Highlights
-
•
Acute OP poisoning complicated with pulmonary thrombosis during the first week of poisoning.
-
•
Antidote treatment included atropine, whereas diazepam was administered in the first 48 h.
-
•
There was no administration of oximes due to unavailability.
-
•
Prolonged hypoxemia in acute OP intoxication indicates exclusion of thrombotic pulmonary event.
Keywords: Organophosphates, Poisoning, Coagulation, Pulmonary thrombosis, Atropine
Abstract
Objective
Acute organophosphate (OP) poisonings are presented with acetylcholine-receptor overstimulation. There have been a few case reports of thrombotic complications in acute OP poisonings, as well as prolonged thrombosis preconditions in patients who survived this type of intoxications. The paper presents a case with pulmonary thrombosis (PT) that develops in the subacute phase of intentional acute OP poisoning, treated only with atropine, as well as a literature overview of OP-induced prothrombotic toxicity.
Case report
A middle aged woman was brought to the hospital after ingestion of unknown insecticide with suicidal intentions. She had a history of HTA (arterial hypertension), hyperlipidemia and untreated depression. The clinical features of poisoning were miosis, vomiting, dizziness, abdominal cramps and diarrhea. Soon after admission, she developed difficulties in breathing with decrease of serum pseudocholinesterase (2590...1769...1644...800 U/l), bibasal pulmonary crackles, drop of SpO2 to 84%. Antidote treatment included carbo medicinalis, atropine, and diazepam, without use of oximes. The seventh day pseudocholinesterase, the levels started to rise but the patient’s hyposaturation (SpO2 86-88%) persisted. Chest ultrasound detected hypoechoic subpleural lesion to the right. Haemostatic tests showed increased D-Dimmer (2312 ng/ml) with hypercoagulability. The CT pulmonary angiography confirmed PT and after the administration of low molecular heparin, her clinical condition improved.
Conclusion
Acute organophosphate poisoning treated with atropine showed a potential for inducing prothrombotic coagulation abnormalities, presented with PT. This life-threatening complication may additionally contribute to prolonged morbidity and mortality in OP poisonings, especially in patients with medical history of comorbidites.
1. Introduction
OPs (Organophosphates) are worldwide used as efficient insecticides in agriculture. The easy accessibility and high toxicity make them very powerful means for performing suicide attempts as a global medical problem. OPs inactivate acetilcholinesterase (AChE), which result in toxicity associated with high concentrations of internal acetylcholine (ACh) and receptor overstimulation. Clinical presentation of acute OP (Organophosphate) poisoning includes muscarinic, nicotinic and central nervous system symptoms. Treatment protocol consists of AChE reactivator (oximes), atropine and diazepam. While acute intoxications with OPs induce cholinergic crisis and respiratory depression, chronic exposure is associated with development of wide series of toxic effects such as hepatotoxicity [1], nephrotoxicity [2], cardiotoxicity [3], neurotoxicity, embriotoxicity [4], reduced fertility [5], with particularly increased susceptibility during exposure in the pre-pubertal period. Some of the involved mechanisms of toxicity were oxidative stress (OS), genotoxicity, and chronic inflammation processes [1,6].
OP poisonings are associated with “high morbidity and death hazard, with the ratio of death being 2.4 times higher than comparisons” [7]. The susceptibility to OPs toxicity is modulated by the activity of xenobiotic metabolizing enzymes, such as paraoxonase -1(PON-1). Its activity may be variable due to the genetic PON1-55 and -192 polymorphisms with great interethnic and individual variability in detoxifying various OPs [8]. Lately, a nationwide prospective cohort study pointed out increased prothrombotic diathesis in patients who survived acute OP intoxication [9]. Thrombotic complications associated with non-target tissues of ACh receptor overstimulation were also described, such as myocardial infarction [10] and “upper limb venous thrombosis” [11]. In the published cases so far, there was no reported thrombosis of pulmonary circulation developed during subacute phase of OP (Organophosphate) poisoning.
This paper presents a case with pulmonary thrombosis (PT) in subacute phase of intentional acute OP poisoning treated only with atropine, as well as literature overview of novel perspectives in prothrombotic mechanisms of OP toxicity.
