Abstract
Primary lung adenocarcinoma, diffuse pneumonic type, can mimic the clinical presentation of an infectious or inflammatory lung disease, which can represent a diagnostic challenge. We present an unusual case of adenocarcinoma of the lung refractory to treatment, associated with rapid deterioration of respiratory status, ARDS requiring intubation and ultimately death.
Keywords: Lung adenocarcinoma, Diffuse pneumonic-type, Primary adenocarcinoma, Consolidation
1. Introduction
Primary lung adenocarcinoma, diffuse pneumonic type, can mimic the clinical presentation of an infectious or inflammatory lung disease. Thus, it can represent a diagnostic challenge, especially in the setting of rapidly progressive respiratory failure.
1.1. Case presentation
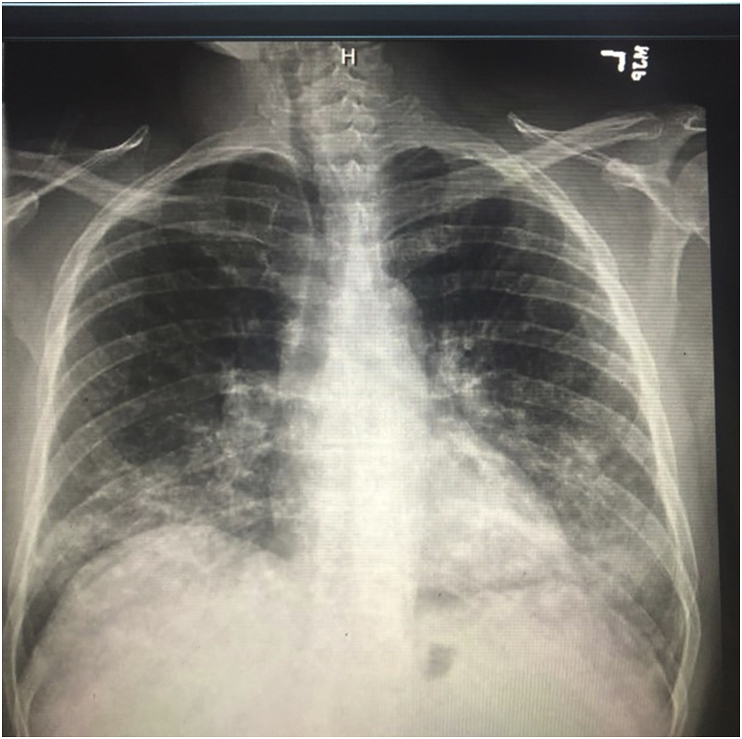
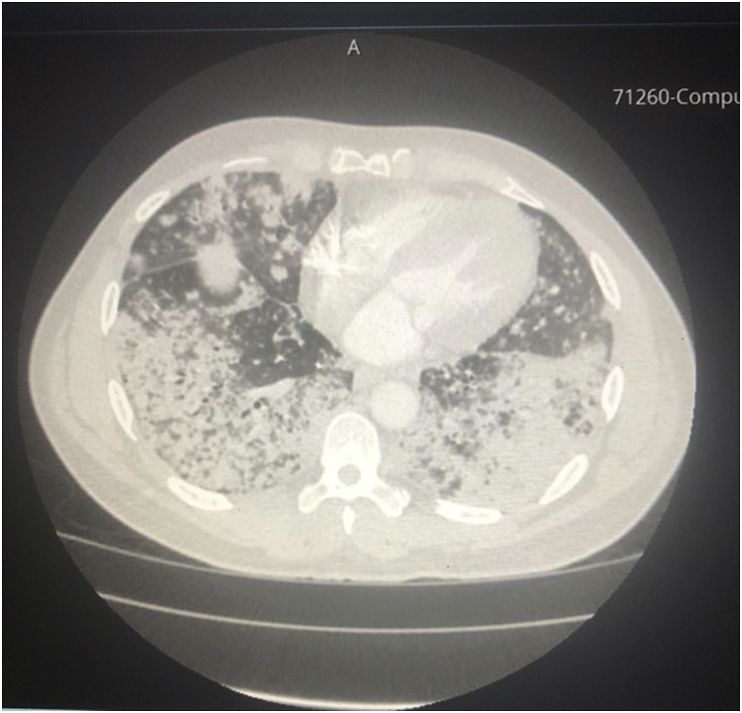
A 55-year-old male patient with a history of essential hypertension, chronic obstructive lung disease (20 pack-year smoking history), untreated hepatitis C and active cocaine use was admitted for evaluation of progressive shortness of breath of one-month duration, associated with productive cough of yellowish sputum. On presentation, he was afebrile and had an oxygen saturation of 96% on 2L/minute by nasal cannula. Physical examination revealed bilateral rhonchi and crackles mainly on auscultation of the left lower lobe. Laboratory results were significant for a normal white blood cell count and a troponin I level of 0.2 ng/mL with no ischemic changes on EKG. A bedside 2D-echo showed no evidence of heart failure or wall-motion abnormalities. A Chest X-ray (Fig. 1) showed diffuse bilateral upper and lower lobe patchy airspace opacities with nodular appearance suggestive of multilobar pneumonia. Consequently, the patient was admitted to the hospital and was started on broad spectrum antibiotics. Shortly after admission, he required intubation for hypoxic respiratory failure. A CT scan of the chest (Fig. 2) showed extensive bilateral parenchymal infiltrates with mediastinal lymphadenopathy.
