Abstract
Background
Attention deficit hyperactivity disorder (ADHD) in children is associated with hyperactivity and impulsivity, attention problems, and difficulties with social interactions. Pharmacological treatment may alleviate the symptoms of ADHD but this rarely solves difficulties with social interactions. Children with ADHD may benefit from interventions designed to improve their social skills. We examined the benefits and harms of social skills training on social skills, emotional competencies, general behaviour, ADHD symptoms, performance in school of children with ADHD, and adverse events.
Objectives
To assess the beneficial and harmful effects of social skills training in children and adolescents with ADHD.
Search methods
In July 2018, we searched CENTRAL, MEDLINE, Embase, PsycINFO, 4 other databases and two trials registers.We also searched online conference abstracts, and contacted experts in the field for information about unpublished or ongoing randomised clinical trials. We did not limit our searches by language, year of publication, or type or status of publication, and we sought translation of the relevant sections of non‐English language articles.
Selection criteria
Randomised clinical trials investigating social skills training versus either no intervention or waiting‐list control, with or without pharmacological treatment of both comparison groups of children and adolescents with ADHD.
Data collection and analysis
We conducted the review in accordance with the Cochrane Handbook for Systematic Reviews of Intervention. We performed the analyses using Review Manager 5 software and Trial Sequential Analysis. We assessed bias according to domains for systematic errors. We assessed the certainty of the evidence with the GRADE approach.
Main results
We included 25 randomised clinical trials described in 45 reports. The trials included a total of 2690 participants aged between five and 17 years. In 17 trials, participants were also diagnosed with various comorbidities.
The social skills interventions were described as: 1) social skills training, 2) cognitive behavioural therapy, 3) multimodal behavioural/psychosocial therapy, 4) child life and attention skills treatment, 5) life skills training, 6) the "challenging horizon programme", 7) verbal self‐instruction, 8) meta‐cognitive training, 9) behavioural therapy, 10) behavioural and social skills treatment, and 11) psychosocial treatment. The control interventions were no intervention or waiting list.
The duration of the interventions ranged from five weeks to two years. We considered the content of the social skills interventions to be comparable and based on a cognitive‐behavioural model. Most of the trials compared child social skills training or parent training combined with medication versus medication alone. Some of the experimental interventions also included teacher consultations.
More than half of the trials were at high risk of bias for generation of the allocation sequence and allocation concealment. No trial reported on blinding of participants and personnel. Most of the trials did not report on differences between groups in medication for comorbid disorders. We used all eligible trials in the meta‐analyses, but downgraded the certainty of the evidence to low or very low.
We found no clinically relevant treatment effect of social skills interventions on the primary outcome measures: teacher‐rated social skills at end of treatment (standardised mean difference (SMD) 0.11, 95% confidence interval (CI) 0.00 to 0.22; 11 trials, 1271 participants; I2 = 0%; P = 0.05); teacher‐rated emotional competencies at end of treatment (SMD −0.02, 95% CI −0.72 to 0.68; two trials, 129 participants; I2 = 74%; P = 0.96); or on teacher‐rated general behaviour (SMD −0.06 (negative value better), 95% CI −0.19 to 0.06; eight trials, 1002 participants; I2 = 0%; P = 0.33). The effect on the primary outcome, teacher‐rated social skills at end of treatment, corresponds to a MD of 1.22 points on the social skills rating system (SSRS) scale (95% CI 0.09 to 2.36). The minimal clinical relevant difference (10%) on the SSRS is 10.0 points (range 0 to 102 points on SSRS).
We found evidence in favour of social skills training on teacher‐rated core ADHD symptoms at end of treatment for all eligible trials (SMD −0.26, 95% CI −0.47 to −0.05; 14 trials, 1379 participants; I2= 69%; P = 0.02), but the finding is questionable due to lack of support from sensitivity analyses, high risk of bias, lack of clinical significance, high heterogeneity, and low certainty.
The studies did not report any serious or non‐serious adverse events.
Authors' conclusions
The review suggests that there is little evidence to support or refute social skills training for children and adolescents with ADHD. We may need more trials that are at low risk of bias and a sufficient number of participants to determine the efficacy of social skills training versus no training for ADHD. The evidence base regarding adolescents is especially weak.
Plain language summary
Social skills training for children aged between 5 and 18 with attention deficit hyperactivity disorder (ADHD)
Review question
What are the benefits and harms of social skills training for children and adolescents with attention deficit hyperactivity disorder (ADHD)?
Background
Children and adolescents with ADHD experience hyperactivity, impulsivity, attention problems, and difficulties with social interactions. Social skills training for ADHD seeks to improve and maintain social interaction and prevent interpersonal difficulties. Programs tend to focus on problem solving, control of emotions, and improving verbal and non‐verbal communication. We examined the benefits and harms of social skills training on the following outcomes: social skills, emotional competencies, general behaviour, ADHD symptoms, and performance in school.
Study characteristics
We found 25 randomised clinical trials (studies where participants with ADHD were randomly assigned to one of two or more groups) involving a total of 2690 participants. The trials lasted between five weeks and two years. The social skills training generally focused on teaching the children how to 'read' the subtle cues in social interaction, such as learning to wait for their turn, knowing when to shift topics during a conversation, and being able to recognise the emotional expressions of others. Social skills training often consists of role play, exercises and games, as well as homework. Children in the control groups either received no intervention or were placed on a waiting list.
Key results
We found no significant differences between social skills training versus controls on social skills, emotional competencies, and general behaviour as assessed by teachers. Compared with the children who had no social skills training, teachers rated those who had been in the social skills groups as having fewer ADHD symptoms at the end of treatment.. However, this finding was questionable because our other analyses did not support it. We found no indications of harmful effects.
All trials suffered from methodological problems such as overestimation of benefits and underestimation of harms. Many studies were also difficult to compare because they involved different interventions. The results from some trials were not very precise, which means it is difficult to be confident in the results. In seven trials, study authors were board members of pharmaceutical companies, had received funding from such companies, or had performed previous research on the topic.
Intepretation
We are unable to conclude whether social skills training is beneficial or not for children with ADHD. We need more randomised clinical trials on social skills training for children and adolescents with ADHD that have a sufficient number of participants and higher methodological quality.The evidence base regarding adolescents is especially weak. We found no adverse treatment effects.
Summary of findings
Summary of findings for the main comparison. Social skills training compared to no intervention.
| Social skills training compared to no intervention | ||||||
| Patient or population: children aged five to 18 years with ADHD Settings: outpatient clinic; inpatient hospital wards; elementary schools; community mental health centre Intervention: social skills training Comparison: no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainity of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Social skills training | |||||
|
Teacher‐rated social skills Measured by: Conners Behavior Rating Scale: Social Problems Index; Strength and Difficulties Questionnaire: Prosocial Behaviour Subscale (teacher‐rated); Social Skills Improvement System; Social Skills Rating Scale: Coorperation Subscale Follow‐up: at end of treatment |
‐ | The mean score for teacher‐rated social skills at end of treatment in the intervention groups was 0.11 standard deviations higher (0.00 lower to 0.22 higher)e | ‐ | 1271 (11 studies) | ⊕⊝⊝⊝
Very low a,b,c |
Social skills training may have no effect on teacher‐rated social skills |
|
Parent‐rated social skills Measured by: Social Skills Rating Scale; Weiss Functional Impairment Scale: Social Acitivities Domain (parent‐rated); Strength and Difficulties Questionnaire: Prosocial Behavior Subscale; Social Skills Improvement System Follow‐up: at end of treatment |
‐ | The mean score for parent‐rated social skills at end of treatment in the intervention groups was 0.19 standard deviations higher (0.06 higher to 0.32 higher) | ‐ | 1609 (15 studies) | ⊕⊝⊝⊝
Very low a,b,c |
Social skills training may have no effect on parent‐rated social skills |
|
Teacher‐rated emotional competencies Measured by: Strengths and Difficulties Questionnaire: Emotional Symptoms Subscale; Conners Behavior Rating Scale: Emotional Index Score Follow‐up: at end of treatment |
‐ | The mean score for teacher‐rated emotional competencies at end of treatment in the intervention groups was 0.02 standard deviations lower (0.72 lower to 0.68 higher) | ‐ | 129 (two studies) | ⊕⊝⊝⊝
Very low a,b,c |
Social skills training may have no effect on teacher‐rated emotional competencies |
|
Teacher‐rated general behaviour Measured by: Self‐Control Rating Scale; Conners Behavior Rating Scale: Aggressiveness Index; Disruptive Behavior Disorders Rating Scale; Conners Teacher Rating Scale: Conduct Problems Index; Strengths and Difficulties Questionnaire: Conduct Problems Subscale (teacher‐rated); Child Symptom Inventory: ODD Scale (teacher‐rated); Child Behavior Checklist Follow‐up: at end of treatment |
‐ | The mean score for teacher‐rated general behaviour at end of treatment in the intervention groups was 0.06 standard deviations lower (0.19 lower to 0.06 higher) | ‐ | 1002 (eight studies) | ⊕⊕⊝⊝
Low a,d |
Social skills training may have no effect on teacher‐rated general behaviour |
|
Parent‐rated general behaviour Measured by: Strengths and Difficulties Questionnaire (parent‐rated; total scores); Conners Behavior Rating Scale: Aggressiveness Index; Disruptive Behavior Disorders Rating Scale; Behavior Rating Inventory of Executive Function; SDQ: Conduct Problems Subscale (parent‐rated); Child Symptom Inventory; Child Behavior Checklist Follow‐up: at end of treatment |
‐ | The mean score for parent‐rated general behaviour at end of treatment in the intervention groups was 0.38 standard deviations lower (0.61 lower to 0.14 lower) | 995 (eight studies) | ⊕⊝⊝⊝
Very low a,b,c,d |
Social skills training may slightly improve parent‐rated general behaviour | |
|
Teacher‐rated ADHD symptoms Measured by: Disruptive Behavior Disorders Rating Scale; ADHD Rating Scales: Hyperactivity and Impulsivity Subscales (total scores); Conner Teacher Rating Scale: Hyperactivity Index; Strengths and Weaknesses of ADHD Symptoms and Normal Behaviors; ADHD Symptom Checklist; Child Symptom Inventory (ADHD (inattention) scale score); SNAP‐IV (teacher rating scale) Follow‐up: at end of treatment |
‐ | The mean score for teacher‐rated ADHD symptoms at end of treatment in the intervention groups was 0.26 standard deviations lower (0.47 lower to 0.05 lower) | ‐ | 1379 (14 studies) | ⊕⊝⊝⊝ Very lowa,b,c | Social skills training may slightly improve teacher‐rated ADHD symptoms |
|
Parent‐rated ADHD symptoms Measured by: Conners Parent Rating Scale: Hyperkinesis Index; Disruptive Behavior Disorders Rating Scale; Strengths and Weaknesses of ADHD Symptoms and Normal Behaviors; Sluggish Cognitive Tempo; ADHD Symptom Checklist; ADHD Rating Scales; Child Symptom Inventory: Inattention; SNAP‐IV (teacher rating scale); Child Attention Profile Follow‐up: at end of treatment |
‐ | The mean score for parent‐rated ADHD symptoms at end of treatment in the intervention groups was 0.54 standard deviations lower (0.81 lower to 0.26 lower) | ‐ | 1206 (11 studies) | ⊕⊝⊝⊝ Very lowa,b,c | Social skills training may slightly improve parent‐rated ADHD symptoms |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% Cl). | ||||||
| ADHD: Attention deficit hyperactivity disorder; CI: Confidence interval; ODD: Oppositional defiant disorder; SNAP‐IV: Swanson, Nolan and Pelham rating scale ‐ Fourth Version. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to high risk of bias (systematic errors leading to overestimation of benefits and underestimation of harms) in several 'Risk of bias' domains, including lack of sufficient blinding and selective outcome reporting (many of the included studies did not report on this outcome) bDowngraded one level due to inconsistency: moderate statistical heterogeneity (I2 = 30% to 50%) c Downgraded one level due to imprecision: wide CI dDowngraded one level due to indirectness (children's general behaviour was assessed by different types of rating scales, each with a different focus on behaviour)
eThe effect on the primary outcome, teacher‐rated social skills at end of treatment, corresponds to a MD of 1.22 points on the social skills rating system (SSRS) scale (95% CI 0.09 to 2.36). The minimal clinical relevant difference (10%) on the SSRS is 10.0 points (range 0 to 102 points on SSRS).
Background
Description of the condition
Attention deficit hyperactivity disorder
Attention deficit hyperactivity disorder (ADHD) affects 3% to 5% of all children (Polanczyk 2007; Thomas 2015). The main symptoms of ADHD include problems with attention, impulsiveness, and hyperactivity (Sergeant 2003; Pasini 2007). Individuals with ADHD also present with difficulties in the domains of attentional and cognitive functions, such as problem‐solving, planning, orienting, flexibility, sustained attention, response inhibition, and working memory (Sergeant 2003; Pasini 2007). Other difficulties involve affective components such as motivation delay and mood regulation (Nigg 2005;Castellanos 2006;Schmidt 2009). These latter difficulties are closely related to the condition and are the fundamental basis for these children's problems with social skills (Whalen 1985; Landau 1991),
Prevalence estimates for ADHD vary across the international literature. A large survey in the UK found that 3.6% of boys aged five to 15 years had ADHD; for girls of the same age, this study reported a prevalence of 0.9% (Ford 2003). In one study from Columbia, the reported prevalence was considerably higher: 19.9% for boys and 12.3% for girls (Pineda 2003). A systematic review on the prevalence of ADHD reported a mean proportion of 5.3% children and adolescents having ADHD overall, and concluded that much of the variation is derived from differences in methods used to diagnose the condition (Polanczyk 2007). Among US children and adolescents, the estimated prevalence of diagnosed attention deficit hyperactivity disorder increased from 6.1% in 1997‐1998 to 10.2% in 2015‐2016 (Xu 2018).
The aetiology of ADHD involves genetic, environmental, and social factors that are not clearly understood. Family and twin studies have shown a high heritability and with no sex differences of heritability (Neale 2010; Franke 2012). Furthermore, genetic factors may be involved in determining the persistence of ADHD into adulthood (Faraone 2000; Franke 2012). Although family studies have shown high heritability, and there are many candidate genes that may be involved in the disorder (Neale 2010), genome‐wide studies have yet to find any clear associations. Environmental risk factors include prenatal substance exposures, heavy metal and chemical exposures, nutritional factors, and lifestyle/psychosocial factors (Froehlich 2011).
A diagnosis of ADHD is made through recognition of excessive inattention, hyperactivity, and impulsivity (according to the presence of 18 symptoms) in a child, before 12 years of age, that causes impairment to his/her functioning or development (DSM‐5; ICD‐10). The principal classification systems for diagnosing ADHD are: International Classification of Diseases ‐ 10th Version (ICD‐10); and the Diagnostic and Statistical Manual of Mental Disorders (DSM) Fourth Edition (DSM‐IV), Fourth Edition ‐ Text Revision (DSM‐IV‐TR), andFifth Edition (DSM‐5).
In the DSM‐IV and DSM‐5, there are three different subdiagnoses, where particular symptoms are identified and classified: the 'predominantly inattentive type'; the 'predominantly hyperactive‐impulsive type'; and the 'combined' type, which presents with both hyperactive‐impulsive and inattentive symptoms (Willcut 2012).
Comorbid disorders, such as behavioural disorders (e.g. oppositional defiant disorder, conduct disorder), depression, anxiety, bipolar disorder, tics, motor skill development disturbance, learning difficulties, and verbal and cognitive difficulties are common in ADHD (Newcorn 2008; Schmidt 2009; Yoshimasu 2012; Czamara 2013; Perroud 2014).
ADHD is associated with negative social outcomes such as severe social incompetence, and displays of off‐task, disruptive and rule‐violating behaviour (Kolko 1990; Landau 1991), health problems such as abuse of drugs or alcohol, and criminality later in life (Barkley 2002; Dalsgaard 2002; Storebø 2014; Koisaari 2015).
ADHD is also associated with negative psychological outcomes such as an increased risk of developing personality disturbances and possibly psychotic conditions (Keshavan 2003; Storebø 2014).
Excessive weight and obesity are found in children with ADHD compared to children without ADHD (Cortese 2016). ADHD is associated with both early‐onset tobacco and alcohol use (Chang 2012). Similarly, ADHD comorbidity with conduct disorder can lead to adverse outcomes in academic achievement, failure to complete high school, criminality, substance use disorder, and unemployment (Erskine 2016).
ADHD seems to increase premature mortality by 50%, compared to individuals without ADHD, in a 24.9 million person‐years Danish cohort study (Dalsgaard 2015). A weakness of this study is that it did not include medical treatment of ADHD in the analysis as a possible confounder for the relationship between ADHD and mortality (Dalsgaard 2015). There have been some reports of sudden death in children and adults treated with stimulant treatment but it is unclear if these are related directly to methylphenidate (US FDA 2011). More research is being conducted on this topic.
Pharmacological management of ADHD
The drug most commonly used for the treatment of ADHD in children and adolescents is methylphenidate (a stimulant); atomoxetine, and dexamphetamine (another stimulant) are used less often (NICE 2009; NICE 2018). Storebø and colleagues conducted a comprehensive Cochrane Review investigating the short‐term benefits and harms of methylphenidate for children and adolescents. This review concluded that there is a possible small beneficial effect on ADHD symptoms, general behaviour, and quality of life, and that methylphenidate does not seem to increase the risk of serious adverse events in the short‐term but is associated with a relatively high risk of non‐serious adverse events in general (Storebø 2015). However, there were a number of limitations in the included trials such as lack of blinding in spite of placebo use, outcome reporting bias, and heterogeneity, which resulted in the evidence being rated as low to very low. The authors concluded that there is high need for long‐term randomised placebo tablet ('active placebo')‐controlled clinical trials, without risks of systematic errors, that investigate the effect of methylphenidate treatment for children and adolescents with ADHD (Storebø 2015). Whilst medication can help in the management of core behavioural symptoms, it is not designed to address skills deficits.
The most common adverse effects associated with methylphenidate are: headache, sleep problems, tiredness, and decreased appetite (Storebø 2018). Methylphenidate also affects the children's height and weight curves (Schachar 1997; Swanson 2007). Dexamphetamine seems to affect children's sleep and can result in dry mouth, thirst, weight loss, decreased appetite and stomach ache, and increase the risk of regressive, dependent behaviour and psychosis (Punja 2016). Atomoxetine is associated with pain, nausea and vomiting, decreased appetite with associated weight loss, dizziness, and slight increases in heart rate and blood pressure (Wolraich 2007).
Research on the neurochemical basis of ADHD has primarily focused on the neurotransmitters noradrenaline and dopamine, and their receptors in the central nervous system. Although the neurophysiological mechanism of the medications are not clearly known, it is presumed that their effects on symptoms of ADHD are explained primarily by the stimulant effects of dopaminergic and, to some extent, noradrenergic neurotransmission(Kadesjö 2002). Selective noradrenaline‐acting tricyclic medications and alpha‐2‐adrenergic agonists have also been observed to reduce symptoms of ADHD in children (Zametkin 1987).
Description of the intervention
Social skills training is developed with the characteristics of ADHD in mind in order to improve and maintain the individual’s social skills and prevent or alleviate social difficulties. Social skills are complex and involve different aspects of cognitions, emotions, and behaviour. Programmes vary in their focus on different aspects of social skills but tend to focus on problem‐solving, control of emotions, and verbal and non‐verbal communication. The training generally focuses on teaching the children how to 'read' the subtle cues in social interaction such as learning to wait for their turn, knowing when to shift topics during a conversation, and being able to recognise the emotional expressions of others (Fohlmann 2009a;Fohlmann 2009b [pers comm]). The children may be taught to practice how to adjust their verbal and non‐verbal behaviour in their social interactions. It may also include efforts to change the children’s cognitive assessment of the 'social world'. Social skills training also includes teaching social norms, social 'rules', and expectations of others (Liberman 1988).
Training may also focus on emotion regulation, such as the child's ability to deal with, manage, express, and control his or her emotions. An inability to regulate both positive and negative emotion has been associated with disorders such as ADHD and conduct disorder (Walcott 2004). Emotional self‐regulation is an important aspect of resilience. Children who have effective strategies for dealing with disappointment, loss, and other upsetting events are more likely to bounce back from adversity than those who do not. Managing positive emotion is also important. Success socially and at school depends on the ability to control exuberance, when appropriate.
Social skills training often consists of role play, exercises and games, as well as homework. Social skills training is often taught in groups and is a relatively short intervention typically lasting between eight and 12 weeks. The duration of each group session is usually 50 to 90 minutes. Treatment frequency can range from a couple of times per month to several times a week. Often the programme also involves parents or teachers. Parental groups are typically included to give the parents the opportunity to support the children's training in the social skills groups by understanding the nature of ADHD and the content of the treatment programme. Teachers are often included to facilitate learning objectives and to coordinate social skills training domains, such as homework.
How the intervention might work
The effect of the intervention may be measured by looking at social skills per se, or by looking at a more global assessment of psychological functioning such as the quality of peer relationships, emotional competences, and general behaviour. Social skills training includes procedures to identify problems and set goals in collaboration with the participant. Through role play, exercises and games, participants demonstrate the required skills, and positive or corrective feedback is given to them accordingly. By social modelling and behavioural practice, participants observe and repeat the skills until they become more generalised. Homework assignments are then given to motivate participants to implement these communications in real‐life situations (Almerie 2015).
Why it is important to do this review
Several randomised clinical trials suggest that social skills training may help children with ADHD (Pfiffner 1997; Antshel 2003; Pfiffner 2007). Social skills training may be effective alone, as an adjunct to medication, or both. However, the evidence on social skills training is unclear, and systematic reviews are necessary to evaluate its effectiveness and potential harms. It is always important to investigate the benefits and harms of interventions in order to not waste valuable resources in clinical practice.
Like medical treatments, the effects of social skills training do not always appear to endure. Some trials indicate that not all children benefit from social skills training, potentially due to lack of parental engagement during treatment (Kadesjö 2002). Some have argued that social skills training groups can have a negative effect on children with behavioural problems because the children’s aggressive and restless behaviour can limit their ability to learn social skills. This, paradoxically, can increase negative behaviour (Mager 2005).
This review is an update of our systematic review published in 2011, which, at the time, was the only high‐quality review on the topic (Storebø 2011). Many new trials have been conducted since 2011 and this update includes more than the double number of trials compared to our original review.
We have identified two meta‐analyses and one review investigating the efficacy of social skills or psychosocial training for children with ADHD. Two of these studies found a significant effect of social skills and psychosocial treatment (De Boo 2007;Majewicz‐Hefley 2007) and one did not find any significant effect (Van der Oord 2008). We also found a meta‐analysis which assessed the effectiveness of social skills training for students with behaviour disorders (Kavale 1997). This meta‐analysis also did not find any significant benefit from social skills training. The review and these meta‐analyses have serious methodological deficits. None of them were systematic reviews like ours and they all lacked a published protocol before they were conducted. Furthermore, none of them systematically evaluated systematic errors (bias) or random errors (play of chance), and therefore their results are questionable. A systematic review published in 2019 on stand‐alone social skills training for youth with ADHD concluded that social skills training implemented without additional treatment components like parent support, showed improvements on some areas of social functioning (Willis 2019). However, this review suffered from a very limited search strategy and did not evaluate systematic errors (bias) in the included trials. In our Cochrane review in 2011 on the topic, we were unable to demonstrate clear benefits or harms of social skills training (Storebø 2011).
Objectives
To present the available evidence on the beneficial and harmful effects of social skills training in children and adolescents with ADHD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials (RCTs) investigating social skills training alone or as an adjunct to pharmacological treatment compared to pharmacological treatment. The comparison groups were no intervention or waiting‐list control.
Types of participants
Children and adolescents between five and 18 years of age and diagnosed with ADHD according to the DSM‐IV, DSM‐IV‐TR or DSM‐5, or with hyperkinetic disorder according to the ICD‐10. The main term used in the DSM‐IV is 'ADHD 314', which is divided into three subdiagnoses: 'predominantly inattentive type' (314.00), 'predominantly hyperactive/impulsive type' (314.01), and 'combined type' (314.02). We also included trials that used the DSM‐IV diagnosis of 'ADHD unspecified' (314.9), as well as other diagnostic categories from earlier DSM systems (DSM‐III; DSM‐IV‐R), and 'hyperkinetic disorder' from the ICD‐9.
In addition, we included participants with a diagnosis of ADHD based on a cut‐off score from a validated diagnostic assessment instrument: for example, Conners’ Parent Rating Scales (Conners 1998). We also included participants with different kinds of comorbidity such as conduct or oppositional disorders, depression, attachment disorder, or anxiety disorders.
Types of interventions
We considered all forms of social skills training where training focused on behavioural and cognitive‐behavioural efforts to improve social skills and emotional competence. This included behavioural and cognitive treatments focusing on teaching the children how to 'read' the subtle cues in social interaction, such as learning to wait for their turn, knowing when to shift topics during a conversation, and being able to recognise the emotional expressions of others, as well as social 'rules', and expectations of others.
We included trials comparing social skills training versus either no intervention or waiting‐list control. We considered these control groups to be equal. Therefore, we did not distinguish between the control groups, but analysed the trials with relevant outcomes together in the same comparison. We also included trials with concurrent medical treatment if the medication was administered equally in both groups. In further updates of the review, we will include trials with social skills training versus placebo or sham intervention, as described in our protocol (Storebø 2010).
Types of outcome measures
Primary outcomes
Social skills in school or at home, measured at post‐treatment and longest follow‐up, by well‐established and validated instruments such as the Social Skills Rating System (SSRS; Gresham 1990) or Conners' Behaviour Rating Scales (CBRS;Conners 2008a).
Emotional competencies in school or at home, measured at post‐treatment and longest follow‐up, by well‐established and validated instruments such as the Emotion Regulation Checklist (ERC; Hannesdottir 2017).
General behaviour in school or at home, measured at post‐treatment and longest follow‐up, by well‐established and validated instruments such as the Achenbach Child Behavior Checklist (Achenbach 1991).
Secondary outcomes
Core ADHD symptoms of inattention, impulsivity, and hyperactivity, measured at post‐treatment and longest follow‐up, by well‐established and validated instruments such as Conners' Parents’ Rating Scales (Conners 1998; Conners 2008b).
Performance and grades in school, measured at post‐treatment and longest follow‐up, by well‐established and validated instruments.
Participant or parent (or both) satisfaction with treatment, measured as continuous outcomes by psychometrically validated instruments such as the Client Satisfaction Questionnaire (Attkisson 1982).
Adverse events. We included both severe and non‐severe adverse events. We defined serious adverse events as any event that led to death, was life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability, or any important medical event that may have jeopardised the participant's health or required intervention to prevent it (ICH 1996). We considered all other adverse events as non‐serious.
Search methods for identification of studies
We ran searches for the previous review up to March 2011, using the search strategies reported in Storebø 2011. For this update, we made some changes to the databases we searched (see Differences between protocol and review).
Electronic searches
For this update, we searched the following electronic databases up to July 2018.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6), in the Cochrane Library (searched 11 July 2018).
MEDLINE Ovid (1948 to 11 July 2018).
Embase Ovid (1980 to 11 July 2018).
ERIC EBSCOhost (Education Resources Information Center; 1966 to 11 July 2018).
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1980 to 11 July 2018).
PsycINFO Ovid (1806 to 11 July 2018).
Sociological Abstracts ProQuest (1952 to 11 July 2018).
ProQuest Dissertations & Theses Global (searched 11 July 2018).
ClinicalTrials.gov (clinicaltrials.gov; searched 12 July 2018).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en; searched 12 July 2018).
The search strategies for this update are shown in Appendix 1. We did not limit our searches by language, year of publication, or type or status of publication. We sought translation of the relevant sections of non‐English language articles.
Searching other resources
We searched the following online proceedings for potentially relevant conference abstracts.
2nd International Congress on ADHD: from childhood to adult disease; 2009 May 21 to 24; Vienna, Austria (International Congress on ADHD 2009).
3rd International Congress on ADHD: from childhood to adult disease; 2011 May 26 to 29; Berlin, Germany (World Congress on ADHD 2011).
4th World Congress on ADHD: from childhood to adult disease; 2013 June 6 to 9; Milan, Italy (World Congress on ADHD 2013).
5th World Congress on ADHD: from child to adult disorder; 2015 May 28 to 31; Glasgow, Scotland (World Congress on ADHD 2015).
6th World Congress on ADHD: from child to adult disorder; 2017 Apr 20 to 23; Vancouver, Canada (World Congress on ADHD 2017).
Eunethydis 1st International ADHD Conference: from data to best clinical practice; 2010 May 26 to 28; Amsterdam, The Netherlands (Eunethydis 2010).
Nordic ADHD Konference: livslange perspektiver og specielle behov [lifelong perspectives and special needs]; 2010 May 19 to 20; Aalborg, Denmark (Nordic ADHD konference 2010).
InternationaI Society for Research in Child and Adolescent Psychopathology (ISRCAP) conference; 2009 June 17‐20; Seattle, Washington, USA.
CADDRA: 14th Annual ADHD Conference; 2018 Nov 10 to 11; Calgary, Canada.
In addition, we contacted 176 experts in the field for information about possible unpublished or ongoing RCTs, and received responses from 15 (a list of those contacted is available from the review contact author).
Data collection and analysis
We conducted the review according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In the following section, we report only the methods that we were able to use in this update. Methods that we had planned to use as per our published protocol (Storebø 2010), but could not (e.g. cluster‐randomised trials), are reported in Table 2.
1. Methods not used in this update.
| Section | Protocol | Review |
| Types of outcome measures | We did not define what we meant by adverse events. | We added a definition of adverse events according to the International Committee of Harmonization guidelines (ICH 1996), because many of the studies included pharmaceutical treatment and it is not known whether social skills training might have adverse events. |
| We stated that we would measure the three primary and the first two secondary outcomes at short‐term (up to six months), medium‐term (six to 12 months), and long‐term (more than 12 months) follow‐up. | We changed this to end of treatment and at the longest follow‐up because we did not have data for the planned three time points. | |
| We did not prespecify the most important comparisons for the 'Summary of findings' table. | We reported a total of seven outcomes in the 'Summary of findings' table as per Cochrane recommendations; three primary outcomes (social skills, emotional competencies and general behaviour) and the first secondary outcome (ADHD symptoms). | |
| Assessment of risk of bias in included studies | We had not planned to evaluate blinding of participants and personnel. | We assessed the blinding of participants and personnel, as this is also important to assess in trials investigating psychosocial interventions, even if it is very difficult to do in these types of trials. |
| We stated that we would only use studies at low risk (or lower risk) of bias in the meta‐analysis. | We changed the decision to restrict the meta‐analysis to studies at comparable risk of bias (for example, all low risk of bias, all unclear risk of bias, or all high risk of bias), and performed sensitivity analyses accordingly. We decided to change this as there were very few trials at low risk of bias in this field. | |
| We stated that we would assess 'baseline imbalance' and 'early stopping' as risk of bias domains. | We did not assess these baseline domains. The randomisation procedure should give an even distribution of confounding factors and baseline imbalance. | |
| Dealing with missing data | We intended to assess the impact of missing dichotomous data in the results by applying procedures for 'intention‐to‐treat' and 'best‐case/worst‐case scenarios'. | We were unable to perform this analysis as there were no dichotomous data. |
| Measures of treatment effect |
Dichotomous data We planned to analyse dichotomous data as risk ratios and present these with 95% confidence intervals (CIs), and to calculate the risk difference and, where there was a significant effect with the intervention and reasonable homogeneity of studies (that is, clinical, methodological, or statistical heterogeneity was within reasonable limits), the number needed to treat for an additional beneficial outcome (Higgins 2011, Section 9.2). |
We did not do this as there were no dichotomous data. |
| Unit of analysis issues |
Cluster‐randomised studies We stated that we thought investigators would have presented their results after appropriately checking for clustering effects (robust standard errors or hierarchical linear models). We planed to contact the investigators for further information if this was unclear. Where appropriate checks were not used, we planned to request and re‐analyse individual participant data using multilevel models that check for clustering. Following this, we planned to analyse effect sizes and standard errors in RevMan 5 (Review Manager 2014), using the generic inverse method (Higgins 2011, Section 9.3.2). If there was insufficient information to check for clustering, we would have entered outcome data into RevMan 5 using individuals as the units of analysis, and then conducting a sensitivity analysis to assess the potential biasing effects of inadequately controlled clustered studies (Donner 2002). See 'Sensitivity analysis' below. |
We did not find any cluster‐randomised trials. |
| Assessment of reporting biases | We did not state that we would use Egger's test to test for small‐study effects. | We performed Egger's statistical test for small‐study effects. |
| Subgroup analysis and investigation of heterogeneity | We planned to perform subgroup analyses according to the following categories.
|
We were not able to perform these subgroup analyses due to lack of sufficient data. |
| Sensitivity analysis | We stated that we would repeat the analysis taking into consideration the different methods used to handle the missing data and the potential biasing effects of inadequately controlled clustered studies. | We did not perform this analysis due to a lack of necessary data and, consequently, have analysed the data as reported. |
ADHD: attention deficit hyperactivity disorder.