2. Case report
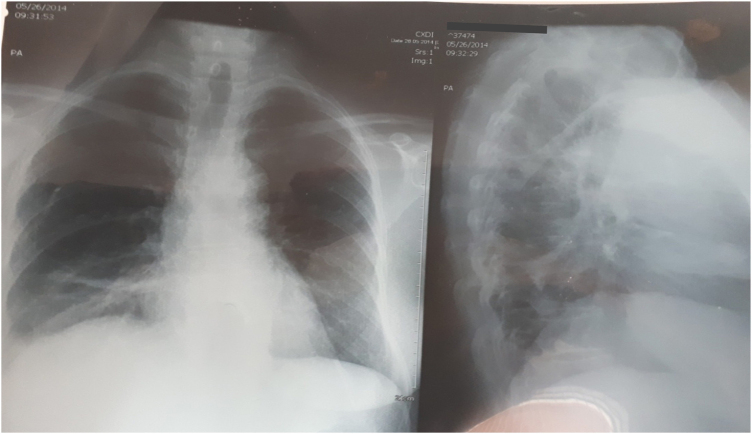
A 52 years old woman was admitted at our clinic after one and half hour of unknown insecticide ingestion in a suicidal attempt. She was a cigarette smoker, who had one year old history of HTA and hyperlipidemia with regular drug control (nifedipine, losartan, hydrochlorothiazide, and statines), untreated depression and no history of either circulatory complications or thromboembolism. She was already treated with atropine 1 mg iv by the emergency medical service. She complained of vomiting, dizziness, abdominal cramps and diarrhea. At admission she was alert, oriented, with miosis, blood pressure 135/90 mm Hg, electro cardiogram (ECG): sinus rhythm, HR 100/min with normal axis, rare pulmonary bibasal crackles and SpO2 96%. The laboratory findings at admission showed normal blood count with increased white blood cell count (WBC) 15,9 × 109/l, neutrophil 91,5%, glycemia 8,7 mmol/l, normal hepatic and pancreatic enzyme status, BUN, creatinine and electrolytes. The pseudocholinesterase (butyrylcholinesterase) concentration was at lower range of reference values: 3828 U/l (4000–12000 U/l).Treatment included gastric lavage, single dose of carbo medicinalis, intravenous atropine, diazepam, diuretics, antibiotics (ceftriaxone), antihypertensive, statins, antiarrhythmic (verapamil) therapy. Oxime therapy was not included, as a result of antidote deficiency in the country. Intravenous atropine bolus was started reaching full atropinization at 12 mg and continued with maintenance atropine. Diazepam dose regime was 0,25 mg/kg im for the first 48 h. After the first couple of hours she developed difficulties in breathing, followed by a decrease of serum pseudocholinesterase during the next two hours (2590…1769…800 U/l), bibasal pulmonary crepitations, drop of SpO2 to 84% with HR 120/min and biphasic T waves in inferiors ECG leads with simultaneously increasing atropine dosage that reached 36 mg per day. Despite the atropinization and clear lungs, the referent patient’s SpO2 was maintained only with oxygen therapy. The seventh day of stay, serum pseudocholinesterase levels began to normalize with a daily increase over the following four days as follows: 1872…3431…3981…4324U/l, but the patient’s dyspnoea and hyposaturation (SpO2 86–88%) without oxygen therapy persisted. The laboratory findings showed WBC 8,1 × 109/l, C-reactive protein 8,0 mg/l, normal protein status and negative microbiological findings (sputum and pneumoslide panel test), ECG presentation as HR 70–80/min (under antiarrhythmic therapy) with 1–2 mm ST segment depression, biphasic T waves in D2, D3, aVF, V4-V6. The chest radiography revealed elevated right hemidiaphragm with atelectasis (Fig. 1).
Fig. 1.
Chest radiography with elevated right hemidiaphragm and atelectasis.
The chest ultrasound detected hypoechoic subpleural lesion right. Suspecting PT, haemostasis tests were performed revealing increased D-Dimer (2312 ng/ml) and hypercoagulability (Table 1).
Table 1.
Haemostasis tests with D-dimer.
| Pl t (x109/l) |
PT (s) |
aPTT (s) |
TT(s) |
DD(ng/ml) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Plt | N | Pt | N | Pt | N | Pt | N | Pt | N |
| 232 | 150–450 | 11 | 13 | 25 | 33 | 20 | 21 | 2312 | <500 |
Plt-platelets, N-normal, PT-prothrombin time, aPTT-activated partial thromboplastin time, TT-thrombin time, DD-D-dimer.
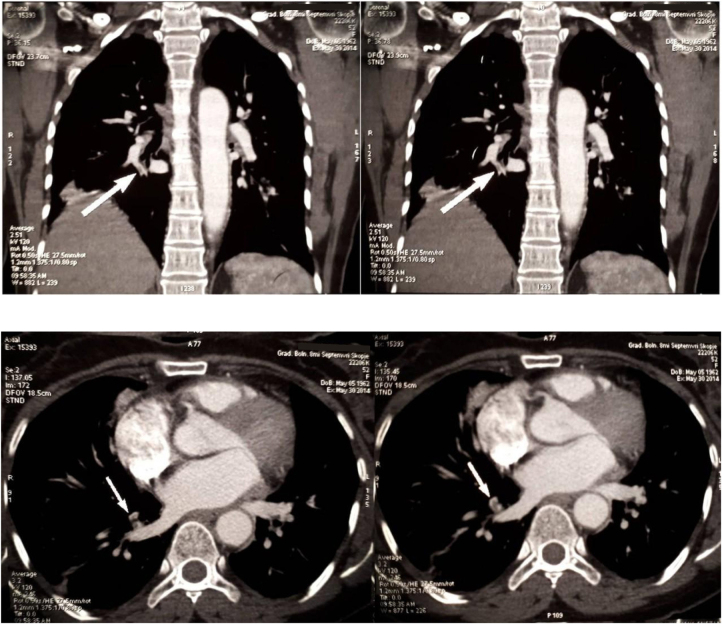
Her vital parameters began stabilizing 2 days after the start of low molecular heparin therapy (enoxaparin 1 mg/kg sc. Tid). The Computed Tomographic (CT) angiography of lungs (10th day of hospitalization) showed suboclusive thrombus in the artery of the right anterior basal pulmonary segment with the post-ischemic parenchyma zone confirming pulmonary embolism (Fig. 2).
