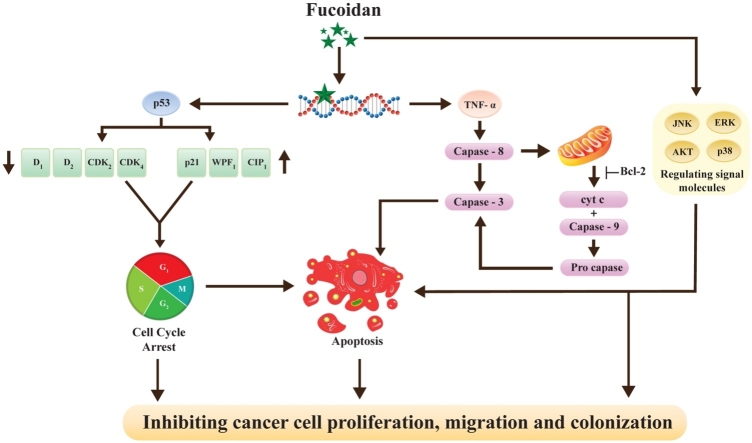
Graphical abstract
Keywords: Fucoidan, HepG2 cells, Cell cycle, Genetic damage, Apoptosis, Seaweed, Natural biopolymer
Highlights
-
•
The centre of the attraction of this article is inevitably associated with fucoidan polymers in terms of brown seaweed such as Turbinaria conoides.
-
•
Fucoidan in various cancer types exhibited by targeting apoptotic molecules and mitigate the toxicity of chemotherapeutic agents and radiation.
-
•
To treat deadly liver cancer by identifying bioactive compounds available in the dietary supplement that rekindles the direction of research against cancer diseases.
-
•
Fucoidan in a HepG2 cell line was studied with typical techniques such as cell viability, colony formation, cell migration, cell cycle progression, genetic damage and apoptosis along with their nuclear morphology and mitochondrial membrane potential.
-
•
This study concluded that the fucoidan contain brown seaweeds consumed as dietary supplement not predispose to liver cancer.
Abstract
The centre of the attraction of this article is inevitably associated with fucoidan polymers in terms of brown seaweed such as Turbinaria conoides. Fucoidan is a sulphated polysaccharide constitutes fucose as a major principle sugar along with other monosugars such as glucuronic acid, xylose and galactose. The core value of fucoidan in terms of various cancer types were substantially exhibited through targeting the key apoptotic molecules and subsequently mitigate the toxicity that are essentially included in the chemotherapeutic agents and radiation. The pragmatic investigation about the anti-cancer effect of fucoidan in a hepatoblastoma-derived (HepG2) cell line was thoroughly analyzed by the typical techniques such as cell viability, colony formation, cell migration, cell cycle progression, genetic damage and apoptosis along with their nuclear morphology and mitochondrial membrane potential. Following the analyzes, the cell viability was precisely evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. And hence, cell cycle arrest and apoptosis was appropriately examined staining with propidium iodide (PI) and annexin V-fluorescein isothiocyante (FITC) by flowcytometer, respectively. Primarily, genetic damage by fucoidan in HepG2 cell line was evaluated by following Trevigen’s comet assay kit. In addition, alteration of nuclear content and mitochondrial membrane potential were also detected with Hoechst and mitochondrial membrane potential dye (JC-1: 5,5′6,6′-tetrachloro-1,1′3,3′tetraethylbenzimi-dazolycarbocyanine iodide) by fluorescence microscopy, respectively. The results of the present study showed that cells constituted with fucoidan/quercetin standard at 50, 100 and 200 μg/ml exhibited cell viability about 71, 60 & 40/80, 65 & 45%, respectively. The above recorded effect of fucoidan was a concentration-dependant inhibition on the basis of decline in colony forming and cell migration potential of HepG2 cancer cells. Compared with untreated control, fucoidan consituted cells were significantly (p ≤ 0.05) accumulated proliferative cells in the G0/G1 phase of the cell cycle in a concentration dependent manner. Increasing concentration of fucoidan (50,100 and 200 μg/ml) was remarkably enhanced the DNA damage which reflected through tail moment value of 3.8, 7.1 & 12.8 folds with respect to the untreated control. Fucoidan induced total apoptotic cells were observed ∼20–40% at 50–200 μg/ml concentrations. The apoptotic cell formation effected by change in the nuclear content and mitochondrial membrane potential was confirmed in HepG2 cancer cells under fluorescence microscopy. It was eventually concluded that the fucoidan display promising anti-cancer activity against HepG2 cancer cells by promoting the inhibition of cell proliferation, migration and cell arrest on concentration dependent-manner that was well correlated with genetic damage and apoptosis.
1. Introduction
On the top of the serious array of hepatocellular carcinoma (HCC) ranks standstill among the most common cancers and causes massive death in the world population [1]. It is mainly due to lack of potential drugs as well as adverse side effects by available therapeutics. One of the new strategy to treat this deadly liver cancer [2,3] is identifying natural bioactive compounds available in the dietary supplement that rekindles the direction of research against cancer diseases [4].
Brown seaweeds are one of the major widespread groups of marine macroalgae that prevail vast resorts of novel and rich bioactive compounds in a large extend. More predominately, the major cell wall component of brown seaweeds such as polysaccharide, alginate and mainly included fucoidan which is so called sulfated polysaccharide with fucose as a major principle sugar along with other monosugar like glucuronic acid, xylose and galactose. In addition, brown seaweeds have contained other elements such as minerals, tannins, polyphenols, vitamins along with proteins, lipids and carotene pigments [[5], [6], [7], [8], [9]]. These sorts of brown seaweed are being broadly used in East Asian countries as a supplement in terms of functional foods, healthcare, medicinal foods and pharmaceuticals [9,10]. There are viable in-vitro studies demonstrated that the fucoidan act against various cancers including hepatocarcinoma and melanoma [[10], [11], [12]]. Furthermore, in-vitro and in-vivo studies that proved the fucoidan displaying wide range of biological activities such as anticoagulant, antithrombotic, antivirus, anti-inflammatory, antioxidant, anti-complementary, pro-survival mechanisms and immunomodulatory activities and so on [[13], [14], [15]]. And also, recent and recent past reports had substantiated the evidence relay on anticancer effects of fucoidan by activating through apoptosis, suppression of metastasis and angiogenesis in different cancer cell types [16,17] and quite interestingly the molecular mechanisms of actions have not been fully clarified in a greater extend [9]. However, anticancer properties of the fucoidan were meticulously documented in the following cancer cells such as lung, breast, liver, colon, prostate and bladder [18]. A study suggested that fucoidan supplement has been improved and promoted in the deep-seated area of the fecal microbiota composition and repaired intensively the intestinal barrier function that could probably be used as an intestinal flora modulator for preventing further cancer burgeoning [19]. While compared to medications in terms of food supplement, fucoidan can be utilized as an underlying complementary alternative therapeutics without being intolerable side effects for treating cancer [20,21].
