Abstract
Introduction
Treatment of paediatric heart failure is based on paradigms extensively tested in the adult population assuming similar underlying pathophysiological mechanisms. Angiotensin converting enzyme inhibitors (ACEI) like enalapril are one of the cornerstones of treatment and commonly used off-label in children. Dose recommendations have been extrapolated from adult experience, but the relationship between dose and pharmacokinetics (PK) in (young) children is insufficiently studied. Furthermore, appropriate paediatric formulations are lacking. Within the European collaborative project LENA, a novel formulation of enalapril orodispersible minitablets (ODMT), suitable for paediatric administration, will be tested in (young) children with heart failure due to either dilated cardiomyopathy or congenital heart disease in two pharmacokinetic bridging studies. Paediatric PK data of enalapril and its active metabolite enalaprilat will be obtained. In a follow-up study, the safety of enalapril ODMTs will be demonstrated in patients on long-term treatment of up to 10 months. Furthermore, additional information about pharmacodynamics (PD) and ODMT acceptability will be collected in all three studies.
Methods and Analysis
Phase II/III, open-label, multicentre study. Children with dilated cardiomyopathy (DCM) (n = 25; 1 month to less than 12 years) or congenital heart disease (CHD) (n = 60; 0 to less than 6 years) requiring or already on ACEI will be included. Exclusion criteria include severe heart failure precluding ACEI use, hypotension, renal impairment, hypersensitivity to ACEI. For those naïve to ACEI up-titration to an optimal dose will be performed, those already on ACEI will be switched to an expected equivalent dose of enalapril ODMT and optimised. In the first 8 weeks of treatment, a PK profile will be obtained at the first dose (ACEI naïve patients) or when an optimal dose is reached. Furthermore, population PK will be done with concentrations detected over the whole treatment period. PD and safety data will be obtained at least at 2-weeks intervals. Subsequently, an intended number of 85 patients will be followed-up up to 10 months to demonstrate long-term safety, based on the occurrence of (severe) adverse events and monitoring of vital signs and renal function.
Ethics and dissemination
Clinical Trial Authorisation and a favourable ethics committee opinion were obtained in all five participating countries. Results of the studies will be submitted for publication in a peer-reviewed journal.
Trial registration numbers
EudraCT 2015-002335-17, EudraCT 2015-002396-18, EudraCT 2015-002397-21.
Keywords: Clinical pharmacology, Paediatric cardiology, Heart failure, Dilated cardiomyopathy, Congenital heart disease
1. Introduction
Heart failure in children has been defined as a clinical and pathophysiologic syndrome resulting from ventricular dysfunction, volume, or pressure overload, or a combination of these causes. Characteristic signs and symptoms are poor growth, feeding difficulties, respiratory distress, and fatigue. In line with the pathophysiology of heart failure in adults, an elevation of numerous neuro-humoral and inflammatory mediators, such as the renin-angiotensin-aldosterone system (RAAS) and natriuretic peptides have been found in children [1,2]. Heart failure in children has a variety of aetiologies, including congenital heart disease (CHD) and cardiomyopathies [3].
More than 50% of paediatric patients have heart failure due to CHD, with this aetiology being disproportionately high in the first year of life [4,5]. It is associated with ventricular dysfunction, volume or pressure overload. Heart failure in paediatric patients with CHD has various aetiologies and the risk of ventricular dysfunction depends on the specific type of malformation [6].
Dilated cardiomyopathy (DCM) refers to a large group of heterogeneous myocardial disorders that are characterised by a decreased systolic function, ventricular dilation and reduced cardiac output. Clinical onset is most often seen in infancy and although it accounts for only a small proportion of heart failure cases in children, it is particularly relevant as it is the main indication for paediatric heart transplantation [7].
To date, the pharmacological therapy of paediatric heart failure is mainly informed by empirical evidence and data extrapolation from studies in the adult population. Systematic dose-finding and long-term safety studies in children have been hampered by methodological and practical difficulties. Therefore, appropriate doses that are safe and efficacious in different paediatric age groups are not well established.
Heart failure medications including angiotensin converting enzyme (ACE) inhibitors have shown efficacy in controlled randomised clinical trials in adults with heart failure. ACE inhibitor (ACEI) therapy in adults should be initiated at low doses followed by up-titration if lower doses have been well tolerated. At optimal doses, ACE inhibitor therapy reduces symptoms and improves survival of adult heart failure patients. Dose-limiting adverse events in adults include hypotension, worsening renal function and hyperkalaemia [8].
Enalapril is an ACE inhibitor commonly administered off-label to young children with heart failure using extemporaneous formulations. It is not labelled for patients below the age of 6 (<20 kg) in the EU [9]. The beneficial effects of enalapril are assumed to be similar to those in adults with heart failure due to cardiomyopathy or coronary heart disease, resulting from afterload reduction improving cardiac output and preventing cardiac hypertrophy and remodelling, as well as long term inhibition of salt and water retention [10,11]. The European Medicine Agency Expert Group Meeting on Paediatric Heart Failure considered enalapril a first-line treatment for chronic heart failure in children [12]. However, no age-appropriate and stable formulation exists in Europe and paediatric pharmacokinetic (PK) and safety data for any formulation is insufficient. Pharmacodynamics (PD) and efficacy data for paediatric cardiac indications are limited [[13], [14], [15]].
