Abstract
Salinity stress is a major abiotic stress for plants worldwide. This study was carried out to determine the variation in salt tolerance for 12 different genotypes belonging to three different tomato species: Solanum lycopersicum (L), S. peruvianum (L) and S. pimpinellifolium (L). Shoot apices and callus cultures were exposed to different levels of salinity stress ranging from no salt (control) to 100, 200 and 300 mmol L−1 NaCl. All growth and physiological parameters were significantly affected by salt stress. Most shoot apices of S. lycopersicum did not develop roots when exposed to low NaCl levels, whereas apices of S. peruvianum and S. pimpinellifolium developed roots when exposed to all salt levels. This difference in salt tolerance was clearly shown on the basis of root fresh weights and root surface areas. Callus growth in response to increased salinity was much greater in S. peruvianum and S. pimpinellifolium than in S. lycopersicum. The Cl− and Na+ concentrations increased significantly with increasing salt in the three species, although the S. peruvianum lines accumulated more ions compared with the others. As the salt concentration increased, less K+ accumulated in S. lycopersicum compared to the related wild species. The results obtained in this study suggest that S. peruvianum line 0043-1 was the accession with the best salt tolerance. The most tolerant cultivated tomato (S. lycopersicum) cultivar was ‘Rutgers.’ Both S. peruvianum line 0043-1 and S. lycopersicum ‘Rutgers’ are good candidates for inclusion in tomato breeding programs for salt-tolerance.
Keywords: cultivated tomato, physiological parameters, salinity stress, salinity tolerance index, wild species
Environmental conditions that are not optimum for plant growth or production are defined as stress factors (Thornton 2002). Abiotic stresses such as salinity, drought, temperature, and flooding are some of the main causes of crop yield loss worldwide. Abiotic stresses can reduce the average yields of most major crops by more than 50% (Bray et al. 2000; Flowers and Yeo 1995; Flowers et al. 2000). In particular, high salinity is a serious threat to crop production. As the world population continues to grow, food production needs to be increased by increases in cultivated land and/or by increases in crop productivity. According to the FAO Land and Nutrition Management Service (2008), over 6% of the world’s land, more than 800 million ha, is affected by either salinity or sodicity. The percentage of cultivated land affected by salt is even greater with 23% of the cultivated land being saline and 20% of the irrigated land suffering from secondary salinization (Flowers 2004; Flowers and Yeo 1995; Ghassemi et al. 1995). Population growth and land degradation by salinization have led plant researchers to the concept of developing salt-tolerant crops by different approaches (Cuartero et al. 2006; Munns 2005; Munns et al. 2006; Yamaguchi and Blumwald 2005). The physiological, biochemical and molecular mechanisms of salt tolerance in plants are not sufficiently understood; hence, progress in developing salt-tolerant crops has been slow.
Salinity stress affects plants through morphological, physiological and metabolic changes in their organs (Amini and Ehsanpour 2005; Zhang et al. 2004). Perhaps the best investigated of the traits related to salt tolerance are those associated with the ion content of plants grown in the presence of salt stress (Abel 1969; Tal and Shannon, 1983). Essential mechanism of tolerance involves the ability to reduce the ionic stress on the plant by minimizing the amount of Na+ that accumulates in the cytosol of cells, particularly those in transpiring leaves. In the majority of plant species grown under saline conditions, Na+ appears to reach a toxic concentration before Cl− does, and so most studies have concentrated on Na+ exclusion and the control of Na+ transport within the plant (Munns 2002). The mechanism of Na+ exclusion allows the plant to avoid or postpone the problem related to ion toxicity, but if Na+ exclusion is not compensated for by the uptake of K+, there becomes a greater demand for organic solutes for osmotic adjustment. The synthesis of organic solutes jeopardizes the energy balance of the plant. Thus, the plant must cope ion toxicity on the one hand, and turgor loss on the other (Bernstein, 1963; Munns and Tester 2008; Phills et al. 1979).
Tomato (Solanum lycopersicum L.) is one of the most important vegetable crops worldwide. Tomato is a member of the Solanaceae family that includes several other economically important crops such as potato, pepper, and eggplant. In terms of human health, tomato fruit is a major component of daily meals in many countries and constitutes an important source of minerals, vitamins, and antioxidant compounds (Naika et al. 2005). Nevertheless, areas providing optimal growing conditions for tomatoes are becoming more restricted around the world because of salt affected soils. The growth and yield of tomato is significantly reduced by high salinity (Feigin et al. 1987; Shalhevet and Hsiao 1986; Smith et al. 1992). The response of tomato to salinity is variable depending upon the line or cultivar (Shannon et al. 1987). Compared with Arabidopsis, there is much less information on breeding for salt tolerant tomato cultivars (Cuartero et al. 2006).
Salinity tolerance by plants depends primarily on the genotype that determines such processes as uptake and transport of salt by roots in combination with metabolic and physiological events (Foolad and Lin 1997; Winicov 1993). Classic screening methods for the selection of salt tolerant variants are difficult and time consuming. In vitro techniques make it possible to screen the required number of genotypes rapidly since in vitro plant cultures, even at different stages of development, may exhibit their capacity to withstand the stress (Tewary et al. 2000; Zaki et al. 2016). Several researchers have proposed using in vitro screening to identify tomato germplasm with salinity tolerance (Amini and Ehsanpour 2005; Cano et al. 1996; Cano et al. 1995; Cuartero et al. 2006; Cuartero et al. 1992; Emilio et al. 1998; Garcia-Reina 1988). Fewer of these studies have examined the salinity tolerance in cultivated tomatoes and their wild species. Cuartero et al. (1992) attempted to enhance the salt-tolerance of the cultivated tomato using the tolerance of wild species of Lycopersicon, suggesting that in vitro screening of large numbers of wild species for salinity tolerance is need. Emilio et al. (1998) also found the possibility of using in vitro shoot apex culture to evaluate salt tolerance of cultivated (Lycopersicon esculentum Mill.) and wild (Lycopersicon pennellii (Correll) D’Arcy) tomato species and reported relation to the response obtained by callus culture.
Therefore, it is very important to screen the available cultivated and wild species for their salt tolerance in order to recommend cultivars that can be cultivated in high saline soil conditions or to use salt-tolerant genotypes in breeding programs. The objective of this study was to determine the extent of genetic variability for salinity tolerance among different cultivated and wild tomato genotypes using tissue culture techniques. Shoot apex and callus growth and physiological characteristics were determined for twelve genotypes in the absence and presence of NaCl to evaluate the varying degrees of salt tolerance among the genotypes.
Materials and methods
Some parts of this study were conducted at the Laboratory of Plant Breeding, Faculty of Agriculture, Iwate University, Morioka, Iwate, Japan during the period of 26 November 2013 to 25 May 2014.
Plant materials
Salt responses were studied in shoots and callus tissues of twelve tomato genotypes. The species used in this study included six tomato cultivars Solanum lycopersicum (L) (‘Moneymaker’, ‘Aichi-first’, ‘Ailsa Craig’, ‘M82’, ‘Rutgers’ and ‘Ponderosa’) and two accessions of the wild species Solanum peruvianum (L) (lines 0043-1 and 0046) and four accessions of the wild species Solanum pimpinellifolium (L) (lines 0041w1, 0043, 0048 and 0049-w1). Seeds of all genotypes were obtained from the Tomato National Bioresource Project (NBRP), Japan.
