Abstract
Oxytocin is a complex molecule involved in a variety of biological processes at both the central and the peripheral level. Although its role was initially associated almost exclusively with birth and breastfeeding, recent studies are suggesting that in fact oxytocin could be involved in many other physiological and pathological processes. In this way, lately there is a growing interest towards a possible involvement of oxytocin in many etiopathogenic and psychopathological processes, as for example in the affective disorders, where the roles of oxytocin are not yet clearly understood. In this paper we shortly describe the main aspects regarding the relevance of oxytocin administration or its mechanisms in the affective disorders, as well as its relations with the hypothalamic-pituitary-adrenal axis and cortisol secretion. It seems that although the researches on the importance of oxytocin in the affective disorders are rather at the beginning, an increasing number of evidence is supporting the involvement of oxytocin in the pathogenic processes of these psychiatric disorders. Still, the studies covering this topic are still in their early days, and the results that are trying to understand if there is a major role of oxytocin in affective disorders are not consistent enough to draw definitive conclusions and establish with certainty where the place of oxytocin in the affective disorders pathology is.
Keywords: oxytocin, affective disorders, depression, cortisol, HPA axis
INTRODUCTION
Lately there is an increased interest in understanding the mechanisms involved in the pathological manifestations from the main neuropsychiatric disorders (1-3). In this way, oxytocin is a complex molecule involved in a variety of biological processes at both the central and the peripheral level. Although its role was initially associated almost exclusively with birth and breastfeeding, recent studies are suggesting that in fact oxytocin could be involved in many other physiological and neuropathological/ psychiatric processes (4, 5).
In fact, as we will show in the present paper, the role of oxytocin is extremely dependent on the environmental context in which the organism is at any given time and should be also considered in a dynamic relation with other molecules essentially important for the balanced functioning of a biological system, such as vasopressin, serotonin, dopamine or cortisol (6, 7).
From the structural point of view, oxytocin is a neuropeptide consisting of nine amino acids. Moreover, the pharmacokinetic data on the oxytocin metabolism showed that endogenous oxytocin, as well as the exogenously (e.g. intranasally, intramuscular or intravenous) administered oxytocin is entering the systemic circulation and attach to the circulating proteins in a proportion of 30% (4). Also, oxytocin half-life depends on the administration form (e.g. 1-6 min intravenous and 2 hours intranasally), while its metabolisation in the liver is done by the oxytocinase enzyme (8). It is also important to mention at this time that the oxytocin intranasal form of administration is able to cross the blood brain barrier, reaching the brain, where it acts on specific central receptors with some psychoactive effects (6), on which we are going to insist later in this report.
Regarding its production, oxytocin is mainly produced in the hypothalamus, by the magnocellular neurons of the supraoptic and paraventricular nuclei, being synthesized from an inactive precursor in the neurohypothalamus and excreted into the neurohypophysis where it is stored along with its carrier neurophysin I. From the hypophysis, oxytocin either enters the systemic circulation or is transported to other brain structures by various oxytocinergic pathways and regulates the activity of other neurotransmitters (10).
Also, other central sources for oxytocin production are the amygdala, some cortical areas, the basal ganglia, limbic system, the thalamus, the brainstem and the spinal cord (11, 12), while on the peripheral level the oxytocin is secreted on sites such as the circulatory system (13), the reproductive system (14) or the kidneys (15).
Introducing cortisol and the stress system
As mentioned before, it is classically known that on the peripheral level oxytocin is involved in many biological processes, mostly related to reproductive function, such as the stimulation of the uterine smooth muscle contraction at birth, milk ejection during lactation, the regulation of the menstrual cycle, could be a luteinisation factor or intervene in the ovarian steroidogenesis, spontaneous erection, ejaculation and orgasm (16) plus other functions such as natriuresis, kaliuresis, lowering the blood pressure, faster healing of wounds or reducing serum cortisol levels (12).
