Key Teaching Points.
-
•
Atrial standstill is a rare clinical condition characterized by absence of electrical and mechanical atrial activity, and the spatial distribution of atrial lesion can be diffuse or partial.
-
•
In atrial standstill when pacemaker implantations are thought to be necessary, evaluation by electrophysiological study should be considered before implantation.
-
•
Atrial standstill is a progressive disease; therefore, atrial pacing failure might occur. Patients implanted with a pacemaker should be followed up carefully.
Introduction
Atrial standstill (AS) is a rare condition characterized by the absence of electrical and mechanical atrial activity,1 first described by Cushny.2 The absence of P waves and bradycardia, along with a wide QRS complex, are seen on the electrocardiogram (ECG). The clinical course of AS varies greatly; the timeline can be persistent or transient, and the spatial distribution of the atrial lesion can be diffuse or partial.3, 4 In many cases, the AS appears initially in the right atrium (RA) and progresses to the entire left atrium. It occasionally complicates symptomatic sick sinus syndrome.5
We hereby describe a case of AS where a dual-chamber permanent pacemaker implantation was necessary owing to symptomatic sick sinus syndrome. An electrophysiological study (EPS) revealed inactivity throughout almost the entire RA, with the exception of the coronary sinus (CS). A permanent atrial lead was therefore placed in the CS.
Case report
A 67-year-old man was admitted for dyspnea (NYHA class III–IV). He had essential hypertension and paroxysmal atrial fibrillation and was treated with an angiotensin II receptor blocker, calcium blocker, and warfarin. On admission, his blood pressure was 160/98 mm Hg and pulse rate was 118 beats/min. His blood oxygen saturation was 94% on room air. The jugular vein was dilated. A regurgitant systolic murmur at the apex was audible and there was bilateral lower leg edema. The laboratory findings revealed an elevated brain natriuretic peptide level (140.3 pg/mL) and elevated liver function test values (total-value bilirubin 1.96 mg/dL and indirect bilirubin 1.27 mg/dL). The other parameters, however, including the complete blood count, electrolytes, and cardiac enzymes, remained within the normal range. An ECG revealed a tachycardia at a rate between 100 and 150 beats/min with a QRS duration of 0.12 seconds; however, neither visible P waves nor atrial fibrillatory waves were observed (Figure 1A). Marked cardiomegaly and pulmonary congestion were revealed by a chest radiograph. The right and left atria were markedly dilated on echocardiography, which was associated with moderate tricuspid and mitral regurgitation (Figure 1B). The left atrium and ventricle were also enlarged (left atrium dimension 53 mm, left ventricular end-diastolic dimension 60 mm, and left ventricular end-systolic dimension 39 mm). The systolic left ventricular function was normal (ejection fraction: 63%). No mechanical atrial activity was observed in a Doppler study of the mitral and tricuspid inflow regions (Figure 1B).
Figure 1.
A: Electrocardiogram (ECG) on admission. The ECG revealed a tachycardia at a rate between 100 and 150 beats/min with a QRS duration of 0.12 seconds; however neither visible P waves nor atrial fibrillatory waves were observed. B: Echocardiogram on admission. Both atria were enlarged (right panel). The Doppler study of the tricuspid inflow showed no mechanical atrial activity (left panel).
The patient presented with orthopnea and cardiomegaly; therefore, he was diagnosed with heart failure. He was treated with vasodilators and diuretics, and his clinical condition rapidly improved. Frequent pauses were observed on the ECG at the beginning of hospitalization. This included a pause of 8.4 seconds, which was associated with presyncope. Various types of arrhythmia, including tachycardias and bradycardia, were also observed. An EPS was therefore used to determine the treatment strategy and to obtain an accurate diagnosis.