Fig. 1.
Chest X-ray AP view showing interval worsening of the bilateral upper and lower lobe patchy airspace opacities with nodular appearance.
Fig. 2.
CT scan of the chest showing extensive bilateral parenchymal infiltrates with mediastinum lymphadenopathy.
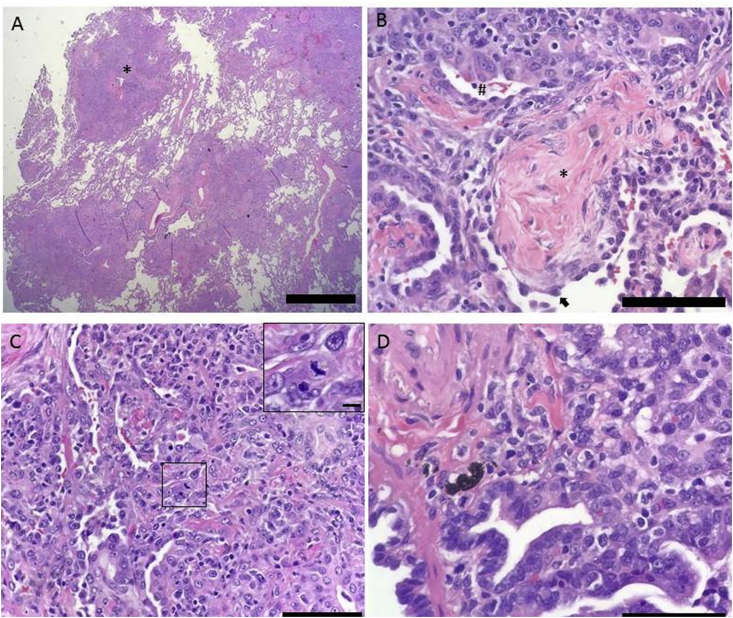
A bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL fluid analysis returned positive for aspergillus antigen and herpes simplex virus – 1 (HSV-1) DNA by PCR. The patient was therefore started on voriconazole and acyclovir in addition to broad spectrum antibiotic therapy. Serum aspergillus antigen was negative. The remainder of the infectious work-up including AFB smear, mycobacterial cultures, BAL fluid bacterial cultures and gram stain, serum cryptococcal antigen and HIV antigen/antibody combo were negative. An autoimmune panel, including ANA, ANCA and myositis autoantibodies, was negative. Due to the progressive respiratory distress with failure to wean off the ventilator, empirical intravenous methylprednisolone was started for possible associated pneumonitis. Transbronchial biopsy or fine needle aspirate could not be performed due to severe acute respiratory distress syndrome (ARDS). A left lobe lung biopsy was obtained by video-assisted thoracoscopic surgery (VATS), and pathology returned positive for adenocarcinoma of the lung of the diffuse pneumonic type. There was no evidence of invasive aspergillosis on microscopy and tissue cultures were negative for aspergillus or HSV (Fig. 3). Despite all efforts, the patient passed away in the intensive care unit.
Fig. 3.