However, lately there is a growing interest towards a possible involvement of oxytocin in many etiopathogenic and psychopathological processes, as for example in the affective disorders, where the roles of oxytocin are not yet clearly understood (4, 5).
Thus, in regards to the connections between oxytocin and the affective disorders, in the present report we mainly considered two aspects: the first one is the complex relationship between oxytocin and the hypothalamic-pituitary-adrenal axis (HPA), with the association of this neuroendocrine axis and the pathophysiology of depression, while the second one is represented by the involvement of cortisol, stress and the HPA axis in the etiopathogeny of depression (17, 18).
It is known that both chronic and acute intense stress cantriggera depressive episode, while the presence of a stress vulnerability trait may be a significant risk factor in the etiopathogenesis of depression (19). Moreover, the importance of stress in depression is supported by numerous reports demonstrating direct and indirect links between stress and depression-like state or indicating chronic stress as a factor in the onset and maintenance of depression (20). Also, stress is important in the recurrence of the depressive episodes and in increasing the actual vulnerability to depression (21, 22). In addition, stressful events (e.g. childhood abuse) cause increased vulnerability to stress in a stressful situation, as compared to subjects without a history of abuse (23).
However, in addition to the psychological traits and environmental stress factors, the HPA axis disturbances and the cortisol levels imbalances are also very important factors in the pathology of depression.
Thus, the relationship between stress, HPA axis and depression is most likely a mechanism that involves the excessive stimulation of the axis against the background of psycho-traumatic events that could lead in the end to complex biochemical reactions that will release high concentrations of cortisol. In this entire context, the role of oxytocin appears to be a modulatory one.
Actually it seems that besides its known peripheral effects, cortisol seems also to have a role in the inhibition of the serotoninergic secretion and in triggering depressive-like symptoms (24). Moreover, this relationship could be mutual, since some studies showed that the administration of oxytocin exerted an inhibitory effect on the secretion of cortisol (25).
Also, other reports showed that more than half of the depressive cases are showing increased level of cortisol in the peripheral blood, but generally the percentage is variable and depending on the type of investigated patients (26). However, hypercortisolemia is not present in all cases of depression, and sometimes the level of cortisol is decreased in depression occurring after psycho-traumatic events (27). Thus, hypercortisolemia in patients with depression could be explained by an exhaustion of the HPA axis, mainly due to a chronic stimulation in the context of the prolonged psychosocial stress (28).
These aspects are suggesting, at least in some of cases, the involvement of cortisol in the depressive pathology and also the probability of some functional disturbances for the HPA axis in patients with depression. Basically, exposure to chronic stress disrupts the normal function of the axis, which can be an important pathophysiological mechanism for a susceptibility in developing depression later in life (29- 31).
The connections between oxytocin and cortisol
Besides the aforementioned aspects, it should be also mentioned that oxytocin was found to be a modulator for the neuro-endocrine axis, by adjusting the cortisol levels especially under stress conditions (32). In this way, Cardoso et al. demonstrated in a study published in 2013 a significant decrease of the salivary cortisol following the intranasal administration of oxytocin in men performing strenuous physical exercises (25). Moreover, the modulation of cortisol level was dose-dependent, since a dose of 48 IU oxytocin produced a greater decrease of cortisol, as compared to 24 IU (25).
In addition, in a study conducted on a group of postmenopausal women, with or without hormone replacement therapy, the exposure to a stressful situation (e.g. Trier social stress test) increased the levels of oxytocin, cortisol and impaired social relations, regardless of whether the patients were receiving or not hormone substitution treatment (33). In this way, the increased oxytocin levels could be explained as the result of some increases in cortisol levels. In other words, cortisol may be a stimulus for the release of oxytocin, which would also modulate the peripheral effects of cortisol.
However, we should mention that the oxytocin functions as anti-stress molecule could also have some other explanations, considering that various papers on the oxytocin-cortisol relationship reported mixed results. In this way, earlier researches in primates, published in 2005, described the effects of chronically administered oxytocin for 8 days in a group of 6 subjects in which the exposure to social stress (such as containment) for the study group and the controls increased the concentration of the adrenocortical hormone only in the control group, with no differences in cortisol levels observed between the 2 groups, suggesting that oxytocin can indirectly alleviate stress reactivity (34).