Electrophysiological study
A quadripolar catheter (5F) was placed into the RA, and pentapolar catheters (5F) were placed in the CS and right ventricle via the right femoral vein and were used for mapping. During EPS, the basal rhythm was a junctional escape rhythm. A nonsustained atrial tachycardia was observed. The maximum sinus node recovery time was >5500 ms, atrioventricular (AV) Wenckebach rate was 110 beats/min, and effective refractory period of the AV node was 410 ms. Although the sinus node function was severely impaired, the AV node function was preserved.
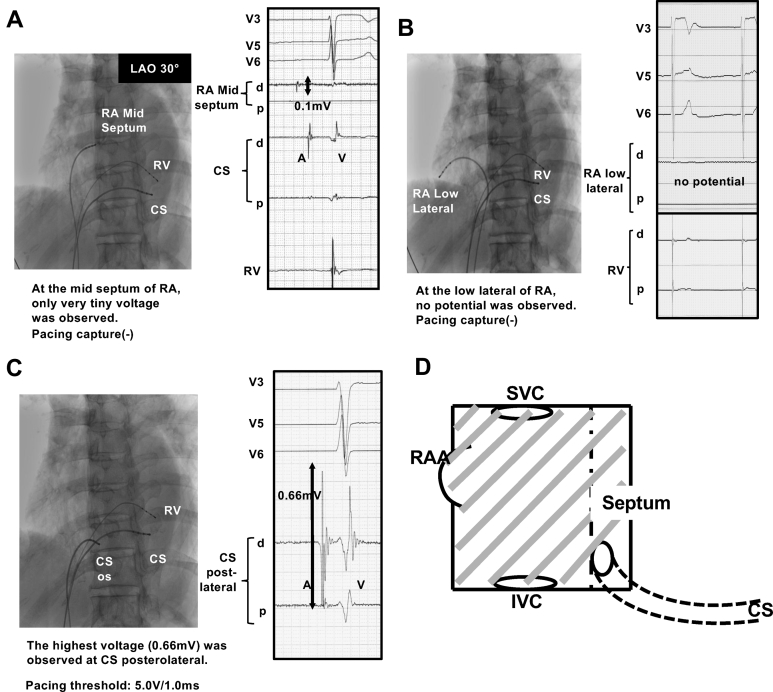
Conventional mapping of the RA and CS was performed. The results of the mapping study are shown in Figure 2A–D. Recording was from multiple sites in the RA and CS. No electrical activity was detected through the entire RA except for the mid septum, where a tiny atrial electrical activity was recorded. However, we were unable to capture the RA by pacing at any right atrial site. On the other hand, a sensing of 0.66 mV and the pacing capture threshold of 5.0 V / 1.0 ms were obtained at a proximal site of the CS (Figure 2C). The EPS confirmed partial AS with sick sinus syndrome and atrial tachycardia.
Figure 2.
A–C: Conventional mapping of the right atrium (RA) and coronary sinus (CS). A: Electrical activity on the midseptum of the RA. Only very tiny atrial electrical activity was recorded; however, pacing could be uncaptured. B: Electrical activity in the low lateral region of RA. No atrial electrical activity was recorded and pacing could be uncaptured. C: Electrical activity at a proximal site of the CS. At a proximal site of the CS, a sensing of 0.66 mV and pacing capture threshold of 5.0 V/1.0 ms were obtained. D: Illustration of the results of conventional mapping and pacing study. There was extensive scarring in the whole RA (shaded area), including the right atrial appendage (RAA). Only inside the CS did atrial electrical activities remain. IVC = inferior vena cava; LAO = left anterior oblique; os = ostium of the CS; RV = right ventricle; SVC = superior vena cava.