Plant micropropagation
Tomato seeds were surface sterilized for 20 s with 90% (v/v) ethanol and for 30 min with 1% (w/v) sodium hypochlorite. Thereafter, the seeds were rinsed in sterile deionized water three times. Seeds were then germinated in vitro on Phytagel-solidified (4.5–5 gL−1) Murashige and Skoog (MS) basic medium (Murashige and Skoog 1962). The medium pH was adjusted to 5.7±0.1 before the addition of Phytagel and subsequent autoclaving at 121°C and 15 psi for 20 min. All cultured seeds were maintained under a 16 h photoperiod, 25±2°C and white cool fluorescent bulbs providing approximately 90 µmol m−2s−1 PPFD. When the plantlets had developed two true leaves (2 weeks after germination) explants were collected.
Shoot apex and callus culture under salinity treatments
Shoot apex culture
Shoot apices 1 cm in length were cut and inserted upright into Magenta GA-7 plant culture boxes (Sigma-Aldrich Co. LLC.) containing 20 ml of medium (Cano et al. 1995). The medium consisted of ½-strength MS mineral salts (Murashige and Skoog 1962), 100 mg L−1 myo-inositol, 1 mg L−1 thiamine HCl, 0.1 mg L−1 indoleacetic acid, 10 g L−1 sucrose, and 4.5–5 g L−1 Phytagel. To compare the genotype tolerance or sensitivity to salinity stress, shoot apices were grown on media supplemented with three different concentrations of NaCl (100, 200 or 300 mmol L−1) and compared with the control (no NaCl). All media were adjusted to pH 5.7±0.1 before autoclaving at 121°C and 15 psi for 20 min. The cultures were maintained under a 16 h photoperiod, 25±2°C and white cool fluorescent bulbs providing approximately 90 µmol m−2s−1 PPFD. After four weeks, plantlets were removed from the culture boxes; plantlet fresh weight, shoot and root fresh weight were measured manually, and root characteristics (total root length, root surface area, and average root diameter) were analyzed by WinRHIZO Basic 2009 image analysis software (Regent Instruments Canada, Inc.). Ten plantlets per genotype were evaluated for each of the salinity treatments and the experiment was repeated at approximately monthly intervals three times.
Callus induction
Callus cultures were initiated from leaves of the same plantlets used for shoot apices culture on MS medium containing macro and micronutrients and supplemented with myo-inositol (100 mg L−1), sucrose (30 g L−1) and solidified with Phytagel (4.5–5 g L−1). Plant growth regulators were 0.4 mg L−1 NAA and 2 mg L−1 kinetin. Three different NaCl concentrations (100, 200 or 300 mmol L−1) were compared with the control (no NaCl) to test the genotypes’ capacities for callus induction under salinity stress. The pH of the medium was adjusted to 5.7±0.1 before autoclaving at 121°C for 20 min. The cultures were maintained in the dark for the first two weeks and subsequently transferred to light conditions for another two weeks. After four weeks, each explant was evaluated for callus fresh weight, percent of explants forming callus and percentage of explants forming roots. Ten calli per genotype were evaluated for each of the salinity treatments and the experiment was repeated three times.
Ion content analysis
Two hundred mg of shoot tissues were transferred to 100 cm3 digestion flasks containing 50 ml 0.1 N HNO3. After 30 min of shaking, the extracts were filtered (Whatman qualitative filter paper, Grade 1) Na+ and K+ were determined by atomic absorption spectrophotometry, whereas Cl− was determined by potentiometric titration with 0.01 N AgNO3. Ions of chloride, sodium and potassium were determined according to the method of Emilio et al. (1998).
Salinity tolerance index (STI)
The Salinity Tolerance Index (STI) for each genotype was calculated following the method of Reddy and Vaidyanath 1982. STI was calculated as the ratio of the trait performance at 100, 200, or 300 mmol L−1 to the trait performance at 0 mmol L−1 NaCl according to the following formula:
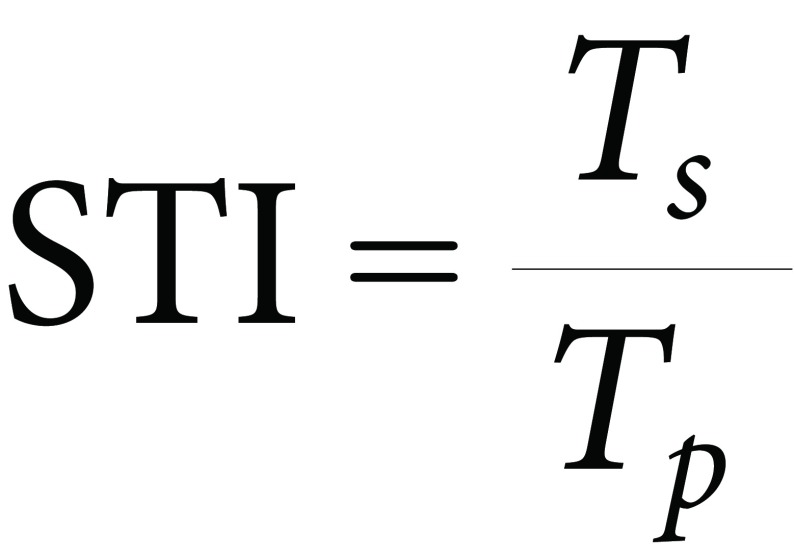 |
Where Ts is the trait of genotype under stress, Tp the trait of genotype under normal conditions.
All STI variables were analyzed and for ease of discussion, the best linear unbiased predictors (BLUPs) illustrated in the Figures are referred to as means and standard errors in the discussion.
Statistical analyses
The experimental design was a 12×4 (genotypes×NaCl concentrations) factorial experiment in a randomized complete block with three replications (n=10). Data obtained from this study were subjected to analysis using MSTAT C software version 4 (1996). Differences among tomato genotypes were tested by an analysis of variance (ANOVA), and mean significant differences were tested by the Least Significant Difference (LSD) test at the 0.05 level of significance.