Thus, as mentioned before, there are some controversies in this area in research, since in general various authors showed a diminished reactivity to stress following the administration of oxytocin, while on the other side some studies support the opposite effect of oxytocin when administered early and chronically. In this way, in an experiment on animal models, the repeated neonatal administration of oxytocin caused disturbances in the functionality of the HPA axis, with a consequent increase in the aggressive behaviour and a low responsiveness of the HPA axis after the administration of dexamethasone (35). This could be perhaps explained by the fact that the early induced imbalance between cortisol and excessive exposure to oxytocin is causing a long-term disruption in the functionality of the axis.
Another aspect which could be mentioned in this context is represented by the fact that the variations of the cortisol levels during the phases of sleep does not correlate with the oxytocin level, since the oxytocin concentration is remaining relatively constant over night (36). On the other hand, it seems that the manipulation of oxytocin receptors by the administration of specific agonists or antagonists in some experimental animals can change the circadian rhythm phases at light exposure (37). This could suggest some possible therapeutic implications of oxytocin in disorders such as depression, which are also characterized by some changes of the circadian rhythm (38). However, the current data on the relevance of oxytocin in insomnia are quite scarce and more complex studies are necessary in order to determine the relevance of oxytocin in sleeping disorders.
In this way, the main connections between oxytocin, cortisol, HPA axis and some neuropsychiatric manifestations are presented in Figure 1.
Figure 1.
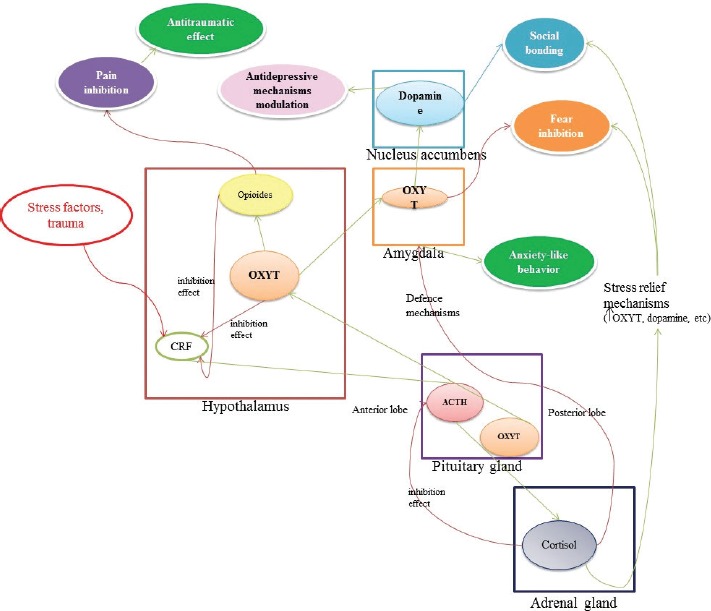
Schematic representation for the main interactions between oxytocin, cortisol, HPA axis and some neuropsychiatric manifestations (modified after 39-43).
Mechanistic aspects
As mentioned before, the impact of oxytocin on the HPA axis could be quite significant, but the manner in which oxytocin is influencing the axis reactivity to stress varies widely, depending on a broad range of factors. In this way, the presence of a stress factor, the intensity and nature of the stressor, the social support, previous functional integrity of the axis, the presence of other medical conditions or the type of medication used could influence in various ways the relationship between oxytocin and the HPA axis. As said above, these aspects generated some contradictory data in this area of research, which could be explained by the fact that the link between oxytocin and the biological stress system, represented by the HPA axis and cortisol, is reflecting only a small part from the dynamics of this relationship in a living organism that is of course subject to constant and various changes.