This lack of age-appropriate formulations and data for paediatric use in general led the EU to implement regulations to encourage the development of medicines and age-appropriate formulations for children and prioritised a list of off-patent paediatric medicinal products to be studied, making them subsequently eligible for a Paediatric Use Marketing Authorisation (PUMA).
The European collaborative project LENA (Labelling Enalapril from Neonates up to Adolescents) aims to address these shortcomings and provide a basis for a future PUMA of enalapril for children with heart failure.
As part of the LENA project, a novel formulation of orodispersible minitablets (ODMT) was developed, small sized tablets (2 mm in diameter), which rapidly dissolve upon contact with water or saliva, suitable for paediatric administration [16]. Subsequently, a bioequivalence study in healthy adults was performed, showing comparable relative pharmacokinetics and drug exposure of the new ODMT and a standard tablet formulation [17].
This manuscript presents the protocols of two clinical phase II/III prospective, open-label, multicentre PK bridging studies in children (1 month to less than 12 years of age) with heart failure due to DCM and CHD and one common follow-up safety study for both populations.
Study patients will be treated with the newly developed ODMTs of enalapril and systematically assessed for PK, PD [[18], [19], [20], [21], [22]], clinical parameters as well as acceptability and palatability. Due to strongly established efficacy of enalapril in adult heart failure and the similarity of the pathophysiology of heart failure in adult and paediatric patients, the Paediatric Committee (PDCO) of the European Medicines Agency (EMA) agreed that PK bridging instead of proof of efficacy would provide an adequate demonstration of treatment suitability of the newly developed enalapril ODMTs in this population. Moreover, the studies will collect safety data over 8 weeks. Subsequently, patients will be included in an open-label, multicentre 10 months follow-up safety study. This will allow for a systematic collection of safety data in paediatric patients under stable optimal dose or no longer under enalapril ODMT treatment who have received at least 3 days of ODMT treatment but then stopped.
In a pharmacogenomics and metabolomics sub-study, we aim to study the metabolite profile of children before and after enalapril therapy, as well as the genes that determine enalapril disposition and effect, for better understanding the underlying disease and the response to enalapril therapy in our population. Several single-nucleotide polymorphisms (SNPs) have been studied in relation to enalapril PK and PD in adults, with some showing promise to individualize enalapril therapy [23].
2. Methods and analysis
2.1. Study design
This manuscript describes the design of two phase II/III prospective, open-label, multicentre PK bridging studies with exploratory PD assessments. Twenty-five children (1 month to less than 12 years of age) with heart failure due to DCM (EudraCT 2015-002335-17) and 60 children (up to less than 6 years of age) with heart failure due to CHD (EudraCT 2015-002396-18) will be treated with newly developed ODMTs of enalapril. A minimum of 37 infants (0 to less than 12 months) must be included.
Additionally, the manuscript presents the protocol of a phase II/III prospective, open-label, safety follow-up study (EudraCT 2015-002397-21) in infants and children with heart failure previously enrolled in the two PK studies, under stable optimal dose or no longer under enalapril ODMT treatment, with exploratory PD assessment in all children and PK assessments in children under ODMT treatment.
2.2. Study objectives
The primary objective of the pharmacokinetic bridging studies is to obtain paediatric PK data of enalapril and its active metabolite enalaprilat in these children in order to characterise the dose exposure in the paediatric population with DCM or CHD. The secondary study objectives are:
-
-
to demonstrate safety, in particular renal safety, of enalapril ODMTs in children with DCM/CHD,
-
-
to characterise the dose/safety relationship from a starting dose to an optimal maintenance dose,
-
-
to explore the dose-exposure-response relationship with PD parameters in the paediatric population with DCM/CHD,
-
-
to investigate the left ventricular shortening fraction (SF) by echocardiography,
-
-
to investigate the acceptability and palatability of enalapril ODMTs in the paediatric population with DCM/CHD.
The objectives of the pharmacogenomics and metabolomics sub-study are to explore the relationship between selected SNPs and enalapril PK and PD, and to explore the metabolite profile in children receiving enalapril ODMT. A comparison of the metabolite profile before and after the start of enalapril, as well as between patients with DCM and patients with CHD, is planned.
The primary objective of the follow-up safety study is to demonstrate the safety of enalapril ODMTs. The secondary objectives are:
-
-
to describe the acceptability and palatability of enalapril ODMTs,
-
-
to collect additional information about PK and PD of enalapril ODMTs during long-term treatment.
The studies were designed by the LENA consortium. The studies are being performed at seven sites in five countries in Europe. The pharmacokinetic bridging studies started in January 2016, the follow-up study started in March 2016. The LENA project is planned to be completed by April 2019. The active treatment period per participant in the pharmacokinetic bridging studies will be a minimum of three days up to eight weeks; the observation period is eight weeks. The follow-up period per participant will be up to 10 months.
2.3. Study population
Inclusion criteria: Patients fulfilling the following inclusion criteria can be enrolled in the pharmacokinetic bridging studies:
-
-
Diagnosis of heart failure due to CHD requiring after load reduction by drug therapy.
or
-
-
Diagnosis of DCM presenting with LV end-diastolic dimension ˃P95 and/or LV shortening fraction ˂25% in patients, resulting from different types of underlying cardiac disease with signs of decreased systolic LV function, and without ACE inhibitor treatment; patients with ACE inhibitor pre-treatment must have documented evidence of having fulfilled these criteria before start of the ACE inhibitor therapy.