Results
Effects of salinity on plantlet growth
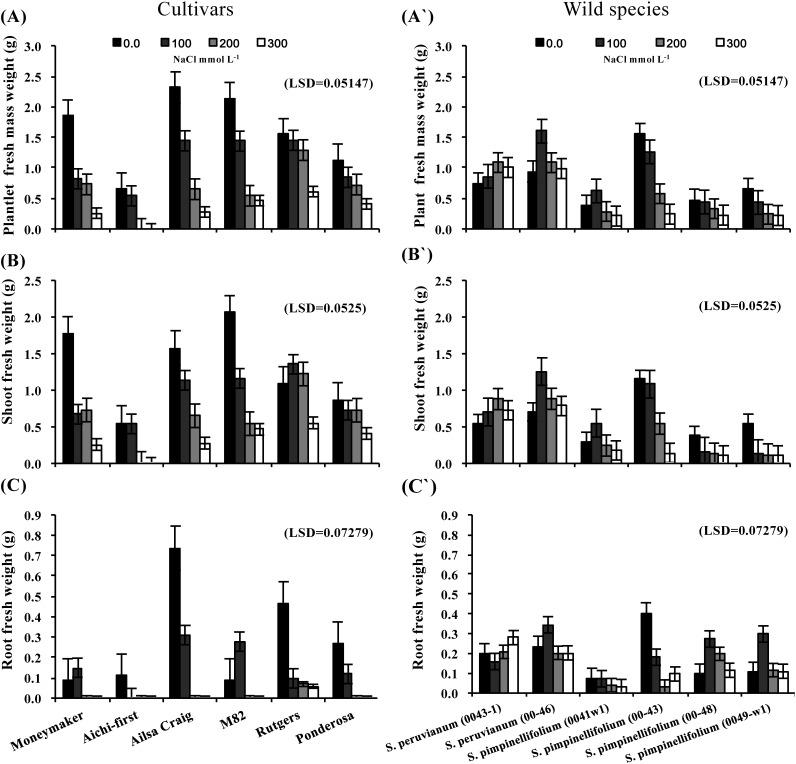
The effects of salinity stress on the fresh weights of plantlets, shoots and roots grown in vitro from shoot apices are shown in Figure 1. All fresh weights decreased as the salinity stress level increased with reductions generally greater in tomato cultivars than in the wild species. In general, most of the S. lycopersicum cultivars showed more plantlet fresh weight and shoot fresh weight at the control level (no NaCl) than any of the other species, although S. peruvianum lines 00-43-1 and 00-46 and S. pimpinellifolium line 00-43 did not differ significantly from the performance of S. lycopersicum. The two S. peruvianum lines had higher plantlet fresh weights (Figure 1A and A′) and shoot fresh weights (Figure 1B and B′) when grown in media with elevated salt concentrations.
Figure 1. Plantlet fresh weight (A and A′), shoot fresh weight (B and B′) and root fresh weight (C and C′) of in vitro-cultured shoot apices of six tomato S. lycopersicum cultivars (‘Moneymaker’, ‘Aichi-first’, ‘Ailsa Craig’, ‘M82’, ‘Rutgers’ and ‘Ponderosa’) and two accessions of the wild species of S. peruvianum (lines 0043-1 and 0046) and four accessions of S. pimpinellifolium (0041w1, 0043, 0048 and 0049-w1). Shoot apices were grown on media supplemented with three different concentrations of NaCl (100, 200 or 300 mmol L−1) and plantlet growth parameters were compared with the control (no NaCl). Values are means ±SE, (n=10) and the experiment was repeated three times.
Root fresh weight declined as a result of salt stress in all S. lycopersicum cultivars (Figure 1C and C′), an expected result since changes in root characteristics are one of the primary responses of plants to salinity stress; however there were clear differences in root growth at the lower NaCl concentrations among the cultivars. With the lowest salt concentration (100 mmol L−1 NaCl), ‘Aichi-first’ did not develop roots at all; however, other cultivars for example, ‘Moneymaker’, ‘Ailsa Craig’, ‘M82’ and ‘Ponderosa’ did develop roots. ‘Rutgers’ even had roots when subjected to the highest level of salt stress (300 mM L−1 NaCl) as clear in Figure 1C. On the other hand, root fresh weight increased with increasing salt concentrations in S. peruvianum (lines 0043-1 and 00-46) and S. pimpinellifolium (lines 00-48 and 0049-w1). Statistical analysis indicated that two accessions of S. peruvianum (lines 0043-1 and 00-46) had significantly heavier root fresh weights when grown in media with high NaCl concentrations than all other accessions and were closely followed by two accessions of S. pimpinellifolium (lines 00-48 and 0049-w1). S. pimpinellifolium line 0041-w1 had the lowest root fresh weight at the highest level of salt stress, closely followed by S. lycopersicum ‘Rutgers’ (Figure 1C′).
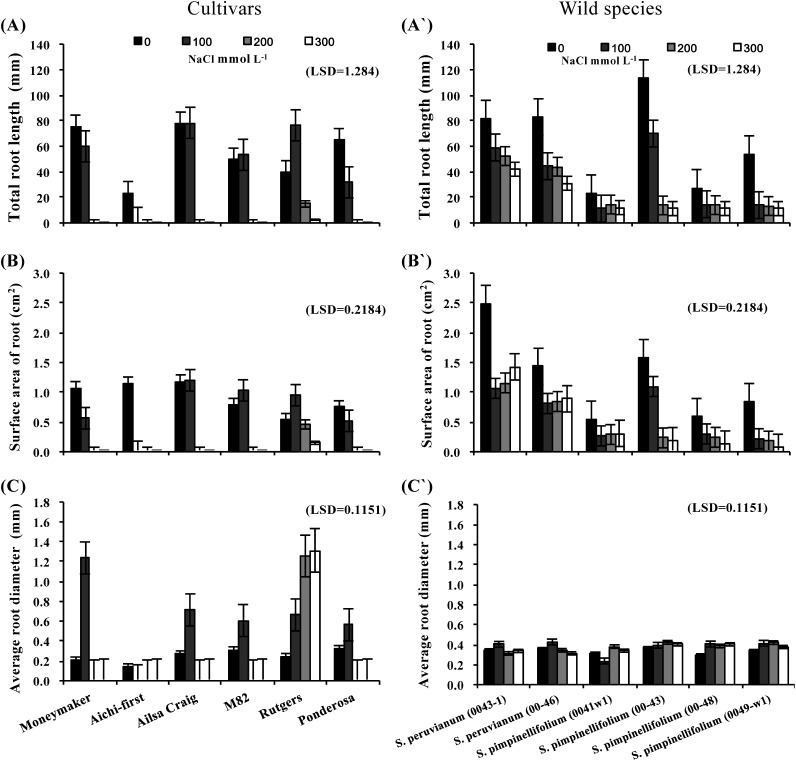
Total root length, root surface area and the average root diameters of tomato cultivars were compared with those of the related Solanum wild species (Figure 2). Overall comparisons of the 12 tested genotypes indicated significant differences among cultivated tomato and wild species in these root properties at all tested NaCl concentrations and the no NaCl control. Total root length and mean root area in most of the genotypes were significantly affected by salinity stress. Several of the S. lycopersicum cultivars had significantly longer roots than the S. peruvianum and S. pimpinellifolium accessions at the lowest level of salinity (100 mmol L−1 NaCl) as shown in (Figure 2A); however, the wild tomato species formed longer roots under high salinity stress than the tomato cultivars. Statistical analysis indicated that S. peruvianum (lines 0043-1 and 00-46) had significantly longer roots under high salinity conditions than accessions of the two other species examined in this study (Figure 2A′).
