Abstract
We report on a case of very rare autosomal recessive cholesteryl ester storage disease due to lysosomal acid lipase deficiency (LALD). LALD is caused by mutations in the lysosomal acid lipase A (LIPA) gene resulting in cholesteryl ester accumulation in liver, spleen, and macrophages. It can lead to liver failure, accelerated atherosclerosis and premature death. Until recently, treatment options were limited to lipid-lowering medications to control dyslipidemia. Presently, a long-term enzyme replacement therapy with Sebelipase alfa, a recombinant human lysosomal acid lipase, is available for patients with LALD.
Our patient's condition became conspicuous at the age of two due to a xanthogranuloma of the chin together with increased lipid levels, elevated liver enzymes and hepatomegaly. It took another five years until our patient was diagnosed with LALD after genetic testing. A bi-weekly therapy with intravenous Sebelipase alfa was started at the age of 26 years. It led to normalization of lipid levels, reduction of liver enzymes and beginning regression of hepatomegaly in the absence of adverse drug reactions after 46 infusions.
Since LALD can take a fatal course even in patients with a long-term stable condition, it is essential to identify affected patients early and to treat them appropriately by enzyme replacement therapy. LALD should be suspected in patients with low high-density lipoprotein cholesterol (HDL-C) and high low-density lipoprotein cholesterol (LDL-C) in conjunction with elevated liver enzymes or hepatomegaly.
A registry for LALD patients shall help to advance our understanding of the disease as well as improve patient care (NCT01633489).
Keywords: Lysosomal acid lipase deficiency (LALD), Cholesteryl ester storage disease, Sebelipase alfa (Kanuma™), LALD registry
Abbreviations: ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; HDL-C, High-density lipoprotein cholesterol; LAL, Lysosomal acid lipase; LALD, Lysosomal acid lipase deficiency; LDL-C, Low-density lipoprotein cholesterol; LIPA, Lysosomal acid lipase A; SA, Sebelipase alfa
1. Background
Lysosomal acid lipase deficiency (LALD) is an autosomal recessive lysosomal storage disorder caused by mutations in the lysosomal acid lipase A (LIPA, OMIM 613497) gene that encodes the lysosomal acid lipase (LAL) enzyme. LAL catalyzes the intracellular hydrolysis of cholesteryl esters and triglycerides in hepatocytes and macrophages [1,2]. LALD is characterized by reduced LAL activity that causes two phenotypes depending on the extent of the deficiency: The more severe form, historically known as Wolman disease, is characterized by no remaining LAL activity. It presents in early infancy and is characterized by failure to thrive, vomiting, diarrhea, hepatomegaly, and malabsorption followed by progressive clinical decline with liver failure. Most patients die within the first twelve months of life [1]. Cholesteryl ester storage disease (CESD), which is seen in children and adults, represents a less severe form of LALD with some remaining LAL activity and slower disease progression [1]. Clinically, patients present with dyslipidemia (elevated plasma levels of low-density lipoprotein cholesterol (LDL-C) and decreased levels of high-density lipoprotein cholesterol (HDL-C)), elevated serum transaminase levels and hepatomegaly. LALD results in accumulation of cholesteryl esters in liver, spleen and macrophages, leading to hepatosplenomegaly and liver fibrosis, liver cirrhosis, and eventually liver failure [2]. Furthermore, patients may suffer from accelerated atherosclerosis [2,3]. With an estimated prevalence of roughly 1 in 175,000, LALD is a very rare condition and often remains undiagnosed in patients with fatty liver disease and dyslipidemia [[2], [3], [4]]. To establish the diagnosis, genetic testing and an enzyme activity assay from dried blood can be employed. If untreated, life expectancy of patients may be shortened but remains highly variable [2]. Recent reports stress that the disease can exacerbate at any point even in patients whose status proved to be stable over a long period [5]. Until the approval of long-term enzyme replacement therapy with the recombinant human LAL enzyme Sebelipase alfa (SA) in August 2015, there was no safe or effective therapy available for LALD. Although hypercholesterolemia responds well to statins, this therapeutic option hardly has any effect on liver injury and progression of fibrosis. However, a recent case series of three young LALD patients showed a significant decrease in alanine aminotransferase (ALT) values and no further progression of liver fibrosis for up to ten years under treatment with ezetimibe [6]. Several patients with advanced liver disease, complications related to portal hypertension, and liver failure have undergone liver transplantation. Unfortunately, outcome data of these patients is sparse. The newly available SA is administered by intravenous infusion bi-weekly and may be used as long-term enzyme replacement therapy in patients of all ages with LALD. The administration of SA reduces lysosomal lipid accumulation and improves dyslipidemia and liver abnormalities [7]. One-year-follow-up data indicate that effects of SA are sustained in the long term [8]. In infants, treatment improved survival, gastrointestinal symptoms, weight gain, biochemical and hematological markers, and reduced hepatosplenomegaly [9]. For better understanding of variability, progression, identification and the natural history of the disease, an observational disease and clinical outcome registry for LALD patients (NCT01633489) has recently been established.
