Abstract
Insecticide resistance across sub-Saharan Africa may impact the continued effectiveness of malaria vector control. We investigated the association between carbamate and pyrethroid resistance with Anopheles gambiae s.l. parity, Plasmodium falciparum infection, and molecular insecticide resistance mechanisms in Guinea. Pyrethroid resistance was intense, with field populations surviving ten times the insecticidal concentration required to kill susceptible individuals. The L1014F kdr-N1575Y haplotype and I1527T mutation were significantly associated with mosquito survival following permethrin exposure (Prevalence Ratio; PR = 1.92, CI = 1.09–3.37 and PR = 2.80, CI = 1.03–7.64, respectively). Partial restoration of pyrethroid susceptibility following synergist pre-exposure suggests a role for mixed-function oxidases. Carbamate resistance was lower and significantly associated with the G119S Ace-1 mutation. Oocyst rates were 6.8% and 4.2% among resistant and susceptible mosquitoes, respectively; survivors of bendiocarb exposure were significantly more likely to be infected. Pyrethroid resistant mosquitoes had significantly lower parity rates than their susceptible counterparts (PR = 1.15, CI = 1.10–1.21). Our findings emphasize the need for additional studies directly assessing the influence of insecticide resistance on mosquito fitness.
Subject terms: Entomology, Genetic markers
Introduction
Malaria remains a leading cause of morbidity and mortality in the tropics, where it is estimated to have resulted in ~445,000 deaths in 2016 alone1. Despite considerable reductions in disease burden achieved by scaling-up the provision of long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS)2, long-term effectiveness of both strategies may be jeopardized by widespread emergence of insecticide resistance in mosquito populations3,4. In response, commercial manufacturers are developing alternate vector control interventions, as well as optimizing use of existing insecticides through combinations and improved formulations. National Malaria Control Programmes (NMCPs) and international policy makers are also taking action to expand resistance monitoring and surveillance5. However, the severity of this threat is currently unknown because there is limited evidence linking the operational failure of available control measures to the presence of resistant mosquito species6–14.
The efficacy of IRS and LLINs is predicated on their ability to reduce the daily survival rate of Anopheles mosquitoes and prevent the completion of parasite development to the infectious stage. One explanation for the sustained effectiveness of LLINs, even in the presence of increased vector tolerance to their insecticidal properties, is that intact nets provide a physical barrier to mosquito feeding. A meta-analysis of field data indicated that treated nets reduced blood feeding and increased mosquito mortality compared to untreated nets, even in areas with the highest levels of resistance4. Furthermore, a recent large-scale, multi-country trial reported no association between malaria disease burden and pyrethroid resistance, with evidence that LLINs continued to provide personal protection across areas of different resistance intensities15. Laboratory studies now suggest that fitness costs associated with insecticide resistance may influence malaria transmission either by directly reducing mosquito life span16 and/or fecundity17, altering host seeking, feeding and mating behaviours18–20 or by impairing parasite development inside vectors21–23. However, to date, few field studies have directly investigated the impact of insecticide resistance intensity on malaria transmission dynamics.
In Guinea, malaria is a serious public health problem, where the entire population of ~11.7 million people is at risk and the nationwide prevalence is estimated at 15%24. Between 2013–2017, the NMCP, with support from the President’s Malaria Initiative (PMI) and the Global Fund, have procured and distributed over 27.6 million pyrethroid LLINs25. Because the current vector control strategy relies almost exclusively on LLIN use26, nationwide pyrethroid resistance is of concern27. To better inform future malaria control efforts in Guinea, there is a need to characterize levels of operationally-significant insecticide resistance, as well as determine the effect this phenomenon has on the vectorial capacity of local mosquitoes to transmit malaria.
Results
Mosquito abundance and species identification
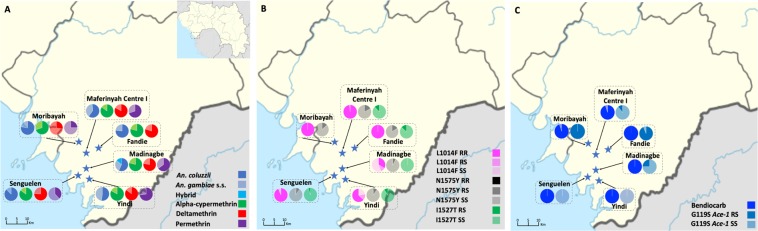
A total of 3962 female An. gambiae s.l. mosquitoes were captured from six sites in Maferinyah subprefecture, in Forecariah Prefecture (Senguelen = 766; Yindi = 755; Maferinyah Centre I = 660; Madinagbe = 608; Fandie = 608 and Moribayah = 565) over 25 days by manual aspiration from houses and human landing catches (HLCs). A subsample (n = 181) was selected for molecular form identification, of which 68% (123/181) and 31% (56/181) were determined to be An. coluzzii (M molecular form) and An. gambiaes.s. (S molecular form), respectively; two hybrid forms (An. gambiae-An. coluzzii) were also identified. While both species were sympatric across Forecariah Prefecture, proportions of An. coluzzii differed among study villages (Fisher’s Exact test; p = 0.007), potentially attributable to varying local ecological factors28 (Fig. 1A).
Figure 1.
(A) Map of Forecariah Prefecture, Guinea displaying proportions of An. gambiae s.l. molecular forms and susceptibility levels to the diagnostic dose (1X) of alpha-cypermethrin, deltamethrin and permethrin, measured using CDC bottle bioassays, at six study sites indicated by stars (Fandie, Madinagbe, Maferinyah Centre I, Moribayah, Senguelen and Yindi). Inset map shows the location of Forecariah Prefecture in Guinea. (B) Map of Forecariah Prefecture, Guinea displaying frequencies of L1014F, N1575Y and I1527T resistant (R) and wild type (S) alleles. (C) Map of Forecariah Prefecture, Guinea displaying susceptibility levels to the diagnostic dose (1X) of bendiocarb, measured using CDC bottle bioassays, and frequency of G119S Ace-1 resistant (R) and wild type (S) alleles. For all maps, legend colours referring to insecticides have darker shading denoting average mosquito mortality.