Selection of studies
Eight reviewers (OJS, NP, EGF, MEA, BT, MS, HC and SJ) independently evaluated and selected trials for inclusion. Having removed duplicates, they assessed the titles and abstracts of all records generated by the search and excluded those that clearly did not meet the inclusion criteria: for example, non‐randomised trials or trials with participants outside the specified age range (Criteria for considering studies for this review). Next, they retrieved the full‐text reports for those trials deemed relevant or for which more information was needed to determine relevance and assessed them for eligibility. The review authors discussed differing interpretations regarding eligibility and consulted a third review author (ES) for those cases where they could not reach an agreement.
We have listed relevant RCTs that did not fulfil the inclusion criteria with reasons for exclusion in the Characteristics of excluded studies table. We recorded our selection process in a study flow diagram (Moher 2009).
Dealing with duplicate publication
We collected multiple reports of the same study to maximise data collection.
Data extraction and management
Working in pairs, eight review authors (OJS, MEA, EGF, BT, HC, SJH, NP, and MS) independently extracted data onto a data collection form (Appendix 2). We extracted data on participants, study design and methods, interventions, outcomes, and relevant data for 'Risk of bias' assessments. We resolved differences by discussion.
OJS entered the data into Review Manager 5 (RevMan 5) (Review Manager 2014).
In cases of lack of data, for the use of e.g. tables with sociodemographic data, 'risk of bias' assessment, or the analysis, or where data in the published study reports were unclear, we contacted the trial authors requesting them to clarify the missing information (see Dealing with missing data).
Assessment of risk of bias in included studies
For each included RCT, eight review authors (OJS, MEA, EGF, BT, HC, SJH, NP, and MS), working in pairs, independently evaluated the following 'Risk of bias' components: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective outcome reporting; vested interest bias; and other sources of bias. We assigned trials to one of three categories (low risk of bias, uncertain risk of bias, and high risk of bias), according to guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011, section 8.2.1) and from the Cochrane Hepato‐Biliary Group (Cochrane Hepato‐Biliary Group 2019) (see Appendix 3). We resolved disagreements by discussion. We used the results of the 'Risk of bias' assessment to inform the GRADE assessment.
Measures of treatment effect
Continuous data
We compared the mean score between the two intervention groups to give a mean difference (MD) and presented this with 95% confidence intervals (CIs). We wanted to use the overall MD, where possible, to compare the outcome measures from trials. However, because many of the included trials used different rating scales for measuring the same construct we used the standardised mean difference (SMD) in many analyses. For the primary outcome, teacher‐rated social skills at end of treatment, we transformed the MD and standard deviation (SD) from the different rating scales used in this analysis to the MD and SD of a commonly used scale, namely the Social Skills Rating Scale (SSRS). We reported this MD in the results section as well as in the abstract and we compared it to a plausible minimal relevant difference of 10% on this scale.
Unit of analysis issues
We did not encounter any unit of analysis issues. Our strategies for dealing with these can be found in Table 2 (see also Storebø 2010).
Dealing with missing data
We sought to retrieve any missing data from the trial authors. Overall, we wrote to 17 authors, eight of whom supplied us with missing sociodemographic data and missing information about methodology; some also supplied us with missing statistics. If data remained unavailable, we tried to estimate the missing data using the available information (e.g. if the standard deviation (SD) was missing, we estimated it from the standard error, if reported). When we were not able to obtain missing data, we conducted analyses using available (incomplete) data.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining variability in the participants, interventions, and outcomes described in each included trial. We assessed methodological heterogeneity by inspecting variability in the designs of the trials, and statistical heterogeneity by assessing the difference in the trials' intervention effects. We assessed heterogeneity between trials by visual inspection of the forest plot for overlapping CIs, using the Chi2 test for homogeneity with a significance level of α (alpha) = 0.10, and the I2 statistic for quantifying inconsistency (estimating the percentage of variation in effect estimates due to heterogeneity rather than sampling error). We judged I2 values of 0% to 40% to indicate little heterogeneity; 30% to 60%, moderate heterogeneity; 50% to 90%, substantial heterogeneity; and 75% to 100%, considerable heterogeneity (Higgins 2011). Furthermore, we explored potential reasons for the heterogeneity by examining individual trial characteristics and conducting subgroup analyses (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We handled different forms of reporting bias, especially publication bias and outcome reporting bias, according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011, Section 10.1). We drew funnel plots (estimated differences in treatment effects against their standard error), and we performed Egger's statistical test for small‐study effects (Egger 1997). There are several reasons for the asymmetry of a funnel plot; for example, true heterogeneity, poor methodological quality, or publication bias (Higgins 2011, section 10.4.1).
Data synthesis
We included and analysed trials undertaken in any setting; for instance, in groups, in the home, or at a centre. We summarised data in a meta‐analysis when they were available and if clinical heterogeneity was not excessive (for example, there was not too much variability in participants' characteristics). We performed statistical analysis in RevMan 5 (Review Manager 2014), according to recommendations in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011, Section 9.4.1). We synthesised data by using final values and the inverse variance method in the meta‐analyses. We generally used the random‐effects model because we expected differences in the treatments. The fixed‐effect model is used when there is an assumption that the observed differences between the study results are just due to ‘play of chance’. When there is heterogeneity that cannot be explained as ‘play of chance’, it is common to use the random‐effects model. A random‐effects model has the assumption that apparent differences between study effects are random, but the estimated difference follows a normal distribution. This method gives more weight to small trials, whereas the fixed‐effect model gives more weight to large trials. We therefore conducted both fixed‐effect and random‐effects models, and checked for differences between these methods of analyses (Higgins 2011, Section 9.5.4). If both models gave the same results, we reported the results from the random‐effects model only. For some outcomes we were unable to conduct a meta‐analysis because the outcomes were reported only in a single study. For these outcomes, we provided a narrative description of the results.
Diversity‐adjusted required information size and Trial Sequential Analysis
Trial Sequential Analysis (TSA) is a tool for controlling risks of type I and type II errors in cumulative meta‐analyses, and gives a valuable overview of the number of participants needed to make a firm evaluation of a possible intervention effect (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Wetterslev 2017).
Comparable to the a priori sample size estimation in a single RCT, a meta‐analysis should include an information size (IS) at least as large as the sample size of an adequately powered single study to reduce the risks of random errors. The TSA provides the required information size (RIS) for a meta‐analysis, adjusting the significance level for sparse data and repetitive testing on accumulating data, to avoid the increased risk of random error (Wetterslev 2008; Wetterslev 2009; Wetterslev 2017).
Multiple analyses of accumulating data from new emergent trials leads to ‘repeated significant testing’, and use of the conventional P value is prone to exacerbate the risk of a type I random error (Lau 1995; Berkley 1996). Meta‐analyses that do not reach the RIS are analysed with trial sequential monitoring boundaries, which are analogous to interim monitoring boundaries in a single study (Wetterslev 2008; Wetterslev 2017). This approach is crucial in coming updates of this review.
We used an a priori assumption that the minimal relevant clinical intervention effect was 4.0 points. This is approximately ½ SD on the used scale, which can be used as a minimal clinical relevant difference (Norman 2003).
We calculated the diversity‐adjusted required information size (DARIS; that is the number of participants required to detect or reject a specific intervention effect in a meta‐analysis), and performed a TSA for the primary outcome (teacher‐rated social skills competences) at the end of treatment, based on the following a priori assumptions:
the SD of the primary outcome of 9.5 points;
an anticipated minimal relevant difference (MIREDIF) of 4.0 points;
a maximum type I error of 2.5% (due to three primary outcomes; Jakobsen 2014);
a maximum type II error of 10% (minimum 90% power; Castellini 2018); and
the diversity observed in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analysis both where we found statistically significant differences between intervention groups, and in other cases to make hypotheses about the subgroups mentioned below.
We performed the following subgroup analyses.
Children aged five to 11 years compared to children aged 12 to 18 years;
ADHD with comorbidity compared to ADHD without comorbidity;
Social skills training only compared to social skills training supported by parent training;
Social skills training, parent training and medication compared to social skills training and parent training without medication;
No‐intervention control group compared to waiting‐list control group with possible minor active intervention components.
Sensitivity analysis
We assessed the robustness of the results by conducting sensitivity analyses in which we repeated the analysis:
excluding the trial with longest treatment duration or the largest trial; and
using different statistical models (fixed‐effect or random‐effects models) (Higgins 2011).
'Summary of findings' table
We constructed 'Summary of findings' tables using GRADE software (GRADEpro GDT 2015) for the comparison 'social skills training compared to no intervention'. We included three primary outcomes (social skills, emotional competencies and general behaviour) and one secondary outcome (ADHD core symptoms) assessed at end of treatment in the table.
We used the GRADE approach to assess the quality of the evidence associated with each of these outcomes (Guyatt 2008). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Considerations are due to: within‐study risk of bias; directness of evidence; heterogeneity of the data; precision of effect estimates; and risk of publication bias (Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Andrews 2013a; Andrews 2013b; Brunetti 2013; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Mustafa 2013).
Results
Description of studies
Results of the search
This updated review fully incorporates the results of searches conducted up to July 2018. We carried out electronic searches over five time periods: up to February 2009; February 2009 to June 2010; June 2010 to March 2011; March 2011 to May 2017; and May 2017 to July 2018. The number of unique records (i.e. number of records after duplicates were removed) generated by these searches were as follows.
Up to February 2009 = 2500 (out of 3045);
February 2009 until June 2010 = 200 (out of 643)
June 2010 until March 2011 = 165 (out of 208)
March 2011 until May 2017 = 1616 (out of 3229)
May 2017 until July 2018 = 324 (out of 410)
To date, the electronic searches for this review have found 7535 records, plus an additional four records from searching other resources. Having removed duplicates, we screened 4805 records, and subsequently excluded 4492 as clearly irrelevant on the basis of title and abstract. We retrieved the full texts of the remaining 313 reports, which we assessed for eligibility against our selection criteria (Criteria for considering studies for this review). From these, we excluded 224 as irrelevant, formally excluded a further 39 with reasons (see Excluded studies), and included 25 trials (from 45 reports). We also identified four ongoing trials, and one which is awaiting classification (see Figure 1).
1.
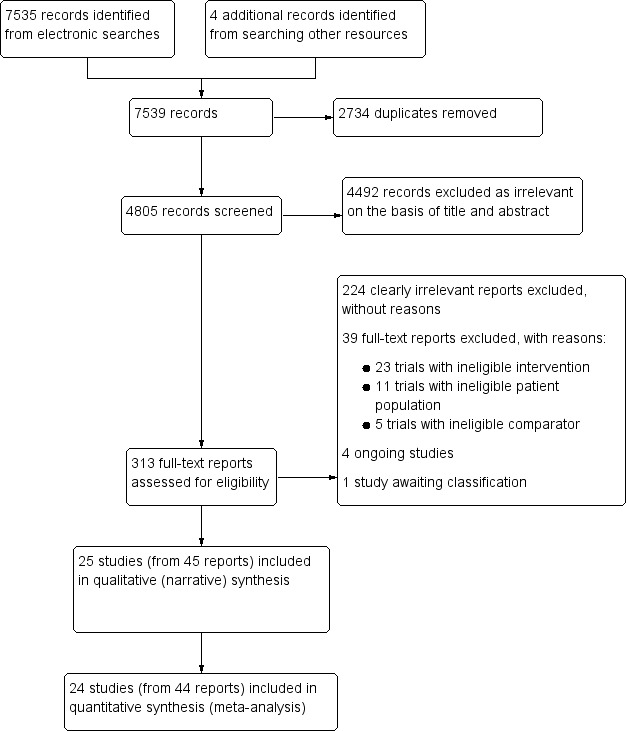
Study flow diagram.
Included studies
This review includes 25 RCTs described in 45 reports. Of these 25 trials, one, Cohen 1981, did not have usable data to be included in the quantitative analysis (meta‐analysis). Another one of the trials had extreme values in some of the outcomes and we did not use the data on these outcomes (Tabaeian 2010).
See Characteristics of included studies tables for further details on each included study.
Setting
Thirteen trials were carried out in North America: 12 in the US (Bloomquist 1991; Pfiffner 1997; MTA 1999; Antshel 2003; Tutty 2003; Abikoff 2004; Pfiffner 2007; Waxmonsky 2010; Pfiffner 2014; Evans 2016; Pfiffner 2016; Waxmonsky 2016). Of the remaining 12 trials, six were carried out in Asia; three in Iran (Tabaeian 2010; Azad 2014; Meftagh 2014a), two in China (Yuk‐chi 2005; Qian 2017), and one in South Korea (Choi 2015). Five trials were conducted in Europe; one apiece in Denmark (Storebø 2012), Iceland (Hannesdottir 2017), Germany (Schramm 2016), The Netherlands (Van der Oord 2007), and one which took place in both Belgium and The Netherlands (Bul 2016): One trial was conducted in Australia (Wilkes Gillan 2016), and one in Canada (Cohen 1981).
The majority of trials were conducted in an outpatient setting; six trials were carried out in a clinical setting (Pfiffner 1997; Tabaeian 2010; Storebø 2012; Azad 2014; Hannesdottir 2017; Qian 2017).
Participants
The 25 RCTs included a total of 2690 participants. The majority of trials included children between five and 13 years of age; a single trial included adolescents between 12 and 17 years of age (Schramm 2016). All participants were diagnosed with ADHD using tools that had been accepted for inclusion in this review. All of these diagnostic tools were based on the international DSM (DSM‐III; DSM‐IV; DSM‐IV‐R; DSM‐IV‐TR; DSM‐5) or ICD diagnostic systems ICD‐10), or a cut‐off score from the Conners' Rating Scale (Conners 1998; Conners 2008a; Conners 2008b).
Six trials did not specify intelligence (IQ) as inclusion or exclusion criteria (Pfiffner 1997; Tutty 2003; Tabaeian 2010; Meftagh 2014a; Azad 2014; Schramm 2016), and the remaining trials excluded children with low IQ (ranging from < 70 to < 90).
All but seven trials (Pfiffner 1997; Antshel 2003; Van der Oord 2007; Azad 2014; Meftagh 2014a; Choi 2015; Schramm 2016) excluded patients with one or more comorbid mental disorders – typically autism spectrum disorder, psychosis, or neurological disorder. Two trials used comorbidity as an exclusion criterion (Tutty 2003; Tabaeian 2010). Eighteen trials reported on different types of comorbidities, such as oppositional defiant disorder, conduct disorder, and anxiety disorder for the children in addition to the ADHD diagnosis.
The distribution of boys to girls was almost equal in two trials (Pfiffner 2014; Choi 2015). In the remaining trials, boys outnumbered girls. The number of boys to girls was: 2:1 in seven trials (Bloomquist 1991; Pfiffner 1997; Pfiffner 2007; Storebø 2012; Evans 2016; Waxmonsky 2016; Hannesdottir 2017); 3:1 in three trials (Antshel 2003; Tutty 2003; Pfiffner 2016); 4:1 in four trials (MTA 1999; Waxmonsky 2010; Bul 2016; Qian 2017); 6:1 in two trials (Schramm 2016; Wilkes Gillan 2016); 7:1 in one trial (Cohen 1981); 9:1 in one trial (Yuk‐chi 2005) and 14:1 in one trial (Abikoff 2004). The participants were all males in one trial (Tabaeian 2010), and three trials provided no information on the sex of the participants (Van der Oord 2007; Azad 2014; Meftagh 2014a).
Participants were between 80% and 100% Caucasian in six trials (Bloomquist 1991; Pfiffner 1997; Antshel 2003; Abikoff 2004; Van der Oord 2007; Waxmonsky 2010). Ethnicity was more mixed in seven other trials: 16% to 74% Caucasian; 3% to 75% Hispanic; 0% to 16% Asian; and 5% to 20% Afro‐American (MTA 1999; Tutty 2003; Pfiffner 2007; Pfiffner 2014; Evans 2016; Pfiffner 2016; Waxmonsky 2016). In four trials, ethnicity was stated with reference to the country of origin, with all or almost all being Canadian (Cohen 1981), Chinese (Yuk‐chi 2005), Iranian (Tabaeian 2010), or Australian (Wilkes Gillan 2016). Ethnicity was not explicitly described in eight trials (Storebø 2012; Azad 2014; Meftagh 2014a; Choi 2015; Bul 2016; Schramm 2016; Hannesdottir 2017; Qian 2017). Eleven trials included and controlled for a measure of socioeconomic status (Pfiffner 1997; Yuk‐chi 2005; Pfiffner 2014; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016; Tutty 2003; Van der Oord 2007; Waxmonsky 2010; Wilkes Gillan 2016).
Sample size
There was considerable variation in sample sizes between trials. The number of participants randomised per study ranged from 24 to 576 participants in all trials. Only three trials reported a sample size calculation before the start of the study (MTA 1999; Storebø 2012; Bul 2016).
Interventions
Experimental
The 25 trials had different but comparable experimental interventions. The interventions named were: social skills training (Pfiffner 1997; Antshel 2003; Tabaeian 2010; Storebø 2012; Choi 2015; Hannesdottir 2017); cognitive behavioural intervention (Cohen 1981; Bloomquist 1991); meta‐cognitive training (Azad 2014); multimodal behavioural/psychosocial therapy (MTA 1999; Abikoff 2004; Van der Oord 2007); behavioural therapy/treatment (Pfiffner 2007; Waxmonsky 2010); behavioural and social skills treatment (Tutty 2003; Waxmonsky 2016); challenging horizon program (CHP; Evans 2016); children's verbal self‐instruction training (Meftagh 2014a); child life and attention skills treatment (CLAS; Pfiffner 2014; Pfiffner 2016), executive skills training (Qian 2017); learning skills training (Schramm 2016); different play or game‐based intervention (Bul 2016; Wilkes Gillan 2016); and psychosocial treatment (Yuk‐chi 2005). We considered that all these interventions were comparable and based on a cognitive behavioural model. Throughout the rest of the review, we referred to the experimental child interventions as 'child social skills training', in accordance with the Description of the intervention section.
The duration of the intervention varied between five and eight weeks in seven trials (Pfiffner 1997; Antshel 2003; Tutty 2003; Waxmonsky 2010; Storebø 2012; Azad 2014; Hannesdottir 2017), and between 10 and 16 weeks in 13 trials (Cohen 1981; Bloomquist 1991; Pfiffner 2007; Van der Oord 2007; Tabaeian 2010; Meftagh 2014a; Pfiffner 2014; Choi 2015; Bul 2016; Pfiffner 2016; Waxmonsky 2016; Wilkes Gillan 2016; Qian 2017). In two trials, the intervention lasted for 24 weeks (Yuk‐chi 2005; Schramm 2016), and, in one trial apiece, the intervention lasted for one year (Evans 2016), 14 months (MTA 1999), and two years (Abikoff 2004).
Five trials used social skills training for children plus parent training (Cohen 1981; Antshel 2003; Tutty 2003; Abikoff 2004; Waxmonsky 2010); one of these trials also administered academic organisational skills training and individual psychotherapy (Abikoff 2004). Seven trials used a combination of social skills training for children, parent training, and teacher consultations in the experimental group (Bloomquist 1991; Yuk‐chi 2005; Pfiffner 2007; Van der Oord 2007; Pfiffner 2014; Pfiffner 2016; Schramm 2016). One trial used social skills training for children, parent training, teacher consultations, and classroom behavioural intervention in the experimental group (MTA 1999), and another used social skills training for children and parent training plus standard treatment in the experimental group (Storebø 2012). One trial used either social skills training for children or social skills training for children plus parent training (Pfiffner 1997).
One trial used social skills training only in the experimental group (Choi 2015). Another trial included the Challenging Horizons Program, which is designed to target different skills such as organisational and social skills (Evans 2016), whereas other trials used life skills (Pfiffner 2014) and group‐based social skills training as the experimental intervention (Hannesdottir 2017). One trial used verbal self‐instruction as the experimental programme (Meftagh 2014a), and another used a play‐based intervention (Wilkes Gillan 2016). Finally, one trial used a specific intervention targeting skills related to mood and behaviour (Waxmonsky 2016).
Two trials used meta‐cognitive training (Azad 2014; Qian 2017); one had a social game intervention, which targeted cooperation and planning skills among others (Bul 2016), and the other used behavioural and cognitive training (Schramm 2016).
Seven trials included concurrent medical treatment with ADHD medication in both the experimental and control groups (Cohen 1981; Antshel 2003; Tutty 2003; Abikoff 2004; Waxmonsky 2010; Tabaeian 2010; Storebø 2012).
Control
Eight trials used medications in the experimental group and as the only treatment in the control group (Cohen 1981; MTA 1999; Antshel 2003; Tutty 2003; Abikoff 2004; Yuk‐chi 2005; Van der Oord 2007; Waxmonsky 2010); one of these trials also included a no‐treatment control group (Cohen 1981). Two trials used standard treatment in the experimental group and as the only treatment in the control group (Storebø 2012; Bul 2016). Nine trials used a waiting‐list or no‐intervention control group without medication in any of the groups (Bloomquist 1991; Pfiffner 1997; Pfiffner 2007; Tabaeian 2010; Azad 2014; Choi 2015; Schramm 2016; Wilkes Gillan 2016; Hannesdottir 2017). One trial used parent training in the experimental group and as the only treatment in the control group (Pfiffner 2014). Four trials used a control group with some active intervention elements, however, the researchers did not provide any direct intervention to the individuals in this condition (Pfiffner 2014; Evans 2016; Waxmonsky 2016; Qian 2017). One trial did not describe the control group (Meftagh 2014a).
Outcome measures
In the following section, we did not describe the measures used in one study, Tabaeian 2010, as we were not able to identify these reliably from the information provided in the translated report.
Social skills
See Table 3.
2. Measures of social skills from included studies.
| Measures | Description | Number of studies | Ratings | |||
| Teacher | Parent | Child | Observer | |||
| Social Skills Rating Scale (SSRS) | Three‐point Likert scale, ranging from zero (never) to two (often); higher scores indicate better social skills | 9 | Pfiffner 1997 | Pfiffner 1997 | ‐ | ‐ |
| MTA 1999 | MTA 1999 | MTA 1999 | ‐ | |||
| Pfiffner 2007 | ‐ | ‐ | ‐ | |||
| ‐ | Antshel 2003 | Antshel 2003 | ‐ | |||
| ‐ | Abikoff 2004 | Abikoff 2004 | ‐ | |||
| Van der Oord 2007 | ‐ | ‐ | ‐ | |||
| Waxmonsky 2010 | ‐ | ‐ | ‐ | |||
| ‐ | Waxmonsky 2016 | ‐ | ‐ | |||
| ‐ | Hannesdottir 2017 | ‐ | ‐ | |||
| SSRS: Cooperation Subscale | Three‐point Likert scale, ranging from zero (never) to two (often); higher scores indicate better cooperation | 1 | Bul 2016 | Bul 2016 | ‐ | ‐ |
| Social Skills Improvement System (SSIS) | Four‐point rating scale, ranging from zero (never) to three (almost always); higher scores indicate better social skills. | 3 | Pfiffner 2014 | Pfiffner 2014 | ‐ | ‐ |
| Evans 2016 | Evans 2016 | ‐ | ‐ | |||
| Pfiffner 2016 | Pfiffner 2016 | ‐ | ‐ | |||
| Teacher Report ‐ Walker‐McConnell Scale of Social Competence and School Adjustment | Five‐point rating scale, ranging from one (never occurs) to five (frequently occurs); higher scores indicate better social skills | 1 | Bloomquist 1991 | ‐ | ‐ | ‐ |
| Weiss Functional Impairment Scale ‐ Parent Form (WFIRS‐P): Social Activities Subscale | Four‐point rating scale, ranging from zero (never or not at all) to three (very often or very much); higher scores indicate better social skills | 1 | ‐ | Qian 2017 | ‐ | ‐ |
| Strengths and Difficulties Questionnaire (SDQ): Prosocial Behavior Subscale | Three‐point rating scale, ranging from zero (not true) to two (certainly true); higher scores indicate better social skills. | 1 | Schramm 2016 | Schramm 2016 | Schramm 2016 | ‐ |
| Conners Behavior Rating Scale (CBRS): Social Problems Subscale | Four‐point rating scale, ranging from zero (not true at all) to three (very much true); higher scores indicate better social skills | 1 | Storebø 2012 | ‐ | ‐ | ‐ |
| Social Interaction Observation Code | Recording frequencies of positive, negative or neutral behaviour, including observations of negative behaviour | 1 | ‐ | ‐ | ‐ | Abikoff 2004 |
| Test of Social Skill Knowledge | Scored from one (low knowledge) to 15 (high knowledge); higher scores indicate better social skills | 1 | ‐ | ‐ | ‐ | Pfiffner 1997 |
| Observation in Classrooms | Observing children for three × eight‐minute periods during a one‐hour period for two categories of behaviour: play behaviour and social behaviour | 1 | ‐ | ‐ | ‐ | Cohen 1981 |
| Test of Playfulness: Skillfulness | Four‐point rating scale, ranging from zero (unskilled) to three (highly skilled); higher scores indicate better social skills | 1 | ‐ | ‐ | ‐ | Wilkes Gillan 2016 |
Nineteen trials measured social skills using a variety of scales (Cohen 1981; Bloomquist 1991; Pfiffner 1997; MTA 1999; Antshel 2003; Abikoff 2004; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Storebø 2012; Pfiffner 2014; Bul 2016; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016; Wilkes Gillan 2016; Hannesdottir 2017; Qian 2017). Ten trials used the Social Skills Rating Scale (Pfiffner 1997; MTA 1999; Antshel 2003; Abikoff 2004; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Bul 2016; Waxmonsky 2016; Hannesdottir 2017); one trial used the Cooperation Subscale (Bul 2016), whereas the nine remaining trials used the full Social Skills Rating Scale. Three trials used the Social Skills Improvement System (Pfiffner 2014; Evans 2016; Pfiffner 2016). The remaining studies each used different measures to assess social skills.
Seven of the 19 trials used more than one informant to measure social skills: two used teacher, parent and observer ratings (Pfiffner 1997; Abikoff 2004); one used teacher, parent and child ratings (Schramm 2016); and four used teacher and parent ratings (MTA 1999; Antshel 2003; Pfiffner 2014; Bul 2016). Of the 12 remaining trials, nine used only teacher ratings (Bloomquist 1991; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Storebø 2012; Evans 2016; Pfiffner 2016; Waxmonsky 2016; Hannesdottir 2017), two used only observer‐ratings (Cohen 1981; Wilkes Gillan 2016), and one used only parent ratings (Qian 2017).
Emotional competencies
See Table 4.
3. Measures of emotional competencies from included studies.
| Measures | Description | Number of studies | Ratings | |||
| Teacher | Parent | Child | Observer | |||
| Emotion Expression Scale for Children | Five‐point Likert scale, ranging from one (not at all) to five (extremely true); higher scores indicate poorer emotion awareness and greater reluctance to express emotion | 1 | ‐ | ‐ | Choi 2015 | ‐ |
| Emotion Regulation Checklist (ERC): Emotion Regulation Subscale | Four‐point rating scale, ranging from one (never) to four (almost always); higher scores indicate better emotional regulation | 1 | ‐ | Hannesdottir 2017 | ‐ | ‐ |
| Behavior Rating Inventory of Executive Function (BRIEF): Emotion Control Subscale | Three‐point rating scale, ranging from one (never) to three (often); lower scores indicate better emotional control. | 1 | ‐ | Qian 2017 | ‐ | ‐ |
| Conners Behavior Rating Scale (CBRS): Emotional Index | Four‐point rating scale, ranging from zero (not true at all) to three (very much true); higher scores indicate better emotional competence | 1 | Storebø 2012 | ‐ | ‐ | ‐ |
| Strengths and Difficulties Questionnaire (SDQ): Emotional Symptoms Subscale | Three‐point rating scale, ranging from zero (not true) to two (certainly true); higher scores indicate lower emotional competence | 1 | Schramm 2016 | Schramm 2016 | Schramm 2016 | ‐ |
| Richman‐Graham Scale | Three‐point rating scale, ranging from zero (no difficulties) to two (occurs frequently). Higher scores indicate lower emotional competence. | 1 | ‐ | Cohen 1981 | ‐ | ‐ |
Six trials measured emotional competencies, each using a different measure (Cohen 1981; Choi 2015; Storebø 2012; Schramm 2016; Hannesdottir 2017; Qian 2017). Of these, only one trial, Schramm 2016, used ratings from more than one type of informant: teacher, parent and child. The five remaining trials used only parent ratings (Cohen 1981; Hannesdottir 2017; Qian 2017), teacher ratings (Storebø 2012), or child ratings (Choi 2015). No trials used observer ratings.
General behaviour
See Table 5.
