Abstract
Campylobacter infections sourced mainly to poultry products, are the most important bacterial foodborne zoonoses worldwide. No effective measures to control these infections in broiler production exist to date. Here, we used passive immunization with hyperimmune egg yolks to confer broad protection of broilers against Campylobacter infection. Two novel vaccines, a bacterin of thirteen Campylobacter jejuni (C. jejuni) and C. coli strains and a subunit vaccine of six immunodominant Campylobacter antigens, were used for the immunization of layers, resulting in high and prolonged levels of specific immunoglobulin Y (IgY) in the hens’ yolks. In the first in vivo trial, yolks (sham, bacterin or subunit vaccine derived) were administered prophylactically in the broiler feed. Both the bacterin- and subunit vaccine-induced IgY significantly reduced the number of Campylobacter-colonized broilers. In the second in vivo trial, the yolks were administered therapeutically during three days before euthanasia. The bacterin IgY resulted in a significant decrease in C. jejuni counts per infected bird. The hyperimmune yolks showed strong reactivity to a broad representation of C. jejuni and C. coli clonal complexes. These results indicate that passive immunization with hyperimmune yolks, especially bacterin derived, offers possibilities to control Campylobacter colonization in poultry.
Subject terms: Public health, Zoology, Vaccines, Bacterial infection
Introduction
Campylobacteriosis is one of the most important foodborne bacterial diseases worldwide and has been the most commonly reported zoonosis in the EU since 20051. Clinical symptoms such as fever and diarrhoea are usually self-limiting, although in rare cases complications can occur, leading to reactive arthritis2, Guillain-Barré syndrome (GBS)3 and inflammatory bowel disease (IBD)4. The disease is mainly caused by Campylobacter jejuni (C. jejuni) and Campylobacter coli (C. coli)1 and contaminated chicken meat is considered a major source of infection5. Worldwide, over 50% of poultry meat is contaminated with Campylobacter6. However, no effective measures to limit Campylobacter infections in primary broiler chicken production exist to date7. Once a chicken is infected, the pathogen rapidly spreads infecting almost 100% of the flock within a week8.
Interestingly, chickens are only colonized from the age of two to three weeks onwards9,10, which is presumably due to the protection by maternal IgY antibodies (MAB)11–13. These antibodies are transferred from the serum of the mother to the egg yolk, protecting the chicks during the first weeks when their immune system is not yet fully developed13. From two weeks onward, the blood concentration of MAB against Campylobacter drops significantly, which coincides with an increased colonization susceptibility of the chickens. As a measure, pure MAB or egg yolks of immunized chickens containing pathogen specific MAB can be added to the feed of the chicks to prolong this effect13,14. Previously, Hermans et al.15 immunized laying hens with a whole-cell lysate of C. jejuni or its hydrophobic protein fraction, and successfully used their eggs to protect young chickens against Campylobacter infection. As such, passive immunization of broiler chickens using egg yolk IgY offers possibilities to control C. jejuni colonization in broiler flocks.
The vaccines tested by Hermans et al.15 were based on one single C. jejuni strain, which is not representative for the field situation with many genetically different strains16. A bacterin containing heterogeneous Campylobacter strains might offer a much broader target reactivity. Also, Hermans et al.15 identified several immunodominant C. jejuni antigens. A subunit vaccine containing a mix of broadly conserved, immunodominant proteins could lead to a well-defined and standardized vaccine.
We developed two vaccines to immunize laying hens against C. jejuni and C. coli to obtain IgY-rich eggs that confer broad protection of chickens against C. jejuni and C. coli infection: a bacterin consisting of genetically heterogeneous Campylobacter strains relevant to the field situation and a subunit vaccine containing multiple recombinant immunodominant antigens of C. jejuni strain KC4015. Egg yolks of hens immunized with these vaccines were used for passive oral immunization of broiler chickens to investigate their prophylactic and therapeutic efficacy against experimental Campylobacter infection in broiler chickens. Finally, the reactivity of these egg yolks to a variety of C. jejuni and C. coli strains, belonging to different clonal complexes was tested as a proxy for the breadth of protection.
Results
Immunodominant antigens are highly prevalent and highly conserved in C. jejuni
A PCR analysis, amplifying AtpA, Ef-Tu, GroEL, Tig, CheV and LivJ encoding gene fragments, resulted in positive PCR products in every C. jejuni strain screened. Sequence analysis of the PCR products and translation of the nucleotide sequences into protein sequences showed conservation levels of 97–100% for both gene and protein sequences (sequence data published elsewhere17). Screening the C. coli strains, positive PCR products were only obtained for LivJ, CheV and Ef-Tu with conservation levels of 80%, 96% and 99%, respectively, for both gene and protein sequences (sequence data published elsewhere17).
Preparation of recombinant C. jejuni antigens
Gene copies of C. jejuni KC40 AtpA, Ef-Tu, GroEL, Tig, CheV and LivJ were cloned successfully in an entry vector and the pDEST™17 destination vector and expressed in BL21-AI One Shot® E. coli transformants. Results of the SDS-PAGE analysis of recombinant C. jejuni antigens are shown in Fig. 1. All proteins were detected at their corresponding length.
Figure 1.
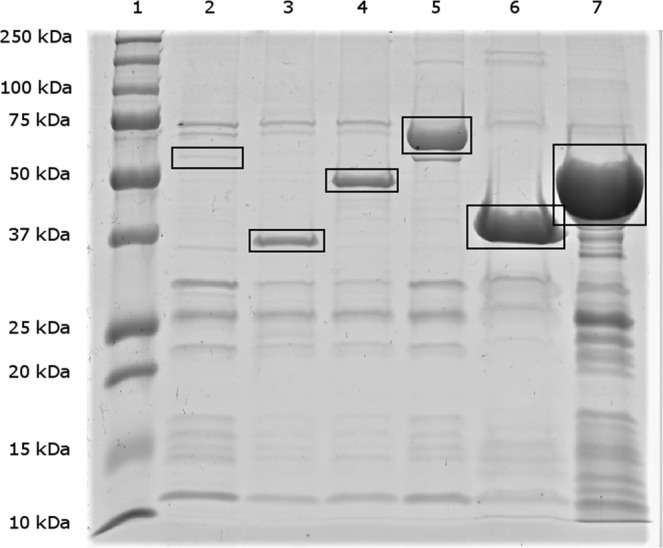
SDS-PAGE analysis visualized by Brilliant Blue G-Colloidal coloring of recombinant C. jejuni proteins. Column 1: protein marker with size labelling in kilodalton (kDa) at the left, 2: AtpA (54.8 kDa), 3: CheV (35.8 kDa), 4: EfTu (43.6 kDa), 5: GroEL (58.0 kDa), 6: LivJ (40.1 kDa), 7: Tig (51.0 kDa).