Moreover, the analysis of the relationship between oxytocin and depression should also include the specific clinical features of depression. Thus, perhaps the most relevant clinical changes in depression are mainly referring to the social behaviour, which marked by obvious social withdrawal, preference for isolation, diminished communication and interpersonal interaction (44). Thus, the involvement of oxytocin in certain mental processes altered in depression and its psychoactive effects could be quite relevant in this context.
In this way, the importance of oxytocin in social behavior is well known (45, 46), as it is involved in prosocial behaviours such as affiliation, trust, seeking social support, that are of course disturbed in depression (47). Moreover, oxytocin mediates social behaviour causing positive social attachments, prosocial behaviour, positive social communication (eye contact, positive nonverbal behaviour, disclosure of the self, empathy, imitation), trust and emotional bounding (4, 5).
Also, classical clinical studies demonstrated before that exogenous administration of oxytocin leads to pro-social behavior (10) (we will insist on this aspect in the last chapter of the present report). In fact, it seems that the biological substrate for the oxytocin effects in social attachment could be explained through the cortico-mesolimbic circuit mediation of the social stimuli (48).
In addition, by following the same line, the social dysfunction seen in depression and other psychiatric disorders could be explained by the disruption of the reward mechanism for the social interactions. Moreover, previous reports showed that the effectiveness of this reward mechanism is ensured through the coordinated activity of both oxytocin and serotonin in the nucleus accumbens (49, 50).
Also, oxytocin is a major mediator for the mesocorticolimbic dopaminergic circuit, which is quite important in the reward mechanisms and exerts various implications in the addictive behaviours, motivation, survival, reproduction, eating, drinking or in the sexual activity (51). In fact, these processes are also greatly affected in depression, where the patients are generally showing avolition, decreased motivation, decreased appetite, reduced libido, anhedonia or even suicidal behaviour (52).
Moreover, in depression there is a serious disturbance in interpreting the environmental conditions, as for example a neutral environment could be seen as uncertain, negative or hostile. This could be quite relevant, since some previous studies showed that oxytocin mediates the interaction of environmental factors with the individual factors, leading to a certain type of prosocial behaviour (4, 5). In this way, when the environment is interpreted as secure, the pro-social behaviors are stimulated, while in hostile, insecure or negative environmental conditions the oxytocin could even mediate aspects of antisocial behaviour (53).
In addition, the oxytocin relevance for the social behaviour seems to be influenced by some contextual factors (such as the presence of a stranger or a friend), as well as by individual demographic or psychological factors (e.g. gender, the type of attachment or the presence of some psychiatric symptoms).
We should also mention that oxytocin interacts with other hormones and neurotransmitters, or with the inflammatory processes which are believed to be involved in depression (54, 55). In fact, there are studies supporting the idea that oxytocin may be an important mediator between stressors and biological/ psychological responses in depression (56). Also, things can get even more complicated in this matter, since it appears that there are differences between individuals in terms of sociability, the volume of the amygdala and the risk of developing disorders such as autism, depression or anxiety, that can be caused by the actual polymorphisms of the oxytocin receptor gene interacting with the environmental factors (57).
In this way, in a study by Lawson et al. regarding the correlation between anorexia nervosa and anxious/depressive-like symptoms, it was highlighted that under food deprivation, the abnormal secretion of oxytocin correlates with the severity of depressive/ anxiety symptoms. In fact, the study suggested antidepressant and anxiolytic effects of oxytocin in food deprivation conditions (58).
In addition, a study published in 2013 by Turan and his colleagues suggested that oxytocin could be a biological marker also in the bipolar disorders. In this way, the authors measured the plasma oxytocin in bipolar patients which were in the manic phase, in the depressive phase or in remission, as compared to control subjects, their results showing a significant increase in the oxytocin levels for the bipolar patients, as compared with controls, with the largest increase being recorded in the patients with mania (59).
Even more, it was previously shown that in unipolar depression the oxytocin level are decreased, as compared to people without depression (59).
