Summary
Quality control of offspring is important for the survival of cells. However, the mechanisms by which quality of offspring cells may be checked while running genetic programs of cellular differentiation remain unclear. Here we investigated quality control during sporulating in Bacillus subtilis by combining single-cell time-lapse microscopy, molecular biology, and mathematical modeling. Our results revealed that the quality control via premature germination is coupled with the electrical polarization of outer membranes of developing forespores. The forespores that accumulate fewer cations on their surface are more likely to be aborted. This charge accumulation enables the projection of multi-dimensional information about the external environment and morphological development of the forespore into one-dimensional information of cation accumulation. We thus present a paradigm of cellular regulation by bacterial electrical signaling. Moreover, based on the insight we gain, we propose an electrophysiology-based approach of reducing the yield and quality of Bacillus endospores.
Subject Areas: Microbiology, Microbial Physiology, Bioinformatics, Mathematical Biosciences
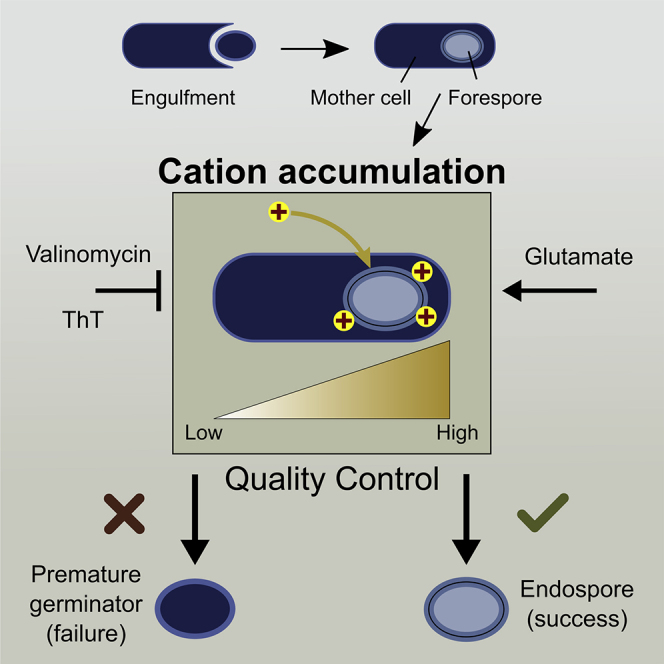
Graphical Abstract
Highlights
-
•
Quality control during bacterial sporulation is coupled with cation accumulation
-
•
Cation accumulation prevents premature germination
-
•
Cation accumulation integrates information on morphological defects and environments
-
•
Spores are less fit when sporulated with Thioflavin T
Microbiology; Microbial Physiology; Bioinformatics; Mathematical Biosciences
Introduction
The significances of ion dynamics in bacterial signaling have been uncovered in the last few years (Lee et al., 2017). Cells within Bacillus subtilis biofilms can communicate with each other through electrical signaling mediated by the gating of K+ channels (Prindle et al., 2015). This bacterial electrical signaling increases the fitness of the population by enabling metabolic co-dependence within a biofilm (Liu et al., 2015) and nutrient time sharing of distant biofilms (Liu et al., 2017). A recent study also revealed that the bacterial electrical signaling can attract motile cells in a species-independent manner (Humphries et al., 2017). In E. coli, rapid change in membrane potential mediated by the opening of Ca2+ channels is crucial for the response to mechanical stresses (Bruni et al., 2017). As a result of these pioneering studies, bacterial electrical signaling concerning the ion dynamics has become an exciting avenue of research, which is, however, still largely uncharted. A particularly important unanswered question is how electrical dynamics interplays with complex genetic signaling processes (e.g., cellular differentiation). How genetically regulated processes interplay with environmental factors and physiological states is a timely and important research topic broadly in biology (Prindle et al., 2012, Taheri-Araghi et al., 2015, Willis et al., 2016). This is because, although molecular biological interactions are well studied in controlled experimental condition, still little is known about the interplay between genetic programs and physiological states. Having this in mind, we investigated the B. subtilis spore formation with a focus on the dynamics of cations and quality control.
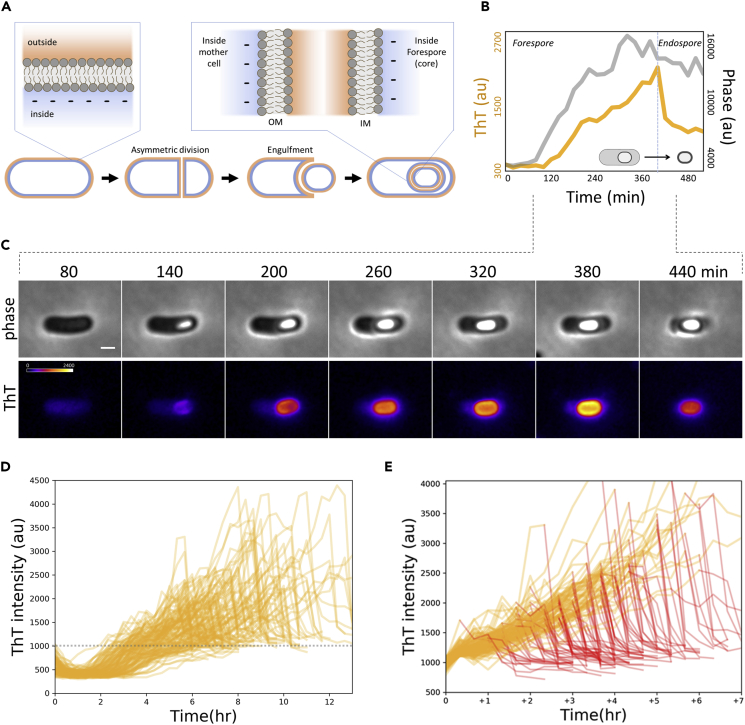
Sporulation of B. subtilis is among the best-characterized bacterial cellular differentiation processes (Lopez-Garrido et al., 2018, Narula et al., 2016). Over five decades of in-depth genetic studies and high-throughput analyses have identified the genes and proteins governing this differentiation process (Eijlander et al., 2014, Mao et al., 2011). The principles of the cellular decision making, leading to the commitment to sporulation, have been deciphered by single-cell time-lapse microscopy and mathematical modeling (Kuchina et al., 2011, Narula et al., 2015). These extensive bodies of research have resulted in a good understanding of the genetic regulatory system driving sporulation. Briefly, the differentiation into endospores begins with phosphorylation of the master transcription factor Spo0A, which leads to the “commitment” defined by the irreversible formation of an asymmetric septum. The septum formation triggers the multistage differentiation program regulated by compartment-specific sporulation sigma factors, resulting in the genetically regulated developmental processes: forespore engulfment, cortex synthesis, coat assembly, and mother-cell lysis. Owing to the mode of formation, the outer spore membrane has opposite polarity with respect to the inner spore membrane (Wilkinson et al., 1975) (Figure 1A).
Figure 1.
Single-cell Dynamics of a Cationic Dye Revealed a Gradual Increase on Forespores and Sudden Drop upon Mother-Cell Lysis
(A) Illustrative diagram showing the membrane polarities of a sporulating cell. Engulfment process results in forespore membranes with opposite polarities to each other. Quality control works during late sporulation (stage IV-VI) where protective layers are assembled and matured.
(B) Time series of the mean intensities of phase contrast (gray) and ThT (orange) in spore region. The data correspond to the panel (C). ROIs (region of interest) used for the measurements are shown in Figure S1. ThT was added at 10 μM.
(C) Film-strip images showing the phase-contrast (upper) and ThT (lower) of a live single-cell sporulating cell. Scale bar, 1 μm. Color scale for ThT intensity is shown in the left-end panel for ThT. Film-strip is a representative of successfully sporulating cells from twenty-nine independent experiments.