Immunization of layers with the bacterin and subunit vaccine dramatically induces Campylobacter-specific egg yolk IgY titers
The bacterin- and subunit vaccine-induced Campylobacter-specific IgY titers in the egg yolks, determined by ELISA, are given in Tables 1 and 2. These yolk titers were maintained for at least two years after final immunization.
Table 1.
Bacterin- and subunit vaccine-induced egg yolk IgY titers against the bacterin and individual Campylobacter strains used in this study, as determined by ELISA.
| Strain | Antibody titers induced by | |
|---|---|---|
| Bacterin | Subunit | |
| Bacterin | 1:65,536 | 1:16,384 |
| KC40b | 1:65,536 | 1:16,384 |
| 10kf-1.16b | 1:65,536 | 1:512 |
| 7P6.12b | 1:65,536 | 1:16,384 |
| 10C-6.1b | 1:65,536 | 1:16,384 |
| 10kf-4.12b | 1:65,536 | 1:65,536 |
| 10VTDD-8b | 1:65,536 | 1:16,384 |
| T124b | 1:65,536 | 1:16,384 |
| T84b | 1:65,536 | 1:65,536 |
| T70b | 1:65,536 | 1:65,536 |
| 2012/3291b | 1:65,536 | 1:2,048 |
| 5970b | 1:65,536 | 1:16,384 |
| 2013/2711b | 1:65,536 | 1:16,384 |
| 2012/3250b | 1:65,536 | 1:32,768 |
| 5CT13 | 1:65,536 | 1:4,096 |
| 3CT13 | 1:32,768 | <1:32 |
| 1CT117 | 1:32,768 | 1:32 |
| 1CT51 | 1:32,768 | <1:32 |
bstrains incorporated in the bacterin.
Table 2.
Bacterin- and subunit vaccine-induced egg yolk IgY titers against the subunit vaccine and its individual antigen compounds, as determined by ELISA.
| Antigen | Antibody titers induced by | |
|---|---|---|
| Bacterin | Subunit | |
| Subunit | 1:256 | 1:65,536 |
| AtpA | 1:512 | 1:32,768 |
| CheV | <1:32 | 1:32,768 |
| EfTu | 1:512 | 1:32,768 |
| GroEL | <1:32 | 1:32,768 |
| LivJ | 1:128 | 1:32,768 |
| Tig | 1:128 | 1:32,768 |
The bacterin-induced IgY titers against the bacterin and the different Campylobacter bacterin strains were all 1:65,536. Also against the Campylobacter strains belonging to different clonal complexes (CC) than the bacterin Campylobacter strains, IgY titers were remarkably high (1:32,768 to 1:65,536). The subunit vaccine-induced IgY titer against the bacterin was 1:16,384. The subunit vaccine-induced IgY titers against the different Campylobacter bacterin strains varied from 1:65,536 (10kf-4.12, T84, T70) to 1:512 (10kf-1.16). For the Campylobacter strains belonging to different CC’s than the bacterin Campylobacter strains, a strong reaction was observed for one strain only (1:4,096; 5CT13). For the subunit vaccine-induced IgY antibodies, a titer of 1:65,536 was obtained against the subunit vaccine and titers of 1:32,768 against each recombinant antigen, separately. The bacterin-induced IgY antibodies showed a much lower reaction, with titers varying from 1:512 (AtpA, EfTu) to non-detectable (<1:32; CheV, GroEL) against the separate recombinant antigens and a titer of 1:256 against the subunit vaccine.
Prophylactic passive immunization of broilers with bacterin and subunit vaccine derived hyperimmune egg yolk significantly reduces the number of C. jejuni colonized birds
In the first in vivo trial, the prophylactic effect of hyperimmune egg yolks from immunized laying hens administered to the feed of broiler chickens was investigated. C. jejuni counts per gram (g) cecal content after euthanasia of the chickens are summarized in Table 3. Posterior distributions of the estimated probabilities of C. jejuni colonization and mean colonization load are added as Supplemental Fig. S1. Both the number of Campylobacter-positive birds and the mean C. jejuni titers of these positive birds should be considered when interpreting the data about the global Campylobacter populations.
Table 3.
Number of positive birds and mean cecal C. jejuni counts of colonized broilers receiving standard feed supplemented with 5% (wt/wt) egg yolk from either bacterin-immunized, subunit vaccine-immunized or sham-immunized (control) layers, from day 1 until day 16 (the day of euthanasia).
| Number of positive birds | Mean C. jejuni counts of positive birds | |||||
|---|---|---|---|---|---|---|
| Bacterin | Subunit | Control | (log10(cfu/g cecal content)) (Standard deviation) | |||
| Bacterin | Subunit | Control | ||||
| All birds | ||||||
| Group 1 | 2/9 | 4/9 | 5/9 | 4.48 (0.25) | 3.64 (2.18) | 3.81 (1.53) |
| Group 2 | 2/9 | 4/9 | 8/9 | 3.50 (0.71) | 5.71 (1.83) | 4.31 (1.74) |
| Group 3 | 0/9 | 4/9 | 8/9 | - (−) | 5.08 (1.88) | 4.59 (1.80) |
| Treatment | 4/27 a | 12/27 a | 21/27 b | 3.99a (0.71) | 4.81a (2.00) | 4.30a (1.66) |
| Seeders | ||||||
| Group 1 | 0/3 | 3/3 | 3/3 | - (−) | 3.85 (2.62) | 4.39 (1.60) |
| Group 2 | 1/3 | 2/3 | 3/3 | 4.00 (−) | 6.01 (2.39) | 5.76 (2.09) |
| Group 3 | 0/3 | 1/3 | 3/3 | - (−) | 5.04 (−) | 4.81 (2.31) |
| Treatment | 1/9 a | 6/9 ab | 9/9 b | 4.00a (−) | 4.77a (2.24) | 4.99a (1.85) |
| Sentinels | ||||||
| Group 1 | 2/6 | 1/6 | 2/6 | 4.48 (0.25) | 3.00 (−) | 2.94 (1.33) |
| Group 2 | 1/6 | 2/6 | 5/6 | 3.00 (−) | 5.41 (2,00) | 3.45 (0.81) |
| Group 3 | 0/6 | 3/6 | 5/6 | - (−) | 5.10 (2.30) | 4.46 (1.72) |
| Treatment | 3/18 a | 6/18 a | 12/18 b | 3.98a (0.87) | 4.85a (1.94) | 3.79a (1.38) |
At 11 days of age, 3 seeder birds per group were inoculated with approximately 105 cfu C. jejuni KC40. A random effect was included in the statistical model at the subgroup (pen) level to account for clustering.