Figure 2. Total root length (A and A′), root surface area (B and B′) and average root diameter (C and C′) of in vitro-cultured shoot apices from six S. lycopersicum cultivars (‘Moneymaker’, ‘Aichi-first’, ‘Ailsa Craig’, ‘M82’, ‘Rutgers’ and ‘Ponderosa’) and two accessions of the wild species of S. peruvianum (lines 0043-1 and 0046) and four accessions of S. pimpinellifolium (lines 0041w1, 0043, 0048 and 0049-w1). Shoot apices were grown on media supplemented with three different concentrations of NaCl (100, 200 or 300 mmol L−1) and root growth parameters were compared with the control (no NaCl). Values are means ±SE, (n=10) and the experiment was repeated three times.
The mean root surface area for most of the examined genotypes differed significantly under high salt levels (Figure 2B and B′). Only S. lycopersicum ‘Rutgers’ had significantly more root area than the other tomato cultivars grown on media containing 300 mmol L−1 NaCl (Figure 2B). On the other hand, wild tomato species had much less root surface area under salt stress. The root surface area of S. peruvianum lines increased with increasing salinity levels, but root surface areas remained lower than the control (Figure 2B′). Overall, the mean root surface area ranged from 0.0 to 2.49 cm2 among the 12 accessions, an approximately 2.5-fold difference.
In contrast, mean root diameter increased with increasing salt concentration in all tested tomato cultivars and most of the wild species accessions. Interestingly, the two S. peruvianum lines formed thinner roots under the highest level of stress used; however, this difference in root diameter was not significant (Figure 2C and C′).
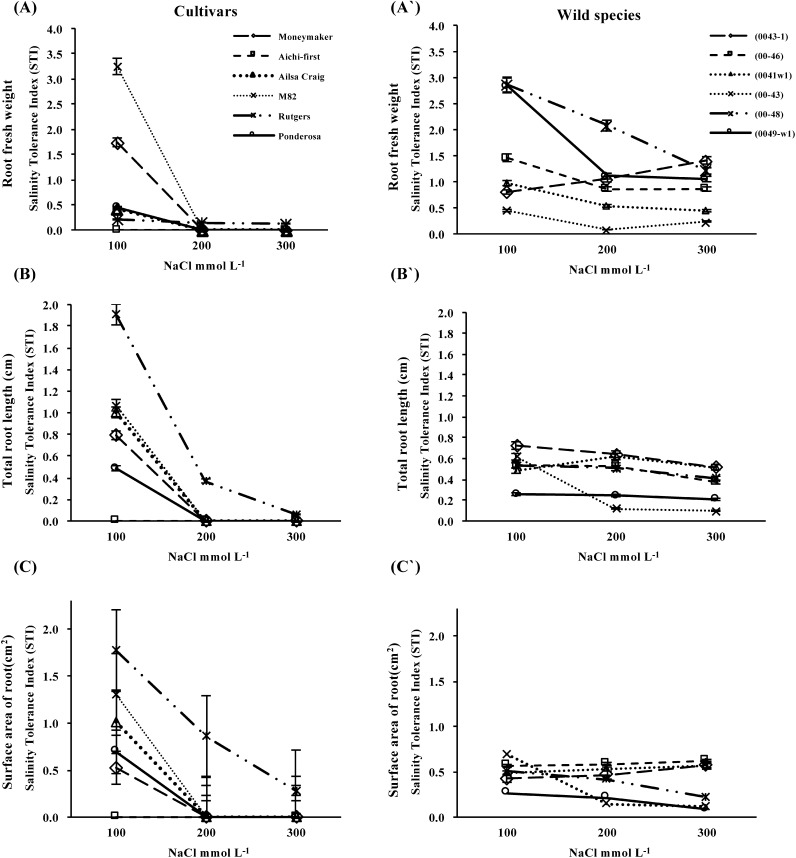
Salt tolerance, as expressed by the salt tolerance index (STI) is shown in Figure 3. The STIs of root fresh weight (Figure 3A and A′), total root length (Figure 3B and B′) and root surface area (Figure 3C and C′) revealed a greater tolerance to salt in the wild species accessions compared with tomato cultivars. The STIs decreased with increasing salinity in all examined cultivars. Variations in the STI among cultivars were noticed from low to high salinity; however, the STI decreased with increasing salinity stress. In contrast, wild species accessions were more tolerant than the tomato cultivars under high salt stress conditions (Figure 3A′, B′ and C′). Two accessions of S. peruvianum, lines 0043-1 and 00-46, and one line of S. pimpinellifolium, 0041-w1, had better STI values across all saline levels. Two lines of S. pimpinellifolium, lines 00-48 and 0049-w1, did not show remarkable changes across all saline levels, and line 00-43 preferred slightly or moderately saline conditions. Thus, more accessions were rated salt-tolerant by root fresh weight and root surface area STI values (Figure 3A′ and C′, respectively) than by total root length STI values (Figure 3B′).
Figure 3. Salt tolerance, as expressed by the salt tolerance index (STI) of root fresh weight (A and A′), total root length (B and B′) and root surface area (C and C′) of in vitro-cultured shoot apices from six S. lycopersicum cultivars (‘Moneymaker’, ‘Aichi-first,’ ‘Ailsa Craig’, ‘M82’, ‘Rutgers’ and ‘Ponderosa’) and two accessions of the wild species of S. peruvianum (lines 0043-1 and 0046) and four accessions of S. pimpinellifolium (lines 0041w1, 0043, 0048 and 0049-w1).Values are means ±SE, (n=10) and the experiment was repeated three times.
In general, ‘Rutgers’ remained the most salt-tolerant cultivar among the S. lycopersicum cultivars (Figure 3). ‘Rutgers’ plantlets under different salinity treatments are shown in Figure 4A. S. peruvianum line (0043-1) was identified as the accession with superior root fresh weight STIs when subjected to the highest level of NaCl treatment (Figure 3). Plantlets of line (0043-1) under different salinity treatments are shown in Figure 4B. Several other species accessions were superior at lower levels of NaCl treatment.
Figure 4. Plantlets from the most tolerant S. lycopersicum ‘Rutgers’ (A) and a wild accession S. peruvianum line 0043-1(B). Shoots were cultured on media supplemented with three different concentrations of NaCl (100, 200 or 300 mmol L−1) and compared with the control (no NaCl).