Recent proven study in mainstream of MCF-7 breast cancer cells has been implied that the fucoidan can be a promising candidate for cancer therapy in combination of the cisplatin, doxorubicin and taxol [22]. Further, fucoidan induced apoptosis in PC-3 human prostate cancer cells has also been well documented [23]. Many studies demonstrated that the fucoidan has imperatively suppressed the cancer tumor and comprehensively enhanced the overall survival rate in cancer patients [24]. In the present pragmatic investigation aimed at the anticancer effect of fucoidan in a hepatoblastoma-derived (HepG2) cell line that was thoroughly analyzed by the typical techniques such as cell viability, colony formation, cell migration, cell cycle progression, genetic damage and apoptosis along with their nuclear morphology and mitochondrial membrane potential.
2. Materials and methods
2.1. Chemicals
Bioassay kits such as Trevigen’s comet assay kit, annexin V-FITC assay kit, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Hoechst (33,342) and 5,5′6,6′-tetrachloro-1,1′3,3′tetraethylbenzimi-dazolycarbocyanine iodide (JC-1) staining solution, fucoidan and propidium iodide (PI) were procured from Sigma-Aldrich. The RPMI-1640 medium, fetal bovine serum (FBS) and phosphate-buffered saline (PBS) were procured from Hi-Media (Mumbai, India). All the solvents and chemical were of analytical grade.
2.2. Cell viability assay
By the protocol of MTT assay was performed with MTT dye (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and whereby the MTT was converted into MTT-formazan in mitochondria of HepG2 cells. Based on the experimental process, the cells (1 × 105 cells/well) were seeded in 96-well plates and kept 6 h for adhering. Subsequently, the cells were constituted with fucoidan/quercetin standard at nuance of concentration such as 0, 50, 100 & 200 μg/ml (filtered by 0.2 μ Millipore filter) for 48 h. And then 100 μl of MTT dye (5 mg in 10 ml of serum free medium) was added into each well and kept at 5% CO2 incubator up to 4 h at 37◦C in the dark. Later on, the superficial media were removed and thereby precipitated formazan dissolved in 100 μl of 20% SDS (in 50% dimethyl formamide) and after construed in an ELISA reader at 540 nm [7]. The percentage of inhibition (I%) was calculated using the following equation: I% = (Control-Treated)/Control×100%.
2.3. Colony formation assay
Underlying clonogenic assay was performed to study about the effect of fucoidan in HepG2 cancer cell growth by following the method of Buranrat et al., [25]. The precise concentration of fucoidan (0–200 μg/ml) was added into 24 h grown HepG2 cancer cells in 6-well plates (500 cells/well). Fucoidan constituted cells were washed with PBS in terms of reassigned into fresh medium for impetus of cell growth another 24 h. Subsequently, the cells were washed thrice with PBS by disposal of old medium and fixed in 100% methanol that was stained with 0.05% crystal violet for 1 h at room temperature. After washing with tap water, the colonies were counted and images regarded by inverted microscope linked up with camera. The counted colony values were profoundly expressed in percentile.
2.4. Wound healing assay
This experiment was executed to study about the cell migration by the method of Buranrat et al., [25]. The HepG2 cells were allowed to grow in 6-well plates to form a cell monolayer. Subsequently, scratches were made in the monolayer cells through a sterile 0.2 ml pipette tip that was constituted with different concentration of fucoidan (0–200 μg/ml). After 48 h, the healed wound images were regarded by using the inverted microscope linked up with camera. Wound distance was calculated by comparing with untreated control cells and values were expressed in percentile.
2.5. Flowcytometric analysis of cell cycle and apoptosis
The HepG2 cells were seeded into 10 cm culture plates and exposure to nuance of fucoidan/quercetin concentration (0–200 μg/ml) for 48 h. Then, cells (1 × 106 cells/ml) were harvested and affixed in 70% ice-cold ethanol (v/v) and stored at -20 °C. Before the analyze, ethanol fixed cells were washed twice with PBS and added into 1 ml of PBS (PI 20 μg/ml, 0.1% Triton X-100 (v/v) & 0.1% RNase A) followed by 30 min incubation in the dark at 37 °C [25]. Then, the cells were analyzed by flowcytometer with ∼30,000 events and scattered as a percentage of cells in different stages of cell cycle such as G0/G1, S and G2/M phases using the Cell Quest acquisition software.
According to the manufacture’s protocol, fucoidan induced apoptosis in HepG2 cells were scored in the array of cells staining with annexin-V FITC through the flowcytometer. Similarly, the treatment and cells preparation were performed as like as cell cycle analysis. After washed cells twice with PBS there were added 500 μl of binding buffer (5 μl of annexin-V FITC & 5 μl of PI) and kept in the dark for 15 min at ambient temperature [26]. Subsequently, cells were analyzed by flowcytometer and expressed as a percentage of total apoptotic cells calculated by flowjo software.
2.6. Detection of nuclear condensation and mitochondrial membrane potential
Fucoidan altered nuclear condensation in HepG2 cells were performed by using Hoechst staining assay. The assay was conducted with maximum concentration of fucoidan (200 μg/ml) and kept incubation for 48 h. Then the cells were harvested and thoroughly washed with PBS and firmly fixed in 3:1 ratio of methanol and acetic acid (3:1) for 15 min at room temperature. Before the staining, fixatives were washed away carefully with PBS and stained in 5 μg/ml of Hoechst 33,342 stain for 10 min. By the condensed nuclear morphology of HepG2 cells as a result of fucoidan treatment was regarded and simultaneously imaged using the fluorescence microscope [27].
Similarly, mitochondrial membrane potential (ΔΨm) of HepG2 cells consituted with maximum concentration of fucoidan (200 μg/ml) was also studied by using JC-1 staining assay. The fucoidan (200 μg/ml) constituted HepG2 cells were added with 5 μl of the JC-1 staining solution and incubated in 5% CO2 incubator at 37 °C for 20 min. The stained cells washed twice with PBS solution were regarded and simultaneously imaged using the fluorescence microscope [27].
