Abstract
Cold-pressed juices are claimed to contain higher levels of antioxidants and bioactive compounds compared to normally centrifuged ones. Herein, we evaluated the antioxidant capacity and the bioactive compound contents of some freshly prepared fruit juices, extracted by a cold-pressed juicer and compared them to those prepared by a normal centrifugal juicer. We observed no significant differences between cold-pressed and normal centrifugal juices in terms of the contents of bioactive compounds (ascorbic acid, total phenolic, and total carotenoid) and antioxidant capacity (ferric ion reducing antioxidant power (FRAP) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity). Storage at room temperature (∼28 °C) adversely affected the ascorbic acid, total phenolics, total carotenoids, FRAP and DPPH values of the cold-pressed juices within 48 h. However, under simulated home-refrigerated storage conditions, the antioxidant capacity, contents of bioactive compounds and physicochemical properties of the cold-pressed juices remained unchanged till day 5 post-storage. However, at day 6, most of the parameters exhibited a decreasing trend and reached their lowest values at day 7. Principal component analysis confirmed significant changes in the quality of juices at day 7 of storage related to the first two principal components (ascorbic acid and FRAP). Our results strongly question the claim regarding the superior quality of cold-pressed juices. Moreover, our findings provided compelling evidence regarding the possible adverse effects of long storage under home-refrigerated conditions on the quality of cold-pressed juices.
Keywords: Food science, Antioxidant activity, Bioactive compound content, Cold-pressed juicing, Centrifugal juicing, Home-refrigerated storage, Physicochemical properties
1. Introduction
There is a large body of evidence suggesting an association between a diet rich in fruits and vegetables and fewer risk factors related to major chronic diseases in humans (Slavin and Lloyd, 2012), including cardiovascular disease (CVD) (Bazzano et al., 2002), several common cancers (Van’t Veer et al., 2000), and age-related degeneration (De Mello-Andrade and Fasolo, 2014). Health promotion guidelines and dietary recommendations strongly suggest a regular consumption of pure (100%) fruit juice (PFJ) (Williams, 1995, see review Caswell, 2009). A review of evidence published by Ruxton et al. (2006) recommended that PFJs (without any added components, e.g. sweeteners) retain the major health-promoting compounds of whole fruit; and therefore, can contribute to improved health conditions, justifying the increasing consumption of PFJs in recent years. Health-benefiting properties of fruit juices are ascribed mostly to their bioactive compounds, such as vitamin C, phenolic compounds, carotenoids, and tocopherols (Gardner et al., 2000; Sun et al., 2002).
Lifestyle changes and increased awareness among health-conscious consumers have driven the beverage industry to develop and introduce functional drinks with added nutritional values and health-promoting benefits (Siró et al., 2008). Moreover, the fast-growing demand for freshly squeezed unpasteurized fruit and vegetable juices reflects changes in consumer preferences for beverages without processing (Raybaudi-Massilia et al., 2009). Consequently, some beverage manufacturers have introduced freshly squeezed, unpasteurized, cold-pressed juices and have claimed that they are healthier and could be stored for more days than the normal centrifugal juices. This claim is based on the following justification. Regular fruit juices are extracted by centrifugal juicers that utilize a fast-spinning metal blade juxtaposed against a mesh filter. This process separates the juice extract from fruit flesh by centrifugal force. When the metal blade spins at a very high speed, it generates heat, which can negatively affect the bioactive compound contents of juice. On the contrary, cold-pressed juices are made by cold-pressed extractors that first crush and then press the fruit to extract its juice at a very low speed. This extraction process generates almost no heat and preserves the nutritional quality of the juice. Notably, the market prices of cold-pressed juices are much higher than those of normal centrifuged ones. However, there is no solid scientific basis for this claim. Previous studies have documented the effects of juice extraction methods on the quality of fruit juices. Miguel et al. (2004) investigated the effects of two different juice extraction methods (centrifugation by a Phillips Electric juice centrifuge vs. squeezing by a Phillips Electric lemon squeezer) on the quality of pomegranate juice. Interestingly, it was found that the two extraction methods did not have any effect on the quality parameters measured, including the composition of sugars and amino acids, color, pH, and anthocyanin content. In a study by Rajasekar et al. (2012), blending was reported to be a more effective approach to preserve the nutritional quality (antioxidant capacity, total phenolic, and total monomeric anthocyanin contents) of pomegranate juice compared to that of a mechanical press juice extraction. Pyo et al. (2014) found that Korean kernel fruit juices prepared by blending harbored a significantly stronger antioxidant capacity and higher total phenolic content than those obtained by juicing. However, the ascorbic acid contents of juices made by juicing were significantly higher than those by blending. Kim et al. (2017) compared the nutritional value of grape juice prepared by three different household juicers: low-speed masticating juicer, a high-speed centrifugal juicer, and a blender. It was found that the juice extracted by the low-speed masticating juicer had a higher nutritional quality than that of the other two types.
Another important factor associated with the quality of juices is the effect of storage conditions, mainly temperature and time-period on the nutritional value of juices. During storage, the degradation of some bioactive compounds, such as vitamin C, total phenolic content (TPC), and total carotenoids could occur, which is a critical factor regarding the quality of juices. Generally, beverage manufacturers set a short period, known as expiration date or shelf life, during which the physicochemical properties of juices remain conserved. However, during this period, the stability of bioactive properties, such as antioxidant capacity and the means by which they are affected by storage conditions remain unclear. To address this issue, a few studies have documented the effect of storage conditions on antioxidant capacity and bioactive compounds of some fruit juices (Del Caro et al., 2004; Piljac-Zegarac et al., 2009; Bhardwaj and Nandal, 2014; Mgaya-Kilima et al., 2014; La Cava and Sgroppo, 2015; Touati et al., 2016). However, to the best of our knowledge, no such information is available regarding the effect of storage conditions on the nutritional quality of freshly prepared unpasteurized cold-pressed juices. Moreover, there is no published study comparing the nutritional values of cold-pressed juices to normal centrifuged ones. Pineapple (Ananas comosus), guava (Psidium guajava L.), white-fleshed pitaya (Hylocereus undatus), known as white dragon fruit, and red-fleshed pitaya (Hylocereus costaricensis), known as red dragon fruit are among the highly nutritive and widely popular fruits commonly found in fresh fruit markets of Southeast Asia, including Thailand. These fruits have also gained a fast-growing popularity in international markets. Moreover, carrot (Daucus carota subsp. sativus) is a nutritious root vegetable with a worldwide consumption. Herein, we selected these fruit and vegetable species for the preparation of juices and aimed to evaluate and compare the antioxidant capacity of freshly prepared unpasteurized juices extracted by a cold-pressed juicer, normal centrifugal juicer, and blender. Moreover, we determined the effect of the storage temperature on the quality of cold-pressed juices. Finally, we investigated the impact of simulated home refrigeration-storage conditions of consumers on the physicochemical properties and nutritional quality of cold-pressed juices. The information obtained from this study will increase consumer awareness regarding the content of bioactive compounds and antioxidant capacity of cold-pressed juices and the effect of refrigerated storage on the quality of this type of fruit beverage.