Effects of salinity on callus growth
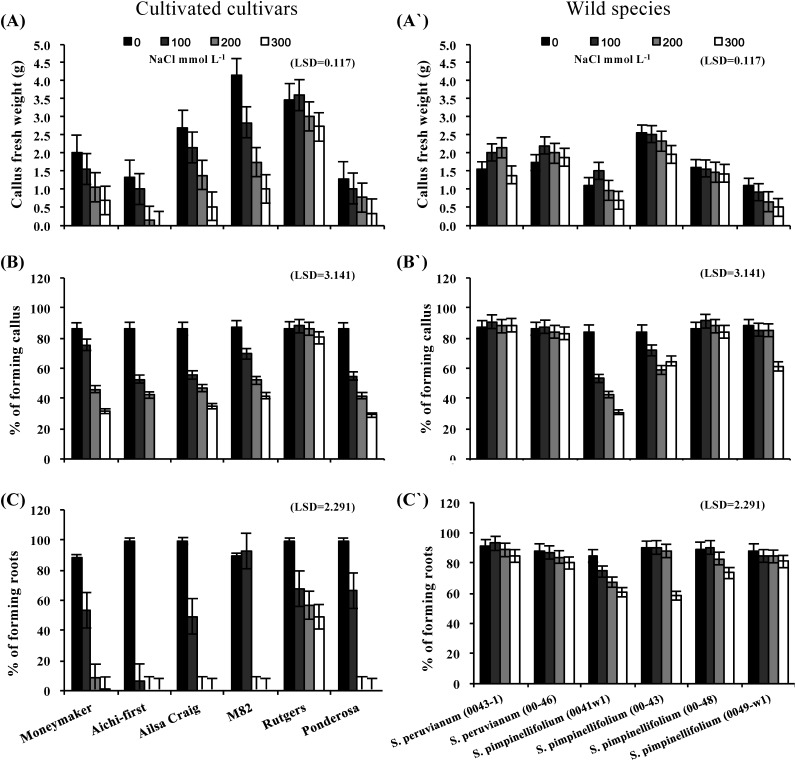
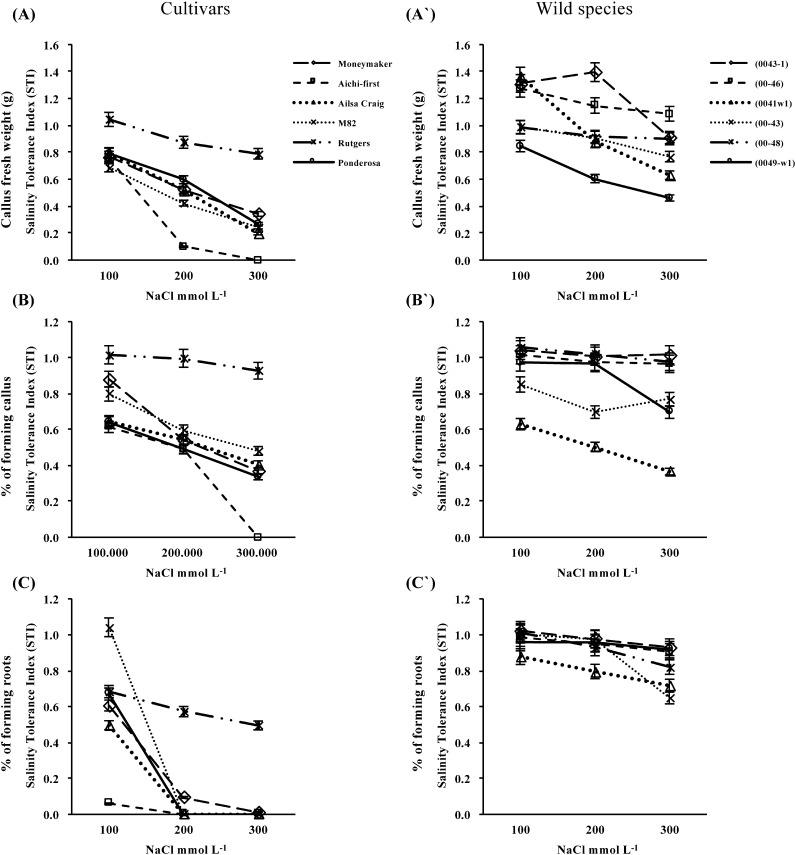
The effects of salinity on callus fresh weight, % of explants forming callus and % of explants forming roots after four weeks are shown in Figure 5. Callus fresh weight was reduced significantly at every salt level for the S. lycopersicum cultivars and S. pimpinellifolium lines 0041-w1, 00-43 and 0049-w1, whereas callus growth was stimulated in the S. peruvianum lines for the lower levels of salt treatment and only reduced at the highest level of salinity stress (Figure 5A and A′). S. pimpinellifolium line 00-48 performed relatively consistently across all saline levels. For the % of explants forming callus, all evaluated species could form callus under different levels of salinity; however, the percentage decreased with increasing salt concentration in all tomato cultivars. Among the cultivars, only ‘Rutgers’ was consistent in its ability to form callus across all salt stress levels. In contrast, most of the wild tomato species formed the highest percentages of callus under the different levels of salt stress (Figure 5B and B′). The percentage of callus that developed roots was significantly affected by salinity stress (Figure 5C and C′). The greater effect was observed on cultivars of the S. lycopersicum; however, ‘Rutgers’ had remained to show the considerable ability forming roots. Accessions from the wild species showed great salt tolerance with 58.3–93.0% of all explants forming roots under high salt stress.
Figure 5. Callus fresh weight (A and A′), % of explants forming callus (B and B′) and % of explants forming roots (C and C′) of callus cultures initiated from leaves of six S. lycopersicum cultivars (‘Moneymaker’, ‘Aichi-first’, ‘Ailsa Craig’, ‘M82’, ‘Rutgers’ and ‘Ponderosa’) and two accessions of the wild species of S. peruvianum (lines 0043-1 and 0046) and four accessions of S. pimpinellifolium (lines 0041w1, 0043, 0048 and 0049-w1). Callus growth was initiated on media supplemented with three different concentrations of NaCl (100, 200 or 300 mmol L−1) and compared with the control (no NaCl). Values are means ±SE, (n=10) and the experiment was repeated three times.
Salt tolerance, as expressed by the salt tolerance index (STI) is shown in Figure 6. The STI for callus fresh weight (Figure 6A and A′) and % of explants forming callus (Figure 6B and B′) decreased with increasing salinity stress. Although reductions in the % of explants forming callus were much greater in tomato cultivars than for the wild species accessions, the trends for the cultivars and accessions did not differ significantly. A significantly higher % of callus formed roots when the callus was initiated from the wild species than the cultivated tomato accessions (Figure 6C and C′).
Figure 6. Salt tolerance, as expressed by the salt tolerance index (STI) of callus fresh weight (A and A′), % of explants forming callus (B and B′) and % of explants forming roots (C and C′) of callus cultures initiated from leaves of six S. lycopersicum cultivars (‘Moneymaker’, ‘Aichi-first’, ‘Ailsa Craig’, ‘M82’, ‘Rutgers’ and ‘Ponderosa’) and two accessions of the wild species of S. peruvianum (lines 0043-1 and 0046) and four accessions of S. pimpinellifolium (lines 0041w1, 0043, 0048 and 0049-w1). Callus growth was initiated on media supplemented with three different concentrations of NaCl (100, 200 or 300 mmol L−1) and compared with the control (no NaCl). Values are means ±SE, (n=10) and the experiment was repeated three times.