The total number of C. jejuni colonized broilers in the groups receiving hyperimmune egg yolk from bacterin-immunized layers (4/27) and subunit vaccine-immunized layers (12/27) was significantly lower than the number of C. jejuni colonized birds in the control subgroups (21/27; resp. p = 0.0030 and p = 0.041), or a reduction from 78% to resp. 15% and 44% infected birds. This also applies for the seeder birds separately (resp. 1/9, 6/9 and 9/9; p = 0.00056 and p = 0.025). For the sentinels, hyperimmune egg yolk from bacterin-immunized layers but not from subunit vaccine–immunized layers significantly reduced the number of C. jejuni colonized broilers compared to the control broilers (resp. 3/18, 6/18, 12/18; p = 0.022 and p = 0.088). The treatments did not significantly differ from each other. No significant differences were observed for the C. jejuni counts in birds positive for colonization.
Therapeutic passive immunization of broilers with bacterin derived hyperimmune egg yolk significantly reduces cecal C. jejuni titers
In the second in vivo trial, the therapeutic potential of hyperimmune egg yolk from immunized laying hens administered to the feed of broiler chickens to reduce cecal C. jejuni colonization was assessed. C. jejuni counts per g cecal content after euthanasia of the chickens are summarized in Table 4. Posterior distributions of the estimated probabilities of C. jejuni colonization and mean colonization load are added as Supplemental Fig. S2.
Table 4.
Number of positive birds and mean cecal C. jejuni counts of colonized broilers receiving standard feed supplemented with 5% (wt/wt) egg yolk from either bacterin-immunized, subunit vaccine-immunized or sham-immunized (control) layers, from day 19 until day 21 (the day of euthanasia).
| Number of positive birds | Mean C. jejuni counts of positive birds | |||||
|---|---|---|---|---|---|---|
| (log10(cfu/g cecal content)) (Standard deviation) | ||||||
| Bacterin | Subunit | Control | Bacterin | Subunit | Control | |
| Group 1 | 7/9 | 9/9 | 7/9 | 3.12 (1.31) | 5.56 (1.28) | 5.64 (1.80) |
| Group 2 | 8/9 | 9/9 | 7/9 | 4.08 (1.59) | 5.19 (1.84) | 4.96 (2.09) |
| Group 3 | 7/9 | 8/9 | 6/9 | 4.74 (1.03) | 5.55 (1.34) | 5.10 (2.11) |
| Treatment | 22/27 a | 26/27 a | 20/27 a | 4.00a (1.44) | 5.43b (1.46) | 5.24b (1.67) |
At 10 days of age, all birds were inoculated with approximately 105 cfu C. jejuni KC40. A random effect was included in the statistical model at the subgroup (pen) level to account for clustering.
Most of the broilers were colonized with C. jejuni and significant differences were not observed concerning the number of colonized animals between groups. Considering C. jejuni counts in the colonized animals, mean cecal C. jejuni numbers in birds receiving hyperimmune egg yolk from bacterin-immunized layers were significantly reduced compared to birds receiving hyperimmune egg yolk from subunit vaccine-immunized layers and control broilers (resp. 4.00, 5.43 and 5.24 log10 cfu/g cecal content; peq = 0.015, p = 0.041). In birds receiving hyperimmune egg yolk from subunit vaccine-immunized layers, mean cecal C. jejuni numbers were not reduced compared to the control birds.
Discussion
Passive immunization of broilers with hyperimmune egg yolk has previously been shown effective at reducing cecal Campylobacter loads when the layer hens were immunized using a whole cell lysate or its hydrophobic protein fraction15. In our study, a bacterin and subunit vaccine were developed for the immunization of the hens. The bacterin was composed of genetically heterogeneous C. jejuni and C. coli strains, as these two species are responsible for up to 99.6% of human campylobacteriosis cases in the EU1. For the subunit vaccine, proteins were selected based on the reactivity of IgY from C. jejuni KC40 immunized layer hens15, their association with the bacterial cell membrane15,18–21 and previous positive results of vaccination studies19,22–26. These proteins function as an ATP synthase subunit (AtpA), a chemotaxis protein associated with transmembrane receptors (CheV), an elongation factor translocated to the surface in several bacteria (EfTu), a heat shock protein shown to mediate Salmonella adhesion (GroEL), an amino acid transporter (LivJ) and in protein transport (Tig)15,18–20,23–25. In this study, the antigens proved to be highly prevalent and conserved in C. jejuni. Both vaccines could therefore be expected to offer protection against a broad range of Campylobacter strains in vivo. Immunization of hens with these vaccines resulted in a high and specific immune response, comparable to the titers obtained by Hermans et al.15. The prolonged response is an economic advantage since the hens would not need to be revaccinated during the production period.
When administered prophylactically, both treatments significantly decreased the number of C. jejuni colonized birds, particularly the bacterin-induced antibodies reduced the overall colonization rate from 78% to 15% infected chickens. The subunit vaccine treatment resulted in a reduction of the overall colonization rate to 44% infected birds. When administered therapeutically, which would be cheaper to apply in practice, the treatments were not able to significantly reduce the number of colonized birds, but the bacterin-induced antibodies were capable of significantly reducing Campylobacter loads in colonized animals, whereas the subunit-induced antibodies did not. These findings indicate that the bacterin-induced egg yolk antibodies yielded better results than the subunit vaccine-induced antibodies in both in vivo trials. Since both vaccines induced a high immune response in laying hens, the difference in protection between both vaccines cannot be attributed to insufficient antibody titers in the subunit yolks. A plausible explanation is that the bacterin contains whole cells and therefore many possible epitopes, while the subunit vaccine only contains the six selected antigens and thus a more limited number of epitopes. Bacterin-induced antibodies were previously shown to protect against Salmonella Enteritidis27,28 and Eimeria sp.29,30, but Wilkie et al.31 found no protective effect against Clostridium perfringens. On the contrary, earlier subunit vaccine-induced antibodies failed at protecting against C. jejuni32 and Salmonella spp.33.
The reduction in cecal C. jejuni titers after therapeutic administration implies that the antibodies must be active in the ceca, since the ceca were already colonized before starting the treatment. However, the site of action may not be restricted to one single gut region. Prophylactic administration possibly allows capturing the bacteria before cecal colonization, which could explain why the prophylactic model resulted in a better overall colonization reduction. Prophylactic and therapeutic passive immunization experiments with MAB carried out by Tsubokura et al.14 led to resp. a >99% and a 80–95% colonization reduction, also indicating an added value of prophylactic compared to therapeutic administration.
During colonization, Campylobacter can be found in the mucus layer34, its site of multiplication, and epithelial cells35, hiding from mucosal clearance36. Hermans et al.15 demonstrated that binding of Campylobacter to chicken intestinal mucus was enhanced by specific IgY. The increased bacterial uptake in the mucus layer may promote mucosal clearance, leading to the reduced colonization rates observed in our experiments.