Insecticide resistance intensity
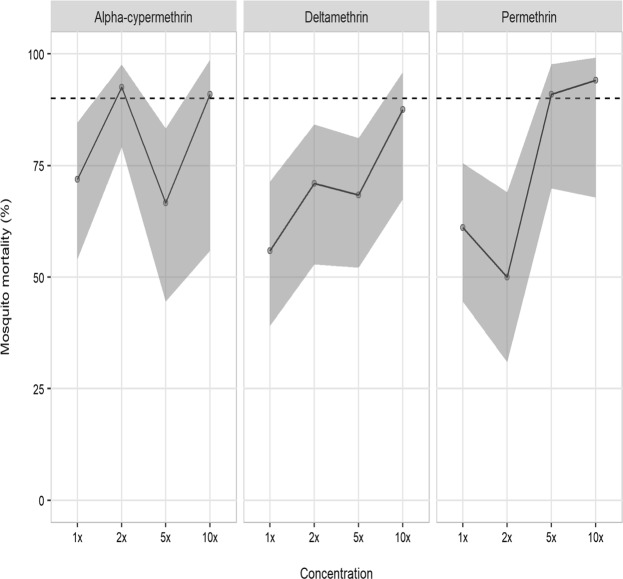
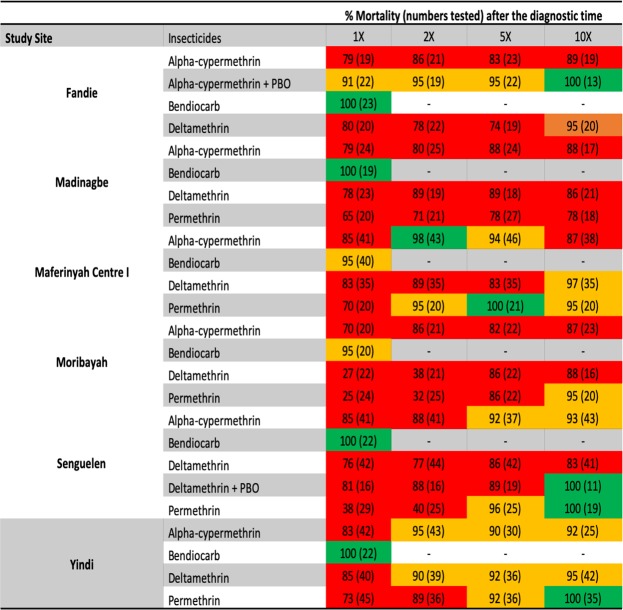
Levels of resistance to four insecticides (alpha-cypermethrin, bendiocarb, deltamethrin and permethrin) were assessed among 2229 female An. gambiae s.l. mosquitoes, collected across six study sites in Forecariah Prefecture (Fig. 1A,C). Local vectors were characterized by intense but highly variable pyrethroid resistance, with all populations demonstrating less than 90% mosquito mortality to the diagnostic doses (1X) of pyrethroids and most areas containing individuals capable of surviving exposure to 10X these insecticide concentrations (Figs 2 and 3). In addition, a subsample of mosquitoes still living following 30 minutes of insecticide exposure were held for up to two hours in treated bottles, with more than 30% of vectors capable of surviving this extended exposure time in Madinagbe (17/55), Moribayah (19/38), Senguelen (30/82) and Yindi (9/27); as compared to alpha-cypermethrin, deltamethrin had higher survival rates (prevalence ratio; PR = 1.60, CI = 1.01–2.57, p = 0.049) but permethrin did not (PR = 1.52, CI = 0.94–2.51, p = 0.09). When considering mosquitoes surviving two hours of pyrethroid exposure, compared to 1X, there was no evidence of lower survival at 2X of extended exposure (PR = 0.68, CI = 0.43–1.05, p = 0.08) or 5X (PR = 0.70, CI = 0.43–1.09, p = 0.09) but there was significantly lower survival at 10X (PR = 0.26, CI = 0.10–0.54, p = 0.001). The highest levels of pyrethroid resistance were observed in Moribayah, where mosquito mortality to two times the diagnostic doses of deltamethrin and permethrin was 38% and 32%, respectively (Fig. 2). Across all sites, levels of resistance of permethrin (PR = 6.67, CI = 2.41–23.76, p = 0.001) and deltamethrin (PR = 3.87, CI = 1.33–14.14, p = 0.02) were higher than alpha-cypermethrin.
Figure 2.
CDC resistance intensity assay data for three pyrethroid insecticides (alpha-cypermethrin, deltamethrin and permethrin) pooled across six study sites in Forecariah Prefecture (Fandie, Madinagbe, Maferinyah Centre I, Moribayah, Senguelen and Yindi). Estimates and confidence intervals are from a binomial regression model. Mortality below 90% (indicated by the dashed line) indicates the presence of resistance.
Figure 3.
Corrected percent mortality (and numbers tested) of Anopheles gambiae s.l. in CDC resistance intensity and synergist bioassays conducted in six sites in Forecariah Prefecture, Guinea.
By comparison, levels of carbamate resistance were low, with complete mosquito susceptibility observed in Fandie, Madinagbe, Senguelen and Yindi; possible resistance (95% mosquito mortality) was restricted to two adjacent villages (Maferinyah Centre I and Moribayah) (Figs 1C and 2).
For all insecticides, there was no difference in ability to survive exposure between An. coluzzii (n = 123) and An. gambiae s.s. (n = 56) (PR = 0.85, CI = 0.70–1.02, p = 0.09 for An. gambiae s.s.) or association with survival and physiological status (i.e. blood-fed, unfed, gravid, etc.) (Fisher’s Exact Test; p = 1.00).
Mosquito parity
Of 737 An. gambiae s.l. mosquitoes tested in resistance bioassays that had their ovaries inspected for parity, 46% were nulliparous (340/737), i.e. had not laid an egg batch, and 54% (397/737) were parous. There was no significant difference in proportion of parous mosquitoes by study village (χ2 = 9.61; p = 0.09) or by month tested (χ2 = 2.18; p = 0.14). However, for all three pyrethroids under evaluation, resistant mosquitoes were significantly more likely to be nulliparous (PR = 1.15, CI = 1.10–1.21, p < 0.001). This association was very similar across insecticides (alpha-cypermethrin: PR = 1.18, CI = 1.06–1.30, p < 0.001; deltamethrin: PR = 1.11, CI = 1.005–1.22, p = 0.04; and permethrin: PR = 1.18, CI = 1.05–1.33, p = 0.002). Small sample sizes precluded direct comparisons between pyrethroid resistance intensity and parity per insecticide. When considering resistance levels of all pyrethroids, nulliparous mosquitoes were more likely to be resistant to 2X (PR = 1.31, CI = 1.11–1.54, p < 0.001) and 10X (PR = 1.22, CI = 1.01–1.47, p = 0.03) but not to 5X (PR = 1.10, CI = 0.94–1.29, p = 0.39). Nulliparous mosquitoes were no more likely to be survivors of exposure to any dose of bendiocarb (1X: PR = 2.47, CI = 0.81–7.50, p = 0.16; 2X: PR = 0.50, CI = 0.11–2.32, p = 0.70; 5X: PR = 1.64, CI = 0.53–5.06, p = 0.73; 10X: PR = 1.63, CI = 0.59–4.49, p = 0.66).
Target site and metabolic mechanisms of resistance
N1575Y mutation screening was undertaken in a subset of 388 An. gambiae s.l. and was detected in 13% (49/388) of samples in both An. coluzzii (59%) and An. gambiae s.s. (41%); only two homozygote individuals were detected (Fig. 1B and Table 1). The frequency of the N1575Y resistant allele did not differ significantly among study districts (Fisher’s Exact Test; p = 0.71) (Table 1 and Fig. 1B) (Table 1). There was no significant association between N1575Y allele frequency and ability of mosquitoes to survive pyrethroid exposure for 30 minutes (alpha-cypermethrin: PR = 0.85, CI = 0.63–1.13, p = 0.42; deltamethrin: PR = 1.06, CI = 0.81–1.37, p = 0.94; and permethrin: PR = 1.26, CI = 0.85–1.87, p = 0.42) or two hours (alpha-cypermethrin: PR = 0.26, CI = 0.03–2.04, p = 0.32; deltamethrin: PR = 0.64, CI = 0.19–2.14, p = 0.76; and permethrin: PR = 1.33, CI = 0.39–4.55, p = 0.92).
Table 1.
N1575Y allele frequencies in An. gambiae s.l. from six study sites in Forecariah Prefecture, Guinea.
| Study Site | Survival Status | # Mosquitoes tested | Homozygote mutation (RR) | Heterozygote mutation (RS) | Homozygote wild type (SS) | N1575Y allele frequency | χ2 Test | P value | |
|---|---|---|---|---|---|---|---|---|---|
| R | S | ||||||||
| Fandie | Alive | 51 | 0 | 8 | 43 | 0.08 | 0.92 | 0.37 | 0.543 |
| Dead | 12 | 0 | 2 | 10 | 0.08 | 0.92 | 0.1 | 0.752 | |
| Madinagbe | Alive | 35 | 0 | 3 | 32 | 0.04 | 0.96 | 0.07 | 0.791 |
| Dead | 5 | 0 | 0 | 5 | 0 | 1.0 | — | — | |
| Maferinyah Centre I | Alive | 43 | 0 | 10 | 33 | 0.12 | 0.88 | 0.74 | 0.390 |
| Dead | 44 | 0 | 5 | 39 | 0.06 | 0.94 | 0.16 | 0.690 | |
| Moribayah | Alive | 38 | 0 | 5 | 33 | 0.07 | 0.93 | 0.19 | 0.663 |
| Dead | 0 | 0 | 0 | 0 | — | — | — | — | |
| Senguelen | Alive | 73 | 1 | 7 | 65 | 0.06 | 0.94 | 2.14 | 0.144 |
| Dead | 18 | 0 | 1 | 17 | 0.03 | 0.97 | 0.01 | 0.920 | |
| Yindi | Alive | 35 | 0 | 3 | 32 | 0.04 | 0.96 | 0.07 | 0.791 |
| Dead | 34 | 1 | 3 | 30 | 0.07 | 0.93 | 4.22 | 0.040 | |
One hundred and twenty-eight base pairs upstream of N1575Y, a second non-synonymous mutation (I1527T) was identified among 10% of individuals (11/109); this mutation was not linked to N1575Y and only one individual from Maferinyah Centre I had both resistance mutations. All individuals with the I1527T resistant allele were heterozygous for this mutation and were identified as An. gambiae s.s. The frequency of the I1527T resistant allele did not vary among study villages (Fisher’s Exact test; p = 0.95) (Table 2 and Fig. 1B). There was an association between I1527T allele frequency and ability of mosquitoes to survive pyrethroid exposure for 30 minutes for permethrin (PR = 2.80, CI = 1.03–7.64, p = 0.04), but not for the other two insecticides (alpha-cypermethrin: PR = 0.94, CI = 0.50–1.79, p = 0.995; and deltamethrin: PR = 1.08, CI = 0.52–2.20, p = 0.99). There were too few positive mosquitos to analyse associations at two hours.