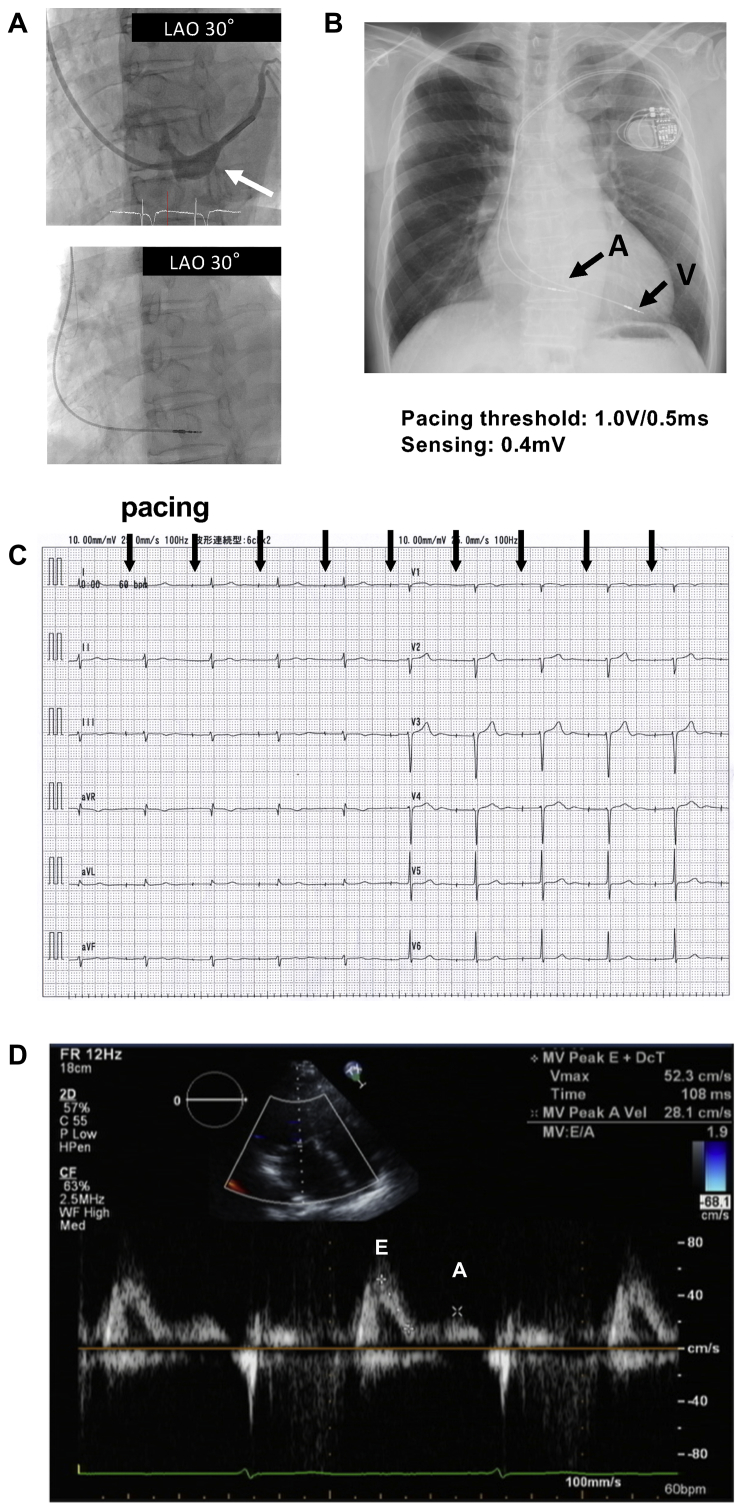
Pacemaker implantation was necessary owing to symptomatic sick sinus syndrome. Considering the results of the EPS, the atrial lead (Capture 5076-65 MRI, Medtronic, Minneapolis, MN) was positioned at the proximal site of the CS with P-wave sensing of 0.4 mV and pacing threshold of 1.0 V at 0.5 ms pulse width (Figure 3A). To the best of our knowledge, there have been no or very few previous reports regarding complications with the fixation of pacemaker leads in the CS. Using a traditional passive fixation lead, there was concern about the dislodgment of the lead. Furthermore, we considered that it would be difficult to control the location of the lead during the process of the lead fixation using a traditional passive fixation lead. Therefore, we chose an active fixation lead (Capture 5076-65 MRI, Medtronic). We succeeded in placing the atrial lead with the initial attempt, and we placed the lead of the pacemaker proximally inside the CS (Figure 3B). After the implantation of the pacemaker (Advisa MRI, Medtronic), surface ECG showed synchronous AV conduction (Figure 3C) and Doppler echocardiography showed A waves of mitral inflow (Figure 3D).
