Abstract
Context
Endometriosis is a common gynecological disease, characterized by ectopic deposits of endometrial tissue outside of the uterine cavity, and it is associated with pelvic pain and infertility, with an important impact on the quality of life. At this point there is a controversy regarding the etiology and pathophysiology of endometriosis and it seems that pro-angiogenic growth factors might be involved, but their role is not completely understood.
Objective
To evaluate the serum concentration of the main growth factors in patients with diagnosed endometriosis compared to healthy controls.
Subjects and methods
A total of 157 women were divided into two study groups (Group I – endometriosis; Group 2 – healthy women). Serum levels of VEGF, G-CSF, GM-CSF, b-FGF, EGF, and HGF were measured with Human Multiplex Cytokine Panels.
Results
VEGF serum levels were significantly lower in women with endometriosis compared to controls (1.924±0.145 compared to 1.806±0.078 pg/mL, p<0.001). Serum levels of GM-CSF, b-FGF, EGF, and HGF respectively did not differ significantly between patients with endometriosis and healthy controls. G-CSF had a very low detection rate.
Conclusions
The present study showed that VEGF serum levels are significantly lower in endometriosis patients compared to healthy controls, indicating a possible role in endometriosis pathogenesis.
Keywords: endometriosis, growth factors, immunology, inflammation
INTRODUCTION
Endometriosis is defined as the presence of endometrial tissue ectopic deposits outside of the uterine cavity, a disease associated with pelvic pain and infertility. It is known as one of the most common diseases in women of reproductive age, with an important impact on the quality of life (1).
The pathogenesis of endometriosis was initially defined as the retrograde passage of endometrial tissue outside the uterine cavityandits subsequent proliferation in this ectopic location (2). Other theories include lymphatic and vascular metastasis, iatrogenic direct implantation, coelomic metaplasia, embryonic rest and mesenchymal cell induction (3). On the other hand, the hormonal milieu (4), aberrant immune responses (5), genetic predisposition (6-8) andenvironmentaltoxicants (9, 10) could contribute to endometriosis susceptibility and progression. Immune system alterations play a key role in endometriosis development, associating immune-inflammatory reaction within the peritoneal cavity of endometriosis patients in which activated immune cells, together with endometriotic implants, produce high amounts of cytokines, growth factors, and angiogenic substances (11). Endometriosis is also considered an angiogenic disease, since the ectopic survival of endometrium requires the formation of new blood vessels. Angiogenesis, therefore, is a critical step in developing endometriotic lesions (12-14), providing substantial background for their further maintenance and growth.
The pivotal angiogenic factor involved in endometriosis pathophysiology and especially in its initiation appears to be Vascular Endothelial Growth Factor (VEGF) (15). There is a controversy in the current literature regarding VEGF serum and peritoneal fluid (PF) levels in endometriosis patients. Some authors have indicated an increased level of VEGF in serum (16-18) and in PF (17, 19, 20), while several others have reported no change in VEGF serum (21-24) and in PF (21, 25) levels in endometriosis patients. Neo- vascularization is essential for a successful implantation of endometrial cells in ectopic sites (11). VEGF is part of a heparin-binding protein family (26), and because the induction of endometrial cell proliferation seems to be functioned by VEGF (27, 28), it is considered an essential factor in uterine angiogenesis (29). Moreover, recent studies, have involved some VEGF specific genetic polymorphisms in endometriosis pathogenesis (7, 8). On the other hand, members of fibroblast growth factor (FGF) family of proteins seem to be involved in vascular remodeling, have been found in endometriosis associated angiogenesis, playing a role in vascular wall formation and migration of endothelial cells and vascular smooth muscle cells (30). Hepatocyte growth factor (HGF), also known as scatter factor, and its receptor c-Met have also been shown to be implicated in endometriosis as well. HGF acts as a mitogen, motogen, and morphogen on endometrial epithelial cells. The expression of c-Met on human endometrial cells has been previously reported (31). Other growth factors recently involved in endometriosis are represented by insulin-like growth factor-I (IGF-I), with higher serum levels in advanced endometriosis, granulocyte macrophage colony-stimulating factor (GM-CSF), epidermal growth factor (EGF), and platelet-derived growth factor (PDGF) (32).