2.7. Comet assay
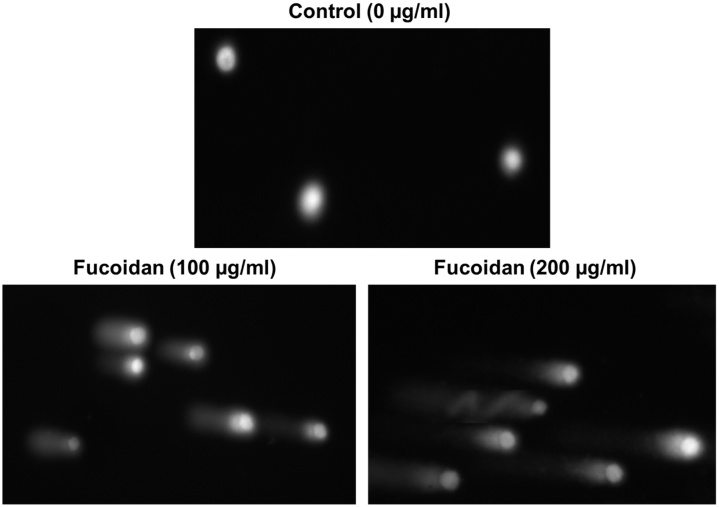
Single cell gel electrophoresis (SCGE) was carried out by Trevigen’s comet assay kit with slight modification. Briefly, HepG2 cell suspension (1 × 105/ml) and molten LM agarose (at 37°C) were prepared in 1:10 (v/v) ratio. From there, instantly 50 μl was poured onto the comet slide pre-coated with 1% normal melting point agarose. Then, the slides were kept at 4°C in the dark for 10 min under low humidity and dust-free environment for their better agarose adhesion on the slides. The slides were placed in an ice-cold lysing solution for 60 min at 4°C. Furthermore, the excess buffer of slides was drained and deeply immersed in freshly prepared alkaline unwinding solution (pH>13: 200 mM NaOH, 1 mM EDTA in 50 ml of dH2O) for 1 h at 4°C in the dark. Subsequently, the slides were placed in an electrophoresis chamber filled with alkaline buffer (200 mM NaOH, 1 mM EDTA in 1 l of dH2O) and it runs with 21 V for 30 min., Excess electrophoresis solution from the slides was drained gently and then a couple time immersed in dH2O and 70% ethanol for each 5 min. Eventually, slides were dried for 10–15 minutes at room temperature and stored with desiccant prior to scoring slides. Silver staining was performed to visualize the comets through transmission light microscope. Randomly, 50 comets per slide were visualized and analyzed by scoring of various parameters such as head, tail and tail moment under light microscope with comet assay software.
2.8. Statistical analysis
The results were elicited with triplicate value and expressed as a mean ± SD. All the data were analyzed statistically by One-way ANOVA and student t-test using SPSS software student’s version-16. A p value <0.05 was considered statistically significant.
3. Results
3.1. Effect of fucoidan on cell viability assay
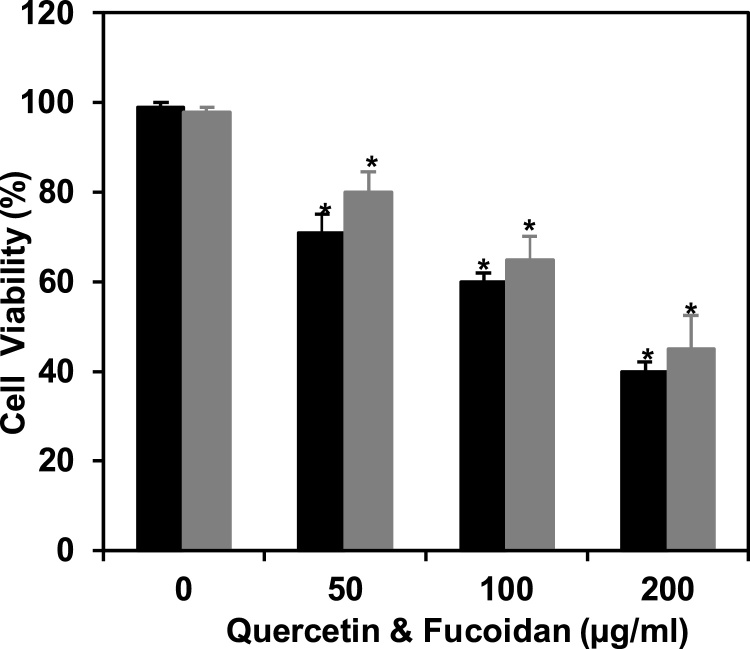
Cell viability by MTT assay was evaluated in HepG2 cancer cells constituted with fucoidan/quercetin at different concentrations (0–200 μg/ml) for 48 h (Fig. 1). The pragmatic results revealed up to 98% cell viability in HepG2 cells cultured without fucoidan/quercetin whereas fucoidan/quercetin at 50, 100 and 200 μg/ml constituted cell viability was about 71, 60 & 40/80, 65 & 45%, respectively. Outcome of the results proved that the fucoidan/quercetin treatment was reduced the cell viability significantly (p ≤ 0.05) as below as 50% and the decline rate was significant in a concentration dependent manner. However, the effect was more pronounced by fucoidan when compared to the quercetin standard (Fig. 1).
Fig. 1.
Inhibition of cell viability in HepG2 cancer cells by fucoidan constituted with nuance of concentration for 48 h. The values are presented as mean ± SD in triplicate and significance (p ≤ 0.05) determined by student t-test between fucoidan treated vs untreated control. Dark bar indicate quercetin and light bar fucoidan.
3.2. Effect of fucoidan on colony formation assay and wound healing assay
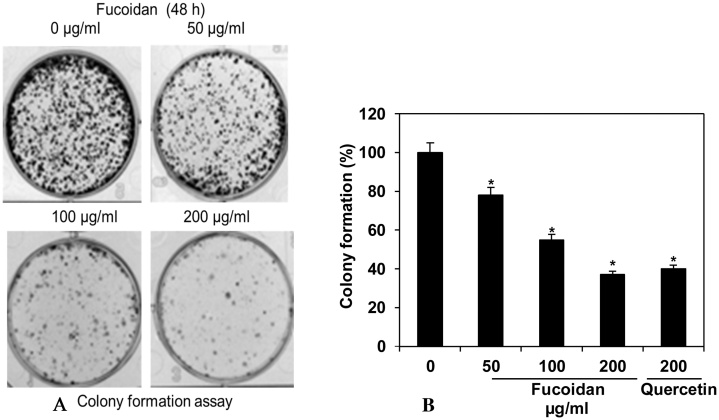
The clonogenic effect of fucoidan in HepG2 cancer cells was examined using colony formation assay. The HepG2 cancer cells were constituted fucoidan with nuance of concentrations (0–200 μg/ml) and maximum concentration of quercetin standard (200 μg/ml). As the response of fucoidan towards colony forming potential of HepG2 cancer cells was found to be 78, 55 & 37% at 200, 100 & 50 μg/ml compared with untreated control (100%), respectively (Fig. 2). Besides, the inhibition effect of fucoidan on colony formation was observed to be more or less equal (37%) to the standard of quercetin (40%) at their maximum concentration (200 μg/ml). Thus, the result clearly revealed that fucoidan has significant (p ≤ 0.05) anti-proliferative effect against HepG2 cancer cells.
Fig. 2.
Fucoidan on clonogenic effect of HepG2 cancer cells was analyzed by colony formation assay. (A) Colony formation image was captured under inverted light microscope. (B) Colony cells calculated and expressed as the percentages of means ± standard deviation of three independent experiments. *P ≤0.05, vs. the untreated control (0 μg/ml).
