Abstract
Paraquat (PQ), a highly effective herbicide, is widely used worldwide. PQ poisoning can cause multiple organ failure, in which the lung is the primary target organ. After PQ poisoning, the patient mortality rate is as high as 90%, and there is currently no specific antidote. The main clinical treatment is the use of glucocorticoids and cyclophosphamide for pulse therapy, but its effectiveness and safety are still uncertain. We investigated the effectiveness and safety of immunosuppressive pulse therapy with glucocorticoids and cyclophosphamide to evaluate the treatment value in patients with acute PQ poisoning. This meta-analysis, combined with seven trials that enrolled a total of 426 patients, showed that immunosuppressive pulse therapy with glucocorticoids and cyclophosphamide for PQ poisoning significantly reduced mortality of the study group (59.3%, 134/226) compared with the control group (81.0%, 162/200). There was no significant difference of hepatitis or renal failure between the control and study groups, indicating that immunosuppressive pulse therapy was relatively safe. Several patients were reported to have leukopenia and returned to normal after 1–2 weeks without any abnormalities. Two cases of non-fatal sepsis were reported and considered to be a side effect of the immunosuppressive pulse therapy. Thus, immunosuppressive pulse therapy can efficiently reduce the mortality of PQ poisoning and it is relatively safe.
Keywords: Paraquat, Glucocorticoids, Cyclophosphamide, Immunosuppressive pulse therapy, Meta-analysis
1. Introduction
Emergency departments often encounter many acute diseases, such as trauma (Zhao and Lu, 2014; Zhu et al., 2015) and acute poisoning (Lu et al., 2011; Jiang et al., 2016). Paraquat (PQ) poisoning is a very common pesticide poisoning in an emergency department. PQ is a high-efficiency herbicide, which is also toxic to the human after ingestion or skin exposure. At present, PQ is still used a lot in agriculture, and PQ poisoning often occurs (Hart, 1987). It is mainly taken in orally and can also be absorbed through skin mucosa and the airway. The early symptoms of PQ intoxication are not obvious: some patients may have no symptoms, or only present with localized corrosive damage such as oropharynx, esophageal mucosa, and contaminated skin; some patients may have nausea and vomiting. However, the disease progresses rapidly after PQ poisoning. Most patients die of severe respiratory failure or multiple organ failure within hours or days. The mechanism of PQ poisoning has not been completely clarified, with possibilities including oxidative damage, inflammatory reaction, apoptosis, imbalance of extracellular matrix metabolism, coagulation disorder, and autophagy (Seidenfeld et al., 1978; Dinis-Oliveira et al., 2008). Since there is no specific antidote, various treatment methods are being explored, such as vitamin C and vitamin E. Recently, lysine acetylsalicylate was found to relieve lung damage caused by PQ (Huang et al., 2011). However, these treatments still need to have clinical trials. At present, the most common treatments used in clinical are hemopurification and immunosuppressive pulse therapy (Wang et al., 2017). Studies have found that immunosuppressive therapy of glucocorticoid combined with cyclophosphamide in large dose of these two drugs may cause serious damage to liver and kidney functions (Sakemi et al., 1993; Weissel and Hauff, 2000; Marino et al., 2004). Therefore, we decided to conduct a meta-analysis to assess whether it is safe and effective to treat PQ poisoning with immunosuppressive pulse therapy.
2. Methods
2.1. Search strategy
We conducted a comprehensive search of the articles on the immunosuppressive pulse therapy after PQ poisoning according to the procedures of the Cochrane Collaboration for Systematic Reviews and Meta-analyses guidelines. The pertinent studies were published before the end of June 2018 as found in PubMed, EMBASE, and Cochrane databases. All these studies were published in English. Search subject keywords were as follows: paraquat, glucocorticoids, and cyclophosphamide.
2.2. Inclusion and exclusion criteria
The included studies must meet the following criteria: (1) prospective study; (2) the patient must be ≥16 years old and PQ was taken orally; (3) the intervention of the experimental group was the pulse therapy of glucocorticoids and cyclophosphamide, and the intervention of the control group was placebo or standard care. Reviews, case reports, letters, and studies without full data were excluded.
2.3. Data extraction and quality assessment
In order to avoid errors and improve the accuracy of the results, two researchers were responsible for the data extraction process. One investigator was responsible for extracting the details from the articles and another investigator verified the extracted data for accuracy. Then, these two investigators independently assessed the risk of bias for included studies. The quality assessment of randomized controlled trials (RCTs) was appraised using the Cochrane Collaboration’s tool for assessing the risk of bias. The Newcastle-Ottawa Scale (NOS) (Stang, 2010) was used to evaluate non-RCTs.
