Abstract
Background
Bilirubin, a by-product of haem catabolism, possesses potent endogenous antioxidant and platelet inhibitory properties. These properties may be useful in inhibiting inappropriate platelet activation and ROS production; for example, during storage for transfusion. Given the hydrophobicity of unconjugated bilirubin (UCB), we investigated the acute platelet inhibitory and ROS scavenging ability of a water-soluble bilirubin analogue, bilirubin ditaurate (BRT) on ex vivo platelet function to ascertain its potential suitability for inclusion during platelet storage.
Methods
The inhibitory potential of BRT (10–100 μM) was assessed using agonist induced platelet aggregation, dense granule exocytosis and flow cytometric analysis of P-selectin and GPIIb/IIIa expression. ROS production was investigated by analysis of H2DCFDA fluorescence following agonist simulation while mitochondrial ROS production investigated using MitoSOX™ Red. Platelet mitochondrial membrane potential and viability was assessed using TMRE and Zombie Green™ respectively.
Results
Our data shows ≤35 μM BRT significantly inhibits both dense and alpha granule exocytosis as measured by ATP release and P-selectin surface expression, respectively. Significant inhibition of GPIIb/IIIa expression was also reported upon ≤35 μM BRT exposure. Furthermore, platelet exposure to ≤10 μM BRT significantly reduces platelet mitochondrial ROS production. Despite the inhibitory effect of BRT, platelet viability, mitochondrial membrane potential and agonist induced aggregation were not perturbed.
Conclusions
These data indicate, for the first time, that BRT, a water-soluble bilirubin analogue, inhibits platelet activation and reduces platelet ROS production ex vivo and may, therefore, may be of use in preserving platelet function during storage.
Keywords: Platelets, Bilirubin ditaurate, Flow cytometry, MitoSOX™ Red, Superoxide, ROS
Graphical abstract
Highlights
-
•
The impact of conjugated bilirubin on platelet function has not been investigated to date.
-
•
Bilirubin ditaurate (BDT) is a water-soluble analogue of conjugated bilirubin.
-
•
BDT attenuates ex vivo platelet activation and ROS generation.
-
•
Conjugated forms of bilirubin might inhibit platelet activation during storage.
1. Introduction
Platelets play a pivotal role in haemostasis, inflammation and wound repair [1]. Thrombocytopenia or impaired platelet function increases bleeding risk with the transfusion of platelets representing an important consideration in the treatment of patients [2]. Clinical demand for platelets is primarily met by the provision of room temperature, liquid stored platelets from public donation [3]. Such platelet products have a limited shelf-life and report deteriorating quality and loss of post transfusion function: termed the platelet storage lesion (PSL) [3]. Deleterious changes to morphological, metabolic and haemostatic characteristics occurs during storage: a shift from typical resting state phenotype towards one of activation and apoptosis and or necrosis [[3], [4], [5]]. Declining mitochondrial function is central to the PSL with loss of mitochondrial membrane potential (Δψm), cytochrome c release, caspase activation and increased ROS production occurring [6,7]. In particular, intracellular superoxide production further promotes the PSL, encouraging platelet degranulation, cytokine release and oxidative damage of key proteins and membranes [5,8,9]. Given the constant demand for platelet products, interventions that preserve haemostatic function, can reversibly inhibit platelet activation and attenuate ROS accumulation during storage may be of significant value.
Inclusion of exogenous antioxidant molecules, such as resveratrol, attenuate aspects of the PSL and preserve haemostatic function [10]. However, to date no investigation of endogenous antioxidants has been undertaken. Unconjugated bilirubin (UCB) is an endogenous antioxidant compound derived from erythrocyte/haem catabolism [11]. We have previously demonstrated the anti-platelet effects of UCB and considering the significant superoxide scavenging capacity of bilirubin (BR) based compounds, inclusion of bilirubin may combat both inappropriate platelet activation and ROS generation during storage [[12], [13], [14], [15]]. The inclusion of a hydrophilic analogue bilirubin ditaurate (BRT) is logistically more favourable than unconjugated bilirubin [16]. No studies to date have investigated the anti-platelet effects of BRT. Therefore, this study determined the impact of acute exposure on platelet function and ROS production, in order to assess potential suitability for inclusion during platelet storage.
2. Materials and methods
2.1. Materials
BRT was purchased from Frontier Scientific Inc. (Logan, UT, USA). All phlebotomy consumables, Annexin V Binding Buffer, Stain Buffer (BSA), Compensation Beads (anti-Mouse Ig, κ/Negative Control) and anti-CD42b-APC (HIP1, 551061), anti-CD42b-PE-Cy5 (HIP1, 551141), anti-CD62P-PE (AK4 555524), anti-PAC-1-FITC (PAC1, 340507) and Annexin V-BV421 (563973) were purchased from Becton Dickinson (Brisbane, Australia). Platelet agonists adenosine diphosphate (ADP), collagen and arachidonic acid (AA) were purchased from Helena Laboratories (Melbourne, Australia) with thrombin receptor activating peptide SFLLRN (TRAP-6) purchased from Haemoview Diagnostics (Brisbane Australia). CHRONO-LUME® and all aggregation consumables were purchased from DKSH Australia (Brisbane, Australia) with MitoSOX™ Red from ThermoFisher Scientific (Brisbane, Australia). Both MitoSPY™ Green FM and Zombie Green™ Fixable Viability Dye were purchased from BioLegend (San Diego, USA). All other reagents were purchased from Sigma Aldrich (Castle Hill, Australia) unless otherwise stated.
2.2. Human volunteers
Griffith University Human Ethics Committee approval and informed consent was obtained prior to both recruitment and blood collection (HREC:2016_605). Volunteers were healthy individuals aged 18–60 years of both sexes with no history of vascular disease, diabetes, bleeding pathologies, were non-smokers and had not ingested medications known to perturb platelet function in the prior two weeks. A full blood examination was undertaken to ensure a normal differential blood cell profile, as assessed by an AIMS accredited medical laboratory scientist (AcT5diff CP or DxH 500, Backman Coulter, Brisbane, Australia).
2.3. BRT preparation
A stock solution of BRT was prepared in DMSO or phosphate buffered saline (PBS) to a final concentration of 10 mM. A clear dark orange solution was obtained with the absence of precipitation confirmed by centrifugation (21500RCF; 5min)[12]. DMSO aliquots were stored at −80 °C for a maximum of 4 weeks whilst PBS suspended BRT solutions were prepared immediately prior to experimentation. The maximum final DMSO concentration in all samples was 0.1% (v/v). Samples were kept covered to prevent degradation during experimentation.
2.4. Platelet preparation
Blood collection was performed using established methods for platelet functional studies [17]. Briefly, whole blood was collected by antecubital venepuncture into 0.109M sodium citrate BD Vacutainers™ with the first 4 mL discarded [18]. Platelet rich plasma (PRP) was obtained via centrifugation (200RCF brake off, 10min) with the remaining blood centrifuged (2000RCF, 10min) to obtain Platelet Poor Plasma (PPP). Platelet isolation from whole blood was undertaken as per Abcam guidelines with minor modifications (Supplementary Material 1) [19]. All platelet samples were standardised to 1 × 108/mL with HEPES Modified Tyrode's Buffer (HMTB) (138 mM NaCl, 5 mM HEPES, 5.5 mM Glucose, 2.6 mM KCl, 0.49 mM MgCl2, 0.36 mM NaH2PO4, 12 mM NaHCO3 pH 7.4) unless otherwise stated and left to rest for 30 min prior to experimentation. Platelets were incubated with varying final concentrations of BRT (1, 10, 35 or 100 μM) and solvent vehicle controls of either 0.1% (v/v) DMSO HEPES Saline (10 mM HEPES, 138 mM NaCl pH 7.4) or PBS at room temperature (22 °C) for 15 minutes prior to experimentation unless otherwise stated.
