Abstract
Phantom limb pain (PLP) following amputation, which is experienced by the vast majority of amputees, has been reported to be relieved with daily sessions of mirror therapy. During each session, a mirror is used to view the reflected image of the intact limb moving, providing visual feedback consistent with the movement of the missing/phantom limb. To investigate potential neural correlates of the treatment effect, we measured brain responses in volunteers with unilateral leg amputation using functional magnetic resonance imaging (fMRI) during a four-week course of mirror therapy. Mirror therapy commenced immediately following baseline scans, which were repeated after approximately two and four week intervals. We focused on responses in the region of sensorimotor cortex corresponding to primary somatosensory and motor representations of the missing leg. At baseline, prior to starting therapy, we found a strong and unexpected response in sensorimotor cortex of amputees to visually presented images of limbs. This response was stronger for images of feet compared to hands and there was no such response in matched controls. Further, this response to visually presented limbs was no longer present at the end of the four week mirror therapy treatment, when perceived phantom limb pain was also reduced. A similar pattern of results was also observed in extrastriate and parietal regions typically responsive to viewing hand actions, but not in regions corresponding to secondary somatosensory cortex. Finally, there was a significant correlation between initial visual responsiveness in sensorimotor cortex and reduction in PLP suggesting a potential marker for predicting efficacy of mirror therapy. Thus, enhanced visual responsiveness in sensorimotor cortex is associated with PLP and modulated over the course of mirror therapy.
Keywords: fMRI, Vision, Amputation, Phantom limb pain, Sensorimotor
Highlights
-
•
Visual responsiveness to the sight of limbs in sensorimotor cortex of leg amputees but not matched controls
-
•
Consistent with prior studies, mirror therapy over 4 weeks reduced phantom limb pain
-
•
Visual responsiveness in sensorimotor cortex of amputees diminished following mirror therapy
-
•
Visual responsiveness in sensorimotor cortex might be useful in predicting the potential efficacy of mirror therapy
1. Introduction
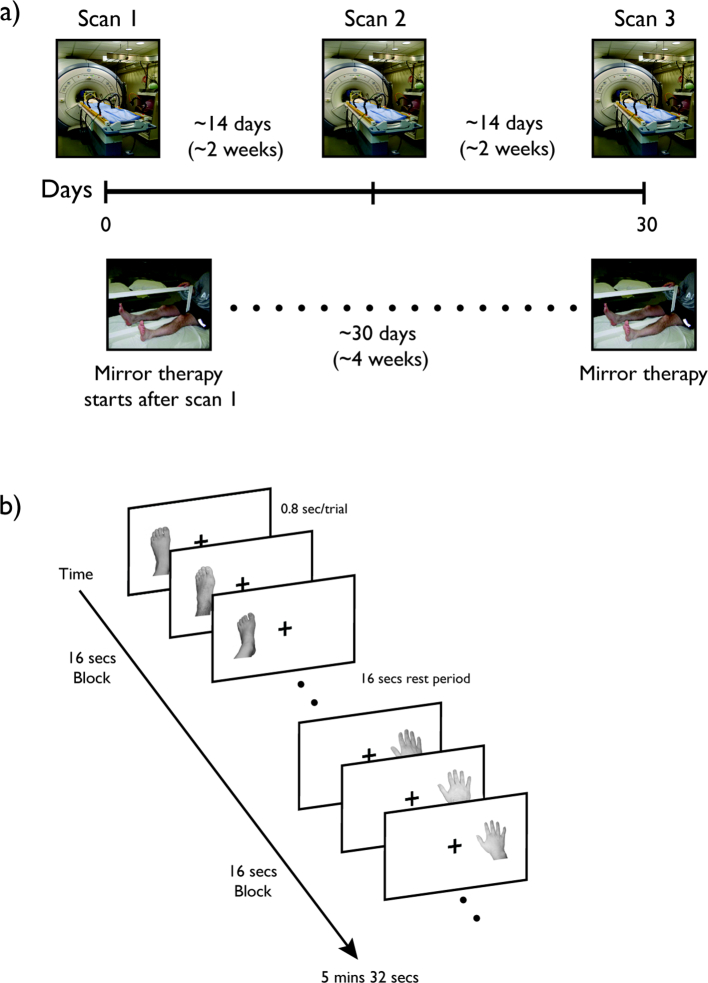
Over 90% of amputees experience vivid pain in the missing limb, frequently referred to as phantom limb pain (PLP), which can be experienced within 24 h following amputation (Borsook et al., 1998). For example, some upper limb amputees have reported that the finger nails of their phantom limb were digging into their palm, while others reported that their phantom hands were stuck in an awkward, immobile position and were hurting (Ramachandran and Blakeslee, 1998). Multiple mechanisms have been hypothesized to account for such pain including a mismatch between visual feedback and proprioceptive representations of the limb (Ramachandran and Rogers-Ramachandran, 1996; Ramachandran and Altschuler, 2009), neuroplasticity (Flor et al., 1995, Flor et al., 2006; Karl et al., 2001; Lotze et al., 2001; but see Borsook et al., 1998), proprioceptive memory (Ramachandran and Rogers-Ramachandran, 1996; Ramachandran and Altschuler, 2009; Anderson-Barnes et al., 2009), or the maintenance of sensorimotor representations of the missing limb (Makin et al., 2013; Kikkert et al., 2016). Importantly, providing visual feedback consistent with the movement of the phantom limb using mirror therapy has been found to dramatically reduce PLP (Ramachandran et al., 1995; MacLachlan et al., 2004; Chan et al., 2007; Sumitani et al., 2008; Mercier and Sirigu, 2009). These ameliorative effects of mirror therapy appear to be specific to the visual feedback, since reductions in pain are not observed when the mirror is covered or the amputees engage in mental visualization of movements only (Chan et al., 2007). To elucidate why mirror therapy reduces PLP and identify potential neural correlates, we conducted an exploratory longitudinal functional magnetic resonance imaging (fMRI) study in 9 lower leg amputees prior to, during, and following four weeks of mirror therapy treatment (Fig. 1).
Fig. 1.
Experimental design. a) Immediately following a baseline fMRI scan, amputee participants started approximately four weeks of mirror therapy. The second and third scan sessions were conducted at approximately two-week intervals. Age- and gender-matched control participants underwent the same series of scans at the same time intervals. b) During each scan session, participants viewed images of hands and feet from the left or right side of the body in a block design. Within each block, limb and side of body were fixed and images from each side were presented in the congruent screen location (e.g., left foot on left side of screen). Only one image was presented at a time, and participants were asked to fixate at the center of the screen throughout each scan.
Prior fMRI studies have reported shifts in somatosensory maps in upper extremity amputees with, for example, movement of the lips producing responses extending into the putative hand representation (Pascual-Leone et al., 1996; Grusser et al., 2001; Lotze et al., 2001; Foell et al., 2014). These extended responses were found to be specific to amputees with PLP compared to those without (Lotze et al., 2001) and have been interpreted as evidence for maladaptive cortical reorganization in the sensorimotor cortex. However, more recent work (Makin et al., 2015a) has reported only a small shift in cortical representations of the lip and no clear relationship between the extent of apparent reorganization and phantom sensations or pain. In contrast to these prior studies, and given the importance of visual feedback for the potential beneficial effect of mirror therapy, we focused primarily on responses to visually presented images of hands and feet rather than motor-related responses. Immediately following a baseline fMRI scan, amputees started 15-min daily sessions of mirror therapy following our previous protocol (Chan et al., 2007). Specifically, during each session, amputees placed a mirror between their intact and missing/phantom leg, and moved both of their legs simultaneously. While looking at the mirror, the reflection of the intact leg moving in the mirror produces visual feedback consistent with the movement of the missing leg. The second and third fMRI sessions were conducted after approximately two-week intervals (Fig. 1). A group of matched control participants without limb amputation underwent the same series of fMRI scans but did not receive mirror therapy. We found strong responses to visually presented limbs in the sensorimotor cortex of amputees that was no longer present by the end of the mirror therapy treatment. Such responses to visually presented limbs were not observed in matched control participants indicating that loss of sensorimotor input such as major limb amputation can modify sensorimotor representations.
2. Material and methods
2.1. Overview
In this study, amputee and control participants participated in three MRI sessions. In each MRI session, we measured the cortical responses to visual presentations of the feet and hands. In particular, we focused on sensorimotor cortex, corresponding to the somatomotor representations of the lower limb. This region of sensorimotor cortex was defined in the control participants using a combination of functional and anatomical markers. Immediately following the first MRI session, amputee participants started on daily sessions of mirror therapy and reported their level of subjective PLP using standard questionnaires. There were three main questions of interest: i) What is the impact of amputation on visual responsiveness in cortex? ii) What is the impact of mirror therapy on visual responsiveness? iii) How does visual responsiveness compare with any reduction in PLP?