2.4. Statistical analysis
All statistical analyses were performed with Review Manager (RevMan) version 5.3 (the Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, Denmark). Heterogeneity of included studies was evaluated with I 2 statistics. If I 2<50% and P>0.1, there was no heterogeneity between the studies, and the fixed effect model was used for statistical analysis. If I 2 ranged from 50% to 75% and P<0.1, the random effect model was used for statistical analysis. If I 2>75% and P<0.1, it indicated that there was obvious heterogeneity between studies, combined analysis cannot be performed, and then only descriptive analysis was performed. Because there were only seven studies included in our meta-analysis, we did not do funnel plots.
3. Results
3.1. Study selection
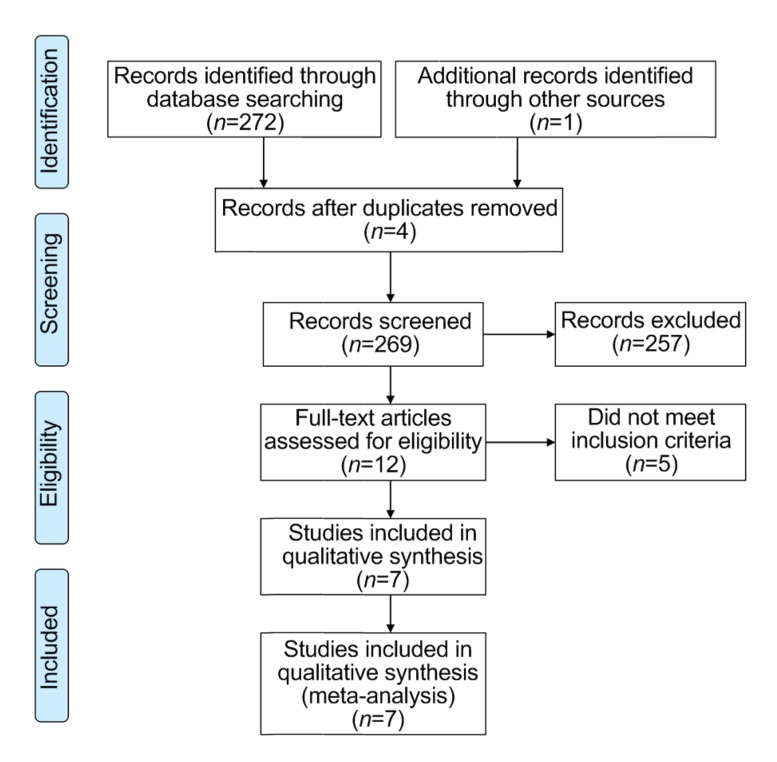
Our search method retrieved 273 studies. The studies identified through search were selected in depth screening, and 266 did not meet the predetermined selection criteria. Finally, seven studies (Perriëns et al., 1992; Lin et al., 1996, 1999, 2006, 2011; Afzali and Gholyaf, 2008; Ghorbani et al., 2015) met the criteria for inclusion in our research (Fig. 1). There are four RCTs and three non-RCTs.
Fig. 1.
Literature search strategy
3.2. Characteristics and methodological quality of the studies
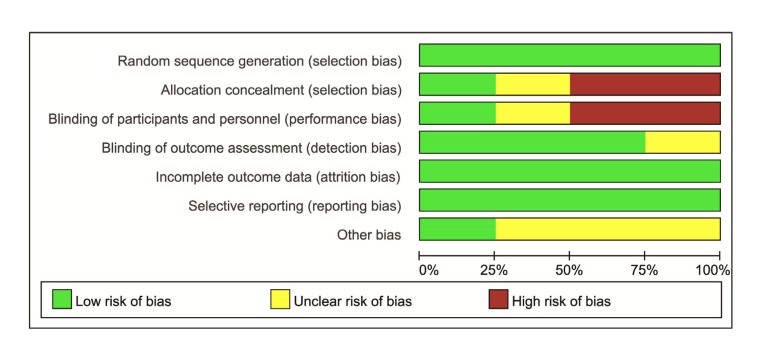
Characteristics of included studies in the meta-analysis are described in Table 1. The seven trials enrolled 426 patients. The characteristics extracted from the included trials were: first author/publication year, area, patient samples, study design, illness severity, outcome measurement, follow-up, and therapy characteristics in the experimental and control groups. All except one trial exceeded one clinical outcomes during the treatment. Mortality data were available in all trials. Three studies were followed up for more than 30 d, one was only followed up for 3 d, and the other three did not report. The quality assessment of RCTs is shown in Fig. 2. Three RCTs (Lin et al., 1999, 2006; Ghorbani et al., 2015) described the special method of randomization. One RCT (Ghorbani et al., 2015) used double blinding. The scores on the NOS scale for the remaining three non-RCTs were 7 (Perriëns et al., 1992), 6 (Lin et al., 2011), and 5 (Lin et al., 1999), respectively.