2. Materials and methods
2.1. Fruit juice preparation
Fresh fruits, including pineapple, guava, carrot, red dragon fruit, and white dragon fruit of similar size and appearance for each type and free of any external defects were purchased from a local market in Bangkok, Thailand. The fruits were fully rinsed with tap water, wiped dry, peeled (except for guava), and cut into small pieces. Equal portions (10 g of fruit pulp from each fruit sample) were prepared prior to juice extraction. Three different juice extraction methods, including cold-pressed juicing, normal centrifugal juicing, and blending were used for each fruit type using a LIVIVE vertical cold-pressed juicer (Foshan Geuwa Electric Appliance Co. Ltd, China), Philips HR1866 centrifugal juicer (Philips, Netherlands), and Otto blender (Otto Kingglass Co. Ltd, Thailand), respectively (Fig. 1). The extracted juices were then filled in sterile 50-mL conical centrifuge tubes and centrifuged at 12000 rpm for 15 min at 4 °C. The supernatants were used for further analyses.
Fig. 1.
Three types of juice extractor, including normal centrifugal juicer, cold-pressed juicer and blender were used in this study. A representative picture of each extractor is presented.
To investigate the effect of storage conditions (temperature and time-period) on the quality of cold-pressed juices, extraction was performed as mentioned above; the extracted fruit juices were stored in sterile 50-mL centrifuge tubes and divided into three groups. The first group was immediately centrifuged, and the supernatant was used for analysis (fresh samples). The second group was stored under dark conditions at RT (∼28 °C) and sampled at 24 and 48 h. The third group was stored at 4 °C (simulated home-refrigerated storage) and sampled every day for a 7-day storage period. The juices were centrifuged immediately after the time-periods mentioned above and the supernatants were used for further analyses.
2.2. Chemicals
All reagents and internal standards used in this study were obtained from Sigma-Aldrich Inc. St. Louis, MO, USA.
2.3. Ascorbic acid content
Ascorbic acid content was determined following the method described by Klein and Perry (1982) with slight modifications. Briefly, a 100-μL aliquot of each juice supernatant was mixed with 50 μL of 3% metaphosphoric acid, and the mixture was incubated for 10 min at RT. The mixture was then added to 150 μL of 0.8 mM 2,6-dichloroindophenol (DCIP) and absorbance was measured within 30 s at 515 nm against a blank (DCIP mixed with acid). Content of ascorbic acid was calculated according to a standard curve derived from authentic L-ascorbic acid (1–50 mg/100 mL). The results were expressed as mg ascorbic acid per 100 g fresh fruit.
2.4. Total phenolic content (TPC)
TPC was measured by the Folin-Ciocalteu's method following a previously described procedure (Swain and Hillis, 1959). A 150 μL aliquot of each supernatant, 2400 μL of ultrapure water, and 150 μL of 0.5 N Folin–Ciocalteu reagent were mixed well using a Vortex mixer and were allowed to react for 30 min at RT (∼28 °C). Later, 300 μL of 1 N Na2CO3 solution was added to the mixture and mixed well. The mixture was then incubated at RT (∼28 °C) for 2 h under dark conditions and the absorbance measured at 725 nm using a microplate reader. The TPCs of juices were expressed as mg gallic acid equivalent (GAE) per 100 g fresh fruit based on a standard curve obtained from authentic gallic acid (0.2–4 mg/100 mL).
2.5. Total carotenoid content
Total carotenoid content was assessed following the spectrophotometric method (A470) described by Talcott and Howard (1999) and expressed as β-carotene equivalent per 100 g fresh fruit using a standard curve of authentic β-carotene (0.1–2 mg/100 mL).
2.6. Antioxidant capacity
Ferric ion reducing antioxidant power (FRAP) assay was performed according to the method described by Benzie and Strain (1996) with some modifications. The FRAP working solution was freshly prepared by mixing 300 mM acetate buffer (3.1 g C2H3NaO2·3H2O and 16 mL C2H4O2; pH 3.6), 10 mM 2, 4, 6-tripyridyl-s-triazine (TPTZ) in 40 mM HCl, and 20 mM FeCl3·6H2O in a 10:1:1 ratio, respectively, and warmed up at 37 °C before use. A 40-μL aliquot of the supernatant was mixed with 160 μL of the FRAP working solution in a 96-well plate and incubated at RT for 30 min under dark conditions. FRAP values (A593) were reported as μmol trolox equivalent (TE) per 100 g fresh fruit based on a standard curve of authentic Trolox (50–1700 μM).
1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity was performed according to a method described previously (Brand-Williams et al., 1995) with some modifications. The DPPH working solution was obtained by mixing 10 mL of the stock solution (24 mg of DPPH dissolved in 100 mL methanol) with 45 mL methanol. Next, the DPPH solution (950 μL) was mixed with 50 μL of each supernatant and incubated for 1 h under dark conditions. The A515 was read, and the DPPH values of the juices were calculated using the following equation:
where Absorbance is the A515 of DPPH solution without supernatant, is the A515 of DPPH solution with supernatant, and is the A515 of the supernatant.
2.7. Total microbial count
Each fruit juice was serially diluted (10−2−10−8) using autoclaved 0.1 % peptone water. Subsequently, a 1-mL aliquot of each diluted sample was placed on a Luria-Bertani (LB) agar plate and incubated at 37 °C for 2 d to obtain a total aerobic bacterial count. For the total yeast and mold count, a 1-mL aliquot was placed on a potato dextrose agar plate and incubated at 30 °C for 3–5 d. The results were expressed as log colony-forming units (CFU) per mL of juice.
2.8. Physicochemical analysis
Total soluble solid (TSS) was measured using a digital refractometer (HANNA HI96801, USA) and the results were reported in standard °Brix units. The pH of juices was determined with a digital benchtop pH meter (METTLER TOLEDO, USA). Color analysis of the juices was performed using a colorimeter (CR-410 chroma meter, Minolta, Japan). Three color parameters, including L* (lightness), a* (redness/greenness), and b* (yellowness/blueness) were measured. The color difference (ΔE) was calculated compared to that of freshly prepared juice as a control using the following equation:
To measure the cloud value representing the degree of turbidity or darkening of juices, A660 was read using a spectrophotometer (Eppendorf BioSpectrometer®, Eppendorf, USA) against a blank (distilled water) (Aadil et al., 2013).
2.9. Statistical analysis
The data obtained in this study were subjected to statistical analysis using SPSS software, version 20 (SPSS Inc., IBM). The data are presented as means ± standard deviation (SD) of three independent replicates. Statistical comparisons of the means were done using a one-way ANOVA, followed by Duncan's multiple range test or Student's t-test at the 0.05 confidence level.