-
-
Male and female patients.
-
-
Age 1 month to less than 12 years for patients with DCM
-
-
Age from birth to less than 6 years for patients with CHD
-
-
Weight greater than 2.5 kg
-
-
Subjects may be naïve to ACE inhibitors.
-
-
Subjects already on ACE inhibitor willing to switch to enalapril ODMTs.
-
-
Written informed consent from parent(s)/legal representative and assent from the patient according to national legislation and as far as achievable from the child.
Exclusion criteria: Patients fulfilling any of the following criteria will be excluded:
-
-
Severe heart failure and/or end stage heart failure precluding introduction or continuation of ACE inhibitor.
-
-
Too low blood pressure, e.g. less than P5 for age.
-
-
Restrictive and hypertrophic cardiomyopathies.
-
-
Obstructive valvular disease (peak echocardiographic gradient more than 30 mm Hg).
-
-
Uncorrected severe peripheral stenosis of large arteries including severe coarctation of the aorta.
-
-
Severe renal impairment with serum creatinine above 2x ULN (Upper Limit of Normal) according to the hospital's test methodology.
-
-
History of angioedema.
-
-
Hypersensitivity to ACE inhibitor.
-
-Concomitant medication:
-
oDual ACE inhibitor therapy
-
oRenin inhibitors
-
oAngiotensin II antagonists
-
oNSAIDs except acetylsalicylic acid only for antiplatelet therapy
-
o
-
-
Already enrolled in an interventional trial with an investigational drug, unless no interference with the current study can be shown.
2.4. Inclusion criteria for follow-up
Patients fulfilling the following inclusion criteria can be enrolled:
-
-
Patients from the preceding pharmacokinetic bridging studies who have been treated with enalapril ODMT and are still under ODMT treatment.
-
-
Patients from the preceding studies who have been treated for at least 3 days with enalapril ODMT and are no longer under ODMT treatment.
-
-
Provided written informed consent by patient and/or parent(s)/legal representative for participation in this follow-up safety study and assent is received from the patient according to national legislation and as far as achievable from the child.
Exclusion criteria for follow-up: As it is the aim of this follow-up study to observe the safety of all patients exposed to enalapril ODMT treatment, no additional exclusion criteria are defined in this protocol. However, adapted to the health situation of the patients, the investigators will decide whether planned study activities can be performed.
2.5. Sample size
The number of patients to be included was previously agreed with the PDCO of the EMA, to provide the best balance between a good representation across the age cohorts where most age-related changes in PK and PD are to be expected due to growth and maturation and recruitment feasibility.
The study was designed to obtain paediatric PK data of enalapril ODMTs in order to describe the dose-exposure in this population, as well as safety data on the use of the IMP in paediatric patients. The sample size was therefore determined taking into consideration these key aims and based on literature PK values for enalapril in paediatric patients and published safety data in paediatric patients treated with enalapril. Only two small studies investigating the PK of enalapril in paediatric heart failure patients have been published; 10 patients aged from 6 weeks to 8 months [13] and 12 patients aged from 10 days to 6.5 years [14]. In contrast, enalapril PK was investigated in 40 hypertension patients aged from 2 months to 15 years, with the evaluation of approximately 10 subjects in different age sub-sets to assess impact of age on the disposition of the drug and its metabolite enalaprilat [15]. Safety experience with enalapril in children is mainly derived from a single clinical study in 110 hypertensive paediatric patients aged 6–16 years [24]. The drug was generally well tolerated, and twelve patients experienced 14 AEs, none of which included renal failure, angioedema or hyperkalaemia. In contrast, post marketing data on the use of the ACEIs enalapril and captopril in neonates and young infants frequently report renal failure (in addition to hypotension) as an AE. Retrospective analyses of AEs in term neonates and infants taking ACEIs have estimated the rate of renal AEs to be between approximately 15 and 20% [25,26]. Hence, taking into consideration the PK studies described above, and the need to ensure sufficient patient numbers to investigate safety, a total of 100 patients were initially planned. The number of patients per age sub-set were based upon the known prevalence of DCM in these age groups. However, it was considered that the disposition of enalapril was unlikely to significantly differ between paediatric DCM and CHD patients, and it would therefore be feasible to combine the PK/PD data generated from paediatric patients with heart failure due to cardiomyopathy and congenital heart disease for PK modelling and simulation.
According to the approved amendment to the Paediatric Investigation Plan (PIP), the minimum number of evaluable patients with DCM to be included across all age groups is 25. The required minimum numbers per age group at the time of inclusion are 9 patients below 12 months (the age range where PK data and safety data are most needed), 8 patients between 12 months and below 6 years and 6 patients between 6 years and below 12 years. The number of evaluable patients with CHD to be included across all age groups is 60 with a minimum number of below 12 months of age is 37. Out of the total number of DCM and CHD patients a sub-set of 10 patients each will be envisaged for the age groups 0–3 months, 4–6 months and 7–12 months respectively. In the follow up study, 40 patients below 12 months of age at minimum need to be monitored during the treatment and at least 1 month after discontinuation of enalapril ODMT. At least 20 patients recruited as neonates or infants in the preceding studies need to be monitored for at least 6 months after first enalapril ODMT administration. Where possible, patients will be monitored for up to 12 months after first enalapril ODMT administration (10 months in this follow-up safety study).