Effects of salinity on the ion content of shoots
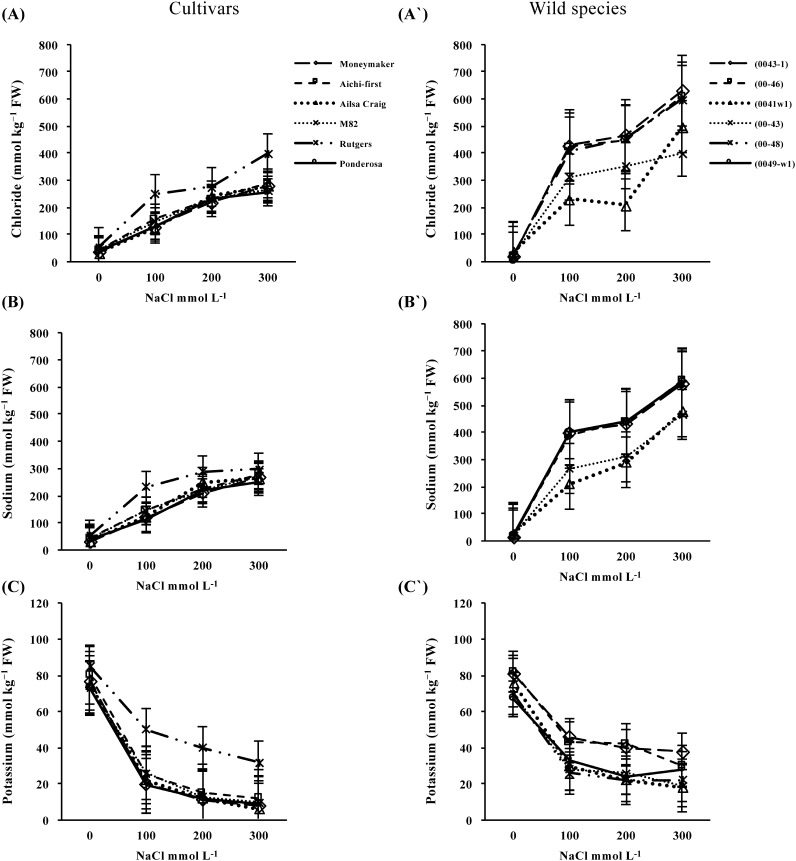
The Cl− and Na+ concentrations (mmol kg−1 FW) in shoots increased significantly with exposure to increasing salt levels in all three species (Figure 7). Large variations in shoot Cl− (Figure 7A and A′) and Na+ (Figure 7B and B′) accumulation by plantlets derived from the wild species were found, although there was a trend toward higher accumulation in the S. peruvianum lines compared with those from S. pimpinellifolium at the highest salt levels. Accumulation of saline ions was greater in ‘Rutgers’ than other cultivars, especially at the higher salt levels. At 100 mmol L−1 NaCl, shoots of S. lycopersicum either accumulated similar levels or more of Cl− and Na+ than those of S. peruvianum and S. pimpinellifolium, whereas the opposite trend was found at the higher salt levels.
Figure 7. Chloride (A and A′), sodium (B and B′) and potassium (C and C′) ion concentrations (mmol kg−1 FW) of shoots from six S. lycopersicum cultivars (‘Moneymaker’, ‘Aichi-first’, ‘Ailsa Craig’, ‘M82’, ‘Rutgers’ and ‘Ponderosa’) and two accessions of the wild species of S. peruvianum (lines 0043-1 and 0046) and four accessions of S. pimpinellifolium (lines 0041w1, 0043, 0048 and 0049-w1). Plants were grown on media supplemented with three different concentrations of NaCl (100, 200 or 300 mmol L−1) and ion concentrations were compared with those of the control (no NaCl). Values are means ±SE, (n=10) and the experiment was repeated three times.
Treatment with increasing salt levels resulted in a significant decrease in K+ in the shoots of all three species, although the K+ reduction was greater for S. lycopersicum than for S. peruvianum and S. pimpinellifolium (Figure 7C and C′). K+ was reduced significantly by salt stress in the shoots of S. lycopersicum started from the treatment using the lowest concentration of NaCl however, ‘Rutgers’ did not show significantly reduction across salinity levels. In all accessions of the wild species, a significant reduction in K+ was observed in the 100 mmol L−1 NaCl treatment, although the decrease was not statistically significant at the highest level of salinity stress.
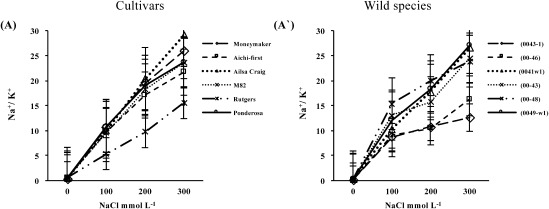
The change in Na+/K+ ratio was calculated from the shoots sodium and potassium concentrations as shown in Figure 8. The greater Na+/K+ ratio was observed on cultivars of the S. lycopersicum; however, ‘Rutgers’ had the lowest ratio under the highest level of salinity (Figure 8A). In wild species; S. peruvianum lines exhibited the lowest Na+/K+ ratio compared with those from S. pimpinellifolium at the highest salt levels (Figure 8A′). S. peruvianum line (0043-1) was identified as the accession with superior tolerance to salt as Na+/K+ ratio was the lowest among all tested genotypes.
Figure 8. Sodium to potassium ratio over three different concentrations of NaCl (100, 200 or 300 mmol L−1) in shoots of (A) six S. lycopersicum cultivars ‘Moneymaker’, ‘Aichi-first’, ‘Ailsa Craig’, ‘M82’, ‘Rutgers’ and ‘Ponderosa’ and (A′) two accessions of the wild species of S. peruvianum (lines 0043-1 and 0046) and four accessions of S. pimpinellifolium (lines 0041w1, 0043, 0048 and 0049-w1). Values are means ±SE, (n=10) and the experiment was repeated three times.
Discussion and conclusions
Tomato (Solanum lycopersicum) is one of the most widely grown and commercially important vegetables throughout the world (Naika et al. 2005). Sources of salt tolerance have been identified among related wild species and primitive cultivars of tomato (Cuartero et al. 1992; Flowers 2004; Jones 1986). Genetic variation in the wild species has already been used to improve salt tolerance in some modern crop cultivars (Zaki et al. 2016). In this work, we determined the tolerance of the tomato cultivars and wild species of Solanum under three different levels of salinity stress in vitro. The wild species were chosen for their expected high salt tolerance at the whole plant level, but the level of tolerance is different among tomato genotypes and/or within genotypes (Bolarin et al. 1991).
In vitro culture, besides its use as a tool for obtaining salt tolerant plants, may offer the potential for quick evaluation of germplasm for salt stress tolerance (Tewary et al. 2000; Zaki et al. 2016). In tomato cultivars, Solanum lycopersicum, a positive correlation between growth of calli and whole plants in saline conditions has been reported (Perez-Alfocea et al. 1994). Screening methods involving in vitro shoot apex culture could provide an efficient protocol for testing and selecting tomato germplasm for salt tolerance (Cano et al. 1996). Callus culture has the advantage that, given the genetic stability of shoots multiplied by apices or auxiliary buds (Hu and Wang 1983), presumably new variability is generated during selection.