Figure 3.
A: Fluoroscopic image in the 30° left anterior oblique (LAO) projection showing the position of the catheters during the electrogram recording. A white arrow indicates the position of the coronary sinus. B: Chest radiograph after the implantation of the pacemaker. Black arrows indicate the atrial (A) and ventricular leads (V). C: Electrocardiogram after the pacemaker implantation showing atrioventricular synchronous conduction. D: Transmitral flows evaluated by an echocardiogram after the pacemaker implantation. A and E waves were clearly detected, indicating a functional contraction of the left atrium.
During the postoperative hospitalization, tachycardia almost completely disappeared. This patient was followed for 2 years and 3 months. In the postoperative pacemaker follow-up, the ventricular pacing was 0.3%, and the atrial pacing burden was 93.7%. The atrial tachyarrhythmia/atrial fibrillation burden was 0.1%. After 2 years and 3 months, device parameters remained stable with atrial sensing of 0.6 mV and atrial pacing threshold of 0.675 V at 0.4 ms. The patient has since remained free of both syncope and dyspnea.
Discussion
AS is a rare clinical condition. A misdiagnosis of AS might often occur because other various clinical conditions also exhibit the absence of P waves on the ECG. Furthermore, the distribution of the AS occasionally spreads into the entire atrium, but it remains partial in some cases. The time course of this disease can be either intermittent or permanent.3, 4 Previous reports showed that AS could be induced by various conditions, including hypoxia, muscular dystrophia, amyloidosis, myocardial infarction, and toxicity from quinidine or digitalis.1, 6, 7, 8, 9 In the present case, however, those systemic diseases and causative agents were undetected.
Nakazato and Nakata10 and Lévy and colleagues11 investigated the time-course changes in the electrophysiological properties in patients with AS by placing electrodes in the RA and CS. They suggested that AS often appears early at the site of the high and midlateral RA, progresses to the entire RA, and then progresses to the left atrium. In the present case, only the septum remained active in the RA.
Bellmann and colleagues5 reported that sick sinus syndrome and AS might share a common underlying pathology of fibrosis. Partial AS sometimes merges with sick sinus syndrome. In their reports, merger, conventional, or electroanatomic mapping was performed in the RA to identify any residual myocardium in 4 cases. In 3 of the cases, dual-chamber pacemakers were implanted. In 1 of their cases, the atrial lead was indwelled in the CS, as in our case. A dual-chamber pacemaker made synchronous AV sequential pacing possible. In their cases, there were no clinical problems such as pacing failure during an average follow-up of 3.5 years. In the present case, the mapping of the atria was performed to identify any electrophysiologically viable areas of the RA to determine if it was possible to implant a dual-chamber pacemaker prioritizing the patient’s own synchronous atrioventricular conduction. Synchronous AV conduction can help avoid any right ventricular apical pacing with a left bundle branch block morphology. It can also help obtain partial diastolic ventricular filling by a partial atrial contraction. In cases with sinus node dysfunction, dual-chamber pacing could reduce the onset of atrial fibrillation, as compared with ventricular pacing,12 since ventricular pacing might cause atrial electrical remodeling and increase the atrial diameter.13, 14 In the present case, the frequency of atrial tachycardia might decrease, as well as atrial fibrillation, owing to a mechanism related to the dual-chamber pacing allowing for the patient’s own synchronous atrioventricular conduction.
Conclusion
In summary, in AS when pacemaker implantations are thought to be necessary, evaluation by an EPS should be considered before the implantation. AS is a progressive disease; therefore, atrial pacing failure might occur. Such cases should therefore be followed up carefully.
