Abstract
We report a case of a 73-year-old man diagnosed with pulmonary pleomorphic carcinoma who showed profound durable response after a single treatment with pembrolizumab. The patient underwent a diagnostic workup in our hospital due to a hoarseness of voice. Chest computed tomography revealed a massive pulmonary tumor in the left upper lobe and multiple nodules in the both lung fields. Histological examination of a transbronchial lung biopsy specimen revealed pulmonary pleomorphic carcinoma. First-line treatment with pembrolizumab was discontinued after a single administration due to treatment-related pneumonitis. However, durable response has been observed over 17 months to date.
Keywords: Durable response, Non-small cell lung cancers, Pembrolizumab, Pulmonary pleomorphic carcinoma, Single administration, Treatment-related pneumonitis
1. Introduction
Pulmonary pleomorphic carcinomas are rare, accounting only for 0.1%–0.4% of all lung carcinoma cases [1,2]. Pulmonary pleomorphic carcinomas commonly cause distant metastases, and are refractory to systemic chemotherapy, resulting in poor prognosis [[1], [2], [3], [4]].
Immune checkpoint inhibitors such as programmed cell death protein-1 (PD-1) inhibitors prevent the downregulation of T-cell effector functions, contributing to tumor cell apoptosis [5,6]. In Japan, two anti-PD-1 blocking monoclonal antibodies, viz., of pembrolizumab (Keytruda Merck Sharp & Dohme Corp) and nivolumab (Opdivo Bristol-Myers Squibb) have been approved for the treatment of non-small-cell lung cancers (NSCLCs).
We report herein the successful treatment of a patient with pulmonary pleomorphic carcinoma via a single pembrolizumab treatment.
2. Case report
In early August 2017, a 73-year-old man who was a current smoker (1 pack a day for 53 years) was referred to our hospital because of continuously elevated white blood cell count for 3 months. Bone marrow aspiration was performed, but yielded inconclusive findings. By mid-August, he developed hoarseness of voice due to left vocal cord dysfunction. A chest roentgenogram showed a massive tumor in the left upper lung field and multiple pulmonary nodules in both lung fields (Fig. 1). Chest computed tomography (CT) revealed a massive 10-cm pulmonary tumor in the left upper lobe and multiple nodules in the both lung fields (Fig. 2). Intense fluorodeoxyglucose (FDG) accumulation in the pulmonary mass of the left upper lobe and the multiple lung nodules and left supraclavicular, mediastinal, hilar lymph nodes, and right adrenal gland was noted on 18-fluorine fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) imaging.
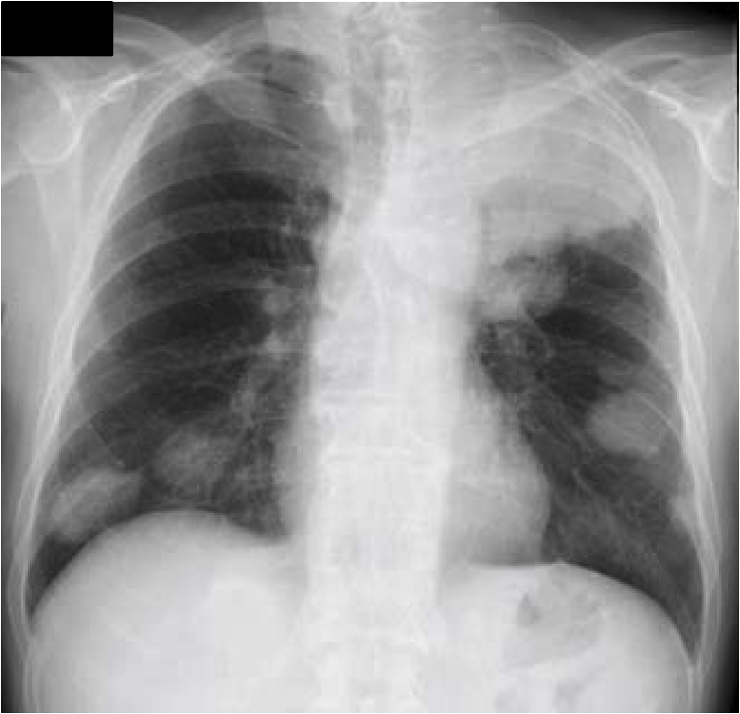
Fig. 1.