4. Measures of general behaviour from included studies.
| Measures | Description | Number of studies | Ratings | |||
| Teacher | Parent | Child | Observer | |||
| Child Behavior Checklist (CBCL) | Three point rating scale, ranging from zero (not true) to two (often true); lower scores indicate better general behaviour | 1 | MTA 1999 | MTA 1999 | ‐ | ‐ |
| Clinical Global Impression (CGI) Scale | Seven‐point rating scale, ranging from one (much worse) to seven (much improved); higher scores indicate improved general behaviour | 2 | Pfiffner 2007 | Pfiffner 2007 | ‐ | Waxmonsky 2010 |
| Disruptive Behavior Disorders Rating Scale: Oppositional Defiant Disorder index (DBDRS‐ODD) | Four‐point Likert scale, ranging from zero (not at all) to three (very much); lower scores indicate better general behaviour | 2 | Evans 2016 | Evans 2016 | ‐ | ‐ |
| Waxmonsky 2016 | ||||||
| Child Symptom Inventory (CSI): Oppositional Defiant Disorder Subscale | Four‐point rating scale, ranging from zero (never) to three (very often); lower scores indicate better general behaviour | 1 | Pfiffner 2016 | Pfiffner 2016 | ‐ | ‐ |
| Behavior Rating Inventory of Executive Function (BRIEF) | Three‐point rating scale, ranging from one (never) to three (often); lower scores indicate better general behaviour | 1 | ‐ | Qian 2017 | ‐ | ‐ |
| Conners Behavior Rating Scale (CBRS): Conduct Problem Subscale | Four‐point rating scale, ranging from zero (not true at all) to three (very much true); lower scores indicate better general behaviour | 1 | Cohen 1981 | Cohen 1981 | ‐ | ‐ |
| CBRS: Aggressiveness Subscale | Four‐point rating scale, ranging from zero (not true at all) to three (very much true); lower scores indicate better general behaviour | 1 | Storebø 2012 | ‐ | ‐ | ‐ |
| Conners Teacher Rating Scale (CTRS) | Four‐point Likert scale, ranging from zero (not at all true) to three (very true); lower scores indicate better general behaviour | 1 | Abikoff 2004 | ‐ | ‐ | ‐ |
| Strengths and Difficulties Questionnaire (SDQ): Total | Three‐point rating scale, ranging from zero (not true) to two (certainly true); lower scores indicate better general behaviour | 1 | ‐ | Hannesdottir 2017 | ‐ | ‐ |
| SDQ: Conduct Problems Subscale | Three‐point rating scale, ranging from zero (not true) to two (certainly true); lower scores indicate better general behaviour | 1 | Schramm 2016 | Schramm 2016 | Schramm 2016 | ‐ |
| Social Skills Rating Scale (SSRS): Problem Behaviour Subscale | Three‐point Likert scale, ranging from zero (never) to two (often); lower scores indicate better general behaviour | 1 | ‐ | Waxmonsky 2016 | ‐ | ‐ |
| Self‐Control Rating Scale | Seven‐point continuum, ranging from one (indicating maximum level of self‐control) to seven (indicating maximum level of impulsivity; lower scores indicate better general behaviour | 1 | Bloomquist 1991 | ‐ | ‐ | ‐ |
Thirteen trials measured general behaviour using a large variety of measures (Cohen 1981; Bloomquist 1991; MTA 1999; Abikoff 2004; Pfiffner 2007; Waxmonsky 2010; Storebø 2012; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016; Hannesdottir 2017; Qian 2017). Of these 13 trials, six used more than one informant; one used teacher, parent and child ratings (Schramm 2016) and five trials used teacher and parent ratings (Cohen 1981; MTA 1999; Pfiffner 2007; Evans 2016; Pfiffner 2016). Of the seven remaining trials, three apiece used only teacher ratings (Bloomquist 1991; Abikoff 2004; Storebø 2012) or parent ratings (Waxmonsky 2016; Hannesdottir 2017; Qian 2017), and one trial used only observer ratings (Waxmonsky 2010).
Core ADHD symptoms
See Table 6.
5. Measures of ADHD symptoms from included studies.
| Measures | Description | Number of studies | Studies reporting ratings from: | |||
| Teacher | Parent | Child | Observer | |||
| Disruptive Behavior Disorders Rating Scale (DBDRS) | Four‐point Likert scale, ranging from zero (not at all) to three (very much); lower scores indicate fewer ADHD symptoms | 4 | Van der Oord 2007 | Van der Oord 2007 | ‐ | ‐ |
| Waxmonsky 2010 | Waxmonsky 2010 | ‐ | ‐ | |||
| Evans 2016 | Evans 2016 | ‐ | ‐ | |||
| Waxmonsky 2016 | Waxmonsky 2016 | ‐ | ‐ | |||
| ADHD Rating Scales (ADHD‐RS) | Five‐point Likert scale, ranging from zero (never) to four (almost always); lower scores indicate fewer ADHD symptoms | 2 | ‐ | Tutty 2003 | ‐ | ‐ |
| ‐ | Qian 2017 | ‐ | ‐ | |||
| ADHD‐RS: Hyperactivity and Impulsivity Subscale | Five‐point Likert scale, ranging from zero (never) to four (almost always); lower scores indicate fewer ADHD symptoms | 1 | ‐ | Hannesdottir 2017 | ‐ | ‐ |
| Child Symptom Inventory (CSI): Inattention Scale | Four‐point rating scale, ranging from zero (never) to three (very often); lower scores indicate fewer ADHD symptoms | 2 | Pfiffner 2007 | Pfiffner 2007 | ‐ | ‐ |
| Pfiffner 2014 | Pfiffner 2014 | ‐ | ‐ | |||
| Child Symptom Inventory (CSI): ADHD Scale | Four‐point scale (never, sometimes, often, very often); lower scores indicate fewer ADHD symptoms | 1 | Pfiffner 2016 | Pfiffner 2016 | ‐ | ‐ |
| Conners Teacher Rating Scale (CTRS) | Four‐point Likert scale, ranging from zero (not at all true) to three (very true); lower scores indicate fewer ADHD symptoms. | 2 | Bloomquist 1991 | ‐ | ‐ | ‐ |
| Abikoff 2004 | ‐ | ‐ | Abikoff 2004 | |||
| Conners Parent Rating Scale (CPRS) | Four‐point Likert scale, ranging from zero (not at all true) to three (very true); lower scores indicate fewer ADHD symptoms | 2 | ‐ | Abikoff 2004 | ‐ | ‐ |
| ‐ | Azad 2014 | ‐ | ‐ | |||
| Conners 3: Hyperactivity/impulsivity Scale | Four‐point Likert scale, ranging from zero (not at all true) to three (very much true); lower scores indicate fewer ADHD symptoms | 1 | Storebø 2012 | ‐ | ‐ | ‐ |
| ADHD Symptom Checklist (Fremdbeurteilungsbogen für Hyperkinetische Störungen) | Four‐point scale ranging from one (not at all) to three (very much); lower scores indicate fewer ADHD symptoms | 1 | Schramm 2016 | Schramm 2016 | Schramm 2016 | ‐ |
| Swanson, Nolan and Pelham Teacher Rating Scale (SNAP) | Four‐point rating scale, ranging from zero (not at al) to three (very often); lower scores indicate fewer ADHD symptoms | 1 | MTA 1999 | MTA 1999 | ‐ | ‐ |
| Child Attention Profile (CAP) | Three‐point rating scale (1 = not true, 2 = sometimes true, 3 = very often true); lower scores indicate fewer ADHD symptoms | 1 | Tutty 2003 | ‐ | ‐ | ‐ |
| Strengths and Weaknesses of ADHD Symptoms and Normal Behaviors (SWAN) | Seven‐point rating scale, including both positive and negative scores to reflect strengths and weaknesses, ranging from three (far below average) to minus three (far above average). Zero = normal/average | 1 | Yuk‐chi 2005 | Yuk‐chi 2005 | ‐ | ‐ |
| Structured Behavioural Observations | Child behaviour coded as 'on task', 'off task', or 'off task/disruptive'; lower scores indicate fewer ADHD symptoms | 1 | ‐ | ‐ | ‐ | Bloomquist 1991 |
| Continuous Performance Test (CPT): Omission Errors | CPT is a computerised test measuring impulse control and attention control based on the child's response to 150 stimuli, including 30 target stimuli. The omission errors reflect degree of inattention; higher score on omission errors indicate higher degree of inattention. | 1 | ‐ | ‐ | ‐ | Meftagh 2014a |
Eighteen trials measured ADHD symptoms using a variety of measures (Bloomquist 1991; Abikoff 2004; MTA 1999; Tutty 2003; Yuk‐chi 2005; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Storebø 2012; Azad 2014; Meftagh 2014a; Pfiffner 2014; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016; Hannesdottir 2017; Qian 2017). Of these 18, 10 trials used ratings from more than one type of informant; 10 trials used both teacher and parent ratings (MTA 1999; Tutty 2003; Yuk‐chi 2005; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Pfiffner 2014; Evans 2016; Pfiffner 2016; Waxmonsky 2016), and one trial apiece used teacher and observer ratings (Bloomquist 1991), parent and observer ratings (Abikoff 2004), or teachers, parents and child ratings (Schramm 2016). Of the five remaining trials, three used only parent ratings (Azad 2014; Hannesdottir 2017; Qian 2017) and one apiece used only teacher ratings (Storebø 2012) or observer ratings ( Meftagh 2014a).
Performance and grades in school
See Table 7.
6. Measures of performance in school from included studies.
| Measure | Description | Numbder of studies | Ratings | |||
| Teacher | Parent | Child | Observer | |||
| Classroom Performance Survey (CPS) | Five‐point Likert scale, ranging from one (always) to five (never); higher scores indicate lower performance in school | 1 | Evans 2016 | ‐ | ‐ | ‐ |
| Conners Behavior Rating Scale (CBRS): Academic Performance Index; | Four‐point rating scale, ranging from zero (not true at all) to three (very much true); higher scores indicate better performance in school | 1 | Storebø 2012 | ‐ | ‐ | ‐ |
| Social Skills Improvement System (SSIS): Academic Competence Scale | Four‐point scale, ranging from zero (never) to three (almost always); higher scores indicate better performance in school | 1 | Pfiffner 2016 | ‐ | ‐ | ‐ |
| Academic Performance Rating Scale (APRS) | Five‐point Likert scale, ranging from one (never or poor) to five (very often or excellent); higher scores indicate better performance in school | 1 | Waxmonsky 2010 | ‐ | ‐ | ‐ |
| Wechsler Individual Achievement Test (WIAT) | WIAT is a clinician‐administered performance test including 16 subtests divided between Oral Reading, Math Fluency and Early Reading Skills; higher scores indicate better performance | 1 | ‐ | ‐ | ‐ | MTA 1999 |
| German teacher‐rated questionnaire for learning and working behaviour (Arbeitsverhalten Lehrer) | German teacher‐rated questionnaire for learning and working behaviour (Arbeitsverhalten Lehrer is a teacher‐rated scale) | 1 | Lauth 2004 | ‐ | ‐ | ‐ |
Six trials measured performance and grades in school, each using a different measure (MTA 1999; Waxmonsky 2010; Storebø 2012; Evans 2016; Schramm 2016; Pfiffner 2016). Five of these trials used teacher ratings (Evans 2016; Pfiffner 2016; Storebø 2012; Schramm 2016; Waxmonsky 2010) while one trial used observer ratings (MTA 1999).
Satisfaction with treatment
Ten trials reported on participants', parents', teachers' and/or mental health professionals' satisfaction with the treatment (Pfiffner 1997; MTA 1999; Yuk‐chi 2005; Pfiffner 2007; Waxmonsky 2010; Storebø 2012; Pfiffner 2014; Bul 2016; Pfiffner 2016; Waxmonsky 2016). Four trials used the Consumer Satisfaction Questionnaire, which is rated on a seven‐point Likert scale (Pfiffner 1997; MTA 1999; Yuk‐chi 2005; Pfiffner 2007). One trial, Pfiffner 2016, developed a seven‐item measure (rated on a five‐point Likert scale) specifically for the study. The five remaining trials measured treatment satisfaction using a single‐item question that was rated on a five‐point Likert scale in two trials (Pfiffner 2014; Waxmonsky 2016), a seven‐point Likert scale in one trial (Waxmonsky 2010), and a 10‐point Likert scale in two trials (Storebø 2012; Bul 2016).
Adverse events
Only two trials reported data on adverse events (Storebø 2012; Bul 2016). They assessed adverse events as spontaneous reporting and reported no adverse events.
Funding
Fourteen studies reported funding sources. Two of these were funded by pharmacological companies (Waxmonsky 2010; Bul 2016) and the remaining 12 trials were funded by university national foundations (Cohen 1981; MTA 1999; Tutty 2003; Pfiffner 2007; Storebø 2012; Meftagh 2014a; Pfiffner 2014; Pfiffner 2016; Evans 2016; Waxmonsky 2016; Wilkes Gillan 2016; Qian 2017). Two studies reported that they did not receive any funding for the trials (Choi 2015; Hannesdottir 2017) and we did not find any information on funding for the nine remaining trials (Bloomquist 1991; Pfiffner 1997; Antshel 2003; Abikoff 2004; Yuk‐chi 2005; Van der Oord 2007; Tabaeian 2010; Azad 2014; Schramm 2016).
Excluded studies
We excluded 263 full‐text reports in total. Of these, we excluded 224 clearly irrelevant reports. We formally excluded a further 39 full‐text reports, providing reasons for exclusion in the Characteristics of excluded studies tables. Of these 39 reports, we excluded 23 trials with ineligible interventions, 11 trials with ineligible patient populations, and five trials with ineligible comparators.
Ongoing studies
We included four ongoing trials (NCT01330849; Yang 2015; IRCT201609186834N11; NCT02937142). All trials used different methods to investigate the benefits of different types of social skills training or comparable cognitive behaviour training for children and adolescents with ADHD.
Studies awaiting classification
We included one study awaiting classification (NCT01019252).
Risk of bias in included studies
We assessed the risk of bias of each included trial using the Cochrane 'Risk of bias' tool (Higgins 2011). A summary of our assessment is provided below, and in Figure 2 and Figure 3. Further details can be found in the 'Risk of bias' tables (in the Characteristics of included studies tables). We also drew a funnel plot to visually assess whether the effect was associated with the size of the trial; it seemed to be symmetrical with no clinical significant effect. Eggers’ test, moreover, was not statistically significant (Egger’s regression intercept (bias) = 1.13 (two‐tailed P = 0.17)) in the conclusion of whether or not there was publication bias in the meta‐analysis on this outcome.
2.
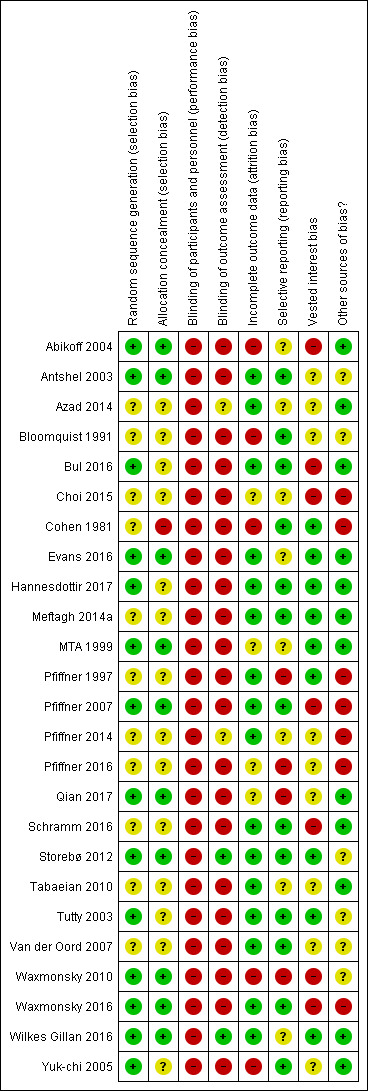
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.
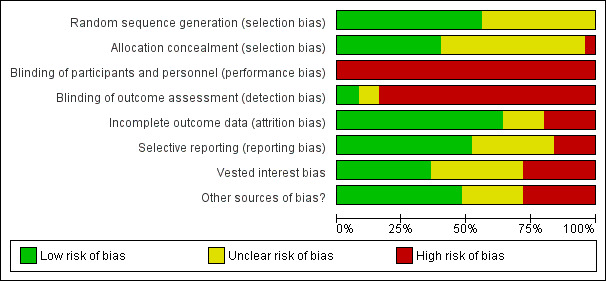
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Generation of the allocation sequence
We considered the random sequence generation to be at low risk of bias in 14 trials that assigned allocation by computer‐generated random numbers derived from a table or by the coin‐toss method (MTA 1999; Antshel 2003; Tutty 2003; Abikoff 2004; Yuk‐chi 2005; Pfiffner 2007; Waxmonsky 2010; Storebø 2012; Bul 2016; Evans 2016; Qian 2017; Waxmonsky 2016; Wilkes Gillan 2016; Hannesdottir 2017). We rated 11 trials that did not state the method used to generate the random sequence to be at unclear risk of bias (Cohen 1981; Bloomquist 1991; Pfiffner 1997; Van der Oord 2007; Tabaeian 2010; Azad 2014; Meftagh 2014a; Pfiffner 2014; Choi 2015; Pfiffner 2016; Schramm 2016). We rated no trials at high risk of bias on this domain.
Allocation concealment
We judged 10 trials to be at low risk of bias due to adequate concealment of the allocation (MTA 1999; Antshel 2003; Abikoff 2004; Pfiffner 2007; Waxmonsky 2010; Storebø 2012; Evans 2016; Waxmonsky 2016; Wilkes Gillan 2016; Qian 2017). Fourteen trials did not describe allocation concealment, so we considered them to be at unclear risk of bias (Bloomquist 1991; Pfiffner 1997; Tutty 2003; Yuk‐chi 2005; Van der Oord 2007; Tabaeian 2010; Azad 2014; Meftagh 2014a; Pfiffner 2014; Choi 2015; Bul 2016; Pfiffner 2016; Schramm 2016; Hannesdottir 2017). We rated one trial, Cohen 1981, to be at high risk of bias because four participants were moved between groups after randomisation due to adverse reactions.
Blinding
We do not believe it is possible to blind participants or personnel involved in the delivery of social skills interventions, and consequently, rated all trials at high risk of performance bias.
It is possible, however, to blind those that perform the ratings and observations. Two trials had blinded ratings and observations and we rated them to be at low risk of detection bias (Storebø 2012; Wilkes Gillan 2016). We rated two other trials to be at uncertain risk of detection bias because it was unclear if raters were blinded (Azad 2014; Pfiffner 2014). We rated the remaining 21 trials as having high risk of detection bias since none of them used blinded ratings and observations for all outcomes (Cohen 1981; Bloomquist 1991; Pfiffner 1997; Abikoff 2004; MTA 1999; Antshel 2003; Tutty 2003; Yuk‐chi 2005; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Tabaeian 2010; Meftagh 2014a; Choi 2015; Bul 2016; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016; Hannesdottir 2017; Qian 2017).
Five trials used blinding for at least one outcome, but we did not use these outcomes in our meta‐analyses as they were not outcomes prespecified for our review.
Incomplete outcome data
We rated 16 trials to be at low risk of attrition bias, as they adequately addressed incomplete outcome data (Pfiffner 1997; Antshel 2003; Tutty 2003; Pfiffner 2007; Van der Oord 2007; Tabaeian 2010; Storebø 2012; Azad 2014; Meftagh 2014a; Pfiffner 2014; Bul 2016; Evans 2016; Schramm 2016; Waxmonsky 2016; Wilkes Gillan 2016; Hannesdottir 2017). We assessed five trials to be at high risk of attrition bias (Cohen 1981; Bloomquist 1991; Abikoff 2004; Yuk‐chi 2005; Waxmonsky 2010). Of these five trials, one reported that 22 out of 103 children failed to complete the trial (Abikoff 2004); another permitted up to 50% missing items on indexes, and dropped participants when there were not enough data (Waxmonsky 2010); while the other three trials did not adequately address incomplete outcome data (Cohen 1981; Bloomquist 1991; Yuk‐chi 2005). We considered the remaining four trials to be at unclear of risk of attrition bias due to a lack of information (MTA 1999; Choi 2015; Pfiffner 2016; Qian 2017).
Selective reporting
We rated 13 trials (which had protocols published before the trial started, and reported on all protocol specified outcomes) to be at low risk of reporting bias (Cohen 1981; Bloomquist 1991; Antshel 2003; Tutty 2003; Yuk‐chi 2005; Pfiffner 2007; Van der Oord 2007; Storebø 2012; Meftagh 2014a; Bul 2016; Schramm 2016; Waxmonsky 2016; Hannesdottir 2017). We rated four trials to be at high risk of reporting bias (Pfiffner 1997; Pfiffner 2016; Qian 2017; Waxmonsky 2010). While most trials reported on all outcomes expected to be addressed as described in their published trial protocol, Pfiffner 1997 did not report on two important outcomes (the Swanson, Nolan and Pelham (SNAP) rating scale and the Conners, Loney, and Milich (CLAM) scale used in pre‐ and post‐treatment assessments) and there was an inconsistency between the published report and the description of the trial (protocol) on clinicaltrials.gov in Waxmonsky 2010, Pfiffner 2016 and Qian 2017. We rated the eight remaining trials to be at unclear risk of reporting bias due to a lack of information (MTA 1999; Abikoff 2004; Tabaeian 2010; Azad 2014; Pfiffner 2014; Choi 2015; Evans 2016; Wilkes Gillan 2016): we were unable to find reports on all prespecified outcomes in one trial (MTA 1999); another trial published reports on both the design of the trial and the results simultaneously (Abikoff 2004); another trial registered the protocol retrospectively (after participant enrolment), and did not report on all prespecified outcomes thus making it difficult to assess if there had been selective reporting (Wilkes Gillan 2016); and five trials had no published design report or trial registration and thus no information to assess this domain (Tabaeian 2010; Azad 2014; Pfiffner 2014; Choi 2015; Evans 2016).
Vested interest
We assessed seven trials to be at high risk of bias on this domain because the trial authors were board members in pharmaceutical companies, had received funding from pharmaceutical companies, or had performed previous research on the topic (Abikoff 2004; Pfiffner 2007; Waxmonsky 2010; Choi 2015; Bul 2016; Schramm 2016; Waxmonsky 2016). We rated nine trials to be at unclear risk of bias because of a lack of information on vested interests (Bloomquist 1991; Antshel 2003; Yuk‐chi 2005; Van der Oord 2007; Tabaeian 2010; Azad 2014; Pfiffner 2014; Pfiffner 2016; Qian 2017). We rated the remaining nine trials to be at low risk of bias (Cohen 1981; Evans 2016; Hannesdottir 2017; Meftagh 2014a; MTA 1999; Pfiffner 1997; Storebø 2012; Tutty 2003; Wilkes Gillan 2016).
Other potential sources of bias
We rated 14 trials to be at low risk of other bias due to no other potential risk of bias (MTA 1999; Abikoff 2004; Yuk‐chi 2005; Tabaeian 2010; Azad 2014; Meftagh 2014a; Bul 2016; Evans 2016; Schramm 2016; Wilkes Gillan 2016; Hannesdottir 2017; Qian 2017).
We considered seven trials to be at high risk of other bias (Cohen 1981; Pfiffner 1997; Pfiffner 2007; Pfiffner 2014; Pfiffner 2016; Choi 2015; Waxmonsky 2016). In five of these trials (Pfiffner 1997; Pfiffner 2007; Pfiffner 2014; Pfiffner 2016; Waxmonsky 2016), the families and teachers were paid for doing the assessment at follow‐up, leading to potential bias from those who were prone to this incentive. In Pfiffner 1997, 44% of participants were medicated with stimulant medication, but the number of medicated children in the comparison group was not stated. One trial provided no information about the between‐group balance of stimulant medication (Cohen 1981), and there was no description of the participant selection procedure in another trial (Choi 2015).
With the exception of one trial where all kinds of medication were balanced between groups (MTA 1999), the remaining trials provided no information about any co‐medication for comorbid disorders.
We judged six trials to be at unclear risk of other bias due to a lack of information (Bloomquist 1991; Antshel 2003; Tutty 2003; Van der Oord 2007; Waxmonsky 2010; Storebø 2012).
We assessed all trials to be at high risk of bias overall.
Effects of interventions
See: Table 1
We present the results for each of the three primary and four secondary outcomes below. We calculated and presented the effect sizes as SMD and, where possible, as MD. We considered a SMD effect size of: 0.15 or less to have no clinically meaningful effect; 0.15 to 0.40 to have a clinical meaningful but small effect ; 0.40 to 0.75 to have a moderate effect; and greater than 0.75 to have a large treatment effect (Thalheimer 2002). We only used the outcomes from included trials, which we had predefined in our protocol that we wanted to use in this review. For those trials for which we were unable to obtain the necessary data to calculate an effect size, or used outcomes that could not be included in the meta‐analyses, we reported the results in the same way as the original report as single study results. We contacted the authors of 17 trials with unclear or missing data and requested the necessary data (some of them several times) (Bloomquist 1991; Pfiffner 1997; MTA 1999; Antshel 2003; Tutty 2003; Abikoff 2004; Pfiffner 2007; Van der Oord 2007; Tabaeian 2010; Waxmonsky 2010; Azad 2014; Choi 2015; Evans 2016; Pfiffner 2016; Wilkes Gillan 2016; Hannesdottir 2017; Qian 2017). We received information back from eight trial groups (Pfiffner 1997; Antshel 2003; Abikoff 2004; Pfiffner 2007; Waxmonsky 2010; Evans 2016; Wilkes Gillan 2016; Hannesdottir 2017).
For 14 trials, we used all of their outcomes in meta‐analyses (Antshel 2003; Pfiffner 2007; Van der Oord 2007; Tabaeian 2010; Storebø 2012; Meftagh 2014a; Pfiffner 2014; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016; Wilkes Gillan 2016; Qian 2017; Hannesdottir 2017). For seven trials, we reported some outcomes separately and used some in meta‐analyses (Bloomquist 1991; Pfiffner 1997; MTA 1999; Tutty 2003; Abikoff 2004; Yuk‐chi 2005; Waxmonsky 2010). Only Cohen 1981 had no outcomes included in a meta‐analysis; we reported all outcomes from this trial separately.
One of the trials did not report means and SD but P values connected to F values (Cohen 1981). We tried to transform these into SD, but this was not possible because we did not have the necessary between‐group values. For one trial, Pfiffner 2007, we received raw data on the SSRS parent‐ and teacher‐rated scores and used these for calculations.
Primary outcomes
Social skills
Twenty trials reported data on social skills (Bloomquist 1991; Pfiffner 1997; MTA 1999; Antshel 2003; Abikoff 2004; Pfiffner 2007; Van der Oord 2007; Tabaeian 2010; Waxmonsky 2010; Storebø 2012; Pfiffner 2014; Choi 2015; Bul 2016; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016; Wilkes Gillan 2016; Hannesdottir 2017; Qian 2017).
Meta‐analysis results
We combined data from 11 eligible trials in a primary meta‐analysis of teacher‐rated social skills at end of treatment (Pfiffner 1997; MTA 1999; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Storebø 2012; Pfiffner 2014; Bul 2016; Evans 2016; Pfiffner 2016; Schramm 2016). We found no evidence of an effect of the intervention (SMD 0.11, 95% CI −0.00 to 0.22; 11 trials, 1271 participants; I2 = 0%; P = 0.05). We rated the certainty of the evidence as very low certainty due to high risk of bias, inconsistency, and imprecision. The primary outcome, teacher‐rated social skills at end of treatment, corresponds to a MD of 1.22 points on the SSRS scale (95% CI 0.09 to 2.36). The minimal clinical relevant difference (10%) on the SSRS is 10.2.
We tested the robustness of this result by conducting a sensitivity analysis in which we excluded the three trials with the longest treatment duration (MTA 1999; Pfiffner 2014; Evans 2016), and again found no evidence of an effect (SMD 0.11, 95% CI −0.05 to 0.27; eight trials, 620 participants; I2 = 0%; P = 0.17; Analysis 1.1).
1.1. Analysis.
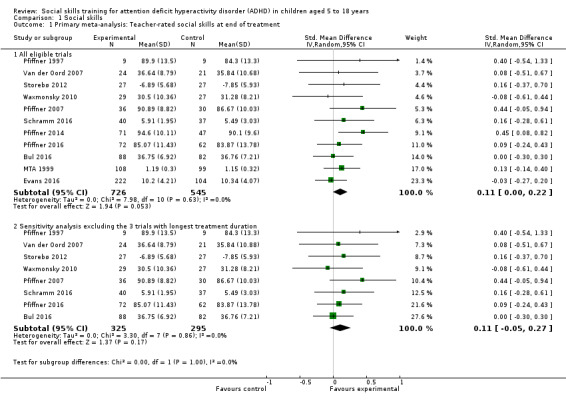
Comparison 1 Social skills, Outcome 1 Primary meta‐analysis: Teacher‐rated social skills at end of treatment.
We conducted four further secondary meta‐analyses (Analysis 1.2), and found that, compared with no intervention, social skills training:
1.2. Analysis.
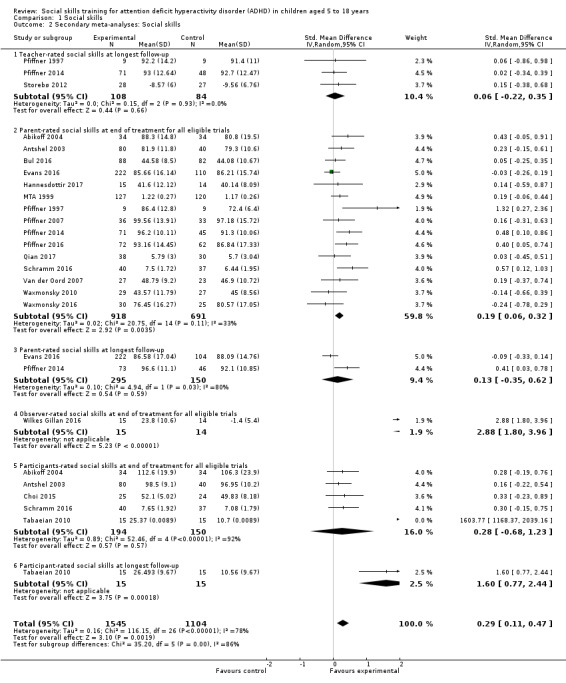
Comparison 1 Social skills, Outcome 2 Secondary meta‐analyses: Social skills.
did not improve teacher‐rated social skills at longest follow‐up (SMD 0.06, 95% CI −0.22 to 0.35; three trials, 192 participants, I2 = 0%; P = 0.66; Pfiffner 1997; Storebø 2012; Pfiffner 2014);
did improve parent‐rated social skills at end of treatment for all eligible trials (SMD 0.19, 95% CI 0.06 to 0.32; 15 trials, 1609 participants; I2 = 33% ; P = 0.003; very low‐certainty evidence; Pfiffner 1997; Abikoff 2004; MTA 1999; Antshel 2003; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Pfiffner 2014; Bul 2016; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016; Hannesdottir 2017; Qian 2017);
did not improve parent‐rated social skills at longest follow‐up (SMD 0.13, 95% CI −0.35 to 0.62; two trials, 445 participants; I2 = 80% ; P = 0.59; Pfiffner 2014; Evans 2016); and
did not improve participant‐rated social skills at end of treatment for all eligible trials (SMD 0.28, 95% CI −0.68 to 1.23; five trials, 344 participants; I2 = 92%; P = 0.57; Abikoff 2004; Antshel 2003; Tabaeian 2010; Choi 2015; Schramm 2016).
We conducted the analyses using a random‐effects model, and obtained similar results when repeating the analyses using a fixed‐effect model.
Single study results
Below, we present the data from six studies, which assessed social skills using different measures and thus could not be included in the aforementioned meta‐analyses (Cohen 1981; Bloomquist 1991; Pfiffner 1997; Abikoff 2004; Tabaeian 2010; Wilkes Gillan 2016).
The Cohen 1981 trial reported no significant group differences from observing children for three eight‐minute periods during a one‐hour period for two categories of behaviour in classrooms: play behaviour and social behaviour.
The Wilkes Gillan 2016 trial reported improved observer‐rated social skills at end of treatment for all eligible trials (SMD 2.88, 95% CI 1.80 to 3.96; one trial, 29 participants; P < 0.001; Analysis 1.2.4).