2.2. Participants
Nine right-handed unilateral lower limb amputees (5 male, 4 female, 3 left lower limb, 6 right lower limb, mean age = 50) were recruited from Walter Reed National Military Medical Center, Bethesda, MD, National Rehabilitation Hospital, Washington, DC, or the metropolitan Washington, DC area. Diabetes, traumatic brain injury, and dysvascular cause of amputation were exclusion factors. All amputees reported taking medications (including opioids, voltage-gated calcium channel modulators and selective serotonin reuptake inhibitors, or norepinephrine reuptake inhibitors). Nonetheless, all reported that pain medication(s) were not effective in treating PLP and all reported ongoing vivid PLP. Participants were asked to maintain a constant medication regime throughout the study. Nine healthy control participants without limb amputation (mean age = 44) were recruited through the National Institutes of Health. We attempted to match for the age and gender of amputees within these control participants to avoid any large demographic differences between groups. All comparisons between amputees and control participants were made at the group level and analyzed in an unpaired manner. See Table 1 for participants' demographics. All participants had normal or corrected-to-normal vision and gave written informed consent. The study was approved by both the National Institutes of Health and Walter Reed National Military Medical Center Institutional Review Boards.
Table 1.
Demographics of amputees and matched control participants.
| Lower limb | Site | Cause | Years Since | Gender | Amputee's | Matched-Control's |
|---|---|---|---|---|---|---|
| Amputee | of Amputation | of Amputation | Amputation | Age When Scanned | Age When Scanned | |
| LAl | Left Above knee | Infection | 2 | M | 52 | 58 |
| LA2 | Right below knee | Trauma | 21 | M | 47 | 42 |
| LA3 | Right Above knee | Trauma | 5 | F | 75 | 55 |
| LA4 | Left Above knee | Cancer | 0.25 | F | 36 | 37 |
| LAS | Right above knee | Trauma | 15 | M | 60 | 58 |
| LA6 | Right Below Knee | Trauma | 1 | F | 45 | 54 |
| LA7 | Right Above knee | Trauma | 0.5 | M | 39 | 38 |
| LA8 | Right Below knee | Trauma | 0 | M | 34 | 36 |
| LA9 | Right at knee | Trauma | 1 | M | 30 | 24 |
In MRI sessions before and after starting mirror therapy (Fig. 1), each participant completed both functional and structural scans. Functional scans included the viewing of visually presented limbs, bodies, or common objects, as well as a motor localizer run to locate the relevant sensorimotor regions.
2.3. Visual representations for hands and feet
A block design experiment containing single-images of hands and feet in an egocentric view was used (Fig. 1). Each stimulus (5 degrees), left hand/ft in the left visual field and right hand/ft in the right visual field, was centered 3 degrees from fixation. Each block lasted 16 s and comprised 20 trials (0.8 s total duration; 0.5 s stimulus plus 0.3 s blank). Within each run a total of 16 stimulus blocks were presented plus a 16 s fixation period every four stimulus blocks as well as at the beginning and end of the run. The total for each run amounted to 5 mins 32 s. Participants were required to maintain fixation throughout the run.
2.4. Localizing the sensorimotor regions
Sensorimotor regions for the foot were defined by a combination of anatomical and functional markers. Although, prior work with amputees has sometimes used the response to movement of the phantom to define regions-of-interest (ROIs), we defined our ROIs using data only from control participants to ensure equivalent ROIs in the two groups. While phantom limb movements can elicit muscle activity similar to controls and engage sensorimotor cortex (Raffin et al., 2012; Reilly et al., 2006), it is not clear that movement of the phantom produces equivalent cortical responses to executed movement from an intact limb. For example, differences in the difficulty of moving the phantom have been associated with the strength of PLP and differing levels of activity in motor cortex (Kikkert et al., 2017, Kikkert et al., 2018). Further, any changes in somatosensory or motor representations as a result of amputation might be reflected in the activity observed with phantom movement in amputees. Here, we are principally interested in changes in visual responses in the cortical region that would have represented the amputated limb prior to amputation and want to compare equivalent regions in the two groups of subjects. Finally, some of the amputees had difficulty moving the phantom in isolation and would sometimes move other associated body parts, which would lead to different activation across sensorimotor cortex compared to controls.
A block design experiment comprising three conditions was used to identify cortical regions with selective activation during motor movement. Participants were prompted by simple instructions in the center of a viewing screen (simple phrases, e.g. left foot, right foot), which were flashed at a rate of one Hz. Participants were required to either gently perform flexion and extension movement with their left or right foot or contract gluteal muscles for 10 s at one Hz. The gluteal movement condition was included to potentially allow isolation of the cortical representation of the body map adjacent to the lower limb representation. However, participants (especially amputees) had a lot of difficulty making these movements without also simultaneously moving their legs and therefore we focused only on the lower limb region.
There were 18 blocks within each run, and each condition appeared six times in a counterbalanced order. Each block lasted for 10 s followed by fixation (rest period 6 or 16 s). In addition a 16 s fixation block was added to the beginning and the end of the scan. In total each run lasted for 4 min 42 s. Participants were instructed to maintain fixation and keep their body still during the rest period.
Functionally, we contrasted Right Foot Movement vs Left Foot Movement at a threshold of p < 10−4 (random effect) from 14 control participants (including the 9 participants who were matched to the amputees) across all three scan sessions (N = 42). Anatomically, we defined a region using the Freesurfer parcellation for the central sulcus, pre-central gyrus and the paracentral gyrus. Individual foot sensorimotor ROIs were created by extracting the intersection of these functionally and anatomically defined regions. The ROIs are shown in Supplementary Fig. 1 and correspond very well to the recently reported 7 T mapping data of the lower limb (Akselrod et al., 2017). These ROIs were then used to extract visual responses from both the control and PLP groups.
2.5. Control ROIs: limb-related regions in ventral and dorsal visual pathways
A block design localizer containing images of limbs and common objects was used to identify cortical regions that are selective to visual presentation of limbs, namely the extrastriate body area (EBA), to serve as a control ROI for the sensorimotor regions. Images were photos of limbs or objects taken from various angles. All stimuli were presented in the center of the screen. Twelve alternating blocks of limbs and object images subtending 5 degrees were presented in the center of the screen. In addition, a fixation block was presented at the beginning and end of the scan. Each block lasted for 16 s, and each localizer run lasted a total of 3 min 44 s. Participants were required to maintain fixation throughout the scan. In addition, limb-related superior and inferior parietal regions along the dorsal pathway were defined using previously identified ROIs (Chan and Baker, 2015). These ROIs (N = 40) were defined by contrasting responses during blocks of a movie clip of a hand being brushed by a paint brush versus blocks of a movie clip of the background (no hand) being brushed with the same motion.
2.6. Imaging acquisition
All participants were scanned on a General Electric 3-Tesla Signa scanner located in the Clinical Research Center at the National Institutes of Health, Bethesda, MD. Whole brain volumes were acquired using an 8-channel head coil (30 slices, 64 × 64 matrix, FoV = 200 × 200 mm, in-plane resolution 3.125 × 3.125 mm, slice thickness 4 mm, 0.4 mm inter-slice gap, TR = 2 s, TE = 30 msec). For each participant, a high-resolution anatomical scan was also acquired. All functional localizer and event-related runs were interleaved.
2.7. fMRI preprocessing
Data were analyzed with the AFNI software package (http://afni.nimh.nih.gov/). Prior to statistical analysis, the first and last eight volumes of each run were removed, and all images were motion-corrected to the eighth volume of the first run. Following motion correction, images from the localizer runs were smoothed with a 5 mm FWHM Gaussian kernel. Cortical surfaces were created using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/) and functional data were then displayed on cortical surfaces using SUMA (http://afni.nimh.nih.gov/afni/suma).
2.8. fMRI statistical analysis
Using independent functional localizers, ROIs were generated using the random effect group analysis from control participants across all three scan sessions. Specifically, ROIs in the sensorimotor regions (primary and secondary) in each hemisphere were created from the motor localizers with the contrast of “Left vs Right Foot Movement” (p < 10−4). Since motor movement from the missing limb could not be reliably produced, this group sensorimotor and SII ROIs from the Controls were served as localizers for the PLP group as well. Further, EBA ROIs from the visual localizer were defined by contrasting limbs versus common objects (p < 10−4). All ROIs were generated from these maps by taking the contiguous clusters of voxels that exceeded threshold and occupied the approximate anatomical location based on prior reports. Using a standard GLM procedure in the experimental runs we examined the magnitude of response (beta values) within each ROI to all conditions. The responses in each experimental condition were then extracted from each ROI and compared using statistical tests, such as ANOVAs and paired t-tests.