Due to existing controversies, our study aimed to determine the serum levels main pro-angiogenic growth factors, VEGF, basic-FGF, HGF, EGF, G-CSF, and GM-CSF in women with endometriosis and to evaluate if any single marker or a combination of markers could be used as a non-surgical predictor for endometriosis.
MATERIALS AND METHODS
Design
We conducted a case-control study, which included 157 patients, divided into two groups, as follows: Group I (endometriosis group) – 77 women with regular menses, and with no history of pelvic infections, autoimmune and neoplastic diseases, undergoing laparoscopy or laparotomy for suspected endometriosis. The evidence of endometriosis was verified by histopathological analysis. The severity of endometriosis was staged according to the revised American Society for Reproductive Medicine (rASRM) classification; all included patients were staged III or IV according to rASRM Group II (control group) - 80 healthy non-pregnant women aged between 18-40 years old, without clinical and para-clinical evidence of endometriosis. Exclusion criteria: patients with previous pelvic surgeries, history of cancer, suspected malignancy, adenomyosis or leiomyoma, pre-surgical suspicion of evidence of premature ovarian failure, or the use of ovarian suppressive drug, such as oral contraceptives, GnRH agonists, progestins or danazol in the preceding 6 months were excluded from the study. None of the patients had taken anti- inflammatory medications or had been diagnosed with an inflammatory or infectious condition for ≥ 6 months before the study.
The study design was approved by the Local Ethics Committee of “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania, and signed informed consent was received from each woman before sample collection. The study was conducted under the tenets of Helsinki Declaration. From each patient, 5 mL of venous blood was collected before breakfast in a covered test tube, which was centrifuged at 2000 x g for 10 minutes and the serum obtained was stored at -70° C for future determinations.
Cytokine Evaluation
We used multiplex cytokine kits (Invitrogen Human Cytokine 30-Plex Panel, LHC6003, LOT 1298425A) in order to measure serum levels of EGF, b-FGF, G-CSF, GM-CSF, HGF and VEGF.
Measurements were performed with a Luminex 200 system (Luminex Corporation, Austin, TX, USA) in accordance with the manufacturer’s specifications (Invitrogen Corporation, Carlsbad, CA, USA). The sensitivity of the test was specified by the manufacturer (Invitrogen Corporation, Carlsbad, CA, USA).
Average sensitivity of the test for EGF was <5 pg/mL with an inter-assay variation coefficient of 4.0%. For b-FGF, the average sensitivity of the test was <10 pg/mL with an inter-assay variation coefficient of 3.0%. The average sensitivity of the test for G-CSF was <10 pg/mL with an inter-assay variation coefficient of 2.4%. In the case of GM-CSF, the average sensitivity of the test was <0.5 pg/mL with an inter-assay variation coefficient of 9.1%. The sensitivity of the test for HGF was <10 pg/mL, and the inter-assay variation coefficient of 4.7%. The test for VEGF revealed an average sensitivity of <5 pg/mL, with inter-assay variation coefficient of 4.8%.
Statistical Analysis
Statistical analyses were performed using Microsoft Excel and IBM SPSS software (version 21.0). Data was presented as mean ± standard deviation (SD) and standard error (SE) for the groups. Kolmogorov- Smirnov test for normality, Levene’s test for equality of variances, t-test were used as statistical tests. P-values less than 0.05 were regarded as significant.
RESULTS
Table 1 presents the descriptive data regarding studied populations. Patients included in the endometriosis group were stage III (30 – 38.96%) or IV (47 – 61.04%) according to rASRM staging criteria. The detection rate for VEGF, b-FGF, HGF, GM-CSF and EGF respectively in the studied groups was 98.70, 85.71, 75.32, 75.32 and 84.41% respectively. GM-CSF was detected in only 6.49% of women, without the possibility of establishing a statistical significance.
Table 1.