2.5. Dense granule exocytosis
The effect of BRT on platelet dense granule release was examined by bioluminescence assay [20]. CHRONO-LUME® is routinely used for the quantification of dense granule ATP release on the CHRONO-LOG® Model 700 platform (CHRONO-LOG Corporation, PA, USA) [20]. However, inconsistent luminescence values upon calibration with known ATP standards in solutions containing BRT necessitated adaption to a plate-based assay. Briefly, 15 minute BRT pre-treated PRP was incubated with CHRONO-LUME® as per manufacturer's instructions, with luminescence recorded immediately following stimulation with 10 μM or 20 μM TRAP-6 (final concentrations), for a period of 6 min (Infinite® 200 PRO, TECAN, Mannedorf, Switzerland). Peak luminescence values were interpolated from treatment specific ATP standard curves (0–2 nM). Platelets treated 10 μM PGE1 (final concentration) served as a positive control for inhibition of ATP release.
2.6. Platelet GPIIb/IIIa receptor upregulation and α granule release
The impact of BRT on platelet activation was investigated using established flow cytometric methods [20,21]. Briefly, both 15 and 60 minute BRT pre-treated PRP was stimulated with a final concentration of 10 μM ADP, 20 μM TRAP-6 or no agonist (NA) for 5 minutes at room temperature. Subsequently, 50 μL was incubated with 5 μL anti-CD42b-APC, 2.5 μL anti-CD62P-PE and 5 μL anti-PAC1-FITC (30min; 22 °C) prior to fixation with 900 μL of 1% (w/v) PFA HEPES Saline (30min, 22 °C). Samples were diluted 1:1 with stain buffer and pelleted (800RCF, 10min) prior to resuspension in 250 μL of stain buffer. Samples were refrigerated at 4 °C prior to analysis by flow cytometry (SORP LSR II Fortessa - Becton Dickinson, NJ, USA) within 24 hours of fixation. Platelets treated with 10 μM PGE1 (final concentration) served as a positive control for inhibition of activation.
2.7. General ROS quantification
The effect of BRT on general platelet ROS production was investigated using the fluorescent probe H2DCFDA [22]. Briefly, BRT pre-treated isolated platelets were diluted 1:10 with HMTB (1.8 mM CaCl2) and incubated with 25 μM H2DCFDA (37 °C; 30min). Samples were treated with a final concentration of 20 μM TRAP-6, 20 μM antimycin or NA (22 °C; 5 min) before 1:5 dilution with HMTB (1.8 mM CaCl2) and immediately assessed via flow cytometry (Guava® easyCyte 5HT – Merk Millipore, MA, USA).
2.8. Scavenging of mitochondrially targeted superoxide
Mitochondrial ROS production was investigated using the superoxide specific fluorogenic dye MitoSOX™ Red [8]. To ensure accurate interpretation, anti-CD42b-APC and MitoSPY™ Green were used to establish platelet population and mitochondrial content respectively [23]. Briefly, 60 minute BRT pre-treated isolated platelets were diluted 1:10 with HMTB (1.8 mM CaCl2), with 100 μL of sample subsequently incubated with 2 μL anti-CD42b-APC and final concentration 50 nM MitoSPY™ Green (15min; 37.4 °C) prior to addition of a final concentration of 2 μM MitoSOX™ Red (15min; 37.4 °C). The mitochondrially targeted antioxidant capacity of BRT was assessed in low, moderate or high oxidative stress conditions induced by 0.2, 2.0 or 20 μM antimycin (5min, 37.4 °C) stimulation respectively [24]. Samples were diluted 1:5 HMTB (1.8 mM CaCl2) and fluorescence immediately assessed by flow cytometry (BD SORP LSR II Fortessa). The fluorescence intensity of the superoxide product (2-OH-Mito-E+) was preferentially analysed via flow cytometry using UV (355 nm) laser line excitation [[24], [25], [26]]. The impact of BRT on basal mitochondrial superoxide production was assessed in unstimulated samples. Platelets incubated with 10 μM Mito-TEMPO (final concentration) served as positive control for scavenging of mitochondrially derived superoxide [27]. Platelet mitochondrial membrane potential was investigated concurrently in antimycin untreated samples with a final concentration of 10 nM TMRE [24,28]. Samples treated with 50 μM CCCP (final concentration) represent positive control for loss of platelet Δψm [24,28].
2.9. Platelet aggregation
The effect of BRT on agonist induced platelet aggregation was assessed by light transmittance aggregometry(CHRONO-LOG® Model 700 Aggregometer, CHRONO-LOG Corporation, PA USA). Briefly, BRT pre-treated platelets were analysed in response to final concentrations of 5 μM ADP, 10 μM TRAP-6, 2 μg/mL collagen or 0.5 mM AA [12,20]. Turbidimetric platelet aggregation was calibrated against a PPP control, including relevant final BRT concentration, representative of 100% aggregation. Aggregation characteristics were recorded over a period of 6 min with all samples analysed in duplicate and magnetic stir bars set to 1000RPM. Platelets treated with 1mM acetylsalicylic acid (final concentration) served as a positive control for inhibition of aggregation.
2.10. Platelet viability
The effect of BRT on platelet viability was assessed using the amine reactive viability dye Zombie Green™. Briefly, isolated platelets were incubated with varying BRT concentrations for a total of 240 minutes. After 15, 60, 120 and 240 minutes a small aliquot was diluted 1:10 with HEPES Saline and 50 μL incubated with 50 μL of Zombie Green™ (1:250 PBS) and 2 μL of anti-CD42b-PE-Cy5 (30min, 22 °C). Samples were fixed with 900μL of 1% (w/v) PFA HEPES Saline (30min, 22 °C), diluted 1:1 with stain buffer and pelleted (800RCF, 10min) prior to resuspension in 250 μL of stain buffer. Samples were refrigerated at 4 °C prior to analysis within 24 hours of fixation by flow cytometry (Guava® easyCyte 5HT). Heat treated platelets (75 °C, 15min) served as positive control for cellular death.
2.11. Phosphatidylserine expression
The effect of BRT on platelet phosphatidylserine expression was undertaken using Annexin V [29]. Briefly, 60 minute BRT pre-treated isolated platelets were diluted 1:10 with HMTB (1.8 mM CaCl2) with 100 μL subsequently incubated with 2 μL anti-CD42b-APC (15min, 37.4 °C) prior to addition of 4 μLAnnexin V-BV421 (15min, 37.4 °C). Samples were diluted 1:5 with Annexin V binding buffer and immediately assessed via flow cytometry (BD SORP LSR II Fortessa). Platelets incubated with 2 μM A23187 (final concentration) served as a positive control for induction of phosphatidylserine expression [29].
2.12. Flow cytometric instrumentation configuration and analysis parameters
Please see Supplementary Material 2.
2.13. Statistical analysis
All values are expressed as mean ± standard deviation (SD). Comparisons between samples from the same volunteer exposed to different BRT treatment conditions were performed using Repeated Measures One-way ANOVA and Bonferroni's post hoc tests for parametric data. The Friedman's test with Dunn's post hoc test was used for non-parametric data with normality assessed by the Shapiro-Wailk test. Statistical analysis was performed using GraphPad PRISM (v8.1.1) and a p < 0.05 was considered significant
3. Results
We aimed to investigate the acute effects of ex vivo BRT exposure on platelet function and ROS production. In response to stimulation platelets release both alpha and dense granule contents, quantifiable by ATP release and P-selectin surface expression respectively, which drives further haemostatic processes [30].