2.9. Visual mirror therapy
Amputee participants were asked to complete 4 weeks of visual mirror therapy, 15 min per day, 5 days/week. Participants were instructed to place a mirror between their lower limbs and to perform movements with their intact and phantom limbs simultaneously while looking at the reflection of the intact limb moving in the mirror. All amputees reported that they could feel their missing leg and were able to control the movement of the missing/phantom limb. The movements were 5 min of foot flexion and extension, 5 min of inward ankle rotation, and 5 min of outward ankle rotation. Participants reported average PLP daily using a 10-cm Visual Analogue Scale (VAS), with one end labeled “no pain” and the other end labeled “most severe pain.”
3. Results
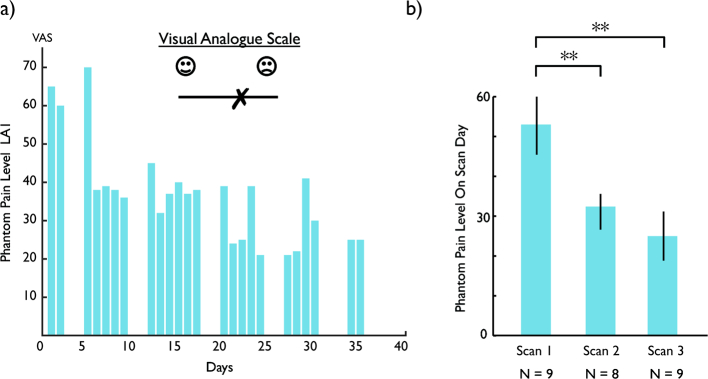
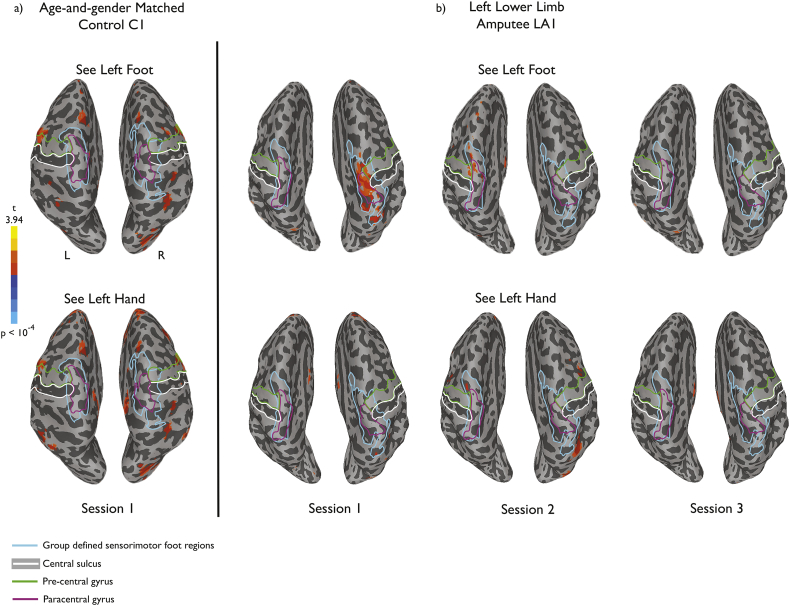
To illustrate the pattern of results we observed, we first present whole-brain data from one representative amputee before presenting data analyses at the group level. LA1 was a 57-year old male at the time of the study, with an amputation below the left knee 2 years previously due to infection. Consistent with prior reports (Ramachandran et al., 1995; Chan et al., 2007), his perceived pain level, as measured using a 10-cm VAS, diminished over the course of mirror therapy (Fig. 2a). During the baseline fMRI scan we observed an unexpected response to visual presentation of left foot images (corresponding to the amputated foot) in sensorimotor cortex, close to the putative foot representation at the dorsal end of the central sulcus, likely corresponding to primary somatosensory and motor cortex (Fig. 3b). The initial measured responses appeared to be specific both to the type of limb (with stronger responses to a foot than a hand) and to the side of the body (with stronger responses to a foot corresponding to the amputated side than to an intact foot from the other side of the body). Further, such responses were not observed in the matched control participant, as expected for static presentation of pictures of limbs (Chan et al., 2005; Chan et al., 2010). This visual response to images corresponding to the amputated limb declined over the course of mirror therapy, and by the third fMRI session, visual responses in sensorimotor cortex were no longer observed.
Fig. 2.
Improvement in pain symptoms. a) Change in Visual Analogue Scale (VAS) score over the course of the mirror therapy in a single participant (LA1). b) Group averaged VAS scores for the days corresponding to the scan sessions, demonstrating a significant reduction in PLP from scan 1 to scans 2 and 3. ** p < .001 indicates significant difference in VAS level between scans. Error bars show the standard error of the mean.
Fig. 3.
Responses to visually presented limbs for a single amputee participant (LA1) and the matched control (C1). a) Baseline scan for the matched control participant showing no significant activation near the sensorimotor foot representation at the dorsal end of the central sulcus (white boundaries). Results were similar for the second and third scan sessions. b) During the baseline scan for the amputee participant, significant activation was observed in and around the central sulcus, corresponding to the typical location of the foot representation in sensorimotor cortex defined in the control group (light blue boundaries). This activation was observed for the visual presentation of a foot, but not hand, from the side of the body corresponding to the amputation (left foot). These responses diminished over the course of the three scan sessions, concomitant with the reduction in pain scores, and there was no significant activation in or around the central sulcus in the third session. Activations are projected onto the inflated brain surface reconstructed from each participant's own brain volume.
This general pattern of visual responsiveness in sensorimotor cortex at baseline that was much reduced by the end of mirror therapy was seen in most amputees, although the effect of type of limb (hand versus foot) and side of body (amputated versus intact) was not consistent across amputees. PLP reduction over the course of mirror therapy was observed in all except one of the amputees. Overall, VAS scores on the day of each fMRI session showed an average of 46% reduction from Session 1 to Session 3 (Fig. 2b). A repeated-measures one-way ANOVA confirmed the reduction in subjective pain over the course of mirror therapy, F(2,14) = 12.237, p < .001.
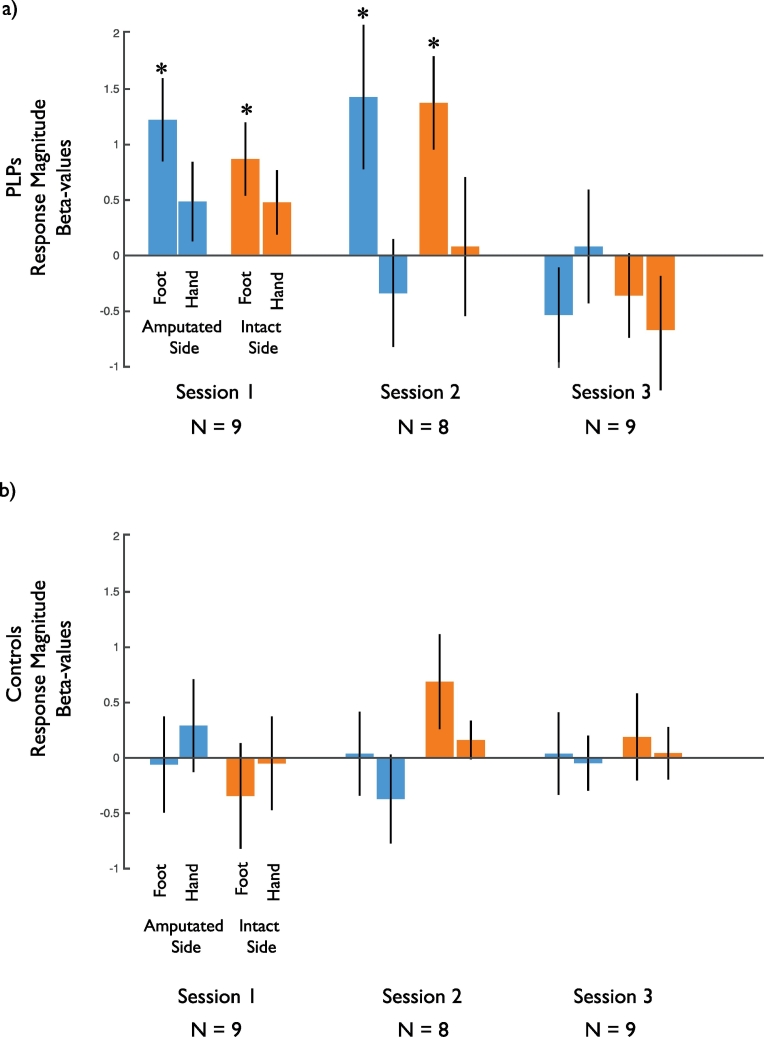
To examine the effects of amputation and mirror therapy on cortical responses across the group of amputees, we identified the putative cortical foot representations in and around the central sulcus using a combination of anatomical and functional constraints (see Methods). This region includes both primary somatosensory and motor areas. Using this ROI we extracted responses to the visual presentation of hands and feet in both amputees and their matched controls. As with the representative amputee presented above, on average across the group we found responses to visually presented limbs in sensorimotor cortex at baseline that were no longer present by the end of mirror therapy (Fig. 4).