3. Results
3.1. Bioactive compound contents and antioxidant capacity of cold-pressed juices compared to normal centrifugal ones
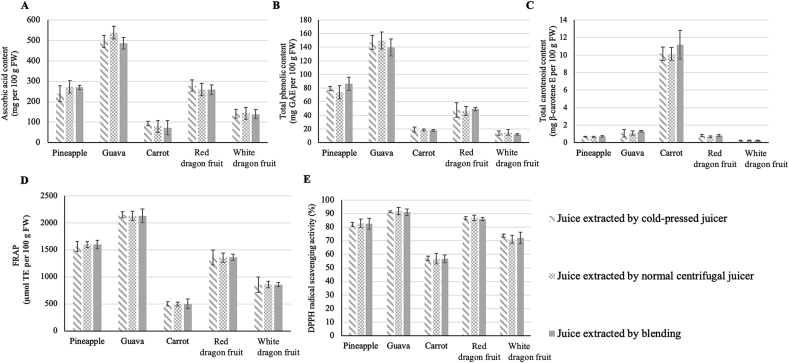
Three different types of juice extractors were used in this study: cold-pressed juicer, normal centrifugal juicer, and blender. Interestingly, as indicated in Fig. 2, no significant differences were observed among the different types of extraction methods in terms of the contents of bioactive compounds (ascorbic acid, total phenolic, and total carotenoid contents) and antioxidant capacity (FRAP and DPPH values) of the juices. Our observation, herein, questioned the claim that cold-pressed juice contains higher antioxidants and bioactive compounds compared to that of the normally extracted juice. Cold-pressed juices are claimed to have a longer shelf life compared to normal centrifugal ones. Therefore, to evaluate this claim, the effect of storage conditions (temperature and time-period) on the quality parameters of cold-pressed juices was investigated.
Fig. 2.
Content of bioactive compounds (ascorbic acid (A), total phenolic (B) and carotenoid (C) contents) and antioxidant capacity (Ferric ion reducing antioxidant power (FRAP) (D) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity (E)) of juices extracted by cold-pressed juicer, normal centrifugal juicer, and blender. Data are presented as mean ± standard deviation (SD) of three independent replicates. For each measured parameter, comparisons are shown for different extraction methods within each fruit juice; no significant differences were observed according to Duncan's multiple-range test (p < 0.05). (GAE: gallic acid equivalent, TE: Trolox equivalent, E: equivalent).
3.2. Effect of storage conditions (temperature and time-period) on the quality attributes of cold-pressed juices
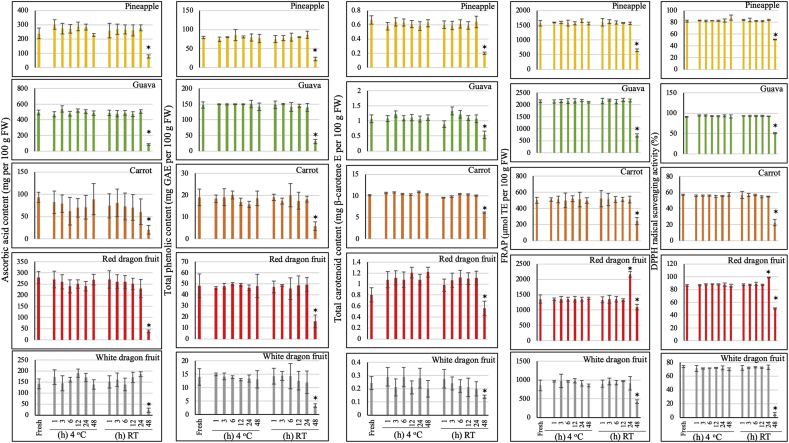
Among health-conscious consumers, there is an ever-existing concern regarding the effect of storage conditions on nutritional qualities of juices. Therefore, we aimed to study this phenomenon by comparing the content of bioactive compounds and antioxidant capacity of five types of cold-pressed juices kept at either RT (∼28 °C) or 4 °C (stored under simulated home-refrigerated conditions) over a period of 48 h. Interestingly, storage at 4 °C and RT during a 24-h period did not have any negative impact on the content of bioactive compounds and antioxidant capacity of all types of juices. The ascorbic acid, TPC, total carotenoids, FRAP and DPPH values of refrigerated juices and those kept at RT, measured at different time-points, did not vary significantly compared to those of the fresh ones, except for the red dragon juice kept at RT (Fig. 3). Notably, the antioxidant capacity (FRAP and DPPH values) of the red dragon juice stored at RT demonstrated a significant increase at 24 h compared to that of the fresh one. However, this phenomenon was not observed for the red dragon juice stored at 4 °C. Additionally, storage for 2 days (48 h) had different effects on the quality of juices depending on the temperature. Storage for 48 h at 4 °C did not have any negative effect on the contents of bioactive compounds and antioxidant capacity of juices. Ascorbic acid, TPC, total carotenoids, FRAP and DPPH values of the juices measured at 48 h did not vary significantly when compared to the fresh ones. However, storage at RT for a 48-h period significantly reduced the bioactive compound contents and antioxidant capacity of juices; all the measured values at 48 h declined significantly compared to those of the fresh juices (Fig. 3). Taken together, our results suggest that storing juices at 4 °C for 48 h can protect the bioactive compounds and antioxidant capacity of juices, whereas keeping at RT can significantly decrease these factors.
Fig. 3.
Effect of storage at room temperature (∼28 °C) and 4 °C on ascorbic acid, total phenolic, and carotenoid contents and antioxidant capacity (FRAP and DPPH) of cold-pressed juices. Bars represent the mean ± standard deviation (SD) of three independent replicates. For each measured value, comparisons are shown between the fresh juice and different time-points during storage of each fruit juice; an asterisk (*) above the bars indicates a significant difference at that time-point compared to the fresh juice (control) (Student's t-test, p < 0.05). (GAE: gallic acid equivalent, TE: Trolox equivalent, E: equivalent).
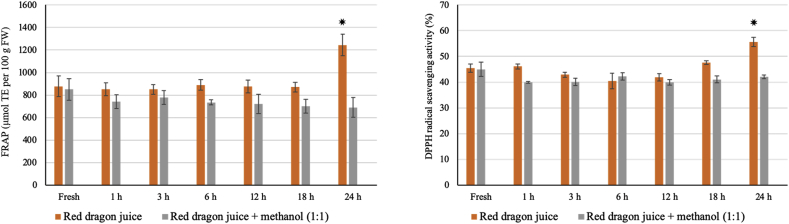
Surprisingly, antioxidant capacity of the red dragon juice stored at RT increased significantly at 24 h compared to that of the fresh one. This phenomenon could have occurred as a result of enzymatic activities during storage at RT. Furthermore, we did not observe any increase in the FRAP and DPPH values of the red dragon juice stored at 4 °C; this observation strengthened the possibility of an enzymatic reaction that could have occurred at a much slower rate at 4 °C. To further examine this possibility, we investigated whether adding pure methanol to the juice can denature proteins and inhibit the enzymatic reaction that is involved in increasing the antioxidant capacity. Interestingly, for the red dragon juice without methanol (control; juice + water, 1:1), both FRAP and DPPH values peaked at 24 h of storage life. However, for the juice mixed with methanol (juice + absolute methanol, 1:1), we did not observe any increase in these values at 24 h of storage (Fig. 4). This result provided convincing evidence that the increase in the antioxidant capacity of the red dragon juice could have occurred as a result of enzymatic reaction(s).