3.1. Effect of BRT on agonist induced ATP release
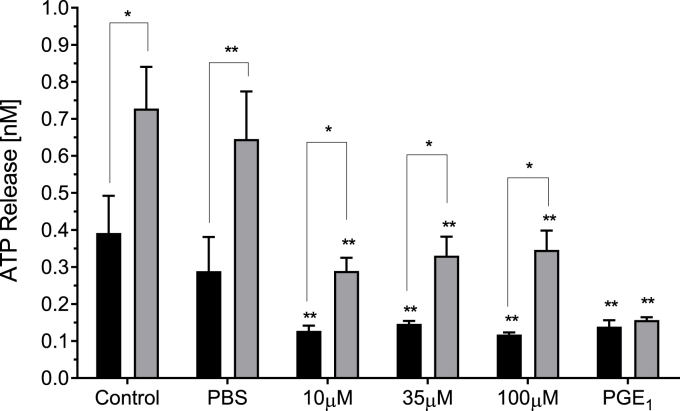
The impact of BRT on platelet dense granule exocytosis was evaluated by bioluminescence assay. BRT (≥10 μM) significantly reduced ATP release in response to both 10 μM TRAP-6 (p < 0.01) and 20 μM TRAP-6 stimulation (p < 0.01) respectively (Fig. 1).
Fig. 1.
The effect of acute BRT exposure on agonist induced platelet ATP release. Data presented as peak concentration following 10 μM TRAP-6 (black bars) or 20 μM TRAP-6 (grey bars) stimulation. PBS is vehicle. 10 μM PGE1 is positive control for platelet inhibition. Data are mean ± SD. n = 3. Statistical significance within agonist concentration treatment compared to PBS vehicle. *p < 0.05 **p < 0.01 Paired t-test between agonist concentrations for the same BRT treatment concentration. Significance at *p < 0.05 **p < 0.01.
Given attenuation of dense granule exocytosis following BRT exposure, we sought to investigate if alpha granule exocytosis was also perturbed. P-selectin redistributes from alpha granules and, along with GPIIb/IIIa expression, is an established marker for platelet activation [31].
3.2. Effect of BRT on platelet α granule exocytosis
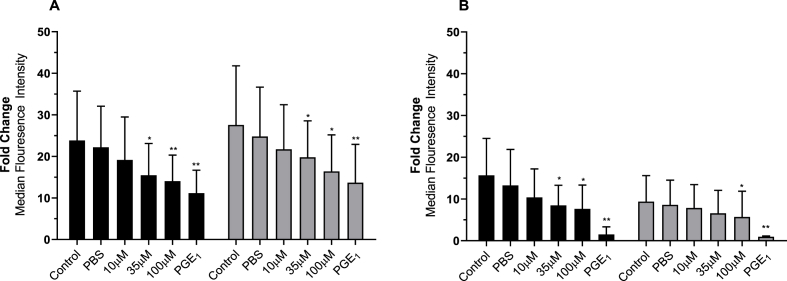
Platelet α granule exocytosis was assessed via flow cytometric evaluation of ADP or TRAP-6 induced P-selectin expression (Fig. 2). Both ADP and TRAP-6 induced P-selectin expression were reduced in a dose dependent manner upon increasing BRT concentrations. ADP induced expression was significantly reduced by 35 μM and 100 μM BRT exposure following 15 (p < 0.05) and 60 minutes (p < 0.05). BRT concentrations (≥35 μM) significantly decreased TRAP-6 induced P-selectin expression following 15 minute (p < 0.05) and 60 minute incubation (p < 0.05). BRT reported no statistically significant impact on basal P selectin expression (Supplementary Material 3).
Fig. 2.
The impact of 15 (black bars) or 60-minute (grey bars) BRT treatment on platelet P-selectin expression following A) 10 μM ADP or B) 20 μM TRAP-6 stimulation. Data reported as fold change of anti-CD62P-PE MFI of agonist treated over the unagonised sample from the same treatment condition. Platelets were identified by characteristics forward and side scatter parameters with a minimum of 50 000 CD42b positive events captured. PBS is vehicle. 10 μM PGE1 is positive control for platelet inhibition. Data are mean ± SD. n = 5 (15 min) and n = 8 (60 min). Statistical significance compared to PBS Vehicle. *p < 0.05 **p < 0.01.
As platelet granule exocytosis was attenuated following BRT exposure, we sought to investigate if integrin GPIIb/IIIa upregulation was impacted. Upon activation, GPIIb/IIIa undergoes a conformational change from low to high affinity, allowing fibrinogen binding and subsequent platelet aggregation [31].
3.3. Effect of BRT on platelet GPIIb/IIIa integrin activation
The effect of BRT on agonist induced platelet integrin GPIIb/IIIa upregulation was investigated by flow cytometric analysis of PAC-1 fluorescence (Fig. 3). Both ADP and TRAP-6 induced expression was reduced in a dose dependent manner with increasing BRT concentrations. Specifically, ADP induced PAC-1 expression was significantly reduced by 35 μM and 100 μM BRT exposure after 15 (p < 0.05) and 60 minutes (p < 0.05). Only 100 μM BRT decreased TRAP-6 induced PAC-1 expression after 15 (p < 0.01) and 60 minutes (p < 0.01) of incubation. 35 μM BRT inhibited integrin GPIIb/IIIa expression after 15 minutes of expsoure only. BRT reported no statistically significant impact on basal GPIIb/IIIa activation (Supplementary Material 3).
Fig. 3.
The impact of 15 (black bars) or 60 minute (grey bars) BRT pre-treatment on platelet GPIIb/IIIa upregulation assessed by PAC-1 expression following A) 10 μM ADP or B) 20 μM TRAP-6 stimulation. Data reported as fold change of anti-PAC-1-FITC MFI of agonist treated over that of the unagonised sample from the same treatment condition. Platelets were identified by characteristics forward and side scatter parameters with a minimum of 50 000 CD42b positive events captured. PBS is vehicle. 10 μM PGE1 is positive control for platelet inhibition. Data are mean ± SD. n = 5 (15 min) and 8 (60 min). Statistical significance compared to PBS vehicle. *p < 0.05, **p < 0.01.
Given the central role of ROS signalling in platelet activation, granule exocytosis, thromboxane A2 synthesis and GPIIb/IIIa upregulation, we sought to examine if the antioxidant properties of BRT, in addition to perturbing activation, attenuated ROS production [32]. Previous publications have noted the impact of antioxidants on platelet function and as such we sought to examine both global and mitochondrially derived ROS production [32].
