Highlights
-
•
Polyneuropathy is a frequent neurological manifestation of B12 hypovitaminosis.
-
•
Thin-myelinated A-delta fibers may be affected in B12 hypovitaminosis.
-
•
CSP testing is a useful diagnostic tool for A-delta fiber function in B12 deficiency.
Keywords: Vitamin B12 deficiency, Polyneuropathy, A-delta fiber, Cutaneous silent period, Nerve conduction, Quantitative sensory testing
Abstract
Objectives
Vitamin B12 deficiency is common in adult and elderly patients and is often underdiagnosed because of its polymorphous manifestations. Neurological symptoms of this condition include subacute combined degeneration and polyneuropathy, with possible affection of thin-myelinated A-delta fibers. Cutaneous silent periods (CSPs) may serve to test small-diameter fiber function non-invasively, using routine electrodiagnostic equipment, but to the best of our knowledge have not been studied so far in vitamin B12 deficiency.
Methods
We report a 49-year-old male patient suffering from B12 hypovitaminosis due to autoantibodies against gastric parietal cells, who underwent neurophysiological investigation to confirm clinically suspected polyneuropathy during the first month of intramuscular vitamin B12 supplementation. We performed standard electroneurography, needle electromyography in tibialis anterior muscle, quantitative sensory testing, and cutaneous silent periods six months after symptom onset and repeated the electrodiagnostic study 21 months later, after intramuscular vitamin B12 supplementation.
Results
Standard electroneurography demonstrated axonal sensory polyneuropathy. Needle electromyography (EMG) in tibialis anterior muscle was unremarkable. Cutaneous silent periods in tibialis anterior muscle after noxious electrical sural nerve stimulation were delayed, with incomplete EMG suppression concurring with dysfunction of thin-myelinated A-delta fibers. Quantitative sensory testing revealed altered cold and warm perception thresholds in both upper limbs, but normal values in both lower limbs. A follow-up electrodiagnostic study after 21 months intramuscular vitamin B12 supplementation revealed improvement of all neurophysiological findings, including normalization of cutaneous silent periods.
Conclusions
Thin-myelinated A-delta fibers may be affected in B12 hypovitaminosis and may show recovery after intramuscular vitamin B12 supplementation. CSP may serve to diagnose small fiber affection in this medical condition and to monitor their recovery after vitamin supplementation.
Significance
CSP testing represents a useful, non-invasive, rapidly available diagnostic and follow-up tool in vitamin B12 deficiency.
1. Introduction
Vitamin B12 (cobalamin) deficiency is common in adult and elderly patients and is often underdiagnosed because of its polymorphous symptoms. It has a prevalence ranging from 5 to 60%, depending on the definition of cobalamin deficiency used in the study (Andrès et al., 2004). The most frequent causes of B12 hypovitaminosis are the food-cobalamin malabsorption syndrome, which leads to the inability to release cobalamin from food or from intestinal transport proteins, and autoantibodies against gastric parietal cells, causing an autoimmune atrophic gastritis and leading to pernicious anemia (Andrès et al., 2004). In the general population, the prevalence of pernicious anemia is 0.1% and reaching 1.9% in subjects over the age of 60 years (Carmel, 2004, Toh et al., 1997). Further causes of B12 hypovitaminosis are dietary deficiency, gastric surgery, malabsorption, drugs, and genetic mutations.
The symptoms of vitamin B12 deficiency vary in severity and include hematological, gastrointestinal, neurological, and psychiatric manifestations. Frequent neurological findings are axonal polyneuropathy and subacute combined degeneration (SCD) of the spinal cord.