In this study, apices and calli were exposed to different levels of salinity stress in vitro ranging from 100 to 300 mmol L−1 NaCl, and growth was compared to control conditions (no NaCl). Other investigators have reported the use of NaCl for in vitro salinity screening in other species of plants (Vijayan et al. 2003; Zhao et al. 2009), but the concentrations used have not been as high as those used in this study. All growth and physiological parameters were significantly affected by salt stress. Root growth is more adversely affected than shoot growth as the salt concentration increases (Cano et al. 1996; Sweby et al. 1994). Similar results were obtained in this study; although both root and shoot growth were inhibited by salt, the effects were more pronounced on root growth, mainly in S. lycopersicum rather than wild species. Only, ‘Rutgers’ developed roots under the highest level of salt stress (Figures 1 and 2). On the other hand, root fresh weight increased with increasing salt concentrations in one accession of S. peruvianum (lines 0043-1) and one accession of S. pimpinellifolium (lines 00-48). These results all suggest that there is considerable variation in response to salt stress among species and within species (Figure 1).
A higher salt tolerance index (STI) was previously reported for wild tomato species compared with that for cultivars of the S. lycopersicum, both at the whole plant level and in callus culture (Perez-Alfocea et al. 1994). In our study, the higher STI of S. peruvianum and S. pimpinellifolium compared with that of S. lycopersicum was clearly shown by the root fresh weight, total root length and root surface area in the 200 and 300 mmol L−1 NaCl conditions (Figure 3); however the STI decreased with increasing salinity stress and ‘Rutgers’ was the most tolerant cultivar among the cultivars. Two lines of S. peruvianum (lines 0043-1 and 00-46) and one line of S. pimpinellifolium (0041-w1) grew better across all saline levels (Figure 3). Thus, on the basis of reduced shoot fresh weight with increasing salinity, the salt tolerance of S. lycopersicum was lower than that of the other two wild species; although no differences were observed as great as those found for the root growth parameters. Thus, root growth, especially root fresh weight and root surface area are good characteristics than shoot growth for evaluating the salt tolerance of tomato genotypes through in vitro shoot apex culture.
Higher salt tolerance has been reported for wild tomato species than for cultivars of the S. lycopersicum in callus culture (Emilio et al. 1998; Perez-Alfocea et al. 1994). The higher salt tolerance of S. peruvianum and S. pimpinellifolium than that of S. lycopersicum was clearly shown by the callus fresh weight, % of explants forming callus and % of explants forming roots in cultures grown from shoot apices after four weeks as shown in Figure 5. The trend for wild species to be more salt tolerant than S. lycopersicum is similar to that previously reported by Cano et al. (1996); however, our study used accessions of different wild species.
Na+ and Cl− accumulations were higher in the wild species than in cultivars of the S. lycopersicum. Accumulation of these ions increased with increasing salinity stress in all species (Figure 7). This fact suggests that the predominant salt tolerance mechanism involves ion accumulation. The interpretation of this data suggests that accessions of wild species were more salt-tolerant, not because they are more capable of restricting Cl− and Na+ uptake at high NaCl levels than cultivars, but because they have a superior ability to tolerate high levels of Cl− and Na+ in their tissues as reported by Bernstein (1963). It is also plausible that high ion accumulation is a necessary consequence of the ability to survive. Chloride and Na+ accumulations in wild species have been reported by other researchers (Dehan and Tal 1978; Phills et al. 1979; Tal and Shannon 1983), suggesting that Na+ leaf concentrations could be used as a key characteristic in evaluation of germplasm for salt-tolerance breeding programs of cultivated tomato (Rush and Epstein 1981). On the other hand, there were small, but significant, differences in the K+ concentrations within shoots treated with the different NaCl levels (Figure 7C and C′). Generally, treatments with higher salt levels resulted in a significant decrease in shoot K+ levels, although the reduction was greater for S. lycopersicum than for S. peruvianum and S. pimpinellifolium. The ability to maintain potassium uptake in the presence of high concentrations of external sodium may be a desirable trait, as suggested by Yeo and Flowers (1989). Ion transport could be essentially influenced by salt stress in a cell-specific manner. Under salinity the increase in cytoplasmic Na+ and reduction of K+ result in changes of membrane potential, osmotic pressure (Munns 2002). Lower Na+/K+ ratio under salt treatment were also found in roots of halophyte compared to glycophyte (Zahran et al. 2007). The lowest Na+/K+ ratio was recorded for S. peruvianum accessions (lines 0043-1 and 00-46), followed by S. lycopersicum ‘Rutgers’ under higher salt level (Figure 8), the genotypes that showed the greatest tolerance to salt as judged by other parameters. Meanwhile, there was overall correlation between growth under saline conditions and the Na+/K+ ratio, these genotypes might be valuable germplasm for breeding.
The results obtained in this study suggest that shoot apex and callus culture may be very useful for rapidly screening and evaluating tomato genotypes. Root characteristics, root fresh weight, surface area of root followed by total root length, are good selection criteria for salt tolerance in tomato. Also, the potassium content and Na+/K+ ratio are important traits. These traits are clearly recognizable and might be transferred to S. lycopersicum during breeding. S. peruvianum line 0043-1 was the accession showing the best salt tolerance overall. ‘Rutgers’ was the most tolerant cultivar among the tested S. lycopersicum cultivars. Both S. peruvianum line 0043-1 and S. lycopersicum ‘Rutgers’ are good candidates for inclusion in breeding programs for salt tolerance.
Acknowledgments
Dr. Zaki is thankful to the Cultural Affairs & Missions, Ministry of Higher Education, Egypt for the award of Post-doctoral Fellowship, and to the Laboratory of Plant Breeding, Faculty of Agriculture, Iwate University, Morioka, Iwate, Japan for the invitation as Visiting Scientist. The tomato resource used in this research was provided by the National Bioresource Project (NBRP), MEXT, JAPAN.
