Key Teaching Points.
-
•
Ventricular arrhythmia storm is a life-threatening clinical event often requiring immediate intervention. Therapeutic strategies include antiarrhythmic drug therapy, deep sedation, cardiac pacing, catheter ablation, and cardiac assist systems.
-
•
Only recently, clinical pilot studies have proven the efficacy of cardiac stereotactic radiotherapy in stabilizing patients with refractory ventricular tachycardia storm.
-
•
We here present the first case of a patient in whom stereotactic radioablation was used to control ventricular fibrillation storm by targeting almost the entire scar.
Introduction
Ventricular arrhythmia storm is a potentially life-threatening clinical event that requires immediate intervention.1, 2, 3 Therapeutic strategies include antiarrhythmic drug therapy, deep sedation, cardiac pacing, catheter ablation, and the use of cardiac assist systems.1, 4 Only recently, a novel innovative therapeutic approach has been successfully established in patients with refractory ventricular tachycardia (VT). Cuculich and colleagues5 presented a series of 5 patients with recurrent VT episodes that could be successfully treated with noninvasive cardiac radiation using a single fraction of 25 Gy targeting the suspected VT exit site and the associated ventricular scar. These first promising results were further validated by a recent prospective study published by the same group that included 17 patients with refractory VT episodes and 2 patients with cardiomyopathy related to premature ventricular contractions.6 However, none of the patients published so far was treated with stereotactic radiotherapy for ventricular fibrillation (VF) storm.
Case report
We present a case of a 53-year-old male patient that was transferred to our center owing to VF storm. A few days earlier the patient had presented to the referring center with subacute anterior wall infarction caused by proximal occlusion of the left anterior descending coronary artery (LAD). The vessel was recanalized successfully using 2 drug-eluting stents and, owing to progressive symptoms of hemodynamic instability, a left ventricular assist device (Impella CP, Abiomed, Danvers, MA) was implanted. Postinterventional episodes of VF required intubation, deep sedation, and, finally, additional venoarterial extracorporeal membrane oxygenation (Cardiohelp, Maquet, Rastatt, Germany).
Under hemodynamic support of both assist systems, the patient was transported in a 2-hour drive to our center. Upon his arrival, the surface electrocardiogram revealed VF. Intravenous (iv) antiarrhythmic drug therapy was established by combining a beta-blocker, amiodarone (starting dose 75 mg/h, iv), and lidocaine (starting dose 83 mg/h, iv). With the help of this multidrug antiarrhythmic regimen, the patient could be successfully converted to sinus rhythm. However, recurrent VF episodes required repetitive defibrillation.
Considering that monomorphic ventricular premature beats consistently preceded VF occurrence, we decided to attempt trigger elimination by catheter ablation (Figure 1A). Since retrograde access to the left ventricle was hindered by the Impella device, a transseptal approach was chosen. A transseptal occluder (Gore, Cardioform Septal Occluder, W. L. Gore & Associates, Flagstaff, AZ) that had been implanted only a few weeks earlier owing to cryptogenic strokes could be passed safely following careful predilation with a noncompliant coronary high-pressure balloon (Pantera Leo 5.0 × 15 mm, Biotronik, Berlin, Germany), as reported by Sang and colleagues.7 As expected, left ventricular endocardial mapping (CARTO 3 V6, Biosense Webster, Diamond Bar, CA) revealed a large scar of the anterior wall of the left ventricle (Figure 1B). The origin of the trigger arrhythmia could be located within the scar using a pace-mapping approach, and extensive catheter ablation (25 minutes) was performed at and around the site of best pace map (90% match) (Figure 1B). However, the trigger arrhythmia, as well as VF, reoccurred early after the procedure (Figure 2A). Considering that ischemia-induced premature beats generally arise from the Purkinje system within the infarcted area, we decided against epicardial mapping. A further attempt to stabilize the patient was performed by percutaneously implanting a right ventricular pacemaker lead and increasing the heart rate to 80 beats/min. Furthermore, class I antiarrhythmic ajmaline (starting dose 30 mg/h, iv) was added temporarily. A comprehensive overview of the full therapeutic regimen is shown in Figure 2B.