The Tabaeian 2010 trial reported a significant difference between the groups in favour of participant‐rated social skills at longest follow‐up (SMD 1.60, 95% CI 0.77 to 2.44; one trial, 30 participants; P=0.0002; Analysis 1.2.6).
The Bloomquist 1991 trial found no significant difference between the groups for social skills assessed using the teacher‐reported version of the Walker‐McConnell Scale of Social Competence and School Adjustment (five‐point scale ranging from 'never occurs' to 'frequently occurs'; higher scores indicate better social skills): MD 1.06 points, 95% CI −0.47 to 2.59; one trial, 46 participants; P = 0.18; Analysis 1.3.(fixed‐effects analysis).
1.3. Analysis.
Comparison 1 Social skills, Outcome 3 Teacher‐reported Walker‐McConnell Scale of Social Competence and School Adjustment.
The Pfiffner 1997 trial found evidence of a large treatment effect in favour of social skills training using the parent‐rated Social Skills Scale (UCI) UC Irvine Health Child Development School (higher scores indicate better social skills): MD 9.70 points, 95% CI 6.07 to 13.33; one trial, 18 participants; P < 0.001; Analysis 1.4. Pfiffner 1997 also found a significant difference between the groups when using the child‐rated Test of Social Skill Knowledge (scored by blinded raters; ranging from one (low knowledge) to 15 (high knowledge); higher scores indicate better social skills): MD 4.20 points, 95% CI 1.99 to 6.41; one trial, 18 participants; P < 0.001; Analysis 1.5.
1.4. Analysis.
Comparison 1 Social skills, Outcome 4 Parent‐rated Social Skills Scale (UCI).
1.5. Analysis.
Comparison 1 Social skills, Outcome 5 Child‐rated Test of Social Skill Knowledge.
Abikoff 2004 reported no significant difference between the groups in negative behaviour assessed by the Social Interaction Observation Code, which records the frequency of positive, negative, or neutral behaviour, including observations of negative behaviour (higher scores equate to more negative behaviour) (MD 0.20 points, 95% CI ‐0.11 to 0.51; one trial, 68 participants; P = 0.21; Analysis 1.6).
1.6. Analysis.
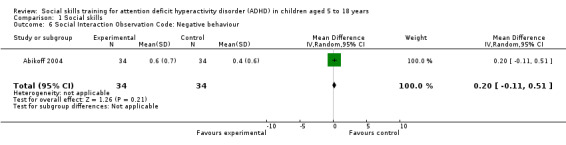
Comparison 1 Social skills, Outcome 6 Social Interaction Observation Code: Negative behaviour.
Emotional competencies
Five trials reported data on emotional competencies (Storebø 2012; Choi 2015; Schramm 2016; Hannesdottir 2017; Qian 2017).
Meta‐analysis results
We combined data from two trials in a meta‐analysis (Storebø 2012; Schramm 2016). We found no evidence of an effect of the intervention for the primary meta‐analysis of teacher‐rated emotional competencies at end of treatment (SMD −0.02, 95% CI −0.72 to 0.68; two trials, 129 participants; I2 = 74%; P = 0.96; Analysis 2.1). We rated the certainty of the evidence as very low due to high risk of bias, inconsistency, and imprecision.
2.1. Analysis.
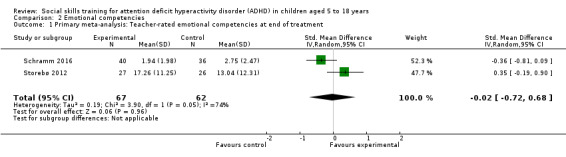
Comparison 2 Emotional competencies, Outcome 1 Primary meta‐analysis: Teacher‐rated emotional competencies at end of treatment.
We also conducted two secondary meta‐analyses (Analysis 2.2), and found no evidence of an effect of the intervention on:
2.2. Analysis.
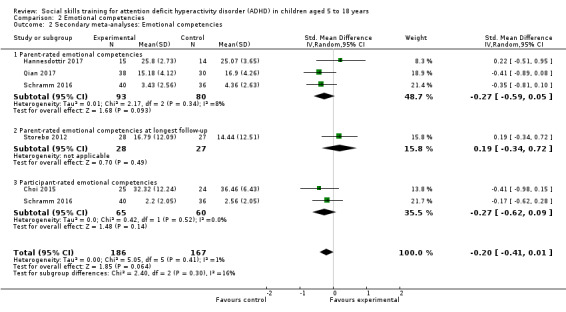
Comparison 2 Emotional competencies, Outcome 2 Secondary meta‐analyses: Emotional competencies.
parent‐rated emotional competencies (SMD −0.27, 95% CI −0.59 to 0.05; three trials, 173 participants; I2 = 8%; P = 0.09; Schramm 2016; Hannesdottir 2017; Qian 2017);
participant‐rated emotional competencies (SMD −0.27, 95% CI −0.62 to 0.09; two trials, 125 participants; I2 = 0% ; P = 0.14; Choi 2015; Schramm 2016).
We conducted the analyses using a random‐effects model, and obtained similar results when repeating the analyses using a fixed‐effect model.
Single study results
The Storebø 2012 trial reported parent‐rated emotional competencies at longest follow‐up (SMD 0.19, 95% CI −0.34 to 0.72; one trial, 56 participants; P = 0.49).
General behaviour
Eleven trials reported data on general behaviour (Bloomquist 1991; MTA 1999; Abikoff 2004; Pfiffner 2007; Storebø 2012; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016; Hannesdottir 2017; Qian 2017).
Meta‐analysis results
We combined data from eight trials in a meta‐analysis (Bloomquist 1991; MTA 1999; Abikoff 2004; Storebø 2012; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016). We found no evidence of an effect for the primary meta‐analysis of teacher‐rated general behaviour at the end of treatment (SMD −0.06, 95% CI −0.19 to 0.06; eight trials, 1002 participants; I2 = 0%; P = 0.33; Analysis 3.1). We rated the quality of the evidence as low due to high risk of bias, inconsistency, and imprecision.
3.1. Analysis.
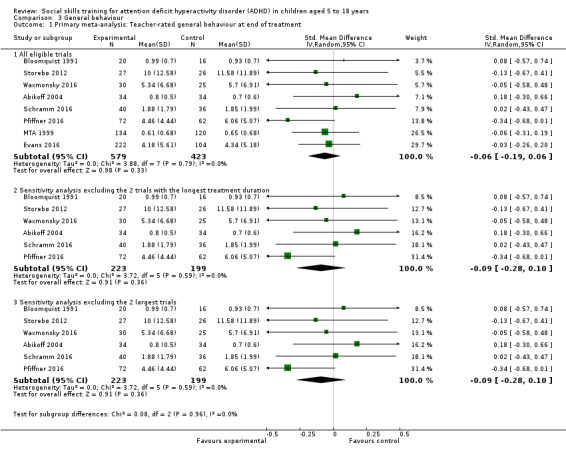
Comparison 3 General behaviour, Outcome 1 Primary meta‐analysis: Teacher‐rated general behaviour at end of treatment.
We tested the robustness of this result by conducting two sensitivity analyses, both of which found no evidence of an effect:
sensitivity analysis excluding the two trials with the longest treatment duration (MTA 1999; Evans 2016): SMD −0.09, 95% CI −0.28 to 0.10; six trials, 422 participants; I2 = 0%; P = 0.36; and
sensitivity analysis excluding the two largest trials (MTA 1999; Evans 2016): SMD −0.09, 95% CI −0.28 to 0.10; six trials, 422 participants; I2 = 0%; P = 0.36.
We also conducted two secondary meta‐analyses (Analysis 3.2), and found that, compared with no intervention, social skills training:
3.2. Analysis.
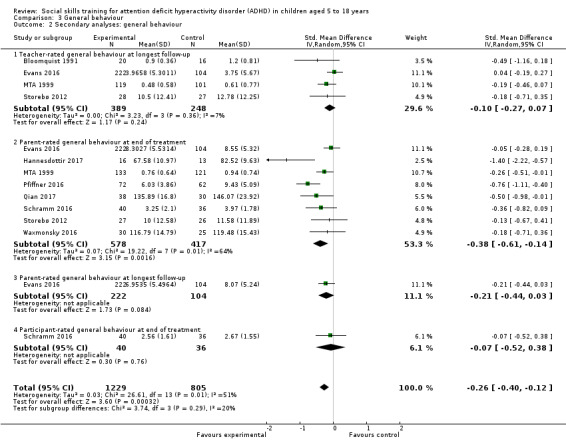
Comparison 3 General behaviour, Outcome 2 Secondary analyses: general behaviour.
did not improve teacher‐rated general behaviour at longest follow‐up (SMD −0.10, 95% CI −0.27 to 0.07; four trials, 637 participants; I2 = 7% ; P = 0.24; Bloomquist 1991; MTA 1999; Storebø 2012; Evans 2016);
did improve parent‐rated general behaviour at end of treatment (SMD −0.38, 95% CI −0.61 to −0.14; eight trials, 995 participants; I2 = 64%; P = 0.002; very low‐quality evidence; MTA 1999; Storebø 2012; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016; Hannesdottir 2017; Qian 2017).
We conducted the analyses using a random‐effects model, and obtained similar results when repeating the analyses using a fixed‐effect model.
Single study results
The Pfiffner 2007 trial measured general behaviour using parent and teacher ratings of the Clinical Global Impression Scale, and found that the intervention group showed significantly greater improvement than the control group (parents: F1, 51 = 28.46, P < 0.001; teachers: F1, 51 = 11.73, P = 0.001; one trial, 69 participants).
The Evans 2016 trial reported parent‐rated general behaviour at longest follow‐up (SMD −0.21, 95% CI 0.44 to 0.03; one trial; 326 participants; P = 0.08).
The Schramm 2016 trial reported participant‐rated general behaviour at end of treatment (SMD −0.07, 95% CI −0.52 to 0.38; one trial, 76 participants; P = 0.76).
Secondary outcomes
Core ADHD symptoms
Nineteen trials reported data on ADHD symptoms (Bloomquist 1991; Tutty 2003; Abikoff 2004; MTA 1999; Yuk‐chi 2005; Pfiffner 2007; Van der Oord 2007; Tabaeian 2010; Waxmonsky 2010; Storebø 2012; Azad 2014; Meftagh 2014a; Pfiffner 2014; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016; Hannesdottir 2017; Qian 2017).
Meta‐analysis results
We combined data from 14 eligible trials in a meta‐analysis of the primary meta‐analysis of teacher‐rated ADHD symptoms at end of treatment (Bloomquist 1991; Abikoff 2004; MTA 1999; Yuk‐chi 2005; Van der Oord 2007; Waxmonsky 2010; Storebø 2012; Pfiffner 2014; Evans 2016; Pfiffner 2016; Schramm 2016; Waxmonsky 2016; Hannesdottir 2017; Qian 2017). We found evidence of an effect in favour of the intervention (SMD −0.26, 95% CI −0.47 to −0.05; 14 trials, 1379 participants; I2 = 69%; P = 0.02; Analysis 4.1). We rated the quality of the evidence as very low due to high risk of bias, inconsistency, and imprecision.
4.1. Analysis.
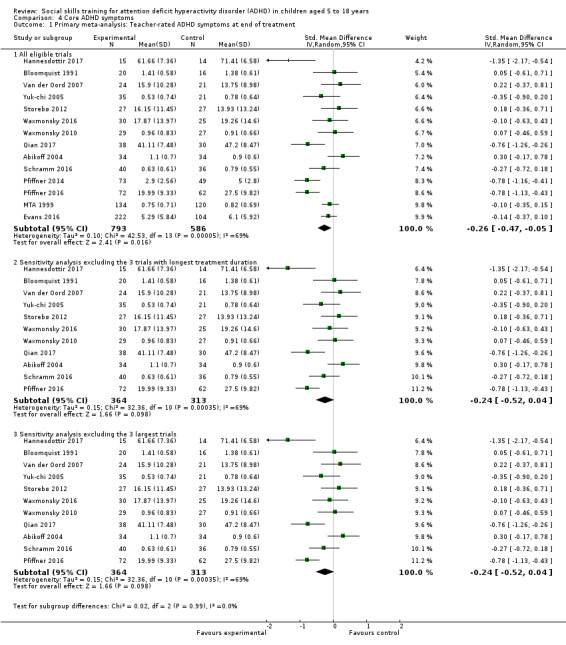
Comparison 4 Core ADHD symptoms, Outcome 1 Primary meta‐analysis: Teacher‐rated ADHD symptoms at end of treatment.
We tested the robustness of this result by conducting two sensitivity analyses, both of which showed no evidence of an effect:
sensitivity analysis excluding the three trials with the longest treatment duration (MTA 1999; Pfiffner 2014; Evans 2016): SMD −0.24, 95% CI −0.52 to 0.04; 11 trials, 677 participants, I2 = 69%; P = 0.10); and
sensitivity analysis excluding the three largest trials (MTA 1999; Pfiffner 2014; Evans 2016): SMD −0.24, 95% CI −0.52 to 0.04; 11 trials, 677 participants; I2 = 69%; P = 0.10).
We also drew a funnel plot to visually assess whether the effect was associated with the size of the trial; it seemed to be symmetrical with no clinically significant effect. Eggers’ test, moreover was not statistically significant (Egger’s regression intercept (bias) = 0.40 (two‐tailed P = 0.78)) for the conclusion whether or not there was publication bias in the meta‐analysis for this outcome.
We conducted five further secondary meta‐analyses (Analysis 4.2), and found that, compared with no intervention, social skills training:
4.2. Analysis.
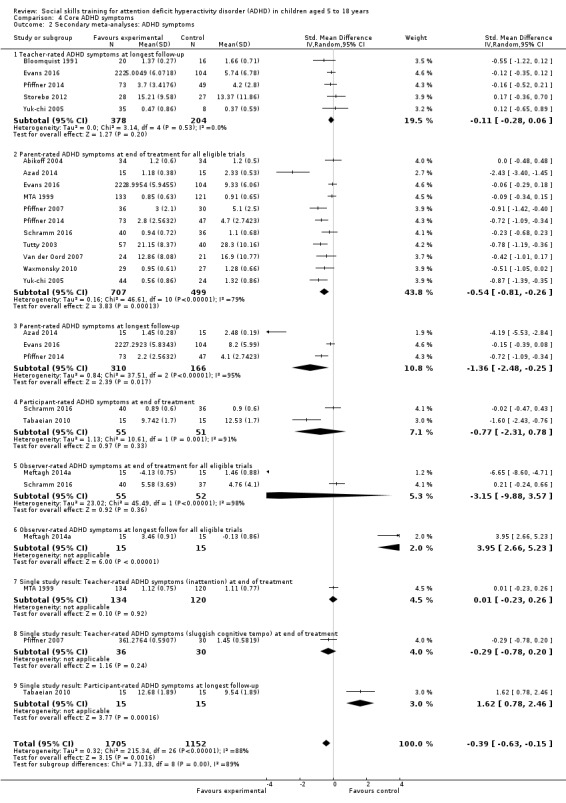
Comparison 4 Core ADHD symptoms, Outcome 2 Secondary meta‐analyses: ADHD symptoms.
did not reduce teacher‐rated ADHD symptoms at longest follow‐up (SMD −0.11, 95% CI −0.28 to 0.06; five trials, 582 participants; I2 = 0%; P = 0.20; Bloomquist 1991; Yuk‐chi 2005; Storebø 2012; Pfiffner 2014; Evans 2016);
did reduce parent‐rated ADHD symptoms at end of treatment for all eligible trials (SMD −0.54, 95% CI −0.81 to −0.26; 11 trials, 1206 participants; I2 = 79%; P < 0.001; very low‐quality evidence; Tutty 2003; Abikoff 2004; MTA 1999; Yuk‐chi 2005; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Azad 2014; Pfiffner 2014; Evans 2016; Schramm 2016);
did reduce parent‐rated ADHD symptoms at longest follow‐up (SMD −1.36, 95% CI −2.48 to −0.25; three trials, 476 participants; I2 = 95%; P = 0.02; Azad 2014; Pfiffner 2014; Evans 2016);
did not reduce participant‐rated ADHD symptoms at end of treatment (SMD −0.77, 95% CI −2.31 to 0.78; two trials, 106 participants; I2 = 91%; P = 0.33; Tabaeian 2010; Schramm 2016); and
did not reduce observer‐rated ADHD symptoms at end of treatment for all eligible trials (SMD −3.15, 95% CI ‐9.88 to 3.57; two trials, 107 participants; I2 = 98%; P = 0.36; Meftagh 2014a; Schramm 2016).
We conducted the analyses using a random‐effects model. We obtained similar results when repeating the analyses using a fixed‐effect model, except for sensitivity analyses 4.1.2 and 4.1.3, both of which showed a statistical significant effect when analysed with fixed‐effect model. However, the random‐effects model is more appropriate because of the heterogeneity in these analyses.
Single study results
The Meftagh 2014a trial found a significant difference between groups for observer‐rated ADHD symptoms at longest follow‐up (SMD 3.95, 95% CI 2.66 to 5.23; one trial, 30 participants; P < 0.001).
The MTA 1999 trial found no significant difference between the groups on teacher‐rated ADHD symptoms (inattention) at end of treatment (SMD 0.01, 95% CI −0.23 to 0.26; one trial, 254 participants; P = 0.92).
The Pfiffner 2007 trial also found no significant difference between the groups on teacher‐rated ADHD symptoms (sluggish cognitive tempo) at end of treatment (SMD −0.29, 95% CI −0.78 to 0.20; one trial, 66 participants; P = 0.24).
The Tabaeian 2010 trial found a significant difference between groups on participant‐rated ADHD symptoms at longest follow‐up (SMD 1.62, 95% CI 0.78 to 2.46; one trial, 30 participants; P < 0.001).
Performance and grades in school
Five trials measured performance in school (MTA 1999; Waxmonsky 2010; Storebø 2012; Evans 2016; Pfiffner 2016).
Meta‐analysis results
We pooled the data in a meta‐analysis and found that social skills training did not improve teacher‐rated performance in school at end of treatment (SMD 0.15, 95% CI ‐0.01 to 0.31; five trials, 642 participants; I2= 0% ; P = 0.07; Analysis 5.1; Waxmonsky 2010; Storebø 2012; Evans 2016; Schramm 2016, Pfiffner 2016) or at longest follow‐up (SMD −0.01, 95% CI −0.22 to 0.20; two trials, 379 participants; I2 = 0%; P = 0.92; Analysis 5.2; Storebø 2012; Evans 2016).
5.1. Analysis.
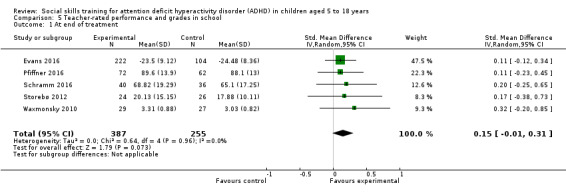
Comparison 5 Teacher‐rated performance and grades in school, Outcome 1 At end of treatment.
5.2. Analysis.
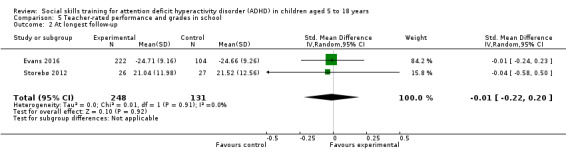
Comparison 5 Teacher‐rated performance and grades in school, Outcome 2 At longest follow‐up.
We conducted the analyses using a random‐effects model, and obtained similar results when repeating the analyses using a fixed‐effect model.
Single study results
The MTA 1999 trial found no significant difference between groups for observer‐rated performance in school (MD 1.50 points, 95% CI −2.06 to 5.06; measured using Wechsler Individual Achievement Test (WIAT); (higher score indicates better performance); one trial, 260 participants; P = 0.41; Analysis 6.1).
6.1. Analysis.
Comparison 6 Observer‐rated performance and grades in school, Outcome 1 Wescheler Individual Achievement Test.
Participant or parent (or both) satisfaction with the treatment
Four trials (233 participants) measured satisfaction with treatment (Pfiffner 1997; Pfiffner 2007; Waxmonsky 2010; Yuk‐chi 2005). Although satisfaction with treatment was high in all four trials, two trials found no significant difference between the intervention and controI groups (Pfiffner 1997; Waxmonsky 2010), and two trials did not report on differences between the groups (Pfiffner 2007; Yuk‐chi 2005).
Adverse events
Only two trials (226 participants) reported data on adverse events (Storebø 2012; Bul 2016).They assessed adverse events as spontaneous reporting and reported no adverse events in the trial.
Trial Sequential Analysis (TSA)
We conducted a TSA of the primary outcome, social skills rated by teachers at end of treatment, with data from four trials (Pfiffner 1997; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010). Using an a priori assumption that the minimal relevant clinical intervention effect was 4.0 points, we found that the intervention effect almost reached the futility area (between the two widening dotted red lines), possibly signalling that the social skills intervention had no effect on teacher‐rated social skills at the end of treatment (MD 1.80, 95% CI ‐1.01 to 4.62; four trials, 185 participants; Analysis 7.1; Figure 4). We used a power of 90% in the analysis and this gives a maximum type II error of 10%, and therefore there is a 10% risk of overlooking a true effect.
7.1. Analysis.
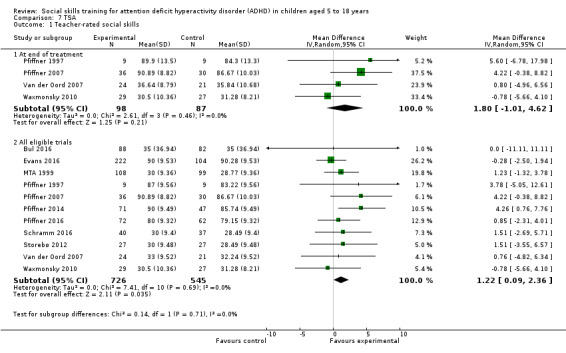
Comparison 7 TSA, Outcome 1 Teacher‐rated social skills.
4.
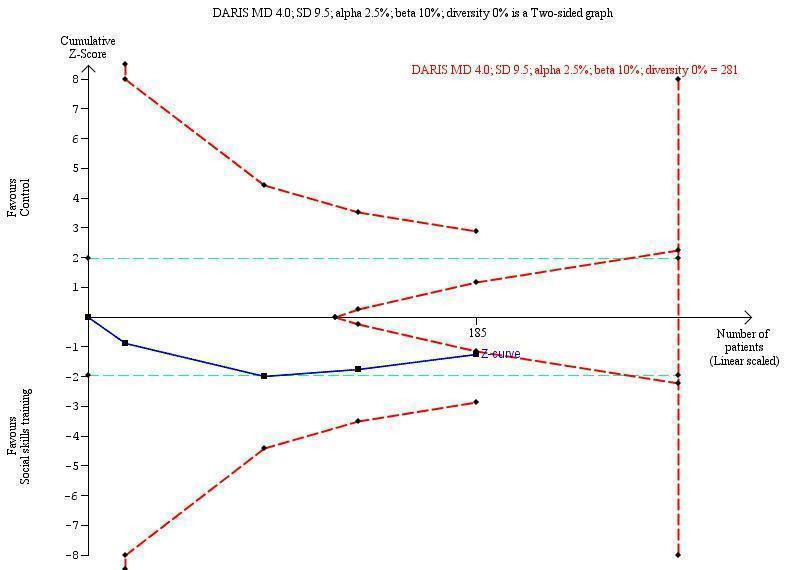
Trial Sequential Analysis of teacher‐rated social skills ‐ SSRS
Footnotes
DARIS: diversity‐adjusted required information size MD: mean difference SSRS: Social Skills Rating Scale
We also conducted a post hoc TSA of social skills rated by teachers for all eligible trials; 11 trials in total provided data (Pfiffner 1997; MTA 1999; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Storebø 2012; Pfiffner 2014; Bul 2016; Evans 2016; Pfiffner 2016; Schramm 2016). To do this, we transformed the MD and SD from the different rating scales used in this analysis to the MD and SD of a commonly used scale, namely the SSRS, using the following formula: MD = SMD*SD (Thorlund 2011). In the TSA we found that the cumulative z scores (blue line) crossed into the areas of futility (in between the two widening dotted red lines) (SMD 0.11, 95% CI −0.00 to 0.22; 11 trials, 1271 participants; Analysis 7.1; Figure 5).
5.
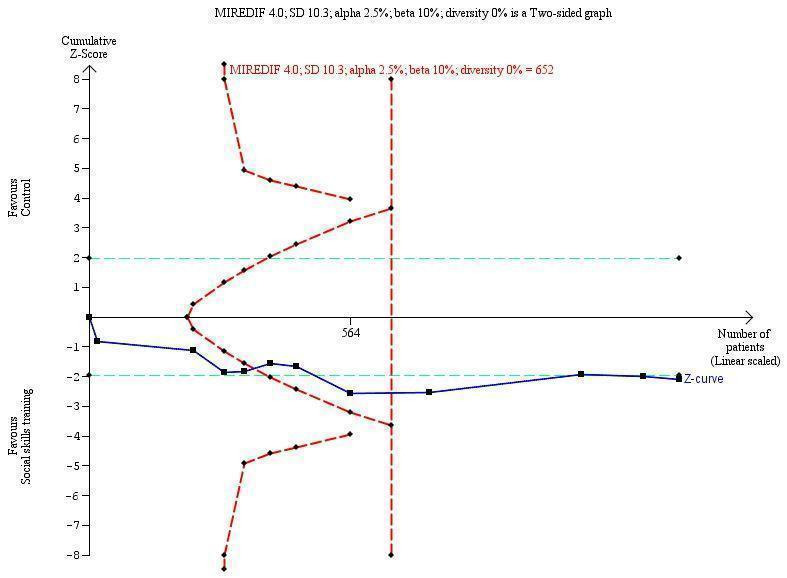
Trial Sequential Analysis of teacher‐rated social skills ‐ all studies transformed to SSRS
Footnotes
MIREDIF: minimum relevant difference SD: standard deviation SSRS: Social Skills Rating Scale
Subgroup analyses
We performed five subgroup analyses, none of which showed significant differences in intervention effects.
Children aged five to 11 years compared to children aged 12 to 18 year:
Teacher‐rated social skills at end of treatment: children aged five to 11 years (10 trials, 1194 participants: Pfiffner 1997; MTA 1999; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Storebø 2012; Pfiffner 2014; Bul 2016; Evans 2016; Pfiffner 2016) compared to children aged 12 to 18 years (one trial, 77 participants: Schramm 2016). Test for subgroup differences: Chi2 = 0.06, df = 1 (P = 0.81), I2 = 0%; Analysis 8.1.
8.1. Analysis.
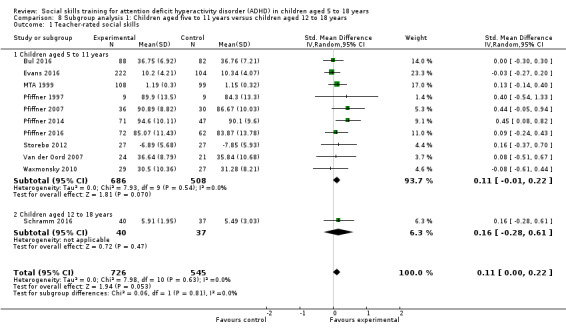
Comparison 8 Subgroup analysis 1: Children aged five to 11 years versus children aged 12 to 18 years, Outcome 1 Teacher‐rated social skills.
ADHD with comorbidity compared to ADHD without comorbidity:
Parent‐rated ADHD symptoms at end of treatment: ADHD with comorbidity (eight trials, 1003 participants: Abikoff 2004; MTA 1999; Yuk‐chi 2005; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Pfiffner 2014; Evans 2016) compared to ADHD without comorbidity (two trials, 173 participants: Tutty 2003; Schramm 2016). Test for subgroup differences: Chi2 = 0.10, df = 1 (P = 0.75), I2 = 0%; Analysis 9.1.
9.1. Analysis.
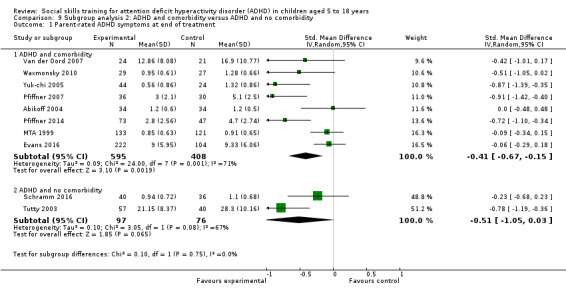
Comparison 9 Subgroup analysis 2: ADHD and comorbidity versus ADHD and no comorbidity, Outcome 1 Parent‐rated ADHD symptoms at end of treatment.
Social skills training only compared to social skills training supported by parent training:
Teacher‐rated social skills at end of treatment: social skills training only (four trials, 336 participants: Pfiffner 1997; Pfiffner 2014; Bul 2016; Evans 2016) compared to social skills training supported by parent training (four trials, 632 participants: Pfiffner 1997; Storebø 2012; Pfiffner 2014; Schramm 2016). Test for subgroup differences: Chi2 = 0.16, df = 1 (P = 0.69), I2 = 0%; Analysis 10.1.
10.1. Analysis.
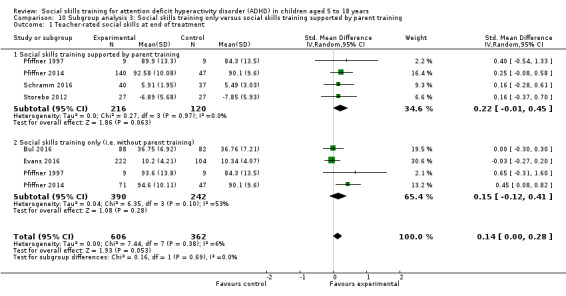
Comparison 10 Subgroup analysis 3: Social skills training only versus social skills training supported by parent training, Outcome 1 Teacher‐rated social skills at end of treatment.
Social skills training, parent training and medication compared to social skills training and parent training without medication:
Parent‐rated social skills at end of treatment: social skills training, parent training and medication (four trials, 299 participants: Abikoff 2004; Antshel 2003; Waxmonsky 2010; Waxmonsky 2016) compared to social skills training and parent training without medication (four trials, 337 participants: Pfiffner 1997; Pfiffner 2007; Pfiffner 2014; Pfiffner 2016). Test for subgroup differences: Chi2 = 2.61, df = 1 (P = 0.11), I2 = 61.6%; Analysis 11.1.
11.1. Analysis.
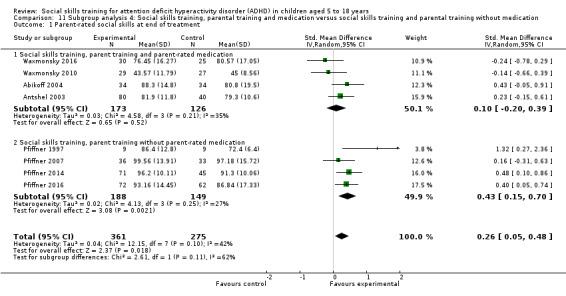
Comparison 11 Subgroup analysis 4: Social skills training, parental training and medication versus social skills training and parental training without medication, Outcome 1 Parent‐rated social skills at end of treatment.