Fig. 2.
CT angiography of PT.
The doppler ultrasound of the lower extremities was normal. The patient was examined by a psychiatrist, who prescribed antidepressants and was then transferred to the Clinic of Pulmonology. She was released in full recovery with oral anticoagulant therapy. The control CT angiography after 6 months showed no residuals of thrombus. But the patient continued the treatment at the Pulmonology Clinic, due to the development of Chronic Obstructive Pulmonary Disease (COPD).
3. Discussion
To our knowledge, this is the first report of acute OP poisoning complicated with PT during the first week of intoxication’s clinical presentation.
Due to the unknown insecticide that she used and the inability to confirm its composition by laboratory analysis, the poisoning with acetylcholinesterase inhibitor in our patient was considered to be of organophosphorus rather than carbamate insecticide; the inhibition of acetylcholinesterase lasted for 7 days, while carbamate poisonings were characterized with
“quick hydrolysis of the inhibited carbamylated acetyicholinesterase to the free active enzyme and carbamic acid” and with clinical signs relieve [12].
Late complications after acute OPs poisoning, such as organophosphate induced delayed polyneuropathy due to neuropathy target esterase inhibition and extrapyramidal syndrome, were frequently described; long term central nervous system toxicity (neuropsychiatric, cognitive and neurobehavioral effects) have also significant presentation in specific population groups of workers who had been at high risk of chronic exposure to OPs including unidentified exposure to low levels of OPs nerve agents [13]. Recently, thrombotic preconditioning has been shown in patients without comorbidites, who had been treated for acute OP poisoning; they presented a” 1.55-fold increased risk for developing DVT compared with the general population” in a one year follow up after the index date (acute OP poisoning); developing pulmonary thromboembolism (PTE) after surviving acute OP poisonings was significantly higher in patients with a history of comorbidites [9]. Also, thrombotic events during acute OP poisoning have been illustrated in several studies; there was a case report that showed upper limb deep vein thrombosis (DVT) as result of a complication of acute suicidal OP poisoning [11] and several cases of acute myocardial infarction, one resolved with fibrinolytic therapy [10]. Mural thrombosis was found at an autopsy in severe OP poisoning [14]. “Cerebral infarcts and gangrene requiring amputation” were described as complications of acute organophosphate poisoning in patients with preexisting vascular disease [15]. There is a case report of poisoning presented as organophosphoros with clinical features of PTE and coronary thrombosis that acutely developed after 20 days (delayed phase) of prolonged intensively treated intoxication [16]. However, the patient had slightly reduced pseudocholinesterase and the poisoning was caused by organic phosphorus fertilizer (mahaphos), the toxicity of which is not associated with AChE inhibition. Hovewer, there are no published reports regarding PT, as a complication or potential organ specific manifestation of acute OP poisoning.
PT is a life-threatening condition with 17% total mortality in patients with confirmed PT for 6 months follow up [17]. Acute pulmonary hypertension is a result of pulmonary artery obstruction (thrombosis) and vasoconstriction, as leading mechanisms inducing acute right ventricle failure and death [18]. The literature overview revealed that pulmonary vasoconstriction and thrombosis in poisonings with acetylcholinesterase inhibitors (AChEI) had a background associated with the activity of several mediators, oxidative stress, endothelial injury and therapy agents.
OP poisoning induced vasoconstriction in pulmonary circulation was mediated by the effects of thromboxane A2 and serotonin, as important pulmonary vasoconstrictors [19]. ACh normally decreases pulmonary vascular resistance, but higher concentrations of ACh in conditions when AChE is inactivated would result in pulmonary artery vasoconstriction [20], [21]. Experimental studies showed that the underlying mechanism of ACh induced vasoconstriction was a generation of tromboxane A2 as a primary pulmonary vasoconstrictor [22]. Acute OP poisonings also activated the “5-HT system with increase in 5-HT activity and resulting 5-HT release” [23] which was a potent pulmonary artery vasoconstrictor [24]. Additionally, atropine as a therapy agent had a potential to induce pulmonary vasoconstriction [25] too, by increasing serotonin levels [26]. Although an experimental study showed that regular doses of atropine can block thromboxane A2 vasoconstrictive effect [22], few studies also presented atropine induced impaired pulmonary ventilation with decreased perfusion, due to vasoconstriction in atropine dose-dependent manner [27]. In addition, in a reported acute OP poisoning treated with excessive doses of atropine, the ischemic extrapulmonary complications were explained with “paradoxical vasoconstriction induced by acetylcholine activity at sites of endothelial injury” [15]. Sympathetic overstimulation as a vasoconstrictive factor should be taken into consideration too, when discussing OP poisoning treated with atropine, as an additional link in the vasoconstrictive cascade.