(D) Time series of single-cell ThT fluorescence dynamics from 122 forespores. Forespore regions of ThT intensity were measured until 1 h after mother-cell lysis. All time series qualitatively follow the pattern represented in panel (C) but a great degree of heterogeneity between cells. The dashed line represents the value used for alignment in panel (E).
(E) The dataset shown in panel (D) was aligned to the frame it first reaches ThT intensity value 1,000. Time series after reaching maximum are highlighted in red. ThT increases at a relatively finite rate. A great degree of heterogeneity in timing of mother-cell lysis was observed (red lines). Histograms of the late-sporulation duration with and without ThT are shown in Figures S3 and S4.
Although it is well established that sporulation is regulated by a complex genetic program, it is also evident that the process is subject to a variety of internal and external conditions. Poorly developing spores are eliminated from the population through the quality control mediated by Clp proteases and GerA-dependent premature germination (Ramírez-Guadiana et al., 2017b, Tan et al., 2015). Intriguingly, the quality (e.g., resistance and germination properties) of endospores differs depending on the environmental conditions during sporulation (Nguyen Thi Minh et al., 2011, Rose et al., 2007). For example, endospores produced at high temperature (∼50°C) exhibit higher resistance against heat (∼100°C) (Palop et al., 1999). A recent study also suggested that the timing of sporulation affects the spore quality (Mutlu et al., 2018). It is worth noting that such condition-dependent variability of spores is recognized as a major challenge in food industries since it precludes the reliable standardization of the sterilization procedures (Eijlander et al., 2011). However, despite these observations demonstrating the phenotypic plasticity of endospores, the mechanism by which diverse environmental factors and morphological properties affect the sporulation process remains unknown.
We hypothesized that diverse morphological properties and environmental factors can be sensed through electrophysiological dynamics of cells during sporulation, which provides an orthogonal dimension to the complex genetic program of spore differentiation. We characterized the dynamics of cationic molecules during late sporulation (stage IV-VI) of B. subtilis. Our single-cell time-lapse microscopy measurements, combined with examinations of genetic mutant strains and minimalistic computational simulations, suggest that the success rate of endospore formation depends on the cation accumulation to the forespore surfaces. Specifically, spore formation is more likely to be completed when forespores accumulate high levels of cations on their surface. Intriguingly, this sensing mechanism enables the quality control to be responsive not only to forespore morphogenetic errors but also to the external environments. Thus, the electrostatic attraction during late sporulation provides a molecular-level insight to the mechanism that enables integrative quality control for spore formation. Finally, in the light of the findings we gained, we succeed in promoting the failure of sporulation using Thioflavin T (ThT) while at the same time decreasing the resistance property of endospores against wet heat.
Results
Single-Cell Measurements of Cation Accumulation Dynamics during Sporulation
To investigate the electrostatic dynamics during late sporulation, we performed time-lapse fluorescence microscopy with sporulating B. subtilis cells. ThT is a membrane-permeable cationic dye that has been used with B. subtilis (Humphries et al., 2017, Lee et al., 2019, Liu et al., 2017, Prindle et al., 2015, Stratford et al., 2019). Hence, we decided to utilize it as an indicator for electrical polarity during forespore development. Single-cell measurements of ThT fluorescence revealed a gradual increase of the signal on a developing forespore, which then rapidly drops upon mother-cell lysis (Figures 1B and 1C, and Video S1). The fluorescence signal was seen intensely on the peripheral regions of the forespores (Figures 1C and S2). The temporal increase in ThT fluorescence was preceded by the increase in phase-contrast channel, which coincides with the pH drop in the core compartment (Figure S3 and Video S2). The rapid drop of the ThT signal upon mother-cell lysis indicates that ThT associates with the forespore surface in a dynamic manner. The experiments with another positively charged lipophilic dye, TMRM (Kralj et al., 2011, Lo et al., 2007), exhibited the same dynamics as ThT (Figure S4, and Video S3, see also Supplemental Information). These results indicate that the forespore surfaces polarize negatively during late sporulation. We note that the forespore surface is defined here as in the electrochemical sense: the membrane can act as an insulator, whereas spore coat and crust layers do not act as diffusion barriers for small ions owing to their porous structures with the exclusion size of 2–8 kDa (Driks, 1999, Plomp et al., 2014).
Fluorescence time-lapse microscopy images corresponding to the main Figures 1B and 1C
Time-lapse microscopy images showing a sporulating cell in phase contrast (left) and pHluorin (right). The intensity in pHluorin images is the ratio of Ex466/Ex400. Lighter colors indicate low pH, and darker colors indicate high pH
Time-lapse microscopy images of a sporulating cell in phase-contrast and TMRM fluorescence
We next performed single-cell tracking of ThT dynamics with the cells that produced phase-bright endospores (n = 122). For individual cells, ThT intensity on the forespore compartment was measured until 1 h after the mother-cell lysis. All time series follow the pattern presented in Figures 1B and 1C; explicitly, ThT intensity increases gradually and drops rapidly upon mother-cell lysis. However, there is a large degree of heterogeneity between individual cells (Figure 1D). To better understand the observed heterogeneity, we analyzed the ThT time traces of individual cells by aligning each of the time trace to the frame where the ThT intensity reaches 1,000 au (arbitrary unit), shown as a dashed horizontal gray line in Figure 1D. This alignment revealed a finite increase rate of fluorescence signal (Figure 1E). On the contrary, the length of time it takes to reach mother-cell lysis varies substantially among cells (Figure 1E; the time traces after mother-cell lysis are highlighted in red). On average, after the ThT value reaches 1,000 au, it takes 3.3 h for the spores to be released from mother cells with a standard deviation of 1.4 h (Figures S5 and S6). As a consequence of the finite gradual increase and heterogeneous timing of “exit,” different cells reach the different level of peak ThT intensities; the longer the forespore persists in the mother cells the greater the ThT accumulation becomes on forespores. The peak ThT intensity levels are, however, only weakly correlated with the intensity levels on the released endospores (Figure S7). This suggests that not only the negative surface potential of forespores but also the internal environment of mother cells may also contribute to the accumulation of ThT on forespore periphery. Altogether, our single-cell analysis revealed that the mother-cell-side surface of outer forespore membranes increasingly becomes negative during late sporulation. This conclusion is consistent with the previous study reporting the negative surface potential of mature endospores (ζ potential = −26 mV at pH 7.0) (Piktel et al., 2017).
Outer Spore Coat Accumulates Positive Molecules on the Forespore Surfaces by Electrostatic Attraction
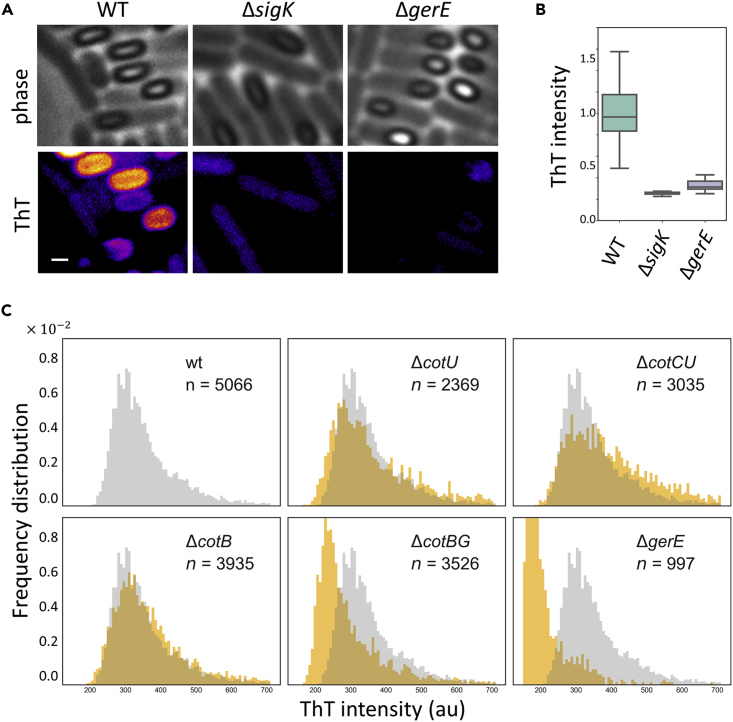
The negative surface potential of developed endospores was implicated with the outer protective layers (Pesce et al., 2014). Therefore, we hypothesized that assembly of outer protective layers (mother-cell side of outer spore membrane) accounts for the negative surface potential of forespores. To examine this conjecture, we measured the ThT dynamics with mutant strains lacking the outer spore coat; specifically, we utilized two deletion mutant strains lacking sigK and gerE. These genes encode late sporulation regulators, which control the expression of coat proteins and cortex synthesis enzymes. The fluorescence intensities on forespores in these mutant strains were clearly lower than in the wild-type strain (Figures 2A and 2B, see also Figure S4C). Furthermore, time-lapse microscopy with cotE deletion strain showed diminished fluorescence intensity increase (Figure S8). These results suggest that the outer layers of forespores are the main contributor to the accumulation of positive ions on the forespore surfaces.