No‐intervention control group compared to waiting‐list control group with possible minor active intervention components:
Teacher‐rated social skills at end of treatment: no‐intervention control group (eight trials, 693 participants: Pfiffner 1997; MTA 1999; Pfiffner 2007; Van der Oord 2007; Waxmonsky 2010; Storebø 2012; Bul 2016; Schramm 2016) compared to waiting‐list control group with possible minor active intervention components (three trials, 578 participants: Pfiffner 2014; Evans 2016; Pfiffner 2016). Test for subgroup differences: Chi2 = 0.02, df = 1 (P = 0.89), I2 = 0%; Analysis 12.1.
12.1. Analysis.
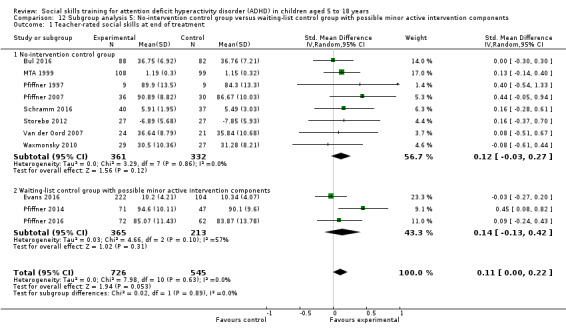
Comparison 12 Subgroup analysis 5: No‐intervention control group versus waiting‐list control group with possible minor active intervention components, Outcome 1 Teacher‐rated social skills at end of treatment.
We conducted the analyses using a random‐effects model, and obtained similar results when repeating the analyses using a fixed‐effect model.
Single study result
One trial (576 participants) reported on subgroup analyses comparing children with ADHD only to children with ADHD and comorbid anxiety disorder (MTA 1999). This analysis found significant differences in teacher‐rated hyperactivity/impulsivity (F = 1.64, P = 0.04) and teacher‐rated social skills (F = 1.68, P = 0.03) between the two subgroups of children in connection with all four active treatments used in this trial. The subgroup with ADHD and comorbidity of anxiety disorder showed better results (MTA 1999).
Discussion
We conducted this systematic review to examine the effects of social skills training for children and adolescents with ADHD. We considered a total of 313 full‐text reports from which we included 25 trials published in 45 articles in this review. Of these 25 trials, we used the results from 24 in meta‐analyses.
Summary of main results
We included 25 trials published in 45 reports. Altogether these trials randomised 2690 participants. All trials included children aged between five and 13 years, except for a single study which included adolescents between 12 and 17 years of age. The duration of the trials ranged from five weeks to two years. Most were conducted in outpatient clinics in the USA, Asia, and Europe. We assessed all trials as having high risk of bias, which might lead to systematic errors: overestimation of benefits and underestimation of harms. We considered the parent‐rated findings to be more questionable than our primary analyses, which were based on teacher‐rated outcomes, due to high risk of systematic errors (lack of blinding) in the parent‐rated outcomes (Daley 2014b).
In accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we combined all relevant trials in a meta‐analysis to investigate common features of treatment effects. We found no significant differences between the group given social skills training and the groups given no intervention or assigned to a waiting list. There was a beneficial effect on some of the parent‐rated primary outcomes and the secondary outcome of teacher‐rated ADHD symptoms at end of treatment, but the finding was questionable due to lack of support from sensitivity analyses with low clinical significance and low‐certainty evidence. We found no indications of harmful effects.
We presented all results using the random‐effects model, thus giving more weight to smaller trials; however, the conclusions on the effect of intervention did not change when we applied a fixed‐effect model. Thus, we conclude that the observed statistical heterogeneity does not seem to be of importance for the results of the present review.
Overall completeness and applicability of evidence
We were able to include most of the data from the trials in our meta‐analyses, which provides a good basis for the evidence in this review. However, the interventions might be considered too heterogeneous to combine in a meta‐analysis and the multiplicity of different outcome measures might limit the external validity of this review. We found a small treatment effect for some of the outcomes, but all of the trials were at high risk of bias.
Components and duration of the interventions
All but three trials (Cohen 1981; Pfiffner 1997; Antshel 2003) used manual‐based interventions. The social skills interventions in the trials were, in general, cognitive behaviour‐based treatments but varied in form, content, and in the use of specific behaviour techniques. Most manuals were structured around specific themes, with most trials focusing on problem‐solving and emotion regulation and a few trials focusing more specifically on academic organisational skills (Evans 2016) or play skills (Wilkes Gillan 2016).
The duration of the treatment also differed greatly, from five weeks to 24 months. However, there were no differences in the results when we excluded the study with the longest intervention from the analyses of social skills and ADHD symptoms.
Parent training
Most trials included specific parent training in the social skills intervention. One study involved parents before the onset of training (Hannesdottir 2017) or as part of the first or last session (Qian 2017), in order that parents might understand the concept of the training and be able to support the children in home assignments or in applying what they learned at home. Other trials did not describe parental involvement in the training (Azad 2014; Meftagh 2014a; Choi 2015; Bul 2016; Evans 2016).
Teacher training
More than half of the trials included teacher training or teacher consultations as part of the social skills training. The inherent differences in the interventions accorded with this review's inclusion criteria (criteria for considering studies for this review), but were likely to produce heterogeneity in the analysis.
Treatment effects
Although the measurable beneficial effects of social skills training were small and questionable due to the low certainty of the evidence, participant, parent, and teacher satisfaction with the intervention was overall in the positive direction, as the level of satisfaction was rated as high in all of the included studies. However, in half of the trials measuring this outcome, there was no significant difference in the level of satisfaction when comparing the experimental and control group. The other half of the trials did not report on between‐group differences. This is a problem as participant satisfaction with treatment is often used as an argument for this kind of treatment.
In the MTA 1999 trial, the multimodal treatment had a superior treatment effect on children with ADHD and comorbid anxiety disorder compared to those without that comorbidity. This was an interesting subgroup finding and suggested that future trials on this topic should investigate these findings further by planning for subgroup analyses on children with and without comorbid anxiety disorder. Moreover, we know very little about the effects of social skills training in adolescents.
Limitations of the evidence
In all meta‐analyses that achieved significant findings with 95% confidence intervals, the findings could be due to bias and overestimation of beneficial intervention effects. We conducted TSA to control the risk of type I and type 2 errors and to estimate how far we were from obtaining the diversity adjusted required information size (DARIS) to detect or reject a certain plausible intervention effect. Moreover, the TSAs showed that the observed intervention effects could be due to type I errors. This highlights the need for more clinical research on this topic without risks of bias. Both the a priori and post hoc TSA showed that there is a need for more participants in order to reach a firm conclusion in the meta‐analyses.
A serious limitation of these types of trials was the lack of blinding or inability to blind. This introduced a high risk of bias in the assessment of outcomes (Schultz 1995; Kjaergard 2002; Wood 2008; Savovíc 2012; Savovic 2018). Statistical heterogeneity was low (0%) in most meta‐analyses, most likely due to inherent features of the trials leading to wide CIs of the estimates, and therefore was not mirroring the clinical and methodological heterogeneity.
RCTs are generally considered to be the highest level of evidence, but most of the trials included in this systematic review were at high risk of bias and the vast majority were at high risk of random errors due to their low sample sizes. Generally, we rated the certainty of the evidence as low or very low using the GRADE approach (GRADE Working Group), downgrading the certainty of the evidence by two or three levels due to high risk of bias, imprecision and inconsistency. Further research may change the estimates of the treatment effect, but such trials ought to be conducted without risk of systematic errors (bias), random errors (play of chance), and design errors (Keus 2010).
Quality of the evidence
Our review has some limitations. Our results were based on only 25 trials with a limited number of participants (n = 2690). Many of the trials were prone to selection bias due to unclear or inadequate generation of the allocation sequence or allocation concealment. All 25 trials had an overall assessment as having 'high risk of bias', so our results might not be robust and reliable (Figure 2).
Funnel Plots
We drew funnel plots of the following two outcomes for all eligible trials to visually assess whether effects were associated with the size of the study: 1) teacher‐rated social skills and 2) teacher‐rated ADHD symptoms. Both outcomes seemed to be symmetrical with no clinically significant effect. An Eggers’ test for both the outcomes was not statistically significant so we were unable to conclude whether or not there was publication bias in the meta‐analysis of these outcomes.
There is, therefore, currently insufficient evidence to draw any conclusions about any form of social skills training as having an effect on ADHD patients.
The important methodological limitations, which have been elaborated above, reduced the reliability of the results of most of the trials included in this review.
Potential biases in the review process
We sought to minimise potential biases in the review process in the following ways. We published a protocol before we embarked on the review itself. We conducted extensive searches of relevant databases. Two review authors, working independently, selected trials for inclusion and extracted data. Disagreements were resolved by discussion with team members. We assessed risk of bias in all trials according to the recommendations provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We recognise, however, that there are some limitations in the review process. In particular, we did not assess quality of life as an outcome and we defined serious adverse events as a secondary outcome when it should have been a primary outcome (though these were reported in the review). Furthermore, we believe that the outcomes of emotional competencies and general behaviour should have been secondary outcomes; we will change this in the next update of this review. Conduct disorder was used in two out of the 10 trials that were included in the analysis of general behaviour, and we took the decision to include this in a meta‐analysis of the impact of social skills training on behaviour more generally. Some might take issue with this, but we believe this was a sensible approach.
Agreements and disagreements with other studies or reviews
Four earlier meta‐analyses examined the effects of social skills training for children with ADHD. Two of these concluded that there was no effect of social skills training for children with ADHD (Kavale 1997; Van der Oord 2008); the other two concluded that there was a beneficial effect (De Boo 2007; Majewicz‐Hefley 2007). The obvious limitation of all four reviews is that all are at least 10 years old, and suffer from several methodological weaknesses; for example, none of these reviews evaluated systematic errors (bias) in the included trials. The conclusion in this update reinforces the conclusion in our original review where we wrote that "there is little evidence to support or refute social skills training for adolescents with ADHD (Storebø 2011). There is need for more trials, with low risk of bias and with a sufficient number of participants, investigating the efficacy of social skills training versus no training for both children and adolescents." (Storebø 2011). A new systematic review published in 2019 investigating the effectiveness of stand‐alone social skills training for youth with ADHD concluded that social skills training implemented without additional treatment components like parent support, showed improvements on some areas of social functioning (Willis 2019). However, this review suffered from a very limited search strategy and did not evaluate systematic errors (bias) in the included trials.
Authors' conclusions
Implications for practice.
We generally found no beneficial effects in favour of social skills training for children and adolescents with ADHD. In the primary analyses, we found no evidence of an effect on social skills, emotional competencies, and general behaviour. In the secondary analyses, we did find favourable effects on ADHD symptoms, indicating that social skills training could exert clinically useful effects in this domain, but we need more evidence to confirm this. Social skills training did not seem to affect child and adolescent school performance and was not associated with any adverse events. We cannot rule out that social skills training is associated with harms. On the basis of the evidence considered in this review, we are unable to conclude whether social skills training is beneficial for children with ADHD or whether it is a waste of scarce resources in clinical practice.
Implications for research.
We need more high‐quality trials that are at low risk of bias and have sufficient sample sizes to investigate social skills training versus no training for children and adolescents with ADHD. Such trials should include adolescents since only one study in this review assessed this group. Future trials should be based on pre‐published protocols to combat the problem of publication bias. Moreover, future trials should be designed according to the SPIRIT statement (Chan 2013), and reported according to the CONSORT statement (Schulz 2010). Social skills problems is an important area of intervention for children and adolescents with ADHD. Untreated social skills problems may decrease self‐confidence in these children due to limited positive social experiences.
Feedback
Comments on protocol by Peter Gøtzche, 16 February 2010
Summary
The Background notes that drugs have a beneficial effect on major symptoms in about 80% of the patients treated. Such a statement is meaningless when we don't know what the effect was in groups treated with placebo. The authors need to rectify this so that the readers can understand what the effect is.
Social skills training is the focus of the review and the authors state that "We have been unable to identify meta‐analyses or systematic reviews on the topic". This statement is a bit surprising. A quick and simple search on PubMed on "(attention deficit hyperactivity disorder children) AND training", limited to meta‐analysis, yielded 7 hits, of which one appears to be highly relevant for the authors' review, as they also want to review combination therapy: Majewicz‐Hefley A, Carlson JS. A meta‐analysis of combined treatments for children diagnosed with ADHD. J Atten Disord. 2007 Feb;10(3):239‐50.
The following reference may also be relevant, particularly as the authors of the Cochrane protocol mention that training may increase negative behaviour, with reference to a single study. In contrast, based on its abstract, this reference seems to be to a meta‐analysis, and had different findings: Weiss B, Caron A, Ball S, Tapp J, Johnson M, Weisz JR. Latrogenic effects of group treatment for antisocial youths. J Consult Clin Psychol. 2005 Dec;73(6):1036‐44.
Reply
We thank Peter Gøtzsche for his interest in our review and for raising the comments.
Point 1
Peter Gøtzsche is correct that only giving the proportion of patients who respond to the active intervention and leaving out the response proportion among placebo‐treated patients does not inform the reader with regard to the relative risk reduction between the two. We will amend the protocol accordingly to make it explicit that the response proportion of response from ‘stimulant’ drugs is about 80% while the placebo response proportion is about 3% to 10%, leading to a relative risk reduction of at least 77%. We thus acknowledge Peter Gøtzsche's vigilance, and have now taken steps to correct the mistake.
Point 2
We would argue, regarding this point, that the truth may be more complex than the statement above. Six of the meta‐analyses identified by Dr Gøtzsche are not relevant to our review. The seventh, to which he makes particular reference, is potentially relevant. This is the meta‐analysis by Majewicz‐Hefley and Carlson (2007). The meta‐analysis includes a total of eight studies. The article divides the outcomes into five different categories of outcome variables. The Social Skills variable was based on four studies. Two of the four studies are not relevant for our review. One concerns behaviour therapy (and not social skills training); the other one is not a randomised clinical trial. That leaves two studies in the meta‐analysis of the social skills outcome variable, which we could have mentioned in the protocol, but chose not to. Both studies will of course be considered for the review, and be cited there.
Point 3
Our point in the protocol here was simply to show that we are aware of the possibility that group training can have adverse effects. We could have found articles (or meta‐analyses) that suggested the opposite, viz., that group training of children with attention deficit hyperactivity disorder (ADHD) or conduct disorder has positive effects, as this finding is more common, but this also would have not been pertinent. Furthermore, the article Peter Gøtzsche refers to concerns antisocial youths, and this population is not the same as that diagnosed with ADHD or conduct disorder.
Contributors
This feedback was prepared by Jane Dennis, feedback editor for CDPLPG, in consultation with the submitter, the authors, the CDPLPG Co‐ordinating Editor Geraldine Macdonald and the former CDPLPG Managing Editor Chris Champion.
What's new
| Date | Event | Description |
|---|---|---|
| 12 October 2018 | New search has been performed | We updated the review following a new search in July 2018. |
| 12 October 2018 | New citation required but conclusions have not changed | We included 14 new studies. |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 12, 2011
| Date | Event | Description |
|---|---|---|
| 14 April 2010 | Amended | US FDA reference corrected |
| 16 March 2010 | Feedback has been incorporated | Feedback comments from Peter Gøtzsche incorporated |
Notes
An administrative error was made in the first published version of the protocol and important information about the declaration of interest of the authors was not included in the publication. This has now been rectified.
Acknowledgements
Trine Lacoppidan Kæstel for her support in conducting the searches and editing the references. We thank Mathilde Holmskov for assessing the Storebø 2012 trial. We thank Psychiatric Research Unit, Region Zealand Psychiatry Denmark, and Institute for Psychology, University of Southern Denmark, Denmark. We also wish to thank Geraldine Macdonald (Co‐ordinating Editor), Joanne Duffield (Managing Editor) and Margareth Anderson (Information Specialist) of the Cochrane Developmental Psychosocial and Learning Problems Group for providing help and support. Finally, we would like to thank Professor M Fitzpatrick, Department of Psychiatry, Trinity College, Dublin 2, and Dr Donna Gillies for their feedback on an earlier version of this review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library
#1 (intellect* disabl*):ti,ab,kw #2 MeSH descriptor Attention Deficit Disorder with Hyperactivity explode all trees #3 (adhd or addh):ti,ab,kw #4 (attention near/3 deficit):ti,ab,kw #5 (hyperactiv*):ti,ab,kw #6 (hyperkinesis*):ti,ab,kw #7 MeSH descriptor Hyperkinesis explode all trees #8 (minimal brain near/3 disorder*):ti,ab,kw #9 ((minimal brain near/3 dysfunction*) or (minimal brain near/3 damage*)):ti,ab,kw #10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 (social skill training):ti,ab,kw #12 (social skills education):ti,ab,kw #13 (social competen*):ti,ab,kw #14 ((behavior regulation) or (behaviour regulation)):ti,ab,kw #15 (social near/10 skills):ti,ab,kw #16 (learning near/25 social):ti,ab,kw #17 (role play*):ti,ab,kw #18 (psychosocial treatment):ti,ab,kw #19 (parent education):ti,ab,kw #20 (educat* near/10 parent*):ti,ab,kw #21 MeSH descriptor Psychotherapy, Group explode all trees #22 (behavior modification):ti,ab,kw #23 (behaviour modification):ti,ab,kw #24 (parent training):ti,ab,kw #25 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) #26 (#10 AND #25)
Medline Ovid
1 exp Attention Deficit Disorder with Hyperactivity/ 2 adhd.mp. 3 addh.mp. 4 (attention adj3 deficit).mp. 5 hyperactiv$.mp. 6 hyperkinesis$.mp. 7 exp Hyperkinesis/ 8 (minimal adj brain adj3 disorder$).mp. 9 (minimal adj brain adj3 dysfunction$).mp. 10 (minimal adj brain adj3 damage$).mp. 11 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 12 social skills training.mp. 13 social skills education.mp. 14 social competenc$.mp. 15 behavior regulation.mp. 16 behaviour regulation.mp. 17 (social adj10 skills).mp. 18 (learning adj25 social).mp. 19 role play$.mp. 20 psychosocial treatment.mp. 21 parent education.mp. 22 (educat$ adj10 parent$).mp. 23 exp psychotherapy, group/ 24 behavior modification.mp. 25 behaviour modification.mp. 26 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27 randomized controlled trial.pt. 28 controlled clinical trial.pt. 29 randomized controlled trials.mp. 30 random allocation.mp. 31 double blind method.mp. 32 single blind method.mp. 33 clinical trial.pt. 34 (clin$ adj25 trial$).ti,ab. 35 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$ or dummy$)).mp. 36 exp clinical trial/ 37 placebos.mp. 38 placebo$.ti,ab. 39 random$.ti,ab. 40 comparative study.mp. 41 evaluation studies as topic/ 42 exp clinical trials as topic/ 43 follow up studies.mp. 44 prospective studies.mp. 45 (control$ or prospectiv$ or volunteer$).ti,ab. 46 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 47 11 and 26 and 46
Embase Ovid
1 exp Attention Deficit Disorder/ 2 adhd.mp. 3 addh.mp. 4 exp Hyperactivity/ 5 Hyperkinesia/ 6 (attention adj3 deficit).mp. 7 hyperactiv*.mp. 8 hyperkinesis*.mp. 9 (minimal adj brain adj3 disorder*).mp. 10 (minimal adj brain adj3 dysfunction*).mp. 11 (minimal adj brain adj3 damage*).mp. 12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13 social skills training.mp. 14 social skills education.mp. 15 social competence*.mp. 16 behavior regulation.mp. 17 behaviour regulation.mp. 18 (learning adj25 social).mp. 19 (social adj10 skills).mp. 20 role play*.mp. 21 psychosocial treatment.mp. 22 parent training.mp. 23 parent education.mp. 24 (educat* adj10 parent*).mp. 25 exp behavior modification/ 26 exp group therapy/ 27 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 28 controlled study.de. 29 clinical trial.de. 30 major clinical study.de. 31 randomized controlled trial.de. 32 double blind procedure.de. 33 clinical article.de. 34 random$.mp. 35 control$.mp. 36 follow up.mp. 37 ((singl$ or doubl$ or tripl$ or trebl$) adj (blind$ or mask$ or dummy)).mp. 38 placebo$.mp. 39 (clinic$ adj (trial$ or study or studies$)).mp. 40 exp comparative study/ 41 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 42 12 and 27 and 41
ERIC EBSCOhost
S1 DE attention deficit disorders S2 DE attention deficit hyperactivity disorder S3 DE hyperactivity S4 TX adhd or addh S5 TX attention within 3 deficit S6 TX attention N3 deficit S7 TX hyperkines* S8 TX minimal N3 brain N3 disorder* S9 TX minimal N3 brain N3 dysfunction* S10 TX minimal N3 brain N3 damage* S11 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 S12 KW randomi* S13 AB random* N3 (allocat* or allot* or assign* or basis or divid* or order*) S14 AB random* N4 (trial* OR study OR studies) S15 AB (control* OR clinic* OR prospectiv*) N5 (trial* OR study OR studies) S16 AB allocat* OR allot* OR assign* OR divid* OR order*) N4 (compar* OR control* OR experiment* OR intervent* OR therap* OR treatment*) N4 (group* OR class*) S17 AB (singl* OR doubl* OR trebl* OR tripl*) N4 (blind* OR mask*) S18 KW placebo* S19 AB placebo* S20 AB (compar* N5 (trial* OR study OR studies) S21 AB (clinic* OR control*) N4 (trial* OR study OR studies) S22 S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 S23 DE "interpersonal competence" or "daily living skills" or "emotional intelligence" or "extraversion introversion" or "interpersonal communication" or "prosocial behavior" or "sharing behavior" or "sensitivity training" or "interpersonal relationship" or "board administrator relationship" or "caregiver child relationship" or "collegiality" or "counselor client relationship" or "dating social" or "employer employee relationship" or "family relationship" or "parent child relationship" or "parent student relationship" or "sibling relationship" or "friendship" or "group unity" or "helping relationship" or "interpersonal attraction" or "interprofessional relationship" or "supervisor supervisee relationship" or "teacher administrator relationship" or "marriage" or "teacher student relationship" or "parent caregiver relationship" or "peer relationship" or "physician patient relationship" S24 DE behavior modification S25 DE "group therapy" or "group counceling" or "sensitivity training" S26 DE "role playing" or "dramatic play" S27 DE "parent education" S28 KW social skills training S29 AB social skills training OR social skills education S30 AB social competenc* S31 AB behavior regulation S32 AB behaviour regulation S33 AB learning N5 social S34 AB social N5 skill* S35 AB psychosocial treatment S36 AB parent training S37 AB educat* N3 parent* S38 S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 S39 S11 AND S22 AND S38
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S42 S20 and S41 S41 S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 S40 TI behaviour modification or AB behaviour modification S39 TI behavior modification or AB behavior modification S38 TI learning N3 social or AB learning N3 social S37 TI social N3 skills or AB social N3 skills S36 TI educat* N2 parent* or AB educat* N2 parent* S35 (MH "Psychotherapy, Group") S34 (MH "Role Playing") S33 TX parent education S32 TX parent training S31 TX psychosocial treatment S30 TX behaviour regulation S29 TX behavior regulation S28 TX social competence* S27 TX social skills education S26 TX social skills training S25 (MH "Social Skills") S24 (MH "Social Behavior+/ED") S23 (MH "Interpersonal Relations+/ED") S22 (MH "Social Skills Training") S21 S9 and S19 S20 S9 and S19 S19 S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S18 TX ( singl* OR doubl* OR tripl* OR trebl* ) and TX ( blind* OR mask* OR dummy* ) S17 TX clin* N25 trial* S16 (MH "Placebos") S15 TX placebo* OR random* S14 TX control* OR prospectiv* OR volunteer* S13 (MH "Evaluation Research+") S12 (MH "Prospective Studies+") S11 PT clinical trial S10 (MH "Clinical Trials+") S9 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 S8 TX minimal N1 brain N3 damage* S7 TX minimal N1 brain N3 dysfunction* S6 TX minimal N1 brain N3 disorder* S5 TX hyperkinesis* S4 TX hyperactiv* S3 TX attention N3 deficit S2 TX adhd or addh S1 (MH "Attention Deficit Hyperactivity Disorder")
PsycINFO Ovid
1 exp attention deficit disorder/ 2 adhd.mp. 3 addh.mp. 4 (attention adj3 deficit).mp. 5 hyperactiv$.mp. 6 hyperkinesis$.mp. 7 exp Hyperkinesis/ 8 (minimal adj brain adj3 disorder$).mp. 9 (minimal adj brain adj3 dysfunction$).mp. 10 (minimal adj brain adj3 damage$).mp. 11 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 12 exp Social Skills Training/ 13 exp Social Skills/ 14 Skill Learning/ 15 exp Human Relations Training/ 16 exp Parent Training/ 17 social skills training.mp. 18 social skills education.mp. 19 social competence$.mp. 20 behavior regulation.mp. 21 (social adj10 skills).mp. 22 (learning adj25 social).mp. 23 role play$.mp. 24 exp Communication skills training/ 25 psychosocial treatment.mp. 26 exp Assertiveness training/ 27 exp Behavior modification/ 28 behaviour regulation.mp. 29 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30 random$.mp. 31 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or dummy or mask$)).mp. 32 placebo$.mp. 33 crossover.mp. 34 assign$.mp. 35 allocat$.mp. 36 ((clin$ or control$ or compar$ or evaluat$ or prospectiv$) adj25 (trial$ or studi$ or study)).mp. 37 exp placebo/ 38 exp treatment effectiveness evaluation/ 39 exp mental health program evaluation/ 40 exp experimental design/ 41 versus.id. 42 vs.id. 43 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 44 11 and 29 and 43
Sociological Abstracts ProQuest
SU.EXACT("Attention Deficit Disorder") OR ((add OR adds) OR (hyperactiv* OR hyperkines*) OR (minimal within 1 brain within 3 disorder) OR (minimal within 1 brain within 3 dysfunction*) OR (minimal within 1 brain within 3 damage) OR (attention within 3 deficit*)) AND if(random* NEAR/4 (trial OR study OR studies)) OR if(randomi*) OR if(random* NEAR/4 (allocat* OR assign* OR divid*)) OR if((control* OR clinic* OR divid*) WITHIN 5 (condition* OR experiment* OR treatment* OR control* OR group*)) OR if((singl* OR doubl*) NEAR/4 (blind* OR mask*)) OR if(placebo*) OR if((crossover OR cross over)) OR if((compar* WITHIN 5 (trial* OR study OR studies)))
ProQuest Dissertations and Theses Global
su(adhd OR addh OR add OR attention deficit hyperactivity disorder OR add OR adds OR attention deficit disorder) AND ab(social skill* OR role play OR psychosocial treatment OR parent education OR group therapy OR behavior modification OR behaviour modfication OR behavior regulation OR behaviour regulation OR social competence)
Clinical Trials (https://clinicaltrials.gov/)
attention deficit hyperactivity disorder OR adhd OR attention OR attention deficit disorder OR hyperactivity OR hyperkin* | social skills training OR social skills education OR social competenc* OR behavior regulation OR social skill* OR role play* OR psychosocial treatment OR parent education OR group therapy OR behavior modification OR behaviour modification | Child |
WHO ICTRP (http://www.who.int/ictrp/en/)
attention deficit hyperactivity disorder OR adhd OR attention OR attention deficit disorder OR hyperactivity OR hyperkin* | social skills training OR social skills education OR social competenc% OR behavior regulation OR social skill* OR role play* OR psychosocial treatment OR parent education OR group therapy OR behavior modification OR behaviour modification | Child |
Appendix 2. Data extraction sheet
Version number: 1:1. 08‐10‐2010
| For notes |
:Source
| Study ID |
| Report ID |
| Year of publication |
| Year of conduct |
| Review author(s) |
| Citation source |
| Authors |
Footnotes
ID: identifier
Eligibility
| Confirm eligibility |
| Reasons for exclusion |
Study
| Design (e.g. randomised, blinded, placebo, etc.) | |
|
| Location (e.g. hospital, outpatient clinic) | ||
| Duration of study | ||
| Inclusion criteria |
||
| Exclusion criteria |
||
| Outcomes | Primary | |
| Secondary | ||
Risk of bias
| Domain | Judgement (low/uncertain/high) | Adequacy (yes/unclear/no) | Descriptions |
| Generation of the allocation sequence | Quote: Comment: |
||
| Allocation concealment | Quote: Comment: |
||
| Blinding of participants and personnel | Quote: Comment: |
||
| Blinding of outcome assessment | Quote: Comment: |
||
| Incomplete outcome data | Quote: Comment: |
||
| Selective outcome reporting | Quote: Comment: |
||
| Vested interest bias | Quote: Comment: |
||
| Other sources of bias: baseline imbalance | Quote: Comment: |
||
| Other sources of bias: early stopping | Quote: Comment: |
Participants
| Sample size or power calculation (yes/no): | Quote: Comment: |
| Total number (sample size): | Pre‐randomisation: |
| Post‐randomisation: | |
| Diagnostic criteria (e.g. ICD‐10 number, DSM‐IV number or by a cut‐off score from report) | |
| Age | |
| Sex | |
| Comorbidity | |
| Sociodemographics (e.g. double or single parent families, low, middle or upper class) | |
| Country/ethnicity | |
| Co‐medication |
Footnotes
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders ‐ Fourth Edition. ICD‐10: International Classification of Diseases ‐ Tenth Revision.
Interventions
| Intervention groups |
| Number of participants allocated per group |
| Number of patients lost to follow‐up per group |
| Format and duration of the intervention (e.g. group base, individual, and setting) |
| Specific intervention (e.g. type of programme) and by whom (e.g. nurse, psychologist, teacher) |
| Content of the intervention |
| Treatment compliance (treatment to manual and participant to treatment) |
Outcomes
| Outcomes specified | Reported (yes/no) | Definition and unit of measurement | Type of scale | Summary statistic for each intervention group (short‐, medium‐ or long‐term) |
Miscellaneous
| Funding source |
| Key conclusions of the study authors |
| References to other relevant studies |
| Correspondence required |
| Miscellaneous comments from the study authors |
| Miscellaneous comments from the review authors |
Appendix 3. Criteria for assigning 'Risk of bias' judgements
We assessed the 'Risk of bias' components as follows.
Generation of the allocation sequence
Low risk of bias: the method used was either adequate (for example, computer‐generated random numbers or table of random numbers), or was unlikely to have introduced selection bias
Uncertain risk of bias: there was not enough information to assess whether the method used could have caused bias
High risk of bias: the method used was improper and likely to have introduced bias
Allocation concealment
Low risk of bias: the method used (for example, central allocation) probably did not bias the observed intervention effect
Uncertain risk of bias: there was not enough information to assess whether the method used could have biased the estimate of effect
High risk of bias: the method (for example, open random allocation schedule) used probably biased the observed intervention effect
Blinding of participants and personnel
Low risk of bias: the method of blinding was described and blinding was carried out satisfactorily
Uncertain risk of bias: there was insufficient information to assess whether the type of blinding used could have biased the estimate of effect
High risk of bias: no blinding or incomplete blinding
Blinding of outcome assessors
Low risk of bias: the method of blinding was described and blinding was carried out satisfactorily
Uncertain risk of bias: there was insufficient information to assess whether the type of blinding used could have biased the estimate of effect
High risk of bias: no blinding or incomplete blinding
Incomplete outcome data
Low risk of bias: the underlying reasons for the missing data probably did not affect the outcome measurement of the effect of the study, or valid methods were used to handle the missing data
Uncertain risk of bias: there was not enough information to assess whether the missing data, or the method used to handle missing data, could have biased the estimate of effect
High risk of bias: the crude estimate of effect was definitely biased due to the underlying reasons for the missing data, or the methods used to handle missing data were unsatisfactory
Selective outcome reporting
Low risk of bias: the study protocol was available, or all prespecified outcomes that were of interest were reported
Uncertain risk of bias: there was not enough information to assess whether the direction and magnitude of the observed effect was related to selective outcome reporting
High risk of bias: not all of the prespecified primary outcomes were reported or participants were excluded after randomisation (selection bias)
Vested interest bias
Low risk of bias: the study's source(s) of funding did not come from any parties that might have had conflicts of interest (for example, a drug or a device manufacturer), or the study author(s) had not conducted previous studies addressing the same interventions
Uncertain risk of bias: the source of funding was not clear, or it was not clear if the study author(s) had conducted previous studies addressing the same interventions
High risk of bias: the study was funded by parties that might have conflicts of interest (e.g. a manufacture of a drug or a device manufacturer), or potential conflicts of interest were reported by study authors
Other sources of bias
Low risk of bias: the study appeared to be free of other sources of bias
Uncertain risk of bias: there was inadequate information and therefore it was not possible to assess other possible sources of bias
High risk of bias: it is likely that potential sources of bias were present; for example, bias related to the specific design used, early termination due to some data‐dependent process, or lack of power calculation, or other bias risks
We defined overall low risk of bias studies as studies that had low risk of bias in all domains. We considered studies with one or more unclear or high risk of bias domains as studies with high risk of bias overall.