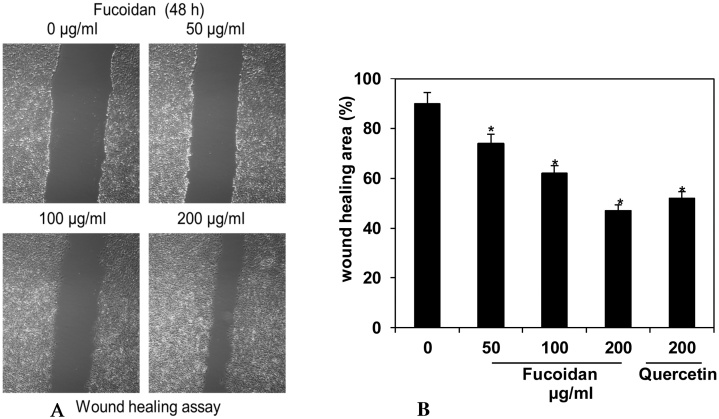
Cell migration is one of the essential processes during the cancer cell development and metastatic spread. The suppressive effect of fucoidan on HepG2 cancer cell migration was determined by wound healing assay. The HepG2 cell migration was estimated with or without fucoidan at different concentrations (50, 100 & 200 μg/ml). Consequently, there was a gradual suppression of HepG2 cell migration observed about 74, 62, and 47% when constituted fucoidan with nuance of concentration 50, 100 & 200 μg/ml compared with untreated control (90%), respectively. However, the effect of fucoidan on HepG2 cell migration suppression was recorded to be concentration-dependent and significantly (p ≤ 0.05) higher (47%) than that of quercetin standard (57%).
3.3. Fucoidan induces G0/G1 arrest in HepG2 cells
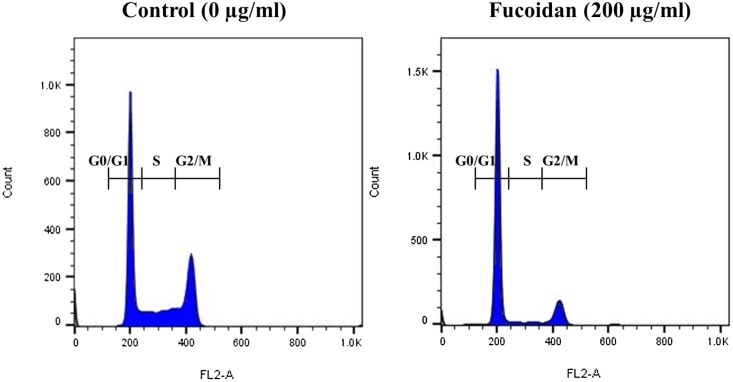
The cell cycle phase distribution of exponentially proliferative HepG2 cancer cells was examined with PI staining by flowcytometer. Histogram of the cell cycle phase illustrated that the HepG2 cancer cells were categorized into G0/G1, S, and G2/M phases (Fig. 4). Compared with untreated control, fucoidan constituted cells had significant (p ≤ 0.05) accumulation of proliferative cells in the G0/G1 phase of the cell cycle in a concentration dependent manner and also significantly (p ≤ 0.05) decreased cells in the S phase of the cell cycle (Table 2). The accumulated proliferative cells in the G0/G1 phase of cell cycle were regarded about 15–35% (50–200 μg/ml) higher than that of untreated control cells (50%) and comparable to the quercetin (87%) at their maximum concentrations (200 μg/ml). Thus, fucoidan also reduced cell population in the G2/M and S phases of cell cycle were about ∼3-4 times lesser than that of untreated control cells (29.4-20.6%). These results substantiated that the fucoidan was appropriately induced HepG2 cancer cell arrest in the G0/G1 phase of the cell cycle.
Fig. 4.
Cell cycle phase distribution of exponentially proliferative HepG2 cancer cells and G1 phase of the cell cycle arrest induced by fucoidan constituted with nuance of concentration for 48 h. Histogram image regarded after cells stained with PI by flowcytometer.
Table 2.
Percentage of genetic damage induced by fucoidan in HepG2 cancer cells.
| Concentration (μg/ml) | Genetic damage (%) |
||
|---|---|---|---|
| Head | Tail | Tail moment | |
| Fucoidan 0 | 96.00 ± 1.00 | 4.00 ± 1.00 | 0.21 ± 0.04 |
| Fucoidan 50 | 89.00 ± 6.56 | 11.00 ± 6.56 | 0.80 ± 0.12 |
| 0.93 | 2.8 | 3.8 | |
| Fucodian 100 | 77.33 ± 7.51 | 22.67 ± 7.51 | 1.49 ± 0.25 |
| 0.81 | 5.7 | 7.1 | |
| Fucoidan 200 | 55.00 ± 6.08 | 45.00 ± 6.08 | 2.68 ± 0.35 |
| 0.57 | 11.25 | 12.76 | |
| Quercetin 200 | 57.00 ± 7.94 | 43.00 ± 7.94 | 2.61 ± 0.43 |
| 0.59 | 10.75 | 12.43 | |
The values are presented as mean ± SD in triplicate and significance (p ≤ 0.05) determined by student t-test between fucoidan treated vs. untreated control.