Table 1.
Characteristics of studies included in meta-analysis
| Author (year) | Area | Study design | Illness severity (control/trial) | No. of patients (male/female) | Intervention |
Outcome measure | Follow up | |
| Trial | Control | |||||||
| Perriëns et al. (1992) | Suriname | Non-RCT | 47(33/14) | MP 8 mg intravenous (iv) qid 14 d; CTX 5 mg/kg iv qd 14 d | Gastric lavage; 10 mg meclopramide iv; Bentonite 30 mg and magnesium sulphate 15 mg in 600 mL water q2 h and meclopramide 10 mg qid | Mortality; respiratory failure; hepatitis; multisystem failure | Not reported | |
| Lin et al. (1996) | Taiwan | Non-RCT | Moderate to fulminant (6NB, 11DB/7NB, 9DB) | 57(29/28) | CTX 1 g iv qd 2 d; MP 1 g iv qd 3 d | Not reported | Mortality; hypoxia; renal failure | Not reported |
| Lin et al. (1999) | Taiwan | RCT | Moderate to fulminant (9NB, 19DB/8NB, 14DB) | 121(56/65) | MP 1 g iv qd 3 d; CTX 15 mg/kg iv qd 2 d; DEX 10 mg iv q8 h 2 weeks | DEX 10 mg iv q8 h | Mortality; hepatitis; renal failure; hypoxia | 3 months |
| Lin et al. (2006) | Taiwan | RCT | Severe (1NB, 6DB/5NB, 11DB) | 23(16/7) | MP 1 g iv qd 3 d; CTX 15 mg/kg iv qd 2 d, followed DEX 20 mg qd until PaO2 >80 mmHg. Repeat doses of MP 1 g iv qd 3 d and CTX 15 mg/kg iv qd 1 d (if PaO2 <60 mmHg) | Gastric lavage with normal saline; activated charcoal was given through a nasogastric tube; two doses of 8 h hemoperfusion therapy; DEX 5 mg iv q6 h until PaO2 ≥80 mmHg | Mortality; hepatitis; renal failure; hypoxia | 6 weeks |
| Afzali and Gholyaf (2008) | Iran | RCT | Moderate to severe (4NB, 7DB/3NB, 6DB) | 20(9/11) | CTX 15 mg/kg qd 2 d; MP 1 g qd 3 d and mesna 15 mg/kg qd for 4 d | Gastric lavage with normal saline; activated charcoal was given through a nasogastric tube | Mortality; hepatitis; hypoxia; renal failure | Not reported |
| Lin et al. (2011) | Taiwan | Non-RCT | Severe* (2.5 (0.2, 21)/1.9 (0.2, 21)) | 111(59/52) | MP 1 g iv qd 3 d; CTX 15 mg/kg iv qd 2 d; DEX 5 mg q6 h until PaO2 >80 mmHg. Repeat doses of MP 1 g iv qd 3 d and CTX 15 mg/kg iv qd 1 d (if PaO2 <60 mmHg) | CTX 2 mg/kg oral qd and DEX 5 mg iv q6 h 14 d | Mortality | 60 d |
| Ghorbani et al. (2015) | Iran | RCT | Moderate to severe (≥NB) | 47(24/23) | MP 1 g iv qd 3 d; CTX 15 mg/kg iv qd 2 d | Gastric lavage; two episodes of 8 h charcoal; hemoperfusion; hemodialysis | Mortality; hepatitis; hypoxia; renal failure | 3 d |
PQ (plasma, mg/L) were presented as median (minimum, maximum); RCT: randomized control trial; MP: methylprednisolone; CTX: cyclophosphamide; DEX: dexamethasone; NB: navy blue color in sodium dithionite; DB: dark blue color; qid: four times a day; qd: once a day; q2 h: every two hours; q8 h: every eight hours; q6 h: every six hours. 1 mmHg=0.133 kPa
Fig. 2.
Quality assessment of the three randomized controlled trials
3.3. Main outcome: mortality
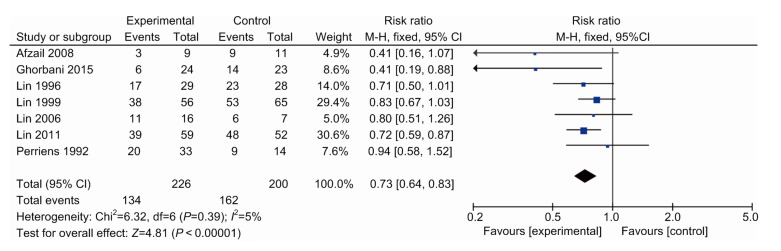
From the data in Fig. 3, we note that the mortality rate of the study group was 59.3% (134/226), while that of the control group was 81.0% (162/200). These results indicated that pulse therapy with the combination of glucocorticoids and cyclophosphamide efficiently improved survival rate (risk ratio (RR), 0.73; 95% confidence interval (CI), 0.64–0.83; P<0.000 01, I 2=5%).