2.6. Study drug and dosing regimen
Enalapril will be administered orally as ODMTs (available in form of 0.25 and 1.0 mg ODMTs). Patients who have been previously treated with an ACE inhibitor will be treated with the same prescribed dose of enalapril ODMT. Patients who are naïve to ACE inhibitors will be uptitrated according to a defined dose titration scheme based on adult data extrapolated to children. Dose-titration aims at achieving optimal efficacy while avoiding adverse events. Daily doses will be increased in 1–7 days intervals, depending on the clinical needs, until the individually defined optimal long-term dose is reached. Uptitration will be subject to agreed stopping rules including sustained hypotension, serum creatinine levels increasing to twofold the baseline level, and potassium levels >5.5 mmol/l. For the initial titration doses in very small children (2.5 kg – max. 7 kg), the ODMT may be dispersed in water according to a defined application procedure via a syringe to allow administration of smaller doses, if deemed appropriate by the investigator.
In the follow-up study, all patients who will be under stable enalapril ODMT treatment at the end-of-study visit in the preceding study, will continue their dosing regimen in this study. Dose adaptation may be required during the maximum of 10 months study duration and will be adapted according to the judgement of the investigator.
2.7. Outcomes and study procedures
The primary outcome of the pharmacokinetic bridging studies is the bioavailability of enalapril and its active metabolite enalaprilat in children with heart failure (area under the curve (AUC) within a dosing interval of 12 h, Cmax and Tmax); descriptive PK investigation.
The secondary outcomes are.
-
1.
The bioavailability of enalapril and its active metabolite enalaprilat in the different age subsets (1 months to less than 12months, 12 months to less than 6 years, 6 years to less than 12 years) of the paediatric population (AUC within a dosing interval of 12 h, Cmax and Tmax); descriptive PK investigation.
-
2.
Markers of the RAAS as exploratory PD investigation.
-
3.
Brain natriuretic peptides (NT-proBNP).
-
4.
Acceptability and palatability of the novel formulation.
-
5.
Safety parameters including blood pressure and renal function.
-
6.
Echocardiography (SF).
-
7.
Rehospitalisation due to heart failure including the need for heart transplantation or the institution of mechanical circulatory support.
-
8.
Death due to worsening of the underlying disease.
-
9.
PD and efficacy endpoints analysis to differentiate high and low output disease.
The outcome parameters of the pharmacogenomics and metabolomics sub-study are selected SNPs in genes relevant for enalapril PK (e.g. CES1, OATP1B1), PD and safety (e.g. angiotensinogen gene, NR3C2), and metabolite profile (untargeted and targeted). As currently new platforms are being developed and new SNPs may emerge, the exact choice of platforms, candidate genes and relevant SNPs will be made at the time of analysis.
2.7.1. Study procedures – PK bridging studies with exploratory PD assessments
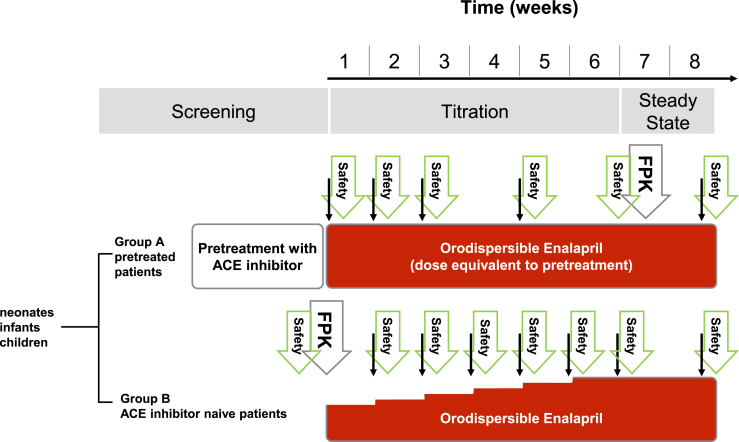
The sampling regimen (Fig. 1) consists of one PK/PD profile day with blood sampling at up to 6 predefined time points over up to 12 h and single PK/PD sampling during titration, dose confirmation and study control visits every two weeks and at the end of the study after 8 weeks. The PK/PD profile can be collected during initial dose visit or at any study control visit, once an optimal dose is reached. PD includes renin, angiotensin I, aldosterone, plasma renin activity as markers of the RAAS. At the screening visit, inclusion and exclusion criteria will be checked, demographic data (age, ethnicity) will be collected and the medical history will be assessed. Echocardiography, ECG, full blood count and measurement of NT-proBNP and body height will be performed at the screening visit and the end-of-study visit.
Fig. 1.
Study design and sampling regimen. The full PK profile (FPK) will be obtained at steady state in patients who have previously been treated with an ACE inhibitor and at initial dose visit in ACE inhibitor naïve patients. Subsequently, trough levels (thin arrows) will be taken before each titration step and at least every two weeks, combined with safety laboratory testing.
Continuous blood pressure and heart rate measurements will be performed every 30 min for 8 h at initial dose, for 4 h at first titration visit and for 2 h as of the next titration visits. At all other visits, blood pressure and heart rate will be measured as part of the routine safety data assessment.