3.4. Effect of BRT on platelet ROS generation
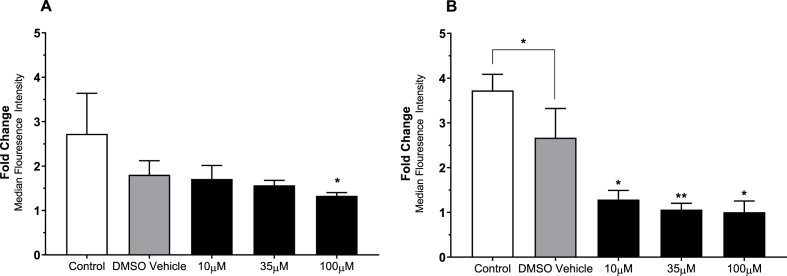
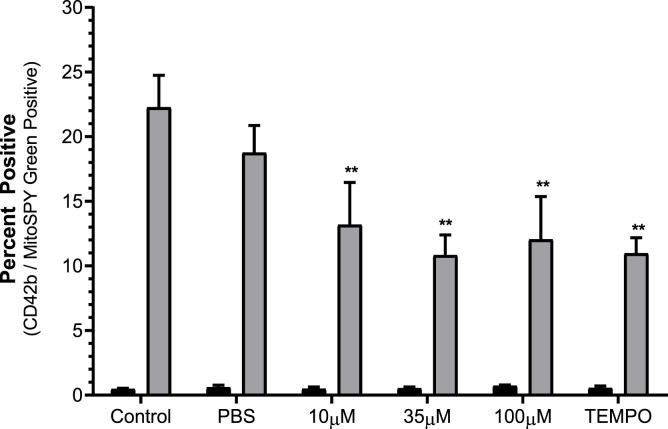
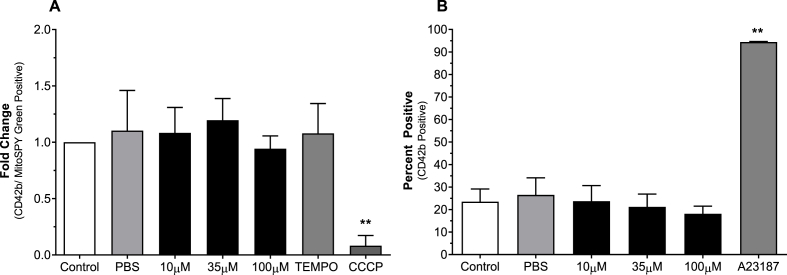
The effect of BRT on general platelet ROS generation was investigated by analysis of H2DCFDA fluorescence following stimulation with the 20 μM TRAP-6 or 20 μM Antimycin (Fig. 4). Upon TRAP-6 stimulation 100 μM BRT treated platelets reported decreased DCF fluorescence (p < 0.05) (Fig. 4A). All treatment conditions significantly reduced fluorescence following antimycin induced ROS generation compared to solvent treatment (p < 0.05), indicating a vehicle independent mechanism exerted by BRT in the presence of antimycin induced ROS production. The impact of BRT on mitochondrially targeted superoxide production was then investigated using the dihydroethidium based fluorogenic dye MitoSOX™ Red (Fig. 5). Mitochondrially derived superoxide was generated using 0.2, 2 or 20 μM antimycin after antimycin loading to induce low, moderate or high levels superoxide production. All BRT concentrations reported decreased fluorescence compared to control (p < 0.01) upon 20 μM antimycin treatment. BRT reported no statistically significant effect on basal H2DCFDA or MitoSOX™ Red superoxide product fluorescence. (Supplementary Material 4).
Fig. 4.
The effect of acute BRT exposure on H2DCFDA fluorescence following stimulation with A) 20 μM TRAP-6 or B) 20 μM antimycin. TRAP-6 and antimycin treatment used to examine physiological and mitochondrial ROS generation respectively. Platelets were identified by characteristic forward and side scatter parameters with a minimum of 20 000 platelet gated events captured. Data reported as fold change of H2DCFDA MFI over an unstimulated sample from the same treatment condition. Vehicle is 0.1% (v/v) DMSO HEPES Saline. Data are mean ± SD. n = 6 Statistical significance compared to DMSO vehicle. *p < 0.05, **p < 0.01.
Fig. 5.
The impact of BRT exposure on platelet mitochondrial superoxide production in response to induced oxidative stress (20 μM antimycin treatment grey bars) or basal state (black bars). Platelets were identified by characteristic forward and side scatter parameters with a minimum of 20 000 CD42b/MSG positive events captured. PBS is vehicle. 10 μM Mito-TEMPO is positive mitochondrial antioxidant control. Data reported as percentage of CD42b/MSG events positive for MitoSOX™ Red superoxide product fluorescence. Data are mean ± SD. n = 4 Statistical significance compared to PBS vehicle. **p < 0.01.
To investigate if the attenuation of platelet activation by BRT resulted in changes to global platelet function, platelet aggregation and parameters relating to viability were investigated.
3.5. Effect of BRT on platelet aggregation
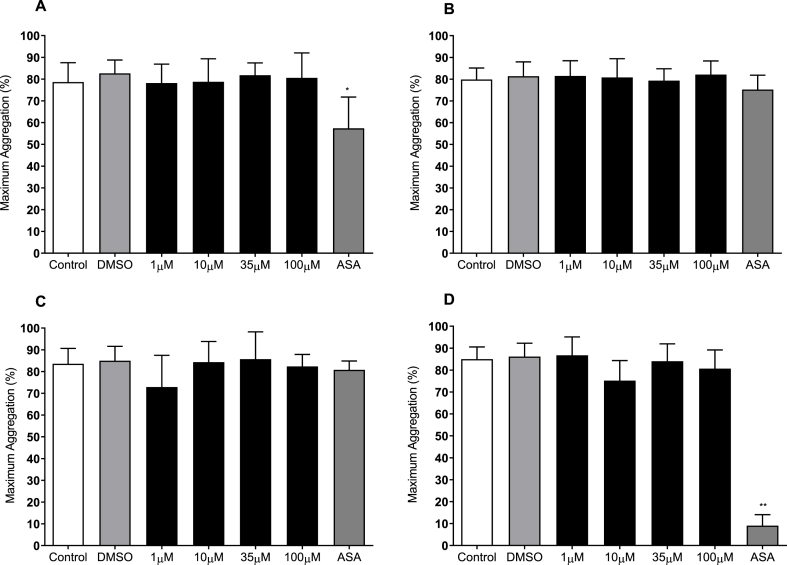
The effect of acute BRT exposure on platelet aggregation was assessed using light transmission aggregometry. Incubation with increasing concentrations of BRT did not affect maximum aggregation in response to 5 μM ADP, 10 μM TRAP-6, 500 μM AA or 2 μg/mL collagen (Fig. 6). Platelet aggregation was significantly reduced upon 1 mM ASA treatment in response to ADP and AA stimulation.
Fig. 6.
The effect of acute BRT exposure on platelet aggregation in response to A) collagen 2 μg/mL, B) ADP 5 μM C) TRAP-6 10 μM or D) Arachidonic Acid 0.5 mM 0.1% (v/v) DMSO HEPES Saline is vehicle. 1 mM acetylsalicylic acid (ASA) is positive control for inhibition of platelet aggregation. Data are mean ± SD. n = 8. Statistical significance compared to vehicle within agonist concentration. *p < 0.05, **p < 0.01.
To differentiate the attenuation of platelet function reported by BRT, from that as a consequence of induced toxicity or stress, platelet viability was investigated.
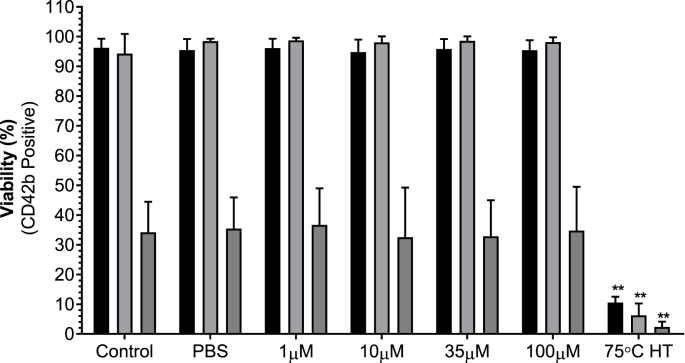
3.6. Effect of BRT on platelet viability
BRT did not impact platelet viability over 240 minutes. Heat treated platelets reported decreased viability at all time points (Fig. 7 and Supplementary Material 5).
Fig. 7.