Figure 2.
ThT Accumulation Is due to the Negative Surface Potential of Spores
(A) ThT fluorescence images with mutant strains lacking spore coats. Microscopy images of phase contrast (upper panels) and ThT fluorescence (lower panels) of wild-type (wt), ΔsigK, and ΔgerE strains. Scale bar, 1 μm.
(B) Box and whisker plot showing peak ThT intensities in wt, ΔsigK, and ΔgerE strains. Thirty cells were analyzed for each strain from two independent experiments. The boxes and whiskers show IQR (interquartile range) and 1.5x IQR, respectively.
(C) Histogram of ThT intensities on endospores with wt, ΔcotU, ΔcotC, ΔcotB, ΔcotBG, and ΔgerE. The number of endospores analyzed from at least two independent experiments is indicated in each panel. Histogram of wt strain is shown in gray in all panels for comparison with mutant strains (shown in orange). ΔcotBG and ΔgerE strains exhibit 13.5% and 43% reduction of average ThT intensity levels from wt.
To further examine the contribution of individual coat proteins, we measured ThT fluorescence with the mutants lacking the structural proteins of the outer spore coat. Specifically, we measured ThT intensities on endospore surfaces with the mutant strains of cotB, cotBG, cotU, and cotCU, as well as the gerE strain. The mutants mostly showed a similar intensity level of ThT compared with wild-type; however, a slight decrease in ThT intensity was observed with the cotBG double deletion strain (Figure 2C). Therefore, the negative surface potential of forespores is likely associated with the multiple components of outer endospores coat, including CotB and CotG. Taking into account the previous studies reporting the negative electric charge of endospores (Pesce et al., 2014, Piktel et al., 2017), our results suggest that the outer protective layers of forespores attract cations as forespores develop into resilient spores on the mother-cell side of outer forespore membranes.
Electrostatic Attraction of Cations on Forespore Surfaces Correlates with the Probability of Premature Germination
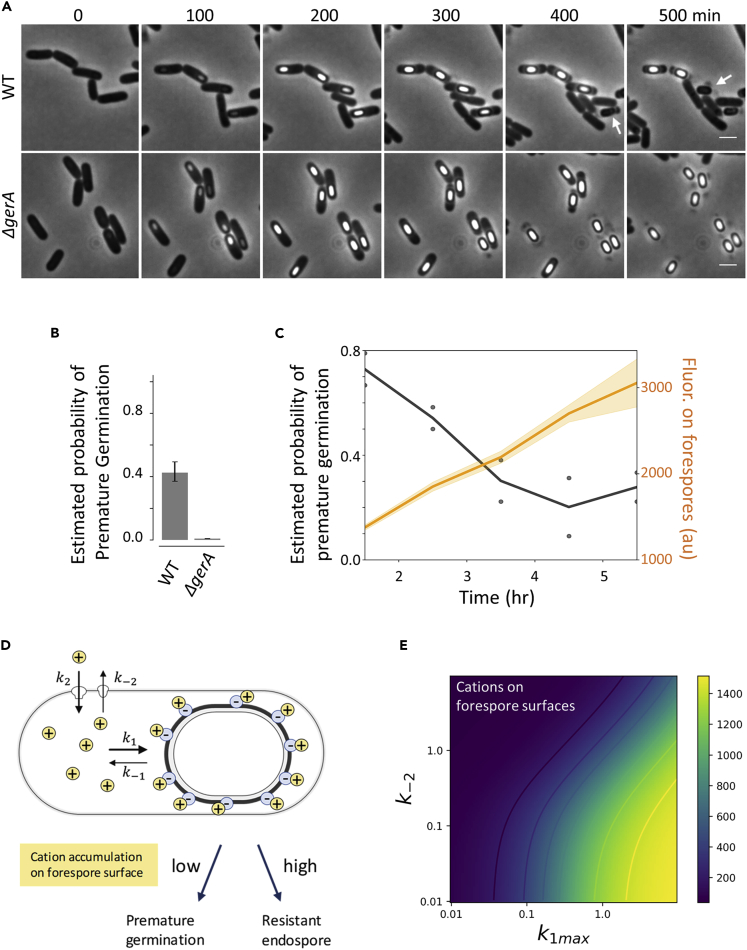
We next wondered if the cation accumulation on the forespore surface has a biological role in sporulation. Our single-cell time-lapse microscopy showed that some phase-bright forespores turned into phase-dark while still inside mother cells (Figure 3A; upper panel, Figure 3B and Video S4). This change in the phase contrast was reminiscent to the germination of endospores. We thus speculated that this change is due to premature germination within mother cells. To test this idea, we conducted time-lapse microscopy with the mutant strain lacking gerA gene, which encodes a main germinant receptor essential for the L-alanine-induced germination. As expected, the deletion of gerA gene eliminates the change of phase brightness with forespores (Figure 3A; lower panel, Figure 3B and Video S5). This observation later appeared complementary to the genetic analyses of a recent paper (Ramírez-Guadiana et al., 2017b). Through extensive genetic analysis, this study by Ramirez-Guadiana et al elegantly demonstrated that GerA-mediated premature germination is coupled with morphogenetic errors. However, how cells are able to couple premature germination with a range of different types of morphogenetic errors, such as synthesis of the endospore protective layers (coat and cortex) and core dehydration, remained unclear. We thus aimed to understand the possible mechanism by which the probability of premature germination is tuned during sporulation.
Figure 3.
Cation Accumulation on Forespore Surfaces Suppresses Premature Germination
(A) Phase-contrast images of wild-type (WT) and ΔgerA strains during sporulation. Scale bar, 2 μm. Premature germination is indicated by white arrows, whereas it is absent with ΔgerA.
(B) Estimated probability of premature germination in wt and ΔgerA strains (at least 351 sporulating cells were analyzed for each strain from two independent experiments). Error bars are 95% confidence intervals for Poisson distribution.
(C) The probability of premature germination and ThT fluorescence (mean ± SEM) over time. Time series of single-cell ThT intensity are aligned as in Figure 1E, and probability of premature germination was calculated for each hour.
(D) A diagram showing the hypothesis that premature germination probability is coupled with the cation accumulation on forespore surfaces. A phenomenological mathematical model was developed accounting the influx and efflux of cations (k2 and k-2) and the binding and unbinding of cations to forespore surfaces (k1 and k-1).
(E) Heatmap showing the simulation results of Cf (cations on forespore surface) computed with various k1max and k-2.
