Abstract
Older adults undergoing age-related decrements in muscle health can benefit substantially from resistance exercise training, a potent stimulus for whole muscle and myofiber hypertrophy, neuromuscular performance gains, and improved functional mobility. With the use of advancing technologies, research continues to elucidate the mechanisms of and heterogeneity in adaptations to resistance exercise training beyond differences in exercise prescription. This review highlights the current knowledge in these areas and emphasizes knowledge gaps that require future attention of the field.
Introduction
Skeletal muscle is a highly adaptable tissue that comprises ~30–40% of total body mass and is remarkably compromised by aging (74). Declines in muscle mass approach nearly 10% per decade and are accelerated with advancing age (51, 87, 88). Muscle-associated clinical pathologies, including sarcopenia and frailty, are more prevalent in individuals in the ninth decade of life (8, 102). Loss of muscle mass is particularly concerning, given its important roles in physiological processes including movement (92), metabolism (154), signaling (107), disease and infection resistance (25), independence (150), and quality of life (9). Furthermore, declines in muscle health have been associated with premature mortality among community-dwelling older adults (16). As a result, exercise training interventions that rescue muscle mass and function have enormous potential to improve the experience of aging and reduce the incidence of age-related conditions that deteriorate quality of life. Progressive resistance exercise training (RT) represents the most widely recognized strategy to combat age-related muscle atrophy and improve overall muscle health on multiple levels: 1) muscle mass, 2) neuromuscular performance (e.g., strength and power), and 3) cellular and subcellular adaptations. The purpose of this review is to 1) summarize the health benefits of RT in the aging neuromuscular system; 2) overview known underlying mechanisms of RT adaptation in the older adult; 3) outline an evidence-based RT prescription proven to promote these adaptations; and 4) highlight key knowledge gaps ripe for future research.
Health Benefits of Resistance Exercise Training in the Aging Neuromuscular System
Muscle Mass
The etiology of age-related muscle atrophy is a multi-faceted degenerative process involving both atrophy of fast (type IIa and IIx) myofibers (78, 97, 108, 135) and a reduction in total myofiber number (85). RT is a potent hypertrophy stimulus for all myofiber types, particularly the type II fibers typically compromised by aging. With RT, older adults exhibit well-characterized shifts in myofiber type distribution (IIx to IIa shift) and concomitant myofiber hypertrophy across fiber types (preferential to type II myofibers) (13, 103, 142, 144). There is no definitive evidence of myofiber hyperplasia in adult humans (although we recognize current limitations in measurement tools). Furthermore, the limited evidence of hyperplasia in animal models is based on an extreme degree of physiological stress to induce myofiber splitting (148). Thus, to our knowledge, no human intervention can restore myofiber number: combating aging muscle atrophy with RT is likely fully dependent on the induction of myofiber hypertrophy. Still, many older adults respond to RT with myofiber hypertrophy sufficient to meet or exceed the type II myofiber size of sex-matched young adults (13, 78).
Neuromuscular Performance
Whereas muscle mass declines at a rate of ~1% per year, accompanying neuromuscular functional reductions (e.g., muscle strength and power) occur more rapidly (92). Longitudinal studies have demonstrated decreases in muscle strength of ~2–4% per year (54, 55), and another study found a reduction of ~8–9% in muscle power over a 3-year period (132). Muscle power is the product of force and contractile velocity; thus age-related loss of muscle power is driven by reductions in the force-generating capacity of muscle, as well as slowing of the rate of force development (RFD) (93). Loss of muscle power is a strong predictor of physical impairment in older adults (26, 42, 98, 131). Contractile properties of single myofibers, when normalized for myofiber size, are not compromised with aging (55, 127, 130, 132), with some data suggesting that heightened fiber function may compensate for deficits at the whole muscle level (61). Although the notable reductions in lower extremity muscle power (27, 122, 130, 132) suggest that whole muscle atrophy is a major driver, muscle atrophy does not account for the entirety of power decline: relative muscle power (i.e., adjusted for muscle mass) is also reduced with advancing age (116, 121, 132, 135), suggesting that a neurological component is also involved. Indeed, older adults may have ~30–40% fewer motor units compared with young adults (76, 79, 108, 119–121), and there is evidence to suggest increased size of surviving motor units [as supported by electromyographic studies (95, 121, 141)] and motor unit remodeling via type I myofiber grouping (67, 77, 135). These findings combined suggest that neural activation of these larger type I motor units may play a role in reducing explosive force and thus power (89).
The effectiveness of RT in reversing age-related reductions in muscle function has been consistently demonstrated. A 25–35% increase in leg muscle strength, measured as one repetition maximum (1RM) (105, 106, 115, 144), and similar improvement in upper body strength (28, 46, 66, 124) occur in healthy older adults with at least 8–12 wk (28, 64, 105, 115, 144) of moderate to high-intensity RT (>70% 1RM) (13, 24, 33, 144). Interestingly, the increases in muscle strength and power with RT occur before and exceed the hypertrophic morphological response (34, 54). This is explained by the early physiological phase of neuroadaptation that normally follows the first weeks of training. These findings support the hypothesis that a main factor of muscle wasting is impaired neurological control—more so than an intrinsic inability of older muscle fibers to generate force—and confirm the effectiveness of RT to improve neuromuscular function in older adults. Moreover, an increase in lower extremity muscle power is accompanied by an improvement in balance (50, 69, 81, 82, 90) and reduced fall risk, which contributes to reduced mortality in older adults (43, 137).
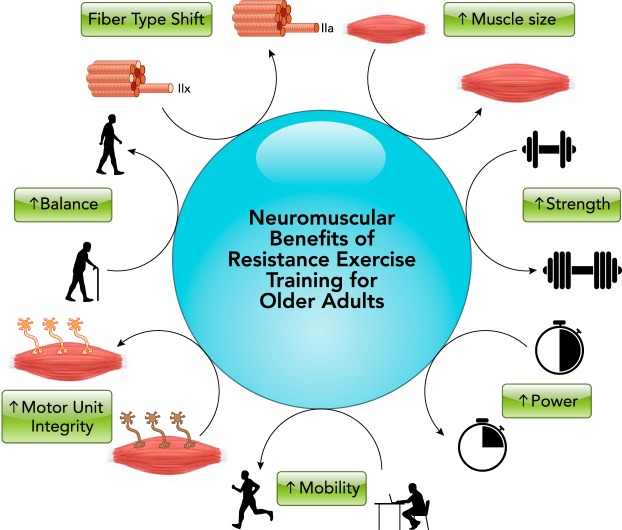
The benefits of RT exceed improvements in skeletal muscle size and strength alone (FIGURE 1). Strength improvement and myofiber hypertrophy due to RT reduce the motor unit activation demand to perform a given submaximal movement, as we (115, 144) and others (71) have shown during a sit-to-stand task. The basis of this may be related to motor unit remodeling that accompanies sedentary aging as an apparent result of denervation-reinnervation events (67, 75, 77). Encouragingly, short-term RT appears to reverse this phenomenon, at least in those individuals with a higher degree of motor unit remodeling (76).
FIGURE 1.
Neuromuscular benefits for older adults undergoing resistance exercise training
The neuromuscular benefits for older adults undergoing resistance exercise training (RT) extend beyond muscle mass and strength. For a summary of RT benefits on other organ systems, see text.
Mechanisms of Resistance Exercise Training Adaptation in the Older Adult
Muscle Protein Synthesis
Skeletal muscle mass is regulated by the fine balance between two cellular processes: protein synthesis and breakdown. A positive net balance is achieved when the rate of protein synthesis exceeds the rate of degradation. Several studies have found that muscle protein synthesis increases after an acute bout of RT (30, 31, 39, 56, 118), and the effect can last up to 48 h. However, more recent findings have also revealed that the acute response to exercise is not always predictive of a long-term adaptation (31, 101). Moreover, the magnitude of the response to RT is different between younger and older adults (40, 80), with aging affecting skeletal muscle tissue sensitivity to anabolic stimuli, such as physical activity and nutrition (104, 138).