Electrophysiological findings in patients with vitamin B12 deficiency typically show a sensorimotor axonopathy (Hemmer et al., 1998). Small-diameter fiber involvement has rarely been described in patients with B12 hypovitaminosis to date (Khan and Zhou, 2012). Cutaneous silent periods (CSPs) may serve to test small-diameter fiber function non-invasively, using routine electrodiagnostic equipment, but to the best of our knowledge have not been studied so far in vitamin B12 deficiency. A CSP is a relative or absolute transient decrease in voluntary EMG activity following noxious stimulation of a nearby cutaneous nerve. This inhibitory reflex is considered to be mediated at the spinal level, with an afferent arch supplied by small-diameter A-delta fibers and an efferent arch supplied by alpha-motoneurons (Kofler et al., 2019a). The CSP onset latency is primarily dependent on the conduction velocity in afferent A-delta fibers and to a minor degree on that in efferent alpha motoneurons and on the spinal synaptic delay. CSP duration, hence also CSP end latency, is a function of the number of intact A-delta fibers (Kofler et al., 2019b).
2. Case presentation
A 49-year-old male patient presented to our neurological clinic with a 6-month history of numbness and tingling of both lower limbs, mild distal tingling in the upper limbs, unsteadiness of gait and blurred peripheral vision. Initially, he was seen by his family physician, who prescribed a multi-vitamin preparation as empirical therapy and subsequently sent him to an orthopedic surgeon, who diagnosed a difference in leg length, but who also initiated further diagnostic studies. A first laboratory examination after three months was normal except for reduced red blood cell count (3.21 T/l) and hyperchromic macrocytosis (mean corpuscular hemoglobin 40.4 pg, mean corpuscular volume 130.2 fl). Vitamin B12 serum levels were at the lower limit of the normal range (242 pg/ml) after intake of oral vitamin B1, B6 and B12 supplementation for three months. Five months after symptom onset, when the patient’s complaints did not ameliorate, he was referred to a practicing internal medicine specialist and to a practicing neurologist. At that time, the patient’s neurological examination showed normal muscle tone and muscle strength in upper and lower limbs, absent pyramidal signs, areflexia in both lower extremities, pallhypesthesia, and reduced temperature sensation in the lower limbs. His gait was slightly unsteady. Meanwhile, the internist had obtained another blood count, which showed slight improvement (red blood cells: 4.05 T/l, mean corpuscular hemoglobin: 36.8 pg, mean corpuscular volume: 111.1 fl), while the patient was still on oral multi-vitamins. Anti-parietal cell antibodies were detected (1:1600), but no antibodies against intrinsic factor, and gastroscopy showed atrophic gastritis. A diagnosis of B12 hypovitaminosis due to autoantibodies against gastric parietal cells was established, and therapy with intramuscular administration of vitamin B12 was initiated with injections twice weekly for the first 4 weeks, then once monthly for three months and later once every two months. A follow-up laboratory investigation after 15 months documented a vitamin B12 incline to 385 pg/ml.
Six months after symptom onset, during the first month of weekly intramuscular vitamin B12 supplementation, the patient was referred to our clinic for confirmation of polyneuropathy.
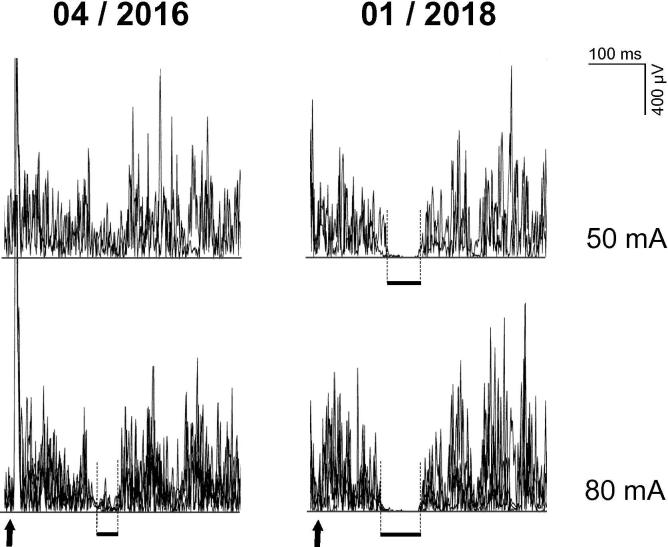
Routine electrodiagnostic equipment was used (Viking EDX System, Natus, Middleton, WI, USA). Standard electroneurography demonstrated axonal sensory polyneuropathy (Table 1). Concentric needle electromyography (EMG) in tibialis anterior muscle was unremarkable. Exteroceptive EMG suppression was assessed according to previously published techniques (Svilpauskaite et al., 2006). CSPs in tibialis anterior muscle after stimulating the sural nerve at the lateral malleolus (0.5 ms stimulus duration, 50 and 80 mA intensity, delivered during 5 s periods of 50% of maximum voluntary contraction every 10 s, filters set at 30 and 10000 Hz, single rectified EMG traces recorded with 500 ms sweep duration) appeared delayed, with incomplete EMG suppression, but normal duration as compared to normative values obtained in 23 healthy subjects (age 20–64 years) (Fig. 1, Table 1). Quantitative sensory testing (Kumru et al., 2013) performed on the same day revealed warm perception thresholds above normal limits in both upper limbs and in the upper normal range in both lower limbs. Cold perception threshold was below normal limits in the left hand and borderline in the right hand. Heat pain perception thresholds were in the upper normal range in the upper limbs, and normal in the lower limbs. Comparative normal values were obtained from 43 healthy subjects (age 18–60 years) (Table 1).