Time-lapse microscopy showing sporulating wild-type cells in phase contrast. Several developing forespores turn phase dark in the mother cells. Scale bar, 2 μm
Time-lapse microscopy showing gerA sporulating cells in phase contrast. No premature germination was observed with this strain. Scale bar, 2 μm
By taking advantage of time-lapse single-cell imaging, we quantified the time evolution of premature germination events. Our data revealed that the probability of premature germination decreases as the level of cation accumulation on the forespore surface increases (Figure 3C). Therefore, we hypothesized that the accumulation of cationic ions on forespore surfaces may prevent the germination of forespores (Figure 3D). Intriguingly, high levels of various cationic ions (e.g., K+, Na+, and Ca2+) prevent the germination of “endo-”spores by limiting the access of L-alanine to the intermembrane space where the germinant recognition sites of GerA exist (Nagler and Moeller, 2015). Inspired by this, we hypothesized that the electrical polarization of the forespore surfaces relates with the membrane transport across the outer membrane, which alters the probability of premature germination. To identify which native cations may be involved in the modulation of premature germination probability, we used fluorescent indicators APG-2 AM and ANG-2 AM to measure K+ and Na+, respectively (Prindle et al., 2015). Both K+ and Na+ appeared to be accumulated on forespore periphery regions (Figures S9 and S10). APG-2 provides a clear signal, whereas the other two reporters are relatively noisy with our experimental setting. Therefore, although we emphasize that we do not exclude the possibilities that other cations (e.g., Mg2+ or Na+) are also accumulated, we concluded that K+ is one of the cations that accumulates highly on the forespore surfaces.
The Premature Germination Probability Can Be Modulated by Chemical Perturbations
To further investigate the hypothesis that the electrical polarization of forespore surfaces suppresses premature germination, we established a minimalistic phenomenological model describing the cation dynamics in cytoplasm (Cc) and forespore-bound (Cf) (Figure 3D). Accounting for the development of negative surface potential of forespores, we assumed that the affinity of cations to forespore surfaces (k1) increases over time and eventually saturates at a value, k1max. Numerical simulations of this model showed that Cf decreases in a monotonic manner when cation accumulation is inhibited (lower k1max) or cation efflux is increased (higher k−2) (Figure 3E).
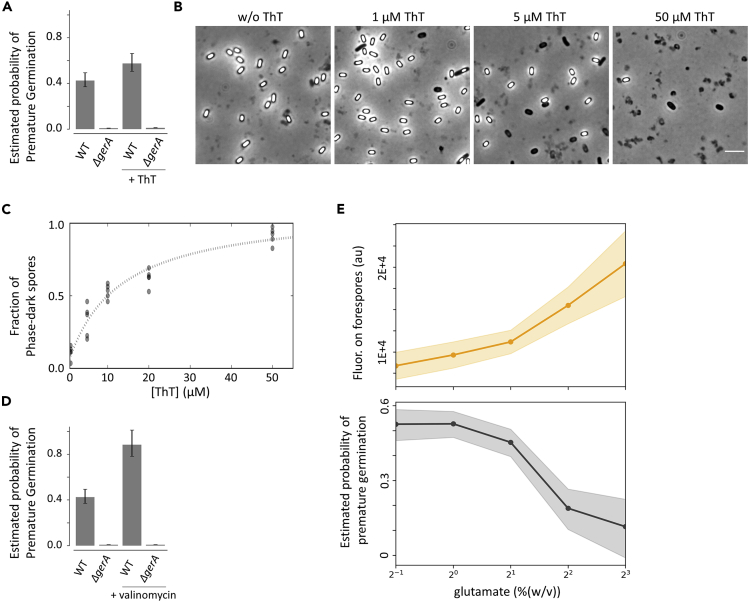
We first examined the prediction from the model regarding the decrease in cation accumulation (lower k1max). By taking into account the charge conservation law, it is expected that exogenous cations should act as a competitive inhibitor for native cations (e.g., K+) in terms of forespore surface accumulation. In fact, we observed decreased accumulation of K+ on forespore surfaces when ThT is present (Figure S9), suggesting that ThT may act as a competitive inhibitor for the cation accumulation. Therefore, our model predicted that addition of ThT should increase the premature germination probability. We estimated the probability of premature germination with and without ThT and found that a greater fraction of forespores, albeit slightly, germinate prematurely when ThT is supplemented to the media (Figure 4A). We confirmed that the premature germination remains absent in ΔgerA strain with or without the presence of ThT (Figure 4A). We also examined whether ThT has a direct interaction with the GerA receptor, by conducting germination assay with purified endospores. The result showed that, unlike premature germination of forespores, the germination of mature endospores is unaffected by ThT (Figure S11). This result indicates that the observed increase of premature germination probability is not due to direct interaction between GerA receptor and ThT. To examine the causality of the ThT effect, we tested different ThT concentrations and measured the premature germination probability. The results showed a clear increase of phase-dark endospore fraction as a function of ThT concentrations (Figures 4B and 4C).
Figure 4.
Chemical Perturbations Alter the Probability of Premature Germination
(A) The probability of premature germination with and without ThT. The premature germination remains absent with gerA deletion strain. At least 351 sporulating cells from two independent experiments were analyzed for each condition/strain. The error bars are 95% confidence intervals for Poisson distribution.
(B) Microscopy images of endospores cultured in liquid RM supplemented without or with ThT at 1, 5, and 50 μM. Scale bar, 5 μm. Phase-dark spores are distinguished from vegetative cells by their size.
(C) Quantification of panel (B). The fraction of germinated (phase-dark) endospores increases with increasing ThT.
(D) Addition of valinomycin to the media increases the probability of premature germination. At least 339 sporulating cells were analyzed for each strain/condition. Error bars represent 95% confidence intervals for Poisson distribution.
(E) The peak fluorescence signal on forespore surfaces (TMRM) and the probability of premature germination drops (lower plot) were plotted with various levels of glutamate (final concentrations). Thirty-one cells from three independent experiments were analyzed for each condition. Shaded regions are standard deviation for the upper plot and Poisson confidence intervals for the lower plot.
We next examined the prediction from the model regarding the increase in cation efflux (higher k−2) by utilizing valinomycin. Valinomycin is a potassium ionophore, which means it increases the membrane permeability of K+, leading to a greater k−2. As can be seen in Figure 3E, the model predicts that valinomycin should increase the probability of premature germination. To minimize the potential global impacts of valinomycin, we exposed cells to valinomycin only after cells reached the commitment stage (cultured in the Resuspension Medium [RM] for 3.5 h). Quantification of single-cell time-lapse microscopy data showed that the probability of premature germination significantly increases by valinomycin (Figure 4D). To check that this is not due to a general toxic effect of valinomycin, committed cells of ΔgerA strain were also exposed to valinomycin. Contrary to the results with wild-type, the sporulation of the ΔgerA strain was unaffected by valinomycin and phase-bright endospores were produced normally (Figures 4D and S12). This result indicates that the crucial role of the transmembrane electrochemical gradient of K+ during late sporulation (stage IV-VI) is to prevent premature germination and not to support harvesting the energy required for the completion of late sporulation process.
Glutamate Availability Influences the Probability of Premature Germination
Although a previous study suggested that the probability of premature germination may be constant in different media (Ramírez-Guadiana et al., 2017b), our model predicted that environmental conditions that affect the mother-cell membrane potential should alter the probability of premature germination. To this end, we focused on glutamate availability because glutamate is a gating molecule for the K+ channels (Liu et al., 2017, Prindle et al., 2015). Thus, according to Figure 3E, our model predicted that the premature germination probability would decrease as a function of glutamate levels in the media (lower k−2).
To test this experimentally, cells committed to sporulation were transferred to the RM containing different levels of monosodium glutamate (final concentrations, 0.5–8.0% [w/v]). Then, the probability of premature germination and the forespore-surface charge were determined by single-cell microscopy (Figure 3B). The results showed that, as predicted by the model, the probability of premature germination decreases as a function of glutamate concentrations, whereas the accumulation of charge is inversely correlated with the glutamate levels (Figure 4E). This result indicates that the probability of premature germination is indeed dependent on the media compositions.
Altogether, our results suggested that, under favorable conditions (e.g., high glutamate concentrations in the media), the probability of premature germination is lower. However, when conditions are not favorable (which results in higher k−2) or when forespores have morphological errors (which results in lower k1), forespores are more prone to germinate prematurely.