Table 2.
I1527T allele frequencies in An. gambiae s.l. from five study sites in Forecariah Prefecture, Guinea.
| Study Site | Survival Status | # Mosquitoes tested | Homozygote mutation (RR) | Heterozygote mutation (RS) | Homozygote wild type (SS) | I1527T allele frequency | χ2 Test | P value | |
|---|---|---|---|---|---|---|---|---|---|
| R | S | ||||||||
| Fandie | Alive | 15 | 0 | 2 | 13 | 0.07 | 0.93 | 0.08 | 0.777 |
| Dead | 0 | 0 | 0 | 0 | — | — | — | — | |
| Madinagbe | Alive | 5 | 0 | 0 | 5 | 0 | 1.0 | — | — |
| Dead | 0 | 0 | 0 | 0 | — | — | — | — | |
| Maferinyah Centre I | Alive | 30 | 0 | 4 | 26 | 0.07 | 0.93 | 0.15 | 0.700 |
| Dead | 19 | 0 | 2 | 17 | 0.05 | 0.95 | 0.06 | 0.806 | |
| Senguelen | Alive | 17 | 0 | 1 | 16 | 0.03 | 0.93 | 0.02 | 0.888 |
| Dead | 2 | 0 | 0 | 2 | — | — | — | — | |
| Yindi | Alive | 16 | 0 | 2 | 14 | 0.06 | 0.94 | 0.07 | 0.791 |
| Dead | 5 | 0 | 0 | 5 | — | — | — | — | |
The L1014F kdr allele was identified in 87% of samples (211/242), with the majority of mosquitoes presenting homozygous kdr profiles (83%; 200/242). The frequency of the L1014F kdr resistant allele varied significantly among study villages (Fisher’s Exact test; p = 0.0005) (Table 3 and Fig. 1B). There were no associations between L1014F kdr allele frequency and ability of mosquitoes to survive insecticide exposure for 30 minutes (alpha-cypermethrin: PR = 0.86, CI = 0.49–1.49, p = 0.88; deltamethrin: PR = 1.59, CI = 0.995–2.55, p = 0.054; and permethrin: PR = 0.64, CI = 0.41–1.004, p = 0.053). There were not enough mosquitoes to assess associations between two hour survival and insecticide. When considering L1014F kdr and N1575Y as a single resistance haplotype, there was an association between presence of these mutations as compared to only the L1014F kdr mutation and ability of mosquitoes to survive permethrin exposure for 30 minutes (PR = 1.92, CI = 1.09–3.37, p = 0.02), but no association with alpha-cypermethrin (PR = 1.05, CI = 0.72–1.54, p = 0.98) or deltamethrin (PR = 1.11, CI = 0.78–1.58, p = 0.87). There was no association with survival data at two hours (alpha-cypermethrin: PR = 0.42, CI = 0.04–4.48, p = 0.76; deltamethrin: PR = 1.08, CI = 0.18–6.53, p = 1.00; and permethrin: PR = 1.58, CI = 0.33–7.50, p = 0.86).
Table 3.
L1014F kdr allele frequencies in An. gambiae s.l. from six study sites in Forecariah Prefecture, Guinea.
| Study Site | Survival Status | # Mosquitoes tested | Homozygote mutation (RR) | Heterozygote mutation (RS) | Homozygote wild type (SS) | L1014F kdr allele frequency | χ2 Test | P value | |
|---|---|---|---|---|---|---|---|---|---|
| R | S | ||||||||
| Fandie | Alive | 29 | 29 | 0 | 0 | 1.0 | 0 | — | — |
| Dead | 9 | 9 | 0 | 0 | 1.0 | 0 | — | — | |
| Madinagbe | Alive | 26 | 8 | 3 | 15 | 0.63 | 0.37 | 14.67 | <0.0001 |
| Dead | 1 | 1 | 0 | 0 | 1.0 | 0 | — | — | |
| Maferinyah Centre I | Alive | 41 | 40 | 0 | 1 | 0.98 | 0.02 | 41.00 | <0.0001 |
| Dead | 29 | 29 | 0 | 0 | 1.0 | 0 | — | — | |
| Moribayah | Alive | 6 | 6 | 0 | 0 | 1.0 | 0 | — | — |
| Dead | 0 | 0 | 0 | 0 | — | — | — | — | |
| Senguelen | Alive | 30 | 28 | 0 | 2 | 0.93 | 0.07 | 30.00 | <0.0001 |
| Dead | 16 | 15 | 0 | 1 | 0.94 | 0.06 | 16.00 | <0.0001 | |
| Yindi | Alive | 32 | 21 | 6 | 5 | 0.75 | 0.25 | 8.00 | 0.005 |
| Dead | 23 | 14 | 2 | 7 | 0.65 | 0.35 | 15.03 | <0.0001 | |
The G119S Ace-1 mutation was detected in 10% of samples (7/67) (Fig. 1C). All individuals with the resistant allele were heterozygous for this mutation and were identified as An. gambiae s.s. Frequencies of the G119S Ace-1 mutation varied significantly among study districts (Fisher’s Exact test; p = 0.029) (Table 4 and Fig. 1C). There was a significant association between presence of the G119S Ace-1 mutation and ability of mosquitoes to survive 30 minute and two hour carbamate exposure (Fisher’s Exact test; p = 0.0047 and Fisher’s Exact test; p = 0.0015, respectively).
Table 4.
G119S Ace-1 allele frequencies in An. gambiae s.l. from six study sites in Forecariah Prefecture, Guinea.
| Study Site | Survival Status | # Mosquitoes tested | Homozygote mutation (RR) | Heterozygote mutation (RS) | Homozygote wild type (SS) | G119S Ace-1 allele frequency | χ2 Test | P value | |
|---|---|---|---|---|---|---|---|---|---|
| R | S | ||||||||
| Fandie | Alive | 9 | 0 | 1 | 8 | 0.06 | 0.94 | 0.03 | 0.862 |
| Dead | 7 | 0 | 0 | 7 | 0 | 1.0 | — | — | |
| Madinagbe | Alive | 3 | 0 | 1 | 2 | 0.17 | 0.83 | 0.12 | 0.729 |
| Dead | 1 | 0 | 0 | 1 | 0 | 1.0 | — | — | |
| Maferinyah Centre I | Alive | 17 | 0 | 2 | 15 | 0.06 | 0.94 | 0.07 | 0.791 |
| Dead | 11 | 0 | 1 | 10 | 0.05 | 0.95 | 0.02 | 0.888 | |
| Moribayah | Alive | 2 | 0 | 2 | 0 | 0.5 | 0.5 | 2.00 | 0.157 |
| Dead | — | — | — | — | — | — | — | — | |
| Senguelen | Alive | — | — | — | — | — | — | — | — |
| Dead | 9 | 0 | 0 | 9 | 0.0 | 1.0 | — | — | |
| Yindi | Alive | — | — | — | — | — | — | — | — |
| Dead | 8 | 0 | 0 | 8 | 0.0 | 1.0 | — | — | |
Across Forecariah Prefecture, significant deviations from Hardy-Weinberg equilibrium were observed for L1014F kdr in Madinagbe, Maferinyah Centre I, Senguelen and Yindi (p < 0.005 for all) (Table 3) and for N1575Y in Yindi (p = 0.040) but not for any other resistance loci.