Despite these caveats, chronic RT increases basal muscle protein synthesis in both young (133) and older adults (103), and this has been shown to correlate with increased skeletal muscle thickness (133) and myofiber cross-sectional area (CSA) (31). The perpetual repetition of training sessions stimulates the activation of the mechanistic target of rapamycin complex 1 (mTORC1), which directly promotes protein synthesis via phosphorylation of specific downstream effectors (S6K1, rpS6, eEF2, and 4EBP1). It is beyond the scope of this review to describe in detail the mechanisms that regulate hypertrophy through mTORC1; however, it is interesting to note that mTORC1 also plays a key role on ribosomal function and biogenesis (21), which are primary promoters of translational capacity. Recent studies have demonstrated that muscle levels of ribosomal RNAs (rRNAs) are highly elevated after resistance exercise in young subjects (49) but not in older adults (15, 143) and that basal levels of upstream mRNAs (ribosome regulators) are predictive of the myofiber hypertrophic response to RT (149). Furthermore, via cluster analysis, differential myofiber hypertrophy among older adults in response to short-term RT appears to be mediated at least in part by differential induction of ribosome biogenesis, enabling heightened capacity for protein synthesis (143).
Several studies have also shown that a single bout of RT can increase the muscle protein anabolic response to nutritional stimuli (17, 37, 41). Exercise, with an increased demand of blood flow and energy, increases the delivery of dietary nutrients and modulates the protein synthetic response to food ingestion (41, 111). Indeed, very old adults undergoing heavy RT benefitted further from the addition of a protein supplement, further highlighting the link between RT-induced demand and nutrient availability in aiding hypertrophy (10). However, it has been recently shown that regular RT increases basal muscle protein synthesis in healthy older adults but does not further improve the muscle protein anabolic response to amino acids (103).
Training also impacts muscle protein breakdown as a physiological response to the damage caused by muscular contraction. Protein breakdown increases following an acute bout of RT, and this response seems to be similar in young and older adults (57). Recently, Damas found that muscle protein breakdown is more pronounced in novice subjects, and this is reduced in favor of an enhanced protein synthetic response with subsequent training (29). These findings underpin the positive effect of RT on muscle repair and regeneration, leading to reinstatement and maintenance of muscle mass throughout life. Thus the primary effects of exercise on muscle protein balance, largely mediated through mTORC1 and ribosomal biogenesis, facilitate a shift to a positive net balance and lead to an increase in muscle mass.
Insights from Exercise -Omics
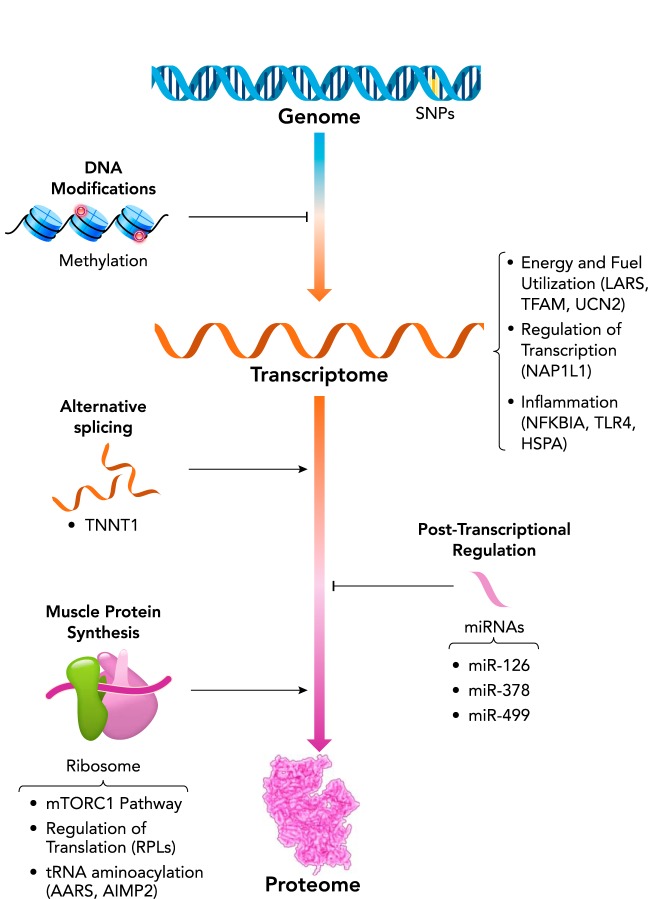
The recent advent of genome-wide and phenome-wide association studies (often termed the GWAS and PheWAS era) has created heightened potential for discovery, providing the opportunity to investigate all possible contributors to RT adaptations. The burgeoning -omics fields are interconnected through their influence on phenotype (FIGURE 2), as recently described in greater detail in the context of exercise physiology (14, 68, 157). These areas represent the new horizon for exercise biology, and the currently available literature has barely begun to scratch the surface of this enormous potential. The field as a whole will be able to leverage the amassed -omics data sets soon to be generated in the ground-breaking NIH Common Fund initiative, the Molecular Transducers of Physical Activity Consortium (MoTrPAC). Early explorations, along with studies targeted at investigating an isolated pathway or biological process, have provided considerable insight into potential mechanisms underlying RT adaptations in older adults. With the continual advancement of available technologies, studies are often not uniform with regard to the selected platform (e.g., targeted PCR to microarray to transcriptomics). However, these methodological differences (including disparities in exercise regimens, biopsy timing, etc.) present a strength, since several recurrent themes are still able to emerge, including mitochondrial health (96, 136), inflammation (128, 149), and regulation of protein metabolism (7, 117, 126, 136, 149).
FIGURE 2.
Regulation of skeletal muscle gene expression and protein expression
Regulation of skeletal muscle gene expression and, ultimately, protein expression, occurs at multiple levels, influencing both baseline phenotype and responsiveness to an RT. Molecular networks of interest, based on current knowledge (32, 36, 117, 128, 134, 136, 149, 155, 156), are highlighted in this diagram and provided in context in the text.
A focal point of exercise and aging research has been the mechanistic investigation into response heterogeneity to exercise training in aging adults by comparing baseline muscle characteristics across a range of hypertrophic responses to resistance exercise. Targeted investigations into single nucleotide polymorphisms in inflammatory cytokines (35, 112) and upstream regulatory elements (112) have shed light on the potential impact of inflammation to blunt adaptive responses to RT (6, 97, 152). More comprehensive approaches using microarray (117, 149) elaborate on the impact of basal gene expression on the training response. For instance, we have found enormous differential gene expression (8,026 genes) between non-responders and extreme responders to RT, predominantly associated with regulation of transcription, muscle function and development, and inflammation (149). Another investigation found increased activity of the rapamycin pathway in individuals who responded best to RT training (117). Beyond this, microRNA (miRNA) abundances (32, 109, 155) and transcript splice variants (156) have been implicated in contributing to the magnitude of RT adaptations, and additional regulatory mechanisms of gene expression (e.g., methylation, acetylation) are also likely involved.
Some studies have demonstrated limited plasticity to exercise training in older adults compared with young adults (59, 127, 139), and this may be the result of a generally less robust adaptive response in basal gene expression (128), gene silencing (via miRNA) (36, 134), and/or another yet unexplored facet of metabolism. Nonetheless, RT is more likely to reverse the expression of age-related genes than those unaffected by aging (96). Raue and colleagues provided early insight into the relationship between the exercise response and changes in gene expression, showing a basal increase in 144 genes after RT training in older women (128). In this study, two inflammation-related genes (TNFRSF12A and NFKBIA) demonstrated significant relationships with changes in skeletal muscle mass and strength throughout training. Likewise, studies have found relationships between RT-induced gains and changes in abundances of miRNAs (32, 134, 155), highlighting the dynamics of posttranscriptional gene expression regulation and interdependence of -omics tracks in adaptations to exercise. In a comparison across three modes of exercise, Robinson and colleagues found that RT increased basal expression of 33 unique genes, along with increased expression of angiogenic factors (e.g., targets of VEGFA) and growth signaling-associated genes across all training modes. In a concomitant proteomics analysis (136), the group found 185 proteins that increased in response to RT, mainly related to translation processes (e.g., tRNA aminoacylation) and ribosome function, highlighting the importance of enhanced translational capacity to support the heightened demands of RT.
