] [Google Scholar]
- Spallarossa P., Altieri P., Barisione C., Passalacqua M., Aloi C., Fugazza G., et al. (2010). p38 MAPK and JNK antagonistically control senescence and cytoplasmic p16INK4A expression in doxorubicin-treated endothelial progenitor cells. PLoS One 5 (12), e15583. 10.1371/journal.pone.0015583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard M. F., Seeger J., Liddell N. E., Hadley T. J., Sullivan D. M., Kupersmith J. (1992). Prolongation of isovolumetric relaxation time as assessed by Doppler echocardiography predicts doxorubicin-induced systolic dysfunction in humans. J. Am. Coll. Cardiol. 20 (1), 62–69. 10.1016/0735-1097(92)90138-D [DOI] [PubMed] [Google Scholar]
- Swain S. M., Whaley F. S., Ewer M. S. (2003). Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97 (11), 2869–2879. 10.1002/cncr.11407 [DOI] [PubMed] [Google Scholar]
- Tacar O., Sriamornsak P., Dass C. R. (2013). Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 65 (2), 157–170. 10.1111/j.2042-7158.2012.01567.x [DOI] [PubMed] [Google Scholar]
- Takemura G., Fujiwara H. (2007). Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 49 (5), 330–352. 10.1016/j.pcad.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Tulubas F., Gurel A., Oran M., Topcu B., Caglar V., Uygur E. (2015). The protective effects of ω-3 fatty acids on doxorubicin-induced hepatotoxicity and nephrotoxicity in rats. Toxicol. Ind. Health 31 (7), 638–644. 10.1177/0748233713483203 [DOI] [PubMed] [Google Scholar]
- Valle-Prieto A., Conget P. A. (2010). Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 19 (12), 1885–1893. 10.1089/scd.2010.0093 [DOI] [PubMed] [Google Scholar]
- Van Berlo J. H., Kanisicak O., Maillet M., Vagnozzi R. J., Karch J., Lin S.-C. J., et al. (2014). C-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509 (7500), 337. 10.1038/nature13309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchi G., Ameri P., Cadeddu C., Ghigo A., Madonna R., Marone G, et al. (2018). Antineoplastic drug-induced cardiotoxicity: a redox perspective. Front. Physiol. 9, 167. 10.3389/fphys.2018.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicinanza C., Aquila I., Cianflone E., Scalise M., Marino F., Mancuso T., et al. (2018). Kit cre knock-in mice fail to fate-map cardiac stem cells. Nature 555 (7697), E1. 10.1038/nature25771 [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Layard M. W., Basa P., Davis H. L., Jr., Von Hoff A. L., Rozencweig M., Muggia F. M. (1979). Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 91 (5), 710–717. 10.7326/0003-4819-91-5-710 [DOI] [PubMed] [Google Scholar]
- Wada T., Stepniak E., Hui L., Leibbrandt A., Katada T., Nishina H., et al. (2008). Antagonistic control of cell fates by JNK and p38-MAPK signaling. Cell Death Differ. 15 (1), 89. 10.1038/sj.cdd.4402222 [DOI] [PubMed] [Google Scholar]
- Xia W., Hou M. (2018. a). Mesenchymal stem cells confer resistance to doxorubicin-induced cardiac senescence by inhibiting microRNA-34a. Oncol. Lett. 15 (6), 10037–10046. 10.3892/ol.2018.8438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W., Hou M. (2018. b). Macrophage migration inhibitory factor rescues mesenchymal stem cells from doxorubicin-induced senescence though the PI3K-Akt signaling pathway. Int. J. Mol. Med. 41 (2), 1127–1137. 10.3892/ijmm.2017.3282 [DOI] [PubMed] [Google Scholar]
- Yu Q., et al. (2014). Impact of repeated intravenous bone marrow mesenchymal stem cells infusion on myocardial collagen network remodeling in a rat model of doxorubicin-induced dilated cardiomyopathy. Mol. Cell. Biochem. 387 (1–2), 279–85. 10.1007/s11010-013-1894-1 [DOI] [PubMed] [Google Scholar]
- Zeng Y.-L., Zheng H., Chen Q.-R., Yuan X.-H., Ren J.-H., Luo X.-F., et al. (2017). Bone marrow-derived mesenchymal stem cells overexpressing MiR-21 efficiently repair myocardial damage in rats. Oncotarget 8 (17), 29161. 10.18632/oncotarget.16254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Liu X., Bawa-Khalfe T., Lu L.-S., Lyu Y. L., Liu L. F., Yeh E. T. (2012). Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nature Medicine 18 (11), 1639. 10.1038/nm.2919 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liang X., Liao S., Wang W., Wang J., Li X., et al. (2015). Potent paracrine effects of human induced pluripotent stem cell-derived mesenchymal stem cells attenuate doxorubicin-induced cardiomyopathy. Sci. Rep. 5, 11235. 10.1038/srep11235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., McLaughlin D., Robinson E., Harvey A. P., Hookham M. B., Shah A. M., et al. (2010). Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with Doxorubicin chemotherapy. Cancer Res. 70 (22), 9287–9297. 10.1158/0008-5472.CAN-10-2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoja C., Garcia P. B., Rota C., Conti S., Gagliardini E., Corna D., et al. (2012). Mesenchymal stem cell therapy promotes renal repair by limiting glomerular podocyte and progenitor cell dysfunction in adriamycin-induced nephropathy. Am. J. Physiol. Renal Physiol. 303 (9), F1370–F1381. 10.1152/ajprenal.00057.2012 [DOI] [PubMed] [Google Scholar]