The prothrombotic milieu in OP poisonings is favored by inducing oxidative stress in the human body, presented with increased production of ROS and RNS, lipid peroxidation and decreased enzymatic (SOD, CAT, GSH-Px) and non-enzymatic (vitamin C, E beta-CAR) antioxidant defense [[28], [29], [30]]. Normally, SOD activity is important for normal platelet function and thrombosis prevention, resulting in maintaining NO levels [31]. In OP intoxications, the resultant oxidative stress is associated with endothelial injury [32] and platelets dysfunction/aggregation [31] as an initial step for developing DVT [33] and PTE [34].The prothrombotic activity in OP poisonings was associated with thromboxane A2 and serotonin activity too. The increased 5-HT promotes platelet aggregation [35] and potentiates platelets procoagulant activity [36].Thromboxane A2, induced by increased ACh [22], stimulates activation of new platelets, as well as increased platelet aggregation [37]. Moreover, there was a case report of angiography verified coronary thrombosis induced by intracoronary acetylcholine injection [38]. Experimentally, some of the OP (soman and paraoxon) poisonings involved increase synthesis of Alpha (1)-acid glycoprothein [39] which induced platelet shape change and activation, indicating the influence of OPs on hemostasis and thrombosis using this mechanism [40]. The ACh induced prothrombotic effects were potentiated in the atherosclerotic vessels [41], giving the potential to OP poisoning to increase the prorthrombotic activity and pulmonary vasoconstrictive tone in patients with comorbidities, as it was the case with our patient with a history of HTA and hyperlypidemia.
An experimental study [42] showed that atropine did not inhibit thrombin activity and did not affect coagulation times (PT, aPTT, TT). Contrary to this, a study was published where atropine manifested increased prothrombotic activity by shortening of the total and thrombin time and lowering of fibrinolysis [43]. Atropine stimulated a rise in serotonin, which “increased procoagulant activity and reduced fibrinolytic activities of endothelial cells through 5-HT2A receptor” [44]. On the other hand, the same study of Golderman V et al. showed that oximes and paraoxon induced inhibition of thrombin with prolongation of coagulation time, but they did not report the coagulation status under simultaneous application of paraoxon, oximes and atropine [42]. When intravenously paraoxon was administered, the hypercoagulability was seen in the sympathomimetic phase while the hypocoagulability was registered in the vagal phase of poisoning [45].
Diazepam has a also a significant place in the antidote palette for treating OP poisoning, with a beneficial outcome especially in seizures and fasciculation treatment [46], decreased development of neuropathy [47], brain and cardiac damage [48]. Several studies have described a significant reduction in acetylcholine effects under the influence of benzodiazepines [49] even when associated with reduced activity of serotonergic system [50], implying the benzodiazepine potential for reduction of prothrombotic acetylcholine/serotonin activity.
Studies of the benzodiazepine effects on platelet aggregation have been published with conflicting conclusions, but also with a different degree of influence on particular benzodiazepines [51]. Contrary to the described inhibitory effect of benzodiazepines on the platelet aggregation, other studies describe stimulating effects. Benzodiazepines inhibit human platelet activation but “significantly higher (357%) doses of diazepam were necessary to inhibit platelet aggregation and serotonin secretion”, compared to other benzodiazepines like flurazepam. Also, “diazepam does not inhibit thrombin-induced release of arachidonic acid, conversion of exogenously added arachidonic acid into MDA, nor the action of prostaglandins” [52]. Considering the neutralization of OP-induced oxidative stress, the use of conventional antidotes (atropine, diazepam) has not yet confirmed a significant impact in the reduction of OS induced by OP poisoning, while the chemical structure of the AChE- reactivator is associated with the potential for induction of OS or antioxidant effects [53]. New treatment considerations emerged after experimentally induced reduction of OS, inflammation and caspase-3 activation in the liver and kidney, using Manganese under conditions of exposure to OPs [54].
Diazepam-induced respiratory depression in OP poisoning has been described in a number of studies in humans and animals [46] versus studies of improving respiratory function under the action of diazepam that are more experimental [55]. Due to the persistence of hypoxemia in our patient, diazepam therapy was discontinued in order to avoid respiratory arrest and mechanical ventilation. The potential benefit from the use of benzodiazepines was additionally lowered as a result of the decrease in plasma concentrations of diazepam, during concomitant use of statins, which our patient regularly received [56]. Several reports and studies indicated an increased occurrence of thrombophlebitis in iv administration of diazepam, which may also be a source of systemic thromboembolism [57], and should be taken in consideration when intravenously administrated diazepam in OP poisoning, although diazepam was administered (im) to our patient. Further studies are necessary to evaluate the individual contribution of these multiple factors and antidotes interactions to coagulation abnormalities and their participation in the high OP- induced morbidity and mortality.
Other case reports of protein coagulation cascade disturbances in OPs poisonings presented opposing conclusions: prolongation of prothrombin time in a 14-month old child successfully corrected with vitamin K administration implying prolonged bleeding time [58] as opposite to the case report of blood coagulation activation, but efficiently treated with heparin implying developing Disseminated Intravascular Coagulation (DIC) [59]. It was found that prolonged bleeding in acute OPs poisonings was associated with more severe intoxications [60]. Finally, there was a case report of OP poisonings where the coagulation status was normal [61]. Studies analyzing predictors of major cardiovascular events also detected that “decline in AChE activity added a risk of adverse outcome” (stroke and myocardial infarction) [62], supporting the theory of prothrombotic preconditioning in parasympathetic dysfunction.
On the other side, we should also consider the association of depression with prothrombotic conditions and impaired fibrinolysis [63], making a significant background for coagulation abnormalities in acute OP poisoning of depressive patients. Respiratory depression in acute OP poisoning may overlap or even cover up PT with atypical presentation, thus postponing diagnosis and treatment of this serious health condition. These findings suggest careful analysis of the coagulation status in acutely poisoned patients with comorbidities and further investigations of the antidotes’ mutual effects on haemostasis.