Descriptive statistics of the endometriosis and control groups
| Variable | Endometriosis group (Mean ± SD) | Control group (Mean ± SD) | Average (Mean ± SD) |
| Age (years) | 30.600±5.486 | 26.350±2.131 | 28.475±4.655 |
| Weight (kg) | 62.050±9.067 | 56.925±8.094 | 59.488±8.920 |
| Height (cm) | 164.725± 5.114 | 167.225±6.773 | 165.975±6.094 |
| BMI (kg/cm2) | 22.912±3.520 | 20.307±2.126 | 21.609±3.173 |
SD - Standard Deviation.
Table 2 shows the statistics regarding studied growth factors in controls and endometriosis group.
Table 2.
Descriptive statistics of the studied growth factors among groups
| Variable | Control group Mean (SEM) ± SD | Median | Endometriosis group Mean (SEM) ± SD | Median |
| VEGF | 1.924 (0.023) ± 0.145 | 1.900 | 1.806 (0.012) ± 0.078 | 1.790 |
| b-FGF | 6.933 (1.254) ± 7.421 | 6.327 | 4.439 (0.666) ± 3.827 | 4.326 |
| HGF | 597.308 (76.629) ± 412.659 | 544.214 | 638.849 (82.817) ± 445.984 | 612.214 |
| GM-CSF | 1.046 (0.100) ± 0.546 | 0.920 | 1.180 (0.139) ± 0.763 | 1.020 |
| EGF | 4.196 (0.075) ± 0.422 | 4.187 | 4.038 (0.089) ± 0.509 | 4.111 |
SD - Standard Deviation; SEM - Standard Error of Mean; VEGF - Vascular endothelial growth factor; bFGF - basic fibroblast growth factor; HGF - Hepatocyte growth factor; GM-CSF- granulocyte macrophage colony-stimulating factor; - EGF - epidermal growth factor.
Table 3 presents the data obtained with the normality test and the distribution normality in the studied groups.
Table 3.
The distribution normality of the studied markers between groups
| Marker | Shapiro-Wilk | |||
| Statistic | df | Sig. | ||
| VEGF | Control | 0.928 | 14 | 0.286 |
| Endometriosis | 0.863 | 11 | 0.063 | |
| b-FGF | Control | 0.943 | 14 | 0.460 |
| Endometriosis | 0.882 | 11 | 0.112 | |
| HGF | Control | 0.862 | 14 | 0.032 |
| Endometriosis | 0.894 | 11 | 0.155 | |
| GM- CSF | Control | 0.918 | 14 | 0.206 |
| Endometriosis | 0.902 | 11 | 0.198 | |
| EGF | Control | 0.956 | 14 | 0.661 |
| Endometriosis | 0.962 | 11 | 0.801 | |
Because the distribution of the studied parameters was abnormal, parametric tests could not be used. As a consequence, for comparing the means, we used Mann Whitney U test, a non-parametric test for independent samples. Also, for a greater accuracy of the comparative statistics, we used Independent samples median test to verify the medians distribution differences between control and endometriosis groups. Table 4 presents the results obtained by comparative statistics, regarding growth factors studied. No significant differences were observed in the serum levels of b-FGF, GM-CSF, EGF and HGF between the studied groups. VEGF has presented a statistical significance between groups with a lower serum level in endometriosis patients.
Table 4.
Comparative statistics of the studied markers
| Independent samples Mann-Whitney U test probability | Independent samples Median test probability | |
| VEGF | <0.001* | <0.001* |
| b-FGF | 0.145 | 0.211 |
| HGF | 0.599 | 0.803 |
| GM-CSF | 0.796 | 0.610 |
| EGF | 0.900 | 0.319 |
*Significant differences; VEGF - Vascular endothelial growth factor; bFGF - basic fibroblast growth factor; HGF - Hepatocyte growth factor; GM- CSF- granulocyte macrophage colony-stimulating factor; - EGF - epidermal growth factor.
Figures 1-2 show the mean serum level of the studied markers between the groups.
Figure 1.
