Abstract
Candida albicans is the fourth most common cause of systemic nosocomial infections, posing a significant risk in immunocompromised individuals. As the majority of systemic C. albicans infections stem from endogenous gastrointestinal (GI) colonization, understanding the mechanisms associated with GI colonization is essential in the development of novel methods to prevent C. albicans-related mortality. In this study, we investigated the role of microbial-derived short-chain fatty acids (SCFAs) including acetate, butyrate, and propionate on growth, morphogenesis, and GI colonization of C. albicans. Our results indicate that cefoperazone-treated mice susceptible to C. albicans infection had significantly decreased levels of SCFAs in the cecal contents that correlate with a higher fungal load in the feces. Further, using in vivo concentration of SCFAs, we demonstrated that SCFAs inhibit the growth, germ tube, hyphae and biofilm development of C. albicans in vitro. Collectively, results from this study suggest that antibiotic-induced decreases in the levels of SCFAs in the cecum enhances the growth and GI colonization of C. albicans.
Subject terms: Fungal pathogenesis, Infection
Introduction
C. albicans, often present in the healthy gastrointestinal (GI) tract, is harmless to the immunocompetent human host with its resident microbiota1,2. Though compelling evidence suggests that disturbances in immune regulation contribute to invasive C. albicans infections, antibiotic-induced gut dysbiosis remains a major risk factor for increased C. albicans colonization and dissemination in immunocompromised patients and individuals with antibiotic-associated diarrhea (AAD)3–7. Administration of broad-spectrum antibiotics increases the risk of C. albicans colonization in the gut and the source of systemic infections is often found to be the GI tract7–10. In addition, more than 60% of individuals with AAD test positive for C. albicans and patients treated with antibiotics for Clostridium difficile often develop an episode of candidemia5,6. Taken together, these studies demonstrate that antibiotic-induced gut dysbiosis in immunocompromised individuals and AAD patients leads to increased colonization of C. albicans and this increased intestinal colonization predisposes high-risk patients to systemic candidiasis11,12. Therefore, understanding the factors involved in antibiotic-induced gut dysbiosis and subsequent GI colonization of C. albicans is critical to treat and prevent C. albicans pathogenesis.
Antibiotic treatment in mice and humans alters the composition of gut microbiota, ultimately leading to changes in the levels of microbial-derived gut metabolites, mainly bile acids and short-chain fatty acids (SCFAs)13–16. Alterations in the normal levels of microbial-derived bile acids and SCFAs have been implicated in the growth, colonization, and pathogenesis of enteric pathogens including C. difficile13,14,16. Moreover, we have recently demonstrated that microbial-derived bile acids play an important role in the GI colonization of C. albicans17,18. However, the role of SCFAs including acetate, propionate, and butyrate–three major fatty acids produced by gut microbiota19–21–in the GI colonization of C. albicans is poorly understood. Given the abundance of SCFAs in the intestine, a natural habitat and invasion site for C. albicans, understanding the role of SCFAs on fungal growth, morphogenesis and colonization will have important implications in C. albicans infections. Therefore, in this study, we aim to understand the role of microbial-derived SCFAs in the GI colonization of C. albicans.
To investigate if antibiotic treatment alters the levels of microbial-derived SCFAs and GI colonization of C. albicans, we treated mice with cefoperazone and determined the levels of SCFAs and the C. albicans load in the cecal and fecal contents, respectively. Furthermore, the role of SCFAs including acetic, butyric, and propionic acid on C. albicans growth and morphogenesis were investigated in vitro. Our results indicate that SCFAs inhibit the growth and morphogenesis of C. albicans and may potentially regulate the GI colonization of this fungal pathogen.
Results
Antibiotic-treated, C. albicans-susceptible mice have significantly reduced levels of SCFAs in the cecum
To determine the impact of antibiotic treatment on cecal SCFA levels and C. albicans load, groups of mice were treated with sterile water with or without cefoperazone for 7 days. After 7 days of antibiotic treatment, mice were euthanized for cecal SCFA analysis. Another set of control or antibiotic-treated mice were infected with C. albicans and their fecal CFU load was determined after 5 days of infection.
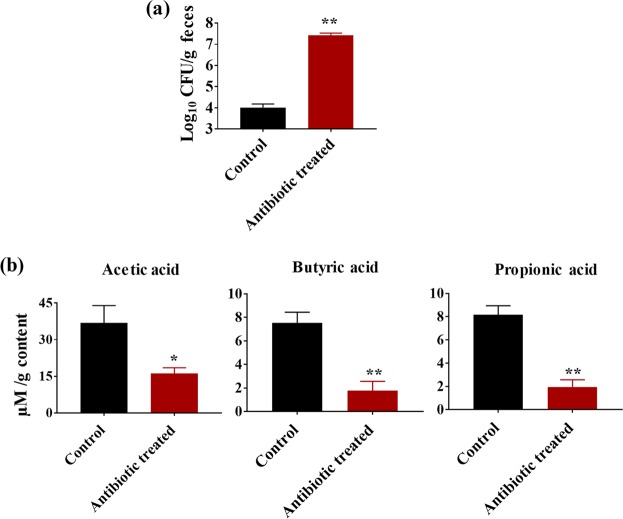
Results indicate that cefoperazone-treated mice had a significantly higher C. albicans load in the feces after 5 days of infection. Cefoperazone-treated mice had an almost 3 log10 increase in fungal load in the feces compared to control groups (Fig. 1a). Next we determined the SCFA levels in cefoperazone-treated C. albicans susceptible group and non-treated control group that are resistant to C. albicans infection. Interestingly, SCFA levels in the cecum of cefoperazone-treated mice were significantly decreased compared to control groups (Fig. 1b). The average concentration of acetic acid, butyric acid and propionic acid in the cecal content of control groups were 36.87 ± 7.11 µmol/g, 7.52 ± 0.92 µmol/g and 8.18 ± 0.77 µmol/g respectively. However, the SCFA levels in the cefoperazone-treated mice that are highly susceptible to C. albicans were acetic acid (16.13 ± 2.39 µmol/g), butyric acid (1.77 ± 0.79 µmol/g) and propionic acid (1.95 ± 0.63 µmol/g) (Fig. 1b). Taken together, these results suggest that cefoperazone-treated mice susceptible to C. albicans GI colonization had significantly decreased levels of SCFAs in the cecal contents.
Figure 1.
Cefoperazone-treated mice susceptible to C. albicans have decreased levels of SCFAs in the cecum. C. albicans SC5314 load in fecal contents after 5 days of infection in mice receiving sterile water with or without cefoperazone. Fungal load (Log10 CFU/g feces) determined by CFU count (a). SCFA levels (µmol/g) in the cecal contents from mice receiving sterile water with or without cefoperazone (b). Data is represented as means ± SEM with n = 5–6 mice in each treatment group. Statistical significance was evaluated using student’s t-test and P values (* ≤ 0.05, ** ≤ 0.01) were considered as significant.