Resistance Training Prescription for the Older Adult
Exercise dosage underlies all potential physiological benefits of RT. Most RT intervention studies have only used one or two training doses, which provides little insight into a dose-response relationship. To our knowledge, only one study has employed four doses (144) in an attempt to titrate the optimal exercise regimen. Nevertheless, a collective agreement in the field is that progressively overloading the muscle through RT is necessary to create continuous adaptations (2). When familiarization to proper exercise technique and structured progression are components of the study design, RT is safe and effective for older adults, with rates of injuries extremely low and similar across all ages and intensities (48, 52). Furthermore, adherence and dropout rates do not differ by training intensity (129), but adherence may be slightly higher on a lower-frequency regimen (144). It is important to note that, even within a given prescription, response heterogeneity is still evident; thus the truly optimal exercise dose is likely variable by individual.
Frequency, Intensity, and Volume
Training dose is dependent on several factors, including number of sets and repetitions, frequency, and intensity. Each of these components is an important consideration in designing an RT regimen. For example, total weekly volume is equal in the following two scenarios: 1) 3 sets of 15 repetitions per set at a 45-kg load repeated 2 days/wk and 2) 3 sets of 6 repetitionss per set at a 75-kg load repeated 3 days/wk. There is considerable variance across studies with regard to exercise programs, but the primary outcome of the study (e.g., hypertrophy, strength, functional mobility) likely dictates the optimal exercise prescription. Although the ACSM recommends that older adults participate in a minimum of two RT sessions per week (3), some researchers use a low frequency as a minimal effective dose (1 day/wk) to promote adaptations (38, 145), and others use a more aggressive approach to maximize changes (3 days/wk) (13, 45, 84, 86, 110). Several have found no difference across training frequencies for strength adaptations (19, 38, 145).
Frequency, however, is not the sole determinant of adaptation. In our recent four-arm, randomized dose-response RT trial among older adults, we noted substantially greater gains in total body lean mass, thigh muscle mass, and isometric strength when the 3 day/week prescription involved a lower intensity “light” day during the midweek session, as compared with high-intensity training all 3 days each week (144). This finding highlights that, in designing exercise prescriptions for older adults, recovery must be considered as carefully as intensity. However, there is no standardized definition of high, moderate, or low intensity in the literature. Two previous analyses have classified >70% 1RM as high intensity, 50–70% 1RM as moderate intensity, and <50% 1RM as low intensity (11, 129). High-intensity RT has been shown to improve lower body strength to a greater degree than moderate or lower intensity, if training volume is equivalent (129). High-intensity RT has also been shown to improve strength, anaerobic power, and mobility, and to increase bone mineral density more than low-intensity RT (47, 147). Outside of intensity, explosive training has also been an interest in the field due to its potential to maximize RFD, which could translate into better functional mobility and balance. One meta-analysis found that there was a similar effect between high-intensity strength training and explosive training (62), although gains in RFD and muscle strength did not always coincide.
Session volume (number of sets and repetitions per session) was found to be positively associated with lean body mass increases in a meta-analysis of 49 studies averaging 20 wk in duration (113). If intensity is held constant, short-term (6–10 wk) gains in muscle mass and strength are similar between high (3 sets) and low (1 set) session volume (18, 125), but a clear benefit appears for the higher session volume prescription by the 20th week of training (123). In a small, 20-wk study of community-dwelling older adults, a high session volume (3 sets vs. 1 set to failure) training program improved muscle strength, endurance, and 400-m walk time. However, both training programs improved functional movements (chair rise, 6-m backward walk, stair climb) and muscular endurance (58). These studies further support the well-accepted concept of progressive overload to continue to promote training adaptations over time (e.g., by manipulating session volume, frequency, and/or intensity).
Detraining
Following cessation of training, some evidence suggests older adults can maintain dynamic strength for several months (86). For example, after 24 wk of RT, only minor changes occurred with 3 wk of detraining, although improvements in walking speed remained elevated (63). Over longer periods, RT adaptations are gradually lost (83, 146). Maintenance of muscle strength and size can be preserved on a minimal dose (one set) after 12 wk of RT (151). In one study, both young and older adults were followed for 32 wk after 16 wk of 3 days/wk RT and were assigned to either no training, 1 day/wk at 1/3 the initial weekly exercise volume (3 sets), or 1 day/wk at 1/9 the initial weekly exercise volume (one set) (13). Without a maintenance dose, loss of RT-induced muscle mass gains was detected in both age groups after only 8 wk of detraining, whereas decrements in 1RM were not detected until 32 wk of detraining. The investigators also found that a maintenance dose of 1/3 or 1/9 the volume preserved strength in both age groups, but, among older adults, only the higher maintenance dose preserved the RT-induced increases in muscle mass, whereas young adults effectively preserved gains in mass on both doses (13). Exercise intensity may also play an important role in preventing detraining, with one study demonstrating that strength and mobility were preserved throughout 2 years of detraining following high-intensity RT (82% 1RM) but not after lower-intensity RT (55% 1RM) (47). Thus the minimal effective dose for increasing and then preserving muscle mass in older adults is likely a very manageable number of muscular contractions.
Response Heterogeneity
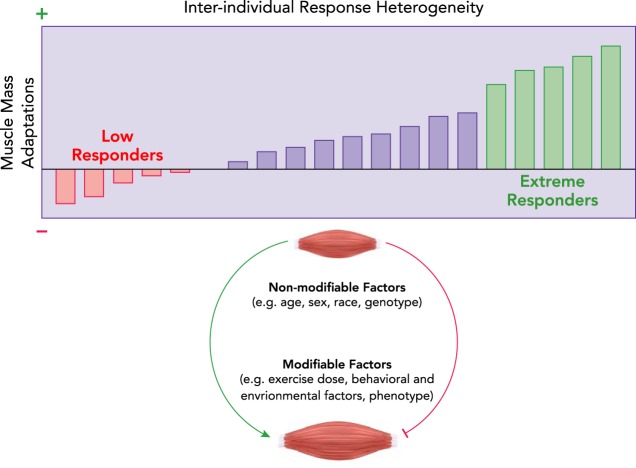
Although all individuals garner some degree of beneficial adaptations to RT, there is appreciable variability in responsiveness to RT when strictly defined as the magnitude of whole muscle or myofiber hypertrophy (1, 4, 23, 32, 44, 70) (FIGURE 3). One approach to classifying and studying response heterogeneity is to group responders using K-means clustering, a method introduced several decades ago (73) and first applied to exercise response heterogeneity in 2007 (7). We have used this method to group subjects by myofiber hypertrophy after a training stimulus (7, 114, 149) and validated the method in subsequent trials (142, 144). Important considerations for clustering include exercise prescription (i.e., a given individual’s response might be poor on a given RT prescription but very good on another) and baseline phenotype [i.e., individuals with poorer muscle mass at the outset may improve more by a regression to the mean phenomenon (22)]. A number of non-modifiable (e.g., sex, race, ethnicity, age, genotype) and modifiable (e.g., co-morbidities, functional capacity, diet, medications, sleep) factors may influence response heterogeneity, but each has yet to be fully explored. To our knowledge, no studies have demonstrated differences in response heterogeneity in response to RT as a direct influence of any of these factors in isolation. Once again, this highlights the potential impact of future GWAS-PheWAS applications to RT trials. Although the causes of response heterogeneity are not fully understood, strategies to attenuate the range of heterogeneity or reduce the number of non-responders may leverage optimization of exercise dose, nutrition, and possibly pharmaceutical adjuvants. For example, response heterogeneity to traditional 3 days/wk RT programs has been noted in older adults (142), but recent work using 2 days/wk of high-resistance concentric-eccentric training and 1 day/wk low-resistance, high-velocity, concentric-only training showed meaningful improvements in thigh muscle mass and myofiber CSA in a high proportion of older adult participants (144).