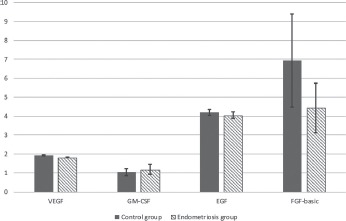
Mean serum levels of VEGF, GM-CSF, EGF and b-FGF between the studied groups. VEGF - Vascular endothelial growth factor; bFGF - basic fibroblast growth factor; GM-CSF- granulocyte macrophage colony-stimulating factor; - EGF- epidermal growth factor.
Figure 2.
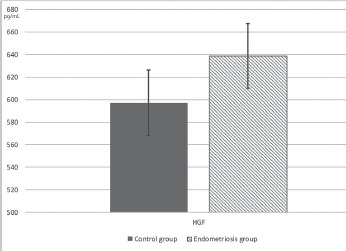
Mean serum levels of HGF between the studied groups HGF - Hepatocyte growth factor.
DISCUSSION
Despite the long years of research, endometriosis is still considered a disease of theories. At this point, there is no single theory that can adequately explain the endometriosis pathophysiology and why the disease is largely associated with infertility. A number of studies involve immune system alterations in the development and progression of endometriosis, together with hormonal, genetic and environmental factors (15, 30). Diagnosis of endometriosis is difficult and mainly postsurgical, because of non-specific symptoms. On the other hand, there are no clinical markers that could be used for a certain pre-operative diagnosis, although some studies have found correlations between elevated serum or peritoneal cytokines and endometriosis (30, 31). As regarding to growth factors, a series of studies are suggesting a key involvement of angiogenic growth factors in the development of endometriotic lesions (15, 32).
The present study was undertaken in order to establish whether serum concentrations of the main growth factors, VEGF, HGF, b-FGF, G-CSF, GM- CSF and EGF, were significantly altered in patients with advanced endometriosis. We found that VEGF serum levels were significantly lower in patients with endometriosis compared to healthy women. At the same time, our study showed that there was no significant difference in HGF, b-FGF, GM-CSF and EGF serum levels between women with endometriosis and healthy controls. On the other hand, G-CSF had a very low detection rate in the studied groups, so we were unable to draw any conclusions regarding its significance.
Endometriosis is characterized by the implantation of exfoliated endometrial tissues on both the peritoneal and ovarian surfaces. It is considered that, besides retrograde menstruation, increased inflammatory activity in the PF, excessive angiogenesis and up-regulation of pro-inflammatory cytokines may facilitate the development of endometriotic deposits (33, 34). This excessive angiogenesis can be promoted and maintained via high PF levels of angiogenic growth factors, such as VEGF (25). At the moment, a series of studies have tried to identify a link between endometriosis and pro-angiogenic factors, and in particular VEGF, with the possibility of using them as diagnosis biomarkers. VEGF is a protein that promotes angiogenesis and vasculogenesis (16, 25). At the same time, other growth factors, such as HGF and FGF2 are considered potent angiogenic factors (30, 35). In the current literature there is an abundance of studies regarding serum and PF levels of VEGF in endometriosis, but with conflicting results. Some of the reviewed studies are indicating high serum and PF levels of VEGF in endometriosis (16, 18), while several others reported no change either in serum or PF levels between patients with endometriosis and healthy women (21, 23, 25). One previous study has found higher serum and PF levels of VEGF in endometriosis women, and concluded that endometriosis was associated with a significant modulation in the circulating levels of VEGF (17). On the other hand, a very recent study has found high VEGF concentration in the PF from women affected by endometriosis, but the VEGF serum levels were similar between the groups (36). Our study is somewhat in contradiction with the previous ones, because we found a lower serum level of VEGF in women with endometriosis. This could be due to the fact that even though VEGF is an indicator of an excessive angiogenic process, the VEGF family comprises members that are not up-regulated during active angiogenesis, and which may impact the total VEGF serum levels. Moreover, a series of studies have shown an anti-angiogenic effect for VEGF isoforms, like VEGF165b, isoforms which could interfere in serum detection of VEGF (37, 38).