Chest roentgenogram at the first presentation showing massive tumor in the left upper lung field and multiple pulmonary nodules in both lung fields.
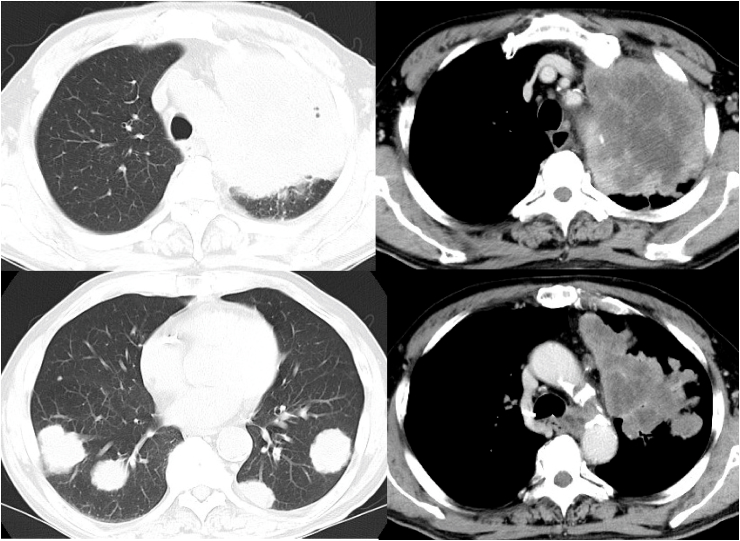
Fig. 2.
Computed tomography at the first presentation showing a 10-cm massive pulmonary tumor in the left upper lobe and multiple nodules in both lung fields.
The white blood cell count was 20.1 × 109/L with 72.0% neutrophils, 3.5% lymphocytes, 7.5% monocytes, and 17.0% eosinophils. The C-reactive protein was elevated to 7.39 mg/dL (normal range, 0–0.3 mg/dL). Further, the levels of carcinoembryonic antigen and cytokeratin 19 fragment were elevated to 6.4 ng/mL (normal range, 0–5.0 ng/mL) and 4.4 ng/mL (normal range, 0–3.5 ng/mL), respectively.
Histological examination of transbronchial lung biopsy specimens obtained from the left pulmonary mass revealed pleomorphic carcinoma of the lung (Fig. 3). Accordingly, the patient was diagnosed with pulmonary pleomorphic carcinoma, clinical T4N3M1c (PUL, ADR), stage IV (the 8th TNM classification of lung cancer). The tumor proportion score of PD-L1 was 100% (Dako 22C3 IHC platform).
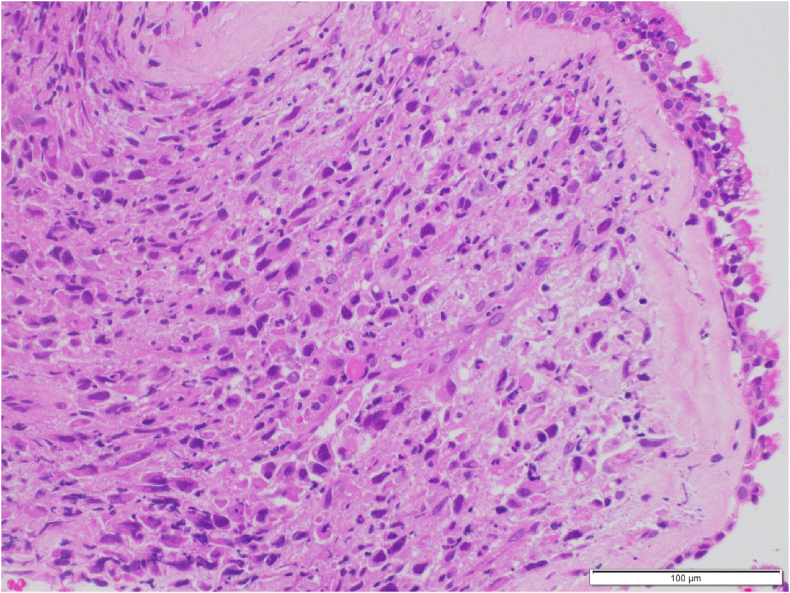
Fig. 3.