2. Case presentation
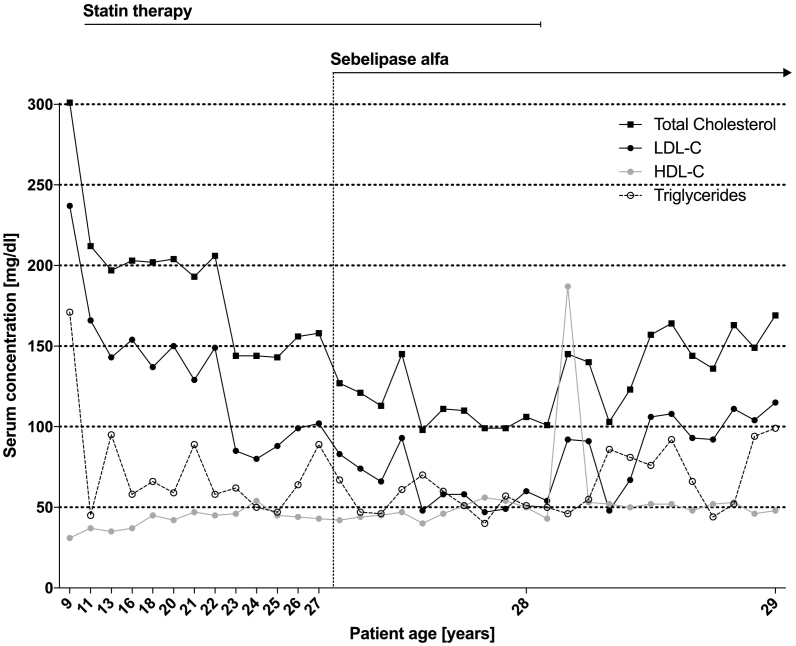
We present the case of a male patient with LALD (height: 184 cm, weight: 68 kg), who visited our outpatient lipid clinic first in 2010 when he was 20 years old. At the age of two years, the patient was in good condition with normal weight and growth development. However, his case was examined further because of a xanthogranuloma of the chin in conjunction with elevated transaminases, increased total cholesterol, and detected hepatomegaly. Four years later, a liver biopsy – originally conducted to exclude Wilson's disease as leading differential diagnosis – demonstrated microvesicular steatosis suggesting the diagnosis of LALD. The LAL activity measurement in dried blood spot testing showed 1.5 U/mg (reference value 32.6 ± 12.2 U/mg). In 1997, a genetic test confirmed the diagnosis of compound heterozygous LALD. The first mutation in the LIPA gene is the common mutation c.894G>A causing a deletion of exon 8 [3]. The second mutation in exon 10 c.980del results in a truncated protein (p.Thr327AsnfsX4). It is presumed that the truncation of 70 terminal residues from human LAL severely compromises the activity, as confirmed by repeated dried blood spot testing. At the age of ten years, an oral medication with low-dose simvastatin was started, increased up to 10 mg and later changed to atorvastatin. Total cholesterol was reduced from 301 mg/dl to 180 mg/dl in the following years (Fig. 1). At the age of 13 years, abdominal ultrasonography showed mild hepato- and splenomegaly without focal lesions.
Fig. 1.
Timeline of the patients' lipid profile (Total Cholesterol, HDL-C, LDL-C and Triglycerides in mg/dl). The initiation of the enzyme replacement treatment is marked by a dotted line and the term ‘Sebelipase alfa’.
In March 2015, the patient presented in our outpatient clinic in good condition. The dyslipidemia was well controlled, but he had continuously elevated transaminases. Duplex carotid ultrasound showed no signs of premature atherosclerosis. The intima-media-thickness was within physiological limits. Liver ultrasonography as well as fibroscan showed no signs of fibrosis nor focal lesions. Physical and mental development was uneventful, the patient remained physically active and pursued his academic career without problems. The patient was promptly included in the LALD registry.