FIGURE 3.
Response heterogeneity to progressive resistance exercise training in older adults
Potential causes of heterogeneity include both non-modifiable (e.g., age, sex, race, genotype) and modifiable (e.g., exercise dose, behavioral and environmental factors, phenotype) factors (5). Titrating the exercise dose has been effective in reducing the number of low responders (144).
Another yet poorly understood component of variability in response to RT is an apparent result of advancing age. Although adults aged 60–75 yr still possess a robust, albeit attenuated, hypertrophic ability following 16 wk of RT (13, 78, 153), octogenarians and very old adults often display a limited or blunted hypertrophic capacity (59, 127, 139). Overall, although response heterogeneity presents a challenge for investigators, it also serves as an opportunity to explore its mechanistic basis, highlighting the importance of continued research into subcellular adaptations to exercise training.
Knowledge Gaps Ripe for Future Research
Mechanistic Knowledge Gaps
Discovery-oriented research may reveal previously unknown molecular transducers of adaptations to RT unique to the individual and perhaps common linkages among phenotypic groups (e.g., age, sex, disease status, or medication profile), creating opportunities to design prescriptions with greater precision. Numerous molecular mapping tools remain largely unexplored in the context of exercise in aging muscle, including but not limited to metabolomics, microbiomics, and ribosome biology. Still, the more commonly used platforms (e.g., RNA-Seq, miRNA-Seq) receive preferential attention in aerobic training study designs, leaving many questions to be answered through RT interventions. For example, the molecular basis of the range of adaptability to short-term RT remains incompletely understood. Examination of transient, exercise-induced changes in gene transcription, translation, and regulatory factors (e.g., methylation, histone modification, gene silencing, and alternative splicing) may provide insight into potential deficiencies in aging skeletal muscle in general or among individuals or subgroups that might be rescued by tailored exercise prescriptions with or without pharmaceutical adjuvant therapies.
Clinical Knowledge Gaps
The benefits of preserving muscle mass through RT during aging extend well beyond strength and power [e.g., heightened exercise tolerance (72); decreased difficulty in activities of daily living (65); enhanced cognition, memory, and mood (12, 91, 94); reduced disease susceptibility (25); improved surgical outcomes (53); and prolonged independence (150) and lifespan (140)], but several unknowns remain. Future studies should be designed to examine the benefits of long-term (i.e., lifelong) resistance exercise training, as has become a recent fascination in the endurance exercise and aging literature (20, 60, 99, 100). Since the popularity of RT as a structured exercise prescription is a relatively recent phenomenon, lagging slightly behind the running boom and other aerobic training trends, individuals with a lifelong RT training background will soon begin to reach retirement age. It would provide considerable insight to investigate not only the skeletal muscle benefits but other health outcomes with lifelong RT. Short-term RT studies have previously demonstrated considerable success in inducing a range of non-muscle health benefits (12, 65, 72, 91, 94). It is attractive to speculate that lifelong RT may not only enhance the degree of these benefits but also prevent other age-related declines that show limited reversibility with short-term exercise training.
In addition to muscle- and performance-specific adaptations to long-term RT and acute responses to a single exposure, it is beneficial to continue to pursue an understanding of the behavioral determinants of participation in and adherence to an RT regimen. For example, aging athletes who are aerobically inclined may engage in RT to forestall overuse injuries, whereas others may be more motivated by non-muscle adaptations to RT (e.g., body composition changes, cognitive function, mobility difficulty, psychological well-being). Through continued pursuit into the molecular basis of RT adaptation, optimization of exercise prescription, and approaches to maximize awareness and adherence, we expect RT to strengthen its position as an effective tool for maximizing healthspan in the aging population.
Acknowledgments
The authors acknowledge support from National Institutes of Health Grants T32 HD-071866 (to K.M.L.), F32 AG-058380 (to B.M.R.), and R01 AR-072061 (to C.S.F.); NIH Common Funds U01 AR-071150 (to B.B.R.) and U01 AR-071133 (to M.M.B.); and NIH NICHD/NCMRR/NINDS/NIBIB P2CHD086851.
No conflicts of interest, financial or otherwise, are declared by the author(s).
K.M.L. and B.M.R. prepared figures; K.M.L., B.M.R., C.S.F., and T.M. drafted manuscript; K.M.L., B.M.R., C.S.F., T.M., B.B.R., and M.M.B. edited and revised manuscript; K.M.L., B.M.R., C.S.F., T.M., B.B.R., and M.M.B. approved final version of manuscript.
References
- 1.Ahtiainen JP, Walker S, Peltonen H, Holviala J, Sillanpää E, Karavirta L, Sallinen J, Mikkola J, Valkeinen H, Mero A, Hulmi JJ, Häkkinen K. Heterogeneity in resistance training-induced muscle strength and mass responses in men and women of different ages. Age (Dordr) 38: 10, 2016. doi: 10.1007/s11357-015-9870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Sports Medicine American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41: 687–708, 2009. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 3.American College of Sports Medicine; Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 41: 1510–1530, 2009. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson G, Batterham AM. True and false interindividual differences in the physiological response to an intervention. Exp Physiol 100: 577–588, 2015. doi: 10.1113/EP085070. [DOI] [PubMed] [Google Scholar]
- 5.Bamman MM, Cooper DM, Booth FW, Chin ER, Neufer PD, Trappe S, Lightfoot JT, Kraus WE, Joyner MJ. Exercise biology and medicine: innovative research to improve global health. Mayo Clin Proc 89: 148–153, 2014. doi: 10.1016/j.mayocp.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bamman MM, Ferrando AA, Evans RP, Stec MJ, Kelly NA, Gruenwald JM, Corrick KL, Trump JR, Singh JA. Muscle inflammation susceptibility: a prognostic index of recovery potential after hip arthroplasty? Am J Physiol Endocrinol Metab 308: E670–E679, 2015. doi: 10.1152/ajpendo.00576.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol (1985) 102: 2232–2239, 2007. doi: 10.1152/japplphysiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147: 755–763, 1998. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 9.Beaudart C, Reginster JY, Petermans J, Gillain S, Quabron A, Locquet M, Slomian J, Buckinx F, Bruyère O. Quality of life and physical components linked to sarcopenia: The SarcoPhAge study. Exp Gerontol 69: 103–110, 2015. doi: 10.1016/j.exger.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Bechshøft RL, Malmgaard-Clausen NM, Gliese B, Beyer N, Mackey AL, Andersen JL, Kjær M, Holm L. Improved skeletal muscle mass and strength after heavy strength training in very old individuals. Exp Gerontol 92: 96–105, 2017. doi: 10.1016/j.exger.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Beneka A, Malliou P, Fatouros I, Jamurtas A, Gioftsidou A, Godolias G, Taxildaris K. Resistance training effects on muscular strength of elderly are related to intensity and gender. J Sci Med Sport 8: 274–283, 2005. doi: 10.1016/S1440-2440(05)80038-6. [DOI] [PubMed] [Google Scholar]
- 12.Best JR, Chiu BK, Liang Hsu C, Nagamatsu LS, Liu-Ambrose T. Long-term effects of resistance exercise training on cognition and brain volume in older women: results from a randomized controlled trial. J Int Neuropsychol Soc 21: 745–756, 2015. doi: 10.1017/S1355617715000673. [DOI] [PubMed] [Google Scholar]
- 13.Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Med Sci Sports Exerc 43: 1177–1187, 2011. doi: 10.1249/MSS.0b013e318207c15d. [DOI] [PubMed] [Google Scholar]