4. Conclusion
Acute OP poisoning treated with atropine showed a potential for inducing prothrombotic coagulation abnormalities presented with PT. Presentation of prolonged hypoxemia should be considered as a diagnostic challenge in acute AChEI intoxication, which requires exclusion of the thrombotic pulmonary event. This life-threatening complication may additionally contribute to the increased morbidity and mortality in OP poisonings, especially in patients with medical history of comorbidites.
Author contribution
Contributed equally to all aspects of this manuscript.
Source of support
This research did not receive any specific grant from funding agencies in the public, commercial or not –for-profit sectors.
Transparency document
Footnotes
Selected images in this article were re-published [in June 2023] for privacy reasons
References
- 1.Tsitsimpikou C., Tzatzarakis M., Fragkiadaki P., Kovatsi L., Stivaktakis P., Kalogeraki A., Kouretas D., Tsatsakis A.M. Histopathological lesions, oxidative stress and genotoxic effects in liver and kidneys following long term exposure of rabbits to diazinon and propoxur. Toxicology. 2013;307:109–114. doi: 10.1016/j.tox.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Georgiadis G., Mavridis C., Belantis C., Zisis I.E., Skamagkas I., Fragkiadoulaki I., Heretis I., Tzortzis V., Psathakis K., Tsatsakis A., Mamoulakis C. Nephrotoxicity issues of organophosphates. Toxicology. 2018;406–407:129–136. doi: 10.1016/j.tox.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Zafiropoulos A., Tsarouhas K., Tsitsimpikou C., Fragkiadaki P., Germanakis I., Tsardi M., Maravgakis G., Goutzourelas N., Vasilaki F., Kouretas D., Hayes A., Tsatsakis A. Cardiotoxicity in rabbits after a low-level exposure to diazinon, propoxur, and chlorpyrifos. Hum. Exp. Toxicol. 2014;33:1241–1252. doi: 10.1177/0960327114532384. [DOI] [PubMed] [Google Scholar]
- 4.Tsiaoussis J., Hatzidaki E., Docea A.O., Nikolouzakis T.K., Petrakis D., Burykina T., Mamoulakis C., Makrigiannakis A., Tsatsakis A. Molecular and clinical aspects of embryotoxicity induced by acetylcholinesterase inhibitors. Toxicology. 2018;409:137–143. doi: 10.1016/j.tox.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Michalakis M., Tzatzarakis M.N., Kovatsi L., Alegakis A.K., Tsakalof A.K., Heretis I., Tsatsakis A. Hypospadias in offspring is associated with chronic exposure of parents to organophosphate and organochlorine pesticides. Toxicol. Lett. 2014;230:139–145. doi: 10.1016/j.toxlet.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Selmi S., Rtibi K., Grami D., Sebai H., Marzouki L. Malathion, an organophosphate insecticide, provokes metabolic, histopathologic and molecular disorders in liver and kidney in prepubertal male mice. Toxicol. Rep. 2018;5:189–195. doi: 10.1016/j.toxrep.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H.-S., Hsu C.-C., Weng S.-F., Lin H.-J., Wang J.-J., Su S.-B., Huang C.-C., Guo H.-R. Acute anticholinesterase pesticide poisoning caused a long-term mortality increase. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dardiotis E., Aloizou A.-M., Siokas V., Tsouris Z., Rikos D., Marogianni C., Aschner M., Kovatsi L., Bogdanos D.P., Tsatsakis A. Paraoxonase-1 genetic polymorphisms in organophosphate metabolism. Toxicology. 2019;411:24–31. doi: 10.1016/j.tox.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Lim Y.-P., Lin C.-L., Hung D.-Z., Ma W.-C., Lin Y.-N., Kao C.-H. Increased risk of deep vein thrombosis and pulmonary thromboembolism in patients with organophosphate intoxication. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S., Diwan S., Dubey S. Myocardial infarction in organophosphorus poisoning: Association or just chance? J. Emerg. Trauma Shock. 2014;7:131. doi: 10.4103/0974-2700.130885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naik M., Bhat T., Mir M., Jalaali U., Bhat A., Gowhar W., Naqash M. Organophosphorus poisoning: a rare case of upper extremity deep vein thrombosis. Indian J. Health Sci. Care. 2016;9:339. doi: 10.4103/2349-5006.196339. [DOI] [Google Scholar]
- 12.Remaley A.T., Hicks D.G., Kane M.D., Shaw L.M. Laboratory assessment of poisoning with a carbamate insecticide. Clin. Chem. 1988;34:1933–1936. http://www.ncbi.nlm.nih.gov/pubmed/3416473 (Accessed 10 March 2019) [PubMed] [Google Scholar]
- 13.Vucinic S., Antonijevic B., Tsatsakis A.M., Vassilopoulou L., Docea A.O., Nosyrev A.E., Izotov B.N., Thiermann H., Drakoulis N., Brkic D. Environmental exposure to organophosphorus nerve agents. Environ. Toxicol. Pharmacol. 2017;56:163–171. doi: 10.1016/j.etap.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Anand S., Singh S., Nahar Saikia U., Bhalla A., Paul Sharma Y., Singh D. Cardiac abnormalities in acute organophosphate poisoning. Clin. Toxicol. 2009;47:230–235. doi: 10.1080/15563650902724813. [DOI] [PubMed] [Google Scholar]