Fig. 4.
Effect of methanol on the antioxidant capacity of red dragon juice stored at room temperature for 24 h. Pure methanol was mixed with freshly prepared red dragon juice at a ratio of 1:1 and stored at room temperature. Ferric ion reducing antioxidant power (FRAP) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity were measured and compared to the control (red dragon juice without methanol; juice + water, 1:1). Bars represent the mean ± standard deviation (SD) of three independent replicates. An asterisk (*) above the bars indicates a significant difference at that time-ponit between red dragon juice with methanol and control (Student's t-test, p <0.05).
3.3. Effects of a 7-day simulated home-refrigerated storage on the quality of the cold-pressed juices
It was observed that storing the juices refrigerated (4 °C) over a 48-h period did not cause any detrimental effect on the quality of cold-pressed juices. This observation prompted us to further examine the effect of a longer refrigerated storage period (a 7-day storage period) on physicochemical properties, content of bioactive compound, and antioxidant capacity of juices.
3.3.1. Effect on total microbial count and physicochemical properties [total soluble solid (TSS), pH, cloud value, and color]
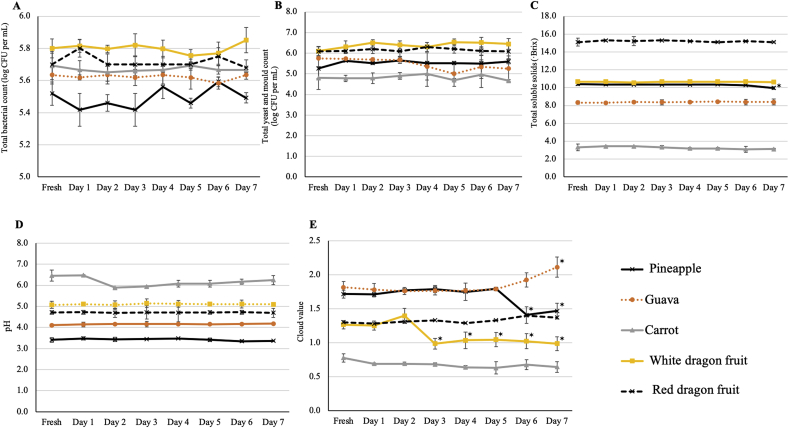
Preserving juices for a 7-day period at 4 °C did not affect the total microbial count of all the juices. The total aerobic bacteria and yeast/mold counts of juices did not vary significantly during the storage period compared to those of the freshly squeezed ones. Moreover, the TSS and pH values of the juices measured during the 7-day storage period did not demonstrate significant changes compared to those of the freshly prepared ones, except for pineapple juice, which showed a significant decrease in the TSS value at day 7 of storage (Fig. 5). Furthermore, cloud value showed significant changes during the storage period for some juices. However, the cloud values of carrot and red dragon juice remained constant during the storage period and showed no significant differences compared to those of the fresh ones. In contrast, the cloud value of white dragon juice declined significantly at day 3 and remained constant during the remaining storage days. Moreover, for the pineapple juice, the cloud value showed a significant decrease at day 6 of storage; however, the guava juice was the only type of juice with an increased cloud value during storage in the refrigerator (Fig. 5) − a 1.16-fold increase at day 7 of storage.
Fig. 5.
Effect of a 7-day simulated home-refrigerated storage at 4 °C on total microbial count [aerobic bacterial (A) and yeast and mold (B) count], total soluble solid (C), pH (D), and cloud value (E) of cold-pressed juices. Data are presented as mean ± standard deviation (SD) of three independent biological replicates. An asterisk (*) on the right top of the time-point indicates its significant difference compared to the fresh juice (control) (Student's t-test, p < 0.05).
With respect to lightness (L*), a significant difference was recorded only for the white dragon juice which showed a slight decrease in lightness at day 7 compared to that of the fresh juice (Table 1). For the redness/greenness (a*), significant changes were observed for the pineapple and carrot juices, whereas guava, white and red dragon fruits did not show significant changes in the a* value during the storage. Notably, the yellowness/blueness (b*) values of all juices did not demonstrate any significant changes during the storage period when compared to those of the freshly prepared ones (Table 1).
Table 1.
Effect of storage under simulated home-refrigerated conditions at 4 °C on color analysis of cold-pressed juices.
| Fresh | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|---|---|
| Lightness (L∗) | ||||||||
| Pineapple | 34.02 ± 0.56ab | 34.49 ± 0.87ab | 35.22 ± 0.59a | 33.98 ± 0.83ab | 34.49 ± 0.76ab | 33.50 ± 1.01b | 35.29 ± 0.78a | 35.45 ± 0.73a |
| Guava | 43.61 ± 0.13b | 44.01 ± 0.6ab | 44.38 ± 0.23a | 44.39 ± 0.43a | 44.46 ± 0.43a | 43.72 ± 0.4b | 43.81 ± 0.32b | 43.65 ± 0.4b |
| Carrot | 35.09 ± 0.54a | 33.54 ± 1.08b | 33.83 ± 0.87b | 33.08 ± 0.65bc | 33.12 ± 0.43bc | 32.49 ± 0.83c | 33.35 ± 0.64b | 35.78 ± 0.43a |