Histologic features of pulmonary adenocarcinoma of the pneumonic type A. Low power view of lung tissue shows neoplastic consolidations surrounding terminal bronchovascular bundles (*) in a pattern reminiscent of bronchopneumonia, from which the entity acquires its adjective “pneumonic”. Scale bar = 2 mm. B. An alveolar space occupied the characteristic fibroblastic plug an organizing pneumonia (*). The arrowhead points to a reactive type II pneumocyte with hobnail morphology. Surrounding the alveoli are malignant gland forming cells (#) of the adenocarcinoma. Scale bar = 0.25 mm C. The adenocarcinoma showing both an acinar (left side) and solid (right side) pattern of growth. The inset demonstrates a mitotic figure. Scale bar = 0.25 mm, inset = 0.025 mm. D. A terminal bronchiole lined by neoplastic epithelium (*) is identified adjacent to adenocarcinoma. Scale bar = 0.25 mm.
2. Discussion
2.1. Definition
Lung cancer can have a wide array of clinical and radiological presentations as well as a variable natural history and disease progression making early recognition and timely diagnosis sometimes challenging. Smoking remains a major risk factor for the development of most types of lung cancer, however, there appears to be a rise in the number of diagnosed cases in non-smokers.
Lung tumors have been classified into small cell lung carcinomas and non-small cell lung carcinomas (NSCLC), with both types combined comprising around 95% of all lung cancers. This classification is useful for guiding staging, treatment and in predicting prognosis. NSCLC comprises around 85% of all lung malignancies and is divided into several histological subtypes, including adenocarcinoma, squamous cell carcinoma and large cell undifferentiated carcinoma among others. Adenocarcinoma represents the most common histological type of lung cancer especially in non-smokers [2].
Previously, the term bronchioalveolar carcinoma (BAC) was used to describe cancers with a bronchioalveolar growth pattern with a characteristic lepidic growth – the pattern of extension without invasion or destruction of alveoli. However, the most recent revised classification of lung tumors (the 2015 WHO classification) is based on immunohistochemistry, small biopsy and cytology findings [1]. The revised classification divides lung adenocarcinomas into: adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA) and invasive adenocarcinoma while abandoning the concept of BAC. The lepidic growth pattern being described now as part of invasive adenocarcinoma [1].
Pneumonic-type lung adenocarcinoma (P-ADC) was defined by Detterbeck et al. [2] as “adenocarcinoma presenting with pneumonia-like areas of infiltrates or consolidations involving a region of the lung”. Histologically, there is predominant lepidic growth, with partial filling of alveolar spaces by mucin or tumor cells [2].
2.2. Histology and molecular characteristics
Based on the 2015 WHO classification, P-ADC were mainly described histologically as mucinous [1]. However, many studies in literature, including one large series (n = 51) [3], reported that around 45% were mucinous, 42% were non-mucinous and around 15% were mixed type (mucinous and non-mucinous) adenocarcinoma. Existing literature suggests a correlation between imaging findings and histological type with mucinous type adenocarcinoma presenting as a consolidative pattern in 33–75% of the cases and as ground glass opacities in 75%. Several studies reported no significant difference on imaging between mucinous and non-mucinous types in terms of nodular versus pneumonic findings. Data on clonality including immunohistochemical analysis is limited with no clear correlation in the literature [2].
Jun-Liu et al. [4] screened around 953 patients with lung cancer between 2011 and 2013, out of which, 85 were found to have P-ADC. The incidence of EGFR mutation in P-ADC was 75% compared to 48.5% in patients with NSCLC of other types. Few studies demonstrated a high frequency of EGFR mutation in lepidic growth pattern, which may be one of the reasons why a high frequency of this mutation is observed in P-ADC [4].
The age of patients diagnosed with P-ADC ranged between 41 and 66 years and the majority were non-smoking females [4]. Several other studies however described no difference in age, sex or smoking status.
2.3. Clinical findings
P-ADC presents as a diffuse non-obstructive solid infiltration of the lung with frequent absence of a single mass lesion. This often makes the diagnosis misleading as it can be confused with infectious or inflammatory diseases of the lung, especially that a large portion of this subtype of adenocarcinoma presents in non-smokers. Cases reported in the literature had a wide range of clinical presentations ranging from dyspnea, cough and fever to acute respiratory failure requiring intubation. In this paper, we report the case of a patient who presented with shortness of breath and productive cough and had diffuse infiltrates on chest X-ray mimicking pneumonia, which was refractory to antimicrobial therapy. The patient's course was complicated by rapidly progressive acute respiratory failure with ARDS requiring mechanical ventilation and death. Table 1 lists a few cases of P-ADC reported in the literature. Almost all cases presented as pneumonia which was refractory to antimicrobial therapy.
Table 1.