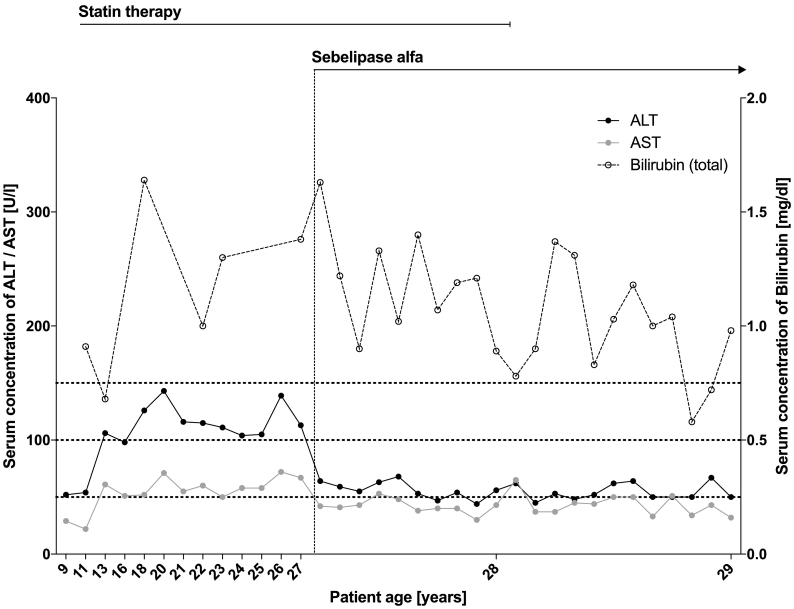
In March 2017, we started a therapy with weight-adapted SA diluted in 0.9% sodium chloride in bi-weekly intervals on top of the lipid lowering therapy with low dose atorvastatin. Up to now, our patient received 46 infusions and did not show any signs of drug intolerance or adverse reactions. After initiation of the therapy, ALT and aspartate aminotransferase (AST) were reduced from 106 U/l and 54 U/l to 55 U/l and 43 U/l (reference ranges were <41 U/l and <50 U/l), respectively (Fig. 2). Similarly, total cholesterol as well as LDL-C dropped significantly so that additional lipid lowering statin therapy with atorvastatin could be terminated in February 2018. Since then, lipid levels remained low with LDL-C at approximately 100 mg/dl. (Fig. 1) A comparison of MRI scans in December 2016 (before treatment) and June 2018 (16 months of treatment) showed a slightly regressive hepatomegaly with the craniocaudal length dropping from 212 mm to 206 mm in conjunction with signs of mild steatosis.
Fig. 2.
Timeline of the patients' transaminases (in U/l) and total Bilirubin (in mg/dl). The initiation of the enzyme replacement treatment is again marked by a dotted line and the term ‘Sebelipase alfa’.
The patient showed no signs of immune impairment or even secondary hemophagocytic lymphohistiocytosis, which has been associated with LALD [10]. Leucocyte count was within physiological limits at all times.
On a side note, the patient's serum levels of creatine kinase were usually measured to be within the reference range (<190 U/l), but responded considerably to any physical activity reaching levels as high as 7875 U/l without the patient reporting any symptoms and subsequent normalization of serum levels within days. The effect persisted even after discontinuation of statin therapy.
3. Discussion and conclusions
Non-alcoholic fatty liver disease (NAFLD) is a very common hepatic disease with a growing number of patients. The prevalence is about 20–30% and reaches 70–90% in obese or diabetic patients. This disease is characterized by excessive hepatic fat accumulation in absence of chronic viral infection or significant alcohol consumption. Later stages are characterized by liver inflammation, formation of irreversible scar tissue and finally liver cirrhosis [9, 11]. In the presented case, liver hypertrophy combined with increased transaminases and elevated lipid levels led to the early diagnosis of LALD, which is a widely under-recognized cause of liver failure and premature atherosclerosis. Since August 2015, the long-term enzyme replacement therapy with SA, a recombinant human lysosomal acid lipase is available. Since there is now an effective and safe treatment of LALD available, it is crucial to identify patients who could benefit from the enzyme replacement therapy. In patients with elevated LDL-C and decreased HDL-C as well as elevated transaminases or hepatomegaly (especially in the absence of obesity), LALD should always be considered as differential diagnosis [3].