| White dragon fruit | 33.56 ± 0.21a | 29.63 ± 0.67c | 30.47 ± 1.32c | 31.87 ± 0.78b | 32.15 ± 0.54b | 31.99 ± 0.32b | 31.97 ± 0.42b | 31.90 ± 0.43b |
| Red dragon fruit | 22.37 ± 0.89a | 21.87 ± 1.07a | 21.98 ± 0.96a | 22.99 ± 1.87a | 22.67 ± 0.95a | 23.08 ± 2.06a | 22.31 ± 0.92a | 22.79 ± 0.64a |
| Redness/greenness (a∗) | ||||||||
| Pineapple | −3.75 ± 0.09a | −3.58 ± 0.08ab | −3.18 ± 0.08b | −3.44 ± 0.09ab | −2.97 ± 0.09c | −3.2 ± 0.1b | −2.91 ± 0.11c | −2.82 ± 0.13c |
| Guava | −5.59 ± 0.11ab | −5.43 ± 0.2b | −5.45 ± 0.09b | −5.57 ± 0.08ab | −5.77 ± 0.08a | −5.36 ± 0.09b | −5.45 ± 0.08b | −5.41 ± 0.09b |
| Carrot | 3.74 ± 0.11b | 3.35 ± 0.2b | 3.59 ± 0.09b | 3.51 ± 0.08b | 3.61 ± 0.098b | 3.74 ± 0.09b | 3.68 ± 0.1b | 4.97 ± 0.21a |
| White dragon fruit | −0.93 ± 0.1ab | −1.03 ± 0.09a | −0.66 ± 0.08bc | −0.76 ± 0.11bc | −0.69 ± 0.089bc | −0.7 ± 0.09bc | −0.67 ± 0.11bc | −0.73 ± 0.13bc |
| Red dragon fruit | 4.92 ± 0.32a | 4.84 ± 0.08a | 5.11 ± 0.53a | 4.88 ± 0.31a | 4.99 ± 0.46a | 4.67 ± 0.11a | 4.78 ± 0.23a | 5.08 ± 0.07a |
| Yellowness/blueness (b∗) | ||||||||
| Pineapple | 6.49 ± 0.1a | 6.50 ± 0.11a | 5.77 ± 0.23b | 5.60 ± 0.21b | 4.56 ± 0.23c | 4.58 ± 0.22c | 2.63 ± 0.1d | 2.75 ± 0.22d |
| Guava | 3.23 ± 0.09a | 3.1 ± 0.08a | 3.09 ± 0.09a | 3.2 ± 0.088a | 3.23 ± 0.08a | 2.4 ± 0.16b | 2.57 ± 0.11b | 2.54 ± 0.09b |
| Carrot | 15.87 ± 0.43bc | 15.71 ± 0.34bc | 16.03 ± 0.45b | 15.16 ± 0.44c | 15.69 ± 0.39bc | 15.50 ± 0.23bc | 15.58 ± 0.32bc | 16.91 ± 0.49a |
| White dragon fruit | −0.97 ± 0.1a | −1.01 ± 0.089a | −1.16 ± 0.09a | −0.22 ± 0.088b | −0.2 ± 0.03b | −0.09 ± 0.007c | −0.08 ± 0.007c | 0.07 ± 0.004c |
| Red dragon fruit | 0.32 ± 0.07ab | 0.27 ± 0.03b | 0.31 ± 0.08ab | 0.32 ± 0.05ab | 0.28 ± 0.03b | 0.33 ± 0.04a | 0.34 ± 0.09a | 0.33 ± 0.05a |
| Color difference (ΔE) | ||||||||
| Pineapple | 1.46 ± 0.11c | 1.58 ± 0.22c | 1.17 ± 0.32c | 2.16 ± 0.27b | 2.16 ± 0.31b | 2.42 ± 0.3b | 3.2 ± 0.15a | |
| Guava | 0.59 ± 0.11a | 0.81 ± 0.13a | 0.78 ± 0.22a | 0.87 ± 0.24a | 0.92 ± 0.31a | 0.75 ± 0.22a | 0.73 ± 0.35a | |
| Carrot | 1.62 ± 0.32ab | 1.29 ± 0.22b | 2.15 ± 0.21ab | 2.00 ± 0.13ab | 2.63 ± 0.32a | 1.79 ± 0.45ab | 1.78 ± 0.76ab | |
| White dragon fruit | 3.93 ± 0.67a | 3.11 ± 0.45a | 1.92 ± 0.33b | 1.66 ± 0.43b | 1.89 ± 0.19b | 1.89 ± 0.42b | 1.96 ± 0.52b | |
| Red dragon fruit | 0.36 ± 0.04a | 0.41 ± 0.09a | 0.35 ± 0.08a | 0.34 ± 0.07a | 0.35 ± 0.06a | 0.32 ± 0.07a | 0.33 ± 0.06a | |
Data are presented as the mean ± standard deviation (SD) of three independent replicates.
For each measured parameter, values in the same row followed by different superscripted letters are significantly different according to Duncan's multiple-range test (p < 0.05).
3.3.2. Effect on bioactive compounds (vitamin C, total phenolic, and total carotenoid contents)
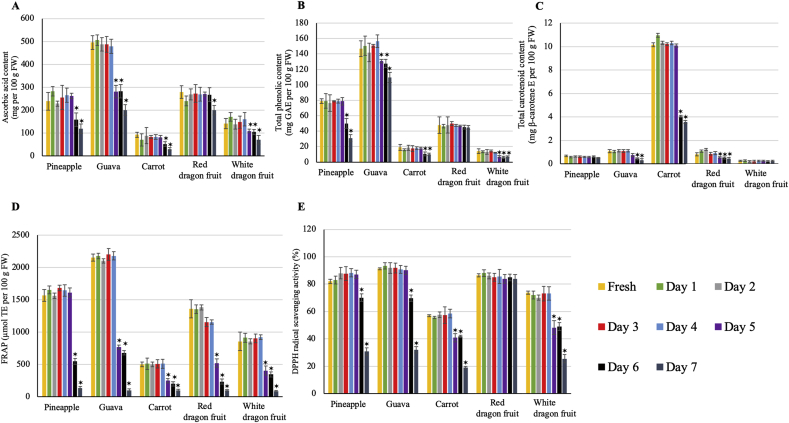
A significant decrease in ascorbic acid and total phenolic contents was observed at day 6 of storage for all juices except for the red dragon juice, which showed a slight decrease in vitamin C content at day 7 of storage but did not show a significant change in TPC during the storage period (Fig. 6). Total carotenoids of carrot, guava, and red dragon juices were decreased significantly at day 6 of storage, whereas for pineapple and white dragon, the total carotenoids did not change significantly during the storage period (Fig. 6).
Fig. 6.
Effect of a 7-day simulated home refrigerated storage at 4 °C on ascorbic acid (A), total phenolic (B) and carotenoid (C) contents and antioxidant capacity (FRAP (D) and DPPH (E)) of cold-pressed juices. Bars represent mean ± standard deviation (SD) of three independent replicates. For each measured value, comparisons are shown between the fresh juice and different time-points during storage of each fruit juice. An asterisk (*) above the bars indicates a significant difference at that time-point compared to the fresh juice (control) (Duncan's multiple-range test, p < 0.05) (GAE: gallic acid equivalent, TE: Trolox equivalent, E: equivalent).
3.3.3. Effect on antioxidant capacity
FRAP and DPPH values of pineapple, guava, carrot, and white dragon juices declined significantly at day 6 of storage. For the red dragon juice, the FRAP value showed a similar pattern to that of other juices, while significant changes in DPPH were not observed during the refrigerated storage (Fig. 6).