- 14.Bouchard C, Rankinen T, Timmons JA. Genomics and genetics in the biology of adaptation to exercise. Compr Physiol 1: 1603–1648, 2011. doi: 10.1002/cphy.c100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol 594: 7399–7417, 2016. doi: 10.1113/JP272857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J Cachexia Sarcopenia Muscle 7: 290–298, 2016. doi: 10.1002/jcsm.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, Cashaback JG, Potvin JR, Baker SK, Phillips SM. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol 588: 3119–3130, 2010. doi: 10.1113/jphysiol.2010.192856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon J, Marino FE. Early-phase neuromuscular adaptations to high- and low-volume resistance training in untrained young and older women. J Sports Sci 28: 1505–1514, 2010. doi: 10.1080/02640414.2010.517544. [DOI] [PubMed] [Google Scholar]
- 19.Carneiro NH, Ribeiro AS, Nascimento MA, Gobbo LA, Schoenfeld BJ, Achour Júnior A, Gobbi S, Oliveira AR, Cyrino ES. Effects of different resistance training frequencies on flexibility in older women. Clin Interv Aging 10: 531–538, 2015. doi: 10.2147/CIA.S77433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrick-Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E, Levine BD. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol (1985) 116: 736–745, 2014. doi: 10.1152/japplphysiol.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaillou T, Kirby TJ, McCarthy JJ. Ribosome biogenesis: emerging evidence for a central role in the regulation of skeletal muscle mass. J Cell Physiol 229: 1584–1594, 2014. doi: 10.1002/jcp.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chmelo EA, Crotts CI, Newman JC, Brinkley TE, Lyles MF, Leng X, Marsh AP, Nicklas BJ. Heterogeneity of physical function responses to exercise training in older adults. J Am Geriatr Soc 63: 462–469, 2015. doi: 10.1111/jgs.13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Churchward-Venne TA, Tieland M, Verdijk LB, Leenders M, Dirks ML, de Groot LC, van Loon LJ. There are no nonresponders to resistance-type exercise training in older men and women. J Am Med Dir Assoc 16: 400–411, 2015. doi: 10.1016/j.jamda.2015.01.071. [DOI] [PubMed] [Google Scholar]
- 24.Cook SB, LaRoche DP, Villa MR, Barile H, Manini TM. Blood flow restricted resistance training in older adults at risk of mobility limitations. Exp Gerontol 99: 138–145, 2017. doi: 10.1016/j.exger.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cosquéric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr 96: 895–901, 2006. doi: 10.1017/BJN20061943. [DOI] [PubMed] [Google Scholar]
- 26.Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci 59: 1200–1206, 2004. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 27.Dalton BH, Power GA, Vandervoort AA, Rice CL. The age-related slowing of voluntary shortening velocity exacerbates power loss during repeated fast knee extensions. Exp Gerontol 47: 85–92, 2012. doi: 10.1016/j.exger.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Daly M, Vidt ME, Eggebeen JD, Simpson WG, Miller ME, Marsh AP, Saul KR. Upper extremity muscle volumes and functional strength after resistance training in older adults. J Aging Phys Act 21: 186–207, 2013. doi: 10.1123/japa.21.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damas F, Nosaka K, Libardi CA, Chen TC, Ugrinowitsch C. Susceptibility to exercise-induced muscle damage: a cluster analysis with a large sample. Int J Sports Med 37: 633–640, 2016. doi: 10.1055/s-0042-100281. [DOI] [PubMed] [Google Scholar]
- 30.Damas F, Phillips S, Vechin FC, Ugrinowitsch C. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med 45: 801–807, 2015. doi: 10.1007/s40279-015-0320-0. [DOI] [PubMed] [Google Scholar]
- 31.Damas F, Phillips SM, Libardi CA, Vechin FC, Lixandrão ME, Jannig PR, Costa LA, Bacurau AV, Snijders T, Parise G, Tricoli V, Roschel H, Ugrinowitsch C. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol 594: 5209–5222, 2016. doi: 10.1113/JP272472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol (1985) 110: 309–317, 2011. doi: 10.1152/japplphysiol.00901.2010. [DOI] [PubMed] [Google Scholar]
- 33.de Souza TMF, Libardi CA, Cavaglieri CR, Gáspari AF, Brunelli DT, de Souza GV, Ugrinowitsch C, Min Li L, Chacon-Mikahil MPT. Concurrent Training with Blood Flow Restriction does not Decrease Inflammatory Markers. Int J Sports Med 39: 29–36, 2018. doi: 10.1055/s-0043-119222. [DOI] [PubMed] [Google Scholar]
- 34.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH; Health, Aging, and Body Composition Study . Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 90: 1579–1585, 2009. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dennis RA, Trappe TA, Simpson P, Carroll C, Huang BE, Nagarajan R, Bearden E, Gurley C, Duff GW, Evans WJ, Kornman K, Peterson CA. Interleukin-1 polymorphisms are associated with the inflammatory response in human muscle to acute resistance exercise. J Physiol 560: 617–626, 2004. doi: 10.1113/jphysiol.2004.067876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Filippo ES, Mancinelli R, Pietrangelo T, La Rovere RM, Quattrocelli M, Sampaolesi M, Fulle S. Myomir dysregulation and reactive oxygen species in aged human satellite cells. Biochem Biophys Res Commun 473: 462–470, 2016. doi: 10.1016/j.bbrc.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 37.Dickinson JM, Gundermann DM, Walker DK, Reidy PT, Borack MS, Drummond MJ, Arora M, Volpi E, Rasmussen BB. Leucine-enriched amino acid ingestion after resistance exercise prolongs myofibrillar protein synthesis and amino acid transporter expression in older men. J Nutr 144: 1694–1702, 2014. doi: 10.3945/jn.114.198671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiFrancisco-Donoghue J, Werner W, Douris PC, McKenna RF. Comparison of once-weekly and twice-weekly strength training in older adults. Br J Sports Med 41: 19–22, 2007. doi: 10.1136/bjsm.2006.029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf) 199: 71–81, 2010. doi: 10.1111/j.1748-1716.2010.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol (1985) 104: 1452–1461, 2008. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol (1985) 111: 135–142, 2011. doi: 10.1152/japplphysiol.01408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 13: 34–39, 2010. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, Tracy JK, Hochberg MC, Rodondi N, Cawthon PM; Study of Osteoporotic Fractures Research Group . Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 62: 744–751, 2007. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 44.Erskine RM, Jones DA, Williams AG, Stewart CE, Degens H. Inter-individual variability in the adaptation of human muscle specific tension to progressive resistance training. Eur J Appl Physiol 110: 1117–1125, 2010. doi: 10.1007/s00421-010-1601-9. [DOI] [PubMed] [Google Scholar]
- 45.Farinatti PT, Geraldes AA, Bottaro MF, Lima MV, Albuquerque RB, Fleck SJ. Effects of different resistance training frequencies on the muscle strength and functional performance of active women older than 60 years. J Strength Cond Res 27: 2225–2234, 2013. doi: 10.1519/JSC.0b013e318278f0db. [DOI] [PubMed] [Google Scholar]
- 46.Fatouros IG, Kambas A, Katrabasas I, Leontsini D, Chatzinikolaou A, Jamurtas AZ, Douroudos I, Aggelousis N, Taxildaris K. Resistance training and detraining effects on flexibility performance in the elderly are intensity-dependent. J Strength Cond Res 20: 634–642, 2006. doi: 10.1519/R-17615.1. [DOI] [PubMed] [Google Scholar]
- 47.Fatouros IG, Kambas A, Katrabasas I, Nikolaidis K, Chatzinikolaou A, Leontsini D, Taxildaris K. Strength training and detraining effects on muscular strength, anaerobic power, and mobility of inactive older men are intensity dependent. Br J Sports Med 39: 776–780, 2005. doi: 10.1136/bjsm.2005.019117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 330: 1769–1775, 1994. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 49.Figueiredo VC, Caldow MK, Massie V, Markworth JF, Cameron-Smith D, Blazevich AJ. Ribosome biogenesis adaptation in resistance training-induced human skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab 309: E72–E83, 2015. doi: 10.1152/ajpendo.00050.2015. [DOI] [PubMed] [Google Scholar]