Therapeutical implications of oxytocin
Considering all the aforementioned aspects regarding the oxytocin effects, there is an increased awareness on the possible relevance of oxytocin exogenous administration in human patients or animal models of psychiatric disorders.
However, before talking about the psycho- active effects of oxytocin, we should initially mention that in analyzing the therapeutic potential of this molecule in the affective disorders, the administration method should be carefully considered, as according to some studies, the oxytocin from the peripheral blood does not cross the blood brain barrier (60). Thus, it is generally believed that the oxytocin administration form should avoid the peripheral path, with intranasal administration being significantly correlated with increased levels of oxytocin in the cerebrospinal fluid (61).
In this way, the possible antidepressant effects of oxytocin are sustained by some referential studies, such as the one from 2010 of Chaviaras group on the antidepressant effect of carbetocin, an agonist of oxytocin. In this way, carbetocin administration in some behavioural animal models of depression showed clearly antidepressant effects, as supported by specific behavioral indices (swimming vs. immobility time) (62), which were similar to those induced by imipramine, a tricyclic antidepressant (63). Also, other preliminary studies showed that sildenafil molecule, mainly used in sexual dysfunctions, is also stimulating the release of neurohypophysial oxytocin and exerts a clear antidepressant effect (64).
At the same time, some clinical studies clearly indicated the involvement of oxytocin in other psychiatric disorders and the possible beneficial effects of oxytocin administration in depression, anxiety (65, 66), schizophrenia (67), autism (68, 69) or substances addiction (70).
Still, the effects of oxytocin in the affective disorders are far from being fully known, although it seems to play a very important role alongside the classic molecules involved in their ethiopatogenesis. This is supported by numerous studies we described in this small report, but also by the information related to the particular roles held by oxytocine in the general protective mechanisms of the organism against the stress factors.
In conclusion, although the researches on the importance of oxytocin in the affective disorders are rather at the beginning, an increasing number of evidence is supporting the involvement of oxytocin in the pathogenic processes of these psychiatric disorders. Still, the studies covering this topic are still in their early days, and the results that are trying to understand if there is a major role of oxytocin in affective disorders are not consistent enough to draw definitive conclusions and establish with certainty where the place of oxytocin in the affective disorders pathology is.
Acknowledgement
Ciobica Alin, Balmus Ioana Miruna and Padurariu Manuela are supported by a research grant PN II PN-II-RU- TE-2014-4-1886 called “A complex study regarding the rel- evance of oxytocin administration in some animal models of neuropsychiatric disorders”.
Conflict of interest
None, except the PN II research grant described above.
References
- 1.Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2010;469(1):6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 2.Padurariu M, Ciobica A, Dobrin I, Stefanescu C. Evaluation of antioxidant enzymes activities and lipid peroxidation in schizophrenic patients treated with typical and atypical antipsychotics. Neurosci Lett. 2010;479(3):317–20. doi: 10.1016/j.neulet.2010.05.088. [DOI] [PubMed] [Google Scholar]
- 3.Stefanescu C, Ciobica A. The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord. 2012;143(1-3):34–8. doi: 10.1016/j.jad.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Levin R, Shany E, Shalev I, Ebstein R, Levy U. Brain Protection in Schizophrenia, Mood and Cognitive Disorders. Amsterdam: Springer; 2010. The role of oxytocin in neuropsychiatric disorders: concepts and mechanisms; pp. 611–635. [Google Scholar]