The impact of acute BRT exposure on platelet viability after 15 (black bars), 60 (grey bars) or 240 minutes (dark grey bars) at 22 °C. PBS is vehicle. 75 °C heat treated platelets is positive control for death. Platelets were identified by characteristic forward and side scatter parameters with a minimum of 50 000 CD42b positive events captured. Viability reported as percentage of Zombie Green™ negative platelets. Data are mean ± SD. n = 5. Statistical significance compared to PBS vehicle. **p < 0.01.
Although BRT had not effect on viability, we sought to further investigate mitochondrial membrane potential and phosphatidylserine expression, as both are critical to haemostatic function [33,34].
3.7. Δψm and phosphatidylserine expression
BRT did not affect platelet Δψm or phosphatidylserine expression (Fig. 8). Treatment with 50 μM CCCP significantly reduced platelet Δψm (p < 0.01) and treatment with 2 μM A23187 signifigantly induced PS expression (p < 0.01).
Fig. 8.
The effect of acute BRT exposure on platelet A) Δψm and B) PS expression following. Vehicle is PBS. 50 μM CCCP is positive control for loss of Δψm. 2 μM A23187 is positive control for PS upregulation. Platelets were identified by characteristics forward and side scatter parameters with a minimum of 20 000 CD42b/MSG positive events captured for (A) and 50 000 CD42b positive events captured for (B). Data presented as A) TMRE MFI fold change over control or B) percentage of Annexin V positive platelets. Data are mean ± SD. n = 4 Statistical significance compared to PBS vehicle **p < 0.01.
4. Discussion
This study demonstrates that the water-soluble conjugated bilirubin analogue, bilirubin ditaurate, acutely attenuates both platelet dense and alpha granule exocytosis and reduces GPIIb/IIIa integrin expression upon agonist stimulation. Furthermore, BRT reported significant antioxidant capacity in conditions of induced oxidative stress with mitochondrially derived superoxide production attenuated by BRT exposure. Finally, despite attenuation of platelet activity, BRT did not alter platelet viability or mitochondrial membrane potential, indicating acute exposure is unlikely to be toxic to platelets.
Platelet granule exocytosis is driven by increased intracellular calcium ion accumulation, resulting in attraction of platelets to sites of vascular injury; propagating the haemostatic response [35]. ATP, the major constituent of platelet dense granules is intrinsic to platelet function [36]. A significant reduction in ATP release following BRT treatment was reported, indicating impaired dense granule exocytosis with additional concentrations above 10 μM reporting no further inhibition. Moreover, greater ATP release was reported upon stimulation with increased agonist concentration. Consistent with dense granule exocytosis inhibition, platelet P-selectin expression was also attenuated by BRT treatment. P-selectin redistributes from the plasma membrane of platelet alpha granules to the surface upon activation and is an accepted marker of platelet activation [37,38]. P-selectin expression was significantly attenuated, and occurred in a dose dependant manner, upon TRAP-6 stimulation following exposure to 10–100 μM BRT concentrations. ADP induced P-selectin expression was attenuated in a dose dependent manner, consistent with previously published data where increasing serum bilirubin concentrations were weakly correlated with reduced ADP induced P-selectin expression [14]. In addition to attenuation of agonist induced P-selectin expression, platelet GPIIb/IIIa receptor upregulation following agonist stimulation was also attenuated by BRT treatment. This is consistent with previous reports of individuals with benign hyperbilirubinemia (Gilbert's syndrome; GS) report a trend towards decreased GPIIb/IIIa integrin upregulation upon agonist stimulation [14]. Interestingly, both ADP induced P-selectin and PAC-1 expression was not significantly attenuated by 10 μM BRT following 15 min, however after 60 minutes both were significantly attenuated. This indicates a time dependent mechanism of inhibition in which lower concentrations of BRT attenuate platelet activation given greater exposure time, potentially advantageous in the context of storage where platelets are stored for up to 5 days [39].
BRT attenuation of platelet granule exocytosis reported here is consistent with similar reports of inhibition by BR based compounds. Perturbation of calcium homeostasis and inhibition of nerve terminal vesicle exocytosis by BR, both through calcium dependent and independent mechanisms, has been previously reported [40]. BR modulation of nicotinic acetylcholine receptor function via the PKA pathway is one hypothesis by which bilirubin may modulate presynaptic neurotransmitter release [40,41]. Concentrations of BR above 3 μM suppressed nicotinic acetylcholine receptor (nAChRα7) channel current in a non-competitive manner [41]. Platelets express nAChRα7 subunits that form functional Ca2+ channels and increase platelet aggregation in response to ADP and TXA2 [42]. Taken together, potential attenuation of nAChRα7 function by BRT may go part way to explaining the reduction in platelet granule exocytosis reported here with further investigations evaluating direct measures of intracellular calcium accumulation a focus for future work. Taken together these data support BRT inhibition of granule exocytosis with subsequent impact on platelet integrin activation.
Despite reported attenuation of platelet granule exocytosis and integrin upregulation, BRT did not perturb aggregation [43]. This is inconsistent with previous findings of inhibited aggregation following antioxidant exposure [12,17,44]. Inducible ROS formation is a key mechanism by which platelet aggregation occurs [45,46]. We first reported the inhibitory effect of UCB on platelet aggregation in response to both collagen and ADP in vitro [12]. This is the first report to investigate the impact of bilirubin ditaurate on platelet aggregation, showing no effect up to a concentration of 100 μM. Although results of global ROS attenuation following TRAP stimulation support BRT activity like that of UCB, work is required to further examine differences in platelet aggregation results seen. However, inclusion of antioxidants during platelet storage reports preserved aggregation characteristics, highlighting the multifaceted, and incompletely understood mechanisms by which oxidative processes within platelets occur [10]. Although the unremarkable impact of BRT on platelet aggregation may be advantageous, to combat inappropriate platelet activation during storage, future studies should study the impact of BRT on additional measures of platelet function.
Linear tetrapyrroles are potent antioxidants therefore any noted attenuation of platelet activation may be a result of such antioxidant capacity [15,47,48]. Inhibition of platelet ROS activity, either through pharmacological agents or genetic deletion of key proteins, demonstrates significant inhibitory effects, both in vitro and in vivo [32,46,49,50]. Bilirubin inhibition of NOX subunit assembly and superoxide production, along with direct and potent scavenging of the superoxide radical cation, strongly supports an effect of bilirubin on ROS production at mildly elevated physiological concentrations [15,48,51,52]. This is consistent with results reported here where BRT attenuated ROS (including superoxide) generation. Non-specific ROS generation was investigated using H2DCFDA fluorescence upon stimulation with various agonists in order to assess the multiple sources of ROS within platelets [53]. ROS production under these conditions was significantly attenuated by both 100 μM BRT pre-treatment. Upon antimycin stimulation, which blocks Q cycling within Complex III thus producing superoxide [54], all BRT concentrations attenuated ROS generation indicating a mitochondrially targeted mechanism of action. This was confirmed where BRT treatmentreduced MitoSOX™ Red oxidation product (2-OH-MitoE+) positive platelets in addition to reducing DCF fluorescence upon antimycin stimulation. The scavenging capacity of BRT, and other bilirubin derivatives, towards superoxide is well established [52,55]. Such characteristics may be considered advantageous in translating BRT for inclusion during platelet storage where ROS accumulation and degradation of mitochondrial stability is noted [9,[56], [57], [58]]. To date a number of studies have investigated ROS generation in platelets during storage [8,9,[56], [57], [58], [59]]. Villarroel et al. reported intracellular mitochondria superoxide generation increased substantially on day 3, peaking after 5 days of storage [8]. This is consistent with the findings of Skripchenko et al. and Ghasemzadeh et al. who demonstrated increased ROS generation in platelets stored over 7 and 5 days respectively [56,57]. Therefore, it is not surprising that inclusion of antioxidants during storage preserves haemostatic function and attenuates of pro-apoptotic phenotype generation [9,10,[60], [61], [62], [63], [64], [65]]. These data, therefore, provide an appropriate justification for testing the efficacy of BRT in stored platelet products.