- 50.Fokkenrood HJ, Bendermacher BL, Lauret GJ, Willigendael EM, Prins MH, Teijink JA. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev (8): CD005263, 2013. doi: 10.1002/14651858.CD005263.pub3. [DOI] [PubMed] [Google Scholar]
- 51.Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism 19: 653–663, 1970. doi: 10.1016/0026-0495(70)90062-4. [DOI] [PubMed] [Google Scholar]
- 52.Foster-Burns SB. Sarcopenia and decreased muscle strength in the elderly woman: resistance training as a safe and effective intervention. J Women Aging 11: 75–85, 1999. doi: 10.1300/J074v11n04_06. [DOI] [PubMed] [Google Scholar]
- 53.Friedman J, Lussiez A, Sullivan J, Wang S, Englesbe M. Implications of sarcopenia in major surgery. Nutr Clin Pract 30: 175–179, 2015. doi: 10.1177/0884533615569888. [DOI] [PubMed] [Google Scholar]
- 54.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985) 88: 1321–1326, 2000. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 55.Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, Fielding RA. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol (1985) 105: 637–642, 2008. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1: 11, 2011. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Volpi E, Rasmussen BB. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci 68: 599–607, 2013. doi: 10.1093/gerona/gls209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galvão DA, Taaffe DR. Resistance exercise dosage in older adults: single- versus multiset effects on physical performance and body composition. J Am Geriatr Soc 53: 2090–2097, 2005. doi: 10.1111/j.1532-5415.2005.00494.x. [DOI] [PubMed] [Google Scholar]
- 59.Greig CA, Gray C, Rankin D, Young A, Mann V, Noble B, Atherton PJ. Blunting of adaptive responses to resistance exercise training in women over 75y. Exp Gerontol 46: 884–890, 2011. doi: 10.1016/j.exger.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 60.Gries KJ, Raue U, Perkins RK, Lavin KM, Overstreet BS, D’Acquisto LJ, Graham B, Finch WH, Kaminsky LA, Trappe TA, Trappe SW. Cardiovascular and skeletal muscle health with lifelong exercise. J Appl Physiol (1985) 125: 1636–1645, 2018. doi: 10.1152/japplphysiol.00174.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grosicki GJ, Standley RA, Murach KA, Raue U, Minchev K, Coen PM, Newman AB, Cummings S, Harris T, Kritchevsky S, Goodpaster BH, Trappe S; Health ABC Study . Improved single muscle fiber quality in the oldest-old. J Appl Physiol (1985) 121: 878–884, 2016. doi: 10.1152/japplphysiol.00479.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guizelini PC, de Aguiar RA, Denadai BS, Caputo F, Greco CC. Effect of resistance training on muscle strength and rate of force development in healthy older adults: a systematic review and meta-analysis. Exp Gerontol 102: 51–58, 2018. doi: 10.1016/j.exger.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 63.Häkkinen K, Alen M, Kallinen M, Newton RU, Kraemer WJ. Neuromuscular adaptation during prolonged strength training, detraining and re-strength-training in middle-aged and elderly people. Eur J Appl Physiol 83: 51–62, 2000. doi: 10.1007/s004210000248. [DOI] [PubMed] [Google Scholar]
- 64.Hangelbroek RWJ, Knuiman P, Tieland M, de Groot LCPGM. Attenuated strength gains during prolonged resistance exercise training in older adults with high inflammatory status. Exp Gerontol 106: 154–158, 2018. doi: 10.1016/j.exger.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Hartman MJ, Fields DA, Byrne NM, Hunter GR. Resistance training improves metabolic economy during functional tasks in older adults. J Strength Cond Res 21: 91–95, 2007. doi: 10.1519/00124278-200702000-00017. [DOI] [PubMed] [Google Scholar]
- 66.Henwood TR, Taaffe DR. Short-term resistance training and the older adult: the effect of varied programmes for the enhancement of muscle strength and functional performance. Clin Physiol Funct Imaging 26: 305–313, 2006. doi: 10.1111/j.1475-097X.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 67.Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594: 1965–1978, 2016. doi: 10.1113/JP270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffman NJ. Omics and exercise: global approaches for mapping exercise biological networks. Cold Spring Harb Perspect Med 7: a029884, 2017. doi: 10.1101/cshperspect.a029884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database Syst Rev (11): CD004963, 2011. doi: 10.1002/14651858.CD004963.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Clarkson PM. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc 37: 964–972, 2005. doi: 10.1249/01.mss.0000170469.90461.5f. [DOI] [PubMed] [Google Scholar]
- 71.Hunter GR, Treuth MS, Weinsier RL, Kekes-Szabo T, Kell SH, Roth DL, Nicholson C. The effects of strength conditioning on older women’s ability to perform daily tasks. J Am Geriatr Soc 43: 756–760, 1995. doi: 10.1111/j.1532-5415.1995.tb07045.x. [DOI] [PubMed] [Google Scholar]
- 72.Hunter GR, Wetzstein CJ, Fields DA, Brown A, Bamman MM. Resistance training increases total energy expenditure and free-living physical activity in older adults. J Appl Physiol (1985) 89: 977–984, 2000. doi: 10.1152/jappl.2000.89.3.977. [DOI] [PubMed] [Google Scholar]
- 73.Jain AK. Data clustering: 50 years beyond K-means. Pattern Recog Lett 31: 651–666, 2010. doi: 10.1016/j.patrec.2009.09.011. [DOI] [Google Scholar]
- 74.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985) 89: 81–88, 2000. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 75.Kanda K, Hashizume K. Changes in properties of the medial gastrocnemius motor units in aging rats. J Neurophysiol 61: 737–746, 1989. doi: 10.1152/jn.1989.61.4.737. [DOI] [PubMed] [Google Scholar]
- 76.Kelly NA, Hammond KG, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Effects of aging and Parkinson’s disease on motor unit remodeling: influence of resistance exercise training. J Appl Physiol (1985) 124: 888–898, 2018. doi: 10.1152/japplphysiol.00563.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelly NA, Hammond KG, Stec MJ, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Quantification and characterization of grouped type I myofibers in human aging. Muscle Nerve 57: E52–E59, 2018. doi: 10.1002/mus.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 101: 531–544, 2006. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 79.Krantic S, Mechawar N, Reix S, Quirion R. Molecular basis of programmed cell death involved in neurodegeneration. Trends Neurosci 28: 670–676, 2005. doi: 10.1016/j.tins.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 80.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587: 211–217, 2009. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lacroix A, Hortobágyi T, Beurskens R, Granacher U. Effects of supervised vs. unsupervised training programs on balance and muscle strength in older adults: a systematic review and meta-analysis. Sports Med 47: 2341–2361, 2017. doi: 10.1007/s40279-017-0747-6. [DOI] [PubMed] [Google Scholar]
- 82.Lacroix A, Kressig RW, Muehlbauer T, Gschwind YJ, Pfenninger B, Bruegger O, Granacher U. Effects of a supervised versus an unsupervised combined balance and strength training program on balance and muscle power in healthy older adults: a randomized controlled trial. Gerontology 62: 275–288, 2016. doi: 10.1159/000442087. [DOI] [PubMed] [Google Scholar]
- 83.Lemmer JT, Hurlbut DE, Martel GF, Tracy BL, Ivey FM, Metter EJ, Fozard JL, Fleg JL, Hurley BF. Age and gender responses to strength training and detraining. Med Sci Sports Exerc 32: 1505–1512, 2000. doi: 10.1097/00005768-200008000-00021. [DOI] [PubMed] [Google Scholar]
- 84.Lera Orsatti F, Nahas EA, Maestá N, Nahas Neto J, Lera Orsatti C, Vannucchi Portari G, Burini RC. Effects of resistance training frequency on body composition and metabolics and inflammatory markers in overweight postmenopausal women. J Sports Med Phys Fitness 54: 317–325, 2014. [PubMed] [Google Scholar]
- 85.Lexell J, Downham D, Sjöström M. Distribution of different fibre types in human skeletal muscles. Fibre type arrangement in m. vastus lateralis from three groups of healthy men between 15 and 83 years. J Neurol Sci 72: 211–222, 1986. doi: 10.1016/0022-510X(86)90009-2. [DOI] [PubMed] [Google Scholar]