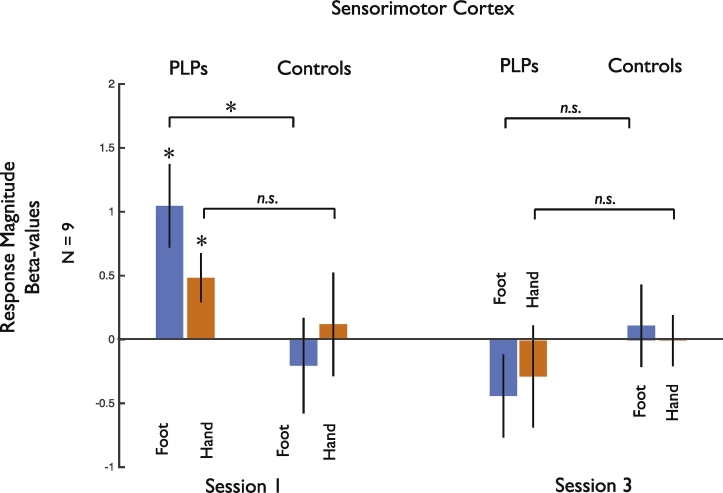
Fig. 4.
Group average changes in visual responsiveness in the sensorimotor foot representation. a) Across the group of amputees there was an elevated response to visually presented limbs in the sensorimotor representation of the foot (identified using foot movements in the matched control participants) during the baseline scan session. These responses were greater for the foot than the hand and were no longer present in Session 3. b) There was no significant response to visually presented limbs in the control participants in any session.* indicates significant difference from zero (p < .05).
To examine the fMRI responses, we conducted statistical analysis across sessions, comparing both PLP and control groups. However, one of our PLP participants was only scanned in Sessions 1 and 3. Given our already limited sample size, we therefore focused on Sessions 1 and 3 only to maximize power (Fig. 5). However, the pattern of results does not change when including only those participants scanned in all 3 Sessions (Supplementary Fig. 2) and details of the statistical analysis across all three Sessions are provided in supplementary material.
Fig. 5.
Summary of the data entered into the main statistical analysis in sensorimotor cortex, collapsed across hemisphere and side. The 5-way ANOVA revealed an interaction between Group, Limb, and Session. Follow-up paired t-tests directly compared responses for Limb across Group within each Session separately. For Foot, there was a significant difference in Session 1 (t(1,8) = 2.59, p < .032, two-tailed) but not Session 3 (t(1,8) = 0.73, p < .49, two-tailed). For Hand, there was no significant difference in either Session (all p < .12, two-tailed). * indicates significant difference between conditions or significant difference from zero, while n.s. indicates non-significant difference between pairs of conditions.
A 5-way ANOVA with Group (Control, PLP), Hemisphere (Contralateral, Ipsilateral to missing limb), Session (Scan 1, 3), Side (Missing, Intact), and Limb (Foot, Hand) as factors revealed a significant three-way interaction between Session, Limb and Group (F(1,16) = 7.07, p < .017). No other main effects or interactions were observed (all F < 3.626, p > .074). There were no effects involving Hemisphere, with similar results in both hemispheres (contra- and ipsilateral to the amputation).
To better understand the nature of this three-way interaction, we conducted follow-up analyses focusing separately on each Group and each Session. First, two-way ANOVAs within each Group with Session and Limb as factors (collapsing across Side and Hemisphere) revealed a significant main effect of Session in the PLP group (F(1,8) = 5.51, p < .047) but not the Control group (F(1,8) = 0.053, p < .82), confirming the reduction in visual responses for the amputees over the course of mirror therapy. However, the interaction between Session and Limb did not reach significance in the PLP group (1,8) = 4.08, p < .078).
Second, separate analyses for each Session, revealed a significant interaction of Limb and Group within Session 1 (F(1,8) = 5.47, p < .048), reflecting stronger responses to the foot than the hand in the amputees but not the controls. However, in Session 3 there were no significant differences between the two groups and no interactions (all F < 0.96, p > .20).
Follow-up paired t-tests directly compared responses to each limb between groups for each Session (see Fig. 5), revealing stronger responses in the PLP compared to Control group for seeing the foot in Session 1 (t(1,8) = 2.59, p 0.032, two-tailed), but not Session 3 (t(1,8) = 0.73, p < .49, two-tailed). Furthermore, there were no significant differences between groups for seeing the hand in either Session 1 or Session 3 (all p > .12, two-tailed).
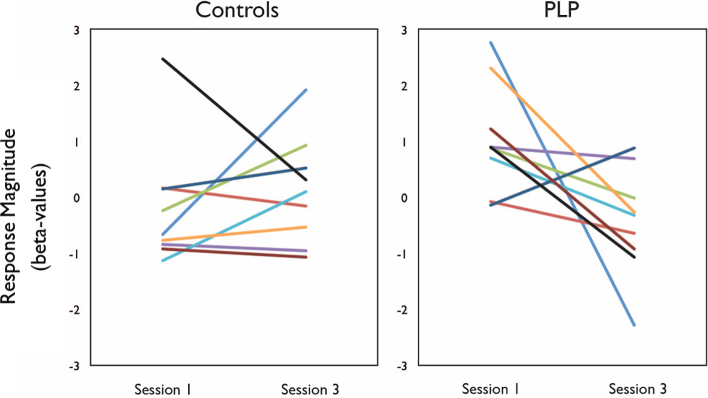
The reduction in visual responsiveness from Session 1 to Session 3 was highly consistent across participants (Fig. 6) and was observed in all but one of the amputees.
Fig. 6.
Individual participants' responses from sensorimotor foot region, illustrating changes in responses across Sessions 1 and 3 in controls and amputees (n = 9). All amputees (except one) showed much sharper reduction in responses from Session 1 to Session 3, relative to controls.
Thus, our results demonstrate an initial enhanced visual responsiveness in the sensorimotor cortex in the PLP group compared to the Control group, which is specific for the amputated (foot) compared to the unaffected limbs (either hand, intact foot) in Session 1. Further, this visual responsiveness was reduced following visual feedback through mirror therapy, suggesting that prolonged visual experience corresponding to the missing limb modulates these atypical visual responses.
To investigate the specificity of our results to the primary sensorimotor regions, we examined.
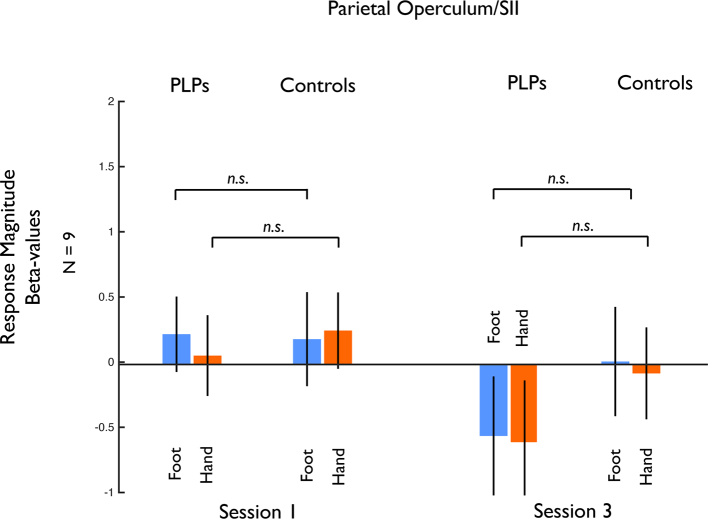
responses in other sensorimotor regions, in particular the secondary somatosensory area (SII). Our localizer for the sensorimotor foot representation also revealed a region close to the parietal operculum, which likely corresponds to SII. We extracted responses from this region and performed the same analyses as for the primary sensorimotor region described above. The 5-way ANOVA with Group (Control, PLP), Hemisphere (Contralateral, Ipsilateral to missing limb), Session (Scan 1, 3), Side (Missing, Intact), and Limb (Foot, Hand) as factors revealed no significant main effects or interactions. Furthermore, responses to the visual presentation of feet and hands in the PLP group were not significantly elevated over baseline (all t < 0.20, p > .23, two-tailed). Thus, we found no evidence of visual responsiveness in SII in either the PLP or the Control groups (Fig. 7). A 6-way ANOVA with ROI (Sensorimotor, SII), Group (Control, PLP), Hemisphere (Contralateral, Ipsilateral to missing limb), Session (Scan 1, 3), Side (Missing, Intact), and Limb (Foot, Hand) as factors showed no significant interaction of ROI x Session x Limb x Group (F(1,16) = 3.380, p < .085). No other main effects or interactions were found.