Renal safety laboratory tests (blood urea nitrogen, creatinine, and potassium), urine analyses for evaluation of microalbuminuria, collection of adverse events (AEs) and adverse reactions (ARs) and concomitant medication, body weight measurement and physical examination will be performed at each visit. The Clinical Heart Failure Score (modified Ross Score) will be calculated.
Acceptability and palatability assessments will be performed at the initial dose visit, at a study control visit, preferably on day 28, and at the end-of-study visit.
Blood sampling for the metabolomics sub-study will be performed at screening visit and end-of-study visit. For genetic analysis a blood sample will be collected at the end-of-study visit but can also be collected any time during the study, as long as the total allowed blood sampling volume is not exceeded.
2.7.2. Study procedures – safety follow-up study
The primary outcome of the follow-up safety study is the safety of enalapril ODMTs in children with heart failure. Collection of information on adverse events will take place at each visit. Study visits will take place at day 0, month 1, 4, 7 and 10. The definition of (serious) AEs will be according to the Medical Dictionary for Regulatory Activities (MedDRA).
As in the preceding pharmacokinetic bridging studies, there are several secondary study parameters including acceptability of enalapril ODMTs, vital parameters, renal function and NT-proBNP, measured at each study visit.
Clinical haematology parameters will be determined and a standard ECG will be performed at the first follow-up and the end of study visit.
In order to monitor potential effects of ongoing or earlier administration of enalapril ODMTs on the RAAS, markers of the RAAS will be measured as part of PD sampling.
In addition, at each visit, a single blood sample will be collected to measure the enalapril and enalaprilat plasma levels during long-term treatment for PK analysis in patients under ongoing enalapril ODMT treatment.
2.8. Early termination and follow-up
In case enalapril ODMT treatment is terminated early for whatever reason, patient and parents will be invited to remain in the study until the end-of-study visit of the follow-up study for safety supervision and PD assessments. In case the patient or the parents want to terminate study participation early, the end-of-study visit assessments should be performed on the day of termination decision or on a separate visit.
2.9. Data analysis plan
Primary end point parameter(s) in the PK bridging studies with exploratory PD assessments will be the assessment of the PK parameters of enalapril (the parent compound) and enalaprilat (the active metabolite). They will be estimated using a non-compartmental analysis from the collected full PK profiles. This include the are under the curve (AUC) within the dosing interval, Cmax, and tmax. Individual PK parameters of AUC and Cmax will be adjusted to weight and dose (and/or body surface area normalized dose) and then compared between the different age subsets using parametric statistical testing (e.g. ANOVA).
The primary endpoint in the follow-up safety study will be the observed adverse events. The observed adverse events will be assessed concerning their causal relationship with ODMT intake and presented descriptively in relation to age and other covariates. AEs and ARs will be coded according to the latest version of MedDRA. Preferred terms of AE/ARs will be calculated as total and relative numbers and categorized by severity, by causality and by dose level.
Secondary endpoints: Markers of the RAAS and NT-proBNP will be calculated as arithmetic mean, standard deviation, median, 1st and 3rd quartile, interquartile-range, minimum and maximum categorized by age-group and by titration-level. Ordinal rating items of the acceptability and palatability assessment will be calculated as median, minimum and maximum. Safety parameters (blood pressure and renal function) will be analysed as individual absolute and relative change from baseline (CFB).
Modelling and Simulation: In addition to the non-compartmental analysis of the full PK profiles, population PK modelling will be performed on all collected PK data from the start to the end of the study. A covariate analysis will be performed to investigate sources of variability of the resulted dose exposure relationship, and covariates shall include the various patient characteristics collected such as age, weight, height, disease score, etc.
3. Ethics and dissemination
Potential benefits for children: Although few clinical studies have been conducted in paediatric patients, enalapril is considered a first-line treatment for chronic heart failure in children [12]. There is currently no licensed formulation of enalapril available in Europe suitable for use in children with heart failure, resulting in the administration of extemporaneous oral preparations. This means that the actual dose received is often not known, as extemporaneous preparations tend to have unknown bioavailability compared to the original product (tablet). Also, the quality attributes (content, stability, etc.) of these preparations are very variable. These clinical studies will contribute to enabling the development of a novel clinically relevant age-appropriate and safe enalapril formulation, with an improved method of administration and ease of dosing without the need for a measuring device compared to products currently available.
The few data currently available on the PK of enalapril in paediatric patients have been generated using outdated assay techniques, and hence the reliability of these data is questionable. The LENA PIP proposes to conduct PK studies on a new paediatric enalapril product (ODMT) in heart failure patients from birth to under 12 years utilising a sensitive and selective assay (HPLC-MS) to enable the dose-exposure relationship of enalapril (and its active metabolite enalaprilat) in paediatric patients to be elucidated. Safety data and explorative PD data on parameters of the RAAS will also be generated. The provision of an age-appropriate enalapril product together with frequent supervision of the treatment and safety parameters will be of benefit for the participating patients and will generate a huge benefit for children with heart failure.
Potential risks for children: Enalapril is currently standard of care in this population. The risks associated with the ODMT formulation of enalapril are managed by the treating investigator and are minimal. The additional risk of participation in the studies may be related to the difference in formulation used (potentially better absorption and higher blood levels), but the risk of overdosing is minimised by the careful up-titration in the pharmacokinetic bridging studies and the option to adapt the dose at any time in the safety follow-up study.