Fig. 3.
Forest plot showing effect of pulse immunosuppressive therapy on mortality of patients with PQ poisoning
3.4. Subgroup analysis
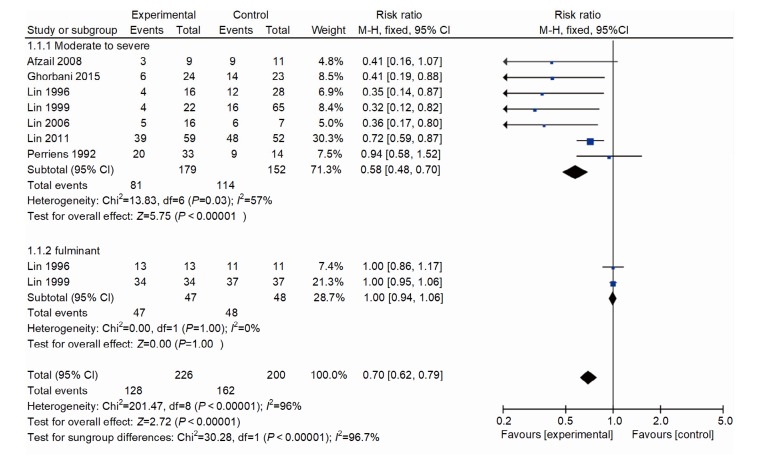
In order to analyze factors affecting heterogeneity, we conducted a subgroup analysis based on the illness severity, in which the study was conducted. There were two studies (Lin et al., 1996, 1999) in the fulminant group (one RCT, one non-RCT; RR, 1; 95% CI, 0.94–1.06; P=1.000; Fig. 4) and it showed no heterogeneity. There were seven studies (Perriëns et al., 1992; Lin et al., 1996, 1999, 2006, 2011; Afzali and Gholyaf, 2008; Ghorbani et al., 2015) in the moderate to severe groups (four RCTs, three non-RCTs; RR, 0.58; 95% CI, 0.48–0.70; P<0.000 01; Fig. 4). The test for heterogeneity was significant (I 2=57%).
Fig. 4.
Forest plot showing effect of pulse immunosuppressive therapy on mortality in fulminant and moderate to severe PQ poisoning patients
3.5. Sensitivity analysis
To explore the potential source of heterogeneity, we performed a sensitivity analysis. In this meta-analysis, the study by Lin et al. (2011) had the largest sample size, which is an important factor in pooled estimates. In another study conducted by Perriëns et al. (1992), the patients did not use hemopurification. Therefore, we performed sensitivity analyses to assess the efficiency of immunosuppressive pulse therapy. We excluded these two studies, which changed RR from 0.58 (0.48–0.70) to 0.37 (0.25–0.55), and there was no heterogeneity in the remaining studies (I 2=0%).
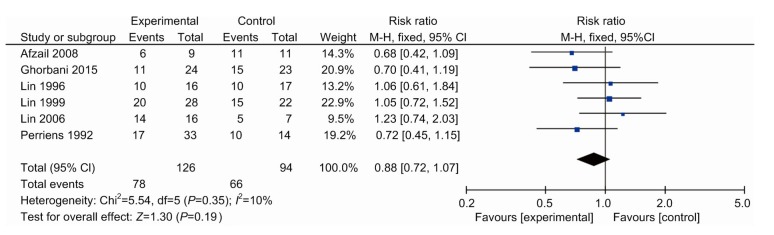
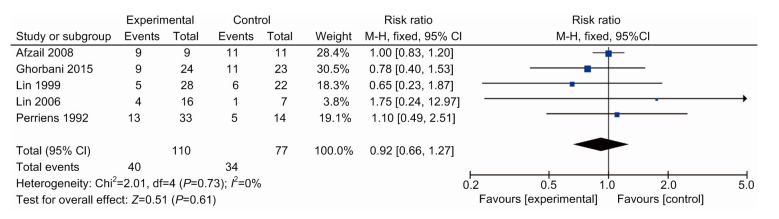
3.6. Secondary outcomes: renal failure, hepatitis, leucopenia, and hypoxia
All except one drug study (Lin et al., 2011) reported complications separately for the treatment and placebo groups (Perriëns et al., 1992; Lin et al., 1996, 1999, 2006; Afzali and Gholyaf, 2008; Ghorbani et al., 2015) (Table 2). Renal failures and hepatitis that occurred within 24 h of admission were considered for non-pharmacological effects and therefore were excluded. Relative risk was calculated for each effect (Figs. 5 and 6). In the remaining studies, there were non-significantly lower rates of renal failure (RR, 0.88; 95% CI, 0.72–1.07; six trials) and hepatitis (RR, 0.92; 95% CI, 0.66–1.27; five trials) when the control group was compared with the study group. Four studies reported that 20 patients suffered from leukopenia, which was significantly higher than the control group (RR, 10.66; 95% CI, 2.18–52.09; four trials). In addition, these patients all spontaneously recovered after 1–2 weeks.