Histological examination of transbronchial lung biopsy specimens obtained from the left pulmonary mass revealing proliferation of polyhedral and spindle atypical cells.
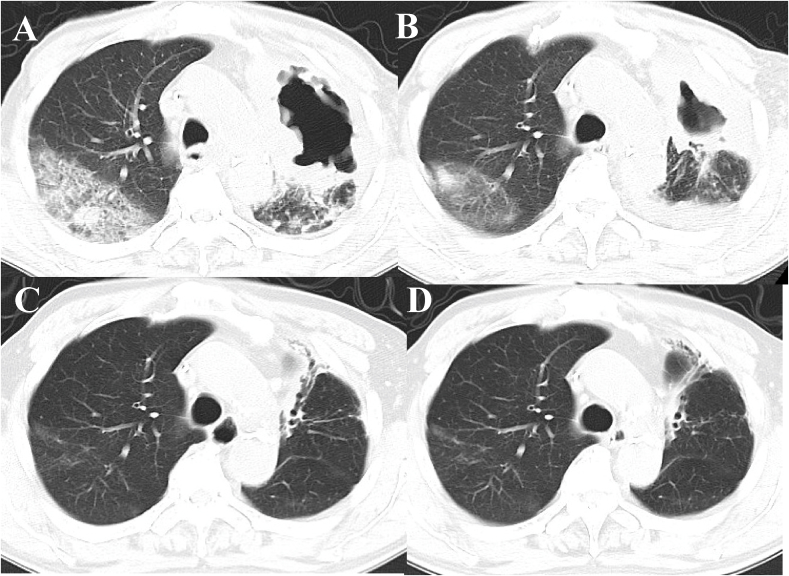
Pembrolizumab (200mg, every 3 weeks) was administered as first-line chemotherapy in September 2017. However, after 14 days of the administration, chest CT images revealed the appearance of a large cavity in the left upper lobe tumor and interstitial lung disease in the right upper lobe (Fig. 4A). The treatment-related pneumonitis in the right lung field was categorized as grade 1 according to the Common Terminology Criteria for Adverse Eents, version 4.0 and therefore the patient did not receive steroid therapy. As the cavity would expand, thoracic empyema was more likely to occur. Therefore, we stopped further pembrolizumab treatment. Follow-up chest CT images revealed gradual tumor shrinkage (Fig. 4). Over 17 months after the discontinuation of pembrolizumab, the primary lung cancer lesion and the lung and adrenal gland metastasis decreased in size without additional treatment.
Fig. 4.
Chest CT images obtained after 2 weeks (A), 2 months (B), 12 months (C), and 17 months (D) in a patient who received single pembrolizumab treatment.
3. Discussion
Pulmonary pleomorphic carcinomas is a rare, aggressive disease characterized by a high rate of early distant metastasis, late recognition, and poor prognosis [[1], [2], [3], [4]]. The response rate to chemotherapy regimens commonly used for NSCLCs is in the range of 0–17% [3,7] and the median survival reported for patients with pulmonary pleomorphic carcinomas was 5–10 months [1,3,7].
The PD-1 receptor is an immune checkpoint inhibitor expressed on activated T cells. Upon binding to its ligands (PD-L1 and PD-L2), which are expressed by tumor cells, stromal cells, or both, the PD-1 receptor inhibits T cell function [8], leading to maintained tolerance to tumor cells. Meanwhile, the blockade of this pathway by the anti-PD-1 antibodies may prevent this downregulation and allow T-cells to maintain their antitumor property and capability to mediate tumor cell death [5,6]. In total, 90.2% of pulmonary pleomorphic carcinomas show high PD-L1 expression [9], and their aggressive behavior could be related to PD-L1-mediated immune escape [10].
There are two anti-PD-1 monoclonal antibodies approved for the treatment of NSCLCs, namely, pembrolizumab and nivolumab. The difference in clinical effects between pembrolizumab and nivolumab has been unclear, and both drugs are considered to be clinically comparable. In the KEYNOTE-001 trial, pembrolizumab showed antitumor activity in patients with advanced NSCLCs, and a significant association was found between PD-L1 expression and pembrolizumab efficacy [11]. Recent case reports also showed a good response at the early course of pembrolizumab (e.g., 3 courses) in patients with pulmonary pleomorphic carcinoma [12,13].