Appendix 4. Glossary
| Attention Control Treatment | ACT |
| Learning Skills Training for Adolescents with ADHD | ADHS‐LeJA |
| ADHD plus Impairments in Mood | AIM |
| Academic Performance Rating Scale | APRS |
| Attention Deficit Hyperactivity Disorder | ADHD |
| Attention Deficit Hyperactivity Disorder ‐ Inattentive subtype | ADHD‐I |
| Attention Deficit Hyperactivity Disorder ‐ Rating Scales | ADHD‐RS |
| Behavior Rating Inventory of Executive Function | BRIEF |
| Child Behavior Checklist | CBCL |
| [Connors] Comprehensive Behavior Rating Scales | CBRS |
| Cognitive Behaviour Therapy | CBT |
| Classroom Challenge | CC |
| Conduct Disorder | CD |
| Clinical Global Impression | CGI |
| Challenging Horizons Program – After School version | CHP‐AS |
| Challenging Horizons Program – Mentoring version | CHP‐M |
| Confidence Interval | CI |
| Collaborative Life skills | CL |
| Clinical Linguistic and Auditory Milestone Scale | CLAM |
| Child Life and Attention Skills [program] | CLAS |
| Community Oriented Parent Education [program] | COPE |
| Conners Parent Rating Scale | CPRS |
| Classroom Performance Survey | CPS |
| Continuous Performance Test | CPT |
| Client Satisfaction Questionnaire | CSQ |
| Conners Teacher Rating Scale | CTRS |
| Comorbid disorder | Additional condition co‐occurring with the primary condition |
| Diagnostic and Statistical Manual of Mental Disorders ‐ Third edition ‐ Revised | DSM‐III‐R |
| Diagnostic and Statistical Manual of Mental Disorders ‐ Fourth edition | DSM‐IV |
| Diagnostic and Statistical Manual of Mental Disorders ‐ Fourth edition ‐ Text Revision | DSM‐IV‐ TR |
| Diagnostic and Statistical Manual of Mental Disorders ‐ Fifth edition | DSM‐5 |
| Disruptive Behavior Disorders Rating Scale | DBDRS |
| Diversity‐Adjusted Required Information Size | DARIS |
| Diagnostic Interview for Children & Adolescents ‐ Revised | DIACA‐R |
| Diagnostic Interview for Children and Adolescents ‐ Revised Parent version | DICA‐R‐P |
| Parent interview with the Diagnostic Interview Schedule for Children | DISC‐P2 |
| Emotion Expression Scale for Children | EESC |
| Emotion Management Training | EMT |
| Emotion Regulation Checklist | ERC |
| Fremdbeurteilungsbogen für Hyperkinetische Störungen | FBB‐HKS |
| Full Scale Intelligence Quotient | FSIQ |
| Information Size | IS |
| Intelligence Quotient | IQ |
| International Classification of Diseases ‐ 10th version | ICD‐10 |
| International Committee of Harmonization guidelines | ICH |
| Impairment rating scale | IRS |
| Intention‐To‐Treat | ITT |
| Kiddie Schedule for Affective Disorders and Schizophrenia ‐ Present and Lifetime version | K‐SADS |
| Mean Difference | MD |
| Matching Familiar Figures Test | MFFT |
| Multimodal Psychosocial treatment | MPT |
| Multimodal Treatment of Attention Deficit Hyperactivity Disorder | MTA |
| Number Needed to Treat | NNT |
| Oppositional Defiant Disorder | ODD |
| Primary Counsellor | PC |
| Parent version of the Children’s Interview for Psychiatric Syndromes | P‐ChIPS |
| Parent‐Focused Treatment | PFT |
| Principal Investigator | PI |
| Participant Intervention Comparison Outcome | PICO |
| Progressive Muscle Relaxation | PMR |
| Randomised Clinical Trial | RCT |
| Required Information Size | RIS |
| Risk Difference | RD |
| Risk Ratio | RR |
| Selective Serotonin Reuptake Inhibitor | SSRI |
| Self‐Control Rating Scale | SCRS |
| Sluggish Cognitive Tempo | SCT |
| Social Skills Improvement System | SSIS |
| Social Skills Rating Scale | SSRS |
| Social skills training | SST |
| Standard Deviation | SD |
| Standardized Mean Difference | SMD |
| Social Skills Training Plus Parental Training Combined with Standard Treatment | SOSTRA |
| Strengths and Difficulties Questionnaire | SDQ |
| Strengths and Weaknesses of ADHD Symptoms and Normal Behaviors | SWAN |
| Swanson, Nolan and Pelham (‐ Revised) rating scale | SNAP (‐R) |
| Treatment As Usual | TAU |
| Trial Sequential Analysis | TSA |
| Wechsler Abbreviated Scale of Intelligence | WASI |
| Wechsler Individual Achievement Test | WIAT |
| WEISS Functional Impairment Scale ‐ Parent form | WFIRS‐P |
| Wechsler Intelligence Scale for Children ‐ Revised edition | WISC‐R |
| Wechsler Intelligence Scale for Children ‐ Third edition | WISC‐III |
| Wechsler Intelligence Scale for Children ‐ Fourth edition | WISC‐IV |
| Wechsler Preschool and Primary Scale of Intelligence | WIPPSI |
| World Health Organization | WHO |
| Waiting List | WL |
Data and analyses
Comparison 1. Social skills.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary meta‐analysis: Teacher‐rated social skills at end of treatment | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All eligible trials | 11 | 1271 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.00, 0.22] |
| 1.2 Sensitivity analysis excluding the 3 trials with longest treatment duration | 8 | 620 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.05, 0.27] |
| 2 Secondary meta‐analyses: Social skills | 19 | 2649 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [0.11, 0.47] |
| 2.1 Teacher‐rated social skills at longest follow‐up | 3 | 192 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.22, 0.35] |
| 2.2 Parent‐rated social skills at end of treatment for all eligible trials | 15 | 1609 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.06, 0.32] |
| 2.3 Parent‐rated social skills at longest follow‐up | 2 | 445 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.35, 0.62] |
| 2.4 Observer‐rated social skills at end of treatment for all eligible trials | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | 2.88 [1.80, 3.96] |
| 2.5 Participants‐rated social skills at end of treatment for all eligible trials | 5 | 344 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.68, 1.23] |
| 2.6 Participant‐rated social skills at longest follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 1.60 [0.77, 2.44] |
| 3 Teacher‐reported Walker‐McConnell Scale of Social Competence and School Adjustment | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 1.06 [‐0.47, 2.59] |
| 4 Parent‐rated Social Skills Scale (UCI) | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 9.70 [6.07, 13.33] |
| 5 Child‐rated Test of Social Skill Knowledge | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 4.20 [1.99, 6.41] |
| 6 Social Interaction Observation Code: Negative behaviour | 1 | 68 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.11, 0.51] |
Comparison 2. Emotional competencies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary meta‐analysis: Teacher‐rated emotional competencies at end of treatment | 2 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.72, 0.68] |
| 2 Secondary meta‐analyses: Emotional competencies | 5 | 353 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.41, 0.01] |
| 2.1 Parent‐rated emotional competencies | 3 | 173 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.59, 0.05] |
| 2.2 Parent‐rated emotional competencies at longest follow‐up | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.34, 0.72] |
| 2.3 Participant‐rated emotional competencies | 2 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.62, 0.09] |
Comparison 3. General behaviour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary meta‐analysis: Teacher‐rated general behaviour at end of treatment | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All eligible trials | 8 | 1002 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.19, 0.06] |
| 1.2 Sensitivity analysis excluding the 2 trials with the longest treatment duration | 6 | 422 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.28, 0.10] |
| 1.3 Sensitivity analysis excluding the 2 largest trials | 6 | 422 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.28, 0.10] |
| 2 Secondary analyses: general behaviour | 9 | 2034 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.40, ‐0.12] |
| 2.1 Teacher‐rated general behaviour at longest follow‐up | 4 | 637 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.27, 0.07] |
| 2.2 Parent‐rated general behaviour at end of treatment | 8 | 995 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.61, ‐0.14] |
| 2.3 Parent‐rated general behaviour at longest follow‐up | 1 | 326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.44, 0.03] |
| 2.4 Participant‐rated general behaviour at end of treatment | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.52, 0.38] |
Comparison 4. Core ADHD symptoms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary meta‐analysis: Teacher‐rated ADHD symptoms at end of treatment | 14 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All eligible trials | 14 | 1379 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.47, ‐0.05] |
| 1.2 Sensitivity analysis excluding the 3 trials with longest treatment duration | 11 | 677 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.52, 0.04] |
| 1.3 Sensitivity analysis excluding the 3 largest trials | 11 | 677 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.52, 0.04] |
| 2 Secondary meta‐analyses: ADHD symptoms | 15 | 2857 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.63, ‐0.15] |
| 2.1 Teacher‐rated ADHD symptoms at longest follow‐up | 5 | 582 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.28, 0.06] |
| 2.2 Parent‐rated ADHD symptoms at end of treatment for all eligible trials | 11 | 1206 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.81, ‐0.26] |
| 2.3 Parent‐rated ADHD symptoms at longest follow‐up | 3 | 476 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐2.48, ‐0.25] |
| 2.4 Participant‐rated ADHD symptoms at end of treatment | 2 | 106 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐2.31, 0.78] |
| 2.5 Observer‐rated ADHD symptoms at end of treatment for all eligible trials | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.15 [‐9.88, 3.57] |
| 2.6 Observer‐rated ADHD symptoms at longest follow for all eligible trials | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 3.95 [2.66, 5.23] |
| 2.7 Single study result: Teacher‐rated ADHD symptoms (inattention) at end of treatment | 1 | 254 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.23, 0.26] |
| 2.8 Single study result: Teacher‐rated ADHD symptoms (sluggish cognitive tempo) at end of treatment | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.78, 0.20] |
| 2.9 Single study result: Participant‐rated ADHD symptoms at longest follow‐up | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 1.62 [0.78, 2.46] |
Comparison 5. Teacher‐rated performance and grades in school.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 At end of treatment | 5 | 642 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.01, 0.31] |
| 2 At longest follow‐up | 2 | 379 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.22, 0.20] |
Comparison 6. Observer‐rated performance and grades in school.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wescheler Individual Achievement Test | 1 | 260 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐2.06, 5.06] |
Comparison 7. TSA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Teacher‐rated social skills | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 At end of treatment | 4 | 185 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐1.01, 4.62] |
| 1.2 All eligible trials | 11 | 1271 | Mean Difference (IV, Random, 95% CI) | 1.22 [0.09, 2.36] |
Comparison 8. Subgroup analysis 1: Children aged five to 11 years versus children aged 12 to 18 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Teacher‐rated social skills | 11 | 1271 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.00, 0.22] |
| 1.1 Children aged 5 to 11 years | 10 | 1194 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.01, 0.22] |
| 1.2 Children aged 12 to 18 years | 1 | 77 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.28, 0.61] |
Comparison 9. Subgroup analysis 2: ADHD and comorbidity versus ADHD and no comorbidity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent‐rated ADHD symptoms at end of treatment | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 ADHD and comorbidity | 8 | 1003 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.67, ‐0.15] |
| 1.2 ADHD and no comorbidity | 2 | 173 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.05, 0.03] |
Comparison 10. Subgroup analysis 3: Social skills training only versus social skills training supported by parent training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Teacher‐rated social skills at end of treatment | 6 | 968 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.00, 0.28] |
| 1.1 Social skills training supported by parent training | 4 | 336 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.01, 0.45] |
| 1.2 Social skills training only (i.e. without parent training) | 4 | 632 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.12, 0.41] |
Comparison 11. Subgroup analysis 4: Social skills training, parental training and medication versus social skills training and parental training without medication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parent‐rated social skills at end of treatment | 8 | 636 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.05, 0.48] |
| 1.1 Social skills training, parent training and parent‐rated medication | 4 | 299 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.20, 0.39] |
| 1.2 Social skills training, parent training without parent‐rated medication | 4 | 337 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.15, 0.70] |
Comparison 12. Subgroup analysis 5: No‐intervention control group versus waiting‐list control group with possible minor active intervention components.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Teacher‐rated social skills at end of treatment | 11 | 1271 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.00, 0.22] |
| 1.1 No‐intervention control group | 8 | 693 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.03, 0.27] |
| 1.2 Waiting‐list control group with possible minor active intervention components | 3 | 578 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.13, 0.42] |
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Cohen 1981.
| Methods | Design: RCT | |
| Participants |
Country: ethnicity: 100 % Canadian, so Canada was assumed Setting: not reported Sample size calculation: not reported Sample size: 24 children Sex: 21 (87.5%) = boys, three (12.5%) = girls Age: range = five‐six years Ethnicity: Canadian Socioeconomic status: not reported IQ: IQ ≥ 80 on WPPSI ADHD diagnosis: subtypes not reported ADHD medication: not reported Comorbidity: not reported Medications for comorbid disorders: not reported Inclusion criteria:
Exclusion criteria: no neurological damage or psychosis Baseline characteristics: no information about medication balance between groups |
|
| Interventions | 24 children allocated to one of four groups
Attendance: not reported |
|
| Outcomes |
Primary outcomes
Outcome assessment: end of treatment |
|
| Notes |
Study ID: not reported Sponsorship source: grants from the Ontario Mental Health Foundation (Grant No. 701‐76/78) and The Hospital for Sick Children Foundation (Grant No. 77‐22) Year conducted: not stated Duration of the study: 15 months Comments: none Lead author's name: NJ Cohen Institution: Psychiatric Research Unit, The Hospital for Sick Children Email: not reported Address: 555 University Avenue, Toronto, Ontario, Canada MSG 1X8. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: unclear |
| Allocation concealment (selection bias) | High risk | Comment: allocation concealment not described, but four patients were moved between groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding on at least one of this review's primary outcomes, but no blinding for the rest of the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: many tables different from text and no explanation provided. Lack of teacher responses |
| Selective reporting (reporting bias) | Low risk | Comment: all apparent assessments were made. |
| Vested interest bias | Low risk | Comment: no other apparent biases, no previous research on the topic |
| Other sources of bias? | High risk | Comment: selection procedure of patients not stated in report |
Bloomquist 1991.
| Methods | Design: RCT | |
| Participants |
Country: USA Setting: outpatient, 3 suburban elementary schools in the same school district Sample size calculation: not reported Sample size: 52 children Sex: 36 (69%) = boys, 16 (31%) = girls Age: range = eight‐nine years Ethnicity: Caucasian = 95% Socioeconomic status: not reported IQ: mental retardation excluded ADHD diagnosis: subtypes not reported ADHD medication: not reported Comorbidity: ODD = 18 (35%) Medications for comorbid disorders: not reported Inclusion criteria:
Exclusion criteria:
Baseline characteristics: groups were highly comparable on descriptive and subjective identification measures; age, IQ, academic achievement, hyperactivity and self‐control behaviour, externalising and internalising behaviour at baseline. |
|
| Interventions | 52 children with ADHD were allocated to one of three groups. Only group one and three were included in the analysis.
Attendance: almost 100% for child and teacher interventions |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: post‐intervention assessment |
|
| Notes |
Study ID: not reported Sponsorship source: not reported Year conducted: not stated Duration of the study: 16 weeks Comments: authors referred to unpublished paper with the treatment manual by Bloomquist and Braswell (Bloomquist 1991) Lead author: Michael L. Bloomquist, PhD Institution: Division of Child and Adolescent Psychiatry Email: not reported Address: Box 95, University of Minnesota Hospital and Clinic, Harvard Street at East River Road, Minneapolis, Minnesota 55455 Year conducted: not reported Duration of the study: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description of the randomisation method used |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of the allocation method used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: observers were blinded to treatment assignment but teachers were not. No blinding on primary outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 16 excluded data sets with much likelihood of biasing results |
| Selective reporting (reporting bias) | Low risk | Comment: all measures of interest reported |
| Vested interest bias | Unclear risk | Comment: no funding sources stated |
| Other sources of bias? | Unclear risk | Comment: no test for compliance of intervention groups |
Pfiffner 1997.
| Methods | Design: RCT | |
| Participants |
Country: USA Setting: university‐based behavioural paediatric clinic specialising in ADHD and related disorders. Participants were recruited from newspaper advertisement and from consecutive referrals. Sample size calculation: not reported Sample size: 27 children Sex: 19 (70%) = boys, eight (30%) = girls Age: range = eight‐10 years Ethnicity: all Caucasian except for one boy, who was African American Socioeconomic status: middle‐ to upper‐middle class; two children were from single‐parent families IQ: not reported ADHD diagnosis: following DSM‐III‐R, 25 children met criteria for ADHD and two met criteria for undifferentiated ADHD ADHD medication: n = 12 (44%) received stimulant medication Comorbidity: ODD = 19, CD = 3, separation anxiety disorder = four, overanxious disorder = five, dysthymic disorder = two Medication for comorbid disorder: no information Inclusion criteria:
Exclusion criteria: not reported. We attempted to get this information from the study investigators but have not succeeded in this attempt. Baseline characteristics: at pretreatment, no significant difference in age, socioeconomic status, medication status and number of symptoms of ADHD, and comorbid disorders or on parent and teacher ratings of social skills and behaviour |
|
| Interventions | 18 participants allocated to one of three groups (see below). Both treatment groups had a protocol and were led by psychologist and the same two therapists taught in the childrens' groups. The two treatment groups attended eight group sessions. Children in both treatment groups received 90‐minute group sessions during consecutive weeks. Assessment was at pre‐ and post‐treatment and follow‐up (3‐4 months post‐treatment).
Attendance: two families each missed one session |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: post‐intervention and follow‐up three to four months after post‐intervention assessment |
|
| Notes |
Study ID: not reported Sponsorship source: not reported Year conducted: not stated Duration of the study: 8 months Comments: none Lead author: Linda J Pfiffner Institution: Department of Psychiatry, The University of Chicago Email: not reported Address: 5841 South Maryland Avenue, MC 3077, Chicago, Illinois 60637 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information given in the article. We requested clarification from the study investigators and they reported in an email on 26 May 2011 that it was not possible to obtain these data at that time (Pfiffner 2011 [pers comm]). |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information given in the article. We requested clarification from the study investigators and they reported in an email 26 May 2011 that it was not possible to find these data at that time (Pfiffner 2011 [pers comm]). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding on at least one of this study's primary outcomes; no blinding for the rest of the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: three participants started medication after post‐intervention assessment but before follow‐up assessment. Individual scores for these participants were replaced by the grand mean of all other participants at follow‐up to avoid possible confounds associated with the medication treatment. |
| Selective reporting (reporting bias) | High risk | Comment: the author informed us in an email (Pfiffner 2011 [pers comm] that the CLAM and SNAP were used post‐treatment, but were not reported in the article. We were not able to get the data because they had been lost over time. |
| Vested interest bias | Low risk | Comment: no other apparent biases, no previous research on the topic |
| Other sources of bias? | High risk | Comment: Teachers were paid $10 for post‐intervention assessment and $25 for follow‐up assessment and families were paid $12 for follow‐up assessment. |
Abikoff 2004.
| Methods | Design: RCT | |
| Participants |
Country: USA and Canada Setting: outpatient clinics in two large medical centres in New York and Montreal Sample size calculation: not reported Sample size: 103 children Sex: 93% = boys, 7% = girls Age: range = 7‐9.9 years Ethnicity: white = 84%, African‐American = 13%, Hispanic = 2%, other = 1% Socioeconomic status: 84 children (81.2%) lived with both parents, 13 (12.6%) with one parent, and six (5.8%) with their mother and stepfather IQ: normal IQ (i.e. WISC‐R ≥ 85) ADHD diagnosis: subtypes not reported ADHD medication: all participants received psychostimulants Comorbidity: ODD = 53.4%, CD = 30%, anxiety disorder = 16.5% Medications for comorbid disorders: not reported Inclusion criteria:
Exclusion criteria:
Baseline characteristics: no between‐group differences except on socioeconomic status, where there were differences between the group given methylphenidate alone and the group given methylphenidate + attention control treatment |
|
| Interventions | 103 participants allocated to one of three groups
Attendance: 75% attendance required. 22 children (methylphenidate = 10, methylphenidate + MPT = 6, methylphenidate + ACT = 6) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: end of treatment |
|
| Notes |
Study ID: not reported Sponsorship source: not reported Year conducted: not stated Duration of the study: 2 years Comments: none Lead author's name: Howard Abikoff Institution: NYU Child Study Center, New York University School of Medicine Email: abikoh01@med.nyu.edu Address: NYU Child Study Center, New York University School of Medicine, New York |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: we requested clarification from one of the study investigators. Howard Abikoff informed us in an email on 28 January 2011 (Abikoff 2011 [pers comm]) that they had used a block randomisation scheme with blocks of four children. The groups were balanced for age, sex, ODD, and ethnicity. |
| Allocation concealment (selection bias) | Low risk | Comment: we requested clarification from 1 of the study investigators. Howard Abikoff informed us in an email as above (Abikoff 2011 [pers comm]) that they had used sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding on at least one of this review's primary outcomes but no blinding for the rest of the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 22 out of 103 children failed to complete the study. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no prior statement of assessment tools. Design article published at the same time as study article |
| Vested interest bias | High risk | Comment: the study was based in two large medical centres and the centres have extensive previous experience with research focused on ADHD and behavioural treatment. Dr Klein is a member of a pharmaceutical board. |
| Other sources of bias? | Low risk | Comment: no other sources identified |
MTA 1999.
| Methods | Design: RCT | |
| Participants |
Country: USA Setting: six multisite outpatient clinics Sample size calculation: 576 participants required Sample size: 576 children Sex: 465 (81%) = boys, 111 (19%) = girls Age: range 7.0 to 9.9 years Ethnicity: white = 61%, African‐American = 20%, Hispanic = 8% Socioeconomic status: not reported IQ: below 80 excluded ADHD diagnosis:DSM‐IV, ADHD combined type ADHD medication: 97% received methylphenidate Comorbidity: anxiety disorder = 33.5%, conduct disorder = 14.3%, ODD = 39.9%, affective disorder = 3.8%, tic disorder = 10.9%, other = 2.2% such as bulimia, enuresis Medications for comorbid disorders: balanced between groups. Inclusion criteria:
Exclusion criteria:
Baseline characteristics: no significant differences among study groups |
|
| Interventions | 576 children allocated to one of four groups
Attendance: in group two and three, the families attended an average of 77.8% of the parent training sessions and 36.2 of 40 possible summer treatment programme days. In the school component, there was an average of 10.7 teacher consultation visits and 47.6 out of 60 possible days of work with a classroom aid |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: post‐intervention, end of treatment and follow‐up data |
|
| Notes |
Study ID:NCT00000388 Sponsorship source: The study was supported by grants from the National Institute of Mental Health (UO1 MH50461, U01 MH50447, U01 MH5044, Uo1 MH50453, U=1 MH 50454 and U01 MH50467). Year conducted: not stated Duration of the study: 38 months Comments: The Multimodal Treatment Study (MTA study) is a cooperative study, performed by six independent research teams in collaboration with the National Institute of Mental Health, Rockville, MD, and the Office of Special Education Programs, US Department of Eduation, Washington, DC. Lead author: MTA Cooperative Group. Corresponding author: Peter S Jensen Institution: Department of Child Psychiatry, Unit 78, Center for the Advancement of Children's Mental Health, New York State Psychiatric Institute/Columbia University Email: jensenp@child.cpmc.columbia.edu Address: 1051 RiversideDr, New York, NY 10032 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: adequate method used |
| Allocation concealment (selection bias) | Low risk | Comment: adequate method used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinded and unblinded raters |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: used imputation |
| Selective reporting (reporting bias) | Unclear risk | Comment: where is the consumer satisfaction and the CBCL data reported? We requested clarification from one of the study investigators, but had received no response when this review was finished. |
| Vested interest bias | Low risk | Comment: no vested interest |
| Other sources of bias? | Low risk | Comment: no other apparent sources of bias |
Tutty 2003.
| Methods | Design: RCT | |
| Participants |
Country: USA Setting: outpatient clinic in Washington Sample size calculation: not reported Sample size: 100 children Sex: 75 (75%) = boys, 25 (25%) = girls Age: range = five‐12 years Ethnicity: (group one: white = 49 (83%), African‐American = 4 (7%), Asian = 5 (8%), Hispanic = 1 (2%); group two: white = 38 (93%), African‐American = 2 (5%), Asian = 1 (2%), Hispanic = 0 (0%)) Socioeconomic status: parent education (group one: grades nine‐12 = nine (15%), grades 13‐16 = 18 (64%), < grade 16 = 12 (20%); group two: grades nine‐12 = 11 (27%), grades 13‐16 = 25 (61%), < Grade 16 = five (12%)) IQ: not reported ADHD diagnosis: (group one: inattentive subtype = 25 (42%), combined subtype = 34 (58%); group two: inattentive subtype = 16 (39%), combined subtype = 25 (61%)) ADHD medication: a new prescription for stimulant medication had been filled in for all children Comorbidity: not reported, but range of comorbid difficulties (see below) were part of exclusion criteria Medications for comorbid disorders: allowed but not stated if it was balanced between groups Inclusion criteria: diagnosis of ADHD (DSM‐IV), and filling in new prescription for stimulant medication (i.e. no stimulant medication use in past 120 days) Exclusion criteria: comorbid CD, ODD, Tourette syndrome, affective disorder, active alcohol or other substance abuse during previously 90 days or chronic mental illness, if children had been enrolled in a child social skills training at the involved centre in the past Baseline characteristics: mean baseline parented ADHD symptom scores were more symptomatic for the intervention group than for the control group, as well as the use of parent discipline practice. These between‐groups differences were adjusted before follow‐up analysis. |
|
| Interventions | 100 participants allocated to one of two groups
Attendance: there was a 95% rate for completing all eight sessions in group one. Blinded follow‐up measures completed by 97% and 98% of parent or guardian participants at three and six months after enrolment, respectively. Follow‐up completion rates for teacher participants yielded 92% and 75% for three and six months after enrolment, respectively. Participants with missing data did not differ from participants with complete data sets across time or any clinical, functional, and demographic variables, according to the authors of the report. For the ADHD Rating Scale outcome, two children were lost to follow‐up. For the Child Attention Profile outcome, 24 children in total were lost to follow‐up (intervention = 16, control = eight). |
|
| Outcomes |
Secondary outcomes
Outcome assessment: at three (post‐intervention) and six months (follow‐up) after enrolment |
|
| Notes |
Study ID: not reported Sponsorship source: the study was supported by the Group Health Cooperative (GHC)/Kaiser Permanente Community Foundation through a grant to the GHC, Center for Attention Deficit Disorders, located in Redmond, Washington Year conducted: not stated Duration of the study: 12 months Comments: there was a third outcome used in this study, but it is not relevant for this review, because it measured the parents' discipline practice. Lead author: Steve Tutty Institution: Group Health Cooperative, Center for Health Studies Email: tutty.s@ghc.org Address: 1730 Minor Avenue, Suite 1600, Seattle, WA 98101 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: study used coin toss method performed by research assistant, which is an adequate method of randomly generating the sequence, according to the Cochrane Handbook of Systematic Reviews of Interventions |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information reported. We requested clarification about the method of allocation concealment from the study investigators but received no information on this topic at the time of the original review. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: outcome assessment by telephone interviews of parents and teacher, which were performed by a blinded research assistant. The parents, however, were not blinded, which is not an adequate method. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: ITT method used |
| Selective reporting (reporting bias) | Low risk | Comment: all measures of interest reported. No protocol identified. Of the measures mentioned in the paper, all measures of interest for the review were reported. |
| Vested interest bias | Low risk | Comment: no apparent source of bias |
| Other sources of bias? | Unclear risk | Comment: co‐medication not specified |
Antshel 2003.
| Methods | Design: RCT | |
| Participants |
Country: USA Setting: outpatient clinic in Kentucky. Participants were recruited from newspaper advertisements and from consecutive referrals to a university‐based behavioural paediatric clinic specialised in ADHD and related disorders. Sample size calculation: not reported Sample size: 120 children Sex: 90 (75%) = boys, 30 (25%) = girls Age: range = 8‐12 years Ethnicity: Caucasian = 112, African‐American = six, Asian‐American = two Socioeconomic status: not reported IQ: IQ > 70 ADHD diagnosis:DSM‐IV (DICA‐R‐P); (inattentive type = 59, combined type = 61) ADHD medication: stimulant medication = 110, SSRIs = 10 Comorbidity: ODD = 53, mood disorders = 29, anxiety disorders = 11, tic disorders = five Medication for comorbid disorders: SSRI balanced between groups Inclusion criteria: children scoring > 1 SD above the mean on the CBCL Attention subscale Exclusion criteria:
Baseline characteristics: no statistically significant between‐group differences in age, sex, or classroom placement, duration and severity of ADHD symptoms, or comorbid conditions. No statistically significant between‐group differences on medication type and dosage |
|
| Interventions | 120 participants allocated to one of two groups
Attendance: mean attendance at the eight sessions was 94% for the diagnostically homogeneous and 92% for the diagnostically heterogenous treatment groups. |
|
| Outcomes |
Primary outcomes
Outcome assessment: post‐treatment assessment eight weeks after pre‐test and follow‐up assessment three months after the post‐test |
|
| Notes |
Study ID: not reported Sponsorship source: not reported Year conducted: not stated Duration of the study: 22 weeks Comments: none Lead author's name: Kevin M Antshel Institution: Children’s Hospital, Boston University of Kentucky Email: Antshelk@upstate.edu Address: SUNY Upstate Medical University, Department of Psychiatry, 750 East Adams Street, Syracuse, NY 13210 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: used a computer‐generated randomisation process. Information received from Kevin Antshel in an email 13 July 2011 (Antshel 2011 [pers comm]) |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation was concealed. Information received from Kevin Antshel in an email 13 July 2011 (Antshel 2011 [pers comm]) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinded outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: stated that there was 100% completion rate |
| Selective reporting (reporting bias) | Low risk | Comment: all measures of interest reported |
| Vested interest bias | Unclear risk | Comment: no funding source reported |
| Other sources of bias? | Unclear risk | Comment: Referral of patients. The selection of ADHD‐I skewed the data. |
Yuk‐chi 2005.