Table 2.
Outcome data of studies included in meta-analysis
| Author (year) | Study group |
Control group |
||||||
| Incidence of renal failure | Incidence of hepatitis | Incidence of hypoxia | Incidence of leukopenia | Incidence of renal failure | Incidence of hepatitis | Incidence of hypoxia | Incidence of leukopenia | |
| Perriëns et al. (1992) | 24/33(72.7%) | 13/33(39.4%) | 17/33(51.5%) | 0/33(0.0%) | 10/14(71.4%) | 5/14(35.7%) | 4/14(28.6%) | 0/14(0.0%) |
| Lin et al. (1996) | 10/16(62.5%) | 3/16(18.8%) | 6/16(37.5%) | 10/17(58.8%) | 3/17(17.6%) | 0/17(0.0%) | ||
| Lin et al. (1999) | 20/28(71.4%) | 5/28(17.9%) | 3/28(10.7%) | 8/28(28.5%) | 15/22(68.2%) | 6/22(27.3%) | 3/22(13.6%) | 0/22(0.0%) |
| Lin et al. (2006) | 14/16(87.5%) | 4/16(25.0%) | 9/16(56.3%) | 6/16(37.4%) | 5/7(71.4%) | 1/7(14.5%) | 4/7(57.1%) | 0/7(0.0%) |
| Afzali and Gholyaf (2008) | 6/9(66.6%) | 9/9(100.0%) | 4/9(44.2%) | 11/11(100.0%) | 11/11(100.0%) | 8/11(72.7%) | ||
| Lin et al. (2011) | ||||||||
| Ghorbani et al. (2015) | 11/24(45.8%) | 9/24(37.5%) | 10/24(41.6%) | 15/23(65.2%) | 11/23(47.8%) | 17/23(73.9%) | ||
Fig. 5.
Hepatitis in patients between the therapy and control groups
Fig. 6.
Renal failure in patients between the therapy and control groups
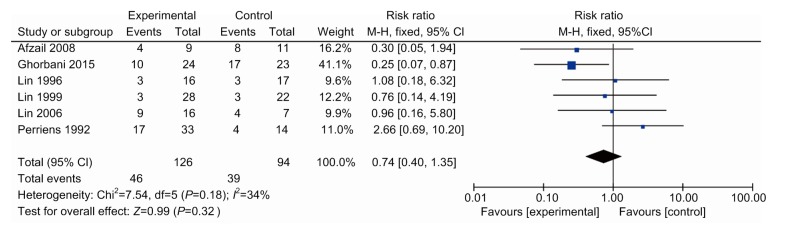
Hypoxia is the most common symptom in patients with PQ poisoning. Six studies (Perriëns et al., 1992; Lin et al., 1996, 1999, 2006; Afzali and Gholyaf, 2008; Ghorbani et al., 2015) reported patients with hypoxia in the experimental and control groups during hospitalization (Fig. 7). We found that the probability of hypoxia in the control group (41.4%) was a little higher than that in the experimental group (36.5%). However, there was no significant difference between the two treatments (RR, 0.74; 95% CI, 0.40–1.35; six trials). It suggested that immunosuppressive pulse therapy may not have an effect on alleviating hypoxia in patients with PQ poisoning.
Fig. 7.