SCFAs inhibit the growth of C. albicansin vitro
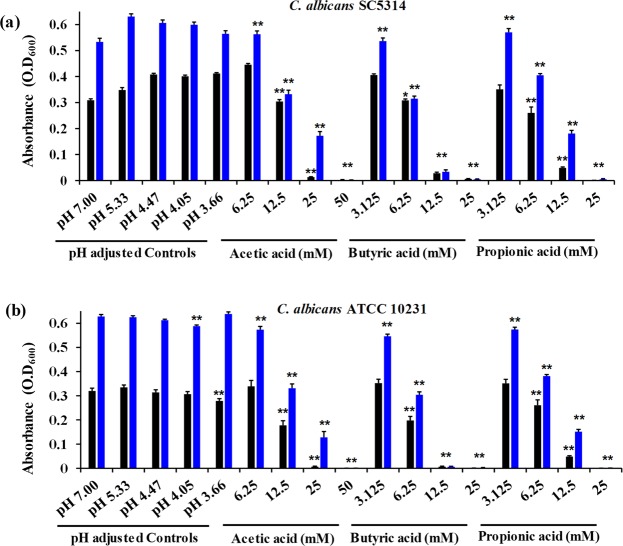
To investigate if in vivo levels of SCFAs in the cecal contents have any potential role in GI colonization of C. albicans, we examined the effect of SCFAs on C. albicans growth in vitro. We used pH-adjusted RMPI media to determine if changes in pH as a result of SCFA treatment have any inhibitory effect on C. albicans growth. RPMI media was titrated with HCl to match the pH values of SCFA treatments (Table 1). Results indicate that C. albicans (ATCC 10231 and SC 5314) strains grown in a pH ranging from 7.00 to 3.65 ± 0.05 experienced strain-dependent changes in growth in different pH-adjusted RPMI media (Fig. 2a,b). C. albicans SC 5314 exhibited a 30% increase in growth at pH 3.65 ± 0.05, 4.12 ± 0.07 and 4.49 ± 0.04, and a 12% increase at pH 5.38 ± 0.05 compared to fungal cells grown in pH 7.00 RPMI control after 24 hours (Fig. 2a). On the other hand, C. albicans ATCC 10231 strain did not show a considerable change in growth at pH values ranging from 7.00 to 4.12 ± 0.07. However, it exhibited a 13% decrease in growth at a pH value of 3.65 ± 0.05 compared to pH 7.00 RPMI control (Fig. 2b). These results indicate that alteration in pH does not considerably inhibit the growth of C. albicans.
Table 1.
Experimental pH of RPMI supplemented with varying concentrations of acetic acid and respective pH of HCl-adjusted pH controls.
| RPMI (for growth and biofilm Assays) | RPMI + 30% FBS (for hyphal and germ tube assays) | ||||
|---|---|---|---|---|---|
| [Acetic Acid] (mM) | Experimental pH | HCl-Adjusted Controls pH | [Acetic Acid] (mM) | Experimental pH | HCl-Adjusted Controls pH |
| 50 | 3.66 ± 0.03 | 3.65 ± 0.05 | 50 | — | — |
| 25 | 4.05 ± 0.03 | 4.12 ± 0.07 | 25 | 4.68 ± 0.02 | 4.69 ± 0.01 |
| 12.5 | 4.47 ± 0.03 | 4.49 ± 0.04 | 12.5 | 5.46 ± 0.05 | 5.52 ± 0.03 |
| 6.25 | 5.33 ± 0.06 | 5.38 ± 0.05 | 6.25 | — | — |
| 3.125 | 6.36 ± 0.03 | 5.85 ± 0.24 | 3.125 | — | — |
| 0 | 7.00 | 7.00 | 0 | >7.00 | 7.01 ± 0.01 |
Values taken as mean ± SEM.
Figure 2.
SCFAs inhibit C. albicans growth in vitro. Growth of C. albicans strains SC 5314 (a) and ATCC 10231 (b) in the presence of SCFAs or in pH-adjusted RPMI media determined by spectrophotometer analysis at an optical density of 600 nm after 24 and 48 hours of incubation. Experiment was repeated three times and the three combined replicates were shown here with total n = 9 for each group. Data is represented as means ± SEM. Statistical significance was evaluated using student’s t-test and P values (* ≤ 0.05, ** ≤ 0.01) were considered as significant. Significance is shown only for data points that exhibited significant decreases in growth compared to respective controls. Significance for pH-adjusted RPMI values was assessed using pH 7.00 as the comparative data set; significance for SCFA-treated conditions was assessed using respective pH controls for each SCFA condition in statistical analyses. Significance (**) for acetic acid (50 mM), butyric acid (12.5 mM and 25 mM), and propionic acid (25 mM) indicates p ≤ 0.01 at both 24 and 48 hours in both strains.
Next, we determined the effect of SCFAs on C. albicans growth. In RPMI media supplemented with average in vivo concentrations of SCFAs, C. albicans exhibited a significant decrease in growth compared to its respective pH-adjusted control groups (Fig. 2a,b). After 24 hours of treatment with acetic acid, C. albicans (SC 5314 and ATCC 10231) exhibited a 25–40% decrease at 12.5 mM, a 95% decrease at 25 mM, and no growth at 50 mM (Fig. 2a,b). Butyric acid exhibited potent inhibitory activity even at lower concentrations. Butyric acid (6.25 mM) reduced growth by 11–41%, followed by reduction of growth by 93–98% and 98–100% at 12.5 mM and 25 mM treatments, respectively (Fig. 2a,b). A similar trend was seen with propionic acid. At a concentration of 6.25 mM, propionic acid exhibited a 22% reduction in growth C. albicans (ATCC 10231). C. albicans (SC 5314 and ATCC 10231) further exhibited a 57–84% and 98–100% decrease in growth in the presence of 12.5 mM and 25 mM of propionic acid, respectively (Fig. 2a,b). Further, we assessed if SCFAs possess an inhibitory activity against C. albicans in RPMI media containing SCFAs buffered to pH 7.00. Surprinsingly, our results indicate that the overall inhibitory effect of SCFAs was abolished when RPMI media containing SCFAs was buffered to pH 7.00 (Supplementary Fig. 1). These trends continued into 48 hours, with strain-dependent significant inhibition of growth at varying concentrations of acetic acid (12.5–50 mM), butyric acid (6.25–25 mM), and propionic acid (6.25–25 mM) (Fig. 2a,b). Overall, our results demonstrate that SCFAs inhibit the growth of C. albicans strains in a concentration-dependent manner and that growth inhibition is not due to changes in pH.