- 5.Cochran DM, Fallon D, Hill M, Frazier JA. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv Rev Psychiatry. 2013;21(5):219–247. doi: 10.1097/HRP.0b013e3182a75b7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neurosci. 2008;3(2):128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baskerville TA, Douglas AJ. Interactions between dopamine and oxytocin in the control of sexual behaviour. Prog Brain Res. 2008;170(1):277–290. doi: 10.1016/S0079-6123(08)00423-8. [DOI] [PubMed] [Google Scholar]
- 8.Huffmeijer R, Alink LR, Tops M. Salivary levels of oxytocin remain elevated for more than two hours after intranasal oxytocin administration. Neuro Endocrinol Lett. 2012;33(1):21–25. [PubMed] [Google Scholar]
- 9.McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? British Journal of Pharmacology. 2008;154(2):358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Insel TR. Oxytocin--a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17(1):3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- 11.Furman DJ, Chen MC, Gotlib IH. Variantinoxytocinreceptorgene is associated with amygdala volume. Psychoneuroendocrinology. 2011;36(6):891–897. doi: 10.1016/j.psyneuen.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tribollet E, Dubois DM, Dreifuss JJ, Barberis C, Jard S. Oxytocin receptors in the central nervous system. Distribution, development, and species differences. Ann NY Acad Sci. 1992;652(12):29–38. doi: 10.1111/j.1749-6632.1992.tb34343.x. [DOI] [PubMed] [Google Scholar]
- 13.Gutkowska J, Jankowski M, Antunes-Rodrigues J. The role of oxytocin in cardiovascular regulation. Braz J Med Biol Res. 2014;47(3):206–214. doi: 10.1590/1414-431X20133309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magon N, Kalra S. The orgasmic history of oxytocin: Love, lust, and labor. Indian J Endocrinol Metab. 2011;15(3):156–161. doi: 10.4103/2230-8210.84851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Wang W, Summer SN, Westfall TD, Brooks DP, Falk S, Schrier RW. Molecular mechanisms of antidiuretic effect of oxytocin. J Am Soc Nephrol. 2008;19(2):225–232. doi: 10.1681/ASN.2007010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thackare H, Nicholson HD, Whittington K. Oxytocin--its role in male reproduction and new potential therapeutic uses. Hum Reprod Update. 2006;12(4):437–448. doi: 10.1093/humupd/dmk002. [DOI] [PubMed] [Google Scholar]
- 17.Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66(6):617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 18.Herbert J. Cortisol and depression: three questions for psychiatry. Psychol Med. 2013;43(3):449–469. doi: 10.1017/S0033291712000955. [DOI] [PubMed] [Google Scholar]
- 19.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 20.Zhong N, Da X, Xue B, Yi XB, van Praag HM. Can stress cause depression? Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(5):891–907. doi: 10.1016/j.pnpbp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Hammen C. Stress generation in depression: reflections on origins, research, and future directions. J Clin Psychol. 2006;62(9):1065–1082. doi: 10.1002/jclp.20293. [DOI] [PubMed] [Google Scholar]
- 22.Liu RT, Alloy LB. Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clin Psychol Rev. 2010;30(5):582–593. doi: 10.1016/j.cpr.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapero BG, Black SK, Liu RT, Klugman J, Bender RE, Abramson LY, Alloy LB. Stressful life events and depression symptoms: the effect of childhood emotional abuse on stress reactivity. J Clin Psychol. 2014;70(3):209–223. doi: 10.1002/jclp.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowen PJ. Cortisol, serotonin and depression: all stressed out? The British Journal of Psychiatry. 2002;180(2):99–100. doi: 10.1192/bjp.180.2.99. [DOI] [PubMed] [Google Scholar]
- 25.Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress: a dose-response study. Psychoneuroendocrinology. 2013;38(3):399–407. doi: 10.1016/j.psyneuen.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Vreeburg SA, Hoogendijk WJ, DeRijk RH, van Dyck R, Smit JH, Zitman FG, Penninx BW. Salivary cortisol levels and the 2-year course of depressive and anxiety disorders. Psychoneuroendocrinology. 2013;38(9):1494–1502. doi: 10.1016/j.psyneuen.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66(6):617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 28.Dziurkowska E, Wesolowski M, Dziurkowski M. Salivary cortisol in women with major depressive disorder under selective serotonin reuptake inhibitors therapy. Arch Womens Ment Health. 2013;16(2):139–147. doi: 10.1007/s00737-013-0329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris MC, Rao U, Garber J. Cortisol responses to psychosocial stress predict depression trajectories: social-evaluative threat and prior depressive episodes as moderators. J Affect Disord. 2012;143(1-3):223–230. doi: 10.1016/j.jad.2012.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 31.Alfonso J, Frasch AC, Flugge G. Chronic stress, depression and antidepressants: effects on gene transcription in the hippocampus. Rev Neurosci. 2005;16(1):43–56. doi: 10.1515/revneuro.2005.16.1.43. [DOI] [PubMed] [Google Scholar]