Table 1.
Results of nerve conduction studies (NCS), cutaneous silent period testing in tibialis anterior muscle following sural nerve stimulation (CSP-TA-sur), and quantitative sensory testing (QST) during first month of vitamin B12 substitution (first examination) and 21 months later (second examination). CMAP = compound muscle action potential, SNAP = sensory nerve action potential, NCV = nerve conduction velocity. Abnormal values are bold.
| First examination | Second examination | Lower normal limit normal range |
|||
|---|---|---|---|---|---|
| NCS | |||||
| Right peroneal nerve | CMAP amplitude | [mV] | 5.7 | 7.3 | 4.0‡ |
| Motor NCV | [m/s] | 46 | 48 | 41‡ | |
| SNAP amplitude | [µV] | 3 | 5 | 5‡ | |
| Sensory NCV | [m/s] | 34 | 42 | 39‡ | |
| Left sural nerve | SNAP amplitude | [µV] | 1 | 7 | 4‡ |
| Sensory NCV | [m/s] | 36 | 41 | 39‡ | |
| Right CSP-TA-sur | Onset latency | [ms] | 140.0 | 112.5 | 83.2–122.4* |
| End latency | [ms] | 178.4 | 172.4 | 128.5–166.1* | |
| Duration | [ms] | 38.4 | 59.9 | 26.5–62.5* | |
| QST | Location (dermatome) | Right/left side | Right/left side | ||
| Warm perception | Hand (C6) | [°C] | 35.0/34.6 | 33.9/33.5 | 32.5–34.0* |
| Foot (L5) | [°C] | 37.9/37.2 | 38.1/36.9 | 32.3–40.8* | |
| Cold perception | Hand (C6) | [°C] | 30.7/30.1 | 30.9/30.3 | 30.3–31.7* |
| Foot (L5) | [°C] | 29.3/29.2 | 27.0/27.2 | 26.9–32.8* | |
| Heat pain perception | Hand (C6) | [°C] | 50.3/47.8 | 48.5/48.4 | 40.1–51.5* |
| Foot (L5) | [°C] | 47.8/46.6 | 47.9/47.7 | 41.4–49.9* | |
Lower normal limit.
Normal range.
Fig. 1.
Cutaneous silent periods in right tibialis anterior muscle after stimulating the right sural nerve at the lateral malleolus with stimulus intensities of 50 mA (two traces superimposed) and 80 mA (four traces superimposed) during the first month of intramuscular vitamin B12 substitution (04/2016) and 21 months later (01/2018). Arrows indicate stimulus onset. Thick horizontal lines indicate CSP onset, end, and duration.
A follow-up electrodiagnostic study 21 months later confirmed normal motor nerve conduction parameters and documented normalization of sensory neurographies with borderline amplitude values for superficial peroneal nerve (Table 1). Likewise, repeat CSP studies revealed normal values and no longer showed dysfunction of thin-myelinated A-delta nerve fibers (Fig. 1, Table 1). Finally, repeat quantitative sensory testing revealed all temperature perception thresholds within normal limits, although cold perception thresholds were just borderline in both upper and lower extremities. (Table 1). At the same time, follow-up laboratory investigation revealed a vitamin B12 level of 440 pg/ml, fully restored erythrocytes (4.98 T/l), normal mean corpuscular hemoglobin (30.9 pg), and normal mean corpuscular volume (89.0 fl).