SCFAs inhibit C. albicans germ tube formation
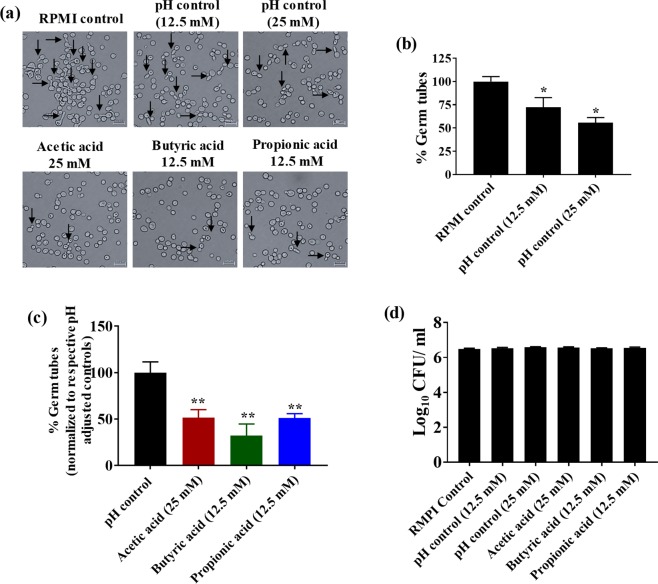
The impact of SCFA treatment on C. albicans germ tube formation was determined using microscopy analysis. pH-adjusted RPMI controls were used to determine if alterations in pH have any effect on germ tube formation. Microscopic imaging revealed that C. albicans considerably reduced the germ tube formation in pH controls for 12.5 mM (pH 5.52 ± 0.03) and 25 mM (pH 4.69 ± 0.01) compared to the RPMI control (pH 7.01 ± 0.01) (Fig. 3a). Quantification of the C. albicans cells that formed germ tubes after 2 hours of incubation revealed a 30% decrease in germ tube formation in pH control 12.5 mM (pH 5.52 ± 0.03) and an almost 50% decrease in pH control 25 mM (pH 4.69 ± 0.01) compared to the RPMI control (pH 7.01 ± 0.01) (Fig. 3b). These results indicate that acidic pH significantly inhibited the germ tube formation in C. albicans.
Figure 3.
SCFAs inhibit germ tube formation. Germ tube formation in C. albicans ATCC 10231 strain in the presence of SCFAs or pH-adjusted media supplemented with 30% FBS. Representative images of germ tubes formed after 2 hours in control and treatment groups determined by microscopic analysis at 40X magnification (a). Quantification of the percent C. albicans cells with germ tubes in pH-adjusted controls; pH-adjusted controls (12.5 mM and 25 mM) were normalized to the RPMI control (pH 7.00) (b). Quantification of the percent C. albicans cells with germ tubes in SCFA treatment groups (c). SCFA treatments were normalized to their respective pH controls. The germ tube experiment was repeated three times and two 40X images were taken from each replicate for each treatment group, with a minimum n = 1000 cells for each group. C. albicans (CFU/mL) viability determined after 2 hours of incubation in germ tube-inducing conditions (d). The experiment was repeated three times with n = 12 for each treatment group. Combined replicates for both experiments are shown here. Data is represented as means ± SEM. Statistical significance was evaluated using student’s t-test and P values (* ≤ 0.05, ** ≤ 0.01) were considered as significant.
Although acidic pH itself was shown to be a factor in inhibiting germ tube formation, we determined the effect of SCFAs on C. albicans germ tube formation. Interestingly, SCFAs were more potent in inhibiting the germ tube formation compared to their respective pH-adjusted controls. Microscopic imaging of C. albicans in the presence of SCFAs revealed a considerable decrease in germ tube formation compared to their respective pH-adjusted controls (Fig. 3a). In addition, quantification of the percentage of germ tubes formed revealed that acetic acid (25 mM) reduced germ tube formation by 50% compared to its pH control (pH 4.69 ± 0.01) (Fig. 3c). Butyric acid (12.5 mM) and propionic acid (12.5 mM) also significantly inhibited germ tube formation compared to their pH control (pH 5.52 ± 0.03), reducing germ tube formation by nearly 70% and 50%, respectively (Fig. 3c).
To determine if germ tube inhibition by SCFAs was not due to fungal cell death, C. albicans cells incubated in the germ tube conditions were determined for cell viability. Results indicated that no significant decrease in fungal cells was noticed after 2 hours of incubation in SCFA-treated or pH-adjusted control groups (Fig. 3d). Assessment of growth by spectrophotometer analysis under germ tube-inducing conditions showed a similar trend (Supplementary Fig. 2a). These results indicate that SCFAs inhibit C. albicans germ tube formation partly by inducing acidic conditions and through other unknown mechanisms.
SCFAs inhibit C. albicans hyphae formation
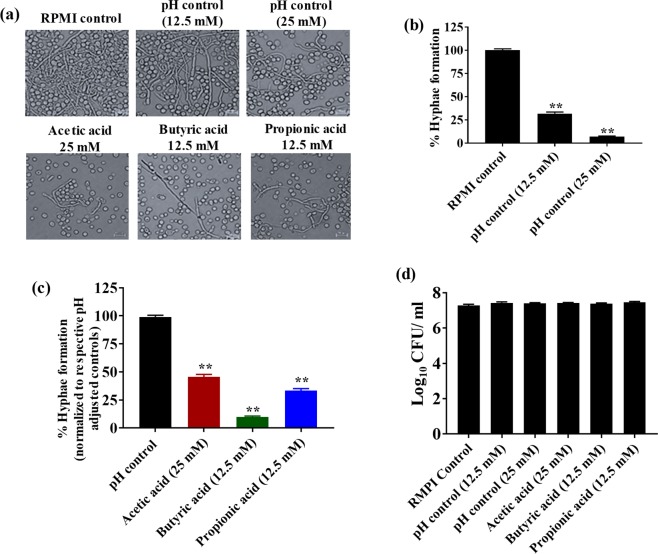
The effect of SCFAs on C. albicans (ATCC 10231 and SC5314) hyphae formation was evaluated using crystal violet and microscopic analyses. We used pH-adjusted RMPI media to determine if changes in pH have any effect on C. albicans hyphae formation. Results indicate that C. albicans ATCC 10231 grown in RPMI media at pH 7.01 ± 0.01 (RPMI control) showed massive hyphae formations (Fig. 4a). However, a considerable decrease in hyphae formation was noticed in the pH-adjusted controls for 12.5 mM (pH 5.52 ± 0.03) and 25 mM (pH 4.69 ± 0.01) treatments (Fig. 4a). Further, a crystal violet assay indicated that the pH-adjusted control 25 mM (pH 4.69 ± 0.01) inhibited 90% of C. albicans hyphae attachment, followed by 75% inhibition in the pH-adjusted control 12.5 mM (pH 5.52 ± 0.03) (Fig. 4b). Taken together, the crystal violet assay complements the findings of microscopic observations, indicating that acidic pH not only decreases hyphae formation but also significantly inhibits C. albicans hyphae attachment to polystyrene plates.
