Abstract
Purpose
Uveal lymphomas are indolent, frequently choroid-involving neoplasms that are mainly CD20-positive B-cell extranodal marginal zone lymphoma. Irreversible visual loss may occur from retinal detachment and/or glaucoma among untreated symptomatic patients, or from radiation-induced changes secondary to external beam radiotherapy. To avoid radiation-induced complications, we used systemic rituximab monotherapy as primary treatment, and present two cases to show its long-term effectiveness for symptomatic primary uveal lymphoma.
Observations
Two elderly men who presented with painless blurred vision were clinically diagnosed with symptomatic primary uveal lymphoma, which were biopsy-confirmed to be marginal zone lymphoma. Both patients with symptomatic, primary marginal zone uveal lymphoma that appeared as multiple yellow, nummular choroidal infiltrates, had complete ocular remission after three and one cycles of systemic rituximab monotherapy (375mg/m2 infused intravenously once weekly for four consecutive weeks), with disappearance of the lesions and improvement of visual acuity. Both patients tolerated systemic monotherapy well without any adverse systemic or ocular effects. There was no local ocular recurrence at 29 and 39 months after the last treatment.
Conclusions
and Importance: Systemic rituximab monotherapy induced complete ocular remission and improved visual acuity, without adverse effects, and without local ocular recurrence of uveal lymphoma 29–39 months following the last treatment. To our knowledge, this is the first manuscript to show long-term effectiveness of systemic rituximab monotherapy as the primary treatment for symptomatic primary uveal lymphoma. Long-term follow-up of this indolent neoplasm is still imperative to monitor its ocular and systemic course.
1. Introduction
Uveal lymphomas are indolent neoplasms, mainly involving the choroid of elderly individuals.1 Although rare, untreated symptomatic lesions may cause irreversible visual loss from retinal detachment and glaucoma.2
External beam radiotherapy (EBRT), averaging a total dose of 30Gy, is the most common treatment used to achieve complete remission of symptomatic, localized primary uveal lymphoma.1,3 However, such EBRT treatment may lead to irreversible visual decline from radiation-induced complications.3
Because most uveal lymphomas are CD20-positive B-cell extranodal marginal zone lymphoma,1,4 we used rituximab, an FDA-approved anti-CD20 monoclonal antibody for treatment of systemic B-cell non-Hodgkin's lymphoma (NHL), as primary treatment for symptomatic primary uveal lymphoma to avoid EBRT-related ocular complications.
2. Findings
2.1. Case #1
A 68-year-old man presented with painless, blurred vision of the right eye for one month. His visual acuity was 20/80 in the right eye and 20/25 in the left eye. Multiple, often confluent, yellow, thickened, nummular infiltrates were present diffusely throughout the right choroid (Fig. 1A) and were associated with intraretinal and subretinal fluid visualized by ultrasonography (Fig. 1B). Numerous smaller, flat, yellow lesions, not associated with edema, were seen diffusely in the left choroid. Ultrasound biomicroscopy revealed bilateral focal areas of iris thickening.
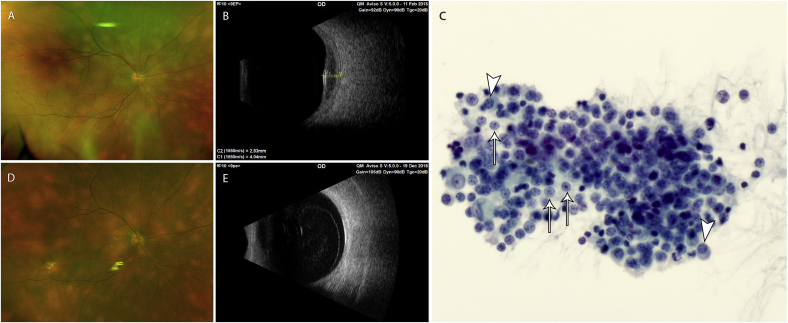
Fig. 1.