Cross-protection for Campylobacter serotypes is one of the major research questions for vaccine development against Campylobacter, as formulated by de Zoete et al.37. The bacterin-induced antibodies strongly reacted to every bacterin and non-bacterin strain, as determined by ELISA. This indicates that passive immunization might protect against the other bacterin strains and suggests a possible cross-protection against heterologous Campylobacter strains, although this should be confirmed in vivo. Nevertheless, these antibodies seem promising at targeting a broad range of Campylobacter strains. In contrast, the subunit vaccine-induced antibodies reacted to the bacterin strains but only to one of the non-bacterin isolates. This can have multiple causes: (1) the genes for the subunit proteins may not be present in these strains, (2) the genes might be present but not expressed or show only a low expression rate38 or (3) the epitopes recognized by the antibodies might be absent or inaccessible39. In vivo protection against these strains using the subunit vaccine-derived antibodies is very unlikely, strengthening the added value of using the bacterin compared to the subunit vaccine.
In this proof of concept study, only young chickens were included in the experiments. The authors acknowledge that additional studies, including experiments in older chickens until slaughter age and field trials should be performed to support our preliminary findings.
In conclusion, two vaccines, a bacterin consisting of thirteen C. jejuni and C. coli strains and a subunit vaccine consisting of six immunodominant Campylobacter antigens, were developed for the immunization of laying hens. Administration of hyperimmune egg yolks induced by these vaccines to the feed of broilers, leads to a reduction of infected birds when used prophylactically and a decrease in Campylobacter titers when used therapeutically. Using one of both strategies, the bacterin treatment resulted in the greatest reduction. Although further research will be needed to provide a treatment protocol fully applicable in the industry, our results indicate that passive immunization of broilers with hyperimmune egg yolks of hens immunized with one of these vaccines, especially the bacterin, offers possibilities to control Campylobacter colonization in poultry.
Methods
Experimental animals
Commercial Lohmann Brown-Classic laying hens, LSL-Classic laying hens and Ross 308 broiler chickens of both sexes were purchased at a local hatchery (layers at De Biest, Kruishoutem, Belgium and broilers at Vervaeke-Belavi, Tielt, Belgium). The animals were provided with a commercial feed and water ad libitum. Husbandry, experimental procedures, euthanasia methods and bio-safety precautions were approved by the Ethical Committee (EC) of the Faculty of Veterinary Medicine, Ghent University, Ghent, Belgium (EC number: 2016/28) and in accordance with the relevant guidelines and regulations. Birds were proved to be free of Campylobacter by examination of mixed fecal samples using standard methods as described by Hermans et al.40.
Bacterial strains and culture conditions
The Campylobacter strains used in this study are listed in Table 5. For all experimental infections in the in vivo trials, C. jejuni reference strain KC40 from poultry origin was used, which colonizes chickens to a high level36. For bacterin composition, Campylobacter strains were kindly provided by Dr. Nadine Botteldoorn (Sciensano, Brussels, Belgium), except for the C. jejuni KC40 reference strain which was previously isolated at the Flanders Research Institute for agriculture, fisheries and food (ILVO, Melle, Belgium). The strains are from chicken origin and were selected based on their genetic heterogeneity based on multilocus sequence typing (MLST), prevalence ratio in broilers16 and relationship with human campylobacteriosis cases41. The remaining Campylobacter strains, used for ELISA crossreaction studies, are from chicken origin and were selected based on their genetic heterogeneity and distinction from the bacterin strains using MLST42.
Table 5.
C. jejuni and C. coli strains from chicken origin used in this study.
| Campylobacter species | Strain | CC | ST | Origin |
|---|---|---|---|---|
| C. jejuni | KC40b | 677 | 794 | Broiler dunghill |
| 10kf-1.16b | 283 | 267 | Carcass | |
| 7P6.12b | 464 | 464 | Feathers | |
| 10C-6.1b | 574 | 305 | Ceca | |
| 10kf-4.12b | 443 | 51 | Carcass | |
| 10VTDD-8b | UA | 905 | Unknown | |
| T124b | 658 | 1044 | Ceca | |
| T84b | 354 | 1073 | Ceca | |
| T70b | 21 | 50 | Carcass | |
| 3291b | 45 | 45 | Carcass | |
| 5970b | UA | 5970 | Carcass | |
| 5CT13 | 48 | 429 | Ceca | |
| 3CT13 | 52 | 600 | Ceca | |
| 1CT117 | 257 | 5742 | Ceca | |
| 1CT51 | 353 | 462 | Ceca | |
| C. coli | 2711b | 828 | 854 | Carcass |
| 3250b | UA | 5163 | Carcass |
CC: Clonal complex; ST: Sequence type; bstrains incorporated in the bacterin; UA: Unassigned.
Bacteria were routinely cultured in Nutrient Broth No. 2 (NB2, CM0067; Oxoid Ltd., Basingstoke, Hampshire, UK) supplemented with Modified Preston Campylobacter-selective supplement (SR0204E; Oxoid) and Campylobacter-specific growth supplement (SR0232E; Oxoid), at 42 °C for 17 h under microaerobic conditions (5% O2, 5% CO2, 5% H2, 85% N2). C. jejuni and C. coli bacteria were enumerated by plating tenfold dilutions in Hank’s Balanced Salt Solution (HBSS; GIBCO-BRL, Invitrogen, Carlsbad, CA) on modified charcoal cefoperazone deoxycholate agar (mCCDA, CM0739; Oxoid) supplemented with CCDA selective supplement (SR0155E; Oxoid) and Campylobacter-specific growth supplement (SR0232E; Oxoid), followed by microaerobic incubation at 42 °C for 22 h.
Prevalence and conservation level of immunodominant Campylobacter antigens
Based on the results of Hermans et al.15, six immunodominant antigens with high reactivity to IgY from eggs of chickens immunized against C. jejuni were selected: AtpA, Ef-Tu, GroEL, Tig, CheV and LivJ. These proteins are known or suggested to be expressed on the bacterial cell surface (EfTu, GroEL) or known to be associated with the cell membrane (AtpA, CheV, LivJ, Tig)15,18–21. Previously, positive results were obtained when vaccinating with these proteins19,22–26.