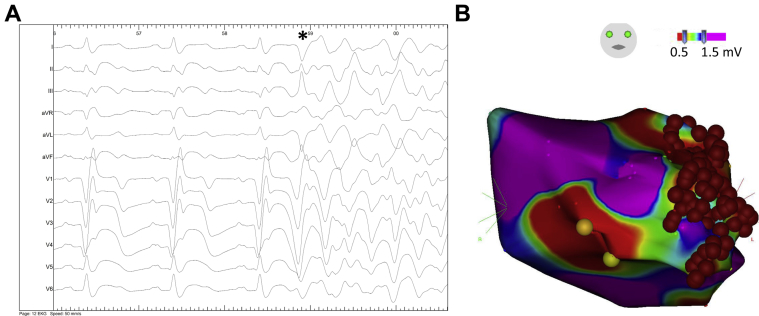
Figure 1.
A: Surface electrogram obtained during the invasive electrophysiological procedure visualizing the initiation of ventricular fibrillation triggered by a ventricular premature beat (*). B: Endocardial left ventricular voltage map reveals large areas of scar including the anterior wall. Areas of normal bipolar voltage amplitude (>1.5 mV) are displayed in purple and areas of scar (<1.5 mV) are displayed in colors ranging from red (<0.5 mV) to blue (see color scale). Catheter ablation (red spheres) was performed at and around the location of best pace map.
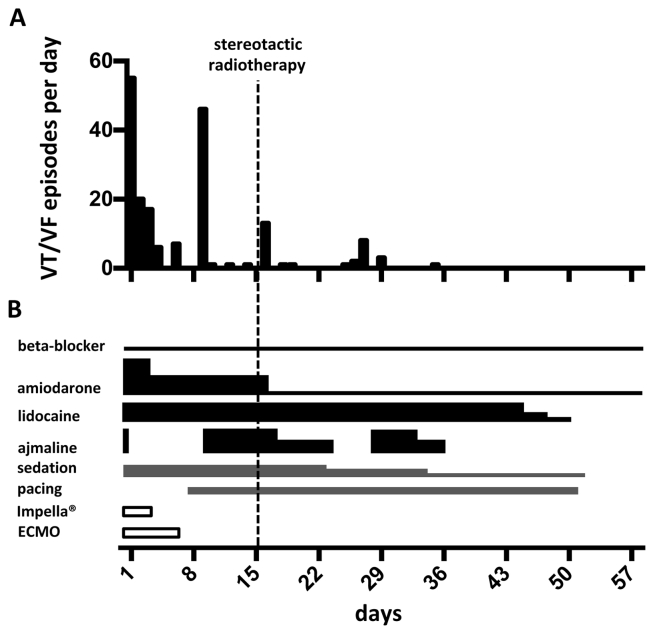
Figure 2.
A: Number of treated arrhythmia episodes per day over a time period of 60 days. B: The corresponding therapeutic regimen. ECMO = extracorporeal membrane oxygenation; VF = ventricular fibrillation; VT = ventricular tachycardia.