Fig. 7.
Responses to visually presented limbs in the parietal operculum region (likely corresponding to SII) between PLP and Control groups. In contrast to primary sensorimotor cortex, there were no significant responses to visually presented limbs in any Session and no change over time. n.s. indicates non-significant difference between pairs of conditions.
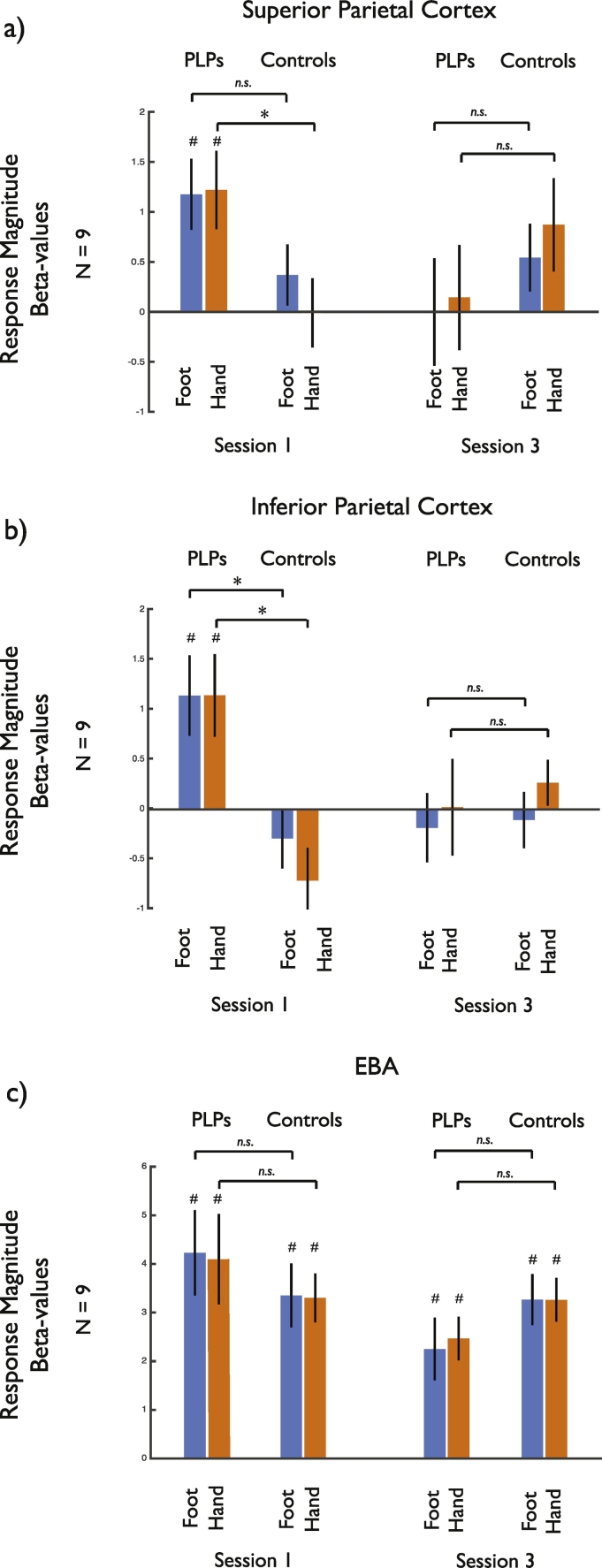
We primarily focused on the sensorimotor cortex given prior results suggesting reorganization in this region in upper limb amputees. To test whether the impact of mirror therapy on the visual responses to limbs in the PLP group was observed only in sensorimotor cortex, we also analyzed the responses in other regions associated with representations of the body. Specifically, we examined the Extrastriate Body Area (EBA; Downing et al., 2001, Downing et al., 2006; Chan et al., 2005; Chan et al., 2010), which is selectively responsive to the sight of body parts, as well as two regions in posterior parietal cortex that are responsive to the sight of the body being touched/brushed, but not to static limb images (Chan and Baker, 2015). Given our prior results which showed no effect of hemisphere or side, for each region (Fig. 8) we conducted a 3-way ANOVA with Group (PLP, Control), Session (1, 3), and Limb (Hands, Feet) as factors. In each region there was a significant interaction of Session and Group (EBA: F(1,16) = 6.327, p < .023; superior parietal: F(1,16) = 6.000, p < .026; inferior parietal: F(1,16) = 5.883, p < .027), reflecting reduced visual responsiveness to images of both hands and feet from Session 1 to Session 3 in the PLP but not the control group. However, in contrast to the sensorimotor cortex, the pattern of results in all three regions was similar for both hands and feet. In the superior and inferior parietal regions, as in sensorimotor cortex, the visual responsiveness observed in Session 1 of amputees was absent in control participants. In EBA, which is normally responsive to images of static body parts, the initial response was not statistically different between the PLP and the control groups. Thus, the enhanced responsiveness to visually presented limbs is also observed in posterior parietal cortex and a change in visual responsiveness over the course of mirror therapy is also found in posterior parietal cortex and EBA, although the specificity to the type of limb (foot versus hand) was only observed in sensorimotor cortex.
Fig. 8.
Responses to visually presented limbs in (a) superior parietal, (b) inferior parietal and (c) EBA regions-of-interest. In all three regions there was a significant interaction between Group and Session, reflecting reduced responses in Session 3 compared to Session 1 for the PLP group. However, in contrast to sensorimotor cortex, the pattern of results was similar for both hands and feet. * indicates significant difference between conditions or significant difference from zero, while n.s. indicates non-significant difference between pairs of conditions.
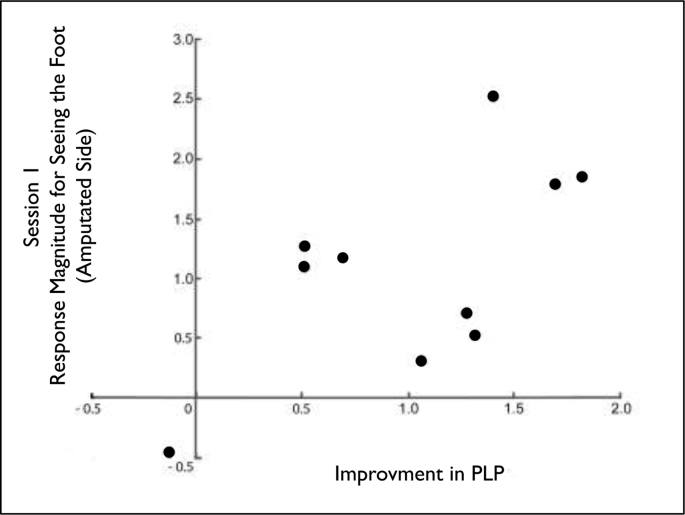
Mirror therapy is not efficacious for all amputees (Chan et al., 2007; Weeks and Tsao, 2010; Foell et al., 2014). If the visual responsiveness of sensorimotor cortex is related to the experience of PLP, it might provide a useful marker for the potential efficacy of mirror therapy. Thus, in an exploratory analysis we investigated whether activation of the sensorimotor ROIs (averaged across the sensorimotor foot ROIs in both hemispheres) in Session 1 for the presentation of an image of the hand or foot (corresponding to the amputated side or intact side) correlated with treatment response, as measured by reduction in PLP (VAS S1 to S3). We found a significant correlation between seeing an image of the foot corresponding to the amputated side and a reduction in PLP (Spearman's r = 0.703, p < .035, two-tailed), suggesting that amputees who have stronger initial responses for viewing the foot image corresponding to their amputated foot have a greater reduction in PLP following four weeks of mirror therapy (Fig. 9). However, there was no correlation between responses for viewing the intact foot and reduction in PLP, despite the lack of an effect of side on fMRI responses (Spearman's r = 0.167, p < .667, two-tailed). We conducted a further exploratory follow-up analysis excluding those amputees who had their amputation surgery >10 years ago (LA 2 and 5) and the correlation between reduction in PLP and visual responsiveness in the sensorimotor region remained significant (Spearman's r = 0.786, p < .036, two-tailed). We did not find any significant correlation between responses in EBA and reduction in PLP (Spearman's r < 0.569, all p > .11, two-tailed).