- 15.Buckley N.A., Dawson A.H., Whyte I.M. Organophosphate poisoning: peripheral vascular resistance--a measure of adequate atropinization. J. Toxicol. Clin. Toxicol. 1994;32:61–68. doi: 10.3109/15563659409000431. http://www.ncbi.nlm.nih.gov/pubmed/8308950 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 16.Umesh Babu R., Krishna Babu B.R., Kumar H., Gayathri B.N. Organophosphorus Poisoning Presenting as Pulmonary Thromboembolism. Medico-Legal Updat. 2012;12:40–41. https://www.researchgate.net/publication/319998172 (Accessed 10 March 2019) [Google Scholar]
- 17.van Beek E.J., Kuijer P.M., Büller H.R., Brandjes D.P., Bossuyt P.M., ten Cate J.W. The clinical course of patients with suspected pulmonary embolism. Arch. Intern. Med. 2019;157:2593–2598. doi: 10.1001/archinte.157.22.2593. http://www.ncbi.nlm.nih.gov/pubmed/9531228 (n.d.), (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 18.Lualdi J.C., Goldhaber S.Z. Right ventricular dysfunction after acute pulmonary embolism: pathophysiologic factors, detection, and therapeutic implications. Am. Heart J. 1995;130:1276–1282. doi: 10.1016/0002-8703(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 19.Smulders Y. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc. Res. 2000;48:23–33. doi: 10.1016/S0008-6363(00)00168-1. [DOI] [PubMed] [Google Scholar]
- 20.Mandel J., Taichman D. Saunders Elsevier; 2006. Pulmonary Vascular Disease. [Google Scholar]
- 21.Bick Rodger L. ASCP Press; Chicago: 1992. Disorders of Thrombosis and Hemostasis: Clinical and Laboratory Practice - Google Books.https://books.google.mk/books?id=d2bQQr3A-iMC&pg=PA53&lpg=PA53&dq=Bick+RL.+Vascular+bleeding+disorders.+In:+Disorders+of+thrombosis+and+homeostasis:+clinical+and+laboratory+practice.+Chicago:+ASCP+Press,1992:35&source=bl&ots=DEHWB97k_W&sig=ACfU3U1UjueoIEX (Accessed 10 March 2019) [Google Scholar]
- 22.Altiere R.J., Kiritsy-Roy J.A., Catravas J.D. Acetylcholine-induced contractions in isolated rabbit pulmonary arteries: role of thromboxane A2. J. Pharmacol. Exp. Ther. 1986;236:535–541. http://www.ncbi.nlm.nih.gov/pubmed/3080588 (Accessed 10 March 2019) [PubMed] [Google Scholar]
- 23.Judge S.J., Savy C.Y., Campbell M., Dodds R., Gomes L.K., Laws G., Watson A., Blain P.G., Morris C.M., Gartside S.E. Mechanism for the acute effects of organophosphate pesticides on the adult 5-HT system. Chem. Biol. Interact. 2016;245:82–89. doi: 10.1016/j.cbi.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacLean M.R., Herve P., Eddahibi S., Adnot S. 5-hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br. J. Pharmacol. 2000;131:161–168. doi: 10.1038/sj.bjp.0703570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritts H.W., Harris P., Clauss R.H., Odell J.E., Cournand A., Cournand A. The effect of acetylcholine on the human pulmonary circulation under normal and hypoxic conditions. J. Clin. Invest. 1958;37:99–110. doi: 10.1172/JCI103590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumari A., Sreetama S., Mohanakumar K.P. Atropine, a muscarinic cholinergic receptor antagonist increases serotonin, but not dopamine levels in discrete brain regions of mice. Neurosci. Lett. 2007;423:100–103. doi: 10.1016/j.neulet.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Gaspari R.J., Paydarfar D. Pulmonary effects of intravenous atropine induce ventilation perfusion mismatch. Can. J. Physiol. Pharmacol. 2014;92:399–404. doi: 10.1139/cjpp-2012-0429. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J.-F., Xu G.-B., Fang W.-J. Relationship between acute organophosphorus pesticide poisoning and damages induced by free radicals. Biomed. Environ. Sci. 2002;15:177–186. http://www.ncbi.nlm.nih.gov/pubmed/12244759 (Accessed 10 March 2019) [PubMed] [Google Scholar]
- 29.Ranjbar A., Solhi H., Mashayekhi F.J., Susanabdi A., Rezaie A., Abdollahi M. Oxidative stress in acute human poisoning with organophosphorus insecticides; a case control study. Environ. Toxicol. Pharmacol. 2005;20:88–91. doi: 10.1016/j.etap.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Giordano G., Afsharinejad Z., Guizzetti M., Vitalone A., Kavanagh T.J., Costa L.G. Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicol. Appl. Pharmacol. 2007;219:181–189. doi: 10.1016/j.taap.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Meng Y.Y., Trachtenburg J., Ryan U.S., Abendschein D.R. Potentiation of endogenous nitric oxide with superoxide dismutase inhibits platelet-mediated thrombosis in injured and stenotic arteries. J. Am. Coll. Cardiol. 1995;25:269–275. doi: 10.1016/0735-1097(94)00349-U. [DOI] [PubMed] [Google Scholar]