3.4. Fucoidan induces genetic damage and apoptosis in HepG2 cells
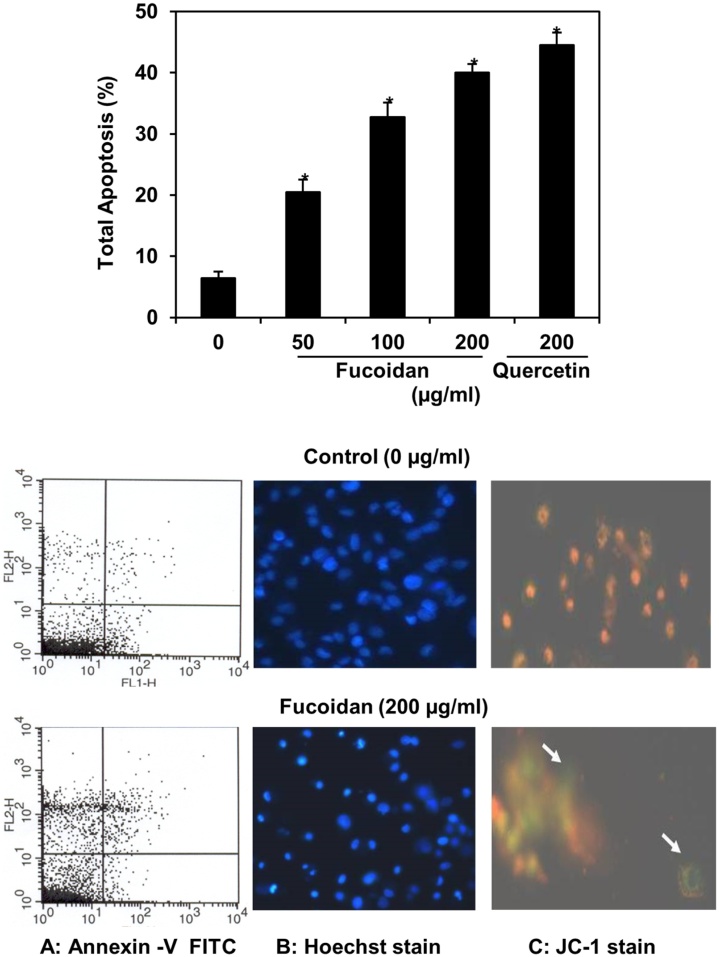
The fucoidan induced DNA damage in HepG2 cells was studied with comet assay kit by single gel electrophoresis (Fig. 5). The occurrence of genetic damage was scoured in terms of various parameters like head, tail and tail moment by comet assay software (Table 1). There was no significant effect on DNA damage in untreated HepG2 cancer cells whereas fucoidan constituted with nuance of concentration (50–200 μg/ml) was observed to be significantly promoted the DNA damage. Hence, the amount of head damage was regarded to be decreased in the range of 0.93-0.59 besides tail amount damage increased in the range of 2.8–11.25 while increasing the concentrations of fucoidan when compared to the untreated control cells. Thus, fucoidan significantly enhanced the tail moment damage was directly reflected in terms of DNA damage occurred in HepG2 cells. The enhanced tail moment value was about 3.8, 7.1 & 12.8 folds higher than that of untreated control cells. The effect of fucoidan on genetic damage in HepG2 cancer cells was recorded as like as quercetin standard. On the other hand, the effect of fucoidan on apoptotic characteristics of the HepG2 cancer cells was examined as fucoidan induced apoptosis in fucoidan constituted HepG2 cancer cells through annexin V-FITC assay kit analyzed in four groups of cells by flowcytometer (Fig. 6A).The total apoptotic cells including the addition of early and late apoptosis were observed after 48 h of the fucoidan treatment. Total apoptotic cells recorded were about 20–40% at 50–200 μg/ml of fucoidan concentration. Further, fucoidan was significantly (p ≤ 0.05) enhanced the total apoptotic cells (40%) better than the standard of quercetin (44%) at their maximum concentration (200 μg/ml). These results indicated that the inhibition and arrest of HepG2 cancer cell proliferation by fucoidan was well correlated with their DNA damage and apoptosis induction. In addition, fucoidan induced DNA damage and apoptosis was further confirmed in terms of change in the morphology of HepG2 cancer cell by the effect of fucoidan on nuclear condensation and shift of mitochondrial membrane potential (ΔΨm) that were detected with Hoechst and JC-1 stains, respectively by fluorescence microscopy (Fig. 6B & C).
Fig. 5.
Genetic damage induction by fucoidan in HepG2 cancer cells detected by comet assay.
Table 1.
Percentage of cell cycle arrest induced by fucoidan in HepG2 cancer cells.
| Concentration (μg/ml) | Cell cycle arrest (%) |
||
|---|---|---|---|
| G0/G1 | S | G2/M | |
| Fucoidan 0 | 50 ± 4.19 | 20.6 ± 2.08 | 29.4 ± 2.07 |
| Fucoidan 50 | 65.3 ± 5.15 | 14.3 ± 3.52 | 20.4 ± 3.00 |
| Fucoidan 100 | 72.6 ± 3.20 | 10.2 ± 1.20 | 17.2 ± 1.44 |
| Fucoidan 200 | 85.3 ± 3.72 | 05.3 ± 1.05 | 09.4 ± 1.91 |
| Quercetin 200 | 87.0 ± 4.06 | 03.7 ± 1.61 | 09.3 ± 1.86 |
The values are presented as mean ± SD in triplicate and significance (p ≤ 0.05) determined by student t-test between fucoidan treated vs. untreated control.
Fig. 6.
Fucoidan induced apoptosis in HepG2 cancer cells (constituted with different concentration of fucoidan for 48 h and staining with annexin-V FITC/PI. The values are presented as mean ± SD in triplicate and significance (p ≤0.05) determined by student t-test between fucoidan treated vs untreated control. A. Each quadrant indicates the percentage of normal cells (annexin V-/PI-), early apoptosis (annexin V+/PI-) and late apoptosis (annexin V+/PI+). B & C. Fucoidan (200 μg/mL) altered nuclear morphology by Hoechst 33,342 stains and mitochondrial membrane potential (ΔΨm) by JC-1stains were observed under fluorescence microscopy (original magnification, 400x). Red fluorescence indicates mitochondria with intact membrane potential and green fluorescence indicates de-energized mitochondria).
4. Discussion
4.1. Anti-cancer effect of fucoidan against HepG2 cancer cell line
In general, mutated gene causes cancer by promoting uncontrolled cell growth and cell division through violating normal cellular function, mainly cell cycle arrest and programmed cell death as well. If not inhibited/restrain the cancer cell growth, the cell has got accelerated the angiogenesis as well as stimulates the metastasis. In fact, dietary plants are encompassed in terms of medical values that can serve as invaluable source for anticancer drug development. Moreover, the anti-cancer drugs available in the dietary supplement are quite safe and effective regulation of normal cell metabolism and also accelerating the programmed cell death by interrupting the uncontrolled cell proliferation [28]. It is well-know that the brown seaweeds are being used as medicinal dietary supplements in most of the Asian countries. All the dietary brown seaweeds have fucoidan, a sulphated polysaccharide which is substantially prevalent in various biological properties such as antioxidant, anticancer, anticoagulant, immunomodulatory, immune-response and anti-inflammatory [29,12]. Such as other brown seaweed, Turbinaria conoides has broadly used as an edible seaweed and also included with fucoidan displaying in different sorts of biological activities that were massively demonstrated under in-vitro/in-vivo [30,7]. In the present pragmatic investigation was carried out in terms of anticancer effect of fucoidan in HepG2 cancer cell line by analyzing cell proliferation, colony formation, cell migration, cell cycle progression, genetic damage and apoptotic cell death. Fucoidan induced genetic damage and apoptosis was further confirmed by visualizing the cell morphological changes by Hoechst staining and alteration of mitochondrial membrane potential by JC-1 staining. The results of cell viability clearly demonstrated that only ≤ 50% cells were found to be viable when HepG2 cells constituted with fucoidan and the effect was merely relay on concentration dependent. As a result, the fucoidan was significantly (p ≤ 0.05) reduced the proliferation of the HepG2 cancer cells and the reduction potential was as comparable as quercetin standard. Similarly, in the recent in-vitro studies demonstrated that fucoidan from T. conoides explored dose-dependent anti-proliferation in EJ and HepG2 cancer cells [29,7].