Figure 4.
SCFAs inhibit C. albicans hyphae formation in vitro. C. albicans ATCC 10231 was grown in the presence of SCFAs or in pH-adjusted RPMI media supplemented with 30% FBS and examined using bright field microscopy at 40× (a). Quantification of C. albicans hyphae attachment to polystyrene plates in pH-adjusted controls; pH-adjusted controls (12.5 mM and 25 mM) were normalized to the RPMI control (pH 7.00) (b). Quantification of C. albicans hyphae attachment to polystyrene plates in SCFA-treatment groups; SCFA treatment groups were normalized to their respective pH controls (c). C. albicans (CFU/mL) viability determined after 12 hours of incubation in hyphae-inducing conditions (d). The experiment was repeated three times with n = 24 for the hyphae formation and n = 12 for the CFU viability determination in each treatment group. Data is represented as means ± SEM. Statistical significance was evaluated using student’s t-test and P values (* ≤ 0.05, ** ≤ 0.01) were considered as significant.
Next, we investigated the effect of SCFAs on C. albicans hyphae formation and attachment. Our results indicate that SCFAs including acetic acid (25 mM), butyric acid (12.5 mM) and propionic acid (12.5 mM) considerably decreased C. albicans hyphae formation compared to their respective pH-adjusted RPMI controls (Fig. 4a). Further, all three SCFAs significantly inhibited C. albicans hyphae attachment to the polystyrene plates (Fig. 4c). Butyric acid (12.5 mM) inhibited 90% of C. albicans hyphae attachment, followed by propionic acid (12.5 mM) and acetic acid (25 mM) by 70 and 50%, respectively compared to their pH-adjusted controls (Fig. 4c).
To rule out if C. albicans hyphae inhibition as a result of SCFA treatment was not due to fungal cell death, C. albicans grown under hyphae-inducing conditions in the presence or absence of SCFAs were plated onto YPD agar plates to determine the CFU count. Results from this experiment suggest that viability of fungal cells was not significantly affected in all treatment conditions compared to RPMI control (pH 7.01 ± 0.01), indicating that decreased hyphae formation and attachment was not due to decrease in cell viability (Fig. 4d). This was consistent with spectrophotometer analysis of cell growth under hyphae-inducing conditions (Supplementary Fig. 2b). A similar trend of SCFAs inhibiting hyphae formation was observed with C. albicans SC5314 strain (Supplementary Fig. 3). Taken together, our results indicate that SCFAs may regulate C. albicans hyphae formation and attachment partly by altering pH levels in addition to other mechanisms.
SCFAs reduce the metabolic activity of fungal cells in C. albicans biofilm
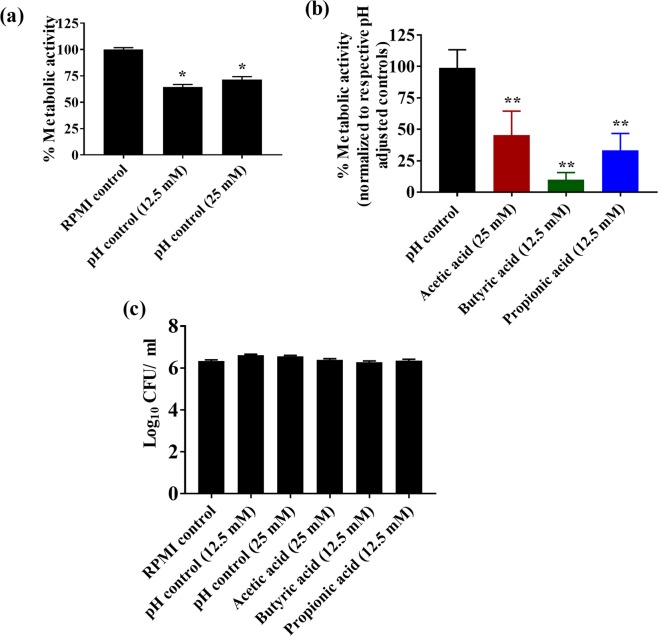
The effect of SCFAs on the metabolic activity of C. albicans (ATCC 10231 and SC5314) in the biofilm was evaluated using an MTS reduction assay. In order to determine if the change in pH has any effect on the metabolic activity of fungal cells in C. albicans biofilm, we used pH-adjusted RMPI media to determine the effect of pH on C. albicans metabolic activity in the biofilm. Results indicate that an acidic pH significantly decreases the metabolic activity of fungal cells in the C. albicans (ATCC 10231) biofilm (Fig. 5a). The metabolic activity of fungal cells in C. albicans biofilm in the pH-adjusted controls 12.5 mM (pH 5.52 ± 0.03) and 25 mM (pH 4.69 ± 0.01) were decreased by 25% compared to the RPMI control (pH 7.00) (Fig. 5a).
Figure 5.
SCFAs reduce the metabolic activity of fungal cells in the C. albicans biofilm. C. albicans ATCC 10231 was grown in the presence of SCFAs or in pH-adjusted RPMI and the metabolic activity of the fungal cells in the biofilm was assessed using MTS assay. Percent metabolic activity of fungal cells in the biofilm formed in pH-adjusted controls was determined; pH adjusted controls (12.5 mM and 25 mM) were normalized to the RPMI control (pH 7.00) (a). Percent metabolic activity of fungal cells in the biofilm formed in SCFA-treatment groups; SCFA treatments groups were normalized to their respective pH controls (b). C. albicans (CFU/mL) viability determined after 48 hours of incubation in biofilm-inducing conditions (c). All experiments were repeated three times, with n = 18 to determine the metabolic activity in the biofilm and n = 12 for the CFU viability analysis in each treatment group. Data is represented as means ± SEM. Statistical significance was evaluated using student’s t-test and P values (* ≤ 0.05, ** ≤ 0.01) were considered as significant.
Next, we assessed the effect of SCFAs on fungal cell metabolic activity in C. albicans biofilm. Results indicate that SCFAs including acetic acid (25 mM), butyric acid (12.5 mM) and propionic acid (12.5 mM) significantly decreased the metabolic activity of fungal cells in C. albicans biofilm compared to their respective pH-adjusted RPMI controls (Fig. 5b). Butyric acid at 12.5 mM decreased the metabolic activity by 90%, followed by propionic acid (12.5 mM) and acetic acid (25 mM) by 70% and 60%, respectively (Fig. 5b). Further, in order to determine if the effect of SCFAs or pH-adjusted RPMI groups on C. albicans metabolic activity was not due to fungal cell death, C. albicans grown under biofilm-inducing conditions were plated onto YPD agar plates to determine the CFU count. Results indicate that the viability of fungal cells was not significantly affected in any treatment condition compared to RPMI control (pH 7.00), suggesting that decreased metabolic activity of fungal cells in C. albicans biofilm was not due to cell viability (Fig. 5c). Growth assessed by spectrophotometer analysis of C. albicans under biofilm conditions showed comparable results (Supplementary Fig. 2c). A similar trend was observed with C. albicans SC5314 strain in the inhibition of biofilm formation by SCFAs (Supplementary Fig. 4). Collectively, results from this experiment indicate that SCFAs regulate the metabolic activity of C. albicans in the biofilm partly by altering pH and through other mechanisms.