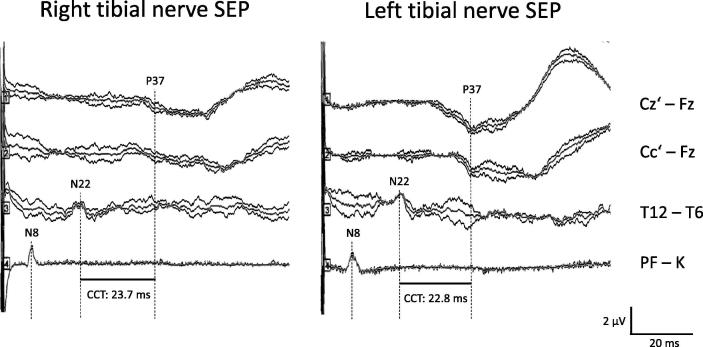
On clinical examination, the patient still had normal muscle tone and strength, and absent pyramidal signs. Muscle reflexes had reappeared; touch, pinprick, and temperature sensation were normal; visual deficits had subsided; and gait was steady. Pallhypesthesia had substantially improved. The patient, however, complained of persisting tingling sensation in both lower limbs with spread above the knee level. Somatosensory evoked potentials (SEPs) were obtained according to published techniques (Kofler et al., 2003) in order to investigate central conduction function along the spinal cord. Median nerve SEPs were unremarkable, but tibial nerve SEPs showed a prolonged central conduction time (Fig. 2), pointing to a possible role of dorsal columns contributing to the patient’s sensory disturbance. Suspecting SCD of the spinal cord, we decided to perform spinal magnetic resonance imaging (MRI) (sagittal T1-weighted turbo spin echo, sagittal and axial T2 weighted turbo spin echo); the results, however, were unremarkable. A cerebral MRI was not obtained, as visual disturbances are a known feature of B12 hypovitaminosis, and visual symptoms had subsided quickly in parallel with amelioration of other symptoms during the course of vitamin B12 supplementation.
Fig. 2.
Tibial nerve somatosensory evoked potentials following right (left panel) and left side stimulation (right panel) at the ankle (square pulses of 0.2 ms duration, 3.7 Hz), obtained 21 months after initiation of intramuscular vitamin B12 substitution. Traces from below to above represent recordings (2 times 150 responses plus grand average superimposed) of the tibial nerve in popliteal fossa (N8, PF – K), of the conus medullaris (N22; twelfth thoracic vertebra versus thoracic reference: T12 – T6), and of leg area of the contralateral somatosensory cortex (P37; cephalic recordings versus frontal reference, Ci’ – Fz, Cz’ – Fz). The bars at the bottom indicate the central conduction time (CCT, i.e., interpeak latency N22-P37), which is prolonged on both sides (upper normal limit 20.1 ms). Note also the untypical configuration of the cortical responses.
3. Discussion
Here we report for the first time abnormal CSPs in a patient suffering from vitamin B12 deficiency and document their normalization with long-term vitamin B12 substitution on follow-up exam some 2 years later.
The exact prevalence of vitamin B12 deficiency in the general population is unknown. In the elderly population it seems to occur in up to 20% of individuals. In the United States, it has been demonstrated that the prevalence of vitamin B12 deficiency varies by age range, affecting at least 3% of those aged 20–39 years old and 4% of those aged 40–59 (Allen, 2009).
The patient we studied was diagnosed with autoimmune chronic atrophic gastritis due to parietal cell antibodies. This clinical condition is one of the most common causes of vitamin B12 deficiency: it accounts for 20–50% of the documented cases of B12 hypovitaminosis in adults (Andrès et al., 2004) but seems to represent a minority of cases in comparison to food-bound cobalamin malabsorption according to a recent review (Shipton and Thachil, 2015).