Supplementary Data
References
- Abel GH (1969) Inheritance of the capacity for chloride inclusion and chloride exclusion by soybeans. Crop Sci 9: 697–698 [Google Scholar]
- Amini F, Ehsanpour AA (2005) Soluble proteins, proline, carbohydrates and Na+/Cl− changes in two tomato (Lycopersicon esculentum Mill.) cultivars under in vitro salt stress. Am J Biochem Biotechnol 1: 212–216 [Google Scholar]
- Bernstein L (1963) Osmotic adjustment of plants to saline media. II. Dinamic phase. Am J Bot 50: 360–370 [Google Scholar]
- Bolarin MC, Fernandez FG, Cruz J (1991) Salinity tolerance in four wild tomato species using vegetative yield-salinity response curves. J Am Soc Hortic Sci 116: 286–290 [Google Scholar]
- Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stress. In: Buchanan B, Gruissem W, Jones R (eds) Biochemistry and Molecular Biology of Plants. The American Society of Plant Physiologist, pp 1158–1203
- Cano EA, Perez-Alfocea F, Moreno V, Bolarin MC (1996) Responses to NaCl stress of cultivated and wild tomato species and their hybrids in callus cultures. Plant Cell Rep 15: 791–794 [DOI] [PubMed] [Google Scholar]
- Cano EA, Perez-Alfocea F, Santa Cruz A, Bolarin MC (1995) Desarrollo in vitro de apices de tomate. Horto Informacion 4: 42–47 [Google Scholar]
- Cuartero J, Bolarin MC, Asins MJ, Moreno V (2006) Increasing salt tolerance in the tomato. J Exp Bot 57: 1045–1058 [DOI] [PubMed] [Google Scholar]
- Cuartero J, Yeo AR, Flowers TJ (1992) Selection of donors for salt-tolerance in tomato using physiological traits. New Phytol 121: 63–69 [Google Scholar]
- Dehan K, Tal M (1978) Salt tolerance in the wild relatives of the cultivated tomato responses of Solanum pennellii to high salinity. Irrig Sci 1: 71–76 [Google Scholar]
- Emilio AC, Francisco PA, Vicente M, Manuel C, Maria CB (1998) Evaluation of salt tolerance in cultivated and wild tomato species through in vitro shoot apex culture. Plant Cell Tissue Organ Cult 53: 19–26 [Google Scholar]
- FAO (2008) Land and plant nutrition management service. htpp://www.fao.org/ag/agl/agll/spush
- Feigin A, Rylski I, Meiri A, Shalhevet J (1987) Response of melon and tomato plants to chloride-nitrate ratio in saline nutrient solution. J Plant Nutr 10: 1787–1794 [Google Scholar]
- Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55: 307–319 [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Koyama ML, Flowers SA, Chinta Sudhaka KP, Shing KP, Yeo AR (2000) QTL: Their place in engineering tolerance of rice to salinity. J Exp Bot 51: 99–106 [PubMed] [Google Scholar]
- Flowers TJ, Yeo AR (1995) Breeding for salinity resistance in crop plants. Where next? Aust J Plant Physiol 22: 875–884 [Google Scholar]
- Foolad MR, Lin GY (1997) Genetic potential for salt tolerance during germination in Lycopersicon species. Hortic Sci 32: 296–300 [Google Scholar]
- Garcia-Reina G, Moreno V, Luque A (1988) Selection for NaCl tolerance in cell culture of three Canary Island tomato land races. II. Inorganic ion content in tolerant calli and somaclones. J Plant Physiol 133: 7–11 [Google Scholar]
- Ghassemi F, Jakeman AJ, Nix HA (1995) Salinization of land and water resources. University of New South Wales Press Ltd., Canberra, Australia, p 562
- Hu CY, Wang PJ (1983) Meristem, shoot tip and bud cultures. In: Evans DA, Sharp WR, Ammirato PV, Yamada Y (eds) Handbook of Cell Culture. Macmillan, New York, pp 177–227
- Jones RA (1986) High salt tolerance potential in Lycopersicon species during germination. Euphytica 35: 575–582 [Google Scholar]
- Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25: 239–250 [DOI] [PubMed] [Google Scholar]
- Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167: 645–663 [DOI] [PubMed] [Google Scholar]
- Munns R, James RA, Lauchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57: 1025–1043 [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Naika S, Lidt J, Goffau M, Hilmi M, Dam B (2005) Cultivation of Tomato Production, Processing and Marketing. 4th ed, Agromisa Foundation and CTA, Wageningen, pp 1–92
- Perez-Alfocea F, Guerrier G, Estañ MT, Bolarin MC (1994) Comparative salt responses at cell and whole-plant levels of cultivated and wild tomato species and their hybrid. J Hortic Sci 69: 639–644 [Google Scholar]
- Phills BR, Perck NH, MacDonald GE, Robinson RW (1979) Differential response of Lycopersicon and Solanum species to salinity. J Am Soc Hortic Sci 104: 349–352 [Google Scholar]
- Reddy PJ, Vaidyanath K (1982) Note on salt tolerance of some rice varieties of Andhra Pradesh during germination and early seedling growth. Indian J Agric Sci 52: 472–474 [Google Scholar]
- Rush DW, Epstein E (1981) Comparative studies on the sodium, potassium and chloride relations of a wild halophytic and a domestic salt-sensitive tomato species. Plant Physiol 68: 1308–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalhevet J, Hsiao TC (1986) Salinity and drought. A comparison of their effects on osmotic adjustment, assimilation, transpiration and growth. Irrig Sci 7: 249–264 [Google Scholar]
- Shannon MC, Gronwald JW, Tall M (1987) Effects of salinity on growth and accumulation of organic and inorganic ions in cultivated and wild tomatoes. J Am Soc Hortic Sci 112: 416–423 [Google Scholar]
- Smith MAL, Spomer LA, Shibli RA, Knight SL (1992) Effect of NaCl salinity on miniature dwarf tomato ‘Micro-Tom’: H. Shoot and root growth responses, fruit production and osmotic adjustment. Plant Nutr 15: 2329–2341 [Google Scholar]
- Sweby DL, Huckett BI, Watt MP (1994) Effects of nitrogen nutrition on salt stressed Nicotiana tabacum var. Samsun in vitro plantlets. J Exp Bot 45: 995–1008 [Google Scholar]
- Tal M, Shannon MC (1983) Salt tolerance in the wild relatives of the cultivated tomato: Response of Lycopersicon esculentum, L. cheesmanii, L. peruvianum, Solanum pennellii and Fl hybrids to high salinity. Aust J Plant Physiol 10: 109–117 [Google Scholar]
- Tewary PK, Sharma A, Raghunath MK, Sarkar A (2000) In vitro response of promising mulberry (Morus sp.) genotypes for tolerance to salt and osmotic stresses. Plant Growth Regul 30: 17–21 [Google Scholar]
- Thornton MK (2002) Effects of heat and water stress on the physiology of potatoes. Presented at the Idaho Potato Conference on January 23
- Vijayan K, Chakraborti SP, Ghosh PD (2003) In vitro screening of mulberry (Morus spp.) for salinity tolerance. Plant Cell Rep 22: 350–357 [DOI] [PubMed] [Google Scholar]
- Winicov D (1993) Gene expression in relation to salt tolerance, In: Basra AS (ed) Stress-induced Gene Expression in Plants. Harwood Academic Publishers, Switzerland, pp 61–130
- Yamaguchi T, Blumwald E (2005) Developing salt-tolerant crop plants: Challenges and opportunities. Trends Plant Sci 10: 615–620 [DOI] [PubMed] [Google Scholar]
- Yeo AR, Flowers TJ (1989) Selections for physiological characters examples from breeding for salt resistance. In: Jones HG, Flowers TJ, Jones MB (eds) Plants under Stress. Cambridge University Press, Cambridge, pp 217–234
- Zahran HH, Marín-Manzano MC, Sánchez-Raya AJ, Bedmar EJ, Venema K, Rodríguez-Rosales MP (2007) Effect of salt stress on the expression of NHX-type ion transporters in Medicago intretexta and Melilotus indicus plants. Physiol Plant 131: 122–130 [DOI] [PubMed] [Google Scholar]
- Zaki HEM, Haynes KG, Vinyard BT (2016) In vitro screening of Solanum chacoense for salinity tolerance. Am J Potato Res 93 (in press) [Google Scholar]
- Zhang F, Yang YL, He WL, Zhao X, Zhang LX (2004) Effects of salinity on growth and compatible solutes of callus induced from Populus euphratica. In Vitro Cell Dev Biol 40: 491–494 [Google Scholar]
- Zhao X, Tan HJ, Liu YB, Li XR, Chen GX (2009) Effect of salt stress on growth and osmotic regulation in Thellungiella and Arabidopsis callus. Plant Cell Tissue Organ Cult 98: 97–103 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.