The risks of ODMT administration in young children are considered to be low by EMA as presented in the EMA guideline on pharmaceutical development of medicines for paediatric use as acceptability and swallowability of 2 mm placebo minitablets have been demonstrated in over 500 children aged between 0 and 6 years without occurrence of any problems, except coughing in two children, in both cases without clinical relevance [[27], [28], [29], [30]]. The enalapril ODMTs administered in these studies will rapidly disperse in the mouth and thus the risk of choking/aspiration is considered negligible [16]. This potential risk is further mitigated by the method of administration, which includes the provision of a drink if required.
A small risk might be sensitivity to the excipients, however, the selected excipients are commonly used and have acceptable safety profiles. The titration phase and the ongoing option for dose adaptation will allow for careful and closely monitored identification of an individual optimal dose over time for the long-term therapy. The option to administer a dispersion of ODMTs as the first dose for very small children (2.5 kg – max. 7 kg) further enhances the flexibility of the investigators to handle the titration very carefully.
Additional risks will be related to blood sampling and are considered minimal, as the blood sampling schedule is designed to be in accordance with the guideline “Ethical Considerations for Clinical Trials on Medicinal Products Conducted with the Paediatric Population, 2008” by applying low volume assays as well as modelling and simulation techniques. The volume of blood draws in paediatric patients of different age groups in these studies will be within the maximum acceptable volumes defined in that guideline.
However, blood sampling is expected to be a burden for the study patients and poses a great challenge on patient and parental consent at time of recruitment and throughout the whole study period. More frequent study visits may also form an obstacle on recruitment. A good relationship and cooperation between patient, parents, treating physicians and the study team is of the utmost importance.
Dissemination: Final clinical study reports presenting primary and secondary outcomes and study performance in comparison to the plans presented in the protocols will be prepared and all results and learning from the studies will be published on conferences and in peer-reviewed journals as well as to the patient population.
The Children's Heart Federation (CHF) will inform both lay people and health professionals about the progress of the studies. Two platforms will be used to share information about the studies' progress: (1) Key CHD conferences and events such as the European Congenital Heart Disease Organisation (ECHDO's) and the British Congenital Cardiac Association (BCCA) annual general meetings; (2) Scheduled quarterly meetings for the congenital heart disease networks requesting CHF's attendance. CHF's on-going links with partner groups and its membership of the UK National Council for Child Health and Wellbeing (NCCHW) also offer a platform and opportunity to share information about the studies. Information from these events will be shared with lay members in local communities.
4. Conclusion
These paediatric clinical studies will enable the characterisation of PK of enalapril and its active metabolite enalaprilat in young children treated for heart failure with a newly developed age-appropriate formulation, orodispersible minitablets. The exploration of the dose to blood level relationships will allow for age-appropriate dose recommendations of enalapril. Furthermore, a wide range of PD parameters in young children treated for heart failure will be evaluated and the acceptability and palatability of enalapril ODMTs will be tested. Efficacy of enalapril for the treatment of heart failure will not be directly demonstrated, but further explored on the basis of the measured pharmacodynamic parameters. The follow-up safety study will demonstrate the long-term safety of enalapril ODMTs in children with heart failure and will collect additional information about PK and PD of enalapril ODMTs. In the sub-study, metabolomics and pharmacogenetics will be evaluated in relation to enalapril PK and PD parameters, improving the understanding of the underlying disease and the individual response to enalapril treatment.
Rank order of authorship
First authorship has been assigned to the three equally contributing EU work package leaders of the paediatric clinical trials. Authorships then follow with the work package leaders according to work package number. Authorships follow then for scientific investigators according to alphabetical order. Last authorship has been assigned to the scientific coordinator of the LENA project. The scientific contributions of authors are listed separately.
Authors’ contributions
Study concept.
SL, JB, JT, FL, CM, MD, SW, MB, IK.
Design, protocol and document editing, generation of software tools.
Study objectives, inclusion, exclusion criteria: MD, SW, MB, IJ, CM, LA, AS, FL, SL.
Acceptability and palatability criteria: LS, IK, JW.
Study drug: JB, IK, LS, KK.
Dosing regimen: SL, MD, SW, IJ, MB, CM, LA, AS.
Pharmacokinetic and pharmacodynamic profile planning, modelling and simulation and blood sampling schedule: SL, BB, WC, FK.
Dose titration scheme: MD, MVM, SW.
Statistical analysis plan: FL, SL, WC, MF, FK, FL, AM (preparation); SW, IK (review)
Translation of trial modules into software (eCRF), supporting tools (visit schedule according to protocol, LENA box): FL, AM, KK, IK, LS, PW, BB.
Scheduling of the study visits: LS, IK, SL.
Metabolomic and pharmagenomic investigations: SW.
Safety parameters: IK, SW.
Preparation, review and submission of EU Paediatric Investigation Plan (PIP): JW (preparation, submission), SL, CM, IK, MD, (intense review and discussion with PDCO) SW, FL, MB, IJ (review)
Writing of study protocols and amendments: IK, LS.
Critical revision of study protocol elements and amendments and participation in weekly and bi-weekly telephone conferences and in LENA project meetings: SL, EO, MD, MB, JB, IK, FL, AM, SW, AKC, HB, CM, IJ, LA, BB, WC, KK, LS, VS, MVM, PW, JW.
Drafting of article: VS, CM.
Revising and accepting the version of the manuscript to be published: LA, SL, MB, MD, CM, SW, IK, LS, JB, BB, EO, AKC, JW, CW, VS, FL.