In the present patient, the diagnosis of B12 hypovitaminosis was made after a complex diagnostic approach, involving family physician, orthopedic surgeon, and internal medicine specialist, leading finally to laboratory investigations and gastroscopy. Previous literature has reported a frequent delay in diagnosis and many unnecessary procedures performed before serum cobalamin levels were finally obtained (Healton et al., 1991, Oo and Rojas-Hernandez, 2017).
Vitamin B12 deficiency is usually associated with hematological, gastrointestinal, neurological, and neuropsychiatric manifestations. Neurological symptoms may be the only manifestation of B12 deficiency, in the absence of anemia (Ralapanawa et al., 2015).
Initially our patient presented with mildly reduced erythrocyte count and showed hyperchromic macrocytosis, in accordance with recent literature (Oo and Rojas-Hernandez, 2017). Later, parietal cell antibodies were positive and gastroscopy showed atrophic gastritis concurring with an autoimmune etiology. His symptoms were in accordance with the known neurological manifestations of vitamin B12 deficiency, including paresthesia of the upper and lower limbs, gait disorder, and visual field defects.
A limitation of our case presentation is the lack of an initial vitamin B12 level, as the first laboratory examination was performed as late as three months after symptom onset, after the patient had already taken oral vitamins for three months. Still, vitamin B12 levels were low at that time. They increased with substitution in parallel with normalization of erythrocyte parameters and of electrodiagnostic findings, along with clinical improvement. This clinical course together with a high titer of parietal cell antibodies concur with a causative role of B12 hypovitaminosis for low red blood cell count, hyperchromic macrocytosis, and large- and small-diameter polyneuropathy.
Polyneuropathy accounts for 30–50% of neurological symptoms associated with vitamin B12 deficiency (Franques and Gazzola, 2013), being most commonly symmetrical, length-dependent axonal sensory or sensorimotor neuropathies (Saperstein et al., 2003, Huang et al., 2011). Others reported axonal polyneuropathies in 76% and demyelinating polyneuropathies in 24% of patients (Puri et al., 2005). Routine electrophysiological findings confirmed the presence of axonal large-diameter fiber sensory polyneuropathy, which improved considerably and even normalized on follow-up after almost 2 years of vitamin B12 substitution in the present patient.
One possible explanation for the recovery is axonal regeneration, which has been described to occur as axons regrow from proximal to distal, concurring with slow improvement of the amplitude in serial sensory and motor neurographies over three years (Huang et al., 2011, Kalita et al., 2014, Franques et al., 2019), similar to our patient. However, the course of improvement is often too fast to be considered pure axonal regeneration. The relatively rapid recovery course in some patients has therefore been considered to be due to remyelination within the central or peripheral nervous system (Fine and Soria, 1991). Histopathological evidence suggests co-occurrence of axonal degeneration, regeneration, demyelination and secondary remyelination with increasing duration of illness (Kalita et al., 2014). These findings are in agreement with reported electrodiagnostic changes of both axonal and demyelinating type, obviously being the case for both large- as well as small-diameter fibers, which also concur at least in part with the variable clinical course of recovery during treatment.
Vitamin B12 deficiency is among the leading causes of secondary small fiber neuropathy (Chan and Wilder-Smith, 2016, Themistocleous et al., 2014), affecting thin-myelinated A-delta fibers or unmyelinated C fibers. A recent study (Güneş et al., 2017) demonstrated a reduced number of small fibers in skin punch biopsy of vitamin B12 deficient patients with or without neuropathic pain. There was, however, no correlation of small fiber loss with vitamin B12 serum levels, duration of neuropathic pain, or subjective intensity of pain obtained with visual analogue scores. To our knowledge, no study to date has investigated CSPs in B12 hypovitaminosis.