Finally, to confirm the potential role of cytochrome P450 enzymes in pyrethroid resistance, mosquitoes collected in Fandie and Senguelen were pre-exposed to piperonyl butoxide (PBO), prior to intensity testing with alpha-cypermethrin and deltamethrin, respectively. In both populations, susceptibility was partially restored at five times the diagnostic dose of pyrethroids (95% and 89% mosquito mortality to alpha-cypermethrin and deltamethrin, respectively) and fully restored at 10X (Fig. 3).
Plasmodium falciparum infection and infectivity
The abdomens and head/thoraxes of 484 An. gambiae s.l. were screened separately to detect the presence of Plasmodium falciparum oocysts and sporozoites, respectively. Overall the proportion of positive mosquitoes for oocysts and sporozoites were 6.0% (29/484) and 0.2% (1/484), respectively. There was no significant difference in abdomen positive proportions among study villages (Fisher’s Exact test; p = 0.21) or molecular forms (n = 179, 63% An. coluzzii and 37% An. gambiae s.s.; PR for An. gambiae s.s. = 1.32, CI = 0.28–5.24, p = 0.70). In mosquitoes that survived insecticide exposure, oocyst rate was 6.8% (23/340) compared to 4.2% (6/144) in their susceptible counterparts (PR = 1.62, CI = 0.72–4.26, p = 0.28). The single sporozoite positive individual was collected from Maferinyah Centre I and died following 2X alpha-cypermethrin exposure.
Considering the interaction between pyrethroid resistance and P. falciparum infection, there was no significant relationship between abdomen positivity rate and survival following insecticide exposure in all pooled data (PR = 1.36, CI = 0.48–5.52, p = 0.61) or per insecticide (alpha-cypermethrin: Fisher’s Exact test; p = 0.65; deltamethrin: Fisher’s Exact test; p = 0.57 and permethrin: Fisher’s Exact test; p = 0.20). Considering the interaction between carbamate resistance and P. falciparum infection, survivors of bendiocarb exposure were significantly more likely to be infected (Fisher’s Exact test; p = 0.049; 4/23 survivors vs. 3/76 susceptible individuals).
No significant associations were observed between presence of target site mutations and abdomen positivity (N1575Y: Fisher’s Exact test, p = 0.76, I1527T: Fisher’s Exact test, p = 1.00, L1014F kdr: Fisher’s Exact test, p = 0.37, and G119S Ace-1: Fisher’s Exact test, p = 0.50).
Discussion
By 2016, resistance to at least one insecticide has been reported from over 80% of malaria endemic countries1, representing a threat to the continued efficacy of key malaria control strategies. However, the relative impact of decreased mosquito susceptibility on vectorial capacity remains unknown, largely due to a paucity of field data. To begin to address this deficit, in an area of high malaria transmission in Guinea, we characterized levels of insecticide resistance and age of local vector populations, in combination with molecular identification of resistance markers and detection of malaria infection.
In Forecariah Prefecture, intense pyrethroid resistance was abundant, evidenced by vector populations that were not only resistant to ten times the insecticide concentration required to kill susceptible individuals, but were also capable of surviving these doses for up to two hours. These observations are of concern given that pyrethroid LLINs are the only malaria vector control intervention deployed in this area. The restoration of mosquito susceptibility following pre-exposure to PBO and the association of mutations in the voltage-gated sodium channel (VGSC; L1014F kdr-N1575Y haplotype and I1527T) with lower mortality to permethrin suggests that both target site mutations and increased activity of P450 monooxygenases are contributing to pyrethroid resistance. This finding aligns with reports from Burkina Faso where upregulated detoxification enzymes were responsible for extreme pyrethroid resistance in An. coluzzii, with N1575Y associated with more limited tolerance to deltamethrin29. It has been proposed that N1575Y may compensate for fitness costs incurred by the L1014F kdr mutation and provide additional pyrethroid resistance30. The I1527T mutation is located within the III S6 helix of the VGSC, adjacent to a predicted pyrethroid/DDT binding site; nearby residues have already been implicated in resistance in other medically-important vector species31,32. However, to date, a role for I1527T in phenotypic resistance in An. gambiae s.l. has not been confirmed, nor has this mutation been detected in Guinea previously.
Of the three pyrethroid insecticides evaluated, resistance levels were often highest to permethrin, despite all mass LLIN campaigns in the country having exclusively distributed deltamethrin-treated products25. This may be explained by L1014F kdr playing a larger contributing role to resistance to type I (permethrin) versus type II (alpha-cypermethrin and deltamethrin) pyrethroids33. Levels of bendiocarb resistance were comparatively lower, which was not unexpected considering IRS is not routinely conducted by the Guinean NMCP. In the case of carbamate resistance, the presence of the G119S Ace-1 mutation was highly predictive of bioassay survivorship and tolerance to increased exposure times. Previous studies have demonstrated that the G119S Ace-1 substitution imposes a high fitness cost34,35 by decreasing affinity of the resistant enzyme for its substrate by more than 60%36; heterogeneous or homogeneous duplication of this locus has been proposed to restore activity37. In Forecariah Prefecture, frequency of the G119S Ace-1 resistant allele (of undetermined copy number) was low and could be a result of selective pressure imposed by unregulated agricultural use of carbamates and organophosphates, which were readily available at the local market (S. Irish, personal communication).
Our results demonstrated that pyrethroid resistance was not associated with malaria prevalence in the mosquitoes that were tested, with no significant differences observed between Plasmodium infection in susceptible or resistant individuals. Our findings contrast with a number of laboratory studies ascribing potential fitness costs to insecticide resistance which may have the collateral benefit of reducing malaria transmission16,18–22. However, in our study, survivors of bendiocarb exposure were significantly more likely to be infected with P. falciparum. Pyrethroid exposure has previously been shown to adversely affect P. falciparum development in L1014F kdr resistant An. gambiae s.s. in Uganda21, which may explain part of our data, if we assume resistant individuals are able to survive contact with LLINs and susceptible vectors are not. Alternative observations have also been reported whereby kdr has been shown to potentiate the vector competence of An. gambiae s.s.23, and Plasmodium infection has partially restored DDT susceptibility in An. gambiae carrying the kdr mutation38 but also reduced the survival of resistant vectors in the absence of insecticide exposure17. The discrepancies between in vitro studies and our field data may reflect more generalised fitness variation between laboratory mosquito strains and wild populations and/or different underlying resistance mechanisms, e.g. target site mutations vs. increased activity of metabolic enzymes, and strongly support the need for additional studies in areas of differing resistance mechanisms and disease transmission intensities.
We examined the impact of resistance on mosquito age as increasing age has been proposed to restore insecticide susceptibility39–46 and insecticide exposure may still reduce resistant vector life-span through delayed mortality effects16. In Forecariah Prefecture, we observed a similar phenomenon, with resistant mosquitoes having significantly lower parity rates than their susceptible counterparts.
Elucidating the interaction between insecticide resistance and vectorial capacity is complex and challenging in field conditions and a number of limitations were encountered during this study. Adult female mosquitoes were sampled using HLCs and via manual aspiration from house walls to maximise the number of individuals available for bioassay testing. However, these strategies may have introduced a bias in species composition collected; previous studies have suggested that proportions of An. gambiae s.s. and An. coluzzii may differ significantly between larval and adult spray catches47. We also encountered issues with individuals that did not amplify with two sub-species PCR assays48,49, potentially reflecting polymorphisms at the primer binding sites and/or the presence of a cryptic sub-species; previously a cryptic subgroup GOUNDRY has been reported, with close genetic affinities to An. coluzzii and enhanced susceptibility to P. falciparum infection50–52. While use of F1 progeny mosquitoes is recommended for resistance testing53,54, to investigate the impact of resistance on Plasmodium infection in the vector, it was necessary to sample wild caught adults of undetermined, mixed age and physiological status; the latter parameter may have varied between sampling methods, with more fed mosquitoes collected from inside houses, compared to unfed, host-seeking mosquitoes in HLCs. As a consequence, mortality in our study may have been under-estimated, considering the proposed inverse relationship between vector resistance and age39–45. Similarly, blood-feeding among resistant mosquitoes has been suggested to increase insecticide tolerance55, however, no association between physiological status and resistance was observed in our dataset. Our study would have also benefitted from larger sample sizes of mosquitoes tested in bioassays, to statistically power comparisons between different resistance intensities. Furthermore, abdomen positive mosquitoes cannot all be assumed to become infective sporozoite-transmitters, particularly if vector life-span is reduced; a positive abdomen can also reflect a recent feed on an infected individual or the passage of sporozoites to the salivary glands. Finally, manual parity dissections have a number of known constraints, including insensitivity, especially in low endemicity areas, and inter-operator subjectivity. While every effort was made to consistently bisect individuals, and our reported sporozoite rate was low, other studies have demonstrated higher number of sporozoite false positives by PCR when abdomens were removed posterior to the junction of the abdomen and thorax56. We also cannot entirely discount natural variation in parity, which may have increased over the sampling period at the beginning of the rainy season.