- 86.Lexell J, Downham DY, Larsson Y, Bruhn E, Morsing B. Heavy-resistance training in older Scandinavian men and women: short- and long-term effects on arm and leg muscles. Scand J Med Sci Sports 5: 329–341, 1995. doi: 10.1111/j.1600-0838.1995.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 87.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 88.Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20-93 yr. J Appl Physiol (1985) 83: 1581–1587, 1997. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 89.Ling SM, Conwit RA, Ferrucci L, Metter EJ. Age-associated changes in motor unit physiology: observations from the Baltimore Longitudinal Study of Aging. Arch Phys Med Rehabil 90: 1237–1240, 2009. doi: 10.1016/j.apmr.2008.09.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu CJ, Chang WP, Araujo de Carvalho I, Savage KEL, Radford LW, Amuthavalli Thiyagarajan J. Effects of physical exercise in older adults with reduced physical capacity: meta-analysis of resistance exercise and multimodal exercise. Int J Rehabil Res 40: 303–314, 2017. doi: 10.1097/MRR.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 91.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med 170: 170–178, 2010. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 3: 9, 2014. doi: 10.1186/2046-2395-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McKinnon NB, Connelly DM, Rice CL, Hunter SW, Doherty TJ. Neuromuscular contributions to the age-related reduction in muscle power: Mechanisms and potential role of high velocity power training. Ageing Res Rev 35: 147–154, 2017. doi: 10.1016/j.arr.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 94.McLafferty CL Jr, Wetzstein CJ, Hunter GR. Resistance training is associated with improved mood in healthy older adults. Percept Mot Skills 98: 947–957, 2004. doi: 10.2466/pms.98.3.947-957. [DOI] [PubMed] [Google Scholar]
- 95.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31: 461–467, 2005. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- 96.Melov S, Tarnopolsky MA, Beckman K, Felkey K, Hubbard A. Resistance exercise reverses aging in human skeletal muscle. PLoS One 2: e465, 2007. doi: 10.1371/journal.pone.0000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Craig Tuggle S, Kosek DJ, Kim JS, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol (1985) 115: 937–948, 2013. doi: 10.1152/japplphysiol.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci 52: B267–B276, 1997. doi: 10.1093/gerona/52A.5.B267. [DOI] [PubMed] [Google Scholar]
- 99.Mikkelsen UR, Agergaard J, Couppé C, Grosset JF, Karlsen A, Magnusson SP, Schjerling P, Kjaer M, Mackey AL. Skeletal muscle morphology and regulatory signalling in endurance-trained and sedentary individuals: The influence of ageing. Exp Gerontol 93: 54–67, 2017. doi: 10.1016/j.exger.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 100.Mikkelsen UR, Couppé C, Karlsen A, Grosset JF, Schjerling P, Mackey AL, Klausen HH, Magnusson SP, Kjær M. Life-long endurance exercise in humans: circulating levels of inflammatory markers and leg muscle size. Mech Ageing Dev 134: 531–540, 2013. doi: 10.1016/j.mad.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 101.Mitchell CJ, Churchward-Venne TA, Parise G, Bellamy L, Baker SK, Smith K, Atherton PJ, Phillips SM. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS One 9: e89431, 2014. doi: 10.1371/journal.pone.0089431. A correction for this article is available at http://dx.doi.org/10.1371/journal.pone.0098731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. J Am Med Dir Assoc 14: 392–397, 2013. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moro T, Brightwell CR, Deer RR, Graber TG, Galvan E, Fry CS, Volpi E, Rasmussen BB. Muscle protein anabolic resistance to essential amino acids does not occur in healthy older adults before or after resistance exercise training. J Nutr 148: 900–909, 2018. doi: 10.1093/jn/nxy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moro T, Ebert SM, Adams CM, Rasmussen BB. Amino acid sensing in skeletal muscle. Trends Endocrinol Metab 27: 796–806, 2016. doi: 10.1016/j.tem.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moro T, Tinsley G, Bianco A, Gottardi A, Gottardi GB, Faggian D, Plebani M, Marcolin G, Paoli A. High intensity interval resistance training (HIIRT) in older adults: Effects on body composition, strength, anabolic hormones and blood lipids. Exp Gerontol 98: 91–98, 2017. doi: 10.1016/j.exger.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 106.Motalebi SA, Cheong LS, Iranagh JA, Mohammadi F. Effect of low-cost resistance training on lower-limb strength and balance in institutionalized seniors. Exp Aging Res 44: 48–61, 2018. doi: 10.1080/0361073X.2017.1398810. [DOI] [PubMed] [Google Scholar]
- 107.Nielsen S, Pedersen BK. Skeletal muscle as an immunogenic organ. Curr Opin Pharmacol 8: 346–351, 2008. doi: 10.1016/j.coph.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 108.Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, van Loon LJ. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48: 492–498, 2013. doi: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 109.Ogasawara R, Akimoto T, Umeno T, Sawada S, Hamaoka T, Fujita S. MicroRNA expression profiling in skeletal muscle reveals different regulatory patterns in high and low responders to resistance training. Physiol Genomics 48: 320–324, 2016. doi: 10.1152/physiolgenomics.00124.2015. [DOI] [PubMed] [Google Scholar]
- 110.Padilha CS, Ribeiro AS, Fleck SJ, Nascimento MA, Pina FL, Okino AM, Venturini D, Barbosa DS, Mayhew JL, Cyrino ES. Effect of resistance training with different frequencies and detraining on muscular strength and oxidative stress biomarkers in older women. Age (Dordr) 37: 104, 2015. doi: 10.1007/s11357-015-9841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 93: 322–331, 2011. doi: 10.3945/ajcn.2010.29649. [DOI] [PubMed] [Google Scholar]
- 112.Pereira DS, Mateo EC, de Queiroz BZ, Assumpção AM, Miranda AS, Felício DC, Rocha NP, da Cruz dos Anjos DM, Pereira DA, Teixeira AL, Pereira LS. TNF-α, IL6, and IL10 polymorphisms and the effect of physical exercise on inflammatory parameters and physical performance in elderly women. Age (Dordr) 35: 2455–2463, 2013. doi: 10.1007/s11357-013-9515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc 43: 249–258, 2011. doi: 10.1249/MSS.0b013e3181eb6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985) 104: 1736–1742, 2008. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 115.Petrella JK, Kim JS, Tuggle SC, Bamman MM. Contributions of force and velocity to improved power with progressive resistance training in young and older adults. Eur J Appl Physiol 99: 343–351, 2007. doi: 10.1007/s00421-006-0353-z. [DOI] [PubMed] [Google Scholar]
- 116.Petrella JK, Kim JS, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol (1985) 98: 211–220, 2005. doi: 10.1152/japplphysiol.00294.2004. [DOI] [PubMed] [Google Scholar]