HGF was first discovered as a mitogen for adult hepatocytes, and it is identical to scatter factor (39). Previous reports, that have investigated HGF relationship with endometriosis, found that HGF levels were elevated in women with endometriosis and did correlate with disease stage (40), while, another study showed no correlation with incidence or stage of disease (41). Same authors have reported that changes in the PF levels of HGF have an association with estradiol and both of them are elevated in the early stage of endometriosis, especially in those women with endometriosis harboring dominant distribution of highly active and blood-filled (opaque lesions) red peritoneal lesions (42, 43). Our results are partially in accordance with the previous studies, as we found a moderate increase in HGF serum levels in patients with advanced endometriosis, but the increase was not statistically significant.
EGF is a single polypeptide chain of 53 amino acid residues and plays a role in many processes such as DNA synthesis, mitogenesis, and proliferation of many cell types, including endometrial cells (44). Previous studies have found no difference in levels of EGF receptor between women with and without endometriosis (16), and serum levels of EGF itself were not found to correlate with the disease (45). Results obtained from our research are in accordance, with similar serum levels of EGF between patients with endometriosis and controls.
FGF is a heparin-binding family of proteins, shown to be growth factors and angiogenesis promoters. One previous study found levels of FGF- 2 to be increased throughout the cycle in women with endometriosis (46). Our results show a mean lower serum level of b-FGF in patients with endometriosis, but with a lack of statistical significance due to the high values of SD.
GM-CSF is a growth factor that stimulates the stem cells to produce granulocytes and monocytes and it functions as a cytokine. Regarding its involvement in endometriosis, in a recent study, levels of GM- CSF were unchanged in women with endometriosis compared with controls (23). In this context, our results are in accordance with the previous study, showing a similar GM-CSF serum level in patients with and without endometriosis.
One major limitation of our study could be represented by the lack of specificity as VEGF is secreted by a large variety of cells in normal conditions, having many isoforms. Moreover, studies have shown that VEGF is stored in α granule of circulating resting platelets and released during clotting, and thus the serum VEGF level may not reflect the level of circulating VEGF produced by peripheral tissues (47). Also, a limitation of the present study could be considered the endometriosis group structure, which has included only patients with stage III or IV according to rASRM. This is due to the fact that, as the study was conducted in a University Clinic, the patients addressing for treatment and included in the study had late stages of endometriosis, thus the low rate of early stages. Probably future studies could include patients presenting for unexplained infertility undergoing laparoscopy, and thus including patients with early stages discovered incidentally. Another limitation could be the analyzed site. We have investigated the serum levels of the above markers without investigating the PF levels at the same time, or their tissue expression. It is well known that in most of cases there is no relationship between the serum level of VEGF and its tissue expression, but at the same time we tried to identify if one of the studied markers could be used as a non-surgical predictor for endometriosis, and as a consequence the PF levels or tissue expression were of no interest. Further studies could be aimed at investigating a relationship between VEGF serum level and its tissue expression in endometriosis.
In conclusion, the present study indicates that VEGF serum levels are significantly lower in endometriosis patients compared to healthy controls, and a moderate change in b-FGF and HGF is present. On the other hand, determining the main growth factors serum levels does not seem to be very helpful in discriminating between patients with endometriosis and healthy women. Further researches involving a more diverse study group are needed to confirm the role of serum growth factors, and particularly of VEGF, in the diagnosis of endometriosis in general population, and if there is a true relationship between VEGF serum level, its tissue expression, and occurrence or progression of endometriosis.
Acknowledgement
This paper was published under the project funded by “Iuliu Hatieganu” University of Medicine and Pharmacy, internal grant no. 1491/7/28.01.2014.
Conflict of interest
The authors declare that they have no conflict of interest concerning this article.