Hypoxia in patients between the therapy and control groups
4. Discussion
PQ is widely used because it is highly efficient and environmentally friendly. Its ready accessibility has led to high human exposure due to unintentional contact or intentional suicide. The survival rate after oral poisoning of PQ is related to the amount of intake and the time of poisoning. There is no specific antidote for PQ poisoning at present. Management of the problem is mainly directed at the following aspects: reducing absorption, increasing excretion, anti-inflammatory, anti-oxidation, and supportive treatment. For the first time, in 1988, a study found that the lung damage caused by PQ was probably due to reactive oxygen species (ROS) production. This finding caused a boom in research directed at anti-oxidation treatment (Smith, 1988). Vitamin E and vitamin C were essential trace elements that scavenged oxygen free radicals and proved to play important roles in the process of PQ poisoning. Studies had demonstrated that vitamin E deficiency aggravated lung damage in rats with PQ poisoning (Block, 1979). Vitamin C could release an electron to neutralize oxygen free radicals and protected biomolecules before it reacted with other ROS. However, there was no benefit when using vitamin E and C to treat normal rats with PQ poisoning. The reason was not clear. Another attempt to treat PQ poisoning was superoxide dismutase (SOD) enzymes. SOD could catalyze the dismutation of peroxy anion to provide protection. The effect of SOD on the treatment of PQ poisoning is still controversial. A study found that injection of SOD could reduce the mortality of rats with PQ poisoning (Wasserman and Block, 1978), but there was no obvious effect on the human body (Block, 1979). This finding might be related to the physical characteristics of its molecules, which were too large to function through the cell membrane. Therefore, studies had been conducted to embed superoxide in various media to enable it to enter the cell membrane to function (Day and Crapo, 1996).
The current main treatment for PQ poisoning is inhibition of inflammatory response and pulmonary fibrosis. Inflammatory cells produced large amounts of ROS. Not only that, they also secreted some hydrolase to damage lung cells. Studies showed that inflammatory cells played an important role in the pathological process of PQ poisoning. Immunosuppressive therapy could reduce the inflammatory response of lungs, thereby reducing ROS production and lung damage. Currently, a combination of glucocorticoids and cyclophosphamide is commonly used in the clinical setting.
This meta-analysis showed that pulse therapy with glucocorticoids and cyclophosphamide after PQ poisoning significantly reduced mortality of the study group compared with the control group.
Except for one study (Perriëns et al., 1992), hemopurification treatment was performed on patients with PQ poisoning in both the control and experimental groups. Interestingly, in a recent study on immunosuppressive pulse therapy on PQ poisoning, neither the experimental group nor the control group had hemopurification treatment, and the results showed that there was no statistically significant difference in mortality of these two groups (Gawarammana et al., 2018). It may be that the effect of immunosuppressive pulse therapy alone on PQ poisoning is not effective. Studies had demonstrated that hemopurification in the early stage of PQ poisoning could reduce the mortality of patients (Wang et al., 2017). The immunosuppressive pulse therapy needs to be combined with hemopurification treatment. This combination therapy is also the conventional treatment of PQ poisoning in most hospitals currently. Some scholars believed that PQ could damage the mitochondria by inducing a large amount of ROS, inducing apoptosis, and so on. However, there was no evidence that methylprednisolone or cyclophosphamide could eliminate ROS in the body to protect cell and mitochondria from damage. Therefore, more research is needed to establish the mechanism and role of immunosuppressive therapy on PQ poisoning.
Although previous studies had shown that the high doses of glucocorticoids and cyclophosphamide might cause adverse effects such as hepatitis, renal failure, and leukopenia in patients (Sakemi et al., 1993; Weissel and Hauff, 2000; Marino et al., 2004), our analysis found that there was no significant difference of hepatitis nor renal failure between the control and study groups, indicating that immunosuppressive pulse therapy was relatively safe.
Although several patients were reported to have leukopenia due to the myelosuppressive effect of cyclophosphamide, they returned to normal after 1–2 weeks without any abnormalities. It reminds us to pay attention to the complete blood count (CBC) of the patients during the treatment. Not only that, the patient’s immune function was inhibited. In one of the studies (Perriëns et al., 1992), two cases of non-fatal sepsis were reported and were considered as a side effect of the immunosuppressive pulse therapy. At present, immunosuppression is the main drug treatment after PQ poisoning, which could also induce the risk of clinical infection while reducing the inflammatory response. In the process of clinical care, more attention should be paid to protect patients from infection.
After PQ poisoning, patients developed hypoxia due to the inflammation of lungs and pulmonary fibrosis. We found that both the study and control groups in our research had high incidences of hypoxia. However, hypoxia in patients treated with the pulse therapy of glucocorticoids and cyclophosphamide did not achieve remission during hospitalization. Two studies mentioned that the respiratory function of patients who used immunosuppressive therapy recovered slowly 3–6 months after PQ poisoning, nearing normal levels. It suggested that methylprednisolone and cyclophosphamide might have little effect on hypoxia caused by early acute inflammation, but might be able to treat advanced pulmonary fibrosis. However, it may need further research.