| Methods | Design: RCT | |
| Participants |
Country: China Setting: community mental health centre: outpatient clinic in Hong Kong Sample size calculation: based on prior research, the assumption was made that effect sizes would be small to moderate, calculations showed that approximately 45 participants were required in each group. Sample size: 90 children Sex: 77 (90%) = boys, nine (10%) = girls (group one: 39 (87%) = boys, six (13%) = girls; group two: 38 (93%) = boys, three (7%) = girls) Age: range = seven to 9.9 years, mean = eight years (SD = 0.95) (group one; mean = 7.87 years (SD = 0.77); group two: mean = 8.15 years (SD = 1.11)) Ethnicity: Chinese children Socioeconomic status: mothers education: elementary or less = 17 (20%), junior high = 20 (23%), high school = 45 (50%), college = four (5%) (group one: elementary or less = six (13%), junior high = 10 (22%), high school = 28 (62%), college = one (2%), group two: elementary or less = 11 (27%), junior high = 10 (24%), high school = 15 (37%), college = three (7%)) IQ: Hong Kong WISC, short form, mean = 111.69 (SD = 13.5) (group one: mean = 111.2 (SD = 13.7); group two: mean = 112.25 (SD = 13.4)) ADHD diagnosis: ADHD combined type required for inclusion ADHD medication: all participants received methylphenidate treatment Comorbidity: anxiety = 29%, depression = 6%, ODD = 50%, CD = 6% (group one: anxiety = 27%, depression = 7%, ODD = 60%, CD = 9%, group two: anxiety = 32%, depression = 5%, ODD = 39%, CD = 2%) Medications for comorbid disorders: not reported Inclusion criteria:
Exclusion criteria: refusal of group one intervention due to parental difficulties to apply leave from work Baseline characteristics: no significant differences between the two treatment groups in demographic and socioeconomic status, comorbid conditions, and additional intervention received in the first six months of the treatment. No information about between group differences in the medical treatment |
|
| Interventions | 90 children with ADHD allocated to one of two groups
Attendance: adherence in group two was defined as taking 80% of prescription without more than one month of discontinuation during school days. In group one, treatment adherence was defined as above in combination with at least 80% attendance in child and parent sessions. *The child training and the parent training were developed to be implemented concomitantly. No protocol violations to the programme were detected in either the child or the parent intervention. |
|
| Outcomes |
Secondary outcomes
Outcome assessment: post‐intervention, six and 12‐month follow‐up |
|
| Notes |
Study ID: not reported Sponsorship source: not reported Year conducted: "this study was planned in 1999" (quote) Duration of the study: 17.5 months Comments: none Lead author: Yuk‐chi So Institution: Chinese University of Hong Kong Email: not reported Address: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: table of random numbers, with block size of two |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding on this review's primary outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: type of imputation method used was unclear |
| Selective reporting (reporting bias) | Low risk | Comment: low risk |
| Vested interest bias | Unclear risk | Comment: no information on funding |
| Other sources of bias? | Low risk | Comment: no other sources of bias identified |
Van der Oord 2007.
| Methods | Design: RCT | |
| Participants |
Country: The Netherlands Setting: five different outpatient clinics Sample size: 50 children* Sample size calculation: not reported Sex: group one =1 (4.2%), group two = 4 (19.4%), in the paper it was not specified which sex these numbers referred to, but it was assumed to be female sex Age: range = eight to 12 years (group one: mean = 9.76 years (SD = 1.13), group two: mean = 9.96 years (SD = 1.31)) Ethnicity: Caucasian = 40 (89%), Caribbean = one (2%), mixed origin = four (9%) Socioeconomic status: Educational level mother (%): low:20%, medium:40%, high:36%. Educational level father (%): low:24%, medium:42%, high:24% IQ: total IQ of 75 or above required for inclusion ADHD diagnosis: subtype not reported ADHD medication: children with a history of methylphenidate treatment were excluded. All children received methylphenidate treatment as part of the study protocol. Comorbidity: (group one: ODD/CD = 10 (41.7%), group two: ODD/CD = 13 (61.9%)) Medications for comorbid disorders: not reported Inclusion criteria:
Exclusion criteria:
Baseline characteristic: one‐way ANOVAs and Chi2 analyses showed no significant differences between the two conditions in terms of baseline demographic characteristics. Furthermore, one‐way ANOVAs showed no significant group differences. The treatment groups did not differ on dose of methylphenidate either at baseline or post‐treatment. *baseline characteristics were reported and analysed for children participating in the pre‐intervention assessment, in total = 45, group one = 24, group two = 21. |
|
| Interventions | 50 children allocated to one of two groups
Attendance: attendance at 75% was set as a criterion for intervention attendance in group one. Mean attendance in the combined condition was 88.6%. Of the 50 randomised children, one declined participation in group two (the methylphenidate‐only group) and two of the children in group one (combined intervention) discontinued the intervention. Furthermore, one child was lost to post‐test and follow‐up in the methylphenidate‐only intervention, and one was omitted from analysis in the combined intervention group as the criteria of 75% attendance was not met. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: post‐intervention |
|
| Notes |
Study ID: not reported Sponsorship source: not reported Year conducted: not stated Duration of the study: 10 weeks Comments: none Lead author: Saskia van der Oord Institution: Department of Clinical Psychology, University of Amsterdam Email: s.vanderoord@uva.nl Address: Roeterstraat 15, 1018 WB Amsterdam, The Netherlands |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information reported. We requested clarification about method of allocation concealment from the study investigators but received no information on this topic at the time of preparing the original review. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information reported. We requested clarification about the method of allocation concealment form the study investigators but received no information on this topic at the time of preparing the original review. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: stated numbers of all participants lost to follow‐up. Lost to follow‐up not believed to influence results |
| Selective reporting (reporting bias) | Low risk | Comment: no |
| Vested interest bias | Unclear risk | Comment: funding source not reported |
| Other sources of bias? | Unclear risk | Comment: co‐medication not specified |
Pfiffner 2007.
| Methods | Design: RCT | |
| Participants |
Country: USA Setting: outpatient clinic Sample size calculation: not reported Sample size: 69 children Sex: 46 (67%) = boys, 23 (33%) = girls Age: range = seven to 11 years Ethnicity: white = 51%, Asian = 16%, Hispanic = 10%, Afro‐American = 6%, mixed = 17% Socioeconomic status: income range: $21,000‐$150,000. Education level measured on scale from 1 (9th grade or less) to 6 (advanced graduate or professional degree), mothers = 5.0, fathers = 4.8 IQ: IQ > 80 on WASI ADHD diagnosis: ADHD‐I required for inclusion ADHD medication: not reported Comorbidity: ODD = 23%, depression = 1%, anxiety = 12% Medications for comorbid disorders: not reported Inclusion criteria:
Exclusion criteria:
Baseline characteristics: no significant differences between groups regarding child age, sex, race, symptoms of hyperactivity/impulsivity, comorbid oppositional defiant disorder, anxiety or depression, IQ, or academic achievement. |
|
| Interventions | 69 participants allocated to one of two groups
Attendance:
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: post‐intervention and follow‐up assessment. Time of follow‐up depended on what time of the school year the intervention ended. |
|
| Notes |
Study ID: not reported Sponsorship source: supported by NIMH grant R21MH065927 Year conducted: not stated Duration of the study: 33 weeks Comments: none Lead author: Linda J. Pfiffner Institution: Department of Psychiatry, University of California, San Francisco Email: lindap@lppi.ucsf.edu Address: 401 Parnassus Avenue, Box 0984, San Francisco, CA 94143 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: random table. Information received from Pfiffner in an email 25 May 2011 (Pfiffner 2011b [pers comm]) |
| Allocation concealment (selection bias) | Low risk | Comment: sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: used imputation |
| Selective reporting (reporting bias) | Low risk | Comment: all apparent assessments made |
| Vested interest bias | High risk | Comment: had done previous studies |
| Other sources of bias? | High risk | Comment: changes in treatment protocol. Timing for follow‐up differed; same school year versus next school year (both approximately three months after treatment but summer break in between). Families and teachers were paid for each of the assessments (teachers = US$50, parents group one = US$30, group two = US$200) and teachers were paid for participating in meetings (US$50‐100). |
Waxmonsky 2010.
| Methods | Design: RCT | |
| Participants |
Country: USA Setting: outpatient Sample size calculation: not reported Sample size: 56 children Age: range = six to 12 years (group one: mean = 8.3 years (SD = 1.6), group two: mean = 8.9 years (SD = 1.5)) Sex: 45 (80%) = boys, 11 (20%) = girls (group one: 24 (82.8%) = boys, 5 (17.2%) = girls; group two: 21 (77.8%) = boys, 6 (22.2%) = girls) Ethnicity: white = 80.4%, African‐American = 10.7%, mixed = 8.9% Socioeconomic status: Nakao and Treas Socioeconomic Index (Nakao 1994): group one: mean = 61 (SD = 17), group two: mean = 53 (SD = 13) IQ: WISC‐III IQ score > 75(group one: mean = 101 (SD = 16), group two: mean = 97 (SD = 13)) ADHD diagnosis:DSM‐IV‐TR; combined type = 48 (85.7%), inattentive type = seven (12.5%), hyperactive/impulsive type = one (1.8%) (group one: combined type = 24 (82.8%), inattentive type = four (13.8%), hyperactive/impulsive type = one (3.4%), group two: combined type = 24 (88.9%), inattentive type = three (11.1%), hyperactive/impulsive type = zero (0%)) ADHD medication: stimulant naive at baseline (group one = 13 (44.8%), group two = eight (29.6%)), all patients received psychostimulant medications as part of the study Comorbidity: CD = 22 (39.3%), ODD = 24 (42.9%), no comorbidity = 10 (17.9%) (group one: CD = 11 (37.9%), ODD = 15 (51.7%), no comorbidity = three (10.3%), group two: CD = 11 (40.7%), ODD = nine (33.3%), no comorbidity = seven (25.9%)) Medications for comorbid disorders: not reported Inclusion criteria: ADHD based on DSM‐IV Exclusion criteria:
Baseline characteristics: no significant between group differences in mean doses of atomoxetine |
|
| Interventions | 56 participants allocated to one of two groups
Attendance: 62% of parents attended eight sessions, 62% attended six or more sessions. The children's attendance in the SST group was not reported; seven children (12.5%) discontinued the study (five from group one (medication + behaviour therapy) and two from group two (medication alone)). |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: post‐treatment |
|
| Notes |
Study ID:NCT00918567 Sponsorship source: investigator‐initiated trial funded entirely by Eli Lilly and Company Year conducted: conducted between 2008 and 2013 Duration of the study: 11 weeks Comments: none Author name: James G Waxmonsky, MD Institution: Center for Children and Families Email: jgw@buffalo.edu Address: 106 Diefendorf Hall, 3435 Main St., Bldg 20, Buffalo, NY 14214 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: clarification requested from one of the study investigators and Dan Waschbusch informed us in an email on 22 June 2011 that they had used a computer‐generated randomisation process (Waschbusch 2011 [pers comm]). |
| Allocation concealment (selection bias) | Low risk | Comment: clarification requested from one of the study investigators and Dan Waschbusch informed us in an email on 22 June 2011 that the clinicians did not know the treatment assignment before it was assigned (Waschbusch 2011 [pers comm]). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: We requested clarification from one of the study investigators and Dan Wascbusch informed us in an email that participants were dropped if there was not sufficient information. Scores in indexes were computed if at least 50% of the items in the index were answered; if not, they were counted as missing. Dan Wascbusch also informed us that they had essentially complete data at pre‐treatment and nearly complete data at post‐treatment. They had a lower response rate for teachers. They included whatever they had in the analyses and dropped participants when there was insufficient information, repeating this for each analysis (Waschbusch 2011 [pers comm] |
| Selective reporting (reporting bias) | High risk | Comment: protocol published in Clinicaltrials.gov after study had been conducted. Publication was not consistent with the report in Clinicaltrials.gov |
| Vested interest bias | High risk | Comment: funding from, and collaboration with, Eli‐Lilly |
| Other sources of bias? | Unclear risk | Comment: co‐medication not specified |
Tabaeian 2010.
| Methods | Design: RCT, parallel group | |
| Participants |
Country: Iran Setting: outpatient clinics Sample size calculation: not reported Sample size: 45 Sex: 100% = boys Age: not reported (based on the educational system in Iran, it should be eight to 10 years old) Ethnicity: reported as Iranian without other information Socioeconomic status: not reported IQ: not reported ADHD diagnosis: not reported ADHD medication: all participants (100%) were taking methylphenidate during the study. Comorbidity: children with comorbidity were excluded. Medication of comorbid disorders: not relevant Inclusion criteria:
Exclusion criteria: comorbidity Baseline assessment: not reported |
|
| Interventions | 45 participants allocated to one of three groups*. Number of participants in each group not reported
Attendance: not reported *the number of participants in each group was not described explicitly but it is assumed that the participants were distributed equally |
|
| Outcomes |
Primary outcomes
Secondary outcomes
*the specific assessment instrument was not possible to confirm based on the description and references in the paper and extracted Outcome assessment: post‐intervention |
|
| Notes |
Study ID: no information Sponsorship source: no funding reported Year conducted: 2010 Duration of the study: 10 weeks Comments: this extraction was based on extraction made by Ghasaleh Aali based on the Persian paper. Lead author's name: SR Tabaeian Institution: University of Isfahan Email: r.tabaeian@yahoo.com Address: |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: 2 centres randomly selected and participants randomly assigned to groups. How this randomisation was done, however, was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described. We contacted the corresponding author for more information but did not receive a reply. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not described but, based on the intervention, we judged that blinding was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: it was not clear what measure was used and if it was possible to blind the assessors. It seemed likely that it was a questionnaire, either for the child or the parents, and that it was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: none reported. There were no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol available. Based on the translation, it was difficult to identify the measure used and judge the appropriateness of the reported outcomes. |
| Vested interest bias | Unclear risk | Comment: funding source not reported |
| Other sources of bias? | Low risk | Comment: none reported |
Storebø 2012.
| Methods | Design: RCT, parallel group | |
| Participants |
Country: Denmark Setting: clinical Sample size calculation: a sample size of 26 children in each group was needed based on a sample size calculation of 80% power in detecting a clinical relevant change of four points on the primary outcome measure of hyperactivity and impulsivity. Sample size: 56 children* Sex: 39 (71%) = boys, 16 (29%) = girls (group one: 19 (67.8%) = boys, nine (32.2%) = girls; group two: 20 (74%) = boys, seven (26%) = girls) Age: (group one: mean = 10.6 years (SD = 1.29); group two: mean = 10.2 years (SD = 1.34)) Ethnicity: Danish 100% Socioeconomic status: not reported IQ: both verbal and nonverbal IQ > 80 (group one: WISC verbal mean = 93.9 (SD = 15.7), group two: WISC non‐verbal mean = 94.8 (SD = 19.0). ADHD diagnosis: group one: inattentive = 10 (35.7%), hyperactive‐impulsive = 0 (0%), combined = 16 (57.2%), not otherwise specified = 2 (7.1%); group two: inattentive = 6 (22.2%), hyperactive‐impulsive = 2 (7.4%), combined = 16 (59.2%), not otherwise specified = 3 (11.1%) ADHD medication: after assessment and confirmation, the family was offered medical treatment for the child following a medication protocol. The children had never previously received medical treatment for ADHD. Comorbidity: (group one: ODD = four (33.3%), anxiety disorder = four (33.3%), depressive disorder = one (8.3%), tics and OCD = zero (0%), enuresis = two (20%), stuttering = one (5%); group two: ODD = four (40%), anxiety disorder = two (20%), depressive disorder = one (10%), tics and OCD = 1 (10%), enuresis = two (20%), stuttering = zero (0%)) Medication of comorbid disorders: not reported Inclusion criteria:
Exclusion criteria:
*baseline characteristics were reported for 55 children as data from the child that withdrew from group two was not allowed to be analysed. Baseline characteristics: no significant difference in baseline demographics between the 2 groups |
|
| Interventions | 56 participants were allocated to one of two groups
Attendance: one participant in each group did not receive the allocated intervention and one participant in group two was lost to follow‐up. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: post‐intervention and follow‐up at three and six months after end of intervention |
|
| Notes |
Study ID:NCT00937469 Sponsorship source: the SOSTRA study was financially supported by Region’s Zealand University Hospital (RESUS), Region Zealand Research Foundation, and Psychiatric Research Unit, Region Zealand. Funding was also received from the Fru C. Hermansens Foundation, Slagtermester Max Wørzner and Inger Wøzners Foundation, and TrygFonden. Year conducted: 2012 Duration of the study: 8 weeks Comments: the study obtained approval from the Regional Ethics Committee of Zealand (SJ‐85), was registered at the Danish Data Protection Agency DO50892, and registered at clinical trials.gov/NCT00937469. Lead author's name: Ole Jakob Storebø Institution: Child Psychiatric Clinic, Child and Adolescent Psychiatric Department, Region Zealand, Denmark Email: ojst@regionsjaelland.dk Address: Birkevænget 3, 4300 Holbæk Denmark |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: adequate method was used. Randomisation was conducted using computer‐generated, permuted randomisation sequences in blocks of four with an allocation ratio of 1:1 stratified for sex and comorbidity. |
| Allocation concealment (selection bias) | Low risk | Comment: adequate method was used. All data that could be used to identify the allocation before data entry was hidden and block size was unknown to the investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was not possible to ‘blind’ participants, parents, treating physicians, or personnel in the Child Psychiatric Clinic in Holbaek. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the involved parties were instructed not to inform the teachers, who rated the primary and secondary outcome measures, of the intervention allocated. The outcome assessors were thus kept unaware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: one child from each group dropped out after the randomisation. Outcome assessment was still obtained from the child allocated to group two. Another child from group two was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: matched study protocol. All outcome measures outlined in protocol were reported. |
| Vested interest bias | Low risk | Comment: no apparent source of bias. The funders of the study did not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. |
| Other sources of bias? | Unclear risk | Comment: no other apparent sources of bias |
Pfiffner 2014.
| Methods | Design: RCT, parallel group | |
| Participants |
Country: USA Setting: outpatient Sample size calculation: not reported Sample size: 125 children in the groups included in the analysis (199 children in the full study) Sex: 58% = boys (group one: 51.4% = boys; group two: 58.8% = boys) Age: mean = 8.6 years (range = seven to 11) (group one: mean = 8.8 years (SD = 1.15); group two: mean = 8.7 group two: mean = 8.4 years (SD = 1.13)) Ethnicity: Caucasian = 54%, Latino = 17%, Asian‐American = 8%, African‐American = 5%, self‐identified as mixed race = 17% (group one: 55.4% Caucasian, 12.2% Latino, 9.5% Asian‐American, 5.4% African‐American, 17.6% self‐identified as mixed race; group two: Caucasian (43.1%), Latino (25.5%), Asian‐American (3.9%), African‐American (3.9%), self‐identified as mixed race (23.5%)) Socioeconomic status: total household income: below US $50,000 = 14.1%, $50,000‐100,000 = 28.3%, $100,000‐150,000 = 28.8%, and more than $150,000 = 28.8% of families. 81.2% of the primary parents reported having graduated from college, 13% of participants were living in single‐parent families (group one: parent education = 83.6% college graduates, single‐parent household = 9.5%; group two: parent education = 78.4% college grads, single‐parent household = 11.8%) IQ: group one: mean = 103.6 (SD = 11.0) on WISC FSIQ; group two: mean = 105.6 (SD = 11.6) on WISC FSIQ ADHD diagnosis: only inattentive subtype ADHD medication: stimulant medication = 4.5% (group one = 9.5%, group two = 2.0%). The small number of children taking stimulant medication (not otherwise specified) completed a one‐week wash‐out to assess behaviour and obtain ratings off‐medication. Comorbidity: group one: anxiety = 6.8%, depression = 1.7%, ODD = 5.1%; group two: anxiety = 5.3%, depression = 2.6%, ODD = 5.3% Medications for comorbid disorders: not reported Inclusion criteria:
Exclusion criteria:
Baseline characteristics: only medication status at randomisation differed across treatment groups (P = 0.04), with significantly more CLAS (Child Life and Attention Skills) children reporting medication use (9.5%) than PFT children (1.4%), but not compared to TAU children (2.0%). |
|
| Interventions | 199 participants allocated to one of three groups. Only group one and group three were included in the analysis.
Attendance:
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: post‐intervention assessment and follow‐up assessment five to seven months after end of intervention |
|
| Notes |
Study ID: not reported Sponsorship source: noncommercial. This research was supported by a grant from the National Institute of Mental Health MH077671. Year conducted: 2014 Duration of the study: 4 years (2009‐2012) Comments: none Lead author's name: Linda J Pfiffner Institution: Department of Psychiatry, University of California, San Francisco Email: linda.pfiffner@ucsf.edu Address: Department of Psychiatry, 401 Parnassus Ave., Box 0984, University of California, San Francisco, San Francisco, CA 94143 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: unclear how randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: number of missing item values were reported as 0.8% at baseline, 3.3% at post‐intervention assessment, and 10.6% at follow‐up with most of the missing values at follow‐up being related to attrition. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol, outcomes not prespecified |
| Vested interest bias | Unclear risk | Comment: study authors had conducted previous studies addressing same intervention. |
| Other sources of bias? | High risk | Comment: families were compensated for completion of post‐intervention (group one and two US$50, group three US$150) and follow‐up assessments (group one and two: US$100, group three: US$150); teachers were compensated at each time point (baseline assessment: US$50, post‐intervention and follow‐up: US$75). Further, the teachers received a total of US$100 for participating in the teacher consultation meetings. |
Azad 2014.
| Methods | Design: RCT, parallel group | |
| Participants |
Country: Iran Setting: psychology and psychiatry clinic Sample size calculation: not reported Sample size: 30 children Sex: not reported Age: not reported; primary school students Ethnicity: not reported Socioeconomic status: not reported IQ: not reported ADHD diagnosis: subtypes not reported ADHD medication: not reported Comorbidity: not reported Medication for comorbid disorders: not reported Inclusion criteria: not reported Exclusion criteria: not reported Baseline characteristics: not reported |
|
| Interventions | 30 participants allocated to one of two groups
Attendance: not reported |
|
| Outcomes |
Secondary outcomes
Outcome assessment: end of treatment and three month follow‐up |
|
| Notes |
Study ID: not reported Sponsorship source: not reported Year conducted: "This study enrolled the Isfahan primary school students afflicted with ADHD, during the educational years 2012‐2013." (quote) Duration of the study: 5 weeks (15 sessions) Comments: ethics approval not reported Lead author's name: Azad Moslem Asli Institution: Islamic Azad University, Science and Research Branch Esfahan, Esfaran, Iran Email: Azzad2020@gmail.com Address: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: participants were described to be randomly assigned to the groups but no further details were reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details reported and not possible to retrieve further information from the study authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not reported, but probably not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no details reported and it was not possible to retrieve further information from the study authors as the study authors did not respond to our email requests. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: none known. No registered protocol available |
| Vested interest bias | Unclear risk | Comment: no other apparent biases |
| Other sources of bias? | Low risk | Comment: no other apparent biases |
Meftagh 2014a.
| Methods | Design: RCT, parallel group | |
| Participants |
Country: Iran Setting: 15 male elementary schools from 4 educational areas in Shiraz Sample size calculation: not reported Sample size: 51 Sex: not reported Age: mean = 8.98 years (SD = 0.77) Ethnicity: not reported Socioeconomic status: not reported IQ: not reported ADHD diagnosis: subtypes not reported ADHD medication: not reported Comorbidity: not reported Medications for comorbid disorders: not reported Inclusion criteria: ADHD diagnosis in clinical interview with children who had been identified with ADHD based on both teacher and parent rating on Child Symptom Inventory Exclusion criteria: not reported Baseline characteristics: no significant differences among study groups regarding age, family, and education |
|
| Interventions | 51 patients allocated to one of three groups. Only group two and three were included in the analysis.
Attendance: 15 children in each group completed the study. Attendance rate was not reported. |
|
| Outcomes |
Secondary outcomes
Outcome assessment: post‐intervention and longest follow‐up two months after end of intervention. Notes: data corresponded to change from pre‐test to post‐test |
|
| Notes |
Study ID: not reported Sponsorship source: Research Committee of Shiraz University Year conducted: 2014 Duration of the study: 10 weeks intervention plus 4 months follow‐up Comments: none Lead author's name: Sayyed Davood Meftagh Institution: Department of Psychology, Payame Noor University, Iran Email: a_najimi@hlth.mui.ac.ir Address: Department of Health Education Health Promotion , School of Health Isfahan University of Medical Sciences, Isfahan, Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no details on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no description of blinding, probably not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no description of blinding, probably not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no flow chart and no explanation as to why some children did not complete the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Vested interest bias | Low risk | Comment: the study authors declared no conflict of interest, no other sources of bias identified |
| Other sources of bias? | Low risk | Comment: no other apparent sources of bias |
Choi 2015.
| Methods | Design: RCT, parallel group | |
| Participants |
Country: Korea Setting: outpatient Sample size calculation: not reported Sample size: 80 children Sex: 32 (44%) = boys, 40 (56%) = girls Age: mean = 11.2 years (SD = 0.93, range = 9‐13) Ethnicity: not reported Socioeconomic status: not reported IQ: all IQ > 90 ADHD diagnosis: subtypes not reported ADHD medication: all participants were under medication at the time of intervention, type of medication not reported Comorbidity: not reported Medications for comorbid disorders: not reported Inclusion criteria
Exclusion criteria: not reported Baseline characteristics: no significant differences on study background variables or pretest measures |
|
| Interventions | 80 participants allocated to one of three groups
Attendance: at least 12 of 16 sessions of either EMT or SST. Mean number of sessions attended by the 75 programme completers was 14.9 (SD = 1.3), with an overall attendance rate of 90.5%. No group differences in the number of sessions attended |
|
| Outcomes |
Primary outcomes
Outcome assessment: post‐intervention, one week after end of intervention |
|
| Notes |
Study ID: not reported Sponsorship source: the author(s) received no financial support for the research, authorship, and/or publication of this article. Year conducted: 2015 Duration of the study: 16 weeks Comments: ethics approval. The study was reviewed and approved by the Research Ethics Commitee of the university at which the experiment was conducted. Lead author's name: Eun Sil Choi Woo Kyeong Lee Institution: Kyungil University, Korea and Seoul Cyber University, Korea Email: wisemind96@iscu.ac.kr Address: 193‐15, Miadong Kangbuk‐gu, Seoul, 142‐700, Korea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: from descriptions, it was unclear if all children had been included before randomisation. However, the sentence describing how WL children after 16 weeks were pooled with newly selected children indicated that randomisation was made progressively. It was unclear who did the sampling in blocks, how these blocks were generated, and if this process was to be considered random. |
| Allocation concealment (selection bias) | Unclear risk | Comment: it was unclear exactly how allocation concealment was done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: parents, children and trainers were aware of group's status. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the PICO measures of emotion expression and peer relational skills are both self‐report questionnaires. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: two children in the EMT group and three children in the SST group did not complete the study. The numbers of non‐completion were small and balanced. However, while it was stated that the reason for non‐completion was dropout during treatment, the reason for this dropout was not specified. |
| Selective reporting (reporting bias) | Unclear risk | Comment: as we did not locate a study registration, it was unclear whether all planned measures had been reported accordingly. However, the measures presented in the paper were all described in the results section. |
| Vested interest bias | High risk | Comment: it was not specified if the therapist delivering the interventions was also one of the authors. The first author is the author of the manual used in one of the intervention arms. There may have been a bias given the first author's investment in the first study arm programme. |
| Other sources of bias? | High risk | Comment: no information on comorbid disorders. It was mentioned that all participants were under medication at the time of the intervention but it was not clear if this referred to ADHD medication or medication for comorbid disorders. It was not specified whether, for example, autism would be a reason for exclusion. |
Bul 2016.
| Methods | Design: RCT, cross‐over | |
| Participants |
Country: Netherlands, Belgium Setting: outpatient Sample size calculation: 78 participants per group required to detect differences of a medium effect size Sample size: 170 children Sex : 137 (80.6%) = boys, 33 (19.4%) = girls (group one: 70 (79.5%) = boys, 18 (20.5%) = girls, group two: 67 (81.7%) = boys, 15 (18.3%) = girls) Age: mean = 9.85 years (SD = 1.26, range = 8‐12) (group one: mean = 9.89 years (SD = 1.28), group two: mean = 9.82 years (SD = 1.24)) Ethnicity: not reported Socioeconomic status: not reported IQ: mean = 106.18 (SD = 14.79) (group one: mean = 105.40 (SD = 14.46), group two: mean = 107.02 (SD = 15.18)) ADHD diagnosis: subtypes not reported ADHD medication: n = 156 (91.8%) (group one: n = 80 (90.9%), group two: n = 76 (92.7%)) Comorbidity: ODD = 170 (149 (87.6%) = clinical, 21 (12.4%) = subclinical)) (group one: ODD = 88 (74 (84.1%) = clinical, 14 (15.9%) = subclinical), group two: ODD = 82 (75 (91.5%) = clinical, 7 (8.5%) = subclinical)) Medications for comorbid disorders: not reported Inclusion criteria:
Exclusion criteria:
Baseline characteristics: there was no significant difference in baseline demographics between the two groups. |
|
| Interventions | Participants allocated to one of two groups and then crossed over
Attendance: In group one, 77 % attended 20 weeks treatment. In group two, 89% attended 20 weeks treatment. |
|
| Outcomes |
Primary outcomes
Outcome assessment: end of treatment |
|
| Notes |
Study ID:ISRCTN62056259 Sponsorship source: Johnson Johnson was the funding source for game development and consultancy with regard to the design of the study. Flanders’ Care provided funding to perform the study (DEM2012‐02‐07) at the University Hospital Gasthuisberg (Belgium). Year conducted: conducted from January to March 2013 Duration of the study: 20 weeks Comments: none Lead author's name: Kim CM Bul Institution: Department of Psychiatry and Psychology, Maastricht University Email: k.bul@yulius.nl Address: Yulius Academy, Yulius Mental Health Care Organization, Dennenhout 1, Barendrecht, 2994 GC, Netherlands |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: 1:1 ratio and based on a prespecified computer‐generated randomisation list. Allocation was stratified by study site and gender, and arranged in permutated blocks. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Group assignment performed online using the next available number on the randomisation list, which corresponded to the site and gender of the participant. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not possible to blind participants to their treatment allocation. After screening and baseline assessment, parents received an email with notification of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: parent and child self‐report. Detection bias due to child and parent knowledge of received intervention. Full blinding of researchers and teachers not guaranteed as participants could spontaneously talk about the game during the assessment or the study time. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: used intention‐to‐treat analyses and included all randomised participants. Used linear trend at point as an imputation method. |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcome measures reported. Only the satisfaction questionnaire was not mentioned in the protocol. |
| Vested interest bias | High risk | Comment: funders were Janssen‐Cilag (Netherlands) and Flanders Care (Netherlands). Funders may have had an interest as they are in the medical industry. It was not clearly stated if there was a commercial aspect to the computer game. Additionally, two of the authors were employees of Janssen Pharmaceuticals; statistical study experts Luc Janssens (MSc), who works for the Research and Development Department of Janssen Pharmaceuticals in Beerse (Belgium), and Franky de Cooman (MSc), who works for Art Deco. The study was conducted in collaboration with the following partners: Parent Association Centre ZitStil (Belgium), Focuz Treatment Centre for Children and Youth (Rotterdam), Mental Health Care Organization Mondriaan (Heerlen), and the University Hospital Gasthuisberg (Belgium). Johnson & Johnson was the funding source for game development and consultancy with regard to the design of the study. Flanders’ Care provided funding to perform the study (DEM2012‐02‐07) at the University Hospital Gasthuisberg (Belgium). |
| Other sources of bias? | Low risk | Comment: no other sources of bias identified |
Waxmonsky 2016.