Conclusions
These findings present a comprehensive overview of the current levels of insecticide resistance and underlying target site mutations present in Maferinyah, Guinea, an area of high malaria transmission. Local mosquito populations were intensely resistant to pyrethroids (alpha-cypermethrin, deltamethrin and permethrin), associated with high frequencies of the L1014F kdr allele. N1575Y and I1527T mutations in the VGSC gene were present at lower levels and may warrant increased surveillance efforts, particularly as L1014F kdr approaches fixation. Restoration of mosquito susceptibility following pre-exposure to PBO indicates increased activity of detoxification enzymes is also contributing to pyrethroid resistance in this area and requires additional characterization. Despite no ongoing vector control activities using carbamates, bendiocarb resistance was observed, and the G119S Ace-1 allele was detected in a subset of tolerant individuals. Malaria infection was not associated with pyrethroid resistance but it was associated with bendiocarb resistance. In general, resistant vectors were younger than their susceptible counterparts. Further studies are necessary to investigate the impact of insecticide resistance on vector fitness, including mosquito fecundity, egg viability, hatchability and parasite development following an infected blood meal.
Methods
Study area and mosquito collections
Mosquito collections were undertaken in six villages in the Maferinyah sub-prefecture, Forecariah Prefecture (Fandie, Madinagbe, Maferinyah Centre I, Moribayah, Senguelen and Yindi), in Southwest Guinea. Deltamethrin-treated LLINs had been distributed as part of a national mass campaign in Maferinyah in 2016. Sampling was conducted between 22nd June and 17th July 2017, coinciding with the beginning of the long rainy season. Following consent from the household owner, indoor resting, female Anopheles mosquitoes were collected from house walls by manual aspiration between 7:00 h and 12:00 h. In the same villages, mosquitoes were also sampled using HLCs, carried out between 22:00 h and 3:00 h. Mosquitoes were stored in cages with access to 10% sugar solution, prior to transport to the Centre de Formation et de Recherche en Santé rurale de Maferinyah (CNFRSR) for analysis.
CDC resistance intensity and synergist bioassays
All bioassays were performed using mosquitoes identified morphologically as An. gambiae s.l.57; wild caught females were held for a maximum of 48 hours before testing. Centers for Disease Control and Prevention (CDC) resistance intensity bioassays for three pyrethroid insecticides (alpha-cypermethrin, deltamethrin and permethrin) were conducted according to published guidelines53. Stock solutions of 1, 2, 5 and 10 times the diagnostic dose of insecticide (alpha-cypermethrin: 12.5 μg/bottle; deltamethrin: 12.5 μg/bottle; and permethrin: 21.5 μg/bottle), were prepared by diluting technical grade insecticide in 50 ml of acetone. Bioassays for bendiocarb were conducted using the diagnostic dose (1X: 12.5 μg/bottle). The inside of each Wheaton 250 ml bottle along with its cap was coated with 1 ml of stock solution by rolling and inverting the bottles. In each test, a control bottle was coated with 1 ml of acetone. Following coating, bottles were left to dry in a dark box. Approximately 15–25 field-caught adult female An. gambiae s.l. of unknown age and mixed physiological status were introduced into each bottle using a mouth aspirator and mortality was recorded at 15 minute intervals until all were dead or up to two hours; dead individuals at 30 minutes were removed from bottles by aspiration and again after two hours. In each bioassay, a control bottle, coated with acetone, was run in parallel. Mortality in control bottles never exceeded 5%. In select sites with significant pyrethroid resistance, synergist assays were also conducted by pre-exposing mosquitoes to piperonyl butoxide (PBO) for 1 hour (100 µg/bottle) prior to performing pyrethroid bioassays. Multiple replicates were performed per insecticide and study village, depending on mosquito availability, and individual surviving (resistant) and dead (susceptible) mosquitoes were preserved in RNAlater® (Thermo Fisher Scientific, UK) at −20 °C at the London School of Hygiene and Tropical Medicine (LSHTM) and CDC. Prior to molecular analysis, mosquito heads/thoraxes were separated from abdomens under a dissecting microscope and stored separately.
Parity dissection
Ovarian dissection to determine mosquito parity was performed on ten mosquitoes (including both survivors and those that died) selected randomly from each bottle after bioassay completion58. The ovaries of each mosquito were dissected on a sterile microscope slide in distilled water, using a binocular dissection microscope and physiological status was determined. The ovaries were then examined under a light microscope (10X magnification) for the presence of tightly coiled skeins or loose coils, indicative of a nulliparous or parous ovary, respectively. On completion of ovarian dissection, head/thoraxes and abdomens of each mosquito were stored separately in RNAlater® (Thermo Fisher Scientific, UK) at −20 °C.
Molecular species identification
A subset of susceptible and resistant An. gambiae s.l. mosquitoes from all six villages containing both nulliparous and parous individuals were selected for molecular analysis at the LSHTM and CDC. Genomic DNA from dissected body parts was extracted per protocol using Qiagen DNeasy 96 Blood and Tissue kits (Qiagen, UK) at LSHTM or ExtractaTM DNA Prep for PCR-Tissue kits (QuantaBio, USA) at CDC.
At LSHTM, molecular species identification was performed using a multiplex TaqMan real time PCR assay to detect and discriminate An. gambiae and An. arabiensis59. PCR reactions were prepared using Qiagen Quantitect Probes Master mix (Qiagen) with each reaction containing 6.25 µl of master mix, a final concentration of 0.8 µM of each primer, 0.2 µM of probe An. arabiensis (Cy5), 80 nM of probe An. gambiae (FAM) and 1 µl of template DNA for a final reaction volume of 12.5 µl. Prepared reactions were run on a Stratagene Mx30005P QPCR system for 10 min at 95 °C, followed by 40 cycles of 95 °C for 25 sec and 66 °C for 60 sec. The increases in the species-specific FAM and Cy5 fluorophores were detected in real-time at the end of each cycle and results were analysed using Stratagene MxPro QPCR software. Positive controls from gDNA extracted from known An. gambiae and An. arabiensis individuals were included in each run, in addition to no template controls (NTCs). Sub-samples of individuals from each district confirmed as An. gambiae were further distinguished as An. coluzzii or An. gambiae s.s by targeting a SINE200 insertion only present in An. coluzzii48. PCR reactions were prepared using Hot Start Taq 2X Master Mix (New England Biolabs, UK) with each reaction containing 12.5 µl of master mix, a final concentration of 1 µM of each primer, 2 µl template DNA for a final reaction volume of 25 µl. Prepared reactions were amplified using a BIORAD T100 Thermal Cycler for 10 min at 95 °C, followed by 35 cycles of 94 °C for 30 sec, 54 °C for 30 sec, 72 °C for 1 min and a final extension of 72 °C for 10 min. PCR products were separated and visualised using 2% Egel EX agarose gels (Invitrogen, UK) with SYBR safe and an Invitrogen E-gel iBase Real-Time Transilluminator. An. coluzzii individuals with the insertion resulted in a single PCR product of 479 bp and An. gambiae s.s. a PCR product of 249 bp.