Shows the fundus photo and B-scan ultrasound images before systemic rituximab monotherapy (Fig. 1A–B), the photomicrograph of the FNAB of the choroid infiltrate prior to monotherapy (Fig. 1C), and the fundus photo and B-scan ultrasound images 29 months after systemic rituximab monotherapy (Fig. 1D–E). Fig. 1A. Fundus photo of the right eye before systemic rituximab monotherapy. There are multiple, often confluent, yellow, thickened, nummular infiltrates diffusely present throughout the choroid. Fig. 1B. B-scan ultrasound image of the right eye before systemic rituximab monotherapy. The image shows acoustically hollow choroidal infiltrate with subretinal fluid. Fig. 1C. Photomicrograph (600x magnification, Pap stain cytology) of the FNAB of the right choroid infiltrate before systemic rituximab monotherapy. There are highly cellular neoplastic pleomorphic small-to medium-sized lymphocytes with prominent stippled chromatin pattern, and monocytoid (arrows) and plasmacytoid (arrow heads) appearing lymphocytes, with features consistent with marginal zone B-cell lymphoma. Fig. 1D. Fundus photo of the right eye taken 29 months after the last systemic rituximab monotherapy. There is complete remission of the infiltrates throughout the choroid which are seen as nummular, more distinct but smaller, yellow RPE changes. Fig. 1E. B-scan ultrasound image of the right eye taken 29 months after the last systemic rituximab monotherapy. There is remission, without recurrence, of the acoustically hollow choroidal infiltrate. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fine needle aspiration biopsy (FNAB) of the right choroid revealed features of marginal zone lymphoma (Fig. 1C). Systemic evaluation by an experienced medical oncologist, including computed tomography (CT) scan of the chest, abdomen, and pelvis, was negative. Rituximab (standard dose per cycle: 375mg/m2 infused intravenously once weekly for four consecutive weeks) was given every six months. The retinal fluid and iris thickening resolved after one cycle. Complete bilateral ocular remission occurred six months after the third cycle, without recurrence at twenty-nine months after the last treatment (Fig. 1D–E). The final visual acuity was 20/40 in the right eye and 20/25 in the left eye.
2.2. Case #2
A 68-year-old man had painless blurred vision and metamorphopsia for two years. His visual acuity was 20/60 in the right eye and 20/25 in the left eye. Fundus examination showed multiple yellow, nummular infiltrates that were more prominent in the right than the left eye. Systemic evaluation by an experienced medical oncologist, including computed tomography (CT) scan of the chest, abdomen, and pelvis, was negative. He chose observation of the clinically diagnosed uveal lymphoma. Five months later, the visual acuity worsened to 20/100 in the left eye. Cystoid retinal edema and subretinal fluid appeared over the choroidal lesions (Fig. 2A–B).
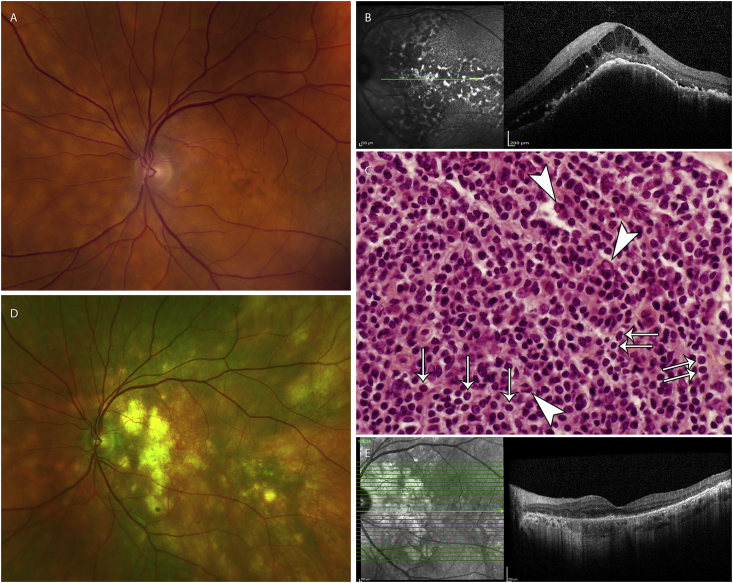
Fig. 2.