Fig. 9.
Visual responsiveness in sensorimotor cortex predicts pain relief from mirror therapy. Within each amputee, visual responses to the foot extracted from sensorimotor cortex correlates with the reduction in PLP (normalized VAS z-scores Session1 minus Session 3), demonstrating that the stronger the initial visual responses in Session 1 for seeing the amputated foot, the better the therapeutic outcome (Spearman's r = 0.70, p < .035, two-tailed).
4. Discussion
Collectively, our findings provide insight into the impact of amputation and the previously reported beneficial effect of mirror therapy. First, consistent with prior reports, we found that mirror therapy reduced perceived PLP, where we have previously found that mirror therapy was more effective than imagined movement or movement of the phantom limb in the absence of the mirror component (Chan et al., 2007). Second, we demonstrate an atypical responsiveness to visually presented limbs in sensorimotor cortical regions corresponding to the somatosensory and motor representations of the foot. Third, visual responsiveness was also enhanced in posterior parietal cortex but we found no evidence for visual activation in SII. Fourth, the visual responsiveness decreased by the end of the treatment when PLP was also reduced. Finally, the extent of visual responsiveness at baseline correlated with the reduction in PLP, suggesting that it could potentially be a marker for the likely efficacy of mirror therapy.
Visual responses are not typically reported in sensorimotor cortex of healthy individuals, especially to static images. There have been reports of responses to the sight of touch in somatosensory areas (Keysers et al., 2004; Schaefer et al., 2009, Schaefer et al., 2013; Kuehn et al., 2013, Kuehn et al., 2014, Kuehn et al., 2018), but in an earlier study we found that the sight of a hand being brushed elicited responses in regions of posterior parietal cortex that did not overlap with the somatosensory representations of the hand (Chan and Baker, 2015), with similar results recently reported in monkeys (Fiave et al., 2018). There is also some evidence for decoding of visual objects based on the pattern of responses in somatosensory regions (e.g., Meyer et al., 2011; Smith and Goodale, 2013), but the univariate responses in these studies are very weak. Consistent with this prior literature (Chan and Baker, 2015; Kilintari et al., 2016; Fiave et al., 2018), we did not observe any responses to the sight of static hands and feet in sensorimotor cortex of our control participants in any session. Thus, the strong responsiveness to visually presented limbs (particularly the amputated limb) appears to be a specific effect following major limb amputation. This atypical visual responsiveness following amputation may reflect unmasking of pre-existing connections between visual areas in parietal cortex and sensorimotor cortices (Keysers et al., 2010), rather than being due to functional re-purposing by other modalities (i.e., the sensorimotor cortex now becoming a visual region). Such unmasking has also been suggested to underlie changes in receptive field properties in visual cortex following deprivation of visual input (Masuda et al., 2008; Baker et al., 2005). Consistent with this view, visual perception can be disrupted very rapidly following the onset of deprivation, too quickly for any structural changes to arise in the cortex (Dilks et al., 2009).
Our results suggest that visual responsiveness in sensorimotor cortex may be a consequence of amputation. However, we do not think there is a simple, one-to-one causal relationship between visual responsiveness and the level of PLP. For example, while we observed a clear drop in PLP from Session 1 to Session 2, there was not an equivalent drop in the visual responsiveness observed. However, the increased responsiveness was largely specific to primary sensorimotor regions and was not observed in the putative SII. Somewhat surprisingly, we found no difference in sensorimotor cortex response between the hemispheres, although it might have been expected that any effects would be specific to the hemisphere contralateral to the amputation. This lack of hemispheric specificity may reflect strong connectivity between the sensorimotor cortices via the corpus callosum (Ragert et al., 2011; Zarei et al., 2006; Clarey et al., 1996). We also observed enhanced responsiveness to visually presented limbs in posterior parietal cortex, but this effect was not specific to the limb, in contrast to our results in primary sensorimotor regions. While our findings do not provide a direct link between the atypical visual responsiveness in sensorimotor cortex and PLP, this visual responsiveness was reduced over the course of treatment, and by the end of treatment both visual responsiveness and pain were reduced. Further, the magnitude of visual responsiveness at baseline correlated with the amount of pain reduction following 4 weeks of mirror therapy. This reduction in visual responsiveness suggests that one impact of mirror therapy may be to modulate visual influences on sensorimotor cortex, perhaps by altering intracortical inhibition (Longo et al., 2011; Cardini et al., 2011).
It is important to consider alternative interpretations of our findings. One possibility is that the presence of chronic pain associated with the amputation has increased the salience of visually presented limbs corresponding to the amputated limb, increased visual attention. The fact that increased responses were observed in multiple regions in both hemispheres with some decrease over the sessions could be taken to support this view. However, we do not think that a change in salience causing a gain in responses provides an easy explanation for our results. First, it is important to note that there is no observable response to the visual stimuli in the sensorimotor cortex of the control participants, so no pre-existing response for saliency to enhance. Second, the effects we observed were not uniform across all regions. In particular while responses were stronger for feet over hands in sensorimotor cortex, in other regions there was no obvious difference between feet and hands. Another possibility is that amputees may have been more likely to mentally image a limb moving when viewing images of limbs producing activation in sensorimotor cortex. However, we think this is unlikely. First, the visual stimulation was of static limbs, which should have minimized any tendency to imagine movement. Second, it's not clear to us that any such tendency should have been greater in the amputees compared to the controls. Finally, any tendency to imagine a limb moving would, if anything, have increased over the course of the mirror training as amputees were trained to move their phantom simultaneous with visual stimulation. Nevertheless, future work will need to test these potential hypotheses.
Prior work has investigated whether PLP is related to plasticity of somatosensory and motor maps (Lotze et al., 2001) with a shift in the cortical representation of the lips toward the representation of the missing hand. However, the extent of these effects has recently been questioned (Makin et al., 2015b), and fine-scale topography of the hand appears to be maintained in somatosensory and motor cortex following hand amputation (Kikkert et al., 2016). Our findings, combined with evidence for network-level reorganization with amputation (Makin et al., 2015b), suggest there may be widespread changes following loss of sensory input, but in the context of maintained cortical representation of the missing limb. The tendency for representation to be maintained has recently been linked to the severity of PLP (Makin et al., 2013) and the visual responses we observed could potentially be one driving force for the maintenance of this representation.
While our study provides some insight into the effects of amputation on sensorimotor cortex and suggests potential neural correlates of pain reduction through visual training (i.e. mirror therapy), there are a number of limitations that should be taken into account. First, our sample size (9 amputees for Sessions 1 and 3, 8 in all three Sessions) is quite small and while we did observe significant and consistent visual responsiveness in sensorimotor cortex of amputees in Session 1 and reductions in visual responsiveness from Session 1 to Session 3 in all but one amputee, there is also quite a lot of variability in the data. Thus, we may not have had sufficient power to detect smaller effects in the data, and the correlation we observed between visual responsiveness in Session 1 and efficacy of mirror therapy remains preliminary. Further, this small sample size means that we cannot meaningfully ask questions about the effects of factors such as age and time since amputation on our results. Second, in contrast to much of the prior published literature examining changes in upper limb amputees, we focused on lower limb amputees. Given the smaller cortical representation of the leg and foot compared to the arm and hand, we may not have had sufficient sensitivity to detect changes and it is difficult to directly relate our findings to those in arm amputees. Third, we had no amputee control group receiving other treatments. Thus we cannot rule out that the effects we observed would also have occurred with those alternative treatments or the effect of time. Nevertheless, our findings highlight the potential importance of examining visual responses to the body in amputees and future work will be needed to establish the robustness of our findings and whether similar effects are observed in arm amputees.