Tumor mutation burden (TMB) is defined as the total number of somatic mutations in the tumor exome [14] and Carbone et al. reported that there was no significant association between TMB and PD-L1 expression [15]. Sarcomatoid tumors, which include pleomorphic carcinoma [16], have a higher number of somatic mutations [17,18]. Rizvi et al. showed that in NSCLCs treated with pembrolizumab, an elevated TMB is strongly associated with clinical efficacy [19]. Schrock et al. reported the case of a durable response to pembrolizumab in a patient with pulmonary pleomorphic carcinoma. This patient had a high TMB as well as positive PD-L1 staining with a tumor proportion score of 80%, and they suggested that pembrolizumab may be beneficial in this population [17].
Drug-induced pneumonitis is considered to be one of the important immune-related adverse events (irAEs) because it is potentially life threatening. In the KEYNOTE-024 trial, 9 (5.8%) of the 154 patients with advanced NSCLCs who received pembrolizumab developed pneumonitis, and 4 of them (2.6%) was grade 3 or higher [20]. Our case had Grade 1 pneumonitis as irAEs, and we discontinued pembrolizumab therapy after a single injection.
There have been several studies about the development of irAEs and the clinical efficacy of the anti-PD-1 antibody nivolumab in patients with NSCLCs [[21], [22], [23], [24]]. In their retrospective study of patients with NSCLCs, Haratani et al. showed that patients who developed irAEs at 6 weeks after treatment with nivolumab had favorable efficacy compared with those without irAEs (overall response rate (ORR): 52% versus 28%; progression-free survival: 9.2 months versus 4.8 months) [21]. Teraoka et al. reported that an early onset of irAEs 2 weeks after commencement of nivolumab treatment was associated with a durable response and clinical benefit to nivolumab in patients with NSCLCs [23]. Tachihara et al. reported that patients with partial response (PR) prior to the onset of irAEs tended to show a durable response after the discontinuation of anti-PD-1 antibodies, whereas most cases with stable disease at 8–12 weeks did not show PR, even if the antibodies were continued afterward [24]. A single injection of pembrolizumab caused pneumonitis as irAEs in our case, but patient had profound durable response after an early onset of pneumonitis and marked tumor reductions at the time of the onset of pneumonitis.
Anti-PD-1 antibodies have the potential for long-term disease control through the activation of the patients' own immune system against NSCLCs [11,[25], [26], [27], [28], [29]]. Recently, Osa et al. reported that prolonged nivolumab binding to T cells was detected more than 20 weeks after the last infusion, showing the possibility of lasting therapeutic efficacy [30]. Clinically, Gettinger et al. reported that 18 patients with NSCLCs who received nivolumab treatment discontinued nivolumab for reasons other than progressive disease and nine patients (50%) had treatment response lasting > 9 months after their last dose [28]. However, a certain duration of anti-PD-1 antibody treatment may be required to achieve long-term disease control. Tachihara et al. reported that even if there was a good antitumor effect initially, most patients developed aggravated disease if treatment was stopped within 8 weeks [24]. In the present case, a single treatment of pembrolizumab caused our ongoing durable response of 17 months. Further studies are required to better understand the mechanism by which patients achieve durable response to anti-PD-1 antibodies after discontinuing them.