The impact of BRT on platelets reported here, is unlikely to be result of cytotoxicity. Platelet viability was not affected over 240 minutes, contrary to previous work indicating a shift towards a pro-apoptotic phenotype upon UCB treatment of platelets [66]. Rangappa et al. reported reduced mitochondrial membrane potential, upregulation of PS and increased ROS production upon treatment of platelets with high (50–300 μM) concentrations of UCB in vitro [66]. Unconjugated bilirubin may induce cytotoxicity through a mitochondrially dependent mechanism, as reported in HCT15 cells [67]. In both platelets and HCT15 cells, UCB treatment reported mitochondrial membrane perturbation and upregulation of caspase activity [66,67]. However, BRT treatment failed to alter HCT15 cell viability or perturb of mitochondrial membrane potential in the study by Keshavan et al., with Rangappa et al. only investigating UCB and not including BRT [66,67]. A possible explanation for this divergent effect might be related to the hydrophilic nature of BRT, versus hydrophobic nature of UCB, and as such may influence cellular and absorption and distribution [68]. Despite the differences in hydrophobicity between UCB and BRT, BRT did not perturb mitochondrial membrane potential or induce platelet death in this study. However, BRT treatment did not significantly increase platelet viability compared to control or vehicle, indicating that although not directly cytotoxic, BRT viability appears indifferent over 240 minutes. However, further assessment of viability in a model of platelet storage, where longer exposure times would be investigated, is required.
4.1. Limitations
The experimental design of this study aimed to assess the acute effects of BRT on platelet activation and functional responses, with viability assessed over a priod of 240 minutes. It should be acknowledged that although the current data demonstrate inhibition of platelet activation and ROS production by BRT, which could be of potential utility within conditions of storage, they do not accurately reflect the storage conditions employed clinically (i.e. in terms of storage duration, inclusion of platelet storage solutions/buffers, use of agitation and oxygen permeable storage bags). Future studies are required to determine the efficacy of BRT use in relevant storage conditions and whether inhibition might be reversible, which would provide a stronger basis for use in clinical practice.
5. Conclusion
This is the first study to investigate the impact of BRT on ex vivo platelet function. Acute BRT platelet exposure attenuated ROS production and perturbed granule exocytosis with inhibition of integrin activation following agonist stimulation. Platelet aggregation was not affected by BRT treatment which also did not induce cytotoxicity or perturb mitochondrial membrane potential. The precise mechanism by which BRT acts on platelets remains to be elucidated however, these data taken together indicate significant antioxidant scavenging upon induced oxidative stress and reductions in granule release and activation upon agonist stimulation. These results form a sound rationale for testing the efficacy of BRT in stored platelet products, with the aim of preserving function.
Author contribution
E.N. Pennell designed and performed research, analysed data and wrote the manuscript. K–H Wagner assisted in revising this manuscript. S. Mosawy and A.C. Bulmer assisted in research design and revised/wrote the manuscript. All authors approved the final revision and submission of this manuscript.
Funding
This work was supported by internal funding provided the Health Group, Griffith University.
Disclosure of conflict of interests
All authors declare no conflicts of interest.
Acknowledgements
The authors thank Dr Jelena Vider MD, Mr Ryan Shiels and Mr Josif Vidimce for their assistance. The authors would also like to thank the volunteers who donated blood.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101250.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Golebiewska E.M., Poole A.W. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29:153–162. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumberg N., Cholette J.M., Schmidt A.E., Phipps R.P., Spinelli S.L., Heal J.M., Pietropaoli A.P., Refaai M.A., Sime P.J. Management of platelet disorders and platelet transfusions in ICU patients. Transfus. Med. Rev. 2017;31:252–257. doi: 10.1016/j.tmrv.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Seghatchian J., Krailabsiri P. The platelet storage lesion. Clin. Lab. Med. 2010;30:475–487. doi: 10.1016/j.cll.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Cookson P., Sutherland J., Turner C., Bashir S., Wiltshire M., Hancock V., Smith K., Cardigan R. Platelet apoptosis and activation in platelet concentrates stored for u p to 12 days in plasma or additive solution. Transfus. Med. 2010;20:392–402. doi: 10.1111/j.1365-3148.2010.01034.x. [DOI] [PubMed] [Google Scholar]
- 5.Jackson S.P., Schoenwaelder S.M. Procoagulant platelets: are they necrotic? Blood. 2010;116:2011. doi: 10.1182/blood-2010-01-261669. [DOI] [PubMed] [Google Scholar]
- 6.Schubert P., V Devine D. Towards targeting platelet storage lesion-related signaling pathways. Blood Transfus. 2010;8(Suppl 3):s69–72. doi: 10.2450/2010.011S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinalducci S., Zolla L. Biochemistry of storage lesions of red cell and platelet concentrates: a continuous fight implying oxidative/nitrosative/phosphorylative stress and signaling. Transfus. Apher. Sci. 2015;52:262–269. doi: 10.1016/j.transci.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Villarroel J.P.P., Figueredo R., Guan Y., Tomaiuolo M., Karamercan M.A., Welsh J., Selak M.A., Becker L.B., Sims C. Increased platelet storage time is associated with mitochondrial dysfunction and impaired platelet function. J. Surg. Res. 2013;184:422–429. doi: 10.1016/j.jss.2013.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghasemzadeh M., Hosseini E. Platelet granule release is associated with reactive oxygen species generation during platelet storage: a direct link between platelet pro-inflammatory and oxidation states. Thromb. Res. 2017 doi: 10.1016/j.thromres.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Lannan K.L., Refaai M.A., Ture S.K., Morrell C.N., Blumberg N., Phipps R.P., Spinelli S.L. Resveratrol preserves the function of human platelets stored for transfusion. Br. J. Haematol. 2015 doi: 10.1111/bjh.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mölzer C., Huber H., Steyrer A., Ziesel G., Ertl A., Plavotic A., Wallner M., Bulmer A.C., Wagner K.-H. Antioxidant capacity and antigenotoxic properties of protoporphyrin and structurally related tetrapyrroles. Free Radic. Res. 2012;46:1369–1377. doi: 10.3109/10715762.2012.715371. [DOI] [PubMed] [Google Scholar]
- 12.Kundur A.R., Bulmer A.C., Singh I. Unconjugated bilirubin inhibits collagen induced platelet activation. Platelets. 2014;25:45–50. doi: 10.3109/09537104.2013.764405. [DOI] [PubMed] [Google Scholar]