While a number of studies and groups have independently demonstrated a benefit from using mirror therapy in the treatment of phantom limb pain (Ramachandran and Rogers-Ramachandran, 1996; MacLachlan et al., 2004; Chan et al., 2007; Sumitani et al., 2008; Mercier and Sirigu, 2009; Finn et al., 2017; Ol et al., 2018), other groups have not reported similar successes (Brodie et al., 2007; Thieme et al., 2016; Richardson and Kulkarni, 2017; Wareham and Sparkes, 2018). In this study we followed mirror therapy treatment protocols that had previously been shown to be effective compared with limb movements alone (mirror covered) or imagined movement in a randomized, controlled, cross-over design (Chan et al., 2007). It is worth noting that the impact of mirror therapy may not be entirely consistent across amputees due to the heterogeneity of the population, and it is known that some amputees do not benefit from mirror therapy even after 4 weeks of treatment (Chan et al., 2007; Griffin et al., 2017). There are wide variations in the location, timing, cause, and age at the time of amputation. All of these may have influence on the effectiveness of mirror therapy. It is also worth noting that those groups which did not report PLP reduction employed only a single session of mirror therapy (Brodie et al., 2007; Wareham and Sparkes, 2018), whereas the studies demonstrating efficacy of the therapy had sustained treatment sessions of a period of time. Recently, it was reported that amputee with greater severity of PLP require a longer duration of treatment (more sessions) before an effect can be seen, compared to fewer treatment sessions required for pain reduction in amputees with less severe phantom pain (Griffin et al., 2017), and this finding likely affects the negative conclusions drawn from analyses of prior studies (Thieme et al., 2016; Richardson and Kulkarni, 2017). Future studies should also examine why some amputees do not derive benefit from mirror therapy whereas many others experience complete pain relief.
In conclusion, we report atypical responsiveness to visually presented limbs in sensorimotor cortex that reduces over the course of 4 weeks of mirror therapy seems to associate with a reduction in phantom pain. Such visual responsiveness found in the PLP group could potentially be a neural correlate or biomarker for quantifying the impact of major limb amputation and the likely efficacy of visual mirror therapy.
Acknowledgments
Acknowledgements
This study was supported by the intramural program of the National Institute of Mental Health (CIB), ZIA-MH-002893, the Postdoctoral Intramural Research Training Award in NIMH (AC), a grant from the David Mahoney Neuroimaging Program of the Dana Foundation (CIB, JWT), and the Center for Rehabilitation Sciences Research at the Uniformed Services University of the Health Sciences (PFP, JWT). We thank Dr. Howard Gilmer (National Rehabilitation Hospital, Washington, DC) for assistance with recruiting amputee participants, Beth Aguila and Marcie King for assistance with fMRI data collection, and Jennifer Henry for consolidating demographic data. We also thank members in the Laboratory and Brain Cognition (NIMH) for helpful discussions and comments on the study. Author contributions: Conceptualization, AC and CIB, with early input from JWT; Design and materials: AC and CIB; Data curation, AC and CIB; Formal analysis: AC and CIB; Investigation: AC and CIB; Methodology: AC and CIB; Recruitment: all authors contributed; Writing – review & editing AC, CIB, and JWT. Writing – original and final drafts: AC and CIB.
Disclaimer
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Navy or the Department of Defense.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101882.
Appendix A. Supplementary data
Supplementary material
References
- Akselrod M., Martuzzi R., Serino A., van der Zwaag W., Gassert R., Blanke O. Anatomical and functional properties of the foot and leg representation in areas 3b, 1 and 2 of primary somatosensory cortex in humans: a 7T fMRI study. Neuroimage. 2017;159:473–487. doi: 10.1016/j.neuroimage.2017.06.021. Oct 1. [DOI] [PubMed] [Google Scholar]
- Anderson-Barnes V.C., McAuliffe C., Swanberg K.M., Tsao J.W. Phantom limb pain – a phenomenon of proprioceptive memory? Med. Hypotheses. 2009;73:555–558. doi: 10.1016/j.mehy.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Baker C.I., Peli E., Knouf N., Kanwisher N.G. Reorganization of visual processing in macular degeneration. J Neurosci. 2005 Jan 19;25(3):614–618. doi: 10.1523/JNEUROSCI.3476-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D., Becerra L., Fishman S., Edwards A., Jennings C.L., Stojanovic M. Acute plasticity in the human somatosensory cortex following amputation. Neuroreport. 1998;9(6):1013–1017. doi: 10.1097/00001756-199804200-00011. [DOI] [PubMed] [Google Scholar]
- Brodie E.E., Whyte A., Niven C.A. Analgesia through the looking-glass? A randomized controlled trial investigating the effect of viewing a 'virtual' limb upon phantom limb pain, sensation and movement. Eur. J. Pain. 2007 May;11(4):428–436. doi: 10.1016/j.ejpain.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Cardini F., Longo M.R., Haggard P. Vision of the body modulates somatosensory intracortical inhibition. Cereb. Cortex. 2011;21(9):2014–2022. doi: 10.1093/cercor/bhq267. Sep. [DOI] [PubMed] [Google Scholar]
- Chan A.W., Baker C.I. Seeing is not feeling: posterior parietal but not somatosensory cortex engagement during touch observation. J. Neurosci. 2015;35:1468–1480. doi: 10.1523/JNEUROSCI.3621-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A.W., Peelen M.V., Downing P.D. The effect of viewpoint on body representation in the extrastriate body area. Neuroreport. 2005;15:2407–2410. doi: 10.1097/00001756-200410250-00021. [DOI] [PubMed] [Google Scholar]
- Chan B.L., Witt R., Charrow A.P., Magee A., Howard R., Pasquina P.F. Mirror therapy for phantom limb pain. New Engl. J. Med. 2007;357(21):2206–2207. doi: 10.1056/NEJMc071927. Letter to the Editor. [DOI] [PubMed] [Google Scholar]
- Chan A.W., Kravitz D.J., Truong S., Arizpe J., Baker C.I. Cortical representations of bodies and faces are strongest in commonly experienced configurations. Nat. Neurosci. 2010;13:417–418. doi: 10.1038/nn.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarey J.C., Tweedale R., Calford M.B. Interhemispheric modulation of somatosensory receptive fields: evidence for plasticity in primary somatosensory cortex. Cereb. Cortex. 1996;6(2):196–206. doi: 10.1093/cercor/6.2.196. Mar-Apr. [DOI] [PubMed] [Google Scholar]
- Dilks D.D., Baker C.I., Liu Y., Kanwisher N. "Referred visual sensations": rapid perceptual elongation after visual cortical deprivation. J. Neurosci. 2009;29(28):8960–8964. doi: 10.1523/JNEUROSCI.1557-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing P.E., Jiang Y., Shuman M., Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Downing P.E., Chan A.W., Peelen M.V., Dodds C.M., Kanwisher N. Domain specificity in visual cortex. Cereb. Cortex. 2006;16:1453–1461. doi: 10.1093/cercor/bhj086. [DOI] [PubMed] [Google Scholar]
- Fiave P.A., Sharma S., Jastorff J., Nelissen K. Investigating common coding ofobserved and executed actions in the monkey brain using cross-modal multi-variate fMRI classification. Neuroimage. 2018;178:306–317. doi: 10.1016/j.neuroimage.2018.05.043. Sep. [DOI] [PubMed] [Google Scholar]
- Finn S.B., Perry B.N., Clasing J.E., Walters L.S., Jarzombek S.L., Curran S., Rouhanian M., Keszler M.S., Hussey-Andersen L.K., Weeks S.R., Pasquina P.F., Tsao J.W. A randomized, controlled trial of Mirror therapy for upper extremity phantom limb pain in male amputees. Front. Neurol. 2017;8:267. doi: 10.3389/fneur.2017.00267. Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H., Elbert T., Knecht S., Wienbruch C., Pantev C., Birbaumer N. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375(6531):482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- Flor H., Nikolajsen L., Staehelin Jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat. Rev. Neurosci. 2006;7(11):873–881. doi: 10.1038/nrn1991. [DOI] [PubMed] [Google Scholar]
- Foell J., Bekrater-Bodmann R., Diers M., Flor H. Mirror therapy for phantom limb pain: brain changes and the role of body representation. Eur. J. Pain. 2014;18:729–739. doi: 10.1002/j.1532-2149.2013.00433.x. [DOI] [PubMed] [Google Scholar]
- Grusser S.M., Winter C., Muhlnickel W., Denke C., Karl A., Villringer K. The relationship of perceptual phenomena and cortical reorganization in upper extremity amputees. Neuroscience. 2001;102(2):263–272. doi: 10.1016/s0306-4522(00)00491-7. [DOI] [PubMed] [Google Scholar]