Shows the fundus photo and OCT images of the left eye before systemic rituximab monotherapy (Fig. 2A–B), the photomicrograph of the biopsy of the inguinal lymph node (Fig. 2C), and the fundus photo and OCT images of the left eye 39 months after systemic rituximab monotherapy (Fig. 2D–E). Fig. 2A. Fundus photo of the left eye before systemic rituximab monotherapy. There are multiple yellow, nummular infiltrates diffusely present throughout the choroid, most prominent underneath the macula. Fig. 2B. OCT image of the left eye before systemic rituximab monotherapy. Cystoid retinal edema and subretinal fluid appeared over the choroidal lesions underneath the macula. Fig. 2C. Photomicrograph (600x magnification, H&E stain) of the enlarged right inguinal lymph node biopsy. There is dense proliferation of small-to medium-sized neoplastic lymphocytes with numerous monocytoid lymphocytes (arrows) and Dutcher bodies (arrow heads), features consistent with marginal zone B-cell lymphoma. Fig. 2D. Fundus photo of left right eye taken 39 months after the last systemic rituximab monotherapy. There is complete remission of the infiltrates, seen as RPE changes, throughout the choroid, most prominent underneath the macula. Fig. 2E. OCT image of the left eye taken 39 months after the last systemic rituximab monotherapy. There is complete remission of the choroid infiltrates, and improvement of the overlying cystoid retinal edema and subretinal fluid. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
He chose empiric rituximab (standard dosage) over EBRT and/or choroidal biopsy. One month after treatment, the retinal fluid started to resolve. Seven months after treatment, right inguinal lymphadenopathy was palpated, confirmed by CT scan, and biopsied to reveal marginal zone lymphoma (Fig. 2C). Both the medical oncologist and the patient opted to observe the inguinal lymphadenopathy. The uveal lymphoma did not recur thirty-nine months after one cycle of rituximab (Fig. 2D–E). The final visual acuity improved to 20/30–2 in the left eye and 20/15 in the right eye.
3. Discussion
Rituximab was FDA-approved in 1997 as monotherapy for indolent systemic B-cell NHL.5 Its mechanisms of action include antibody-dependent cellular toxicity and complement-mediated cell lysis.6 Both patients with symptomatic, primary marginal zone uveal lymphoma that appeared as multiple yellow, nummular choroidal infiltrates, had complete ocular remission after three and one cycles of rituximab treatment, with disappearance of the lesions and improvement of visual acuity. Both patients tolerated systemic monotherapy well without any adverse systemic or ocular effects, while avoiding EBRT-related ocular complications.
Previous reports of rituximab monotherapy for ocular lymphoma have not described results for those only involving the uvea, except for a single case reported by Pelegrin et al.7 who did not provide long term results following initial complete remission. Rituximab monotherapy of ocular adnexal lymphomas, some with uveal involvement, have been reported with mixed results in two different studies by the same authors.1,8 One of their retrospective studies8 showed progression or recurrence in 7 of 11 patients treated with systemic rituximab monotherapy. In that study, eighteen (18) of the ninety-five (95) patients had uveal involvement, but the results of treatment on the uveal component of the lymphomas were not reported. In a descriptive study1 of 34 eyes with uveal lymphoma and concurrent systemic, bilateral, or ocular adnexal lymphoma, 5 patients were treated with systemic rituximab monotherapy resulting in disease stability and partial remission in 1 and 2 patients, respectively. Thus, the result of systemic rituximab monotherapy on uveal lymphoma alone could not be deduced. Two studies by the same authors3,9 described the use of systemic rituximab monotherapy for primary uveal lymphoma, but results of the treatment were not given in any of the 5 patients in the two studies.
To our knowledge, this is the first manuscript describing long-term results for systemic rituximab monotherapy as primary treatment for uveal marginal zone lymphoma. Similar to a previous report,7 we found that systemic rituximab monotherapy resulted in complete ocular response. In addition, we found that it prevented recurrence of uveal marginal zone lymphoma in two patients, with over 29 months of follow-up since the last systemic treatment.