Essentially, colony formation assay was conducted further to acquire the anti-cancer effect of fucoidan based on the proliferative potential and prolong viability of HepG2 cancer cells. It was observed that fucoidan caused a concentration-dependent decline in the colony forming ability of HepG2 cancer cells and found more effective than that of quercetin standard. Decline of colony formation ability by various concentration of fucoidan was exactly about 1.3, 1.8 and 2.7 fold higher than that of untreated control (100%; Fig. 2). This result clearly divulged that fucoidan possess significant (p ≤ 0.05) anti-proliferative effect against HepG2 cancer cells. Indeed, cell migration is one the essential processes during the cancer cell development and metastatic spread [31]. In this study, percentage of cell migration was suppressed in response to an increase in fucoidan concentration (50, 100, 200 μg/ml) and the suppressive effect was about 1.2, 1.5 and 1.9 fold over the untreated control (90%), respectively (Fig. 3). The wound healing effect of fucoidan was also exhibited in a concentration-dependent manner and significantly (p ≤ 0.05) higher than that of quercetin standard (57%) (Fig. 3). In-vitro anticancer effect of fucoidan on the colorectal carcinoma cells, DLD-1 and HCT-116 was reported to be inhibited the colony formation of cancer cells at the concentration of 200 μg/ml and found no cytotoxicity up to 400 μg/ml [32]. Recent studies revealed that most of the marine plant derived anticancer drugs including fucoidan acted in dose-dependent decline of colony formation ability and cell migration in HepG2 cancer cells. In the present study was totally obsessed with similar result, regarding the effect of fucoidan on colony formation and cell migration assays as reported elsewhere [33,28,31]. This dietary brown seaweed was eventually proven and encompassed with active compounds against tumor cells [34].
Fig. 3.
Fucoidan inhibited HepG2 cancer cell migration was analyzed by wound healing assay. (A) Wound healing image was captured under inverted light microscope. (B) Wound healing calculated and expressed as the percentages of means ± standard deviation of three independent experiments. *p ≤ 0.05, vs. the untreated control (0 μg/ml).
4.2. Fucoidan act as anticancer drug by arresting cell cycle of HepG2 cancer cells
It is well established that the cancer cells have ability to go for continuous proliferation which is essentially associated with the deregulation of the cell cycle progress and promotion of invasion [31]. Fucoidan constituted HepG2 cancer cells that were showed significant (p ≤ 0.05) accumulation of proliferative cells in the G0/G1 phase of the cell cycle in a concentration dependent manner. The observed cell accumulation in the G0/G1 phase of cell cycle was about 65–85% (50–200 μg/ml) over the untreated control cells (50%) and comparable to the quercetin standard (87%) (Fig. 4). Moreover, fucoidan significantly arrested the EJ cell in G1 phase of the cell cycle with concentration dependent manner and stated that growth inhibitory effect of fucoidan in EJ cells was reflected in terms of arresting the G1 phase of the cell cycle [28]. In previous study depicted that the ethyl acetate fraction of brown seaweed, T. conoides arrested the G0/G1 phase of cell cycle about 1.3–1.7 fold higher than the untreated control cells [7]. Most of reports also highlighted the similar sort of results that were depicted the functional role of fucoidan in cell cycle arrest at G1 phase. It was consistently defined due to the down regulation of cyclin (D1, D2, CDK2 & CDK4) and up regulation of p21/WAF1/CIP1 in various cancer cells. In addition, increased level of CDK inhibitors like p21 and phosphorylation inhibition played an imperative role in G1 arrest however the cell cycle arrested by fucoidan differ from some extent based on the type of cancer cells [18,24].
4.3. Understanding the anti-cancer effect of fucoidan through apoptosis induction, genetic damage and other associated characters in HepG2 cancer cells
One or other way of the cancer cell initiation and continuous progression are due to alteration in apoptotic pathway and predominantly in hepatocarcinogenesis through dysregulation of apoptosis [35]. Therefore, it is essential to inhibit/restrain cancer cells development by adapting naturally occurring dietary plants which have enormous potential to induce apoptosis in cancer cells [36]. In the present pragmatic study with respect to fucoidan induced genetic damage and apoptosis in HepG2 cancer cells were analyzed with comet assay and annexin V-FITC assay kit, respectively. Genetic damage was scoured in terms of various factor such as head, tail and tail moment by comet assay software whereas apoptosis analyzed in four groups of cells by flowcytometer (Fig. 5, Fig. 6&A). The fucoidan induced genetic damage and apoptosis in HepG2 cancer cells were regarded to be 45–50% when compared to the untreated control cells (Table 2 & Fig. 6). The degree of genetics damage enhanced by fucoidan was reflected in terms of tail moment that were revealed about 12.8, 7.1 & 3.8 folds higher than that of untreated control cells (Table 2). On other hand, fucoidan enhanced total apoptotic cells were statistically (p ≤ 0.05) significant and effectively boosted than the standard of quercetin at their maximum concentration (44%). These results concluded that the inhibition of HepG2 cancer cell proliferation and cell arrest by the effect of fucoidan can be significantly well correlated with their genetic damage and apoptosis. Eventually, genetic damage played major role on cell death/senescence that can either be dependent or independent of the immune system [37]. However, the natural link between early tumorigenic events and the induction of the p53-mediated checkpoints that were constituted a barrier to tumour progression [38]. In the recent study was stated about 14–42% of apoptosis by altering nuclear condensation and also by mitochondrial membrane potential (ΔΨm) in HepG2 cancer cells constituted with fucoidan of T.conoides [7]. Other reports on different cancers such as hematopoietic, lung, breast and colon have clearly exhibited that fucoidan induced the apoptosis through cytoplasmic shrinkage and chromatin condensation [18]. Another report also proved regarding fucoidan induced apoptosis through activation of caspase-cascade path and regulation of signaling molecules such as c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), p38 signaling, Bcl-2 protein expression and Akt signaling. Further, fucoidan provoked apoptosis by shifting mitochondrial functions and accumulated dead cells/apoptotic cells in the G0/G1 phase of the cell cycle [24,18]. Apart, anticancer potential of fucoidan was explored by the increase of immune response signaling molecules like interleukins (IL-2, IL-12) and interferon gamma (IFN-γ) [12]. Thus, immune-therapy potential of fucoidan was proven through intruding cells proliferation by increasing anti-inflammatory (IL-2, IL-4, and IL-10) and reducing pro-inflammatory (IL-6 and TNF-α) cytokines in gastric and mammary cancers [39,40]. In addition, fucoidan exhibited immune therapy was takes place through the programmed cell death activated by 1/PDL1 signaling pathway [41]. In the present study explored that the apoptotic cell morphology altered by fucoidan in terms of nuclear condensation was further confirmed by Hoechst 33,342 staining (Fig.6. B). It was clearly illustrated the differences in HepG2 cancer cells constituted with fucoidan (bright chromatin condensation and nuclear fragmentation) than that of untreated one (weak homogeneous blue). Similarly, altered mitochondrial membrane potential as result of fucoidan treatment was examined with JC-1 staining and it was also evidenced further for the fucoidan induced apoptosis in HepG2 cancer cells (Fig. 6C). Hence, the alteration in terms of significant increase in the green fluorescence intensity occurred due to oxidative stress and subsequent mitochondrial damage [42,43]
5. Conclusion
In the present study clearly shows ∼50% of HepG2 cancer cell proliferation was inhibited by the fucoidan and the effect was accordingly regarded by way of concentration. Based on the previous and present studies, the fucoidan apparently promoted cells accumulation in the G1 phase of the cell cycle. In addition, fucoidan inhibited cell proliferation, colony formation and cell migration were significantly well associated with G1 phase of the cell cycle arrest and apoptosis induction. On other hand, the quantified genetic damage induced by fucoidan was significantly correlated with apoptosis as well. The fluorescence microscopy study was eventually proved further that fucoidan promoted the apoptotic cell formation in HepG2 cancer cells in terms of change in the nuclear content and mitochondrial membrane potential. The recent systematic and typical reports clearly supported for the present study that the effect of combinatory mycotoxin (Aflatoxin and Sterigmatocystin) on HepG2 cells were regard as like as fucoidan in terms of cytotoxicity and apoptosis associated endpoints, cell cycle arrest, mitochondria integrity, and apoptosis related proteins such as caspase-3 and p53 [44]. In the present pragmatic study concluded that the fucoidan contain brown seaweeds consumed as dietary supplement not predispose to liver cancer however further in-vivo investigation would certainly strengthen this results.