The prevalence and the conservation level of the genes coding for these immunodominant proteins were determined in the Campylobacter strains selected for constructing the bacterin using PCR. Because of the genetic heterogeneity, separate primers were developed for C. jejuni and C. coli strains (http://www.ncbi.nlm.nih.gov/gene/) (Table S1, Supplementary Materials). Campylobacter strains were plated on Columbia Sheep Blood agar (CSB, Oxoid) and incubated overnight at 37 °C under microaerobic conditions (5% O2, 5% CO2, 5% H2, 85% N2). For DNA extraction, colonies were incubated with 20 µL lysis buffer (1/40 10% SDS, 1/20 1N NaOH in AquaDest) until the formation of slime was visible, and afterwards incubated at 95 °C for 10 min. After cooling to condense the water vapor and short centrifugation, 80 µl high performance liquid chromatography (HPLC, Merck, VWR, Amsterdam, Netherlands) grade water was added. The lysate was centrifuged at 13000 rpm for 5 min and the supernatant was stored at −20 °C. The amplification of DNA was performed in a Mastercycler (Eppendorf AG, Hamburg, Germany) in a volume of 25 µL with 1X mastermix [dNTP’s, MgCl and NA polymerase of Bioline (Luckenwalde, Germany)] and 0.5 µM of each primer. C. jejuni strain KC40 was used as a positive control and blanc HPLC water was added to the mix as a negative control. The PCR program was set at 4 min at 95 °C, 35 cycles (1 min at 94 °C, 1 min at 57 °C, 1 min 30 s at 72 °C) and a final elongation step of 15 min at 72 °C. The PCR reaction products were analyzed with gel electrophoresis. Sequencing analysis was performed to determine the degree of conservation of the prevalent encoding proteins. For genes consisting of more than 1000 base pairs, multiple primer pairs were developed (Table S1). The DNA amplification and gel electrophoresis were performed as described above. After checking the purity of the bands, sequencing analysis was performed by Eurofins Genomics (Ebersberg, Germany). Data were analyzed using Nucleotide BLAST (https://blast.ncbi.nlm.nih.gov/Blast) for comparison of the nucleotide sequences, ExPASy Bioinformatics Resource Portal (http://web.expasy.org/translate/) to translate the nucleotide sequences into protein sequences and Protein BLAST (https://blast.ncbi.nlm.nih.gov/Blast) for comparison of the protein sequences.
Preparation of recombinant C. jejuni antigens
For recombinant production of the immunodominant antigens, derived from the C. jejuni reference strain KC40, the E. coli Expression System using Gateway® Technology (Invitrogen) was used. Signal peptides in the coding regions, which were screened by using the SignalP 4.1 server (http://www.cbs.dtu.dk/services/SignalP/), were removed. The coding regions were then amplified by PCR, using Pwo polymerase with proofreading activity (Roche Applied Science, Mannheim, Germany) according to the manufacturer’s instructions and with the primers given in Table S1. The resulting PCR products were cloned into the pENTR™/TEV/D-TOPO® vector (AtpA, EF-Tu and GroEL) or the pENTR™/SD/D-TOPO® vector (Tig, CheV and LivJ) using the Topo TA cloning kit (Invitrogen) according to the manufacturer’s instructions. Next, the genes were transferred into the pDEST™17 destination vector and the resulting expression clones were transformed into BL21-AI One Shot® chemocompetent E. coli cells (Invitrogen).
A fresh transformed E. coli culture was grown in 100 mL Luria Broth medium (LB, Oxoid) supplemented with 50 µL/mL carbenicillin at 37 °C with shaking until an OD600 of 0.6–1.0 was reached. The culture was inoculated in 6 × 200 mL fresh LB medium supplemented with 50 µL/mL carbenicillin at an OD600 of 0.05–0.1 and grown at the same circumstances until an OD600 of 0.4 was obtained. Next, 0.2% L-arabinose was added to induce expression of the recombinant antigens. After 6 h of incubation, the cultures were centrifuged (30 min, 4500 rpm) and the pellets were resuspended in binding buffer (40 mM imidazole, 10 mL binding buffer per 1 g pellet). Next, 100 µL lysozyme (20 µg/ml), 200 µl DNase (Sigma Aldrich, Steinheim, Germany), 50 µl 200 × MgCl2 and 100 µl protease inhibitor (Sigma) were added and the mixture was shaken (30 min). After sonication (7x, 15 sec, maximal amplitude), the lysate was centrifuged (30 min, 4500 rpm). The supernatant was purified on Ni-sepharose columns (His GraviTrap; GE Healthcare Bio-science AB, Uppsala, Sweden) according to the manufacturer’s instructions. Bound proteins were eluted with 3 mL elution buffer (20 mM sodium phosphate, 500 mM NaCl, 500 mM imidazole, pH 7.4) and collected in 17 mL HBSS. The eluate was concentrated to a final volume of 1.5 mL using ultrafiltration (VIVASPIN 20, 5000 MCWO; Sartorius Stedem Biotech, Goettingen, Germany) and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), followed by Brilliant Blue G-Colloidal (Sigma) coloring and Western blotting.
For the Western blot, separated proteins were electrotransferred from SDS-PAGE gels onto nitrocellulose membranes (Bio-Rad, Nazareth, Belgium) as described previously43. Membranes were blocked in 5% skimmed milk in phosphate buffer saline (PBS) (blocking buffer), incubated overnight with mouse monoclonal antibody to hexahistidine tag (1/3000 in blocking buffer, Icosagen Cell Factory, Tartu, Estonia) at room temperature (RT), rinsed in PBS with 0.3% Tween-20 (wash buffer) and incubated for 1 h at RT with rabbit anti-mouse IgG (whole molecule)–peroxidase antibody (1/30 000 in blocking buffer, Sigma-Aldrich). After a wash step in wash buffer, 10 × CN/DAB Concentrate in Stable Peroxide Substrate Buffer (Thermo Scientific) was added for immunodetection of proteins. Protein patterns were scanned using the GS-800 Calibrated Densitometer (Bio-Rad). The protein concentrations were determined using the RC DC Protein Assay (Bio-Rad) and the purified proteins were stored at −80 °C until further use.
Bacterin and subunit vaccine preparation
The bacterin was composed as follows: 13 Campylobacter strains (Table 5) were grown separately in NB2 until 9 log10 colony forming units (cfu)/mL and killed by overnight incubation with 5 mL 36% formaldehyde/L (Sigma-Aldrich) at 37 °C. After centrifugation (30 min at 5000 rpm at 20 °C), the pellets were resuspended in 5 mL 36% formaldehyde/L PBS and incubated overnight at 37 °C. After plating on CSB agar and overnight incubation at 37 °C to check that all the cells were killed, the suspensions were stored at 4 °C. A mix of the 13 Campylobacter suspensions was made, so that each bacterin dose consisted of 8.1 log10 cfu inactivated Campylobacter (i.e. 7 log10 cfu/Campylobacter strain).
For the subunit vaccine, 75 µg protein (i.e. 12.5 µg of each recombinant antigen) was supplemented with HBSS until a volume of 125 µL/vaccine dose. For sham immunization, 125 µL HBSS was used (negative control).
Each immunization dose consisted of 250 µL of a 1:1 mixture of the inoculum with Freund’s Complete Adjuvant (FCA, Sigma-Aldrich) for the first immunization and Freund’s Incomplete Adjuvant (FIA, Sigma-Aldrich) for the boosters.