The ability of rituximab to prevent or delay onset of systemic marginal zone lymphoma has yet to be determined. In our experience, no ocular recurrence occurred 29 and 39 months after the last systemic rituximab monotherapy. Both patients are alive 40 months after their first monotherapy. Isolated inguinal lymphadenopathy, which developed in the second patient with bilateral uveal lymphoma seven months after one cycle of rituximab, allowed a more accessible biopsy site to establish his diagnosis.
4. Conclusion
In both cases of symptomatic primary uveal lymphoma, rituximab induced complete ocular remission and improved visual acuity, without adverse effects, and without local ocular recurrence 29–39 months following treatment. To our knowledge, this is the first manuscript to show long-term effectiveness of systemic rituximab monotherapy as the primary treatment for symptomatic primary uveal lymphoma.1,3,7, 8, 9 Long-term follow-up of this indolent neoplasm is still imperative to monitor its ocular and systemic course.
Acknowledgments
The authors would like to acknowledge Timothy S. Costello for preparing the photos based on the specifications of AJO Case Reports. Dr. Hakan Demirci is supported by the Richard N. and Marilyn K. Witham Professorship, which funded the processing fee of the manuscript for this journal. The endowment has no effect on the design, conduct, and results of the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2019.100484.
Contributor Information
Honeylen Maryl Tiu Teo, Email: teo.honeylen@gmail.com.
Süleyman Çiftçi, Email: ciftci1977@hotmail.com.
Victor Maurice Elner, Email: velner@med.umich.edu.
Hakan Demirci, Email: hdemirci@med.umich.edu.
Patient consents
The study complied with U.S. Health Insurance Portability and Accountability Act of 1996. Consents to publish the cases were not obtained. This manuscript does not contain any personal information that could lead to the identification of the patients.
Funding
Dr. Hakan Demirci is supported by the Richard N. and Marilyn K. Witham Professorship, which funded the processing fee of the manuscript for this journal. The endowment has no effect on the design, conduct, and results of the study.
The following authors have no financial disclosures related to this research: HMT, SC, VME.
Conflicts of interest
There is no conflict of interest from any of the authors.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Aronow M.E., Portell C.A., Sweetenham J.W., Singh A.D. Uveal lymphoma: clinical features, diagnostic studies, treatment selection, and outcomes. Ophthalmology. 2014;121(1):334–341. doi: 10.1016/j.ophtha.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Gass J.D. Retinal detachment and narrow-angle glaucoma secondary to inflammatory pseudotumor of the uveal tract. Am J Ophthalmol. 1967;64(3):612–621. [PubMed] [Google Scholar]
- 3.Mashayekhi A., Hasanreisoglu M., Shields C.L., Shields J.A. External beam radiation for choroidal lymphoma: efficacy and complications. Retina. 2016;36(10):2006–2012. doi: 10.1097/IAE.0000000000001026. [DOI] [PubMed] [Google Scholar]
- 4.Coupland S.E., Foss H.D., Hidayat A.A., Cockerham G.C., Hummel M., Stein H. Extranodal marginal zone B cell lymphomas of the uvea: an analysis of 13 cases. J Pathol. 2002;197(3):333–340. doi: 10.1002/path.1130. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=103705
- 6.Golay J., Zaffaroni L., Vaccari T. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95(12):3900–3908. [PubMed] [Google Scholar]
- 7.Pelegrín L., Adán A., López-Guillermo A., Martinez A., Shields C.L. An old disease in an atypical place. Surv Ophthalmol. 2014;59(6):660–663. doi: 10.1016/j.survophthal.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Portell C.A., Aronow M.E., Rybicki L.A., Macklis R., Singh A.D., Sweetenham J.W. Clinical characteristics of 95 patients with ocular adnexal and uveal lymphoma: treatment outcomes in extranodal marginal zone subtype. Clin Lymphoma, Myeloma & Leukemia. 2014;14(3):203–210. doi: 10.1016/j.clml.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Mashayekhi A., Shukla S.Y., Shields J.A., Shields C.L. Choroidal lymphoma: clinical features and association with systemic lymphoma. Ophthalmology. 2014;121(1):342–351. doi: 10.1016/j.ophtha.2013.06.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.