Spores Produced in Media Containing ThT Are less Resistant against Wet Heat
Considering that the quality/quantity control of endospores poses a challenge in our society (Nguyen Thi Minh et al., 2011), we wondered if the aforementioned understanding enables us to propose a way of controlling the quality and quantity of endospores.
SpoVV is a concentrative nucleoside transporter that translocates dipicolinic acid (DPA; pyridine-2,6-dicarboxylic acid) against the concentration gradient across the outer forespore membrane from the mother-cell compartment to the intermembrane space (Ramírez-Guadiana et al., 2017a). The deletion of spoVV dramatically increases the probability of premature germination (Ramírez-Guadiana et al., 2017b). The reduction of DPA accumulation into spore core is typically associated with wet-heat resistance. We speculate that ThT treatment during sporulation could reproduce the phenotype of the spoVV deletion.
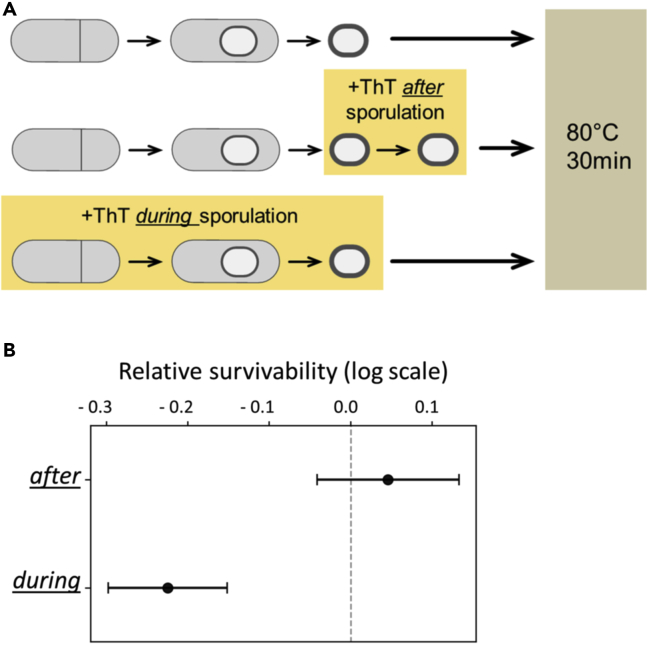
To test this, we prepared endospores using the sporulation media RM with and without 10 μM ThT and conducted the wet-heat resistance assays at 80°C for 30 min (Figure 5A). As a control, we also treated endospores with ThT after completion of spore formation (Figure 5A; middle). The survivability of endospores was measured for each sample and normalized to the survivability of endospores prepared without ThT (Figure 5A; top). The endospores exposed to ThT after completing sporulation (Figure 5A; middle) exhibit the survivability comparable with that of the endospores without ThT (Figure 5B). However, the endospores prepared with ThT are more sensitive to the wet-heat treatment than the endospores prepared without ThT (Figure 5B). This result suggests that supplementation of ThT not only reduces the yield of endospores (Figure 4B) but also diminishes the wet-heat resistance property (Figures 5B and 5C).
Figure 5.
ThT Decreases the Wet-heat Resistance Level of Endospores
(A) Diagram describing the wet-heat resistance assay shown in panel (B). Cells were committed to sporulation (culture for 3.5 h in RM), then moved to RM with and without ThT. Spores were treated at 80°C for 30 min. See Methods for details.
(B) Survivability of endospores relative to spores produced without ThT. Wet-heat survivability is lower when spores are formed in the presence of 10 μM ThT. Survivability is comparable when ThT is added to the endospores already prepared in RM without ThT. Error bars are 95% confidence intervals for Poisson distribution based on the number of spores observed. Data from three technical replica and three independent biological replicates.
Discussion
By measuring the cation dynamics during late sporulation, we demonstrated that forespores poorly accumulating cations preferentially germinate while still inside the mother cells. Our results revealed that the premature germination process is coupled with the external and internal conditions during late sporulation through the cation accumulations on forespore surfaces. More specifically, environmental factors affect the cytoplasmic cation levels and the establishment of protective layers and molecule transport across outer membranes alter the accumulation of cations. This mechanism could add plasticity to the genetically regulated processes of sporulation.
Cation Accumulation as an Integrative Indicator for Various Internal and External Environments
A universal challenge of quality control is to integratively monitor the diverse internal and environmental factors that may be influential for the offspring survival. This is a challenging task to be achieved by specific sensor proteins because it requires a wide variety of sensors and integration of inputs. In the case of B. subtilis sporulation, our data suggested that the cation accumulation enables coupling of internal and external conditions with the quality control system via premature germination. This mechanism presents an elegant solution to the challenge of sensing diverse environmental factors and morphological errors. This finding also raised the possibility that concentrations of cations may influence the assembly of protective layers, which can result in phenotypic plasticity. It would be interesting to experimentally examine this possibility. Another important unanswered question is the driving force of SpoVV transporter. If the asymmetric surface potential across the outer membrane contributes to the driving force for SpoVV, it may be expected that it is an antiporter. Biophysically analyzing the SpoVV transporter to examine the direction of ion flux and its driving force will be an important topic of research.
Our results suggest that K+ is an important cation accumulated on forespore surfaces. However, it remains unclear if the accumulation of other cation species (e.g., Na+) is equally or more crucial for the premature germination control. Since various cations are shown to suppress endospore germination (Nagler et al., 2014), it is conceivable that accumulation of different ion species may have different impacts to assembly of coat proteins.
Potential Roles of Premature Germination in Biofilm Colonies
Our study presents a perspective in the emerging research field of bacterial electrical signaling (Lee et al., 2017). We showed that the probability of premature germination depends on the mother cells' ability to take up cations from their environment. Because the efficiency of cation uptake can be altered by the biofilm electrical signaling (Prindle et al., 2015), electrical signaling should, in theory, alter the probability of premature germination. Intriguingly, in B. subtilis biofilms, sporulation is regulated both in space and time (Branda et al., 2001). However, the mechanism by which this pattern emerges remains unclear. Based on the insight we gained in this study, we suspect that the spatiotemporal organization of sporulation during biofilm formation may also be regulated by electrical signaling and premature germination. Cell lysis after premature germination may also provide some benefits to the surrounding cells by providing nutrients or physical space. Such interactions would be pronounced when cells are structurally organized, such as in biofilms (Asally et al., 2012, Momeni et al., 2013). Hence, it is plausible to speculate that cell death through premature germination may provide more significant population-level impacts in biofilms. In our future research, we shall determine the potential roles of biofilm electrical signaling to sporulation, and vice versa.
ThT as a Potential Chemical to Prevent the Formation of Resistant Endospores
We showed that ThT decreases the yield of endospores by promoting the premature germination, while at the same time lowering the wet-heat resistance of endospores. These results propose a usage of ThT as an agent to limit the formation of resistant endospores. We believe this is an attractive possibility since ThT is likely non-toxic to humans. Intriguingly, ThT at 50 μM has been shown to prevent the disruption of muscle sarcomeres during aging and extend the median lifespan of C. elegans (Alavez et al., 2011). Coincidentally, 50 μM is the ThT concentration at which we observed almost complete abolishment of phase-bright endospore formation. Therefore, ThT at a concentration around 50 μM may bring multiple benefits to the industries where endospore formation poses problems. We also note that ThT is a relatively inexpensive chemical; a liter of 50 μM ThT solution would cost approximately 0.05 US dollars. Bacterial spores of other species are also negatively charged (Pesce et al., 2014, Piktel et al., 2017), which suggests that the mechanism may be conserved among species. As such, systematic investigation of the impact of ThT in various spore-forming bacterial species, such as B. anthrax and C. difficile, would be an important avenue of further research.