At CDC, An. coluzzii and An. gambiae s.s. specimens were differentiated by targeting single nucleotide polymorphisms (SNPs) present in rDNA49. PCR reactions were prepared using 20–40 ng of DNA, 5X Green GoTaq® Reaction Buffer (Promega, USA), 25 mM MgCl2, 2.5 mM of each dNTP, 1 U GoTaq® DNA polymerase and 25 pmol/μl of primers IMP-UN, AR-3T, GA-3T, IMP-S1 and IMP-M1 in a final volume of 25 µl. PCR reaction conditions were 95 °C for 5 min, followed by 30 amplification cycles (95 °C for 30 sec, 58 °C for 30 sec, 72 °C for 30 sec) and a final elongation step at 72 °C for 5 min. Amplified PCR products were visualized on 1.5% agarose gels, stained with GelRedTM (Biotium, USA). An. arabiensis Dongola, An. coluzzii AKDR and An. gambiae s.s. RSP-ST strains from the Malaria Research and Reference Reagent Resource Center (MR4), were used as positive controls. Amplification products of 463 bp and 333 bp or 463 bp and 221 bp were indicative of An. coluzzii or An. gambiae s.s., respectively.
Insecticide target site mutation detection
Detection of the L1014F West African kdr mutation was performed on a subset of individuals from all six districts according to the adapted protocol for allele-specific PCR developed by Martinez-Torres et al.60. At both LSHTM and CDC, PCR reactions were prepared using Hot Start Taq 2X Master Mix (New England Biolabs) with each reaction containing 12.5 µl of master mix and variable final concentrations of primers (IPCF 0.1 µM, AltRev 0.1 µM, West WT 1 µM, West West 1.1 µM) for a final reaction volume of 25 µl. Prepared reactions were run on a BIO-RAD QPCR system for 5 min at 95˚C, followed by 35 cycles of 95˚C for 30 sec, 59˚C for 30 sec and 72˚C for 30 sec and a final extension of 72˚C for 5 min. PCR products were separated and visualised using 2% Egel EX agarose gels (Invitrogen) with SYBR safe and an Invitrogen E-gel iBase Real-Time Transilluminator. A PCR product of 214 bp indicated the susceptible wild type allele and a PCR product of 156 bp indicated the resistant allele. An. coluzzii AKDR and An. gambiae s.s. RSP-ST were used as positive and negative controls for L1014F kdr, respectively.
At LSHTM, a larger sub-sample of individuals from all six districts was chosen to be screened for the N1575Y mutation (previously shown to have low prevalence in West African populations) using the TaqMan real time PCR assay developed by Jones et al.30. PCR reactions were prepared using Qiagen Quantitect Probes Master mix (Qiagen) with each reaction containing 10 µl of master mix, a final concentration of 1 µM of each primer and 0.5 µM of each probe, 5 µl of PCR grade water and 2 µl of template DNA, for a final reaction volume of 20 µl. Prepared reactions were run on a Roche LightCycler 96 System for 15 min at 95˚C, followed by 35 cycles of 94˚C for 15 sec and 60˚C for 60 sec. Positive controls from gDNA extracted from known An. gambiae s.s. with or without the N1575Y mutation were included on each run in addition to no template controls (NTCs). PCR results were analysed using the LightCycler 96 software (Roche Diagnostics).
At CDC, to detect the N1575Y mutation, a 218 bp fragment of the VGSC channel spanning domains III-IV was sequenced. PCR reactions were prepared using 25 µl of 2X AccuStartTM II PCR SuperMix, a final concentration of 20 pmol/μl of primers Exon_29_F (5′-AAATGCTCAGGTCGGTAAACA-3′) and Exon_29_R (5′-GCCACTGGAAAGAATGGAAA-3′)30 and 2 μl of template DNA, for a final reaction volume of 50 μl. PCR reaction conditions were 95 °C for 3 min, followed by 35 amplification cycles (95 °C for 30 sec, 58 °C for 30 sec, 72 °C for 30 sec) and a final elongation step at 72 °C for 5 min. Amplified PCR products were visualized on 2% agarose gels, stained with GelRedTM (Biotium, USA) and purified using the 96-well MilliporeTM MultiScreenTM HTS vacuum manifold system. Bi-directional sequencing was performed using primers Exon_29_F and Exon_29_R with the BigDye® Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, USA) according to the manufacturer’s protocol. Sequencing reactions were purified using the Big Dye® XterminatorTM Purification Kit (Applied Biosystems, USA), according to the manufacturer’s protocol and data were generated using a 3500xL Genetic Analyzer (Applied Biosystems, USA). Sequences were assembled manually in BioEdit v7.0.9.0 sequence alignment editor software (Ibis Biosciences, USA) and unambiguous consensus sequences were produced for each individual. Consensus sequences are available from GenBank under the accession numbers MH929325 - MH929433.
The presence of the G119S Ace-1 mutation was determined using PCR restriction fragment length polymorphism analysis61. At both LSHTM and CDC, PCR reactions were prepared using Hot Start Taq 2X Master Mix (New England Biolabs) with each reaction containing 12.5 µl of master mix, a final concentration of 1 µM of each primer, 2 µl template DNA, to a final reaction volume of 25 µl. Prepared reactions were run on a BIO-RAD QPCR system for 5 min at 95˚C, followed by 30 cycles of 95˚C for 30 sec, 52˚C for 30 sec and 72˚C for 1 min and a final extension of 72˚C for 5 min. PCR products were digested using the AluI restriction enzyme through incubation at 37˚C for 16 hr followed by 65˚C for 20 min. DNA fragments were visualised on 2% Egel EX agarose gels (Invitrogen) with SYBR safe and an Invitrogen E-gel iBase Real-Time Transilluminator. 194 bp undigested PCR products indicated the susceptible allele and 74 bp and 120 bp digested fragments indicated the presence of the resistant allele. Presence of all three product sizes indicated that the sample was heterozygous.
Plasmodium falciparum detection
Bisected mosquito head/thoraxes and abdomens from bioassay individuals were screened separately for P. falciparum infection, by targeting a 120 bp sequence of the P. falciparum cytochrome c oxidase subunit 1 (cox1) mitochondrial gene62. At LSHTM real-time PCR reactions were prepared using FastStart SYBR Green Master mix (Roche Diagnostics) with each reaction containing 5 µl of master mix, a final concentration of 1 µM of each primer, 1 µl of PCR grade water and 2 µl template DNA, to a final reaction volume of 10 µl. Prepared reactions were run on a Roche LightCycler 96 System for 15 min at 95˚C, followed by 35 cycles of 95˚C for 15 sec and 58˚C for 30 sec. Amplification was followed by a dissociation curve (95˚C for 10 sec, 65˚C for 60 sec and 97˚C for 1 sec) to ensure the correct target sequence was amplified. Positive controls from gDNA extracted from a cultured P. falciparum-infected blood sample (parasitaemia of ~10%) were included on each run, in addition to no template controls (NTCs). PCR results were analysed using the LightCycler® 96 software (Roche Diagnostics).
At CDC, a conventional PCR was used for cox1 detection63. PCR reactions were prepared using 6.25 µl of2X AccuStartTM II PCR SuperMix, a final concentration of 5 µM of primers COX-IF (5′-AGAACGAACGCTTTTAACGCCTG-3′) and COX-IR (5′-ACWGGATGGACTTTATATCCACCATTAAGT-3′)63 and 2 μl of template DNA, for a final reaction volume of 12.5 μl. PCR reaction conditions were 94 °C for 5 min, followed by 40 amplification cycles (94 °C for 1 min, 62 °C for 1 min, 72 °C for 1.5 min) and a final elongation step at 72 °C for 10 min. Amplified PCR products were visualized on 2% agarose gels, stained with GelRedTM (Biotium, USA), alongside the same positive and negative controls used in the real-time assay. A positive head/thorax or abdomen was assumed to indicate the presence of sporozoites or oocysts, respectively.
Data analysis
Percent mosquito mortality for each insecticide dose was interpreted using the WHO criteria: 98–100% mortality at 30 minutes of exposure indicates ‘susceptibility’, 90–97% mortality suggests ‘possible resistance’ and <90% indicates the presence of ‘resistance’54. Where molecular species ID data were available, results are reported for An. coluzzii and An. gambiae s.s. separately; otherwise individuals are referred to as An. gambiae s.l. Pearson’s Chi squared tests and Fisher’s exact tests (when sample sizes were small) were used to investigate the statistical association between survival by site, allele frequencies, and deviations from Hardy-Weinberg equilibrium. Other analyses utilized quasi-Poisson regression to estimate prevalence ratios (PRs) of mosquito survival, except the model in Fig. 2, which used a binomial model to ensure confidence intervals did not extend beyond 100%. Outcomes from pyrethroid bioassays were evaluated separately to carbamate assays, unless otherwise specified. The endpoint of all bioassays was considered to be 30 minutes (the diagnostic time for the insecticides under evaluation), unless otherwise specified. All statistical analyses were performed in R version 3.5.164, with the level of significance set at α = 0.05.