4. Discussion
Dietary guidelines include frequent consumption of pure fruit juices, the health-promoting properties of which are attributed to their rich content of bioactive compounds, including vitamin C and phenolic compounds. Over the past few years, there is an ever-growing demand for the consumption of freshly prepared unpasteurized fruit juices with no or minimal processing, due to its freshness, higher vitamin content, and high nutritional value. Traditional thermal treatments, including pasteurization adversely affect the nutritional values and sensory characteristics of foods and beverages (Wolbang et al., 2008; Rawson et al., 2011; Pilavtepe-Celik, 2013), and sometimes fail to produce a microbiologically stable product (Mohamed and Eissa, 2012). Cold-pressed juices have been introduced as a new generation of fruit beverages and are claimed by beverage manufacturers to have higher contents of bioactive compounds compared to those of regular (normal) centrifugal juices (The wonderful benefits of cold Pressed Juice, 2018; The real benefits of cold-pressed juice, 2018). However, our findings in the present study strongly question this claim. Five types of juices, which are commonly found in the Thai markets, were prepared using centrifugal (regular) and cold-pressed juicers. Numerous research studies have estimated FRAP and DPPH values as indicators of antioxidant capacity and vitamin C, and total phenolic and carotenoid contents as indicators of beneficial value of fruit beverages to health (Piljac-Zegarac et al., 2009; Rajasekar et al., 2012; Mgaya-Kilima et al., 2014; Pyo et al., 2014; Touati et al., 2016; Abountiolas and Nascimento Nunes, 2018). When we measured these values of normal and cold-pressed juices, we consistently found that the antioxidant capacity and content of bioactive compounds of cold-pressed juices were not significantly different from those of regular centrifugal juices (Fig. 2). These results provide convincing evidence that the above-mentioned claim by some beverage manufacturers is misleading. Notably, our study is not the first scientific report, which questions the claim of these beverage producers. Abountiolas and Nascimento Nunes (2018) reported that the labels on some fruit beverages claiming high antioxidant and/or phenolic contents were not consistent with the measured values; thus, these claims were considered misleading. An important factor to be considered about our results is the condition for preparing the juices. We used a short time (30 seconds) for preparing each juice using the centrifugal juicer. However, if a longer time is used for juice extraction using the centrifugal juicer, the quality of the extracted juice might differ due to the greater amount of heat produced by the juicer. In other words, juicing conditions, including the juicing time and the juicer specifications (e.g., rotating speed) might affect the quality of the extracted juices. This phenomenon could explain the observation by Kim et al. (2017), who found a higher nutritional quality of grape juice extracted by a low-speed masticating juicer compared to the juice extracted by a high-speed centrifugal one.
Interestingly, among the different types of juices, the guava juice harbored the highest levels of ascorbic acid, TPC, FRAP, and DPPH. Thaipong et al. (2006) also reported that ascorbic acid, total phenolic and total carotenoid contents of guava extract were significantly higher than those of other fruit crops.
Storage conditions (storage temperature and time-period) could affect the content of bioactive compounds and antioxidant capacity of fruit juices. Previous studies have addressed this phenomenon and have found that storing juices under refrigerated conditions (4 °C) could strongly protect the nutritional quality and antioxidant capacity of juices compared to storing them at RT (Bhardwaj and Nandal, 2014; Mgaya-Kilima et al., 2014; Touati et al., 2016). Consistently, in our study, storage at RT adversely affected the quality of cold-pressed juices within 48 h (Fig. 3), possibly due to the degradation of bioactive compounds, including ascorbic acid, total phenolics and carotenoids, and/or reactions with other compounds. However, for the juices stored at 4 °C, no changes were observed for all the measured values at 48 h. Our results clearly confirmed that similar to regular juices (Bhardwaj and Nandal, 2014; Mgaya-Kilima et al., 2014; Touati et al., 2016) the quality of cold-pressed juices is negatively affected by storage at RT. Interestingly, the antioxidant capacity (FRAP and DPPH) of red dragon juice stored at RT was significantly increased at 24 h of storage, whereas we did not observe any increase for the one stored at 4 °C. This increase in antioxidant capacity of red dragon juice could possibly be due to enzymatic reactions that were much slower at 4 °C than at RT. The intense red color of red dragon juice is attributed to its rich level of betacyanin, a nitrogenous red-violet pigment (see review Polturak and Aharoni, 2018). A possible enzymatic reaction by β-glucosidase, which converts betacyanin to its aglycone form, betanidin (Gandía-Herrero et al., 2004), might explain the increase in antioxidant content of the red dragon juice. The high antioxidant capacity of some aglycones has been previously reported (Kähkönen and Heinonen, 2003).
Storing fruit juices in a refrigerator at home and consuming as desired is a regular practice. However, as reported by Piljac-Zegarac et al. (2009), this type of storage could affect the TPC and antioxidant capacity of juices. Moreover, a slight decrease in ascorbic acid and total carotenoid content of some fruit beverages kept in a refrigerator for a period of 8 d was reported by Castro-López et al. (2016). Due to the possible adverse effect of storage conditions on the safety and quality of fresh unpasteurized fruit juices, gaining a better understanding of this phenomenon is of paramount importance. There is no definite guideline regarding the exact shelf life of freshly prepared unpasteurized juices kept in home-refrigerated conditions. However, most juice manufacturers suggest a shelf life of 3–5 d (Understanding shelf life of cold-pressed juice, 2016). Therefore, we investigated the effect of storage under home- refrigerated conditions on the quality of cold-pressed juices for a period of 7 d [we designed our experiment to include sampling of the juices shortly before (1–2 d) and after (6–7 d) the commonly suggested shelf life]. No changes were observed for the total microbial (bacterial and yeast) count during the 7-d storage at 4 °C for all the juices, indicating stability of the juices during the storage period. Moreover, physicochemical properties (TSS and pH) of the juices measured in this study remained constant for all the juices, excluding pineapple juice, which exhibited a decreased value of TSS at day 7 (Fig. 5).
Cloud value (clarity) is ascribed to particles in the juice, such as cellulose, hemicellulose, proteins, lipids, and some other minor particles (Baker and Cameron, 1999). For fruit juices, stability of the cloud value is a visual quality factor associated with the flavor and color and is interconnected to consumer acceptance (Beveridge, 2002). The cloud value of carrot and red dragon did not exhibit any changes during the storage, which clearly confirmed the stability of these juices during the storage at 4 °C. However, a significant decrease in the cloud value of white dragon and pineapple was observed (Fig. 5), which indicated less turbidity of these juices compared to that of the fresh ones. This phenomenon could have occurred as a result of fewer suspended particles, possibly due to reactions among the particles. Nevertheless, guava juice was the only type of juice that showed a significant increase of its cloud value at day 7. This increase could be due to the breakdown of larger molecules resulting in higher numbers of suspended particles at day 7 of storage than those in the fresh juice. Color stability is an important quality characteristic of juices during storage. We observed the greatest value of color difference (ΔE) for pineapple (3.2) than for other juices, which indicated a medium color difference obvious to an untrained eye (2 <ΔE< 3.5). This obvious change in the color of pineapple juice was mainly attributed to the significant decrease in the +b* value (yellowness), which might be due to the degradation of pigments during the storage period. The change in the color of pineapple juice, which coincided, with a significant decrease in the clarity and TSS values clearly confirmed the impact of the storage on the quality of pineapple juice. For the carrot and white dragon juices, a very small color difference, obvious only to a trained eye was observed (1 <ΔE< 2). Meanwhile, the guava juice also demonstrated a much smaller color difference (invisible difference) (0 <ΔE< 1); however, the smallest difference in color was observed for the red dragon juice (0.33) (Table 1). Taken together, because of the measured parameters, the red dragon juice exhibited the greatest storage stability with minimum changes during storage in a refrigerator compared to that of the other types of juices.