A CSP is identified by a relative or absolute transient decrease in the voluntary EMG activity following noxious stimulation of a nearby cutaneous nerve. This robust inhibitory reflex is considered to be mediated at the spinal level, with an afferent arch supplied by A-delta fibers and an efferent arch supplied by alpha motoneurons (Kofler et al., 2019a). Hence, CSP abnormalities may reflect spinal and peripheral nerve dysfunction (Kofler et al., 2019b). Due to lack of habituation of the CSP (Uncini et al., 1991, Serrao et al., 2001) only very few synapses seem to be involved (Kofler et al., 2003). Putative spinal circuitry has been suggested by Kofler et al. (2003).
Observed CSP abnormalities in the present patient, i.e. delayed onset but normal duration, is actually in agreement with demyelination of A-delta fibers (Lopergolo et al., 2015), while incomplete EMG suppression rather suggests profound axonal loss of A-delta fibers. The fact that CSP abnormalities returned to entirely normal values after 21 months of intramuscular vitamin B12 substitution also concurs with demyelination and secondary remyelination (Fine and Soria, 1991, Kalita et al., 2014), which tends to recover better than axonal loss. Furthermore, spinal centromedullary pathology seems to be an unlikely cause of the observed CSP abnormalities, as clinical symptomatology pointed to distal peripheral nerve involvement rather than spinal cord dysfunction, tibial nerve SEPs documented dorsal column dysfunction, but no centromedullary dysfunction, and spinal MRI was unremarkable.
Quantitative sensory testing also showed abnormal results initially, compatible with hypothermesthesia due to small fiber dysfunction, concurring with previous literature (Saperstein et al., 2003): Notably, results improved with vitamin B12 substitution, as did the other electrodiagnostic findings.
The normalized neurophysiological findings, however, were not paralleled by complete clinical improvement. Although touch, pinprick, and temperature sensation returned to normal, and visual and gait disturbance subsided, the patient reported persistent paresthesia progressing proximally to the knees two years after initial symptoms. Abnormally slowed central conduction time, as evident in tibial nerve SEPs (Fig. 2), may have contributed to the patient’s complaints. Similar findings have previously been described in patients with SCD (Karnaze and Carmel, 1990). Spinal MRI could not detect any T2-hyperintensity typical for SCD. However, sensitivity of spinal cord MRI for SCD lesions is low (14.8% according to the study of Jain et al., 2014). Furthermore, spinal cord abnormality seems not to correlate with clinical severity of vitamin B12 deficiency (Jain et al., 2014). The resolution over time of spinal cord signal abnormalities may occur more quickly than clinical normalization, and clinical signs may persist despite complete resolution of imaging abnormalities (Locatelli et al., 1999). Therefore, a normal spinal MRI does not exclude the presence of SCD.
Although most reports describe a rapid reduction of clinical symptoms after intramuscular vitamin B12 substitution, there is evidence that neurological manifestations may only partially regress despite prolonged and high-dose vitamin B12 therapy, possibly leading to irreversible sequelae (Healton et al., 1991, Martin et al., 1992).
In conclusion, investigation of small-diameter fiber function may be meaningful in B12 hypovitaminosis as A-delta and C fibers can be affected in this medical condition. Thin- and unmyelinated fibers cannot be assessed by routine nerve conduction studies, but CSP testing represents a simple non-invasive neurophysiological technique which only requires routine electrodiagnostic equipment and offers a useful additional diagnostic and follow-up tool.
Informed consent
A written informed consent was obtained from the patient for the publication of this case report.
Author contributions statement
EF and MK performed the acquisition of data, drafting and revising of the manuscript and accepted responsibility for conduct of research and final approval. LS revised the manuscript and accepted responsibility for conduct of research and final approval.
Funding
No funding was obtained for this research.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors express their gratitude to Hermine Reindl, MD, for referring the patient, to Elke Gizewski, MD, for magnetic resonance imaging, to Maria Hoch, for technical assistance with neurophysiological testing, and to Ellen Quirbach, for her help with editing the manuscript.