References
- 1.Rižner TL. Noninvasive biomarkers of endometriosis: myth or reality? Expert Rev Mol Diagn. 2014;14(3):365–385. doi: 10.1586/14737159.2014.899905. [DOI] [PubMed] [Google Scholar]
- 2.Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93–110. [PMC free article] [PubMed] [Google Scholar]
- 3.Maruyama T, Yoshimura Y. Stem cell theory for the pathogenesis of endometriosis. Front Biosci. 2012;4:2854–2863. doi: 10.2741/e589. [DOI] [PubMed] [Google Scholar]
- 4.Balasch J, Creus M, Fábregues F, Carmona F, Ordi J, Martinez-Román S, Vanrell JA. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod. 1996;11(2):387–391. doi: 10.1093/humrep/11.2.387. [DOI] [PubMed] [Google Scholar]
- 5.Baldi A, Campioni M, Signorile PG. Endometriosis: pathogenesis, diagnosis, therapy and association with cancer (review) Oncol Rep. 2008;19(4):843–846. [PubMed] [Google Scholar]
- 6.Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15(10):587–607. doi: 10.1093/molehr/gap064. [DOI] [PubMed] [Google Scholar]
- 7.Henidi B, Kaabachi W, Naouali A, Kaabachi S, Zhioua A, Haj Sassi F, Hamzaoui K. Vascular endothelial growth factor (-460 C/T, +405 G/C, and +936 C/T) polymorphisms and endometriosis risk in Tunisian population. Syst Biol Reprod Med. 2015;61(4):238–244. doi: 10.3109/19396368.2015.1041622. [DOI] [PubMed] [Google Scholar]
- 8.Szczepańska M, Mostowska A, Wirstlein P, Skrzypczak J, Jagodziłski PP. Involvement of vascular endothelial growth factor -460 C/T, +405 G/C and +936 C/T polymorphisms in the development of endometriosis. Biomed Rep. 2015;3(2):220–224. doi: 10.3892/br.2014.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anger DL, Foster WG. The link between environmental toxicant exposure and endometriosis. Front Biosci. 2008;13:1578–1593. doi: 10.2741/2782. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Chun S, Jang JY, Chae HD, Kim CH, Kang BM. Increased plasma levels of phthalate esters in women with advanced-stage endometriosis: a prospective case–control study. Fertil Steril. 2011;95(1):357–359. doi: 10.1016/j.fertnstert.2010.07.1059. [DOI] [PubMed] [Google Scholar]
- 11.Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001;76(1):1–10. doi: 10.1016/s0015-0282(01)01816-7. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann NY Acad Sci. 2002;955:89–100. doi: 10.1111/j.1749-6632.2002.tb02769.x. 118,396-406. [DOI] [PubMed] [Google Scholar]
- 13.Groothuis PG, Nap AW, Winterhager E, Grümmer R. Vascular development in endometriosis. Angiogenesis. 2005;8(2):147–156. doi: 10.1007/s10456-005-9005-x. [DOI] [PubMed] [Google Scholar]
- 14.Taylor RN, Yu J, Torres PB, Schickedanz AC, Park JK, Mueller MD, Sidell N. Mechanistic and therapeutic implications of angiogenesis in endometriosis. Reprod Sci. 2009;16(2):140–146. doi: 10.1177/1933719108324893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YZ, Wang LJ, Li X, Li SL, Wang JL, Wu ZH, Gong L, Zhang XD. Vascular endothelial growth factor gene polymorphisms contribute to the risk of endometriosis: an updated systematic review and meta-analysis of 14 case-control studies. Genet Mol Res. 2013;12(2):1035–1044. doi: 10.4238/2013.April.2.20. [DOI] [PubMed] [Google Scholar]
- 16.Matalliotakis IM, Goumenou AG, Koumantakis GE, Neonaki MA, Koumantakis EE, Dionyssopoulou E, Athanassakis I, Vassiliadis S. Serum concentrations of growth factors in women with and without endometriosis: the action of anti-endometriosis medicines. Int Immunopharmacol. 2003;3(1):81–89. doi: 10.1016/s1567-5769(02)00216-3. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Gorpudolo N, Li Y, Feng D, Wang Z, Zhang Y. Elevated vascular endothelia growth factor-A in the serum and peritoneal fluid of patients with endometriosis. J Huazhong Univ Sci Technolog Med Sci. 2009;29(5):637–641. doi: 10.1007/s11596-009-0520-7. [DOI] [PubMed] [Google Scholar]