Transparency document
Declaration of Competing Interest
There was no competing interest.
Acknowledgment
This contributed research work was supported by University Grant Commission (UGC), Government of India, New Delhi (Ref. No. TAM-8496).
References
- 1.Jemal F., Bray J., Ferlay E.Ward, Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kurahashi N., Inoue M., Iwasaki M., Tanaka Y., Mizokami M., Tsugane S. Vegetable, fruit and antioxidant nutrient consumption and subsequent risk of hepatocellular carcinoma: a prospective cohort study in Japan. Br. J. Cancer Suppl. 2009;100:181–184. doi: 10.1038/sj.bjc.6604843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharati S., Rishi P., Koul A. Azadirachta indica exhibits chemopreventive action against hepatic cancer: studies on associated histopathological and ultrastructural changes. Microsc. Res. Tech. 2012;75:586–595. doi: 10.1002/jemt.21095. [DOI] [PubMed] [Google Scholar]
- 4.Gupta P., Bansal M.P., Koul A. Spectroscopic characterization of lycopene extract from Lycopersicum esculentum (Tomato) and its evaluation as a chemopreventive agent against experimental hepatocarcinogenesis in mice. Phytother. Res. 2013;27:448–456. doi: 10.1002/ptr.4741. [DOI] [PubMed] [Google Scholar]
- 5.Eluvakkal T., Sivakumar S.R., Arunkumar K. Fucoidan in some Indian brown seaweeds found along the coast of Gulf of mannar. Int. J. Bot. 2010;6:176–181. [Google Scholar]
- 6.Moghadamtousi S.Z., Karimian H., Khanabdali R., Razavi M., Firoozinia M., Zandi K., Kadir H.A. Anticancer and antitumor potential of fucoidan and fucoxanthin, two main metabolites isolated from brown algae. Sci. World J. 2014;2:1–10. doi: 10.1155/2014/768323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arumugama P., Kavipriya R., Murugan M., Ramar M., Kamalakannan S., Murugan K. Antibacterial, antioxidant and anticancer properties of Turbinaria conoides (J. Agardh) Kuetz. Clin. Phytosci. 2017;3:1–10. [Google Scholar]
- 8.Ale M.T., Mikkelsen J.D., Meyer A.S. Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs. 2011;9:2106–2130. doi: 10.3390/md9102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park H.Y., Kim G.-Y., Moon S.-K., Kim W.J., Yoo Y.H., Choi Y.H. Fucoidan inhibits the proliferation of human urinary bladder cancer T24 cells by blocking cell cycle progression and inducing apoptosis. Molecules. 2014;19:5981–5998. doi: 10.3390/molecules19055981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z., Teruya, Yoshida T., Eto H., Shirahata S. Fucoidan extract enhances the anti-cancer activity of chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer cells. Mar. Drugs. 2013;11:81–98. doi: 10.3390/md11010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagamine T., Hayakawa K., Kusakabe T., Takada H., Nakazato K., Hisanaga E., Iha M. Inhibitory effect of fucoidan on Huh7 hepatoma cells through down regulation of CXCL12. Nutr. Cancer. 2009;6:340–347. doi: 10.1080/01635580802567133. [DOI] [PubMed] [Google Scholar]
- 12.Ale M.T., Maruyama H., Tamauchi H., Mikkelsen J.D., Meyer A.S. Fucose containing sulfated polysaccharides from brown seaweeds inhibit proliferation of melanoma cells and induce apoptosis by activation of caspase-3 in-vitro. Mar. Drugs. 2011;9:2605–2621. doi: 10.3390/md9122605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitton J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs. 2011;9:1731–1760. doi: 10.3390/md9101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W., Wang S.X., Guan H.S. The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar. Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kan J., Hood M., Burns C., Scholten J., Chuang F., Tian F., Pan X., Du J., Gui M. A novel combination of wheat peptides and fucoidan attenuates ethanol-induced gastric mucosal damage through anti-oxidant, anti-inflammatory, and pro-survival mechanisms. Nutrients. 2017;9:978. doi: 10.3390/nu9090978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyung J., Kim D., Park D., Yang Y.H., Choi E.K., Lee S.P., Kim T.S., Lee Y.B., Kim Y.B. Synergistic anti-inflammatory effects of Laminaria japonica fucoidan and Cistanche tubulosa extract. Lab. Anim. Res. 2012;28:91–97. doi: 10.5625/lar.2012.28.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senthilkumar K., Manivasagan P., Venkatesan J., Kim S.K. Brown seaweed fucoidan: biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013;60:366–374. doi: 10.1016/j.ijbiomac.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Atashrazm F., Lowenthal R.M., Woods G.M., Holloway A.F., Dickinson J.L. Fucoidan and cancer: a multifunctional molecule with anti-tumor potential. Mar. Drugs. 2015;13:2327–2346. doi: 10.3390/md13042327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue M., Ji X., Liang H., Liu Y., Wang B., Sun L., Li W. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food Funct. 2018;9:1214–1223. doi: 10.1039/c7fo01677h. [DOI] [PubMed] [Google Scholar]
- 20.Ohnogi H., Nakade Y., Takimoto Y., Sekiya A., Kawashima T., Schneider A., Arai T., Uebaba K., Suzuki N. Safety of fucoidan from Gagome kombu (Kjellmaniella crassifolia) in healthy adult volunteers. Japanese J. Complement. Altern. Med. 2011;8:45–53. [Google Scholar]
- 21.Suzuki N., Uebaba K., Song H., Takimoto Y., Suzuki R., Kawabata T., Xu F.H., Ohnogi H., Nakai M. The safety of long-term ingestion of Fucoidan from Gagome kombu (Kjellmaniella Crassifolia) on cancer patients. Japanese J. Complement. Altern. Med. 2013;10:17–24. [Google Scholar]
- 22.Abudabbus A., Badmus J.A., Shalaweh S., Bauer R., Hiss D. Effects of fucoidan and chemotherapeutic agent combinations on malignant and non-malignant Breast cell lines. Curr. Pharm. Biotechnol. 2017;18:748–757. doi: 10.2174/1389201018666171115115112. [DOI] [PubMed] [Google Scholar]