Conflicts of interest
The authors declare that they have no conflicts of interest (COI).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2019.100879.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fishback N.F., Travis W.D., Moran C.A., Guinee D.G., Jr., MaCarthy W.F., Koss M.N. Pleomorphic (spindle/giant cell) carcinoma of the lung A clinicopathologic correlation of 78 cases. Cancer. 1994;73:2936–2945. doi: 10.1002/1097-0142(19940615)73:12<2936::aid-cncr2820731210>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Chang Y.L., Lee Y.C., Shih J.Y., Wu C.T. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicoathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer. 2001;34:91–97. doi: 10.1016/s0169-5002(01)00224-0. [DOI] [PubMed] [Google Scholar]
- 3.Bae H.M., Min H.S., Lee S.H. Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer. 2007;58:112–115. doi: 10.1016/j.lungcan.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Mochizuki T., Ishii G., Nagai K. Pleomorphic carcinoma of the lung: clinicopathologic characteristics of 70 cases. Am. J. Surg. Pathol. 2008;32:1727–1735. doi: 10.1097/PAS.0b013e3181804302. [DOI] [PubMed] [Google Scholar]
- 5.Ribas A. Tumor immunotherapy directed at PD-1. N. Engl. J. Med. 2012;366:2517–2519. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 6.Topalian S.L., Hodi F.S., Brahmer J.R. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong J.Y., Choi M.K., Uhm J.E. The role of palliative chemotherapy for advanced pulmonary pleomorphic carcinoma. Med. Oncol. 2009;26:287–291. doi: 10.1007/s12032-008-9117-4. [DOI] [PubMed] [Google Scholar]
- 8.Swart M., Verbrugge I., Beltman J.B. Combination approaches with immune-checkpoint blockade in cancer therapy. Front. Oncol. 2016;6:223. doi: 10.3389/fonc.2016.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S., Kim M.Y., Koh J. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: comparison of sarcomatous and carcinomatous areas. Eur. J. Cancer. 2015;51:2698–2707. doi: 10.1016/j.ejca.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Chang Y.L., Yang C.Y., Lin M.W., Wu C.T., Yang P.C. High co-expression of PD-L1 and HIF-1α correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur. J. Cancer. 2016;60:125–135. doi: 10.1016/j.ejca.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Garon E.B., Rizvi N.A., Hui R. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 12.Ikematsu Y., Yoneshima Y., Ijichi K. Marked response to pembrolizumab in a patient with pulmonary pleomorphic carcinoma highly positive for PD-L1. Lung Cancer. 2017;112:230–231. doi: 10.1016/j.lungcan.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto Y., Miura T., Horiuchi H., Usui K. The successful treatment of pulmonary pleomorphic carcinoma with pembrolizumab: a case report. Case Rep. Oncol. 2017;10:752–757. doi: 10.1159/000479552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarchoan M., Hopkins A., Jaffee E.M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carbone D.P., Reck M., Paz-Ares L. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Travis W.D., Brambilla E., Nicholson A.G. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 17.Schrock A.B., Li S.D., Frampton G.M. Pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J. Thorac. Oncol. 2017;12:932–942. doi: 10.1016/j.jtho.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Fallet V., Saffrory R., Girard N. High-throughput somatic mutation profiling in pulmonary sarcomatoid carcinomas using the LungCartaTM Panel: exploring therapeutic targets. Ann. Oncol. 2015;26:1748–1753. doi: 10.1093/annonc/mdv232. [DOI] [PubMed] [Google Scholar]
- 19.Rizvi N.A., Hellmann M.D., Snyder A. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reck M., Rodríguez-Abreu D., Robinson A.G. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 21.Haratani K., Hayashi H., Chiba Y. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato K., Akamtsu H., Murakami E. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–74. doi: 10.1016/j.lungcan.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Teraoka S., Fujimoto D., Morimoto T. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J. Thorac. Oncol. 2017;12:1798–1805. doi: 10.1016/j.jtho.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Tachihara M., Negoro S., Inoue T. Efficacy of anti-PD-1/PD-L1 antibodies after discontinuation due to adverse events in non-small cell lung cancer patients (HANSHIN 0316) BMC Cancer. 2018;18:946. doi: 10.1186/s12885-018-4819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gettinger S., Horn L., Jackman D. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J. Clin. Oncol. 2018;36:1675–1684. doi: 10.1200/JCO.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 26.Brahmer J., Reckamp K.L., Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borghaei H., Paz-Ares L., Horn L. Nivolumab versus docetaxel in advanced nonsquamus non-small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gettinger S.N., Horn L., Gandhi L. Overall survival and long-term safety of nivolumab (Anti-Programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbst R.S., Baas P., Kim D.W. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer. (KEYNOTE-010): a randomized controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 30.Osa A., Uenami T., Koyama S. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight. 2018;3 doi: 10.1172/jci.insight.59125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.