Discussion
The gut microbiota plays a major role in the colonization resistance to enteric bacterial and fungal pathogens including C. albicans22–25. While mechanisms of colonization resistance to enteric pathogens by commensal bacteria are speculated to include immune responses, competition for nutrients, pH modulation, and synthesis of antimicrobial and antifungal compounds17,24–27, the mechanisms associated with colonization resistance to C. albicans remain poorly understood. The development of effective preventative and therapeutic treatments thus necessitates a deeper understanding of the innate mechanisms of colonization resistance to C. albicans. Commensal bacteria produce a variety of bioactive molecules; however, SCFAs have emerged as key regulators of gut homeostasis for colonization resistance against enteric pathogens14,16,24. Several preliminary studies have highlighted the antifungal potential of select SCFAs28–34. However, using in vivo concentrations, the role of SCFAs on C. albicans growth, morphogenesis and GI colonization is poorly understood. Importantly, though the inhibitory effects of SCFAs on enteric pathogens have been speculated to occur due to alterations in pH levels, this hypothesis has not been investigated in detail, particularly in C. albicans35,36.
SCFAs including acetate, butyrate and propionate are produced as a result of bacterial fermentation in the cecum20,21,37. SCFAs are abundant microbiota metabolites in the GI tract luminal microenvironment, where C. albicans colonization takes place9,10,38. Acetate is the predominant SCFA present in the cecum, followed by butyrate and propionate39–41, with the molar ratio of acetate, butyrate, and propionate typically being 60:20:20 in the human intestinal tract42,43. In mice (cecum) and humans (feces), the concentration of acetate ranges from 30.09 ± 2.09–40.66 ± 0.122 µmol/g to 69.1 ± 5.0–73.7 ± 21.5 µmol/g39–42. Butyrate concentrations in mice and humans remain relatively consistent, ranging from 18.52 ± 4.92–35.9 ± 10.2 µmol/g, although concentrations as low as 2.59 ± 0.31 µmol/g have been reported39–42. Propionate has been reported to range from 7.43 ± 0.16–25.3 ± 3.7 µmol/g39–42. In this study, we report that concentrations of SCFAs in untreated mice are in agreement with these findings39–42. Since antibiotic-induced gut dysbiosis is an important factor for the GI colonization of C. albicans44,45, we examined if antibiotic-induced alterations in the levels of SCFAs play a role in the GI colonization of this fungal pathogen. In this study, we found that cefoperazone-treated mice susceptible to C. albicans infection had significantly decreased levels of SCFAs in the cecal contents, which is in agreement with previous findings14.
Next, we examined if alterations in the levels of SCFAs as a result of antibiotic treatment actually play a role in the growth and morphogenesis of C. albicans, leading to increased GI colonization in cefoperazone-treated mice. The ability for C. albicans to cause infection is associated with its morphological switching from yeast to virulent hyphae and, therefore, inhibition of the morphological plasticity of C. albicans would substantially reduce its pathogenic potential46–52. While the reported cecal concentrations of SCFAs vary considerably based on different factors including detection methods in addition to the age and diet of mice53,54, we decided to use the average in vivo concentrations of SCFAs for the in vitro assays that correlate to published in vivo levels found in the cecum of mice and humans39–42. Our results indicate that average in vivo concentration of SCFAs (acetic acid: 25 mM, butyric acid: 12.5 mM, and propionic acid: 12.5 mM)39–42 found in the control mice exhibit significant inhibitory effects on C. albicans growth in vitro, whereas the in vivo concentrations of SCFAs found in the antibiotic-treated mice (acetic acid: 12.5 mM, butyric acid: 3.25 mM, and propionic acid: 3.25 mM)14 in the in vitro assays had only minimal effects on the growth of C. albicans. Using in vivo cecal concentrations of SCFAs found in C. albicans-resistant mice, we also found significant inhibition of the morphogenesis of C. albicans in vitro39–42. Collectively, our in vivo and in vitro results along with previous findings demonstrate that antibiotic-induced decreases in the levels of SCFAs in the cecum potentially allow C. albicans to grow and colonize in the gut14.
SCFAs inducing an acidic environment due to their dissociative properties55 may be a factor in their inhibitory effects on C. albicans growth and morphogenesis. Therefore, we investigated if inhibitory effects of SCFAs on C. albicans growth and morphogenesis are due to changes in environmental pH levels. Our results along with previous findings indicate that C. albicans can actively neutralize an environmental pH and a SCFA-induced change in pH levels does not considerably affect C. albicans growth2,56. Previous studies also indicate that under neutral conditions, SCFAs exist in the anion form and do not exhibit antimicrobial activity, which is in agreement with the lack of growth inhibition observed in RPMI media containing SCFAs that was buffered to pH 7.0057–59. Taken together, our results along with previous findings2,56–59 suggest that (i) SCFA-mediated inhibition of C. albicans growth was not likely due to an alteration in environmental pH levels and (ii) the undissociated acidic form of SCFAs is required for inhibitory activity.
Next, we examined the effect of pH on C. albicans morphogenesis. We found that acidic pH significantly inhibits C. albicans morphogenesis, which is in agreement with reports that highlight the dynamic of acidic conditions impeding C. albicans morphogenesis, while neutral and alkaline conditions induce morphogenesis47,56,60–62. SCFA-induced inhibition of C. albicans morphogenesis may be due in part to the generation of an acidic environment; however, we report a significant difference in the morphogenic inhibition of SCFAs when compared to pH-adjusted RPMI control groups. Taken together, these results indicate that the inhibitory effects of SCFAs on C. albicans morphogenesis are not limited to alteration in environmental pH levels, and other unknown mechanisms are involved. Although SCFA-induced fungal cell death was not a potential inhibitory mechanism in the hyphae and biofilm formation, it remains unclear if the fungistatic effects of SCFAs may play a role in inhibiting hyphae and biofilm formation as a function of general growth arrest mechanisms30. Therefore, future studies to understand the mechanisms behind the SCFA-mediated effects on C. albicans growth and morphogensis are important to understand its pathogenesis and to develop novel therapeutic approaches.