References
- 1.Wooliscroft J., Tuna N. Permanent atrial standstill: the clinical spectrum. Am J Cardiol. 1982;49:2037–2041. doi: 10.1016/0002-9149(82)90226-0. [DOI] [PubMed] [Google Scholar]
- 2.Cushny A. Optical isomerism, and the mechanism of drug action. J Hist Biol. 1975;8:145–165. doi: 10.1007/BF00130436. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez Reyes H., Cruz F., Iturralde Torres P., de Micheli A., González, Hermosillo J.A. Persistent atrial paralysis: reported of 2 cases. Arch Inst Cardiol Mex. 1997;67:498–502. [PubMed] [Google Scholar]
- 4.Nakamoto K. Relation between the duration of pancardiac asystole and the post systolic atrial cycle and the effects of orciprenaline on the atrioventricular conduction in a patient with 2:1 A-V block and intermittent cardiac standstill. Jpn Circ J. 1969;33:823–832. doi: 10.1253/jcj.33.823. [DOI] [PubMed] [Google Scholar]
- 5.Bellmann B., Roser M., Muntean B., Tscholl V., Nagel P., Schmid M., Schauerte P. Atrial standstill in sinus node disease due to extensive atrial fibrosis: impact on dual chamber pacemaker implantation. Europace. 2016;18:238–245. doi: 10.1093/europace/euv098. [DOI] [PubMed] [Google Scholar]
- 6.Wolff L., White P.D. Auricular fibrillation. Results of seven years’ experience with quinidine sulphate therapy (1921–1928) Arch Intern Med. 1928;43:653. [Google Scholar]
- 7.Surawicz B. Electrolytes and the electrocardiogram. Am J Cardiol. 1963;12:656. doi: 10.1016/0002-9149(63)90255-8. [DOI] [PubMed] [Google Scholar]
- 8.James T.N. Myocardial infarction and atrial arrhythmias. Circulation. 1961;24:761. doi: 10.1161/01.cir.24.4.761. [DOI] [PubMed] [Google Scholar]
- 9.Wentworth D.C., Rice G.J., Lowe G.W. Persistent atriai standstill in a family with myocardial disease. Am J Med. 1969;47:775–784. doi: 10.1016/0002-9343(69)90170-3. [DOI] [PubMed] [Google Scholar]
- 10.Nakazato Y., Nakata Y. Clinical and electrophysiological characteristics of atrial standstill. Pacing Clin Electrophysiol. 1995;18:1244–1254. doi: 10.1111/j.1540-8159.1995.tb06964.x. [DOI] [PubMed] [Google Scholar]
- 11.Lévy S., Pouget B., Bemurat M., Lacaze J.C., Clementy J., Bricaud H. Partial atrial electrical standstill: Report of three cases and review of clinical and electrophysiological features. Eur Heart J. 1980;1:107–116. doi: 10.1093/oxfordjournals.eurheartj.a061104. [DOI] [PubMed] [Google Scholar]
- 12.Lamas G.A., Lee K.L., Sweeney M.O. Ventricular pacing or dual-chamber pacing for sinus-node-dysfunction. N Engl J Med. 2002;346:1854–1862. doi: 10.1056/NEJMoa013040. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen J.C., Andersen H.R., Thomsen P.E. Heart failure and echocardiographic changes during long-term follow-up patients with sick sinus syndrome randomized to single-chamber atrial or ventricular pacing. Circulation. 1998;97:987–995. doi: 10.1161/01.cir.97.10.987. [DOI] [PubMed] [Google Scholar]
- 14.Sparks P.B., Mond H.G., Vohra J.K., Jayaprakash S., Kalman J.M. Electrical remodeling of the atria following loss of atrioventricular synchrony: a long-term study in humans. Circulation. 1999;100:1894–1900. doi: 10.1161/01.cir.100.18.1894. [DOI] [PubMed] [Google Scholar]