Since all available cardiologic therapeutic strategies failed to free the patient from VF reoccurrence and the patient still needed deep sedation, and prognosis was judged to be poor, we decided, as a therapy of last resort, to attempt trigger elimination and scar homogenization using stereotactic ablative radiotherapy as reported by Cuculich and colleagues.5 Therefore, at day 15 a 4-dimensional (4D) computed tomography (CT) was performed to apply radiation planning. Based on all available information from coronary angiography, left ventricular angiography, and the 3-dimensional electroanatomic map, we defined a 3-cm-wide stripe parallel and left lateral to the LAD over a length of 8 cm as clinical target volume (33.2 mL) (Figure 3). From the 4D CT information we extracted movement and deformation induced by heartbeats and mechanical lung ventilation. These data were added as safety margins to the clinical target volume, leading to an internal target volume of 55.8 mL. Finally, we generated the planning target volume (PTV) by adding an isotropic 2-mm safety margin to the internal target volume to compensate for uncertainties in the beam setup. This final volume comprised 82.4 mL. The maximum dose in the PTV reached 30 Gy and was well aligned with the proposed range of 25–35 Gy, as suggested before.5 Stereotactic ablative radiotherapy was performed as volumetric modulated arc therapy (3 arcs, 6 MeV, flattening filter free, 4990 monitor units, dose rate 500–1400 monitor units/min) using a linear accelerator (Elekta Versa HD, Stockholm, Sweden). Treatment time was 30 minutes and comprised 20 minutes for positioning the deeply sedated patient and reprogramming of mechanical ventilation to resimulate the situation of the planning CT that was created 6 hours earlier, 5 minutes for image guidance by 4D kV cone beam CT and portal imaging to verify correct patient positioning, and finally a further 5 minutes as pure irradiation time.
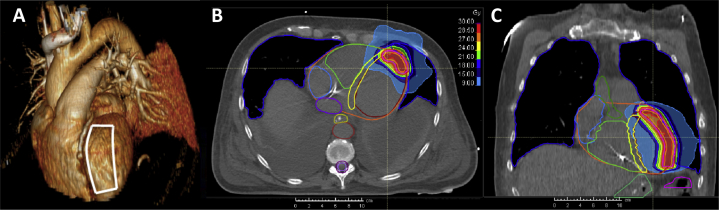
Figure 3.
A: Target area drafted on a 3-dimensional cardiovascular reconstruction of the planning computed tomography (CT). Dose distribution of radiotherapy in an axial CT slice (B) and a frontal reconstruction (C) showing the planning target volume–encompassing 80% isodose in yellow (24 Gy) and the 95% isodose in red (28.5 Gy). Lowest depicted dose is the 30% isodose corresponding to 9 Gy.
After radiotherapy the antiarrhythmia medication, as well as the degree of sedation, could be reduced stepwise (Figure 2). The last arrhythmia episode occurred 2 weeks after radiation. After complete cessation of all VF episodes, an implantable cardioverter-defibrillator was implanted and the patient could be discharged from the intensive care unit in full consciousness and without any neurologic deficit. Holter electrocardiogram revealed as few as 6 single ventricular premature beats over a time period of 24 hours. Cardiac echocardiography at the beginning and at the end of hospital stay showed no difference with respect to left ventricular ejection fraction and motion of the anterior wall.
Discussion
To the best of our knowledge, this is the first documented case in which stereotactic radiotherapy has been applied to stabilize a patient with life-threatening refractory VF storm. This case exemplarily shows that noninvasive stereotactic ablative radiotherapy is feasible and can be performed safely and promptly in an emergency setting. Furthermore, considering the complete abolishment of arrhythmia burden starting 2 weeks after radiation, our data point to a potential effectiveness of stereotactic radiotherapy in electrically stabilizing a proarrhythmic postischemic infarction zone. Similar to both recently published studies, we decided to target the entire infarcted area of the anterior wall of the left ventricle.5, 6 This approach was chosen because the probability of a functional recovery of the akinetic scar was deemed very low. Furthermore, the trigger arrhythmia resided deep within the center of the scar, and owing to absence of VT a clear exit site could not be defined. In contrast to Cuculich and colleagues,5 we decided to prescribe a single dose of 24 Gy to the PTV-encompassing 80% isodose, resulting in a maximum dose of 30 Gy as normalization dose. This concept leads to a steeper dose gradient outside the PTV and is commonly used in stereotactic radiotherapy, especially if the PTV is in close proximity to organs or structures at risk, like the LAD. In previously published cases the dose was prescribed homogeneously to the PTV. We chose a different approach in order to reduce the dose to the surrounding healthy tissue, taking into account the comparably large target volume.