Diverse microbes in the gut possess the ability to produce SCFAs. Among these, Bacteroides, Ruminococcaceae, Lachnospiraceae, Clostridia, Prevotella, Oscillospira and Verrucomicrobia (Akkermansia muciniphila), and Faecalibacteria are most commonly associated with the production of SCFAs21,37. Fan et al. observed that bacteroides including Blautia producta and Bacteroides thetaiotaomicron directly affect C. albicans colonization through SCFAs2. Our recent studies found that the probiotics interventions increase the SCFAs production by modulating human and mice gut microbiome63. The role of microbial-derived SCFAs in contributing to the host’s defense mechanisms against enteric pathogens has been previously reported,20,37 highlighting the importance of the ability for SCFAs to inhibit pathogenic growth by maintaining the pH gradient in the colon, among other factors including immune cell homeostasis. Therefore, future studies including characterization of probiotic or commensal bacteria to enhance the abundance of SCFA levels may form a novel approach to prevent and treat C. albicans colonization and subsequent pathogenesis.
Antibiotic treatment significantly alters SCFA levels in the gut; however, the composition and concentration of other critical gut metabolites including bile acids are also affected13,14,17,18. Recently, we have shown that bile acids play an important role in controlling C. albicans growth and morphogenesis17,18. We previously demonstrated that treatment with in vivo concentrations of the primary conjugated bile acid taurocholic acid (TCA) (0.0125%) promotes C. albicans growth and morphogenesis, whereas treatment with in vivo concentrations of secondary bile acids deoxycholic acid (DCA [0.05%]) and lithocholic acid (LCA [0.01%]) inhibit C. albicans growth and morphogenesis in vitro17,18. Therefore, understanding the role of various gut metabolites in the GI colonization of C. albicans will expand our knowledge on C. albicans pathogenesis. In addition, this may form a strong foundation for efforts to use commensal bacteria to modulate gut metabolites to prevent and treat C. albicans infections.
Materials and Methods
Strains and reagents
Candida albicans ATCC 10231 was purchased from ATCC. Candida albicans SC5314 was kindly provided by Dr. Andrew Koh from University of Texas Southwestern Medical Center2. Media used in this study included RPMI 1640 (Gibco, MA), MOPS (Sigma, MO), YPD Agar (BD Biosciences, CA), and Fetal Bovine Serum (Atlanta Biologicals, GA). Short-chain fatty acids (acetic acid, butyric acid, and propionic acid) were purchased from Sigma Aldrich (MO). Mice were purchased from The Jackson Laboratory (ME). Cefoperazone was purchased from Sigma Aldrich (MO). Other materials were purchased as indicated: mouse oral gavages (Kent Scientific, MA), vancomycin (Alfa Aesar, MA), gentamicin (Acros Organics, NJ), paraformaldehyde (Alfa Aesar, MA), and glycerol (DOT Scientific, MI).
Cefoperazone treatment and C. albicans infection in mice
Female C57BL/6J mice (5–6 mice per group) were supplemented with sterile water with or without cefoperazone (0.5 mg/mL)64. Cefoperazone water was replaced every two days. After 7 days of antibiotic treatment, mice were either sacrificed for SCFA metabolite analysis or infected with C. albicans SC5314 via oral gavage at a dose of 4.25 × 108 CFU per mice31. After 5 days of infection, fecal samples were collected from individual mice to determine the fungal load. Briefly, fecal pellets were weighed and homogenized in PBS and the supernatant was plated onto YPD agar plates containing 0.1 mg/mL gentamicin and 0.010 mg/mL vancomycin2. After 24 hours of incubation, the colonies were counted and the fungal load (CFU/gram) was determined for individual mice. The Institutional Animal Care and Use Committee (IACUC) at Midwestern University approved this study under MWU IACUC Protocol #2894. The MWU animal care policies follow the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals and the policies laid out in the Animal Welfare Act (AWA). Trained animal technicians performed animal husbandry in our IACUC monitored animal care facility in the Foothills Science Building.
Quantifying SCFAs levels in the cecal content
Equal amount of snap frozen cecal content was weighed, dissolved in HPLC grade water and supernatants were collected after centrifugation (12,000 g, 10 min), while processing on ice. Concentrations of SCFAs (acetate, propionate and butyrate) were determined using a HPLC system (Waters-2695 Alliance HPLC system, Waters Corporation, Milford, MA, USA) equipped with Aminex HPX-87H column (Bio-Rad Laboratories, Hercules, CA) and DAD detector (210 nm), and eluting with H2SO4 (0.005 N) mobile phase with a flow rate of 0.6 ml/min at 25 °C, as described elsewhere63,65,66.
Growth assay
The growth of C. albicans ATCC 10231 and SC 5314 was measured as previously described,17 using pH-adjusted controls titrated with HCl as described below (Table 1).
pH-adjusted control RPMI media for in vitro assays
pH-adjusted controls were used for all in vitro experiments involving SCFAs. Briefly, pH was measured using a Fisherbrand Accumet AE150 pH meter (Thermo Fisher, MA). The pH meter was calibrated each time before use using Orion calibration buffers (Thermo Fisher, MA). pH was adjusted for short-chain fatty acid treatments using acetic acid as the reference point since acetic, butyric, and propionic acid share similar experimental pKas (4.76, 4.83, and 4.87, respectively55), with acetic acid having the lowest pKa and thus most potent effect on pH via dissociation55. pH was adjusted using HCl for in vitro assays as described in Table 1. For growth and biofilm assays, RPMI media was adjusted with HCl to match the pH values of SCFAs. Similarly, for hyphae and germ tube assays, RPMI media supplemented with 30% FBS was adjusted with HCl as described in Table 1.
Biofilm assay
C. albicans (ATCC 10231 and SC5314) with an inoculum size of 1.7 × 106–3.2 × 106 CFU/mL was used to form the biofilm and the metabolic activity of fungal cells in the biofilm was carried out using MTS assay as previously described17. The effect of SCFAs on C. albicans cell viability was assessed by incubating fungal cells with the indicated concentration of SCFAs at 37 °C for 48 hours in 4-mL tubes. Appropriate pH-adjusted RPMI control media was used as described in Table 1. After 48 hours of incubation, the cell suspension was plated onto YPD agar plates and the CFUs were counted to determine the effect of SCFAs on C. albicans cell viability.
Germ tube and hyphae assays
The effect of SCFAs on C. albicans germ tube and hyphae formation was assessed as previously described17. For the germ tube assay, C. albicans (ATCC 10231) with an inoculum size of 3.48 × 106 CFU/mL was incubated with or without SCFAs for 2 hours and the percentage of germ tubes formed were quantified at 20X magnification17. Similarly, C. albicans ATCC 10231 and SC 5314 strains (3.34 × 107–4.60 × 108 CFU/mL) were incubated with or without SCFAs and the C. albicans hyphae formation and attachment were determined using bright field microscopy and crystal violet assay as described before17. The crystal violet assay was adopted using a protocol defined by Abe et al.67,68 Further, the effect of SCFAs on C. albicans cell viability under germ tube and hyphae assay conditions was assessed by incubating fungal cells with the indicated concentration of SCFAs at 37 °C for 48 hours in 4-mL tubes. After 48 hours of incubation, cell suspensions were plated onto YPD agar plates and the CFUs were counted to determine the effect of SCFAs on C. albicans cell viability. Appropriate pH-adjusted RPMI control media was used as described in Table 1.