- 32.Turner RA, Altemus M, Enos T, Cooper B, McGuinness T. Preliminary research on plasma oxytocin in normal cycling women: investigating emotion and interpersonal distress. Psychiatry. 1999;62(2):97–113. doi: 10.1080/00332747.1999.11024859. [DOI] [PubMed] [Google Scholar]
- 33.Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom Med. 2006;68(2):238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- 34.Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30(9):924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Rault JL, Carter CS, Garner JP, Marchant-Forde JN, Richert BT, Lay DC., Jr Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiol Behav. 2013;112-113(1):40–48. doi: 10.1016/j.physbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Blagrove M, Fouquet NC, Baird AL, Pace-Schott EF, Davies AC, Neuschaffer JL, Henley-Einion JA, Weidemann CT, Thome J, McNamara P, Turnbull OH. Association of salivary-assessed oxytocin and cortisol levels with time of night and sleep stage. J Neural Transm. 2012;119(10):1223–1232. doi: 10.1007/s00702-012-0880-1. [DOI] [PubMed] [Google Scholar]
- 37.Gannon RL. Non-peptide oxytocin receptor ligands and hamster circadian wheel running rhythms. Brain Res. 2014;1585(1):184–90. doi: 10.1016/j.brainres.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 38.Azorin JM, Kaladjian A. Depression and circadian rhythm. Encephale. 2009;35(2):68–71. doi: 10.1016/S0013-7006(09)75537-X. [DOI] [PubMed] [Google Scholar]
- 39.Välimäki SD. Duodecim. 2009. Endokrinologia. ISBN 978-951-656-290-5. [Google Scholar]
- 40.Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philosophical transactions of the royal society B –Biological Sciences. 2006;361(1476):1–10. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez-Bolado G, Grinevich V, Puelles L. Development of the hypothalamus. Front Neuroanat. 2015;9(1):83. doi: 10.3389/fnana.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemaire JJ, Nezzar H, Sakka L. Maps of the adult human hypothalamus. Surgical Neurology International. 2013;4(3):156–163. doi: 10.4103/2152-7806.110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jankowski M, Wang D, Hajjar F, Mukaddam-Daher S, McCann SM, Gutkowska J. Oxytocin and its receptors are synthesized in the rat vasculature. Proc Natl Acad Sci U S A. 2000;97(11):6207–11. doi: 10.1073/pnas.110137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steger MF, Kashdan TB. Depression and Everyday Social Activity, Belonging, and Well-Being. J Couns Psychol. 2009;56(2):289–300. doi: 10.1037/a0015416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz JA, Yee JR, van Zuiden M. The role of oxytocin in social bonding, stress egulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38(9):1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 46.Romero T, Nagasawa M, Mogi K, Hasegawa T, Kikusui T. Oxytocin promotes social bonding in dogs. Proc Natl Acad Sci U S A. 2014;111(25):9085–9090. doi: 10.1073/pnas.1322868111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McQuaid RJ, McInnis OA, Abizaid A, Anisman H. Making room for oxytocin in understanding depression. Neurosci Biobehav Rev. 2014;45(3):305–322. doi: 10.1016/j.neubiorev.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003;79(3):351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 49.Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lefter R., Ciobica A, Hritcu L, Stoica B, Cojocaru D, Olteanu Z. The effects of a 6-OHDA induced lesion in murine nuccleus accumbens on memory and oxidative stress status. Central European Journal of Medicine. 2013;8(4):443–449. [Google Scholar]