Limitations of the Study
The exact molecular mechanisms by which premature germination is coupled with cation accumulation remain unclear. Additional experiments are needed to quantitatively determine the electrical potential across outer and inner spore membranes and to understand their roles in sporulation and germination. Physicochemical modeling framework is still to be done to understand the electrical potential dynamics during sporulation.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank GM Süel, P Schäfer, DY Lee, T Çağatay, A Kuchina, A Saggese, and the members of the Asally laboratory (J Stratford, M Delise, I Lopez-Grobas, and C Edwards) for their comments to the drafts of the manuscript; the anonymous reviewers for constructive criticism; E Ricca, L Baccigalupi, and R Isticato for generously providing bacterial strains; and the reviewers for their constructive criticisms. This study was supported by the start-up fund from University of Warwick, SLS Pump priming fund, and the Royal Society Research Grant to M.A., BBSRC/EPSRC grant to WISB (BB/M017982/1), and the Spanish Ministry of Economy and Competitiveness and FEDER (FIS2015-66503-C3-1-P) and the Maria de Maeztu Program for Units of Excellence (project MDM-2014-0370) to J.G.-O. J.M.B. acknowledges the funding by the MRC Doctoral Training Partnership (MR/N014294/1). P.B. was supported by a scholarship from the Region Auvergne-Rhone-Alpes and the Université Grenoble Alpes.
Author Contributions
T.S. and P.B. prepared samples for experiments and conducted experiments. T.S. and M.A. designed experiments and analyzed experimental data. J.M.B. analyzed the data and electrochemical models. J.G.-O. developed theoretical formalism and performed numerical simulations. M.A. wrote the manuscript with input from all other authors. All authors provided critical feedback to the manuscript and discussed the results.
Declaration of Interests
The authors declare no competing interests.
Published: June 28, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.05.044.
Supplemental Information
References
- Alavez S., Vantipalli M.C., Zucker D.J.S., Klang I.M., Lithgow G.J. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature. 2011;472:226–229. doi: 10.1038/nature09873. [DOI] [PMC free article] [PubMed] [Google Scholar]; Alavez, S., Vantipalli, M.C., Zucker, D.J.S., Klang, I.M., Lithgow, G.J.., 2011. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature 472, 226-229. [DOI] [PMC free article] [PubMed]
- Asally M., Kittisopikul M., Rue P., Du Y., Hu Z., Cagatay T., Robinson A.B., Lu H., Garcia-Ojalvo J., Suel G.M. Localized cell death focuses mechanical forces during 3D patterning in a biofilm. Proc. Natl. Acad. Sci. U S A. 2012;109:18891–18896. doi: 10.1073/pnas.1212429109. [DOI] [PMC free article] [PubMed] [Google Scholar]; Asally, M., Kittisopikul, M., Rue, P., Du, Y., Hu, Z., Cagatay, T., Robinson, A.B., Lu, H., Garcia-Ojalvo, J., Suel, G.M.., 2012. Localized cell death focuses mechanical forces during 3D patterning in a biofilm. Proc. Natl. Acad. Sci. U S A 109, 18891-18896. [DOI] [PMC free article] [PubMed]
- Branda S.S., Gonzalez-Pastor J.E., Ben-Yehuda S., Losick R., Kolter R., González-Pastor J.E., Ben-Yehuda S., Losick R., Kolter R. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. U S A. 2001;98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]; Branda, S.S., Gonzalez-Pastor, J.E., Ben-Yehuda, S., Losick, R., Kolter, R., Gonzalez-Pastor, J.E., Ben-Yehuda, S., Losick, R., Kolter, R.., 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. U S A 98, 11621-11626. [DOI] [PMC free article] [PubMed]
- Bruni G.N., Weekley R.A., Dodd B.J.T., Kralj J.M. Voltage-gated calcium flux mediates Escherichia coli mechanosensation. Proc. Natl. Acad. Sci. U S A. 2017;114:9445–9450. doi: 10.1073/pnas.1703084114. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bruni, G.N., Weekley, R.A., Dodd, B.J.T., Kralj, J.M.., 2017. Voltage-gated calcium flux mediates Escherichia coli mechanosensation. Proc. Natl. Acad. Sci. U S A 114, 9445-9450. [DOI] [PMC free article] [PubMed]
- Driks A. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 1999;63:1–20. doi: 10.1128/mmbr.63.1.1-20.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]; Driks, A.., 1999. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 63, 1-20. [DOI] [PMC free article] [PubMed]
- Eijlander R.T., Abee T., Kuipers O.P. Bacterial spores in food: how phenotypic variability complicates prediction of spore properties and bacterial behavior. Curr. Opin. Biotechnol. 2011;22:180–186. doi: 10.1016/j.copbio.2010.11.009. [DOI] [PubMed] [Google Scholar]; Eijlander, R.T., Abee, T., Kuipers, O.P.., 2011. Bacterial spores in food: how phenotypic variability complicates prediction of spore properties and bacterial behavior. Curr. Opin. Biotechnol. 22, 180-186. [DOI] [PubMed]
- Eijlander R.T., De Jong A., Krawczyk A.O., Holsappel S., Kuipers O.P. SporeWeb: an interactive journey through the complete sporulation cycle of Bacillus subtilis. Nucleic Acids Res. 2014;42:685–691. doi: 10.1093/nar/gkt1007. [DOI] [PMC free article] [PubMed] [Google Scholar]; Eijlander, R.T., De Jong, A., Krawczyk, A.O., Holsappel, S., Kuipers, O.P.., 2014. SporeWeb: an interactive journey through the complete sporulation cycle of Bacillus subtilis. Nucleic Acids Res. 42, 685-691. [DOI] [PMC free article] [PubMed]
- Humphries J., Xiong L., Liu J., Prindle A., Yuan F., Arjes H.A., Tsimring L., Süel G.M. Species-Independent attraction to biofilms through electrical signaling. Cell. 2017;168:200–209.e12. doi: 10.1016/j.cell.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; Humphries, J., Xiong, L., Liu, J., Prindle, A., Yuan, F., Arjes, H.A., Tsimring, L., Suel, G.M.., 2017. Species-Independent attraction to biofilms through electrical signaling. Cell 168, 200-209.e12. [DOI] [PMC free article] [PubMed]
- Kralj J.M., Hochbaum D.R., Douglass A.D., Cohen A.E. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science. 2011;333:345–348. doi: 10.1126/science.1204763. [DOI] [PubMed] [Google Scholar]; Kralj, J.M., Hochbaum, D.R., Douglass, A.D., Cohen, A.E.., 2011. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science 333, 345-348. [DOI] [PubMed]
- Kuchina A., Espinar L., Çağatay T., Balbin A.O., Zhang F., Alvarado A., Garcia-Ojalvo J., Süel G.M. Temporal competition between differentiation programs determines cell fate choice. Mol. Syst. Biol. 2011;7:1–11. doi: 10.1038/msb.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kuchina, A., Espinar, L., Cağatay, T., Balbin, A.O., Zhang, F., Alvarado, A., Garcia-Ojalvo, J., Suel, G.M.., 2011. Temporal competition between differentiation programs determines cell fate choice. Mol. Syst. Biol. 7, 1-11. [DOI] [PMC free article] [PubMed]