Immunization of layers
Thirty Campylobacter-free commercial Lohmann Brown-Classic (LBC) and thirty Lohmann LSL-Classic (LLC) layer hens were assigned to the following immunization groups at the age of 20 weeks: bacterin (n = 20 LLC hens), subunit (n = 20 LBC hens) and control (n = 10 LLC hens; n = 10 LBC hens). Chickens were immunized by intramuscular injection in the pectoral muscle with the vaccines composed as described above. Three booster immunizations were given in a two-weekly time interval. Starting from one week after the last immunization, eggs were collected and stored at 4 °C.
Determination of egg yolk IgY titers
Campylobacter-specific IgY titers in egg yolks were determined as previously described by Hermans et al.15 with minor changes to the protocol. Egg yolks were diluted 1/5 (vol/vol) in HBSS, mixed thoroughly and incubated overnight at 4 °C. The supernatant, containing the water-soluble fraction of the egg yolk, was collected for IgY quantification using enzyme-linked immunosorbent assay (ELISA). To determine egg yolk IgY titers against the complete bacterin and the complete subunit vaccine, 96 well flat bottom plates (Nunc MaxiSorp, Nalge Nunc Int., Rochester, NY, USA) were coated (24 h, 4 °C) with 106 cfu bacterin or 3 µg of a mixture of subunit antigens diluted in 50 µL coating buffer (2.16 g Na2CO3.10H2O, 1.935 g NaHCO3 in 500 mL H2O). To determine egg yolk IgY titers against each recombinant antigen, separately, plates were coated with 3 µg of AtpA, CheV, EfTu, GroEL, LivJ or Tig diluted in 50 µL coating buffer. To determine egg yolk IgY titers against the different Campylobacter strains, plates were coated with 106 cfu/strain, diluted in 50 µL coating buffer. After washing (3x HBSS, 1x washing buffer: 0,1% Tween-20 in PBS), the wells were blocked (1 h, room temperature) with 100 µl blocking buffer [1% bovine albumin serum (BSA) in washing buffer]. Next, 100 µL of a 1/2 dilution series of the supernatant of the mixed egg yolks was incubated during 60 min at room temperature. Plates were washed as described above and incubated with 100 µL 1/10,000 horseradish peroxidase (HRP)-labelled anti-chicken IgY (Sigma Aldrich) in washing buffer during 90 min at room temperature. After washing as described above, the plates were incubated with 50 µl 3,3′,5,5′-tetramethyl benzidine (TMB) substrate (Sigma Aldrich) for 10 min at room temperature in the dark. Next, 50 µL 0.5 M H2SO4 was added to each well and the absorbance at 450 nm (OD450) was measured using an automated spectrophotometer (Pharmacia LKB Ultrospec III, Gemini BV, Apeldoorn, Nederland). The IgY titers from yolks of immunized hens were reported as the highest dilution where the OD450 was greater than the OD450+ three standard deviations of wells containing yolk originating from sham vaccinated birds15.
Prophylactic efficacy of in-feed supplementation of bacterin and subunit vaccine derived hyperimmune egg yolk on transmission of and cecal colonization with C. jejuni in broilers
In trial 1, 81 day-of-hatch Campylobacter free broilers were raised in three randomly assigned treatment groups (n = 27/group) and housed in separate isolation units. From the day of hatch until the end of the experiment, the chicks were provided with feed containing 5% (wt/wt) egg yolk (mixed manually through the feed) from hens immunized with the bacterin (group 1), subunit vaccine (group 2) or sham-immunized with HBSS (group 3). Equal amounts of feed and drinking water were provided for each group during treatment and care was taken that all animals had unlimited access to the feed and water. At 10 days of age, the chicks of each group were randomly assigned to three subgroups (n = 9/subgroup) and housed in separate isolation units. At 11 days of age, three seeder chicks of each subgroup were randomly selected and orally inoculated with approximately 1 × 105 cfu of C. jejuni strain KC40. The birds that were not inoculated are referred to as contact animals or sentinels. Using this model, the Campylobacter infection will spread from the seeders to the other animals of the same group reproducing the natural way of infection in the stable and prevention of infection and transmission can be investigated15. At day 16, all animals were euthanized by injection of an overdose (100 mg/kg) sodium pentobarbital (Kela, Hoogstraten, Belgium) in the wing vein and the cecal content was collected for C. jejuni enumeration (as described below).
Therapeutic efficacy of in-feed supplementation of bacterin and subunit vaccine derived hyperimmune egg yolk on cecal C. jejuni colonization in broilers
In trial 2 a therapeutic model was used to test the effect of treatments in birds already colonized with Campylobacter. For this, 81 day-of-hatch Campylobacter free broilers were raised in three randomly assigned groups (n = 27/group) and housed in separate isolation units. At 9 days of age, the chicks of each group were randomly assigned to three subgroups (n = 9/subgroup) and housed in separate isolation units. At 10 days of age, all chicks were orally inoculated with approximately 1 × 105 cfu of C. jejuni strain KC40, similar to the inoculation during the first trial. From day 19 to 21, the chicks were provided with feed containing 5% (wt/wt) egg yolk (mixed manually through the feed) from hens immunized with the bacterin (subgroups 1, 2, 3), subunit vaccine (subgroups 4, 5, 6) or sham-immunized with HBSS (subgroups 7, 8, 9). Since the therapeutic effect on colonized broilers was to be investigated, all birds were inoculated and sufficient time was given between inoculation and the beginning of the treatment to obtain high Campylobacter titers in the gut, comparable to the field situation. At day 22, all animals were euthanized (as described above) and the cecal content was collected for C. jejuni enumeration (as described below).
Cecal Campylobacter jejuni enumeration
Cecal contents were weighed and diluted 1:9 (wt/vol) in NB2 with supplements. A 10-fold dilution series was made in HBSS and 100 µl of each dilution was spread on mCCDA plates. Colonies were counted after 24 h and 48 h incubation at 42 °C under microaerobic conditions. The diluted samples in NB2 were incubated overnight at 42 °C under microaerobic conditions for enrichment. Samples were plated on mCCDA and further incubated. After 24 h and 48 h, the plates were examined for the presence or absence of C. jejuni. Samples negative after titration and enrichment were considered to be free of Campylobacter (<102 cfu/g cecal content, limit of detection). Samples negative after titration but positive after enrichment were considered to contain 102 cfu/g cecal content.
Statistical analysis
Data of the in vivo trials were analyzed using R 3.3.1. Before statistical analysis, C. jejuni counts were transformed to log10 counts. The colonization data were analyzed using a hurdle model44,45, a class of model that assumes that the data are generated by two processes. First, the event that an individual is colonized (i.e. returning a non-zero count) follows a Bernoulli distribution. Given colonization, its intensity or load is a random variable following a discrete or continuous distribution; in this case, a gamma distribution was assumed.