Recently, it has been found clinically that some acquired immune deficiency syndrome (AIDS) patients had survived after taking a lethal dose of PQ
(Shang and Lu, 2015; Lu, 2018). It was well known that a loss of CD4+ T lymphocytes was the immune compromise caused by human immunodeficiency virus (HIV). Therefore, we hypothesized that the CD4+ T lymphocytes cell might play a critical role in the mechanism of body damage caused by PQ (Zhang et al., 2012; Feng et al., 2018). The proposed use of CD4+ T lymphocytes as a target to guide immunosuppressive therapy to improve the prognosis might be the future direction for the treatment of PQ poisoning.
There were several limitations to our meta-analysis. First, unfortunately not all studies were randomized; some studies had small sample sizes and might increase statistical variance. However, in clinical practice, patients with PQ poisoning were too rare to conduct a large-scale clinical controlled study. Even so, most of the enrolled studies were high-quality clinical trials. Second, there was no uniform standard for the severity of PQ poisoning for patients included in the study, which might increase the heterogeneity between studies and impact on the results. Third, the definition of immunosuppressive pulse therapy was inconsistent. In addition, several known complications of glucocorticoid and cyclophosphamide such as hyperglycemia, gastrointestinal ulcer, avascular necrosis of the femoral head, or tumor were not discussed in these included studies. Fourth, some studies were not followed up for more than four weeks and could not fully reflect the real effects of immunosuppressive pulse therapy. Despite these limitations, our meta-analysis incorporated all currently available evidence concerning pulse therapy on the treatment of PQ poisoning, and it might have directive sense to clinical work.
5. Conclusions
Based on seven studies of immunosuppressive pulse therapy on PQ poisoning, this systematic review and meta-analysis revealed a 21.7% mortality reduction when patients were treated with immunosuppressive pulse therapy (RR, 0.73; 95% CI, 0.64–0.83; P<0.000 01, I 2=5%). There was no significant difference of hepatitis or renal failure between the control and study groups, indicating that immunosuppressive pulse therapy was relatively safe. Several patients were reported to have leukopenia and returned to normal after 1–2 weeks without any abnormalities. Two cases of non-fatal sepsis were reported and considered as a side effect of the immunosuppressive pulse therapy.
Footnotes
Project supported by the Foundation of Key Discipline Construction of Zhejiang Province for Traditional Chinese Medicine (No. 2017-XK-A36), the Medical and Health Science Foundation of Zhejiang Province (No. 2019327552), the Foundation of Key Research Project of Zhejiang Province for Traditional Chinese Medicine (No. 2019ZZ014), and the Foundation of Science and Technology Department of Zhejiang Province for Beneficial Technology Research of Social Development (No. 2015C33146), China
Contributors: Ying-ge XU and Yuan-qiang LU designed the study, and participated in the data collection and analyses. Ying-ge XU participated in the drafting of the manuscript. Both authors read and approved the final manuscript.
Compliance with ethics guidelines: Ying-ge XU and Yuan-qiang LU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by either of the authors.
References
- 1.Afzali S, Gholyaf M. The effectiveness of combined treatment with methylprednisolone and cyclophosphamide in oral paraquat poisoning. Arch Iran Med. 2008;11(4):387–391. [PubMed] [Google Scholar]
- 2.Block ER. Potentiation of acute paraquat toxicity by vitamin E deficiency. Lung. 1979;156(1):195–203. doi: 10.1007/BF02714010. [DOI] [PubMed] [Google Scholar]
- 3.Day BJ, Crapo JD. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced lung injury in vivo . Toxicol Appl Pharmacol. 1996;140(1):94–100. doi: 10.1006/taap.1996.0201. [DOI] [PubMed] [Google Scholar]
- 4.Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, et al. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38(1):13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 5.Feng MX, Li YN, Ruan WS, et al. Predictive value of the maximum serum creatinine value and growth rate in acute paraquat poisoning patients. Sci Rep, 8:11587. 2018 doi: 10.1038/s41598-018-29800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gawarammana I, Buckley NA, Mohamed F, et al. High-dose immunosuppression to prevent death after paraquat self-poisoning–a randomised controlled trial. Clin Toxicol (Phila) 2018;56(7):633–639. doi: 10.1080/15563650.2017.1394465. [DOI] [PubMed] [Google Scholar]