- Lee D.D., Galera-Laporta L., Bialecka-Fornal M., Moon E.C., Shen Z., Briggs S.P., Garcia-Ojalvo J., Süel G.M. Magnesium flux modulates ribosomes to increase bacterial survival. Cell. 2019;177:352–360.e13. doi: 10.1016/j.cell.2019.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee, D.D., Galera-Laporta, L., Bialecka-Fornal, M., Moon, E.C., Shen, Z., Briggs, S.P., Garcia-Ojalvo, J., Suel, G.M.., 2019. Magnesium flux modulates ribosomes to increase bacterial survival. Cell 177, 352-360.e13. [DOI] [PMC free article] [PubMed]
- Lee D.D., Prindle A., Liu J., Süel G.M. SnapShot: electrochemical communication in biofilms. Cell. 2017;170:214–214.e1. doi: 10.1016/j.cell.2017.06.026. [DOI] [PubMed] [Google Scholar]; Lee, D.D., Prindle, A., Liu, J., Suel, G.M.., 2017. SnapShot: electrochemical communication in biofilms. Cell. 170, 214-214.e1. [DOI] [PubMed]
- Liu J., Martinez-Corral R., Prindle A., Lee D.D., Larkin J., Gabalda-Sagarra M., Garcia-Ojalvo J., Süel G.M. Coupling between distant biofilms and emergence of nutrient time-sharing. Science. 2017;356:638–642. doi: 10.1126/science.aah4204. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu, J., Martinez-Corral, R., Prindle, A., Lee, D.D., Larkin, J., Gabalda-Sagarra, M., Garcia-Ojalvo, J., Suel, G.M.., 2017. Coupling between distant biofilms and emergence of nutrient time-sharing. Science 356, 638-642. [DOI] [PMC free article] [PubMed]
- Liu J., Prindle A., Humphries J., Gabalda-Sagarra M., Asally M., Lee D.D., Ly S., Garcia-Ojalvo J., Süel G.M. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature. 2015;523:550–554. doi: 10.1038/nature14660. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu, J., Prindle, A., Humphries, J., Gabalda-Sagarra, M., Asally, M., Lee, D.D., Ly, S., Garcia-Ojalvo, J., Suel, G.M.., 2015. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature 523, 550-554. [DOI] [PMC free article] [PubMed]
- Lo C.J., Leake M.C., Pilizota T., Berry R.M. Nonequivalence of membrane voltage and ion-gradient as driving forces for the bacterial flagellar motor at low load. Biophys. J. 2007;93:294–302. doi: 10.1529/biophysj.106.095265. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lo, C.J., Leake, M.C., Pilizota, T., Berry, R.M.., 2007. Nonequivalence of membrane voltage and ion-gradient as driving forces for the bacterial flagellar motor at low load. Biophys. J. 93, 294-302. [DOI] [PMC free article] [PubMed]
- Lopez-Garrido J., Ojkic N., Khanna K., Wagner F.R., Villa E., Endres R.G., Pogliano K. Chromosome translocation inflates Bacillus forespores and impacts cellular morphology. Cell. 2018;172:758–770.e14. doi: 10.1016/j.cell.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lopez-Garrido, J., Ojkic, N., Khanna, K., Wagner, F.R., Villa, E., Endres, R.G., Pogliano, K.., 2018. Chromosome translocation inflates Bacillus forespores and impacts cellular morphology. Cell 172, 758-770.e14. [DOI] [PMC free article] [PubMed]
- Mao L., Jiang S., Wang B., Chen L., Yao Q., Chen K. Protein profile of Bacillus subtilis spore. Curr. Microbiol. 2011;63:198–205. doi: 10.1007/s00284-011-9967-4. [DOI] [PubMed] [Google Scholar]; Mao, L., Jiang, S., Wang, B., Chen, L., Yao, Q., Chen, K.., 2011. Protein profile of Bacillus subtilis spore. Curr. Microbiol. 63, 198-205. [DOI] [PubMed]
- Momeni B., Waite A.J., Shou W. Spatial self-organization favors heterotypic cooperation over cheating. Elife. 2013;2:e00960. doi: 10.7554/eLife.00960. [DOI] [PMC free article] [PubMed] [Google Scholar]; Momeni, B., Waite, A.J., Shou, W.., 2013. Spatial self-organization favors heterotypic cooperation over cheating. Elife 2, e00960. [DOI] [PMC free article] [PubMed]
- Mutlu A., Trauth S., Ziesack M., Nagler K., Bergeest J.P., Rohr K., Becker N., Höfer T., Bischofs I.B. Phenotypic memory in Bacillus subtilis links dormancy entry and exit by a spore quantity-quality tradeoff. Nat. Commun. 2018;9:69. doi: 10.1038/s41467-017-02477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mutlu, A., Trauth, S., Ziesack, M., Nagler, K., Bergeest, J.P., Rohr, K., Becker, N., Hofer, T., Bischofs, I.B.., 2018. Phenotypic memory in Bacillus subtilis links dormancy entry and exit by a spore quantity-quality tradeoff. Nat. Commun.. 9, 69. [DOI] [PMC free article] [PubMed]
- Nagler K., Moeller R. Systematic investigation of germination responses of Bacillus subtilis spores in different high-salinity environments. FEMS Microbiol. Ecol. 2015;91:1–10. doi: 10.1093/femsec/fiv023. [DOI] [PubMed] [Google Scholar]; Nagler, K., Moeller, R.., 2015. Systematic investigation of germination responses of Bacillus subtilis spores in different high-salinity environments. FEMS Microbiol. Ecol.. 91, 1-10. [DOI] [PubMed]
- Nagler K., Setlow P., Li Y.Q., Moeller R. High salinity alters the germination behavior of Bacillus subtilis spores with nutrient and nonnutrient germinants. Appl. Environ. Microbiol. 2014;80:1314–1321. doi: 10.1128/AEM.03293-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nagler, K., Setlow, P., Li, Y.Q., Moeller, R.., 2014. High salinity alters the germination behavior of Bacillus subtilis spores with nutrient and nonnutrient germinants. Appl. Environ. Microbiol. 80, 1314-1321. [DOI] [PMC free article] [PubMed]
- Narula J., Fujita M., Igoshin O.A. Functional requirements of cellular differentiation: lessons from Bacillus subtilis. Curr. Opin. Microbiol. 2016;34:38–46. doi: 10.1016/j.mib.2016.07.011. [DOI] [PubMed] [Google Scholar]; Narula, J., Fujita, M., Igoshin, O.A.., 2016. Functional requirements of cellular differentiation: lessons from Bacillus subtilis. Curr. Opin. Microbiol. 34, 38-46. [DOI] [PubMed]
- Narula J., Kuchina A., Lee D.D., Fujita M., Süel G.M., Igoshin O.A. Chromosomal arrangement of Phosphorelay genes couples sporulation and DNA replication. Cell. 2015;162:328–337. doi: 10.1016/j.cell.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Narula, J., Kuchina, A., Lee, D.D., Fujita, M., Suel, G.M., Igoshin, O.A.., 2015. Chromosomal arrangement of Phosphorelay genes couples sporulation and DNA replication. Cell 162, 328-337. [DOI] [PMC free article] [PubMed]
- Nguyen Thi Minh H., Durand A., Loison P., Perrier-Cornet J.-M., Gervais P. Effect of sporulation conditions on the resistance of Bacillus subtilis spores to heat and high pressure. Appl. Microbiol. Biotechnol. 2011;90:1409–1417. doi: 10.1007/s00253-011-3183-9. [DOI] [PubMed] [Google Scholar]; Nguyen Thi Minh, H., Durand, A., Loison, P., Perrier-Cornet, J.-M., Gervais, P., 2011. Effect of sporulation conditions on the resistance of Bacillus subtilis spores to heat and high pressure. Appl. Microbiol. Biotechnol. 90, 1409-1417. [DOI] [PubMed]
- Palop A., Mañas P., Condón S. Sporulation temperature and heat resistance of Bacillus spores: a review. J. Food Saf. 1999;19:57–72. [Google Scholar]; Palop, A., Mañas, P., Condon, S.., 1999. Sporulation temperature and heat resistance of Bacillus spores: a review. J. Food Saf. 19, 57-72.