References
- Allen L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009;89(2):693S–696S. doi: 10.3945/ajcn.2008.26947A. [DOI] [PubMed] [Google Scholar]
- Andrès E., Loukili N.H., Noel E., Kaltenbach G., Abdelgheni M.B., Perrin A.E. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ. 2004;171(3):251–259. doi: 10.1503/cmaj.1031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel R. Pernicious anemia. In: Johnson L.R., editor. Encyclopedia of Gastroenterology. Academic Press; Waltham, MA: 2004. pp. 170–171. [Google Scholar]
- Chan A.C., Wilder-Smith E.P. Small fiber neuropathy: getting bigger! Muscle Nerve. 2016;53(5):671–682. doi: 10.1002/mus.25082. [DOI] [PubMed] [Google Scholar]
- Fine E.J., Soria E.D. Myths about vitamin B12 deficiency. South. Med. J. 1991;84(12):1475–1481. doi: 10.1097/00007611-199112000-00016. [DOI] [PubMed] [Google Scholar]
- Franques J., Gazzola S. Metabolic and nutritional neuropathies: update in diabetes, vitamin B12 and copper deficiency. Rev. Neurol. (Paris) 2013;169(12):991–996. doi: 10.1016/j.neurol.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Franques J., Chiche L., De Paula A.M., Grapperon A.M., Attarian S., Pouget J. Characteristics of patients with vitamin B12-responsive neuropathy: a case series with systematic repeated electrophysiological assessment. Neurol. Res. 2019;1–8 doi: 10.1080/01616412.2019.1588490. [DOI] [PubMed] [Google Scholar]
- Güneş H.N., Bekircan-Kurt C.E., Tan E., Erdem-Özdamar S. The histopathological evaluation of small fiber neuropathy in patients with vitamin B12 deficiency. Acta Neurol. Belg. 2017;118(3):405–410. doi: 10.1007/s13760-017-0847-y. [DOI] [PubMed] [Google Scholar]
- Healton E.B., Savage D.G., Brust J.C., Garrett T.J., Lindenbaum J. Neurologic aspects of cobalamin deficiency. Medicine. 1991;70(4):229–245. doi: 10.1097/00005792-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Hemmer B., Glocker F.X., Schumacher M., Deuschl G., Lücking C.H. Subacute combined degeneration: clinical, electrophysiological, and magnetic resonance imaging findings. J. Neurol. Neurosurg. Psychiatry. 1998;65(6):822–827. doi: 10.1136/jnnp.65.6.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.R., Chang W.N., Tsai N.W., Lu C.H. Serial nerve conduction studies in vitamin B-12 deficiency-associated polyneuropathy. Neurol. Sci. 2011;32(1):183–186. doi: 10.1007/s10072-010-0428-9. [DOI] [PubMed] [Google Scholar]
- Jain K.K., Malhotra H.S., Garg R.K., Gupta P.K., Roy B., Gupta R.K. Prevalence of MR imaging abnormalities in vitamin B12 deficiency patients presenting with clinical features of subacute combined degeneration of the spinal cord. J. Neurol. Sci. 2014;342(1–2):162–166. doi: 10.1016/j.jns.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Kalita J., Chandra S., Bhoi S.K., Agarwal R., Misra U.K., Shankar S.K. Clinical, nerve conduction and nerve biopsy study in vitamin B12 deficiency neurological syndrome with a short-term follow-up. Nutr. Neurosci. 2014;17(4):156–163. doi: 10.1179/1476830513Y.0000000073. [DOI] [PubMed] [Google Scholar]
- Karnaze D.S., Carmel R. Neurologic and evoked potential abnormalities in subtle cobalamin deficiency states including deficiency without anemia and with normal absorption of free cobalamin. Arch. Neurol. 1990;47:1008–1012. doi: 10.1001/archneur.1990.00530090082017. [DOI] [PubMed] [Google Scholar]
- Khan S., Zhou L. Characterization of non-length-dependent small-fiber sensory neuropathy. Muscle Nerve. 2012;45(1):86–91. doi: 10.1002/mus.22255. [DOI] [PubMed] [Google Scholar]