- 18.García-Manero M, Alcazar JL, Toledo G. Vascular endothelial growth factor (VEGF) and ovarian endometriosis: correlation between VEGF serum levels, VEGF cellular expression, and pelvic pain. Fertil Steril. 2007;88(2):513–515. doi: 10.1016/j.fertnstert.2006.11.117. [DOI] [PubMed] [Google Scholar]
- 19.McLaren J, Prentice A, Charnock-Jones DS, Smith SK. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod. 1996;11(1):220–223. doi: 10.1093/oxfordjournals.humrep.a019023. [DOI] [PubMed] [Google Scholar]
- 20.Oosterlynck DJ, Meuleman C, Sobis H, Vandeputte M, Koninckx PR. Angiogenic activity of peritoneal fluid from women with endometriosis. Fertil Steril. 1993;59(4):778–782. doi: 10.1016/s0015-0282(16)55859-2. [DOI] [PubMed] [Google Scholar]
- 21.Dziunycz P, Milewski Ł, Radomski D, Barcz E, Kamiński P, Roszkowski PI, Malejczyk J. Elevated ghrelin levels in the peritoneal fluid of patients with endometriosis: associations with vascular endothelial growth factor (VEGF) and inflammatory cytokines. Fertil Steril. 2009;92(6):1844–1849. doi: 10.1016/j.fertnstert.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Gagné D, Pagé M, Robitaille G, Hugo P, Gosselin D. Levels of vascular endothelial growth factor (VEGF) in serum of patients with endometriosis. Hum Reprod. 2003;18(8):1674–1680. doi: 10.1093/humrep/deg326. [DOI] [PubMed] [Google Scholar]
- 23.Othman Eel D, Hornung D, Salem HT, Khalifa EA, El-Metwally TH, Al-Hendy A. Serum cytokines as biomarkers for nonsurgical prediction of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2008;137(2):240–246. doi: 10.1016/j.ejogrb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Pellicer A, Albert C, Mercader A, Bonilla-Musoles F, Remohí J, Simón C. The follicular and endocrine environment in women with endometriosis: local and systemic cytokine production. Fertil Steril. 1998;70(3):425–431. doi: 10.1016/s0015-0282(98)00204-0. [DOI] [PubMed] [Google Scholar]
- 25.Pupo-Nogueira A, de Oliveira RM, Petta CA, Podgaec S, Dias JA, Jr, Abrao MS. Vascular endothelial growth factor concentrations in the serum and peritoneal fluid of women with endometriosis. Int J Gynaecol Obstet. 2007;99(1):33–37. doi: 10.1016/j.ijgo.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Gordon JD, Shifren JL, Foulk RA, Taylor RN, Jaffe RB. Angiogenesis in the human female reproductive tract. Obstet Gynecol Surv. 1995;50(9):688–697. doi: 10.1097/00006254-199509000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 28.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84(5):1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimwood J, Bicknell R, Rees MC. The isolation, characterization and culture of human decidual endothelium. Hum Reprod. 1995;10(8):2142–2148. doi: 10.1093/oxfordjournals.humrep.a136250. [DOI] [PubMed] [Google Scholar]
- 30.Hayrabedyan S, Kyurkchiev S, Kehayov I. FGF1 and S100A13 possibly contribute to angiogenesis in endometriosis. J Reprod Immunol. 2005;67(1-2):87–101. doi: 10.1016/j.jri.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Khoshdel Rad N, Salehi Z, Mashayekhi F, Abbasi O, Mirzajani E. Soluble c-Met expression in the peritoneal fluid and serum of patients with different stages of endometriosis. Arch Gynecol Obstet. 2014;289(5):1107–1112. doi: 10.1007/s00404-013-3082-7. [DOI] [PubMed] [Google Scholar]
- 32.May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010;16(6):651–674. doi: 10.1093/humupd/dmq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May K, Becker CM. Endometriosis and angiogenesis. Minerva Ginecol. 2008;60(3):245–254. [PubMed] [Google Scholar]