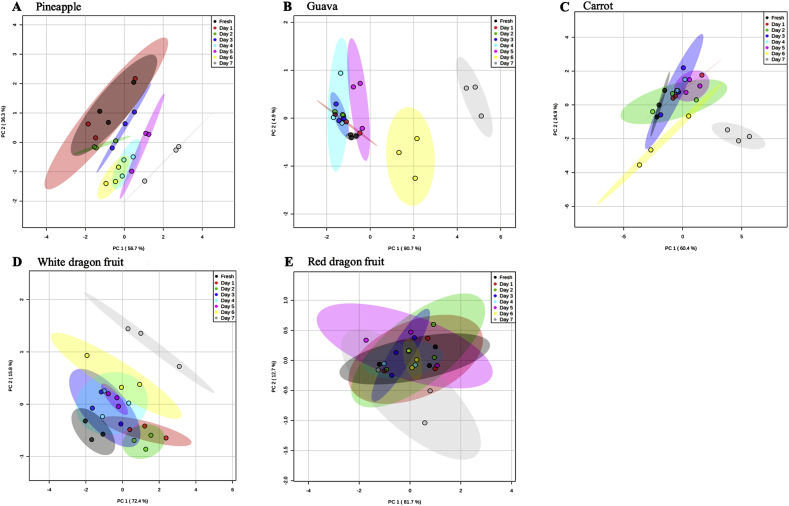
Antioxidant capacity is widely used as a tool to evaluate the quality of fruit beverages after processing and during storage. Accordingly, in our study, we measured FRAP, DPPH, ascorbic acid, total phenolic and carotenoid contents of cold-pressed juices during the 7-day storage at 4 °C. Most of the measured values started to show similar declining trends at day 6 of storage and reached their lowest values at day 7 (Fig. 6). However, this trend was quite different for the red dragon juice, in which the TPC and DPPH remained unchanged during the storage period and ascorbic acid showed a slight decrease only at day 7 of storage (Fig. 6). Our findings indicated that different storage conditions could have different effects on the quality of juices depending on the type of the fruit, possibly due to different compositions of bioactive compounds in different fruit types. This phenomenon has previously been reported by some studies (Castro-López et al., 2016; Abountiolas and Nascimento Nunes, 2018). In terms of the effect of storage on the quality of juices and in accordance with the measured values, principal component analysis (PCA) of pineapple, guava, carrot, and white dragon revealed a similar pattern and clearly indicated significant changes in the quality of juices at day 6 and especially at day 7 of storage. PCA of each fruit juice classified the samples into eight groups (fresh, day 1, day 2, day 3, day 4, day 5, day 6, and day 7). Groups of fresh to day 5 were close together, which indicated that there were no significant changes in the quality of juices until day 5 post-storage. However, samples at day 6 and especially at day 7 were clearly separated, which pointed to the significant changes in the quality of juices at days 6 and 7 of storage (Fig. 7). For the red dragon juice, PCA revealed a different trend compared to that for the other juices. All groups (fresh to day 7) were located in close proximity to each other, indicating minimal changes in the quality of red dragon juice during the storage period (Fig. 7). Taken together, PCA strongly suggested that the storage conditions affected fruit juices differently, based on the fruit type – the red dragon juice was the most stable type during the storage period.
Fig. 7.
Principal component analysis (PCA) of nutritional (ascorbic acid, total phenolic and carotenoid contents), antioxidant (FRAP and DPPH), and physicochemical (pH, total soluble solid, clarity, and color) properties of fruit juices during a 7-day simulated home-refrigerated storage at 4 °C. Each spot represents one independent biological replicate. PCA was performed using a web-based tool MetaboAnalyst 4.0.
During juice extraction, cells from the fruit pulp are disrupted. This phenomenon could bring enzymes previously localized at different subcellular compartments (e.g., cell-wall-localized β-glucosidase) and substrates in close proximity to each other and subsequently stimulate enzymatic reactions. These enzymatic reactions could be responsible for the possible changes in the quality attributes of fruit juices during storage. Further studies, investigating these biochemical reactions at molecular levels by using state-of-the-art mass spectrometers coupled with cutting-edge metabolomic technologies, could provide new insights into the effects of storage on the nutritional quality of fruit juices. Maintaining the quality of fruit juices during storage is of utmost importance and a better understanding in this area should lead to improvements to obtain optimal storage conditions. Sensory analysis is widely used in food science and technology to assess the quality of a product with respect to mouth feel, odor, taste, color, and creaminess. In this regard, studying the sensory characteristics of freshly squeezed unpasteurized juices during home-refrigerated storage is of great interest for obtaining optimal storage conditions that would satisfy the general consumer acceptance. Hence, the parameters measured in this study would provide in-depth knowledge regarding the effect of storage conditions on the quality of cold-pressed juices. However, to expand our knowledge, metabolomics and sensory analyses could be a subject for future investigation.
In summary, we compared the content of bioactive compounds and antioxidant capacity of cold-pressed juices to those of the regular (normal) ones and found no significant differences between the two. Our results thus provide compelling evidence that the claim regarding the higher nutritional quality of cold-pressed juices could be misleading and should be questioned. Moreover, the physicochemical properties, antioxidant capacity and content of bioactive compounds of cold-pressed juices remained unchanged until day 5 of storage under home-refrigerated conditions. However, at day 6, most of the measured values started to decline and reached their lowest levels at day 7 of storage. This observation clearly confirmed that storage of juices in refrigerators could negatively affect the quality of cold-pressed juices, thus questioning the claim regarding the longer shelf life of cold-pressed juices kept in home refrigerators.
Declarations
Author contribution statement
Gholamreza Khaksar: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Kitipong Assatarakul: Contributed reagents, materials, analysis tools or data.
Supaart Sirikantaramas: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research was financially supported by Chulalongkorn research funding (GRU 6203023003-1) to S.S., and Ratchadapisek Somphot Fund for Postdoctoral Fellowship, Chulalongkorn University to G.K.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Ms. Krongkan Thongmat for her assistance in physicochemical analysis (color measurement).