All authors approved the final manuscript as submitted and they agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgement
We thank Prof. Dr. Jochen Theis, InHeCon, Germany for participation in the development of the study concept, Dr. Feras Khalil, PharmD, Germany for support in the development of the dosing regimen, PK sampling schedule and Dr. Agnes Maria Ciplea for supporting the development of the quality system (both former Heinrich-Heine Universität Düsseldorf). We thank Dr. Angelika Moder (Paracelsus Medizinische Privatuniversität, Salzburg, Austria) for supporting the development of the statistical tools especially for the development of the eCRF.
We thank all consortium members and investigators of the LENA project for supporting the study protocol development of the paediatric clinical trials:
Coordination of the LENA project: SL.
WP leaders: Pharmaceutical Development and Regulatory Interaction JB, Clinical Trial Management and Pharmacovigilance IK, Data management and Statistics FL, Pharmacokinetics, Modelling and Simulation SL, Dilated Cardiomyopathy Paediatric Trial MD, Congenital Heart Disease paediatric trial MB, Longterm follow up safety trial SW+, Patient and parent organisation AKC.
Principal investigators: HB, CM, IJ, MDj$, TSM°, SA, VV#
Advanced Scientists: LA (investigator), BB (bioanalytics), WC (modelling and simulation), KK (quality), EO (assistant to coordinator), LS (trial manager), VS (clinical investigator), MVM (investigator), PW (informatics), JW (regulatory)
$ Prof. Dr. Milan Djukic (1)
#Prof. Dr. Vladislav Vukomanović, Mother and Child Health Care Institute “Dr Vukan Cupic”, Belgrade, Serbia.
°Prof. Dr. Thomas Mir Universitäres Herzzentrum Hamburg, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100393.
Funding statement
This study is part of the project “Labelling of Enalapril from Neonates up to Adolescents” (LENA) and has received funding from the EU’s Seventh Framework Programme (FP7/2007–2013) under grant agreement number 602295 (LENA).
Conflicts of interest
No competing interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ross R.D., Daniels S.R., Schwartz D.C. Plasma norepinephrine levels in infants and children with congestive heart failure. Am. J. Cardiol. 1987;59(8):911–914. doi: 10.1016/0002-9149(87)91118-0. [DOI] [PubMed] [Google Scholar]
- 2.Auslender M., Artman M. Overview of the management of pediatric heart failure. Prog. Pediatr. Cardiol. 2000;11(3):231–241. doi: 10.1016/s1058-9813(00)00055-2. [DOI] [PubMed] [Google Scholar]
- 3.Kirk R., Dipchand A.I., Rosenthal D.N. The International Society of Heart and Lung Transplantation Guidelines for the management of pediatric heart failure: executive summary. J. Heart Lung Transplant. 2014;33:888–909. doi: 10.1016/j.healun.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Sommers C., Nagel B.H., Neudorf U. Congestive Heart Failure in Childhood. An Epidemiologic Study; Herzinsuffizienz im Kindesalter. Eine epidemiologische Studie. Herz. 2005 Oct 1;30(7):652. doi: 10.1007/s00059-005-2596-6. [DOI] [PubMed] [Google Scholar]
- 5.Massin M.M., Astadicko I., Dessy H. Epidemiology of heart failure in a tertiary pediatric center. Clin. Cardiol. 2008;31(8):388–391. doi: 10.1002/clc.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinton R.B., Ware S.M. Heart failure in pediatric patients with congenital heart disease. Circ. Res. 2017;120(6):978–994. doi: 10.1161/CIRCRESAHA.116.308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews R.E., Fenton M.J., Ridout D.A. New-onset heart failure due to heart muscle disease in childhood: a prospective study in the United Kingdom and Ireland. Circulation. 2008;117(1):79–84. doi: 10.1161/CIRCULATIONAHA.106.671735. [DOI] [PubMed] [Google Scholar]
- 8.Yancy C.W., Jessup M., Bozkurt B. ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J. Am. Coll. Cardiol. 2013;62(16):1495–1539. doi: 10.1016/j.jacc.2013.05.019. 2013. [DOI] [PubMed] [Google Scholar]
- 9.EMA . 2003. Committee for Proprietary Medicinal Products (CPMP) Summary Information of Referral Opinion Pursuant to Article 30 of Council Directive 2001/83/EC for Renitec and Associated Names (See Annex I), London 4 December 2003.https://www.ema.europa.eu/documents/referral/summary-information-referral-opinion-pursuant-article-30-council-directive-2001/83/ec-renitec-associated-names-see-annex-i-international-non-proprietary-name-inn-enalapril-background_en.pdf Available at: (accessed November 2018) [Google Scholar]
- 10.Cleland J.G., Dargie H.J., Ball S.G. Effects of enalapril in heart failure: a double blind study of effects on exercise performance, renal function, hormones, and metabolic state. Heart. 1985;54(3):305–312. doi: 10.1136/hrt.54.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt S.A. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure) J. Am. Coll. Cardiol. 2005;46(6):e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 12.EMA . 2010. Report on the Expert Group Meeting of Paediatric Heart Failure, London 29 November 2010.https://www.ema.europa.eu/documents/other/report-expert-group-meeting-paediatric-heart-failure-london-29-november-2010_en.pdf Available at: (accessed November 2018) [Google Scholar]