- 13.Kundur A.R., Singh I., Bulmer A.C. Bilirubin, platelet activation and heart disease: a missing link to cardiovascular protection in Gilbert's syndrome? Atherosclerosis. 2015;239:73–84. doi: 10.1016/j.atherosclerosis.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 14.Kundur A.R., Santhakumar A.B., Bulmer A.C., Singh I. Mildly elevated unconjugated bilirubin is associated with reduced platelet activation-related thrombogenesis and inflammation in Gilbert's syndrome. Platelets. 2017;28:779–785. doi: 10.1080/09537104.2017.1280146. [DOI] [PubMed] [Google Scholar]
- 15.Bakrania B., Bulmer A.C., Wagner K.-H., Du Toit E.F., Powell L.W., Clark P.J., Headrick J.P., Boon A.-C. Bilirubin acts as a multipotent guardian of cardiovascular integrity: more than just a radical idea. Am. J. Physiol. Cell Physiol. 2018;315:H429–H447. doi: 10.1152/ajpheart.00417.2017. [DOI] [PubMed] [Google Scholar]
- 16.Neubrand M.W., Carey M.C., Laue T.M. Self-assembly of aqueous bilirubin ditaurate, a natural conjugated bile pigment, to contraposing enantiomeric dimers and M(-) and P(+) tetramers and their selective hydrophilic disaggregation by monomers and micelles of bile salts. Biochemistry. 2015;54:1542–1557. doi: 10.1021/bi501251v. [DOI] [PubMed] [Google Scholar]
- 17.Mosawy S., Jackson D.E., Woodman O.L., Linden M.D. The flavonols quercetin and 3’,4’-dihydroxyflavonol reduce platelet function and delay thrombus formation in a model of type 1 diabetes. Diabetes Vasc. Dis. Res. 2014;11:174–181. doi: 10.1177/1479164114524234. [DOI] [PubMed] [Google Scholar]
- 18.Taylor M.L., Misso N.L.A., Stewart G.A., Thompson P.J. Differential expression of platelet activation markers in aspirin-sensitive asthmatics and normal subjects. Clin. Exp. Allergy. 1996;26:202–215. doi: 10.1111/j.1365-2222.1996.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 19.Abcam Isolation of Human Platelets from Whole Blood. 2015. http://docs.abcam.com/pdf/protocols/isolation-of-human-platelets-from-whole-blood.pdf
- 20.Mosawy S., Jackson D.E., Woodman O.L., Linden M.D. Inhibition of platelet-mediated arterial thrombosis and platelet granule exocytosis by 3’,4’-dihydroxyflavonol and quercetin. Platelets. 2013;24:594–604. doi: 10.3109/09537104.2012.749396. [DOI] [PubMed] [Google Scholar]
- 21.Kicken C.H., Roest M., Henskens Y.M.C., De Laat B., Huskens D. Application of an optimized flow cytometrybased quantification of Platelet Activation (PACT): monitoring platelet activation in platelet concentrates. PLoS One. 2017;12:1–12. doi: 10.1371/journal.pone.0172265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heger M., Egmond M.R., van Golen R.F., Bonnet S., Reiniers M.J., Broekgaarden M., van Gulik T.M. Preparation and practical applications of 2′,7′-dichlorodihydrofluorescein in redox assays. Anal. Chem. 2017;89:3853–3857. doi: 10.1021/acs.analchem.7b00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bynum J.A., Adam Meledeo M., Getz T.M., Rodriguez A.C., Aden J.K., Cap A.P., Pidcoke H.F. Bioenergetic profiling of platelet mitochondria during storage: 4°C storage extends platelet mitochondrial function and viability. Transfusion. 2016;56:S76–S84. doi: 10.1111/trf.13337. [DOI] [PubMed] [Google Scholar]
- 24.Polster B.M., Nicholls D.G., Ge S.X., Roelofs B.A. first ed. Elsevier Inc.; 2014. Use of Potentiometric Fluorophores in the Measurement of Mitochondrial Reactive Oxygen Species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zielonka J., Srinivasan S., Hardy M., Ouari O., Lopez M., Vasquez-vivar J., Avadhani N.G., Kalyanaraman B. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic. Biol. Med. 2008;44:835–846. doi: 10.1016/j.freeradbiomed.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalyanaraman B., Dranka B.P., Hardy M., Michalski R., Zielonka J. HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes - the ultimate approach for intra- and extracellular superoxide detection. Biochim. Biophys. Acta Gen. Subj. 2014;1840:739–744. doi: 10.1016/j.bbagen.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy C., Kenny L.C. Therapeutically targeting mitochondrial redox signalling alleviates endothelial dysfunction in preeclampsia. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cottet-Rousselle C., Ronot X., Leverve X., Mayol J.F. Cytometric assessment of mitochondria using fluorescent probes. Cytometry. 2011;79 A:405–425. doi: 10.1002/cyto.a.21061. [DOI] [PubMed] [Google Scholar]
- 29.Ramstrom S., O'Neill S., Dunne E., Kenny D. Annexin v binding to platelets is agonist, time and temperature dependent. Platelets. 2010;21:289–296. doi: 10.3109/09537101003660564. [DOI] [PubMed] [Google Scholar]
- 30.Gibbins J.M. Platelet adhesion signalling and the regulation of thrombus formation. J. Cell Sci. 2004;117:3415–3425. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 31.Koedam J.A., Cramer E.M., Briend E., Furie B.C., Furie B.C., Wagner D.D. P-selectin, a granule membrane protein of platelets and endothelial cells, follows the regulated secretory pathway in AtT-20 cells. J. Cell Biol. 1992;116:617–625. doi: 10.1083/jcb.116.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Violi F., Pignatelli P. Platelet NOX, a novel target for anti-thrombotic treatment. Thromb. Haemostasis. 2014;111:817–823. doi: 10.1160/TH13-10-0818. [DOI] [PubMed] [Google Scholar]
- 33.Leytin V., Allen D.J., Mutlu A., Gyulkhandanyan A.V., Mykhaylov S., Freedman J. Mitochondrial control of platelet apoptosis: effect of cyclosporin a, an inhibitor of the mitochondrial permeability transition pore. Lab. Investig. 2009;89:374–384. doi: 10.1038/labinvest.2009.13. [DOI] [PubMed] [Google Scholar]
- 34.Gyulkhandanyan A.V., Mutlu A., Freedman J., Leytin V. Selective triggering of platelet apoptosis, platelet activation or both. Br. J. Haematol. 2013;161:245–254. doi: 10.1111/bjh.12237. [DOI] [PubMed] [Google Scholar]
- 35.Estevez B., Du X. New concepts and mechanisms of platelet activation signaling. Physiology. 2017;32:162–177. doi: 10.1152/physiol.00020.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heijnen H., van der Sluijs P. Platelet secretory behaviour: as diverse as the granules... or not? J. Thromb. Haemost. 2015;13:2141–2151. doi: 10.1111/jth.13147. [DOI] [PubMed] [Google Scholar]
- 37.Paniccia R., Priora R., Liotta A.A., Abbate R. Platelet function tests: a comparative review. Vasc. Health Risk Manag. 2015;11:133–148. doi: 10.2147/VHRM.S44469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stenberg P.E., McEver R.P., Shuman M.A., Jacques Y.V., Bainton D.F. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J. Cell Biol. 1985 doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson L., Reade M.C., Hyland R.A., Tan S., Marks D.C. In vitro comparison of cryopreserved and liquid platelets: potential clinical implications. Transfusion. 2015;55:838–847. doi: 10.1111/trf.12915. [DOI] [PubMed] [Google Scholar]