Clinical presentations of P-ADC; Case reports.
| First author | Symptoms on presentation |
|---|---|
| Raminderiit Sekhon et al [5] | Shortness of breath, recurrent episodes of pneumonia refractory to antibiotics. |
| Yokouchi et al [6] | Recurrent productive cough, refractory to antibiotics. Consolidation of left lower lobe on Chest X-ray. |
| Yuan et al. [7] | Recurrent cough, consolidation of left lower lobe on Chest X-ray, refractory pneumonia for 8 years until adenocarcinoma diagnosis. |
| Zielonka et al [8] | Bronchorrhea, cough, fever and weight loss with infiltrative changes on right lung lobe on Chest X-ray |
| Farjo et al [9] | Progressive dyspnea, productive cough, low grade fever and consequent acute hypoxic respiratory failure |
2.4. Diagnosis and imaging
Both clinical and pathological criteria exist for the diagnosis of pneumonic type adenocarcinoma as described in an article by Detterbeck et al. (Table 2). These criteria help guide clinicians to distinguish P-ADC from other subtypes of adenocarcinoma with similar clinical and radiological findings [2].
Table 2.
Criteria identifying the pneumonic-type adenocarcinoma.
| Clinical criteri |
|
| Pathologic criteria |
|
Criteria as described by Detterbeck el al [2].
Chest X-ray findings are usually significant for infiltration or consolidation mimicking pneumonia. In a case series of 51 patients by Wislez el al., P-ADC was diagnosed by routine chest radiography in 17% of cases (n = 9) [3]. Chest CT scan is the preferred modality for diagnosis. It is also sensitive for the detection of pulmonary extension of the disease. Several studies described findings on CT chest which could favor P-ADC over infectious pneumonia. Aquino et al. [10,11] reported that CT findings of peripheral consolidative pneumonia with surrounding nodules favored pneumonic-type adenocarcinoma over pneumonia. Similarly, Jung et al. [11,12] reported that CT findings of air-filled bronchus with stretching, squeezing, widening of the branching angle or bulging of the interlobar fissure, favor the diagnosis of pneumonic-type BAC over infectious pneumonia. Bronchoscopy with BAL, transbronchial biopsy and transthoracic pulmonary biopsy usually provide definitive diagnosis [11].
2.5. TNM classification
The classic TNM classification for lung cancer would apply to P-ADC with a single area of tumor involvement (T for size, N and M for nodal and extrathoracic metastasis respectively). However, it has been challenging to classify tumors with many pulmonary sites of involvement, especially given the nature of P-ADC where boundaries could be difficult to identify. Table 3 describes a proposal for TNM classification of P-ADC as described by Detterbeck et al. [2].
Table 3.
TNM classification of P-ADC.
| Tumor Involvement | T | N | M |
|---|---|---|---|
| Confined to one lobe | T3 | – | – |
| Involving different lobes in one lung, extension of tumor to adjacent lobe or discrete separate area from adjacent lobe | T4 | – | – |
| Tumor involving both lungs | Designated to the side of lung with the larger size of tumor (T3 if one lobe and T4 if more than one lobe) | – | M1a |
| Pleural, pericardial or distant metastasis | – | – | M1a or M1b |
Wislez et al. [3] reported that patients with P-ADC usually present without nodal or extrathoracic metastasis.
Note: this classification was proposed by Detterbeck et al. and applies to all subtypes of pneumonic-type adenocarcinoma; mucinous, non-mucinous and mixed [2].
2.6. Treatment and survival rates
Lobectomy, bilobectomy or pneumonectomy, have the best curative results so far. Differences in survival rates between the different types of surgery, independent from TNM staging have been reported in few studies. For example, patients who underwent lobectomy and bilobectomy were found to have longer survival time compared to those treated with pneumonectomy [6,13].
Although there are case reports of successful treatment with oral epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors or pemetrexed, an effective therapeutic strategy for this type of lung cancer has not been established. One of the clinical cases of non-mucinous type of pneumonic-type adenocarcinoma showed prompt response to targeted therapy with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI), erlotinib or gefitinib. This could be explained by the fact that this type of adenocarcinoma was five times more likely to carry the EGFR gene mutation compared to conventional lung adenocarcinomas [14].
In one study, Wislez et al. [3] (n = 52) reported that bronchorrhea and crepitant rales were two main independent factors associated with shorter survival rates and that the outcome of patients treated by lobectomy or bilobectomy was significantly better than those treated with pneumonectomy, chemotherapy, or best supportive care [3]. Okubo et al. [15] (n = 25) reported that patients with no symptoms and findings of a mass lesion on chest radiograph had a better prognosis compared to those who were symptomatic and had infiltrative findings on radiography. In addition, Oktubo et al. [15] reported that complete surgical resection in the absence of evidence lymph node metastases conferred a good prognosis.