- 32.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekim M., Sekeroglu M.R., Balahoroglu R., Ozkol H., Ekim H. Roles of the oxidative stress and ADMA in the development of deep venous thrombosis. Biochem. Res. Int. 2014;2014 doi: 10.1155/2014/703128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halici B., Sarinc Ulasli S., Günay E., Nural S., Sen S., Akar O., Celik S., Unlu M. Assessment of inflammatory biomarkers and oxidative stress in pulmonary thromboembolism: follow-up results. Inflammation. 2014;37:1186–1190. doi: 10.1007/s10753-014-9844-y. [DOI] [PubMed] [Google Scholar]
- 35.Peters J.R., Grahame-Smith D.G. Human platelet 5HT receptors: characterisation and functional association. Eur. J. Pharmacol. 1980;68:243–256. doi: 10.1016/0014-2999(80)90522-1. http://www.ncbi.nlm.nih.gov/pubmed/7202490 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Vilchez I., Diaz-Ricart M., White J.G., Escolar G., Galan A.M. Serotonin enhances platelet procoagulant properties and their activation induced during platelet tissue factor uptake. Cardiovasc. Res. 2009;84:309–316. doi: 10.1093/cvr/cvp205. [DOI] [PubMed] [Google Scholar]
- 37.Paul B.Z., Jin J., Kunapuli S.P. Molecular mechanism of thromboxane A(2)-induced platelet aggregation. Essential role for p2t(ac) and alpha(2a) receptors. J. Biol. Chem. 1999;274:29108–29114. doi: 10.1074/jbc.274.41.29108. http://www.ncbi.nlm.nih.gov/pubmed/10506165 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 38.Endoh Y., Shibata N., Takeichi K., Shinya W. 1995. Coronary Thrombosis Induced by Intracoronary Acetylcholine Injection in a Patient With Normal Coronary Myocardial Infarction.https://www.jstage.jst.go.jp/article/internalmedicine1992/34/3/34_3_171/_pdf (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 39.Ivanović-Matić S., Poznanović G., Grigorov I., Dinić S., Mihailović M., Grdović N., Uskoković A., Martinović V., Arambašić J., Petrović M., Bogojević D. The organophosphate-induced acute-phase response is characterized by synthesis ofα1-acid glycoprotein that exhibits an immunomodulatory effect. J. Appl. Toxicol. 2008;28:63–71. doi: 10.1002/jat.1254. [DOI] [PubMed] [Google Scholar]
- 40.Gunnarsson P., Levander L., Påhlsson P., Grenegård M. α1-acid glycoprotein (AGP)-induced platelet shape change involves the Rho/Rho kinase signalling pathway. Thromb. Haemost. 2009;102:694–703. doi: 10.1160/TH09-03-0156. [DOI] [PubMed] [Google Scholar]
- 41.Ludmer P.L., Selwyn A.P., Shook T.L., Wayne R.R., Mudge G.H., Alexander R.W., Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N. Engl. J. Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 42.Golderman V., Shavit-Stein E., Tamarin I., Rosman Y., Shrot S., Rosenberg N., Maggio N., Chapman J., Eisenkraft A. The organophosphate paraoxon and its antidote obidoxime inhibit thrombin activity and affect coagulation in vitro. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalishevskaia T.M., Nikol’skaia M.G. Nature of vagotomy and atropine hypercoagulation. Fiziol. Zh. SSSR Im. I. M. Sechenova. 1979;65:398–404. http://www.ncbi.nlm.nih.gov/pubmed/456658 (Accessed 10 March 2019) [PubMed] [Google Scholar]
- 44.Kawano H., Tsuji H., Nishimura H., Kimura S., Yano S., Ukimura N., Kunieda Y., Yoshizumi M., Sugano T., Nakagawa K., Masuda H., Sawada S., Nakagawa M. Serotonin induces the expression of tissue factor and plasminogen activator inhibitor-1 in cultured rat aortic endothelial cells. Blood. 2001;97:1697–1702. doi: 10.1182/blood.v97.6.1697. http://www.ncbi.nlm.nih.gov/pubmed/11238110 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 45.Petroianu G., Toomes M., Maleck W., Bergler W., Rüfer R. Intravenous paraoxon (POX) exposure: coagulation studies in mini pigs. Chem. Biol. Interact. 1999;119–120:489–495. doi: 10.1016/s0009-2797(99)00062-9. http://www.ncbi.nlm.nih.gov/pubmed/10421487 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 46.Marrs T.C. Diazepam in the treatment of organophosphorus ester pesticide poisoning. Toxicol. Rev. 2003;22:75–81. doi: 10.2165/00139709-200322020-00002. http://www.ncbi.nlm.nih.gov/pubmed/15071817 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 47.Murphy M.R., Blick D.W., Dunn M.A., Fanton J.W., Hartgraves S.L. Diazepam as a treatment for nerve agent poisoning in primates. Aviat. Space Environ. Med. 1993;64:110–115. http://www.ncbi.nlm.nih.gov/pubmed/8431183 (Accessed 10 March 2019) [PubMed] [Google Scholar]