- 40.Hansen T.W.R., Mathiesen S.B.W., Sefland I., Walaas S.I. Bilirubin inhibits Ca2+-dependent release of norepinephrine from permeabilized nerve terminals. Neurochem. Res. 1999;24:733–738. doi: 10.1023/a:1020775312214. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C., Wang Z., Dong J., Pan R., Qiu H., Zhang J., Zhang P., Zheng J., Yu W. Bilirubin modulates acetylcholine receptors in rat superior cervical ganglionic neurons in a bidirectional manner. Sci. Rep. 2014;4:1–8. doi: 10.1038/srep07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bugert P., Schedel A., Thornton S., Klüter H., Schloss P. Human platelets express functional α7-nicotinic acetylcholine receptors. Arterioscler. Thromb. Vasc. Biol. 2010;31:928–934. doi: 10.1161/ATVBAHA.110.218297. [DOI] [PubMed] [Google Scholar]
- 43.Shiels R.G., Vidimce J., Pearson A.G., Matthews B., Wagner K.-H., Battle A.R., Sakellaris H., Bulmer A.C. Unprecedented microbial conversion of biliverdin into bilirubin-10-sulfonate. Sci. Rep. 2019;9:2988. doi: 10.1038/s41598-019-39548-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosawy S., Jackson D.E., Woodman O.L., Linden M.D. Treatment with quercetin and 3’,4’-dihydroxyflavonol inhibits platelet function and reduces thrombus formation in vivo. J. Thromb. Thrombolysis. 2013;36:50–57. doi: 10.1007/s11239-012-0827-2. [DOI] [PubMed] [Google Scholar]
- 45.Bedard K., Krause K.-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 46.Walsh T.G., Berndt M.C., Carrim N., Cowman J., Kenny D., Metharom P. The role of Nox1 and Nox2 in GPVI-dependent platelet activation and thrombus formation. Redox Biol. 2014;2:178–186. doi: 10.1016/j.redox.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bakrania B., Du Toit E.F., Wagner K.H., Headrick J.P., Bulmer A.C. Pre- or post-ischemic bilirubin ditaurate treatment reduces oxidative tissue damage and improves cardiac function. Int. J. Cardiol. 2016;202:27–33. doi: 10.1016/j.ijcard.2015.08.192. [DOI] [PubMed] [Google Scholar]
- 48.Jansen T., Hortmann M., Oelze M., Opitz B., Steven S., Schell R., Knorr M., Karbach S., Schuhmacher S., Wenzel P., Münzel T., Daiber A. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J. Mol. Cell. Cardiol. 2010;49:186–195. doi: 10.1016/j.yjmcc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Olas B., Wachowicz B., Saluk-Juszczak J., Zieliński T. Effect of resveratrol, a natural polyphenolic compound, on platelet activation induced by endotoxin or thrombin. Thromb. Res. 2002;107:141–145. doi: 10.1016/s0049-3848(02)00273-6. [DOI] [PubMed] [Google Scholar]
- 50.Vara D., Campanella M., Pula G. The novel NOX inhibitor 2-acetylphenothiazine impairs collagen-dependent thrombus formation in a GPVI-dependent manner. Br. J. Pharmacol. 2013;168:212–224. doi: 10.1111/j.1476-5381.2012.02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwak J.Y., Takeshige K., Cheung B.S., Minakami S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim. Biophys. Acta (BBA)/Protein Struct. Mol. 1991;1076:369–373. doi: 10.1016/0167-4838(91)90478-i. [DOI] [PubMed] [Google Scholar]
- 52.Jansen T., Daiber A. Direct antioxidant properties of bilirubin and biliverdin. Is there a role for biliverdin reductase? Front. Pharmacol. 2012:1–10. doi: 10.3389/fphar.2012.00030. 3 MAR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krötz F., Sohn H.Y., Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler. Thromb. Vasc. Biol. 2004;24:1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 54.Q-cycle A.M., Quinlan C.L., Gerencser A.A., Treberg J.R., Brand M.D. The mechanism of superoxide production by the. J. Biol. Chem. 2011;286:31361–31372. doi: 10.1074/jbc.M111.267898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conti M., Almolki A., Foresti R., Aubier M., Bloc S., Lanone S., El-Benna J., Taillé C., Dureuil B., Boczkowski J., Motterlini R., Callebert J., Goven D. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J. 2005;19:1890–1892. doi: 10.1096/fj.04-2368fje. [DOI] [PubMed] [Google Scholar]
- 56.Ghasemzadeh M., Hosseini E., Roudsari Z.O., Zadkhak P. Intraplatelet reactive oxygen species (ROS) correlate with the shedding of adhesive receptors, microvesiculation and platelet adhesion to collagen during storage: does endogenous ROS generation downregulate platelet adhesive function? Thromb. Res. 2018;163:153–161. doi: 10.1016/j.thromres.2018.01.048. [DOI] [PubMed] [Google Scholar]
- 57.Skripchenko A., Myrup A., Thompson-Montgomery D., Awatefe H., Wagner S.J. Mitochondrial dysfunction of platelets stored in first- and second-generation containers is, in part, associated with elevated carbon dioxide levels. Transfusion. 2011;51:371–379. doi: 10.1111/j.1537-2995.2010.02829.x. [DOI] [PubMed] [Google Scholar]
- 58.Manasa K., Vani R. Influence of oxidative stress on stored platelets. Adv. Hematol. 2016 doi: 10.1155/2016/4091461. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pietraforte D., Vona R., Marchesi A., de Jacobis I.T., Villani A., Del Principe D., Straface E. Redox control of platelet functions in physiology and pathophysiology. Antioxidants Redox Signal. 2014;21:177–193. doi: 10.1089/ars.2013.5532. [DOI] [PubMed] [Google Scholar]
- 60.Matsumura K., Takayama H., Bae J.Y. Preservation of platelets by adding epigallocatechin-3- O -Gallate. 2017;18:521–528. doi: 10.1177/096368970901805-606. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi T., Tanaka S., Hori Y., Hirayama F., Sato E.F., Inoue M. Role of mitochondria in the maintenance of platelet function during in vitro storage. Transfus. Med. 2011;21:166–174. doi: 10.1111/j.1365-3148.2010.01065.x. [DOI] [PubMed] [Google Scholar]
- 62.Warncke U.O., Chalfant C.E., Fowler A.A., III, Wijesinghe D.S., Mohammed B.M., Brophy D.F., Sanford K.W., Martin E.J., Contaifer D., Jr., Natarajan R., Fisher B.J. Impact of high dose vitamin C on platelet function. World J. Crit. Care Med. 2017;6:37. doi: 10.5492/wjccm.v6.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stivala S., Meyer S.C., Reiner M., Luscher T.F., Buser A.S., Beer J.H. Addition of omega-3 α-linolenic acid to platelet apheresis units preserves platelet activatability over time and reduces baseline activation under routine storage conditions: a pilot study. Blood. 2012;120:3433. [Google Scholar]
- 64.Handigund M., Bae T.W., Lee J., Cho Y.G. Evaluation of in vitro storage characteristics of cold stored platelet concentrates with N acetylcysteine (NAC) Transfus. Apher. Sci. 2016;54:127–138. doi: 10.1016/j.transci.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Hosseini E., Ghasemzadeh M., Atashibarg M., Haghshenas M. ROS scavenger, N-acetyl-l-cysteine and NOX specific inhibitor, VAS2870 reduce platelets apoptosis while enhancing their viability during storage. Transfusion. 2019:1–11. doi: 10.1111/trf.15114. [DOI] [PubMed] [Google Scholar]
- 66.NaveenKumar S.K., Thushara R.M., Sundaram M.S., Hemshekhar M., Paul M., Thirunavukkarasu C., Basappa, Nagaraju G., Raghavan S.C., Girish K.S., Kemparaju K., Rangappa K.S. Unconjugated bilirubin exerts pro-apoptotic effect on platelets via p38-MAPK activation. Sci. Rep. 2015;5:15045. doi: 10.1038/srep15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keshavan P., Schwemberger S.J., Smith D.L.H., Babcock G.F., Zucker S.D. Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int. J. Cancer. 2004;112:433–445. doi: 10.1002/ijc.20418. [DOI] [PubMed] [Google Scholar]
- 68.Čvorović J., Passamonti S. Membrane transporters for bilirubin and its conjugates: a systematic review. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.