Ethical approval and consent to participate
The study protocol was reviewed and approved by the Comite National d’Ethique pour la Recherche en Sante (030/CNERS/17) and the institutional review boards (IRB) of the London School of Hygiene and Tropical Medicine (#13612 and #14076) and the Centers for Disease Control and Prevention, USA (2018–086); all study procedures were performed in accordance with relevant guidelines and regulations. Prior to study initiation, community consent was sought from village leaders and written, informed consent was obtained from the heads of all households selected for participation and from all fieldworkers who performed HLCs. Study information was provided to participants in French, Susu, Foula and Malinké. Fieldworkers participating in human landing catches were provided with doxycycline malaria prophylaxis for the duration of the study.
Acknowledgements
The authors express their sincere thanks to the entomology fieldworkers and the residents of Forecariah Prefecture for their study participation; to Luc Djogbenou and Corine Ngufor for providing G119S Ace-1 controls; to Martin Donnelly for providing N1575Y controls; to Dustin Miller for providing An. arabiensis, An. coluzzii and An. gambiae s.s. controls; to Alice Sutcliffe for providing primers and reagents; and to Jo Lines and Cheryl Whitehorn for providing training and support. Study funding was provided by a Royal Society of Tropical Medicine and Hygiene small grant, a Sir Halley Stewart Trust grant, a L’Oreal-UNESCO for Women in Science UK and Ireland Fellowship awarded to LAM, individual Bayer Vector Control Research and Travel grants awarded to EC and NMV, the Helena Vrbova scholarship awarded to NMV and a Wellcome Trust /Royal Society grant awarded to TW (101285/Z/13/Z): http://www.wellcome.ac.uk; https://royalsociety.org. LAM is supported by an American Society for Microbiology/Centers for Disease Control and Prevention Fellowship. SI is supported by the President’s Malaria Initiative (PMI)/CDC. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author Contributions
L.A.M., S.I., T.W., M.S. and A.H.B. designed the study and were responsible for data analysis and interpretation. E.C., N.M.V. and M.S. led the entomology field activities and participated in data collection. E.C., N.V.M., T.W., J.O., L.A.M. and G.L. performed the molecular assays. R.E.W. provided statistical expertise and data analysis support. L.A.M., T.W., S.I., E.C. and N.M.V. drafted the manuscript which was revised by co-authors. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Emma Collins and Natasha M. Vaselli contributed equally.
References
- 1.Organization, W. H. World Malaria Report 2017 (2017).
- 2.World Health Organization. World Malaria Report 2016. World Health Organization, 10.1071/EC12504 (2016).
- 3.Ranson HN’guessan, R. Lines JM, N. Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Strode, C., Donegan, S., Garner, P., Enayati, A. A. & Hemingway, J. The Impact of Pyrethroid Resistance on the Efficacy of Insecticide-Treated Bed Nets against African Anopheline Mosquitoes: Systematic Review and Meta-Analysis. PLoS Med. 11 (2014). [DOI] [PMC free article] [PubMed]
- 5.Hemingway J, et al. Tools and Strategies for Malaria Control and Elimination: What Do We Need to Achieve a Grand Convergence in Malaria? PLoS Biol. 2016;14:1–14. doi: 10.1371/journal.pbio.1002380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinschmidt I, et al. Design of a study to determine the impact of insecticide resistance on malaria vector control: a multi-country investigation. Malar. J. 2015;14:282. doi: 10.1186/s12936-015-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindblade KA, et al. A cohort study of the effectiveness of insecticide-treated bed nets to prevent malaria in an area of moderate pyrethroid resistance, Malawi. Malar. J. 2015;14:31. doi: 10.1186/s12936-015-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry MC, et al. Protective efficacy of lambda-cyhalothrin treated nets in Anopheles gambiae pyrethroid resistance areas of Cote d’Ivoire. Am J Trop Med Hyg. 2005;73:859–864. doi: 10.4269/ajtmh.2005.73.859. [DOI] [PubMed] [Google Scholar]
- 9.Asidi A, N’Guessan R, Akogbeto M, Curtis C, Rowland M. Loss of household protection from use of insecticide-treated nets against pyrethroid-resistant mosquitoes, Benin. Emerg. Infect. Dis. 2012;18:1101–1106. doi: 10.3201/eid1807.120218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochomo E, et al. Insecticide-Treated Nets and Protection against Insecticide- Resistant Malaria Vectors in Western Kenya. Emerg. Infect. Dis. 2017;23:158–764. doi: 10.3201/eid2305.161315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damien GB, et al. Malaria infection and disease in an area with pyrethroid-resistant vectors in southern Benin. Malar. J. 2010;9:380. doi: 10.1186/1475-2875-9-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokponnon FT, et al. Impact of long-lasting, insecticidal nets on anaemia and prevalence of Plasmodium falciparum among children under five years in areas with highly resistant malaria vectors. Malar. J. 2014;13:76. doi: 10.1186/1475-2875-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanda E, et al. Insecticide resistance and the future of malaria control in Zambia. PLoS One. 2011;6:1–9. doi: 10.1371/journal.pone.0024336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Protopopoff N, et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two fact. Lancet. 2018;391:1577–1588. doi: 10.1016/S0140-6736(18)30427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinschmidt, I. et al. Articles Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: a WHO-coordinated, prospective, international, observational cohort study. 3099, 1–10 (2018). [DOI] [PMC free article] [PubMed]
- 16.Viana M, Hughes A, Matthiopoulos J, Ranson H, Ferguson HM. Delayed mortality effects cut the malaria transmission potential of insecticide-resistant mosquitoes. Proc. Natl. Acad. Sci. 2016;113:8975–8980. doi: 10.1073/pnas.1603431113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alout H, et al. Interactive cost of Plasmodium infection and insecticide resistance in the malaria vector Anopheles gambiae. Sci. Rep. 2016;6:29755. doi: 10.1038/srep29755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegert PY, Walker E, Miller JR. Differential behavioral responses of Anopheles gambiae (Diptera: Culicidae) modulate mortality caused by pyrethroid-treated bednets. J. Econ. Entomol. 2009;102:2061–2071. doi: 10.1603/029.102.0607. [DOI] [PubMed] [Google Scholar]
- 19.Diop MM, et al. Behavioral cost & overdominance in anopheles gambiae. PLoS One. 2015;10:1–12. doi: 10.1371/journal.pone.0121755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platt N, et al. Target-site resistance mutations (kdr and RDL), but not metabolic resistance, negatively impact male mating competiveness in the malaria vector Anopheles gambiae. Heredity (Edinb). 2015;115:243–252. doi: 10.1038/hdy.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristan M, et al. Exposure to deltamethrin affects development of Plasmodium falciparum inside wild pyrethroid resistant Anopheles gambiae s.s. mosquitoes in Uganda. Parasit. Vectors. 2016;9:100. doi: 10.1186/s13071-016-1384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alout H, et al. Insecticide exposure impacts vector-parasite interactions in insecticide-resistant malaria vectors. Proc. R. Soc. B Biol. Sci. 2014;281:20140389–20140389. doi: 10.1098/rspb.2014.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alout Haoues, Ndam Nicaise Tuikue, Sandeu Marcel Maurice, Djégbe Innocent, Chandre Fabrice, Dabiré Roch Kounbobr, Djogbénou Luc Salako, Corbel Vincent, Cohuet Anna. Insecticide Resistance Alleles Affect Vector Competence of Anopheles gambiae s.s. for Plasmodium falciparum Field Isolates. PLoS ONE. 2013;8(5):e63849. doi: 10.1371/journal.pone.0063849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paludisme, M. de la S. D. N. de la P. et de la S. C. P. N. de L. C. le. Plan strategique national de lutte contre le paludisme 2018–2022 (2017).
- 25.Initiative, P. M. Guinea Malaria Operational Plan FY 2017 (2017).