- 48.McDonough J.H., Jaax N.K., Crowley R.A., Mays M.Z., Modrow H.E. Atropine and/or diazepam therapy protects against soman-induced neural and cardiac pathology. Fundam. Appl. Toxicol. 1989;13:256–276. doi: 10.1016/0272-0590(89)90262-5. http://www.ncbi.nlm.nih.gov/pubmed/2792594 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 49.Rada P., Hoebel B.G. Acetylcholine in the accumbens is decreased by diazepam and increased by benzodiazepine withdrawal: a possible mechanism for dependency. Eur. J. Pharmacol. 2005;508:131–138. doi: 10.1016/j.ejphar.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 50.Petkov V., Georgiev V.P., Getova D., Petkov V.V. Effects of some benzodiazepines on the acetylcholine release in the anterior horn of the lateral cerebral ventricle of the cat. Acta Physiol. Pharmacol. Bulg. 1982;8:59–66. http://www.ncbi.nlm.nih.gov/pubmed/6133407 (Accessed 10 March 2019) [PubMed] [Google Scholar]
- 51.Rajtar G., Zółkowska D., Czechowska G., Kleinrok Z. Effects of antiepileptic drugs on rat platelet aggregation: ex vivo and in vitro study. Epilepsy Res. 2001;43:59–66. doi: 10.1016/s0920-1211(00)00176-5. http://www.ncbi.nlm.nih.gov/pubmed/11137387 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 52.Romstedt K., Huzoor-Akbar Benzodiazepines inhibit human platelet activation: comparison of the mechanism of antiplatelet actions of flurazepam and diazepam. Thromb. Res. 1985;38:361–374. doi: 10.1016/0049-3848(85)90135-5. http://www.ncbi.nlm.nih.gov/pubmed/2990062 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 53.Vanova N., Pejchal J., Herman D., Dlabkova A., Jun D. Oxidative stress in organophosphate poisoning: role of standard antidotal therapy. J. Appl. Toxicol. 2018;38:1058–1070. doi: 10.1002/jat.3605. [DOI] [PubMed] [Google Scholar]
- 54.Owumi S.E., Dim U.J. Manganese suppresses oxidative stress, inflammation and caspase-3 activation in rats exposed to chlorpyrifos. Toxicol. Rep. 2019;6:202–209. doi: 10.1016/j.toxrep.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dickson E.W., Bird S.B., Gaspari R.J., Boyer E.W., Ferris C.F. Diazepam inhibits organophosphate-induced central respiratory depression. Acad. Emerg. Med. 2003;10:1303–1306. doi: 10.1111/j.1553-2712.2003.tb00001.x. http://www.ncbi.nlm.nih.gov/pubmed/14644779 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 56.Słupski W., Trocha M., Rutkowska M. Pharmacodynamic and pharmacokinetic interactions between simvastatin and diazepam in rats. Pharmacol. Rep. 2017;69:943–952. doi: 10.1016/j.pharep.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell P.F. Diazepam-associated thrombophlebitis: a review and discussion of possible prevention. J. Am. Dent. Assoc. 1980;101:492–495. doi: 10.14219/jada.archive.1980.0299. http://www.ncbi.nlm.nih.gov/pubmed/6932436 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 58.Murray J.C., Stein F., McGlothlin J.C., McClain K.L. Prolongation of the prothrombin time after organophosphate poisoning. Pediatr. Emerg. Care. 1994;10:289–290. doi: 10.1097/00006565-199410000-00012. http://www.ncbi.nlm.nih.gov/pubmed/7845858 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 59.Jastrzebski J., Złotorowicz M., Szczepański M. Activation of blood coagulation induced by organophosphate pesticide. Mater. Med. Pol. 2019;26:33–34. http://www.ncbi.nlm.nih.gov/pubmed/7808040 (n.d.), (Accessed 10 March 2019) [PubMed] [Google Scholar]
- 60.Ziemen M. Thrombocyte function and plasma coagulation following poisoning with organophosphates. Klin. Wochenschr. 1984;62:814–820. doi: 10.1007/BF01711857. http://www.ncbi.nlm.nih.gov/pubmed/6482318 (Accessed 10 March 2019) [DOI] [PubMed] [Google Scholar]
- 61.Pieris R.R., Fernando R. Coronary artery bypass grafting in a patient with organophosphate poisoning. Heart Surg. Forum. 2015;18 doi: 10.1532/hsf.1370. E167–170. [DOI] [PubMed] [Google Scholar]
- 62.Arbel Y., Shenhar-Tsarfaty S., Waiskopf N., Finkelstein A., Halkin A., Revivo M., Berliner S., Herz I., Shapira I., Keren G., Soreq H., Banai S. Decline in serum cholinesterase activities predicts 2-year major adverse cardiac events. Mol. Med. 2014;20:1. doi: 10.2119/molmed.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lahlou-Laforet K., Alhenc-Gelas M., Pornin M., Bydlowski S., Seigneur E., Benetos A., Kierzin J.-M., Scarabin P.-Y., Ducimetiere P., Aiach M., Guize L., Consoli S.M. Relation of depressive mood to plasminogen activator inhibitor, tissue plasminogen activator, and fibrinogen levels in patients with versus without coronary heart disease. Am. J. Cardiol. 2006;97:1287–1291. doi: 10.1016/j.amjcard.2005.11.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.