Until today, almost 100 disease-causing LIPA variants have been identified [4]. Though, the mutation c.894G>A found in one allele in our patient accounts for 50–60% of all cholesteryl ester storage disease cases [12]. The frameshift mutation c.980del in Exon 10 has not yet been described, but the resulting truncation presumably compromises the enzyme's activity [4]. Results from repeated dried blood spot tests of our patient showing very low LAL activity confirm this presumption. Fortunately, even before treatment, our patient did not suffer from a progressive course of the disease and showed only mild clinical signs of LALD. However, a recent report demonstrated that in LALD also a long-term stable condition can exacerbate suddenly and fatally [5]. Therefore, even in mild manifestations of the disease, the long-term enzyme replacement therapy with SA should be recommended.
Consistent with our patient's development, a phase III trial testing SA against placebo has recently shown increased rates for normalized ALT and ALT levels as well as improved lipid levels in the SA group. Furthermore, the beginning regression of our patient's hepatomegaly is in line with results from the same trial showing a significant reduction in hepatic fat content for patients receiving SA compared to placebo [7].
Due to generally low patient numbers, registries are among the most meaningful instruments to collect health data and to improve patient care in rare diseases [13]. LALD registry data contribute to expand our knowledge on LALD and may help to learn more about the long-term outcome of the newly available therapy with SA.
Acknowledgements
We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Funds of Charité - Universitätsmedizin Berlin.
References
- 1.Pericleous Marinos. Wolman's disease and cholesteryl ester storage disorder: the phenotypic spectrum of lysosomal acid lipase deficiency. The Lancet Gastroenterology & Hepatology. 2017;2(9):670–679. doi: 10.1016/S2468-1253(17)30052-3. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman E.P., Barr M.L., Giovanni M.A., Murray M.F. Lysosomal acid lipase deficiency. In: Adam M.P., editor. GeneReviews®. University of Washington; Seattle: 1993. [Google Scholar]
- 3.Reiner Ž. Lysosomal acid lipase deficiency--an under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis. 2014;235:21–30. doi: 10.1016/j.atherosclerosis.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Carter A., Brackley S.M., Gao J., Mann J.P. The global prevalence and genetic spectrum of lysosomal acid lipase deficiency: a rare condition that mimics NAFLD. J. Hepatol. 2018 doi: 10.1016/j.jhep.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Canbay A., Müller M.N., Philippou S., Gerken G., Tromm A. Cholesteryl ester storage disease: fatal outcome without causal therapy in a female patient with the preventable sequelae of progressive liver disease after many years of mild symptoms. Am. J. Case Rep. 2018;19:577–581. doi: 10.12659/AJCR.907755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Rocco M., Pisciotta L., Madeo A., Bertamino M., Bertolini S. Long term substrate reduction therapy with ezetimibe alone or associated with statins in three adult patients with lysosomal acid lipase deficiency. Orphanet J. Rare Dis. 2018;13(24) doi: 10.1186/s13023-018-0768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton B.K. A phase 3 trial of sebelipase alfa in lysosomal acid lipase deficiency. N. Engl. J. Med. 2015;373:1010–1020. doi: 10.1056/NEJMoa1501365. [DOI] [PubMed] [Google Scholar]
- 8.Valayannopoulos V. Sebelipase alfa over 52 weeks reduces serum transaminases, liver volume and improves serum lipids in patients with lysosomal acid lipase deficiency. J. Hepatol. 2014;61:1135–1142. doi: 10.1016/j.jhep.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones S.A. Survival in infants treated with sebelipase Alfa for lysosomal acid lipase deficiency: an open-label, multicenter, dose-escalation study. Orphanet J. Rare Dis. 2017;12(25) doi: 10.1186/s13023-017-0587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomaraschi M., Bonacina F., Norata G.D. Lysosomal acid lipase: from cellular lipid handler to immunometabolic target. Trends Pharmacol. Sci. 2018;40(2):104–115. doi: 10.1016/j.tips.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Sporea I. Nonalcoholic fatty liver disease: status quo. J. Gastrointest. Liver Dis. JGLD. 2018;27:439–448. doi: 10.15403/jgld.2014.1121.274.quo. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein D.L., Hülkova H., Bialer M.G., Desnick R.J. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J. Hepatol. 2013;58:1230–1243. doi: 10.1016/j.jhep.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Steinhagen-Thiessen E. The role of registries in rare genetic lipid disorders: review and introduction of the first global registry in lipoprotein lipase deficiency. Atherosclerosis. 2017;262:146–153. doi: 10.1016/j.atherosclerosis.2016.08.023. [DOI] [PubMed] [Google Scholar]