- 117.Phillips BE, Williams JP, Gustafsson T, Bouchard C, Rankinen T, Knudsen S, Smith K, Timmons JA, Atherton PJ. Molecular networks of human muscle adaptation to exercise and age. PLoS Genet 9: e1003389, 2013. doi: 10.1371/journal.pgen.1003389. A correction for this article is available at http://dx.doi.org/10.1371/annotation/0dd3671e-1460-48fa-9d6a-2865dce78c07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 273: E99–E107, 1997. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 119.Piasecki M, Ireland A, Coulson J, Stashuk DW, Hamilton-Wright A, Swiecicka A, Rutter MK, McPhee JS, Jones DA. Motor unit number estimates and neuromuscular transmission in the tibialis anterior of master athletes: evidence that athletic older people are not spared from age-related motor unit remodeling. Physiol Rep 4: e12987, 2016. doi: 10.14814/phy2.12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Piasecki M, Ireland A, Jones DA, McPhee JS. Age-dependent motor unit remodelling in human limb muscles. Biogerontology 17: 485–496, 2016. doi: 10.1007/s10522-015-9627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Piasecki M, Ireland A, Stashuk D, Hamilton-Wright A, Jones DA, McPhee JS. Age-related neuromuscular changes affecting human vastus lateralis. J Physiol 594: 4525–4536, 2016. doi: 10.1113/JP271087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Power GA, Dalton BH, Rice CL, Vandervoort AA. Power loss is greater following lengthening contractions in old versus young women. Age (Dordr) 34: 737–750, 2012. doi: 10.1007/s11357-011-9263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Radaelli R, Botton CE, Wilhelm EN, Bottaro M, Brown LE, Lacerda F, Gaya A, Moraes K, Peruzzolo A, Pinto RS. Time course of low- and high-volume strength training on neuromuscular adaptations and muscle quality in older women. Age (Dordr) 36: 881–892, 2014. doi: 10.1007/s11357-013-9611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Radaelli R, Fleck SJ, Leite T, Leite RD, Pinto RS, Fernandes L, Simão R. Dose-response of 1, 3, and 5 sets of resistance exercise on strength, local muscular endurance, and hypertrophy. J Strength Cond Res 29: 1349–1358, 2015. doi: 10.1519/JSC.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 125.Radaelli R, Wilhelm EN, Botton CE, Rech A, Bottaro M, Brown LE, Pinto RS. Effects of single vs. multiple-set short-term strength training in elderly women. Age (Dordr) 36: 9720, 2014. doi: 10.1007/s11357-014-9720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci 62: 1407–1412, 2007. doi: 10.1093/gerona/62.12.1407. [DOI] [PubMed] [Google Scholar]
- 127.Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol (1985) 106: 1611–1617, 2009. doi: 10.1152/japplphysiol.91587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol (1985) 112: 1625–1636, 2012. doi: 10.1152/japplphysiol.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raymond MJ, Bramley-Tzerefos RE, Jeffs KJ, Winter A, Holland AE. Systematic review of high-intensity progressive resistance strength training of the lower limb compared with other intensities of strength training in older adults. Arch Phys Med Rehabil 94: 1458–1472, 2013. doi: 10.1016/j.apmr.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 130.Reid KF, Doros G, Clark DJ, Patten C, Carabello RJ, Cloutier GJ, Phillips EM, Krivickas LS, Frontera WR, Fielding RA. Muscle power failure in mobility-limited older adults: preserved single fiber function despite lower whole muscle size, quality and rate of neuromuscular activation. Eur J Appl Physiol 112: 2289–2301, 2012. doi: 10.1007/s00421-011-2200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40: 4–12, 2012. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, Frontera WR, Fielding RA. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol 114: 29–39, 2014. doi: 10.1007/s00421-013-2728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reidy PT, Borack MS, Markofski MM, Dickinson JM, Fry CS, Deer RR, Volpi E, Rasmussen BB. Post-absorptive muscle protein turnover affects resistance training hypertrophy. Eur J Appl Physiol 117: 853–866, 2017. doi: 10.1007/s00421-017-3566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rivas DA, Lessard SJ, Rice NP, Lustgarten MS, So K, Goodyear LJ, Parnell LD, Fielding RA. Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling. FASEB J 28: 4133–4147, 2014. doi: 10.1096/fj.14-254490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roberts BM, Lavin KM, Many GM, Thalacker-Mercer A, Merritt EK, Bickel CS, Mayhew DL, Tuggle SC, Cross JM, Kosek DJ, Petrella JK, Brown CJ, Hunter GR, Windham ST, Allman RM, Bamman MM. Human neuromuscular aging: Sex differences revealed at the myocellular level. Exp Gerontol 106: 116–124, 2018. doi: 10.1016/j.exger.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR, Nair KS. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab 25: 581–592, 2017. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing 35, Suppl 2: ii37–ii41, 2006. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 138.Shad BJ, Thompson JL, Breen L. Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am J Physiol Endocrinol Metab 311: E803–E817, 2016. doi: 10.1152/ajpendo.00213.2016. [DOI] [PubMed] [Google Scholar]
- 139.Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol 295: R273–R280, 2008. doi: 10.1152/ajpregu.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med 127: 547–553, 2014. doi: 10.1016/j.amjmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stålberg E, Fawcett PR. Macro EMG in healthy subjects of different ages. J Neurol Neurosurg Psychiatry 45: 870–878, 1982. doi: 10.1136/jnnp.45.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab 310: E652–E661, 2016. doi: 10.1152/ajpendo.00486.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stec MJ, Mayhew DL, Bamman MM. The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J Appl Physiol (1985) 119: 851–857, 2015. doi: 10.1152/japplphysiol.00489.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stec MJ, Thalacker-Mercer A, Mayhew DL, Kelly NA, Tuggle SC, Merritt EK, Brown CJ, Windham ST, Dell’Italia LJ, Bickel CS, Roberts BM, Vaughn KM, Isakova-Donahue I, Many GM, Bamman MM. Randomized, four-arm, dose-response clinical trial to optimize resistance exercise training for older adults with age-related muscle atrophy. Exp Gerontol 99: 98–109, 2017. doi: 10.1016/j.exger.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Taaffe DR, Duret C, Wheeler S, Marcus R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc 47: 1208–1214, 1999. doi: 10.1111/j.1532-5415.1999.tb05201.x. [DOI] [PubMed] [Google Scholar]
- 146.Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology 55: 217–223, 2009. doi: 10.1159/000182084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taaffe DR, Pruitt L, Pyka G, Guido D, Marcus R. Comparative effects of high- and low-intensity resistance training on thigh muscle strength, fiber area, and tissue composition in elderly women. Clin Physiol 16: 381–392, 1996. doi: 10.1111/j.1475-097X.1996.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 148.Taylor NA, Wilkinson JG. Exercise-induced skeletal muscle growth. Hypertrophy or hyperplasia? Sports Med 3: 190–200, 1986. doi: 10.2165/00007256-198603030-00003. [DOI] [PubMed] [Google Scholar]
- 149.Thalacker-Mercer A, Stec M, Cui X, Cross J, Windham S, Bamman M. Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genomics 45: 499–507, 2013. doi: 10.1152/physiolgenomics.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med 337: 1279–1284, 1997. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 151.Trappe S, Williamson D, Godard M. Maintenance of whole muscle strength and size following resistance training in older men. J Gerontol A Biol Sci Med Sci 57: B138–B143, 2002. doi: 10.1093/gerona/57.4.B138. [DOI] [PubMed] [Google Scholar]
- 152.Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 300: R655–R662, 2011. doi: 10.1152/ajpregu.00611.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Welle S, Totterman S, Thornton C. Effect of age on muscle hypertrophy induced by resistance training. J Gerontol A Biol Sci Med Sci 51: M270–M275, 1996. doi: 10.1093/gerona/51A.6.M270. [DOI] [PubMed] [Google Scholar]
- 154.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 84: 475–482, 2006. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 155.Zhang T, Birbrair A, Wang ZM, Messi ML, Marsh AP, Leng I, Nicklas BJ, Delbono O. Improved knee extensor strength with resistance training associates with muscle specific miRNAs in older adults. Exp Gerontol 62: 7–13, 2015. doi: 10.1016/j.exger.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang T, Choi SJ, Wang ZM, Birbrair A, Messi ML, Jin JP, Marsh AP, Nicklas B, Delbono O. Human slow troponin T (TNNT1) pre-mRNA alternative splicing is an indicator of skeletal muscle response to resistance exercise in older adults. J Gerontol A Biol Sci Med Sci 69: 1437–1447, 2014. doi: 10.1093/gerona/glt204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zierath JR, Wallberg-Henriksson H. Looking ahead perspective: Where will the future of exercise biology take us? Cell Metab 22: 25–30, 2015. doi: 10.1016/j.cmet.2015.06.015. [DOI] [PubMed] [Google Scholar]