Statistical analyses
The Student t-test was utilized for statistical analyses using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA) with p-values of (* ≤ 0.05, ** ≤ 0.01) being considered significant.
Supplementary information
Acknowledgements
We thank Dr. Andrew Koh from University of Texas Southwestern Medical Center for providing Candida albicans SC5314 strain. This work was supported by Midwestern University College of Veterinary Medicine Start-up Fund to Dr. Thangamani.
Author Contributions
S.T.: Conceived the idea, help to design experiments, analyze and interpret data, and overall supervision of the project; J.G.: Carried out in vitro experiments and analyzed data; S.W. and H.Y.: Carried out S.C.F. As analysis; J.G., S.W., H.Y., T.H. and S.T.: Reviewed data and wrote the manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-45467-7.
References
- 1.Neville B. Anne, d'Enfert Christophe, Bougnoux Marie-Elisabeth. Candida albicanscommensalism in the gastrointestinal tract. FEMS Yeast Research. 2015;15(7):fov081. doi: 10.1093/femsyr/fov081. [DOI] [PubMed] [Google Scholar]
- 2.Fan D, et al. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nature medicine. 2015;21:808–814. doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Low CY, Rotstein C. Emerging fungal infections in immunocompromised patients. F1000 Med Rep. 2011;3:14. doi: 10.3410/M3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huppert M, Macpherson DA, Cazin J. Pathogenesis of Candida albicans infection following antibiotic therapy. I. The effect of antibiotics on the growth of Candida albicans. J Bacteriol. 1953;65:171–176. doi: 10.1128/jb.65.2.171-176.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause R, et al. Role of Candida in antibiotic-associated diarrhea. J Infect Dis. 2001;184:1065–1069. doi: 10.1086/323550. [DOI] [PubMed] [Google Scholar]
- 6.Guastalegname M, Russo A, Falcone M, Giuliano S, Venditti M. Candidemia subsequent to severe infection due to Clostridium difficile: is there a link? Clin Infect Dis. 2013;57:772–774. doi: 10.1093/cid/cit362. [DOI] [PubMed] [Google Scholar]
- 7.Samonis G, et al. Prospective evaluation of effects of broad-spectrum antibiotics on gastrointestinal yeast colonization of humans. Antimicrob Agents Chemother. 1993;37:51–53. doi: 10.1128/AAC.37.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy MJ, Volz PA, Edwards CA, Yancey RJ. Mechanisms of association of Candida albicans with intestinal mucosa. J Med Microbiol. 1987;24:333–341. doi: 10.1099/00222615-24-4-333. [DOI] [PubMed] [Google Scholar]
- 9.Miranda LN, et al. Candida colonisation as a source for candidaemia. J Hosp Infect. 2009;72:9–16. doi: 10.1016/j.jhin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Nucci M, Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis. 2001;33:1959–1967. doi: 10.1086/323759. [DOI] [PubMed] [Google Scholar]
- 11.Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5:161–169. doi: 10.4161/viru.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole GT, Halawa AA, Anaissie EJ. The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside. Clin Infect Dis. 1996;22(Suppl 2):S73–88. doi: 10.1093/clinids/22.Supplement_2.S73. [DOI] [PubMed] [Google Scholar]
- 13.Theriot, C. M., Bowman, A. A. & Young, V. B. Antibiotic-Induced Alterations of the Gut Microbiota Alter Secondary Bile Acid Production and Allow for Clostridium difficile Spore Germination and Outgrowth in the Large Intestine. mSphere1, 10.1128/mSphere.00045-15 (2016). [DOI] [PMC free article] [PubMed]
- 14.Theriot CM, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nature communications. 2014;5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young VB, Schmidt TM. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol. 2004;42:1203–1206. doi: 10.1128/JCM.42.3.1203-1206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seekatz Anna M., Theriot Casey M., Rao Krishna, Chang Yu-Ming, Freeman Alison E., Kao John Y., Young Vincent B. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe. 2018;53:64–73. doi: 10.1016/j.anaerobe.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guinan, J., Villa, P. & Thangamani, S. Secondary bile acids inhibit Candida albicans growth and morphogenesis. Pathogens and disease76, 10.1093/femspd/fty038 (2018). [DOI] [PubMed]
- 18.Guinan, J. & Thangamani, S. Antibiotic-induced alterations in taurocholic acid levels promote gastrointestinal colonization of Candida albicans. FEMS Microbiology Letters, fny196-fny196, 10.1093/femsle/fny196 (2018). [DOI] [PubMed]
- 19.Topping DL. Short-chain fatty acids produced by intestinal bacteria. Asia Pacific journal of clinical nutrition. 1996;5:15–19. [PubMed] [Google Scholar]
- 20.den Besten G, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason KL, et al. Interplay between the Gastric Bacterial Microbiota and Candida albicans during Postantibiotic Recolonization and Gastritis. Infection and Immunity. 2012;80:150–158. doi: 10.1128/iai.05162-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason KL, et al. Candida albicans and Bacterial Microbiota Interactions in the Cecum during Recolonization following Broad-Spectrum Antibiotic Therapy. Infection and Immunity. 2012;80:3371–3380. doi: 10.1128/IAI.00449-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawley TD, Walker AW. Intestinal colonization resistance. Immunology. 2013;138:1–11. doi: 10.1111/j.1365-2567.2012.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nature reviews. Immunology. 2013;13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS microbiology reviews. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Winston JA, Theriot CM. Impact of microbial derived secondary bile acids on colonization resistance against Clostridium difficile in the gastrointestinal tract. Anaerobe. 2016;41:44–50. doi: 10.1016/j.anaerobe.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noverr MC, Huffnagle GB. Regulation of Candida albicans Morphogenesis by Fatty Acid Metabolites. Infection and Immunity. 2004;72:6206–6210. doi: 10.1128/IAI.72.11.6206-6210.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoberg KA, Cihlar RL, Calderone RA. Inhibitory effect of cerulenin and sodium butyrate on germination of Candida albicans. Antimicrob Agents Chemother. 1983;24:401–408. doi: 10.1128/AAC.24.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cottier F, Tan AS, Xu X, Wang Y, Pavelka N. MIG1 Regulates Resistance of Candida albicans against the Fungistatic Effect of Weak Organic Acids. Eukaryot Cell. 2015;14:1054–1061. doi: 10.1128/EC.00129-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan D, et al. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nature medicine. 2015;21:808–814. doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lastauskiene E, Zinkeviciene A, Girkontaite I, Kaunietis A, Kvedariene V. Formic acid and acetic acid induce a programmed cell death in pathogenic Candida species. Current microbiology. 2014;69:303–310. doi: 10.1007/s00284-014-0585-9. [DOI] [PubMed] [Google Scholar]
- 33.Yun JiEun, Lee Dong Gun. A novel fungal killing mechanism of propionic acid. FEMS Yeast Research. 2016;16(7):fow089. doi: 10.1093/femsyr/fow089. [DOI] [PubMed] [Google Scholar]