The influence of treatment was assessed by specifying predictors for the Bernoulli probability of occurrence (i.e. probability of colonization, modelled as a logistic function of covariates) and the rates of the gamma distribution (average C. jejuni counts given colonization, modelled as a log-linear function of covariates). In both functions treatment was included as a categorical covariate (bacterin/subunit/control). The sample size prevented the inclusion of an additional covariate for individual type (seeder/sentinel) and the associated interaction term for the first in vivo trial. Instead, the analysis was repeated for all birds and for seeders and sentinels separately. A random effect was included at the subgroup (pen) level to account for clustering.
The model was implemented in a Bayesian framework using JAGS46. Uninformative, flat priors were used for all parameters. Over three Markov chains, 100.000 iterations were run, discarding the first 50.000 as a burn-in. Convergence was assessed by visual inspection of the chain histories and using the Gelman-Brooks-Rubin statistic47. The model was used to estimate the probability of C. jejuni colonization and the mean C. jejuni numbers in the cecal content of colonized birds for each treatment level. Next, the pairwise differences between those, and the proportion of the respective posterior distributions that had the same sign as the mean were calculated. If working in a null-hypothesis significance testing framework, this can be interpreted as a one-sided test (broilers treated with bacterin-induced antibodies versus control birds, broilers treated with subunit vaccine-induced antibodies versus control birds), estimating the probability that the true difference between treatments is zero or greater (if negative) or smaller (if positive), and thus the level of confidence that the null hypothesis can be rejected. The broilers treated with bacterin- and subunit vaccine-induced antibodies were compared with the equivalent of a two-sided test; the null hypothesis was retained when the posterior distribution of the difference did not encompass zero between the 2.5% and 97.5% quantiles.
Supplementary information
Acknowledgements
This work was financed by a grant of Federal Public Service for Health, Food Chain Safety and Environment (FOD, Brussels, Belgium), Project RT14/4-Campimmun. We are grateful to Gunther Antonissen and Marc Verlinden for their co-operation during the in vivo trials. Except for the C. jejuni KC40, all Campylobacter strains for bacterin development were kindly provided by Dr. Nadine Botteldoorn (Sciensano, Brussels, Belgium) and originate from the Project RF11/6241-Campytrace, funded by the Federal Public Service for Health, Food Chain Safety and Environment (FOD, Brussels, Belgium).
Author Contributions
A.M., L.D.Z., M.H., F.H., F.P. and A.G. designed the experiment. F.P. and A.G. selected the vaccine constituents. A.G. and N.V.R. developed the vaccines and vaccinated the layer hens. J.V., A.G. and N.V.R. performed the in vivo trials and sample analysis. J.V. and S.C. carried out data analysis. J.V. and A.G. prepared the manuscript. All authors reviewed the data and the manuscript prior to publication.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jasmien Vandeputte, Email: Jasmien.Vandeputte@UGent.be.
An Garmyn, Email: An.Garmyn@UGent.be.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-45380-z.
References
- 1.EFSA The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017;15:5077. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hannu T, et al. Campylobacter-triggered reactive arthritis: a population-based study. Rheumatology. 2002;41:312–318. doi: 10.1093/rheumatology/41.3.312. [DOI] [PubMed] [Google Scholar]
- 3.Nachamkin I. Chronic effects of Campylobacter infection. Microbes Infect. 2002;4:399–403. doi: 10.1016/S1286-4579(02)01553-8. [DOI] [PubMed] [Google Scholar]
- 4.Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. Brit. Med. J. 1997;314:779–782. doi: 10.1136/bmj.314.7083.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphrey T, O’Brien S, Madsen M. Campylobacters as zoonotic pathogens: A food production perspective. Int. J. Food Microbiol. 2007;117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki H, Yamamoto S. Campylobacter contamination in retail poultry meats and by-products in Japan: A literature survey. Food Control. 2009;20:531–537. doi: 10.1016/j.foodcont.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Hermans D, et al. Campylobacter control in poultry by current intervention measures ineffective: Urgent need for intensified fundamental research. Vet. Microbiol. 2011;152:219–228. doi: 10.1016/j.vetmic.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Stern NJ, Cox NA, Musgrove MT, Park CM. Incidence and levels of Campylobacter in broilers after exposure to an inoculated seeder bird. J. Appl. Poultry Res. 2001;10:315–318. doi: 10.1093/japr/10.4.315. [DOI] [Google Scholar]
- 9.Berndtson E, Dainelsson-Tham ML, Engvall A. Campylobacter incidence on a chicken farm and the spread of Campylobacter during the slaughter process. Int. J. of Food Microbiol. 1996;32:35–47. doi: 10.1016/0168-1605(96)01102-6. [DOI] [PubMed] [Google Scholar]
- 10.van Gerwe T, et al. Quantifying transmission of Campylobacter jejuni in commercial broiler flocks. Appl. Environ. Microb. 2009;75:625–628. doi: 10.1128/AEM.01912-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cawthraw SA, Newell DG. Investigation of the presence and protective effects of maternal antibodies against Campylobacter jejuni in chickens. Avian Dis. 2010;54:86–93. doi: 10.1637/9004-072709-Reg.1. [DOI] [PubMed] [Google Scholar]
- 12.Sahin O, Luo ND, Huang SX, Zhang QJ. Effect of Campylobacter-specific maternal antibodies on Campylobacter jejuni colonization in young chickens. Appl. Environ. Microb. 2003;69:5372–5379. doi: 10.1128/AEM.69.9.5372-5379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalghoumi R, Beckers Y, Portetelle D, Thewis A. Hen egg yolk antibodies (IgY), production and use for passive immunization against bacterial enteric infections in chicken: a review. Biotechnol. Agron. Soc. Environ. 2009;13:295–308. [Google Scholar]
- 14.Tsubokura K, et al. Oral administration of antibodies as prophylaxis and therapy in Campylobacter jejuni-infected chickens. Clin. Exp. Immunol. 1997;108:451–455. doi: 10.1046/j.1365-2249.1997.3901288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermans D, et al. Passive immunization to reduce Campylobacter jejuni colonization and transmission in broiler chickens. Vet. Res. 2014;45:27–27. doi: 10.1186/1297-9716-45-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duarte A, et al. Discriminitave power of Campylobacter phenotypic and genotypic typing methods. J. Microbiol. Meth. 2016;125:33–39. doi: 10.1016/j.mimet.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Garmyn, A. et al. Immunization of chickens against Campylobacter infections. FOD, Brussels, Belgium (2016).