- 51.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(1):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Loo HM, de Jonge P, Romeijn JW, Kessler RC, Schoevers RA. Data-driven subtypes of major depressive disorder: a systematic review. BMC Med. 2012;10(1):156. doi: 10.1186/1741-7015-10-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz JA, Yee JR, van Zuiden M. The role of oxytocin in social bonding, stress egulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38(9):1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 54.Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35(1):676–692. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Stefanescu C, Ciobica A. The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord. 2012;143(1-3):34–38. doi: 10.1016/j.jad.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 56.van Loo HM, de Jonge P, Romeijn JW, Kessler RC, Schoevers RA. Data-driven subtypes of major depressive disorder: a systematic review. BMC Med. 2012;10(1):156. doi: 10.1186/1741-7015-10-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brüne M. Does the oxytocin receptor (OXTR) polymorphism (rs2254298) confer vulnerability’ for psychopathology or ‘differential susceptibility’? Insights from evolution. BMC Med. 2012;10(1):38. doi: 10.1186/1741-7015-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawson EA, Holsen LM, Santin M, DeSanti R, Meenaghan E, Eddy KT, Herzog DB, Goldstein JM, Klibanski A. Postprandial oxytocin secretion is associated with severity of anxiety and depressive symptoms in anorexia nervosa. J ClinPsychiatry. 2013;74(5):451–457. doi: 10.4088/JCP.12m08154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turan T, Uysal C, Asdemir A, Kılıç E. May oxytocin be a trait marker for bipolar disorder? Psychoneuroendocrinology. 2013;38(12):2890–2896. doi: 10.1016/j.psyneuen.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 60.Harris MC, Jones PM, Robinson IC. Differences in the release of oxytocin into the blood and cerebrospinal fluid following hypothalamic and pituitary stimulation in rats. J Physiol (Lond) 1981;320(1):109–110. [Google Scholar]
- 61.Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB. CSF and Blood Oxytocin Concentration Changes following Intranasal Delivery in Macaque. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0103677. 103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foyet HS, Hritcu L, Ciobica A, Stefan M, Kamtchouing P, Cojocaru D. Methanolic extract of Hibiscus asper leaves improves spatial memory deficits in the 6-hydroxydopamine-lesion rodent model of Parkinson’s disease. J Ethnopharmacol. 2011;133(2):773–779. doi: 10.1016/j.jep.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 63.Chaviaras S, Mak P, Ralph D, Krishnan L, Broadbear JH. Assessing the antidepressant-like effects of carbetocin, an oxytocin agonist, using amodification of the forced swimming test. Psychopharmacology (Berl) 2010;210(1):35–43. doi: 10.1007/s00213-010-1815-x. [DOI] [PubMed] [Google Scholar]
- 64.Matsuzaki M, Matsushita H, Tomizawa K, Matsui H. Oxytocin: a therapeutic target for mental disorders. J Physiol Sci. 2012;62(6):441–444. doi: 10.1007/s12576-012-0232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blume A., Bosch O.J., Miklos S., Torner L., Wales L., Waldherr M., Neumann I.D. Oxytocin reduces anxiety via ERK1/2 activation: Local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27(2):1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- 66.Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B. Anxiolytic-like activity of oxytocin in male mice: Behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185(1):218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- 67.Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68(3):678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 68.Young LJ, Barrett CE. Neuroscience. Can oxytocin treat autism? Science. 2015;347(6224):825–826. doi: 10.1126/science.aaa8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bartz JA, Hollander E. Oxytocin and experimental therapeutics in autism spectrum disorders. Prog Brain Res. 2008;170(5):451–462. doi: 10.1016/S0079-6123(08)00435-4. [DOI] [PubMed] [Google Scholar]
- 70.Sarnyai Z, Kovács GL. Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology. 1994;19(1):85–117. doi: 10.1016/0306-4530(94)90062-0. [DOI] [PubMed] [Google Scholar]