- 34.Kyama CM, Debrock S, Mwenda JM, D’Hooghe TM. Potential involvement of the immune system in the development of endometriosis. Reprod Biol Endocrinol. 2003;1:123–131. doi: 10.1186/1477-7827-1-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siristatidis C, Nissotakis C, Chrelias C, Iacovidou H, Salamalekis E. Immunological factors and their role in the genesis and development of endometriosis. J Obstet Gynaecol Res. 2006;32(2):162–170. doi: 10.1111/j.1447-0756.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- 36.Kianpour M, Nematbakhsh M, Ahmadi SM, Jafarzadeh M, Hajjarian M, Pezeshki Z, Safari T, Eshraghi-Jazi F. Serum and peritoneal fluid levels of vascular endothelial growth factor in women with endometriosis. Int J Fertil Steril. 2013;7(2):96–99. [PMC free article] [PubMed] [Google Scholar]
- 37.Bates DO, MacMillan PP, Manjaly JG, Qiu Y, Hudson SJ, Bevan HS, Hunter AJ, Soothill PW, Read M, Donaldson LF, Harper SJ. The endogenous anti-angiogenic family of splice variants of VEGF, VEGFxxxb, are down-regulated in pre-eclamptic placentae at term. Clin Sci (Lond) 2006;110(5):575–85. doi: 10.1042/CS20050292. [DOI] [PubMed] [Google Scholar]
- 38.Qiu Y, Hoareau-Aveilla C, Oltean S, Harper SJ, Bates DO. The anti-angiogenic isoforms of VEGF in health and disease. Biochem Soc Trans. 2009;37(Pt 6):1207–13. doi: 10.1042/BST0371207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyazawa K, Tsubouchi H, Naka D, Takahashi K, Okigaki M, Arakaki N. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem Biophys Res Commun. 1998;163(2):967–973. doi: 10.1016/0006-291x(89)92316-4. [DOI] [PubMed] [Google Scholar]
- 40.Zong L, Li Y, Ha X. Determination of HGF concentration in serum and peritoneal fluid in women with endometriosis. Di Yi Jun Yi Da Xue Xue Bao. 2003;23(8):757–760. [PubMed] [Google Scholar]
- 41.Khan KN, Masuzaki H, Fujishita A, Kitajima M, Hiraki K, Miura S, Sekine I, Ishimaru T. Peritoneal fluid and serum levels of hepatocyte growth factor may predict the activity of endometriosis. Acta Obstet Gynecol Scand. 2006;85(4):458–466. doi: 10.1080/00016340500432556. [DOI] [PubMed] [Google Scholar]
- 42.Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T. Higher activity by opaque endometriotic lesions than non-opaque lesions in women with endometriosis. Acta Obstet Gynecol Scand. 2004;83(4):375–382. doi: 10.1111/j.0001-6349.2004.00229.x. [DOI] [PubMed] [Google Scholar]
- 43.Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, Masuzaki H. Immunopathogenesis of pelvic endometriosis: role of hepatocyte growth factor, macrophages and ovarian steroids. Am J Reprod Immunol. 2008;60(5):383–404. doi: 10.1111/j.1600-0897.2008.00643.x. [DOI] [PubMed] [Google Scholar]
- 44.Giudice L, Kao L. Endometriosis. Lancet. 2004;364(9447):1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 45.Philippoussis F, Gagne D, Hugo P, Gosselin D. Concentrations of alpha-fetoprotein, insulin-like growth factor binding protein-3, c-erbB-2, and epidermal growth factor in serum of patients with endometriosis. J Soc Gynecol Investig. 2004;11(3):175–181. doi: 10.1016/j.jsgi.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Bourlev V, Larsson A, Olovsson M. Elevated levels of fibroblast growth factor-2 in serum from women with endometriosis. Am J Obstet Gynecol. 2006;194(3):755–759. doi: 10.1016/j.ajog.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 47.Ferrero S, Gillott DJ, Anserini P, Remorgida V, Teisner B, Grudzinskas JG. Methodological concerns regarding levels of vascular endothelial growth factor (VEGF) in serum of patients with endometriosis. Hum Reprod. 2004;19(1):220–221. doi: 10.1093/humrep/deh033. [DOI] [PubMed] [Google Scholar]