- Pesce G., Rusciano G., Sasso A., Isticato R., Sirec T., Ricca E. Surface charge and hydrodynamic coefficient measurements of Bacillus subtilis spore by optical tweezers. Colloids Surf. B Biointerfaces. 2014;116:568–575. doi: 10.1016/j.colsurfb.2014.01.039. [DOI] [PubMed] [Google Scholar]; Pesce, G., Rusciano, G., Sasso, A., Isticato, R., Sirec, T., Ricca, E.., 2014. Surface charge and hydrodynamic coefficient measurements of Bacillus subtilis spore by optical tweezers. Colloids Surf. B Biointerfaces 116, 568-575. [DOI] [PubMed]
- Piktel E., Pogoda K., Roman M., Niemirowicz K., Tokajuk G., Wróblewska M., Szynaka B., Kwiatek W.M., Savage P.B., Bucki R. Sporicidal activity of ceragenin CSA-13 against Bacillus subtilis. Sci. Rep. 2017;7:44452. doi: 10.1038/srep44452. [DOI] [PMC free article] [PubMed] [Google Scholar]; Piktel, E., Pogoda, K., Roman, M., Niemirowicz, K., Tokajuk, G., Wroblewska, M., Szynaka, B., Kwiatek, W.M., Savage, P.B., Bucki, R.., 2017. Sporicidal activity of ceragenin CSA-13 against Bacillus subtilis. Sci. Rep.. 7, 44452. [DOI] [PMC free article] [PubMed]
- Plomp M., Carroll A.M., Setlow P., Malkin A.J. Architecture and assembly of the Bacillus subtilis spore coat. PLoS One. 2014;9:e108560. doi: 10.1371/journal.pone.0108560. [DOI] [PMC free article] [PubMed] [Google Scholar]; Plomp, M., Carroll, A.M., Setlow, P., Malkin, A.J.., 2014. Architecture and assembly of the Bacillus subtilis spore coat. PLoS One 9. e108560. [DOI] [PMC free article] [PubMed]
- Prindle A., Liu J., Asally M., Ly S., Garcia-Ojalvo J., Süel G.M.G.M. Ion channels enable electrical communication in bacterial communities. Nature. 2015;527:59–63. doi: 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prindle, A., Liu, J., Asally, M., Ly, S., Garcia-Ojalvo, J., Suel, G.M.G.M.., 2015. Ion channels enable electrical communication in bacterial communities. Nature 527, 59-63. [DOI] [PMC free article] [PubMed]
- Prindle A., Samayoa P., Razinkov I., Danino T., Tsimring L.S., Hasty J. A sensing array of radically coupled genetic “biopixels.”. Nature. 2012;481:39–44. doi: 10.1038/nature10722. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prindle, A., Samayoa, P., Razinkov, I., Danino, T., Tsimring, L.S., Hasty, J.., 2012. A sensing array of radically coupled genetic “biopixels.” Nature 481, 39-44. [DOI] [PMC free article] [PubMed]
- Ramírez-Guadiana F.H., Meeske A.J., Rodrigues C.D.A., Barajas-Ornelas R.D.C., Kruse A.C., Rudner D.Z. A two-step transport pathway allows the mother cell to nurture the developing spore in Bacillus subtilis. PLoS Genet. 2017;13:e1007015. doi: 10.1371/journal.pgen.1007015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ramirez-Guadiana, F.H., Meeske, A.J., Rodrigues, C.D.A., Barajas-Ornelas, R.D.C.. Kruse, A.C., Rudner, D.Z., 2017a. A two-step transport pathway allows the mother cell to nurture the developing spore in Bacillus subtilis. PLoS Genet. 13, e1007015. [DOI] [PMC free article] [PubMed]
- Ramírez-Guadiana F.H., Meeske A.J., Wang X., Rodrigues C.D.A., Rudner D.Z. The Bacillus subtilis germinant receptor GerA triggers premature germination in response to morphological defects during sporulation. Mol. Microbiol. 2017;105:689–704. doi: 10.1111/mmi.13728. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ramirez-Guadiana, F.H., Meeske, A.J., Wang, X., Rodrigues, C.D.A., Rudner, D.Z.., 2017b. The Bacillus subtilis germinant receptor GerA triggers premature germination in response to morphological defects during sporulation. Mol. Microbiol. 105, 689-704. [DOI] [PMC free article] [PubMed]
- Rose R., Setlow B., Monroe A., Mallozzi M., Driks A., Setlow P. Comparison of the properties of Bacillus subtilis spores made in liquid or on agar plates. J. Appl. Microbiol. 2007;103:691–699. doi: 10.1111/j.1365-2672.2007.03297.x. [DOI] [PubMed] [Google Scholar]; Rose, R., Setlow, B., Monroe, A., Mallozzi, M., Driks, A., Setlow, P.., 2007. Comparison of the properties of Bacillus subtilis spores made in liquid or on agar plates. J. Appl. Microbiol. 103, 691-699. [DOI] [PubMed]
- Stratford J.P., Edwards C.L.A., Ghanshyam M.J., Malyshev D., Delise M.A., Hayashi Y., Asally M. Electrically induced bacterial membrane-potential dynamics correspond to cellular proliferation capacity. Proc. Natl. Acad. Sci. U S A. 2019;116:9552–9557. doi: 10.1073/pnas.1901788116. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stratford, J.P., Edwards, C.L.A., Ghanshyam, M.J., Malyshev, D., Delise, M.A., Hayashi, Y., Asally, M.., 2019. Electrically induced bacterial membrane-potential dynamics correspond to cellular proliferation capacity. Proc. Natl. Acad. Sci. U S A 116, 9552-9557. [DOI] [PMC free article] [PubMed]
- Taheri-Araghi S., Brown S.D., Sauls J.T., McIntosh D.B., Jun S. Single-cell physiology. Annu. Rev. Biophys. 2015;44:123–142. doi: 10.1146/annurev-biophys-060414-034236. [DOI] [PMC free article] [PubMed] [Google Scholar]; Taheri-Araghi, S., Brown, S.D., Sauls, J.T., McIntosh, D.B., Jun, S.., 2015. Single-cell physiology. Annu. Rev. Biophys. 44, 123-142. [DOI] [PMC free article] [PubMed]
- Tan I.S., Weiss C.A., Popham D.L., Ramamurthi K.S. A quality-control mechanism removes unfit cells from a population of sporulating bacteria. Dev. Cell. 2015;34:682–693. doi: 10.1016/j.devcel.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tan, I.S., Weiss, C.A., Popham, D.L., Ramamurthi, K.S.., 2015. A quality-control mechanism removes unfit cells from a population of sporulating bacteria. Dev. Cell 34, 682-693. [DOI] [PMC free article] [PubMed]
- Wilkinson B.J., Deans J.A., Ellar D.J. Biochemical evidence for the reversed polarity of the outer membrane of the bacterial forespore. Biochem. J. 1975;152:561–569. doi: 10.1042/bj1520561. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wilkinson, B.J., Deans, J.A., Ellar, D.J.., 1975. Biochemical evidence for the reversed polarity of the outer membrane of the bacterial forespore. Biochem. J. 152, 561-569. [DOI] [PMC free article] [PubMed]
- Willis L., Refahi Y., Wightman R., Landrein B., Teles J., Huang K.C., Meyerowitz E.M., Jönsson H. Cell size and growth regulation in the Arabidopsis thaliana apical stem cell niche. Proc. Natl. Acad. Sci. U S A. 2016;113:E8238–E8246. doi: 10.1073/pnas.1616768113. [DOI] [PMC free article] [PubMed] [Google Scholar]; Willis, L., Refahi, Y., Wightman, R., Landrein, B., Teles, J., Huang, K.C., Meyerowitz, E.M., Jonsson, H.., 2016. Cell size and growth regulation in the Arabidopsis thaliana apical stem cell niche. Proc. Natl. Acad. Sci. U S A 113, E8238-E8246. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescence time-lapse microscopy images corresponding to the main Figures 1B and 1C
Time-lapse microscopy images showing a sporulating cell in phase contrast (left) and pHluorin (right). The intensity in pHluorin images is the ratio of Ex466/Ex400. Lighter colors indicate low pH, and darker colors indicate high pH
Time-lapse microscopy images of a sporulating cell in phase-contrast and TMRM fluorescence
Time-lapse microscopy showing sporulating wild-type cells in phase contrast. Several developing forespores turn phase dark in the mother cells. Scale bar, 2 μm
Time-lapse microscopy showing gerA sporulating cells in phase contrast. No premature germination was observed with this strain. Scale bar, 2 μm