- 23.Rui X., Pan H.-F., Shao S.-L., Xu X.-M. Anti-tumor and anti-angiogenic effects of fucoidan on prostate cancer: possible JAKSTAT3 pathway. Complement. Altern. Med. 2017;17:378. doi: 10.1186/s12906-017-1885-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C., Wang C., Tang S., Sun Y., Zhao D., Zhang S., Deng S., Zhou Y., Xiao X. TNFR1/TNF-α and mitochondria interrelated signaling pathway mediates quinocetone-induced apoptosis in HepG2 cells. Food Chem. Toxicol. 2013;62:825–838. doi: 10.1016/j.fct.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Buranrat L., Senggunprai A., Prawan V., Kukongviriyapan Simvastatin and atorvastatin as inhibitors of proliferation and inducers of apoptosis in human cholangiocarcinoma cells. Life Sci. 2016;153:41–49. doi: 10.1016/j.lfs.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Darzynkiewicz Z., Bedner E., Smolewski P. Flowcytometry in analysis of cell cycle and apoptosis. Sem. Hematol. 2001;38:179–193. doi: 10.1016/s0037-1963(01)90051-4. [DOI] [PubMed] [Google Scholar]
- 27.Liu T.Y., Tan Z.J., Jiang L., Gu J.F., Wu X.S., Cao Y. Curcumin induces apoptosis in gallbladder carcinoma cell line GBC-SD cells. Cancer Cell Int. 2013;13:64. doi: 10.1186/1475-2867-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buranrat B., Mairuae N., Kanchanarach W. Cytotoxic and antimigratory effects of Cratoxy formosum extract against HepG2 liver cancer cells. Biomed. Rep. 2017;6:441–448. doi: 10.3892/br.2017.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park H.Y., Choi I.I.-W., Kim G.-Y., Kim B.W., Kim W.-J., Choi Y.H. Fucoidan induces G1 arrest of the cell cycle in EJ human bladder cancer cells through down-regulation of pRB phosphorylation. Revista Brasileira de Farmacognosia. 2015;25:246–251. [Google Scholar]
- 30.Arumugam P., Murugan M., Ramar M., Murugan K. In-vivo evaluation of antigenotoxic and anti-inflammatory potential of Turbinaria conoides (J. Agardh) Kuetz. Int. J. Drug Devel. Res. 2016;8:010–013. [Google Scholar]
- 31.Lu H., Li X., Zhang J., Shi H., Zhu X., He X. Effects of cordycepin on HepG2 and EA.hy926 cells: potential anti-proliferative, anti-metastatic and anti‑angiogenic effects on hepatocellular carcinoma. Oncol. Lett. 2014;7:1556–1562. doi: 10.3892/ol.2014.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usoltseva R.V., Anastyuk S.D., Ishina I.A., Isakov V.V., Zvyagintseva T.N., Thinh P.D., Zadorozhny P.A., Dmitrenok P.S., Ermakova S.P. Structural characteristics and anticancer activity in-vitro of fucoidan from brown alga Padina boryana. Carbohydr. Polym. 2018;184:260–268. doi: 10.1016/j.carbpol.2017.12.071. [DOI] [PubMed] [Google Scholar]
- 33.Han Y.-S., Lee J.H., Lee S.H. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol. Med. Rep. 2015;12:3446–3452. doi: 10.3892/mmr.2015.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gambato Gabriela, Baroni Érico G., Garcia Charlene S.C., Frassini Rafaele, Frozza Caroline O.S., Moura Sidnei, Pereira Cláudio M.P., Fujii Mutue T., Colepicolo Pio, Lambert Ana Paula F., Henriques João A.P., Roesch-Ely Mariana. Brown algae Himantothallus grandifolius (Desmarestiales, Phaeophyceae) suppresses proliferation and promotes apoptosis-mediated cell death in tumor cells. Adv. Bio. Chem. 2014;4:98–108. [Google Scholar]
- 35.Fabregat Dysregulation of apoptosis in hepatocellular carcinoma cells. World Gastroenterol. J. 2009;15:513–520. doi: 10.3748/wjg.15.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan N., Adhami V.M., Mukhtar H. Apoptosis by dietary agents for prevention and treatment of prostate cancer. Endocr. Relat. Cancer. 2010;17:39–52. doi: 10.1677/ERC-09-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang Q., Xu T.D., Martin M.Z., Li M., Demaria L., Aron T., Lu B.A., Yankner J., Campisi S.J., Elledge The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349 doi: 10.1126/science.aaa5612. aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meek D.W. Tumour suppression by p53: a role for the DNA damage response? Nat. Rev. Cancer. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 39.Han M., Sun P., Li Y., Wu G., Nie J. Structural characterization of a polysaccharide from Sargassum henslowianum and its immunomodulatory effect on gastric cancer rat. Int. J. Biol. Macromol. 2018;108:1120–1127. doi: 10.1016/j.ijbiomac.2017.12.109. [DOI] [PubMed] [Google Scholar]
- 40.Vetvicka V., Vetvickova J. Fucoidans stimulate immune reaction and suppress cancer growth. Anticancer Res. 2017;37:6041–6046. doi: 10.21873/anticanres.12051. [DOI] [PubMed] [Google Scholar]
- 41.Xue M., Liang H., Tang Q., Xue C., He X., Zhang L., Liang Z., Zhang Z., Zhang K., Bian L., Li Z. The protective and immunomodulatory effects of fucoidan against 7,12-dimethyl benz[a]anthracene-induced experimental mammary carcinogenesis through the PD1/PDL1 signaling pathway in rats. Nutr. Cancer. 2017;69:1234–1244. doi: 10.1080/01635581.2017.1362446. [DOI] [PubMed] [Google Scholar]
- 42.Qiu L., Liu M., Pan K. Triple staining method for accurate cell cycle analysis using aultiparameter flowcytometry. Molecules. 2013;18:15412–15421. doi: 10.3390/molecules181215412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das J., Sarkar A., Parames C., Sil Hexavalent chromium induces apoptosis in human liver (HepG2) cells via redox imbalance. Toxicol. Rep. 2015;2:600–608. doi: 10.1016/j.toxrep.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y., Du M., Zhang G. Proapoptotic activity of aflatoxinB1 and sterigmatocystin in HepG2 cells. Toxicol. Rep. 2014;1:1076–1086. doi: 10.1016/j.toxrep.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.