- 26.Paludisme, P. N. de L. contre le. Rapport technique: Etude de sensibilité des vecteurs du paludisme aux insecticides dans le district sanitaire de Boffa (2012).
- 27.Keita K, et al. Species Identification and Resistance Status of Anopheles gambiae s.l. (Diptera: Culicidae) Mosquitoes in Guinea. J. Med. Entomol. 2017;54:677–681. doi: 10.1093/jme/tjw228. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann T, Diabate A. The molecular forms of Anopheles gambiae: A phenotypic perspective Tovi. Infect. Genet. Evol. 2008;8:737–746. doi: 10.1016/j.meegid.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toé KH, N’Falé S, Dabiré RK, Ranson H, Jones CM. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genomics. 2015;16:146. doi: 10.1186/s12864-015-1342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones CM, et al. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc. Natl. Acad. Sci. 2012;109:6614–6619. doi: 10.1073/pnas.1201475109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris AF, Rajatileka S, Ranson H. Pyrethroid resistance in Aedes aegypti from Grand Cayman. Am. J. Trop. Med. Hyg. 2010;83:277–284. doi: 10.4269/ajtmh.2010.09-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, et al. Multi-country Survey Revealed Prevalent and Novel F1534S Mutation in Voltage-Gated Sodium Channel (VGSC) Gene in Aedes albopictus. PLoS Negl. Trop. Dis. 2016;10:1–12. doi: 10.1371/journal.pntd.0004696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimer L, et al. Relationship Between kdr Mutation and Resistance to Pyrethroid and DDT Insecticides in Natural Populations of Anopheles gambiae. J. Med. Entomol. 2008;45:260–266. doi: 10.1603/0022-2585(2008)45[260:RBKMAR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 34.Assogba BS, et al. An ace-1 gene duplication resorbs the fitness cost associated with resistance in Anopheles gambiae, the main malaria mosquito. Sci. Rep. 2015;5:19–21. doi: 10.1038/srep14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Djogbenou L, et al. Costs of insensitive acetylcholinesterase insecticide resistance for the malaria vector Anopheles gambiae homozygous for the G119S mutation. Malar. J. 2010;9:12. doi: 10.1186/1475-2875-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alout H, Djogbénou L, Berticat C, Chandre F, Weill M. Comparison of Anopheles gambiae and Culex pipiens acetycholinesterase 1 biochemical properties. Comp. Biochem. Physiol. - B Biochem. Mol. Biol. 2008;150:271–277. doi: 10.1016/j.cbpb.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Assogba BS, et al. The ace-1 Locus Is Amplified in All Resistant Anopheles gambiae Mosquitoes: Fitness Consequences of Homogeneous and Heterogeneous Duplications. PLoS Biol. 2016;14:1–26. doi: 10.1371/journal.pbio.2000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alout H, et al. Interplay between Plasmodium infection and resistance to insecticides in vector mosquitoes. J. Infect. Dis. 2014;210:1464–1470. doi: 10.1093/infdis/jiu276. [DOI] [PubMed] [Google Scholar]
- 39.Jones CM, et al. Aging partially restores the efficacy of malaria vector control in insecticide-resistant populations of Anopheles gambiae s.l. from Burkina Faso. Malar. J. 2012;11:24. doi: 10.1186/1475-2875-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowland M, Hemingway J. Changes in malathion resistance with age in Anopheles stephensi from Pakistan. Pestic. Biochem. Physiol. 1987;28:239–247. doi: 10.1016/0048-3575(87)90022-8. [DOI] [Google Scholar]
- 41.Lines J, Nassor N. DDT resistance in Anopheles gambiae declines with mosquito age. Med. Vet. Entomol. 1991;5:261–265. doi: 10.1111/j.1365-2915.1991.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 42.MH H, Curtis C. Evaluation of the effect of mosquito age and prior exposure to insecticide on pyrethroid tolerance in Anopheles mosquitoes (Diptera: Culicidae) Bull. Entomol. Res. 1999;89:329–337. doi: 10.1017/S0007485399000462. [DOI] [Google Scholar]
- 43.S R, J B, Ranson H. Mosquito age and susceptibility to insecticides. Trans. R. Soc. Trop. Med. Hyg. 2011;105:247–253. doi: 10.1016/j.trstmh.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Glunt, K. D., Thomas, M. B. & Read, A. F. The effects of age, exposure history and malaria infection on the susceptibility of Anopheles mosquitoes to low concentrations of pyrethroid. PLoS One6 (2011). [DOI] [PMC free article] [PubMed]
- 45.RH H, BD B, C P, LL K, Coetzee M. Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus. Med. Vet. Entomol. 2005;19:271–275. doi: 10.1111/j.1365-2915.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 46.Guedes RNC, Walse SS, Throne JE. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin. Insect Sci. 2017;21:47–53. doi: 10.1016/j.cois.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Coulibaly B, et al. Malaria vector populations across ecological zones in Guinea Conakry and Mali, West Africa. Malar. J. 2016;15:191. doi: 10.1186/s12936-016-1242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santolamazza F, et al. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 2008;7:163. doi: 10.1186/1475-2875-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilkins EE, Howell PI, Benedict MQ. IMP PCR primers detect single nucleotide polymorphisms for Anopheles gambiae species identification, Mopti and Savanna rDNA types, and resistance to dieldrin in Anopheles arabiensis. Malar. J. 2006;5:125. doi: 10.1186/1475-2875-5-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crawford JE, et al. Evolution of GOUNDRY, a cryptic subgroup of Anopheles gambiae s.l., and its impact on susceptibility to Plasmodium infection. Mol. Ecol. 2016;25:1494–1510. doi: 10.1111/mec.13572. [DOI] [PubMed] [Google Scholar]
- 51.Riehle MM, et al. The anopheles gambiae 2La chromosome inversion is associated with susceptibility to Plasmodium falciparum in Africa. Elife. 2017;6:1–24. doi: 10.7554/eLife.25813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riehle MM, et al. A cryptic subgroup of Anopheles gambiae is highly susceptible to human malaria parasites. Science (80-.). 2011;331:596–598. doi: 10.1126/science.1196759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. Guideline for Evaluating Insecticide Resistance in Vectors Using the CDC Bottle Bioassay. CDC Methods 1–28 (2012).
- 54.Kitsche Wolfgang. Operation of a Cryogenic Rocket Engine. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. [Google Scholar]
- 55.Spillings BL, Coetzee M, Koekemoer LL, Brooke BD. The effect of a single blood meal on the phenotypic expression of insecticide resistance in the major malaria vector Anopheles funestus. Malar. J. 2008;7:226. doi: 10.1186/1475-2875-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foley DH, et al. Mosquito bisection as a variable in estimates of PCR-derived malaria sporozoite rates. Malar. J. 2012;11:1–7. doi: 10.1186/1475-2875-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gillies, M. T. & Coetzee, M. A supplement of the Anopheles of Africa south of the Sahara (Afrotropical Region). Publ. South African Inst. Med. Res. No. 55 (1987).
- 58.Detinova TS. Age grading methods in Diptera of medical importance with special reference to some vectors of malaria. Monogr Ser World Heal. Organ. 1962;47:13–191. [PubMed] [Google Scholar]
- 59.Bass C, Williamson MS, Wilding CS, Donnelly MJ, Field LM. Identification of the main malaria vectors in the Anopheles gambiae species complex using a TaqMan real-time PCR assay. Malar. J. 2007;6:155. doi: 10.1186/1475-2875-6-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez-Torres D, et al. P. D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol. Biol. 1998;7:179–84. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 61.Weill M, et al. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol. Biol. 2004;13:1–7. doi: 10.1111/j.1365-2583.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 62.Marie A, et al. Evaluation of a real-time quantitative PCR to measure the wild Plasmodium falciparum infectivity rate in salivary glands of Anopheles gambiae. Malar. J. 2013;12:224. doi: 10.1186/1475-2875-12-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Echeverry, D. F. et al. Fast and robust single PCR for Plasmodium sporozoite detection in mosquitoes using the cytochrome oxidase I gene. Malar. J. 1–8, 10.1186/s12936-017-1881-1 (2017). [DOI] [PMC free article] [PubMed]
- 64.R Core Team. R: A Language and Environment for Statistical Computing (2018).