- Kofler M., Kronenberg M.F., Brenneis C., Felber A., Saltuari L. Cutaneous silent periods in intramedullary spinal cord lesions. J. Neurol. Sci. 2003;216(1):67–79. doi: 10.1016/s0022-510x(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Kofler M., Leis A.A., Valls-Solé J. Cutaneous silent periods – part 2: pathophysiology and clinical utility. Clin. Neurophysiol. 2019;130(4):604–615. doi: 10.1016/j.clinph.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Kofler M., Leis A.A., Valls-Solé J. Cutaneous silent periods – part 1: update on physiological mechanisms. Clin. Neurophysiol. 2019;130(4):588–603. doi: 10.1016/j.clinph.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Kumru H., Kofler M., Flores M.C., Portell E., Robles V., Leon N. Effect of intrathecal baclofen on evoked pain perception: an evoked potentials and quantitative thermal testing study. Eur. J. Pain. 2013;17(7):1039–1047. doi: 10.1002/j.1532-2149.2012.00266.x. [DOI] [PubMed] [Google Scholar]
- Locatelli E.R., Laureno R., Ballard P., Mark A.S. MRI in vitamin B12 deficiency myelopathy. Can. J. Neurol. Sci. 1999;26:60–63. [PubMed] [Google Scholar]
- Lopergolo D., Isak B., Gabriele M., Onesti E., Ceccanti M., Capua G. Cutaneous silent period recordings in demyelinating and axonal polyneuropathies. Clin. Neurophysiol. 2015;126(9):1780–1789. doi: 10.1016/j.clinph.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Martin D.C., Francis J., Protech J., Huff F.J. Time dependency of cognitive recovery with cobalamin replacement: report of a pilot study. J. Am. Geriatr. Soc. 1992;40(2):168–172. doi: 10.1111/j.1532-5415.1992.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Oo T.H., Rojas-Hernandez C.M. Challenging clinical presentations of pernicious anemia. Discov. Med. 2017;24(131):107–115. [PubMed] [Google Scholar]
- Puri V., Chaudhry N., Goel S., Gulati P., Nehru R., Chowdhury D. Vitamin B12 deficiency: a clinical and electrophysiological profile. Electromyogr. Clin. Neurophysiol. 2005;45(5):273–284. [PubMed] [Google Scholar]
- Ralapanawa D.M., Jayawickreme K.P., Ekanayake E.M., Jayalath W.A. B12 deficiency with neurological manifestations in the absence of anaemia. BMC Res. Notes. 2015;8:458. doi: 10.1186/s13104-015-1437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saperstein D.S., Wolfe G.I., Gronseth G.S., Nations S.P., Herbelin L.L., Bryan W.W. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch. Neurol. 2003;60(9) doi: 10.1001/archneur.60.9.1296. [DOI] [PubMed] [Google Scholar]
- Serrao M., Parisi L., Pierelli F., Rossi P. Cutaneous afferents mediating the cutaneous silent period in the upper limbs: evidences for a role of low-threshold sensory fibres. Clin. Neurophysiol. 2001;112(11):2007–2014. doi: 10.1016/s1388-2457(01)00675-7. [DOI] [PubMed] [Google Scholar]
- Shipton M.J., Thachil J. Vitamin B12 deficiency – A 21st century perspective. Clin. Med. (Lond.) 2015;15(2):145–150. doi: 10.7861/clinmedicine.15-2-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svilpauskaite J., Truffert A., Vaiciene N., Magistris M.R. Electrophysiology of small peripheral nerve fibers in man. A study using the cutaneous silent period. Medicina. 2006;42(4):300–313. [PubMed] [Google Scholar]
- Themistocleous A.C., Ramirez J.D., Serra J., Bennett D.L. The clinical approach to small fibre neuropathy and painful channelopathy. Pract. Neurol. 2014;14(6):368–379. doi: 10.1136/practneurol-2013-000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh B.H., Van Driela I.R., Gleeson P.A. Pernicious anemia. N. Eng. J. Med. 1997;337:1441–1448. doi: 10.1056/NEJM199711133372007. [DOI] [PubMed] [Google Scholar]
- Uncini A., Kujirai T., Gluck B., Pullman S. Silent period induced by cutaneous stimulation. Electroencephalogr. Clin. Neurophysiol. 1991;81(5):344–352. doi: 10.1016/0168-5597(91)90023-q. [DOI] [PubMed] [Google Scholar]