- 34.Cottier F, et al. The Transcriptional Stress Response of Candida albicans to Weak Organic Acids. G3: Genes|Genomes|Genetics. 2015;5:497–505. doi: 10.1534/g3.114.015941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricke SC. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poultry science. 2003;82:632–639. doi: 10.1093/ps/82.4.632. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, O’Riordan MXD. Regulation of Bacterial Pathogenesis by Intestinal Short-Chain Fatty Acids. Advances in applied microbiology. 2013;85:93–118. doi: 10.1016/B978-0-12-407672-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 38.Koh AY, Köhler JR, Coggshall KT, Van Rooijen N, Pier GB. Mucosal Damage and Neutropenia Are Required for Candida albicans Dissemination. PLoS Pathogens. 2008;4:e35. doi: 10.1371/journal.ppat.0040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan X-d, Chen F-q, Wu T-x, Tang H-g, Zhao Z-y. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. Journal of Zhejiang University. Science. B. 2009;10:258–263. doi: 10.1631/jzus.B0820261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoverstad T, Midtvedt T. Short-chain fatty acids in germfree mice and rats. The Journal of nutrition. 1986;116:1772–1776. doi: 10.1093/jn/116.9.1772. [DOI] [PubMed] [Google Scholar]
- 41.Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science (New York, N.Y.) 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cummings JH, Hill MJ, Bone ES, Branch WJ, Jenkins DJA. The effect of meat protein and dietary fiber on colonic function and metabolism II. Bacterial metabolites in feces and urine. The American Journal of Clinical Nutrition. 1979;32:2094–2101. doi: 10.1093/ajcn/32.10.2094. [DOI] [PubMed] [Google Scholar]
- 43.Trompette A, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature medicine. 2014;20:159. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 44.Clark JD. Influence of antibiotics or certain intestinal bacteria on orally administered Candida albicans in germ-free and conventional mice. Infect Immun. 1971;4:731–737. doi: 10.1128/iai.4.6.731-737.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koh AY. Murine models of Candida gastrointestinal colonization and dissemination. Eukaryot Cell. 2013;12:1416–1422. doi: 10.1128/EC.00196-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pande K, Chen C, Noble SM. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat Genet. 2013;45:1088–1091. doi: 10.1038/ng.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo HJ, et al. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/S0092-8674(00)80358-X. [DOI] [PubMed] [Google Scholar]
- 48.Carlisle PL, et al. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc Natl Acad Sci USA. 2009;106:599–604. doi: 10.1073/pnas.0804061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mendelsohn, S., Pinsky, M., Weissman, Z. & Kornitzer, D. Regulation of the Candida albicans Hypha-Inducing Transcription Factor Ume6 by the CDK1 Cyclins Cln3 and Hgc1. mSphere2, 10.1128/mSphere.00248-16 (2017). [DOI] [PMC free article] [PubMed]
- 50.Bendel CM, et al. Effects of Alteration of the Candida albicans Gene INT1 on Cecal Colonization in Orally Innoculated Mice. Pediatric Research. 1999;45:156A. doi: 10.1203/00006450-199904020-00929. [DOI] [Google Scholar]
- 51.Gale CA, et al. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science (New York, N.Y.) 1998;279:1355–1358. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- 52.Vila T, et al. Targeting Candida albicans filamentation for antifungal drug development. Virulence. 2017;8:150–158. doi: 10.1080/21505594.2016.1197444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai M, Chandrasekera PC, Barnard ND. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutrition &Amp. Diabetes. 2014;4:e135. doi: 10.1038/nutd.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scalbert A, et al. Mass-spectrometry-based metabolomics: limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics. 2009;5:435–458. doi: 10.1007/s11306-009-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao L, Han J, Tao FM. Correlations and predictions of carboxylic acid pKa values using intermolecular structure and properties of hydrogen-bonded complexes. The journal of physical chemistry. A. 2008;112:775–782. doi: 10.1021/jp710291c. [DOI] [PubMed] [Google Scholar]
- 56.Vylkova S, et al. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio. 2011;2:e00055–00011. doi: 10.1128/mBio.00055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai SK, et al. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. Journal of virology. 2009;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aldunate M, et al. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Frontiers in physiology. 2015;6:164–164. doi: 10.3389/fphys.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lourenco A, Pedro NA, Salazar SB, Mira NP. Effect of Acetic Acid and Lactic Acid at Low pH in Growth and Azole Resistance of Candida albicans and Candida glabrata. Front Microbiol. 2018;9:3265. doi: 10.3389/fmicb.2018.03265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vylkova S. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog. 2017;13:e1006149. doi: 10.1371/journal.ppat.1006149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buffo J, Herman MA, Soll DR. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia. 1984;85:21–30. doi: 10.1007/BF00436698. [DOI] [PubMed] [Google Scholar]
- 62.Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiology and molecular biology reviews: MMBR. 2007;71:348–376. doi: 10.1128/mmbr.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagpal R, et al. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci Rep. 2018;8:12649. doi: 10.1038/s41598-018-30114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Theriot CM, et al. Cefoperazone-treated mice as an experimental platform to assess differential virulence of Clostridium difficile strains. Gut Microbes. 2011;2:326–334. doi: 10.4161/gmic.19142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagpal R, et al. Comparative Microbiome Signatures and Short-Chain Fatty Acids in Mouse, Rat, Non-human Primate, and Human Feces. Front Microbiol. 2018;9:2897. doi: 10.3389/fmicb.2018.02897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagpal R, et al. Gut Microbiome Composition in Non-human Primates Consuming a Western or Mediterranean Diet. Front Nutr. 2018;5:28. doi: 10.3389/fnut.2018.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abe S, Satoh T, Tokuda Y, Tansho S, Yamaguchi H. A rapid colorimetric assay for determination of leukocyte-mediated inhibition of mycelial growth of Candida albicans. Microbiol Immunol. 1994;38:385–388. doi: 10.1111/j.1348-0421.1994.tb01795.x. [DOI] [PubMed] [Google Scholar]
- 68.Wakabayashi H, Abe S, Teraguchi S, Hayasawa H, Yamaguchi H. Inhibition of Hyphal Growth of Azole-Resistant Strains of Candida albicans by Triazole Antifungal Agents in the Presence of Lactoferrin-Related Compounds. Antimicrobial Agents and Chemotherapy. 1998;42:1587–1591. doi: 10.1128/AAC.42.7.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.