3. Conclusion
P-ADC is an uncommon type of lung cancer which is often misdiagnosed due to its unusual presentation mimicking both infectious and inflammatory diseases of the lung. In this paper, we describe the case of a patient with P-ADC who had a rapid deterioration in his respiratory status leading to acute hypoxic respiratory failure and death. It is important for clinicians to keep this type of lung cancer in the differential diagnosis especially in patients presenting with clinical and radiological findings suggestive of pneumonia that is refractory to antimicrobial therapy.
Conflicts of interest
The authors of this article have no conflict of interest to disclose.
Acknowledgments
The authors would like to thank Dr. Kunil Raval from the Pathology department of Detroit Medical Center for providing us with the pathology slides and the necessary description.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2019.100881.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Travis W.D. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 2.Detterbeck F.C. The IASLC Lung Cancer Staging Project: background data and proposals for the application of TNM staging rules to lung cancer presenting as multiple nodules with ground glass or lepidic features or a pneumonic type of involvement in the forthcoming eighth edition of the TNM classification. J. Thorac. Oncol. 2016;11(5):666–680. doi: 10.1016/j.jtho.2015.12.113. [DOI] [PubMed] [Google Scholar]
- 3.Wislez M. Clinical characteristics of pneumonic-type Adenocarcinoma of the lunga. Chest. 2003;123(6):1868–1877. doi: 10.1378/chest.123.6.1868. [DOI] [PubMed] [Google Scholar]
- 4.Liu J. High incidence of EGFR mutations in pneumonic-type non-small cell lung cancer. Medicine (Baltim.) 2015;94(8) doi: 10.1097/MD.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekhon R. Primary lung adenocarcinoma disguised as diffuse parenchymal disease. Chest. 2017;152(4):A684. [Google Scholar]
- 6.Yokouchi H. [A case of diffuse pneumonic type of mucinous adenocarcinoma treated with reduction surgery] Gan To Kagaku Ryoho. 2012;39(12):2396–2398. [PubMed] [Google Scholar]
- 7.Yuan D.M. Recurrent “pneumonia” in left lower lobe lasting for 8 years: a case report. Transl. Lung Cancer Res. 2016;5(3):356–362. doi: 10.21037/tlcr.2016.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zielonka T.M. [Bronchorrhea in a case of pneumonic type of bronchioloalveolar carcinoma] Pol. Arch. Med. Wewn. 2004;112(2):961–967. [PubMed] [Google Scholar]
- 9.Farjo B., Murugan A., Annikka W. A case of severe hypoxic respiratory failure caused by an unusual presentation of primary bronchogenic adenocarcinoma. Chest. 2012;142(4_MeetingAbstracts):602A. [Google Scholar]
- 10.Aquino S.L., Chiles C., Halford P. Distinction of consolidative bronchioloalveolar carcinoma from pneumonia: do CT criteria work? AJR Am. J. Roentgenol. 1998;171(2):359–363. doi: 10.2214/ajr.171.2.9694451. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura S. CT findings of pneumonic adenocarcinoma: comparison between invasive mucinous adenocarcinomna and nonmucinous adenocarcinoma. Glob. J. Med. Res. 2014;14(1):1–4. Version 1.0. [Google Scholar]
- 12.Jung J. CT differentiation of pneumonic-type bronchioloalveolar cell carcinoma and infectious pneumonia. Br. J. Radiol. 2001;74(882):490–494. doi: 10.1259/bjr.74.882.740490. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y.-Y. Prognosis and recurrent patterns in bronchioloalveolar carcinoma. Chest. 2000;118(4):940–947. doi: 10.1378/chest.118.4.940. [DOI] [PubMed] [Google Scholar]
- 14.Svoboda M. EGFR tyrosine kinase inhibitors as a targeted therapy for bronchioloalveolar carcinoma of the lung: a case report of a clinically prompt and intensive response and literature review. Klin. Onkol. 2010;23(4):224–230. [PubMed] [Google Scholar]
- 15.Okubo K. Bronchoalveolar carcinoma: clinical, radiologic, and pathologic factors and survival. J. Thorac. Cardiovasc. Surg. 1999;118(4):702–709. doi: 10.1016/S0022-5223(99)70016-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.