References
- Aadil R.M., Zeng X., Han Z., Sun D. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013;141:3201–3206. doi: 10.1016/j.foodchem.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Abountiolas M., Nascimento Nunes C. d. Polyphenols, ascorbic acid and antioxidant capacity of commercial nutritional drinks, fruit juices, smoothies and teas. Int. J. Food Sci. Technol. 2018;53:188–198. [Google Scholar]
- Baker R.A., Cameron R.G. Clouds of citrus juices and juice drinks. Food Technol. 1999;53:64–69. [Google Scholar]
- Bazzano L.A., Serdula M.K., Liu S. Dietary intakes of F&V and risk of cardiovascular disease. Curr. Atheroscler. Rep. 2002;5:492–499. doi: 10.1007/s11883-003-0040-z. [DOI] [PubMed] [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Beveridge T. Opalescent and cloudy fruit juices: formation and particle stability. Crit. Rev. Food Sci. Nutr. 2002;42:317–337. doi: 10.1080/10408690290825556. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R.L., Nandal U. Effect of storage temperature on physico-chemical and sensory evaluation of kinnow Mandarin juice blends. J. Food Process. Technol. 2014;5:8. [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995;28:25–30. [Google Scholar]
- Castro-López C., Sánchez-Alejo E.J., Saucedo-Pompa S., Rojas R., Aranda-Ruiz J., Martínez-Avila G.C.G. Fluctuations in phenolic content, ascorbic acid and total carotenoids and antioxidant activity of fruit beverages during storage. Heliyon. 2016;2 doi: 10.1016/j.heliyon.2016.e00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell H. The role of fruit juice in the diet: an overview. Nutr. Bull. 2009;34:273–288. [Google Scholar]
- De Mello-Andrade J.M., Fasolo D. Polyphenol antioxidants from natural sources and contribution to health promotion. Polyphenols Hum. Health Dis. 2014;1:253–265. [Google Scholar]
- Del Caro A., Piga A., Vacca V., Agabbio M. Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage. Food Chem. 2004;84:99–105. [Google Scholar]
- Gandía-Herrero F., García-Carmona F., Escribano J. Purification and characterization of a latent polyphenol oxidase from beet root (Beta vulgaris L.) J. Agric. Food Chem. 2004;52:609–615. doi: 10.1021/jf034381m. [DOI] [PubMed] [Google Scholar]
- Gardner P.T., White T.A.C., McPhail D.B., Duthie G.G. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000;68:471–474. [Google Scholar]
- Kähkönen M.P., Heinonen M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003;29:628–633. doi: 10.1021/jf025551i. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Jun J.G., Park S.Y., Choi M.J., Park E., Kim J.I., Kim M.J. Antioxidant activities of fresh grape juices prepared using various household processing methods. Food Sci. Biotechnol. 2017;26:861–869. doi: 10.1007/s10068-017-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B.P., Perry A.K. Ascorbic acid and vitamin A activity in selected vegetables from different geographical areas of the United States. J. Food Sci. 1982;47:941–945. [Google Scholar]
- La Cava E.L.M., Sgroppo S.C. Evolution during refrigerated storage of bioactive compounds and quality characteristics of grapefruit [Citrus paradisi (Macf.)] juice treated with UV-C light. LWT-Food Sci. Technol. 2015;63:1325–1333. [Google Scholar]
- Mgaya-Kilima B., Remberg S.F., Chove B.E., Wicklund T. Influence of storage temperature and time on the physicochemical and bioactive properties of roselle-fruit juice blends in plastic bottle. Food Sci. Nutr. 2014;2:181–191. doi: 10.1002/fsn3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel G., Dandlen S., Antunes D., Neves A., Martins D. The effect of two methods of pomegranate (Punica granatum L.) juice extraction on quality during storage at 4 °C. J. Biomed. Biotechnol. 2004;5:332–337. doi: 10.1155/S1110724304403064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.E.A., Eissa A.H.A. Pulsed electric fields for food processing technology. In: Eissa A.A., editor. Structure and Function of Food Engineering. InTech; Rijeka: 2012. pp. 275–280. [Google Scholar]
- Pilavtepe-Celik M. High hydrostatic pressure (HHP) inactivation of foodborne pathogens in low-acid juices. Int. J. Food Sci. Technol. 2013;48:673–677. [Google Scholar]
- Piljac-Zegarac J., Valek L., Martinez S., Belšcak A. Fluctuations in the phenolic content and antioxidant capacity of dark fruit juices in refrigerated storage. Food Chem. 2009;113:394–400. [Google Scholar]
- Polturak G., Aharoni A. ‘‘La Vie en Rose’’: biosynthesis, Sources, and Applications of Betalain Pigments. Mol. Plant. 2018;11:7–22. doi: 10.1016/j.molp.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Pyo Y.H., Jin Y.J., Hwang J.Y. Comparison of the effects of blending and juicing on the phytochemicals contents and antioxidant capacity of typical Korean kernel fruit juices. Prev. Nutr. Food Sci. 2014;19:108–114. doi: 10.3746/pnf.2014.19.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekar D., Akoh C.C., Martino G.K., MacLean D.D. Physico-chemical characteristics of juice extracted by blender and mechanical press from pomegranate cultivars grown in Georgia. Food Chem. 2012;133:1383–1393. [Google Scholar]
- Rawson A., Patras A., Tiwari B.K., Noci F., Koutchma T., Brunton N. Effect of thermal and non-thermal processing technologies on the bioactive content of exotic fruits and their products: review of recent advances. Food Res. Int. 2011;44:1875–1887. [Google Scholar]
- Raybaudi-Massilia R.M., Mosqueda-Melgar J., Martın-Belloso O. Antimicrobial activity of malic acid against Listeria monocytogenes, Salmonella enteritidis and Escherichia coli O157:H7 in apple, pear and melon juices. Food Control. 2009;20:105–112. [Google Scholar]
- Ruxton C.H., Gardner E., Walker D. Can pure fruit and vegetable juices protect against cancer and cardiovascular disease too? A review of the evidence. Int. J. Food Sci. Nutr. 2006;57:249–272. doi: 10.1080/09637480600858134. [DOI] [PubMed] [Google Scholar]
- Siró I., Kapolna E., Kapolna B., Lugasi A. Functional food. Product development, marketing and consumer acceptance—a review. Appetite. 2008;51:456–467. doi: 10.1016/j.appet.2008.05.060. [DOI] [PubMed] [Google Scholar]
- Slavin J.L., Lloyd B. Health benefits of fruits and vegetables. Adv. Nutr. 2012;3:506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Chu Y.F., Wu X., Liu R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002;50:449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- Swain T., Hillis W.E. The phenolic constituents of Prunus domestica. L.—the quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959;10:63–68. [Google Scholar]
- Talcott S.T., Howard L.R. Phenolic autoxidation is responsible for color degradation in processed carrot puree. J. Agric. Food Chem. 1999;47:2109–2115. doi: 10.1021/jf981134n. [DOI] [PubMed] [Google Scholar]
- Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Hawkins Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006;19:669–675. [Google Scholar]
- The Real Benefits of Cold-Pressed Juice. 2018. https://www.revitasize.ca/blogs/juicy-lifestyle-blog/the-real-benefits-of-cold-pressed-juice/ [Google Scholar]
- The Wonderful Benefits of Cold Pressed Juice. 2018. https://www.puregreen.com/pure-green-magazine/benefits-of-cold-pressed-juice/ [Google Scholar]
- Touati N., Barba F.J., Louaileche H., Frigola A., Esteve M.F. Effect of storage time and temperature on the quality of fruit nectars: determination of nutritional loss indicators. J. Food Qual. 2016;39:209–217. [Google Scholar]
- Understanding Shelf Life of Cold-Pressed Juice. 2016. https://www.goodnature.com/blog/understanding-shelf-life-of-cold-pressed-juice/ [Google Scholar]
- Van’t Veer P., Jansen M., Klerk M., Kok F.J. Fruits and vegetables in the prevention of cancer and cardiovascular disease. Publ. Health Nutr. 2000;3:103–107. doi: 10.1017/s1368980000000136. [DOI] [PubMed] [Google Scholar]
- Williams C. Healthy eating: clarifying advice about fruit and vegetables. Br. Med. J. 1995;310:1453–1455. doi: 10.1136/bmj.310.6992.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbang C.M., Fitos J.L., Treeby M.T. The effect of high pressure processing on nutritional value and quality attributes of Cucumis melo L. Innov. Food Sci. Emerg. Technol. 2008;9:196–200. [Google Scholar]