- 18.Lertsethtakarn P, Ottemann KM, Hendrixson DR. Motility and chemotaxis in Campylobacter and Helicobacter. Annu. Rev. Microbiol. 2011;65:389–410. doi: 10.1146/annurev-micro-090110-102908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieves W, et al. Immunospecific responses to bacterial elongation factor Tu during Burkholderia infection and immunization. PloS One. 2010;5:14361. doi: 10.1371/journal.pone.0014361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsugawa H, Ito H, Ohshima M, Okawa Y. Cell adherence-promoted activity of Plesiomonas shigelloides groEL. J. Med. Microbiol. 2007;29(56):23. doi: 10.1099/jmm.0.46766-0. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, et al. Cloning, expression, and antigenicity of 14 proteins from Campylobacter jejuni. Foodborne. Pathog. Dis. 2012;9:706–712. doi: 10.1089/fpd.2011.1122. [DOI] [PubMed] [Google Scholar]
- 22.Bao Y, et al. Chaperonin GroEL: A novel phylogenetically conserved protein with strong immunoreactivity of avian pathogenic Escherichia coli isolates from duck identified by immunoproteomics. Vaccine. 2013;31:2947–2953. doi: 10.1016/j.vaccine.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 23.Kovach Z, et al. Immunoreactive proteins of Campylobacter concisus, an emergent intestinal pathogen. FEMS Immunol. Med. Microbiol. 2011;63:387–396. doi: 10.1111/j.1574-695X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 24.Ribardo DA, Hendrixson DR. Analysis of the LIV system of Campylobacter jejuni reveals alternative roles for LivJ and LivK in commensalism beyond branched-chain amino acid transport. J. Bacteriol. 2011;193:6233–6243. doi: 10.1128/JB.05473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoaf-Sweeney KD, Larson CL, Tang X, Konkel ME. Identification of Campylobacter jejuni proteins recognized by maternal antibodies of chickens. Appl. Environ. Microbiol. 2008;74:6867–6875. doi: 10.1128/AEM.01097-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan L, et al. Characterization of the chaperonin GroEL in Mycoplasma gallisepticum. Arch. Microbiol. 2015;197:235–244. doi: 10.1007/s00203-014-1047-2. [DOI] [PubMed] [Google Scholar]
- 27.Gürtler M, Methner U, Kobilke H, Fehlhaber K. Effect of orally administered egg yolk antibodies on Salmonella Enteritidis contamination of hen’s eggs. J. Vet. Med. 2004;51:129–134. doi: 10.1111/j.1439-0450.2004.00739.x. [DOI] [PubMed] [Google Scholar]
- 28.Fulton RM, Nersessian BN, Reed WM. Prevention of Salmonella Enteritidis infection in commercial ducklings by oral chicken egg-derived antibody alone or in combination with probiotics. Poult. Sci. 2002;81:34–40. doi: 10.1093/ps/81.1.34. [DOI] [PubMed] [Google Scholar]
- 29.Lee SH, et al. Induction of passive immunity in broiler chickens against Eimeria acervulina by hyperimmune egg yolk immunoglobulin Y. Poult. Sci. 2009;88:562–566. doi: 10.3382/ps.2008-00340. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, et al. Protective effect of hyperimmune egg yolk IgY antibodies against Eimeria tenella and Eimeria maxima infections. Vet. Parasitol. 2009;163:123–126. doi: 10.1016/j.vetpar.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Wilkie DC, Van Kessel AG, Dumonceaux TJ, Drew MD. The effect of hen-egg antibodies on Clostridium perfringens colonization in the gastrointestinal tract of broiler chickens. Prev. Vet. Med. 2006;74:279–292. doi: 10.1016/j.prevetmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Paul N, Al-Adwani S, Crespo R, Shah DH. Evaluation of passive immunotherapeutic efficacy of hyperimmunized egg yolk powder against intestinal colonization of Campylobacter jejuni in chickens. Poult. Sci. 2014;93:2779–2787. doi: 10.3382/ps.2014-04234. [DOI] [PubMed] [Google Scholar]
- 33.Chalghoumi R, Marcq C, Thewis A, Portetelle D, Beckers Y. Effects of feed supplementation with specific hen egg yolk antibody (immunoglobin Y) on Salmonella species cecal colonization and growth performances of challenged broiler. Poult. Sci. 2009;88:2081–2092. doi: 10.3382/ps.2009-00173. [DOI] [PubMed] [Google Scholar]
- 34.Beery JT, Hugdahl MB, Doyle MP. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microb. 1988;54:2365–2370. doi: 10.1128/aem.54.10.2365-2370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knudsen KN, Bang DD, Andresen LO, Madsen M. Campylobacter jejuni strains of human and chicken origin are invasive in chickens after oral challenge. Avian Dis. 2006;50:10–14. doi: 10.1637/7376-051005R.1. [DOI] [PubMed] [Google Scholar]
- 36.Van Deun K, et al. Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Vet. Microbiol. 2008;130:285–297. doi: 10.1016/j.vetmic.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 37.de Zoete MR, van Putten JPM, Wagenaar JA. Vaccination of chickens against Campylobacter. Vaccine. 2007;25:5548–5557. doi: 10.1016/j.vaccine.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Snyder, L., Peters, J. E., Henkin, T. M. & Champness, W. Molecular genetics of bacteria (ed. ASM press) (Washington, DC, 2013).
- 39.Bagnoli, F. & Rappuoli, R. Vaccine design: innovative approaches and novel strategies (ed. Caister Academic Press) (Wymondham Norofolk, UK, 2011).
- 40.Hermans D, et al. The cinnamon-oil ingredient trans-cinnamaldehyde fails to target Campylobacter jejuni strain KC 40 in the broiler chicken cecum despite marked in vitro activity. J. Food Prot. 2011;74:1729–1734. doi: 10.4315/0362-028X.JFP-10-487. [DOI] [PubMed] [Google Scholar]
- 41.Botteldoorn, N. Workshop Campylobacter in het slachthuis en uitsnijderij: risico’s en mogelijkheden voor een betere beheersing. FOD Volksgezondheid, Veiligheid van de Voedselketen en Leefmilieu, Brussels, Belgium (2016).
- 42.Vinueza-Burgos C, et al. Prevalence, antimicrobial resistance and genetic diversity of Campylobacter coli and Campylobacter jejuni in Ecuadorian broilers at slaughter age. Poult. Sci. 2017;96:2366–2374. doi: 10.3382/ps/pew487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Steendam K, et al. Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins. Arthritis Res. Ther. 2010;12:132–132. doi: 10.1186/ar3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cragg JG. Some statistical models for limited dependent variables with application to the demand for durable goods. Econometrica. 1971;39:829–844. doi: 10.2307/1909582. [DOI] [Google Scholar]
- 45.Mullahy J. Specification and testing of some modified count data models. J. Econom. 1986;33:341–365. doi: 10.1016/0304-4076(86)90002-3. [DOI] [Google Scholar]
- 46.Plummer, M. JAGS: just another Gibbs sampler. Proceedings of the 3rd International Workshop on Distributed Statistical Computing, Vienna, Austria (2005).
- 47.Brooks SP, Gelman G. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1998;7:434–455. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.