- 7.Ghorbani A, Masoumi K, Forouzan A, et al. Effect of pulse therapy with glucocorticoids and cyclophosphamide in patients with paraquat poisoning. Hong Kong J Emerg Med. 2015;22(4):235–240. doi: 10.1177/102490791502200405. [DOI] [Google Scholar]
- 8.Hart TB. Paraquat–a review of safety in agricultural and horticultural use. Hum Toxicol. 1987;6(1):13–18. doi: 10.1177/096032718700600103. [DOI] [PubMed] [Google Scholar]
- 9.Huang WD, Wang JZ, Lu YQ, et al. Lysine acetylsalicylate ameliorates lung injury in rats acutely exposed to paraquat. Chin Med J (Engl) 2011;124(16):2496–2501. doi: 10.3760/cma.j.issn.0366-6999.2011.16.018. [DOI] [PubMed] [Google Scholar]
- 10.Jiang JK, Fang W, Gu LH, et al. Early changes of peripheral blood lymphocyte subpopulations in patients with occupational 2,4-dinitrophenol poisoning. Biomed Environ Sci. 2016;29(12):909–914. doi: 10.3967/bes2016.122. [DOI] [PubMed] [Google Scholar]
- 11.Lin JL, Wei MC, Liu YC. Pulse therapy with cyclophosphamide and methylprednisolone in patients with moderate to severe paraquat poisoning: a preliminary report. Thorax. 1996;51(7):661–663. doi: 10.1136/thx.51.7.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JL, Leu ML, Liu YC, et al. A prospective clinical trial of pulse therapy with glucocorticoid and cyclophosphamide in moderate to severe paraquat-poisoned patients. Am J Respir Crit Care Med. 1999;159(2):357–360. doi: 10.1164/ajrccm.159.2.9803089. [DOI] [PubMed] [Google Scholar]
- 13.Lin JL, Lin-Tan DT, Chen KH, et al. Repeated pulse of methylprednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Crit Care Med. 2006;34(2):368–373. doi: 10.1097/01.CCM.0000195013.47004.A8. [DOI] [PubMed] [Google Scholar]
- 14.Lin JL, Lin-Tan DT, Chen KH, et al. Improved survival in severe paraquat poisoning with repeated pulse therapy of cyclophosphamide and steroids. Intensive Care Med. 2011;37(6):1006–1013. doi: 10.1007/s00134-010-2127-7. [DOI] [PubMed] [Google Scholar]
- 15.Lu YQ. HIV and paraquat poisoning: fighting fire with fire? J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2018;19(2):168–170. doi: 10.1631/jzus.B1700567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu YQ, Jiang JK, Huang WD. Clinical features and treatment in patients with acute 2,4-dinitrophenol poisoning. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(3):189–192. doi: 10.1631/jzus.B1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marino M, Morabito E, Brunetto MR, et al. Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophthalmopathy. Thyroid. 2004;14(5):403–406. doi: 10.1089/105072504774193276. [DOI] [PubMed] [Google Scholar]
- 18.Perriëns JH, Benimadho S, Lie Kiauw I, et al. High-dose cyclophosphamide and dexamethasone in paraquat poisoning: a prospective study. Hum Exp Toxicol. 1992;11(2):129–134. doi: 10.1177/096032719201100212. [DOI] [PubMed] [Google Scholar]
- 19.Sakemi T, Fujimoto S, Fujimi S, et al. Difference between renal failure associated with methylprednisolone pulse therapy and deterioration of renal function unrelated to methylprednisolone therapy. Am J Nephrol. 1993;13(2):132–137. doi: 10.1159/000168603. [DOI] [PubMed] [Google Scholar]
- 20.Seidenfeld JJ, Wycoff D, Zavala DC, et al. Paraquat lung injury in rabbits. Br J Ind Med. 1978;35(3):245–257. doi: 10.1136/oem.35.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang AD, Lu YQ. A case report of severe paraquat poisoning in an HIV-positive patient: an unexpected outcome and inspiration. Medicine (Baltimore) 2015;94(8):e587. doi: 10.1097/MD.0000000000000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith LL. The toxicity of paraquat. Adverse Drug React Acute Poisoning Rev. 1988;7(1):1–17. [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 24.Wang HR, Pan J, Shang AD, et al. Time-dependent haemoperfusion after acute paraquat poisoning. Sci Rep. 2017;7(1):2239. doi: 10.1038/s41598-017-02527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasserman B, Block ER. Prevention of acute paraquat toxicity in rats by superoxide dismutase. Aviat Space Environ Med. 1978;49(6):805–809. [PubMed] [Google Scholar]
- 26.Weissel M, Hauff W. 2000. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Wu WZ, Lu YQ, et al. Successful treatment of patients with paraquat intoxication: three case reports and review of the literature. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2012;13(5):413–418. doi: 10.1631/jzus.B1200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao XH, Lu YQ. Multiple embolisms resulted from a huge fishbone piercing the left atrium. Intensive Care Med. 2014;40(4):621–622. doi: 10.1007/s00134-014-3232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu JY, Pan J, Lu YQ. A case report on indirect transmission of human rabies. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(11):969–970. doi: 10.1631/jzus.B1500109. [DOI] [PMC free article] [PubMed] [Google Scholar]