- Karl A., Birbaumer N., Lutzenberger W., Cohen L.G., Flor H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J. Neurosci. 2001;21(10):3609–3618. doi: 10.1523/JNEUROSCI.21-10-03609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C., Kaas J.H., Gazzola V. Somatosensation in social perception. Nat. Rev. Neurosci. 2010;11(6):417–428. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Keysers C., Wicker B., Gazzola V., Anton J.L., Fogassi L., Gallese V. A touching sight: SII/PV activation during the observation and experience of touch. Neuron. 2004 Apr 22;42(2):335–346. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- Kikkert S., Kolasinski J., Jbabdi S., Tracey I., Beckmann C.F., Johansen-Berg H., Makin T.R. Revealing the neural fingerprints of a missing hand. eLife. 2016, Aug 23;5 doi: 10.7554/eLife.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkert S., Mezue M., Henderson Slater D., Johansen-Berg H., Tracey I., Makin T.R. Motor correlates of phantom limb pain. Cortex. 2017, Oct;95:29–36. doi: 10.1016/j.cortex.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkert S., Mezue M., O'Shea J., Henderson-Slater D., Johansen-Berg H., Tracey I., Makin T.R. The neural basis of induced phantom limb pain relief. Ann. Neurol. 2018 doi: 10.1002/ana.25371. (Nov 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilintari M., Narayana S., Babajani-Feremi A., Rezaie R., Papanicolaou A.C. Brain activation profiles during kinesthetic and visual imagery: an fMRI study. Brain Res. 2016 Sep 1;1646:249–261. doi: 10.1016/j.brainres.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Kuehn E., Trampel R., Mueller K., Turner R., Schu¨tz-Bosbach S. Judging roughness by sight—a 7-tesla fMRI study on responsivity of the primary somatosensory cortex during observed touch of self and others. Hum. Brain Mapp. 2013;34:1882–1895. doi: 10.1002/hbm.22031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn E., Mueller K., Turner R., Schutz-Bosbach S. The functional architecture of S1 during touch observation described with 7 T fMRI. Brain Struct. Funct. 2014;219:119–140. doi: 10.1007/s00429-012-0489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn E., Haggard P., Villringer A., Pleger B., Sereno M.I. Visually-driven maps in area 3b. J. Neurosci. 2018;38(5):1295–1310. doi: 10.1523/JNEUROSCI.0491-17.2017. Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo M.R., Pernigo S., Haggard P. Vision of the body modulates processing in primary somatosensory cortex. Neurosci. Lett. 2011;489(3):159–163. doi: 10.1016/j.neulet.2010.12.007. Feb 11. [DOI] [PubMed] [Google Scholar]
- Lotze M., Flor H., Grodd W., Larbig W., Birbaumer N. Phantom movements and pain. An fMRI study in upper limb amputees. Brain. 2001;124(Pt 11):2268–2277. doi: 10.1093/brain/124.11.2268. [DOI] [PubMed] [Google Scholar]
- MacLachlan M., McDonald D., Waloch J. Mirror treatment of lower limb phantom pain: a case study. Disabil. Rehabil. 2004;26(14–15):901–904. doi: 10.1080/09638280410001708913. [DOI] [PubMed] [Google Scholar]
- Makin T.R., Scholz J., Filippini N., Henderson Slater D., Tracey I., Johansen-Berg H. Phantom pain is associated with preserved structure and function in the former hand area. Nat. Commun. 2013;4:1570. doi: 10.1038/ncomms2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin T.R., Scholz J., Henderson Slater D., Johansen-Berg H., Tracey I. Reassessing cortical reorganization in the primary sensorimotor cortex following arm amputation. Brain. 2015;138:2140–2146. doi: 10.1093/brain/awv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin T.R., Filippini N., Duff E.P., Henderson Slater D., Tracey I., Johansen-Berg H. Network-level reorganisation of functional connectivity following arm amputation. NeuroImage. 2015;114:217–225. doi: 10.1016/j.neuroimage.2015.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y., Dumoulin S.O., Nakadomari S., Wandell B.A. V1 projection zone signals in human macular degeneration depend on task, not stimulus. Cereb. Cortex. 2008;18:2483–2493. doi: 10.1093/cercor/bhm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier C., Sirigu A. Training with virtual visual feedback to alleviate phantom limb pain. Neurorehabil. Neural Repair. 2009;23:587–594. doi: 10.1177/1545968308328717. [DOI] [PubMed] [Google Scholar]
- Meyer K., Kaplan J.T., Essex R., Damasio H., Damasio A.R. Seeing touch is correlated with content-specific activity in primary somatosensory cortex. Cereb. Cortex. 2011;21:2113–2121. doi: 10.1093/cercor/bhq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ol H.S., Van Heng Y., Danielsson L., Husum H. Mirror therapy for phantom limb and stump pain: a randomized controlled clinical trial in landmine amputees in Cambodia. Scand J Pain. 2018;18(4):603–610. doi: 10.1515/sjpain-2018-0042. Oct 25. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A., Peris M., Tormos J.M., Pascual A.P., Catala M.D. Reorganization of human cortical motor output maps following traumatic forearm amputation. Neuroreport. 1996;7(13):2068–2070. doi: 10.1097/00001756-199609020-00002. [DOI] [PubMed] [Google Scholar]
- Raffin E., Mattout J., Reilly K.T., Giraux P. Disentangling motor execution from motor imagery with the phantom limb. Brain. 2012;135(Pt 2):582–595. doi: 10.1093/brain/awr337. [DOI] [PubMed] [Google Scholar]
- Ragert P., Nierhaus T., Cohen L.G., Villringer A. Interhemispheric interactions between the human primary somatosensory cortices. PLoS One. 2011;6(2):e16150. doi: 10.1371/journal.pone.0016150. Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V.S., Altschuler E.L. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. 2009;132:1693–1710. doi: 10.1093/brain/awp135. [DOI] [PubMed] [Google Scholar]
- Ramachandran V.S., Blakeslee S. Harper Collins Publishers Inc; New York: 1998. Phantoms in the Brain: Human Nature and the Architecture of the Mind. [Google Scholar]
- Ramachandran V.S., Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc. Biol. Sci. 1996 Apr 22;263(1369):377–386. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- Ramachandran V.S., Rogers-Ramachandran D., Cobb S. Touching the phantom limb. Nature. 1995;377(6549):489–490. doi: 10.1038/377489a0. [DOI] [PubMed] [Google Scholar]
- Reilly K.T., Mercier C., Schieber M.H., Sirigu A. Persistent hand motor commands in the amputees' brain. Brain. 2006 Aug;129(Pt 8):2211–2223. doi: 10.1093/brain/awl154. [DOI] [PubMed] [Google Scholar]
- Richardson C., Kulkarni J. A review of the management of phantom limb pain: challenges and solutions. J. Pain Res. 2017;10:1861–1870. doi: 10.2147/JPR.S124664. Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M., Xu B., Flor H., Cohen L.G. Effects of different viewing perspectives on somatosensory activations during observation of touch. Hum. Brain Mapp. 2009;30:2722–2730. doi: 10.1002/hbm.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M., Rotte M., Heinze H.J., Denke C. Mirror-like brain responses to observed touch and personality dimensions. Front. Hum. Neurosci. 2013;7:227. doi: 10.3389/fnhum.2013.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F.W., Goodale M.A. Decoding visual object categories in early somatosensory cortex. Cereb. Cortex. 2013;25(4):1020–1031. doi: 10.1093/cercor/bht292. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitani M., Miyauchi S., McCabe C.S., Shibata M., Maeda L., Saitoh Y. Mirror visual feedback alleviates deafferentation pain, depending on qualitative aspects of the pain: a preliminary report. Rheumatology. 2008;47(7):1038–1043. doi: 10.1093/rheumatology/ken170. [DOI] [PubMed] [Google Scholar]
- Thieme H., Morkisch N., Rietz C., Dohle C., Borgetto B. The efficacy of movement representation techniques for treatment of limb pain--a systematic review and meta-analysis. J. Pain. 2016 Feb;17(2):167–180. doi: 10.1016/j.jpain.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Wareham A.P., Sparkes V. Effect of one session of mirror therapy on phantom limb pain and recognition of limb laterality in military traumatic lower limb amputees: a pilot study. J. R. Army Med. Corps. 2018 doi: 10.1136/jramc-2018-001001. Nov 14. [DOI] [PubMed] [Google Scholar]
- Weeks S.R., Tsao J.W. Incorporation of another person's limb into body image relieves phantom limb pain: a case study. Neurocase. 2010;16:461–465. doi: 10.1080/13554791003730592. [DOI] [PubMed] [Google Scholar]
- Zarei M., Johansen-Berg H., Smith S., Ciccarelli O., Thompson A.J., Matthews P.M. Functional anatomy of interhemispheric cortical connections in the human brain. J. Anat. 2006;209(3):311–320. doi: 10.1111/j.1469-7580.2006.00615.x. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material