From the time point of radiation it took 2 weeks until the patient was free from any arrhythmia episode requiring therapy. Perhaps the temporary increase in events 10 days after irradiation was triggered by a too-early reduction in sedation or antiarrhythmics. However, this time span is in line with a previous report by Jumeau and colleagues,8 reporting almost immediate reduction of VT episodes that cannot be attributed to radiation-induced fibrosis. But still little is known about the exact biological effects of such high radiation doses on viable or infarcted cardiac tissue. Considering radiation-induced side effects, from our experience in treating lung tumors we expect a pneumonitis of the left upper lung lobe within 1 year and, even later, a residual but local radiation-induced lung fibrosis. In most cases this remains a radiologic finding only, but there is also a risk for a clinically relevant pneumonitis that would require medical treatment, as observed for 2 of 19 patients in a report published by Robinson and colleagues.6 Nevertheless, our case supports the idea that high-dose radiotherapy may trigger a fast consolidation of the infarcted area and might have played a crucial role in the electrical stabilization of the scar area as well. However, it cannot be excluded that consolidation of the infarcted area by healing might have contributed to or even caused the electrical stabilization of the patient as well. Prospective randomized studies are urgently needed to further clarify this aspect. Furthermore, we realize that the follow-up of approximately 60 days seems short. However, considering the dramatic reduction in arrhythmia burden following radioablation, we are convinced that the data still remain relevant.
Conclusion
We present a case with favorable outcome in a patient suffering from severe postischemic VF storm in which stereotactic radiotherapy was paralleled by a rapid decline of arrhythmia burden. This case further strengthens stereotactic radiotherapy as a bailout strategy in patients with refractory ventricular arrhythmias. However, caution is warranted and patients should therefore be selected carefully, since data on long-term toxicity are scarce.
References
- 1.Writing Committee Members. Zipes D.P., Camm A.J. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:746–837. doi: 10.1093/europace/eul108. [DOI] [PubMed] [Google Scholar]
- 2.Exner D.V., Pinski S.L., Wyse D.G., AVID Investigators Antiarrhythmics Versus Implantable Defibrillators. Electrical storm presages nonsudden death: the antiarrhythmics versus implantable defibrillators (AVID) trial. Circulation. 2001;103:2066–2071. doi: 10.1161/01.cir.103.16.2066. [DOI] [PubMed] [Google Scholar]
- 3.Gatzoulis K.A., Andrikopoulos G.K., Apostolopoulos T. Electrical storm is an independent predictor of adverse long-term outcome in the era of implantable defibrillator therapy. Europace. 2005;7:184–192. doi: 10.1016/j.eupc.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Kowey P.R. An overview of antiarrhythmic drug management of electrical storm. Can J Cardiol. 1996;12 Suppl B:3B–8B. discussion 27B–28B. [PubMed] [Google Scholar]
- 5.Cuculich P.S., Schill M.R., Kashani R. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377:2325–2336. doi: 10.1056/NEJMoa1613773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson C.G., Samson P.P., Moore K.M.S. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation. 2019;139:313–321. doi: 10.1161/CIRCULATIONAHA.118.038261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sang C.-H., Dong J.-Z., Long D.-Y. Transseptal puncture and catheter ablation of atrial fibrillation in patients with atrial septal occluder: initial experience of a single centre. Europace. 2018;20:1468–1474. doi: 10.1093/europace/eux282. [DOI] [PubMed] [Google Scholar]
- 8.Jumeau R., Ozsahin M., Schwitter J. Rescue procedure for an electrical storm using robotic non-invasive cardiac radio-ablation. Radiother Oncol. 2018;128:189–191. doi: 10.1016/j.radonc.2018.04.025. [DOI] [PubMed] [Google Scholar]