- 13.Lloyd T.R., Mahoney L.T., Knoedel D. Orally administered enalapril for infants with congestive heart failure: a dose-finding study. J. Pediatr. 1989;114(4):650–654. doi: 10.1016/s0022-3476(89)80715-2. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura H., Ishii M., Sugimura T. The kinetic profiles of enalapril and enalaprilat and their possible developmental changes in pediatric patients with congestive heart failure. Clin. Pharmacol. Ther. 1994;56(2):160–168. doi: 10.1038/clpt.1994.119. [DOI] [PubMed] [Google Scholar]
- 15.Wells T., Rippley R., Hogg R. The pharmacokinetics of enalapril in children and infants with hypertension. J. Clin. Pharmacol. 2001;41(10):1064–1074. doi: 10.1177/00912700122012661. [DOI] [PubMed] [Google Scholar]
- 16.Thabet Y., Walsh J., Breitkreutz J. Flexible and precise dosing of enalapril maleate for all paediatric age groups utilizing orodispersible minitablets. Int. J. Pharm. 2018;541(1–2):136–142. doi: 10.1016/j.ijpharm.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 17.Faisal M., Cawello W., Burckhardt B.B. On behalf of the LENA consortium. Model-dependent pharmacokinetic analysis of enalapril administered to healthy adult volunteers using orodispersible mini-tablets for use in paediatrics. Drug Des. Dev. Ther. 2019 Jan 25;13:481–490. doi: 10.2147/DDDT.S188417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burckhardt B.B., Tins J., Ramusovic S. Tailored assays for pharmacokinetic and pharmacodynamic investigations of aliskiren and enalapril in children: an application in serum, urine, and saliva. J. Pediatr. Pharmacol. Ther. 2015;20(6):431–452. doi: 10.5863/1551-6776-20.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramusovic S., Laeer S. An integrated physiology-based model for the interaction of RAA system biomarkers with drugs. J. Cardiovasc. Pharmacol. 2012;60(5):417–428. doi: 10.1097/FJC.0b013e3182676f06.. [DOI] [PubMed] [Google Scholar]
- 20.Ramusovic S., Thielking G., Läer S. Determination of enalapril and enalaprilat in small human serum quantities for pediatric trials by HPLC-tandem mass spectrometry. Biomed. Chromatogr. 2012;26(6):697–702. doi: 10.1002/bmc.1716. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer J., Burckhardt B.B., Tins J. Validated low-volume aldosterone immunoassay tailored to GCLP-compliant investigations in small sample volumes. Pract. Lab. Med. 2017;27:28–38. doi: 10.1016/j.plabm.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaefer J., Burckhardt B.B., Tins J. Validated low-volume immunoassay for the reliable determination of direct renin especially valuable for pediatric investigations. J. Immunoass. Immunochem. 2017;38(6):579–594. doi: 10.1080/15321819.2017.1350707. [DOI] [PubMed] [Google Scholar]
- 23.Flaten H.K., Monte A.A. The pharmacogenomic and metabolomic predictors of ACE inhibitor and angiotensin II receptor blocker effectiveness and safety. Cardiovasc. Drugs Ther. 2017 Aug 1;31(4):471–482. doi: 10.1007/s10557-017-6733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells T., Frame V., Soffer B. For the Enalapril Pediatric Hypertension Collaborative Study Group. A double-blind, placebo-controlled, dose-response study of the effectiveness and safety of enalapril for children with hypertension. J. Clin. Pharmacol. 2002 Aug;42(8):870–880. doi: 10.1177/009127002401102786. [DOI] [PubMed] [Google Scholar]
- 25.Gantenbein M.H., Bauersfeld U., Baenziger O. Side effects of angiotensin converting enzyme inhibitor (captopril) in newborns and young infants. J. Perinat. Med. 2008;36(5):448–452. doi: 10.1515/JPM.2008.064. [DOI] [PubMed] [Google Scholar]
- 26.Lindle K.A., Dinh K., Moffett B.S. Angiotensin-converting enzyme inhibitor nephrotoxicity in neonates with cardiac disease. Pediatr. Cardiol. 2014 Mar;35(3):499–506. doi: 10.1007/s00246-013-0813-2. [DOI] [PubMed] [Google Scholar]
- 27.EMA . 2013. Guideline on Pharmaceutical Development of Medicines for Paediatric Use. EMA/CHMP/QWP/805880/2012 Rev. 2; 2013.http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/07/WC500147002.pdf Available at: (accessed December 2018) [Google Scholar]
- 28.Spomer N., Klingmann V., Stoltenberg I. Acceptance of uncoated mini-tablets in young children: results from a prospective exploratory cross-over study. Arch. Dis. Child. 2012;97(3):283–286. doi: 10.1136/archdischild-2011-300958. archdischild-2011. [DOI] [PubMed] [Google Scholar]
- 29.Klingmann V., Spomer N., Lerch C. Favorable acceptance of mini-tablets compared with syrup: a randomized controlled trial in infants and preschool children. J. Pediatr. 2013;163(6):1728–1732. doi: 10.1016/j.jpeds.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Klingmann V., Seitz A., Meissner T. Acceptability of uncoated mini-tablets in neonates—a randomized controlled trial. J. Pediatr. 2015;167(4):893–896. doi: 10.1016/j.jpeds.2015.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.