| Methods | Design: RCT, parallel group | |
| Participants |
Country: USA Setting: outpatient Sample size calculation: not reported Sample size: 68 children* Sex: 39 = boys, 17 = girls (group one: 20/31 (65%) = boys; group two: 19/25 (76%) = boys) Age: (group one: mean = 9.3 years (SD = 1.6), mean = 9.4 years (SD = 1.5)) Ethnicity: % defined as racial/ethnic minority: group one: = 12 (39%); group two = 9 (36%) Socioeconomic status: group one: mean = 42.3 (SD = 15.2) on Socioeconomic Index**, group two: mean = 42.03 (SD = 12.8) both on Nakao and Treas Socioeconomic Index (Nakao 1994) IQ: group one: mean = 100.6 (SD = 15.4); group two: mean = 100.7 (SD = 10.6) ADHD diagnosis: not reported ADHD medication: prior to therapy, phase psychostimulant doses were optimised for all participants. Thus, all participants received pharmacological treatment (group one: mean entry stimulant dose = 0.90 mg/kg/day (SD = 0.40); group two: mean entry stimulant dose = 0.90 mg/kg/day (SD = 0.43)) both in methylphenidate equivalents on a mg/kg/day. Comorbidity: (group one: CD = 4 (13%), ODD = 29 (94%), anxiety/subthreshold anxiety = 9 (29%); group two: CD = 1 (4%), ODD = 24 (96%), anxiety/subthreshold anxiety = 11 (44%)) Medications for comorbid disorders: balanced between groups Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 68 participants allocated to one of two groups
Attendance: % of attendance was required. Completers were participants attending at least six of the 11 sessions (n = 29). Mean attendance = 9.7 out of 11 group sessions. All but two participants attended at least half of the group or make‐up sessions. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: assessment at following points (in weeks after baseline): mid‐intervention (6 weeks), post‐intervention (11 weeks) and group one at follow‐up assessment (17 weeks) |
|
| Notes |
Study ID:NCT00632619 Sponsorship source: National Institute of Mental Health (MH080791; Principal Investigator: Waxmonsky) Year conducted: 2016 Duration of the study: 11 sessions Comments: the study was approved by governing institutional review boards (IRBs) at both sites. Lead author's name: James G. Waxmonsky Institution: Pennsylvania State University, Department of Psychiatry Email: jwaxmonsky@hmc.psu.edu Address: Pennsylvania State University, Psychiatry, 500 University Dr. Dept of Psychiatry H073, Hershey Medical Center, Hershey, PA 17033 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomised using a computer‐generated permutated blocking procedure. Randomisation occurred before the medication phase so parents would be aware of therapy status prior to making decisions about medication. |
| Allocation concealment (selection bias) | Low risk | Comment: assumed that the use of a computer blocking procedure would conceal the allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the PICO measures included were rated by parents or teachers. Clinician‐rated assessments were completed by staff masked to therapy status, though this was not relevant for these measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3 dropped out of the CC group due to assignment. All dropout was well described and except the dropout due to assignment, the reasons did not indicate a bias. |
| Selective reporting (reporting bias) | Low risk | Comment: all primary and secondary outcome measures stated in protocol were reported in paper. SSRS was also reported in the paper but this measure was not described in the study registration. |
| Vested interest bias | High risk | Comment: potential vested interest due to relationships with pharmaceutical companies |
| Other sources of bias? | High risk | Comment: potential bias in the data collected. Selection of informants prone to monetary incentive. The referral may have increased the service received in the CC group during the intervention period and thus may have provided a potential bias. At study registration, the authors also mentioned as a limitation that the study therapy group had more contact with study staff, which may have impacted results. Additionally, the first author seemed to be involved in the development of the treatment programme used in the study and thus there might be bias of interests. |
Wilkes Gillan 2016.
| Methods | Design: RCT, cross‐over | |
| Participants |
Country: Australia Setting: outpatient Sample size calculation: not reported Sample size: 31 children Sex: 25 (86%) = boys, four (14%) = girls (group one: 13 (87%) = boys, 2 (13%) = girls; group two: 12 (86%) = boys, 2 (14%) = girls) Age: group one: mean = 8.2 years (SD = 1.5); group two: mean = 8.5 years (SD = 1.7) Ethnicity: 26/29 born in Australia Socioeconomic status: (group one: parent with degree or diploma = 93%, occupation (requiring tertiary qualifications) = 60%; group two: parent with degree or diploma = 87%, occupation (requiring tertiary qualifications) = 57%) IQ: not reported ADHD diagnosis: (group one: predominantly inattentive = 5/15, predominantly hyperactive/impulsive = 1/15, combined subtype = 9/15; group two: predominantly inattentive = 6/14, predominantly hyperactive/impulsive = 0/14, combined subtype = 8/14)) ADHD medication: 20 of 29 (69%), specific type of medication not reported (group one = 60% , group two = 76%) Comorbidity: subscale T‐scores on Connors Comprehensive Behaviour Rating scale, clinical cut‐off T‐score > 70 (group one: oppositional behaviour, mean = 75 (SD = 13.4), generalised anxiety disorder, mean = 71 (SD = 11.5); group two: oppositional behaviour, mean = 76 (SD = 13.0), generalised anxiety disorder, mean = 73 (SD = 12.9)) Medication for comorbid disorders: not reported Inclusion criteria:
Comorbid difficulties accepted (i.e. language difficulties, conduct disorder) and current medication was permitted but asked to be maintained in the study period. The therapist monitored the consistency of the medication use throughout the study period. Exclusion criteria: diagnosed with other major developmental disorders (i.e. intellectual disability, autism spectrum disorder) Baseline characteristics: no significant difference in baseline demographics between the two groups: *baseline characteristics in this table is based on analysis of 29 children followed up in the outcome assessment (group one = 15; group two = 14)". |
|
| Interventions | 31 participants allocated to one of two groups and then crossed over
Attendance: 98.3% (range 88‐100%), no specific % was required, one child in group one never started the intervention, and one child in group two did not finish the wait‐time. |
|
| Outcomes |
Primary outcomes
Outcome assessment: post‐intervention |
|
| Notes |
Study ID:ACTRN12614000973617 Sponsorship source: Rotary Club of Mosman and the University of Sydney’s postgraduate research support scheme Year conducted: 2016 Duration of the study: 10‐week intervention Comments: none Lead author's name: Sarah Wilkes‐Gillan Institution: School of Allied Health, Australian Catholic University, North Sydney, NSW Email: Sarah.WilkesGillan@acu.edu.au Address: North Sydney, NSW, Australia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: simple randomisation used to assign one of each two children who entered to each group (1:1 ratio). Once two parents had booked a baseline assessment, a sealed envelope from each group and the times of the baseline assessments were taken to an academic staff member not involved in the research. The person shuffled the envelopes and used a coin toss to pick one of the two, writing it on the sealed envelopes. The researcher left the room while the academic staff member completed the procedure. |
| Allocation concealment (selection bias) | Low risk | Comment: assessors were blinded to treatment allocation for all participants. While the researchers knew that children in the same family would receive the same allocation, treatment allocation was not revealed to them. The blinded raters were not aware of any familial relationships. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel were blinded at baseline assessment, but participants were then informed of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: assessors were blind at baseline and outcome assessment. Blinded raters were used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: reasons for dropout (death in family and change in sport schedule) unrelated to true outcome |
| Selective reporting (reporting bias) | Unclear risk | Comment: retrospectively registered; protocol submitted 2 September 2014 and approved 10 September 2014. Participants enrolled 1 June 2013 to 7 June 2014 and data collection was finished in October 2014. More measures described in protocol but not reported in paper (potentially published in other paper but we have not been able to identify other papers related to this study during the review process) |
| Vested interest bias | Low risk | Comment: funding organisation not found to have vested interests. A manual and DVD material were described which could indicate a potential commercial interest, however, we were unable to locate information on the material online. |
| Other sources of bias? | Low risk | Comment: no other sources of bias identified |
Schramm 2016.
| Methods | Design: RCT, parallel group | |
| Participants |
Country: Germany Setting: outpatient Sample size calculation: not reported Sample size: 113 children Sex: 85% = boys (group one: 34 (85%) = boys; group two: 32 (86.5%= = boys)) Age: mean = 13.99 years (SD = 1.43) (group one: mean = 14.10 years (SD = 1.42; group two: mean = 13.83 years (SD = 1.28)) Ethnicity: not reported Socioeconomic status: not reported IQ: not reported ADHD diagnosis: not reported ADHD medication: number of children not receiving medication, type not specified = 50% (group one = 16 (40%); group two = 17 (47%) Comorbidity: not reported Medications for comorbid disorders: not reported Inclusion criteria:
Exclusion criteria: meeting criteria for severe comorbid disorders (e.g. psychotic episode) Baseline characteristics: no significant difference found in baseline characteristics |
|
| Interventions | 113 participants allocated to one of three groups. Only group one and group two were included in the review analysis.
Attendance: rate not reported. Four dropped out (group one = two (one = familial difficulties, one = discontinued intervention), group two = two (no longer interested in participating)) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: post‐intervention assessment |
|
| Notes |
Study ID: not reported Sponsorship source: not reported Year conducted: data were collected during the years of 2009‐2012 Duration of the study: 6 months Comments: approved by the Research Board of the Department of Special Education and Rehabilitation, University of Oldenburg, Germany Lead author: Satyam Antonio Schramm Institution: University of Potsdam Email: satyam.schramm@ifs.uni‐hannover.de Address: University of Potsdam, Campus Golm, House 31, Space 2:12, Karl‐Liebknecht‐Strasse 24‐25, 14476 Potsdam, Germany |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: need more information. Trickle processing approach described as often being associated with corruption of assignment |
| Allocation concealment (selection bias) | Unclear risk | Comment: need more information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: trainers and children must have been aware of status, as training, active control or waiting list were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: parent and children were aware of group assignment. Teachers not described as effectively blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: results for all measures noted in methods section reported |
| Selective reporting (reporting bias) | Low risk | Comment: small (n = 2) loss to follow‐up in waiting‐list control group due to participants not wanting to wait anymore. One dropped out of the intervention group due to familial difficulties and one dropped out of the active control group due to an accident.. |
| Vested interest bias | High risk | Comment: last study author was also the author of the published manual of the programme being investigated. |
| Other sources of bias? | Low risk | Comment: moderate level of missing data (6.4%), mostly due to incomplete teacher report (17.6%). Missing items were assumed to be missing at random and were imputed groupwise through expectation maximization algorithm. |
Evans 2016.
| Methods | Design: RCT, parallel group | |
| Participants |
Country: USA Setting: outpatient Sample size calculation: not reported Sample size: 326 children (group one: 112, group two: 110, control :104) Sex: 232 (71%) = boys, 94 (29%) = girls (group one: 79 (71%) = boys, 33 ( 29%) = girls, group two: 76 (69%) = boys, 34 (31%) = girls, group three: 77 (74%), 27 (26%) = girls) Age: mean = 12.1 years (SD: 0.9‐1.0) (group one: mean = 12.1 years (SD = 0.9), group two: mean = 12.1 years (SD = 0.9), group three: mean = 12.1 years (SD = 1.0)) Ethnicity: group one: 7.1% African‐American, 74.1% white, 14.3% biracial, 4.5% other, 2.7% Hispanic, group two: 14.5% African‐American, 78.2% white, 5.5% biracial, 1.8% other, 5.5% Hispanic, group three: 14.4% African‐American, 79.8% white, 4.8% biracial, 1% other, 1% Hispanic Socioeconomic status: (group one: mean 56.500, SD 45.200 in thousand $, group two: mean 61.500, SD 52.400 in thousand $, group three: mean 63.500, SD 55.500 in thousand $) IQ: means: group one: mean = 100.3 (SD = 14.2), group two: mean = 99.2 (SD = 13.1), group three: mean = 101.4 (SD = 13.7)) ADHD diagnosis: children with combined subtype: group one = 55 (49.1%), group two = 55 (50%), group three = 49 (47.1%) ADHD medication: group one: 49 (43.8%), group two: 57 (51.8%), group three 47 (45.4%) Comorbidity: ODD or CD: 55.%, anxiety disorders: 27%, depressive disorders: 13% (group one: anxiety disorder = 19.6%, depression = 8%, mean ODD symptoms = 4.5 (SD = 2.3), mean CD symptoms = 2.1 (SD = 1.9), group two: anxiety disorder = 21.1%, depression = 10.9%, mean ODD symptoms = 4.7 (SD = 2.3), mean CD symptoms = 1.9 (SD = 1.3), group three: anxiety disorder = 17.3%, depression = 7.7%, mean ODD symptoms = 4.4 (SD = 2.2), mean CD symptoms = 1.7 (SD = 1.4) Medication for comorbid disorders: group one: 49 (43.8%), group two: 57 (51.8%), group three: 47 (45.4%) Inclusion criteria:
Exclusion criteria:
Baseline characteristics: there were no statistically significant differences between groups on any demographic variables. |
|
| Interventions | 326 participants allocated to one of three groups
Attendance:
|
|
| Outcomes |
Primary outcomes
Seconardy outcomes
Outcome assessment: post‐intervention and follow‐up six months after end of intervention |
|
| Notes |
Study ID: not identified Sponsorship source: National Insitute of Mental Health (NIMH); R01MH082864, R01MH082865 Year conducted: 2016 Duration of the study: 1 academic year plus 6 months follow‐up Comments: none Lead author's name: Evans SW Institution: Ohio University Email: evanss3@ohio.edu Address: Department of Psychology, Ohio University, Athens, OH45701 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: comment from author (Evans 2017 [pers comm]): "Randomization was conducted after the recruitment of each of the three cohorts. Our statistician (who was not involved in recruitment) generated a string of random numbers that led to the assignment of participants to condition. The statistician sent the PIs at each site the condition allocation for our sites". |
| Allocation concealment (selection bias) | Low risk | Comment: comment from author (Evans 2017 [pers comm]): "The statistician sent the PIs at each site the condition allocation for our sites". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: based on the type of intervention, it was not possible for participants and personnel to be blinded to group status. However, according to the study authors, parents had similar expectations for improvement in both active treatment conditions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the outcomes relevant for this review were parent and teacher ratings. The study author commented (Evans 2017 [pers comm]): "Teachers were minimally aware of condition as they completed assessments, but were not actively involved in any other activities for participants in any condition. Nevertheless, had they wanted to know the services a child was or was not receiving, the information was available to them. Thus, as most, if not all teachers remained unaware, they were not “blinded” to treatment condition". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: used intention‐to‐treat. In total, 12 children were included in the study but were not included in the outcome assessment. Eight children did not start the intervention (seven from group one and one from group two) and four children withdrew from the study (one from group one and three from group three). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study registration found, so it was not possible to judge if all outcome data were reported. Grade point average data were collected, however mean and SD were not reported. |
| Vested interest bias | Low risk | Quote: the research was supported by grants from the National Institute of Mental Health (NIMH; R01MH082864, R01MH082865), no sources of bias identified. |
| Other sources of bias? | Low risk | Comment: no other sources of bias identified |
Pfiffner 2016.
| Methods | Design: Cluster‐RCT, parallel group | |
| Participants |
Country: USA Setting: outpatient Sample size calculation: sample size was based on medium to large effect sizes previously found by the authors. For the sample in the study, the estimated detectable effect size was 0.48. Sample size: 135 children Sex: (group one: 54 (75 %) = boys, 18 (25%) = girls; group two: 42 (67%) = boys, 21 (33%) = girls) Age: (group one: mean = 8.3 years (SD = 1.1); group two: mean = 8.5 years (SD = 1.1)) Ethnicity: (group one: white = 31%, African‐American = 8%, Asian = 22%, Hispanic/Latino = 21%, multiracial/multiethnic = 18%; group two: white = 22%, African‐American = 10%, Asian = 19%, Hispanic/Latino = 27%, multiracial/multiethnic = 22%) Socioeconomic status: % college graduates (group one: 65%; group two 55%) IQ: on WASI FSIQ (group one: mean = 103.0 (SD = 13.0); group two: mean = 101.0 (SD = 14.7)) ADHD diagnosis: group one: combined = 54%, inattentive = 40%, hyperactive‐impulsive = 6%; group two: combined = 62%, inattentive = 38%, hyperactive‐impulsive = none ADHD medication: group one: 9.7%, group two: 7.9% Comorbidity: group one: ODD = 43%, group two: ODD = 59% Medication for comorbid disorders: (balanced between groups) Inclusion criteria:
Exclusion criteria: students with significant visual or hearing impairments,severe language delay, psychosis, or pervasive developmental disorder or who were in full‐day special day classrooms Baseline characteristics: groups did not differ on demographics or medication use at baseline. |
|
| Interventions | 135 participants allocated to one of two groups
Attendance:
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: post‐intervention assessment |
|
| Notes |
Study ID:NCT01686724 Sponsorship source: supported by a grant from the Institute of Education Sciences, US Department of Education to the University of California‐San Francisco (award number R324A120358) Year conducted: 2016 Duration of the study: 12‐week programme Comments: none Lead author's name: Linda J Pfiffner Institution: Department of Psychiatry, University of California San Francisco Email: linda.pfiffner@ucsf.edu Address: Department of Psychiatry, University of California San Francisco, 401 Parnassus Avenue, Box 0984, San Francisco, CA 94143 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: unclear how the randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not mentioned, but it did not seem possible to blind participants and personnel in the study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: outcome measures were based on teacher and parent ratings and the authors commented that this might lead to rater bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not clear |
| Selective reporting (reporting bias) | High risk | Comment: protocol available. The primary and secondary outcomes, including the time frame reported in the study, did not match the protocol. |
| Vested interest bias | Unclear risk | Comment: the study was supported by a grant from the Institute of Education Sciences. Authors had conducted previous studies on the same intervention. |
| Other sources of bias? | High risk | Comment: at each assessment point, parents and teachers were paid US$50 for completing the measurements. |
Qian 2017.
| Methods | Design: RCT, parallel group | |
| Participants |
Country: China Setting: clinical Sample size calculation: not reported Sample size: 86 children Sex: 54 = boys, 14 = girls (group one: 32 (84.2%) = boys, 6 (15.8%) = girls; group two: 22 (73.3%) = boys, 8 (26.7%) = girls) Age: 6‐12 years (group one: mean = 8.3 years (SD = 1.3); group two: mean = 7.8 years (SD = 1.2)) Ethnicity: not reported Socioeconomic status: not reported IQ: group one: mean = 105.7 (SD = 13.9); group two: mean = 101.8 (SD = 10.4) ADHD diagnosis: group one: inattentive = 17 (44.7%), hyperactivity‐impulsivity = 0 (0%), combined = 21 (55.33%); group two: inattentive = 16 (53.3%), hyperactivity‐impulsivity = 1 (3.3%), combined = 13 (43.3%) ADHD medication: group one: 10 participants had maintained steady dosage of medications for more than a half year and remained unchanged during the entire study. New medications could not be initiated during the study. Comorbidity: group one: ODD = 7 (18.4%), learning disorder = 8 (21.1%), special phobia = 5 (13.2%); group two: ODD = 7 (23.3%), learning disorder = 4 (13.3%), special phobia = 2 (6.7%) Medications for comorbid disorders: not reported Inclusion criteria:
Exclusion criteria:
Baseline characteristics: no significant difference found in reported baseline characteristics |
|
| Interventions | 86 participants allocated to one of two groups
*Analysis, including baseline characteristics, was based on the following sample sizes: intervention = 38 participants, waiting‐list control = 30 participants Attendance: 86.4% (38/44) of children in intervention group complied with the training, completing 10 or more sessions in the 12‐session period. All missed group sessions were administered to the trainee individually. The percentage of the number of sessions administered individually was not specified. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: post‐intervention assessment |
|
| Notes |
Study ID:NCT02327585 Sponsorship source: the study was supported by grants from the Beijing Municipal Science and Technology Commission (No. Z151100004015103), the Major State Basic Research Development Program of China (973 Program, No. 2014CB846100), National Key Research Plan of Ministry of Science and Technology of China (No. 2016YFC1306103), and the Capital Health Development Research Fund (No.2011‑4024‑04). Year conducted: 2017 Duration of the study: 12 weeks Comments: the study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Peking University Sixth Hospital. Lead author's name: Ying Qian Institution: Child Psychiatric Research Center, Peking University Sixth Hospital (Institute of Mental Health), National Clinical Research Center for Mental Disorders, Key Laboratory of Mental Health, Ministry of Health (Peing University) Email: yangli_pkuimh@bjmu.edu.cn, corresponding author Dr Li Yang Address: Beijing 100191, China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomised block design with participants randomised to a block that comprised a permutation of four participants, two for each group separately. The design was used to balance the individuals between the intervention and waiting‐list groups. |
| Allocation concealment (selection bias) | Low risk | Comment: randomisation grouping concealed in envelopes and recruited participant notified of his or her group sequentially |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: primarily used parent rating scales and parents were not blinded to group status. Unclear, however, if assessors of executive functioning performance tests were blinded to group status |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 6/44 children in intervention group and 12/42 children in control (waiting‐list) group dropped out. No reasons given for dropout. Baseline data for the 18 children who dropped out not provided and no analysis of attrition |
| Selective reporting (reporting bias) | High risk | Comment: The study registration specified the following secondary outcomes, which were not mentioned or reported in the paper: Conners; and Cambridge Neuropsychological Test Automatic Battery. The following measures were not mentioned in the study registration but were reported in the paper: BRIEF; WEISS Functional Impairment Scale‐Parents. |
| Vested interest bias | Unclear risk | Comment: funding source not reported |
| Other sources of bias? | Low risk | Comment: no other sources of bias identified |
Hannesdottir 2017.
| Methods | Design: RCT, cross‐over | |
| Participants |
Country: Iceland Setting: clinical: Centre for Child Development and Behavior in Reykjavik Sample size calculation: not reported Sample size: 41 Sex: 29 (71%) = boys, 12 (29%) = girls Age: mean = 9.2 years (SD = 0.62, range = 8‐10) Ethnicity: not reported Socioeconomic status: not reported IQ: IQ > 70 Diagnosis: ADHD combined = 36 (88%), ADHD inattentive = four (10%), ADHD hyperactive‐impulsive = one (2%) ADHD medication: intervention = 100%, control = 85.7% Cormorbidity: not reported Medications for comorbid disorders: not reported Inclusion criteria:
Exclusion criteria:
Baseline characteristics: significant difference in number of days between assessment. Concern with regard to practice effect, but this was not the case for the waiting‐list control group, who had the fewest days between measurements. |
|
| Interventions | 30 participants allocated to one of two groups and then crossed over
Attendance: no percentage of attendance reported as requirement. Participants in the intervention group attended more than 90% of the sessions and no child missed more than two sessions. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Outcome assessment: post‐intervention and follow‐up three months after end of intervention |
|
| Notes |
Study ID: none found Sponsorship source: no financial support for the study reported Year conducted: 2014 Duration of the study: 5 weeks plus 3 months follow‐up Comments: study was approved by the National Bioethics Committee in Iceland. We received additional information from authors on the randomisation procedure, allocation concealment, and assessment (Hannesdottir 2018 [pers comm]) and on the intervention and funding (Hannesdottir 2018b [pers comm]). Lead author's name: Hannesdottir DK Institution: Centre for Child Development and Behaviour for the Primary Health Care of the Capital Area Email: dagmar.kristin@gmail.com Address: Dagmar Kristin Hannesdottir, Throska‐ og hegdunarstod, Thonglabakka 1, 109 Reykjavik, Iceland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Parents who contacted the coordinator for their child to participate in the OutSMARTers programme were allocated the next number available (e.g. fifth parent to contact the coordinator got the number five). A computer‐generated list with computer randomisation of numbers (1‐50) allocated participants to either the treatment group or the waiting‐list control group. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was not seen as possible to blind participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: outcomes were parent‐reported (who were not blinded) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only one child in intervention group one did not complete treatment, compared with two children in intervention group two. |
| Selective reporting (reporting bias) | Low risk | Comment: all measures described in methods section were reported in results tables. There was no study registration so it was not possible to judge whether any further measures had been included but not reported. It was not clear on what basis it was decided to use and report total scores or subscale scores for the included measures. |
| Vested interest bias | Low risk | Comment: the authors did not receive any funding for the study. The child centre is run by the government and is part of the primary healthcare service in Iceland. Thus, the government paid for the trainers/instructors and supplied the WISC subtests and other measures. |
| Other sources of bias? | Low risk | Comment: no other sources of bias identified |
See Appendix 4
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
|---|---|
| Wolraich 1978 | Ineligible intervention |
| Rosén 1984 | Ineligible intervention |
| Horn 1990 | Ineligible comparator |
| Kolko 1990 | Ineligible patient population |
| Klein 1997 | Ineligible intervention |
| Miranda 2002 | Ineligible intervention |
| Kolko 1999 | Ineligible intervention |
| Gonzalez 2002 | Ineligible intervention |
| Feinfield 2004 | Ineligible patient population |
| Döpfner 2004 | Ineligible intervention |
| Corkum 2005 | Parent training programme only |
| Gol 2005 | Ineligible comparator |
| Langberg 2008 | Ineligible intervention |
| Molina 2008 | Ineligible comparator |
| Grasmann 2011 | Ineligible patient population |
| Dodge 2011 | Ineligible patient population |
| Lessard 2011 | Ineligible patient population |
| Pumpuang 2012 | Ineligible intervention |
| Power 2012a | Ineligible intervention |
| Cionek Szpak 2012 | Ineligible intervention |
| Jans 2012 | Ineligible intervention |
| ICBM 2012 Meeting | Ineligible intervention |
| Malti 2012 | Ineligible patient population |
| Langberg 2012 | Ineligible intervention |
| Ostberg 2012 | Ineligible intervention |
| ESCAP 2013 | Ineligible intervention |
| Abikoff 2013 | Ineligible intervention |
| Lim‐Ashworth 2013a | Ineligible patient population |
| Cipolla 2013 | Ineligible patient population |
| Sibley 2013 | Ineligible intervention |
| Sibley 2013a | Ineligible intervention |
| Vidal 2015 | Adult population |
| Jans 2015 | Ineligible intervention |
| Sibley 2016 | Ineligible intervention |
| Bussing 2016 | Ineligible patient population |
| Leung 2017 | Ineligible patient population |
| NCT02574273 | Ineligible comparator |
| Pfiffner 2018 | Ineligible comparator |
| NCT03176108 | Ineligible intervention |
Characteristics of studies awaiting assessment [ordered by year of study]
NCT01019252.
| Methods |
Design: RCT Number of arms: two |
| Participants |
Country: USA Sample size calculation: not reported Target sample size: 66 Eligible age: age 14 to 18 years Eligible sex: all Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
Time of assessment: before randomisation, and at follow‐up, four months and eight months after randomisation |
| Notes |
Study ID:NCT01019252 Sponsorship source: R34MH083063, US NIH Grant/Contract; DDTR B4‐TBI, National Institute of Mental Health Study start date: October 2009 Study end date: August 2012 Status: completed Declared conflict of interest: not reported Comments: none Registrant's name: Steven A Safren Institution: Massachusetts General Hospital Address: not reported Email: not reported Telephone: not reported |
ADHD: attention deficit hyperactivity disorder ADHD‐ RS: Attention Deficit Hyperactivity Disorder ‐ Rating Scal CBT: cognitive behavioural therapy CGI: Clincal Global Impressions RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by year of study]
NCT01330849.
| Trial name or title | Toolkit for school behavior modification in children with attention‐deficit/hyperactivity disorder (ADHD) |
| Methods |
Design: RCT Number of arms: two |
| Participants |
Country: Belgium Sample size calculation: not reported Target sample size: 100 Eligible age: five to 13 years old Eligible sex: all Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
Time of assessment: evaluation after using the ADHD Toolkit for a three‐month period |
| Starting date |
Study start date: December 2010 Estimated study completion date: June 2011 Status: unknown. Registered, with June 2011 as the estimated final date of data collection. No changes since registration. First posted 7 April 2011 |
| Contact information |
Registrant name: Marina Danckaerts Institution: Universitaire Ziekenhuizen, Leuven Address: Leuven, Vlaams‐Brabant, Belgium, 3000 Email:marina.danckaerts@uzleuven.be Telephone: (+32)16343821 |
| Notes |
Study ID:NCT01330849 Sponsorship source: Universitaire Ziekenhuizen Leuven Declared conflict of interest: not reported Comments: none |
Yang 2015.
| Trial name or title | Efficacy of an integrated behavioural therapy for children with attention‐deficit/hyperactivity disorder: a randomized controlled trial |
| Methods |
Design: RCT Number of arms: two |
| Participants |
Country: China Sample size calculation: not reported Target sample size: 40 Eligible age: not reported Eligible sex: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions |
|
| Outcomes |
Time of assessment: pre‐ and post‐training |
| Starting date |
Start date: not reported Estimated study completion date: not reported Status: conference abstract but no publication identified |
| Contact information |
Lead author's name: L Yang Institution: not reported Address: Beijing, China Email: not reported Telephone: not reported |
| Notes |
Study ID: not reported Sponsorship source: not reported Declared conflict of interest: not reported Comments: none |
IRCT201609186834N11.
| Trial name or title | The effect of psychoeducational group therapy of emotional intelligence on enhancing of emotional intelligence of adolescents with ADHD |
| Methods |
Design: RCT Number of arms: two |
| Participants |
Country: Iran Sample size calculation: not reported Target sample size: 76 Eligible age: 11 to 16 years old Eligible sex: both Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
Time of assessment: before intervention, one week and three months after finishing the intervention |
| Starting date |
Study start date: recruitment expected to start on 22 September 2016 Estimated study completion date: recruitment expected to end 19 February 2017 Status: no updates made to the registration |
| Contact information |
Registrant name: Hossein Ebrahimi Institution: Tabriz University of Medical Sciences Address: Psychiatric Nursing Group, Tabriz Nursing and Midwifery Faculty, South Shariati Street Email: ebrahimih@tbzmed.ac.ir Telephone: +98 41 1475 1709 |
| Notes |
Study ID:IRCT201609186834N11 Sponsorship source: Research Vice‐ Chancellor, Tabriz University of Medical Sciences Declared conflict of interest: not reported Comments: none |
NCT02937142.
| Trial name or title | Cognitive behavior group therapy in adolescents with attention deficit hyperactivity disorder |
| Methods |
Design: RCT Number of arms: two |
| Participants |
Country: Norway Sample size calculation: not reported Target sample size: 96 Eligible age: 14 to 18 years Eligible sex: all Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Time of assessment: at baseline, immediately after the last group therapy session (after 12 weeks) and at follow‐up (after nine months) |
| Starting date |
Study start date: January 2017 Estimated study completion date: August 2020 Status: last verified January 2019 |
| Contact information |
Registrant name: Torunn Stene Nøvik, MD PhD Institution: St Olavs Hospital; Norwegian University of Science and Technology Address: not reported Email:torunn.stene.novik@stolav.no Telephone: not reported |
| Notes |
Study ID:NCT02937142 Sponsorship source: St Olavs Hospital Declared conflict of interest: not reported Comments: none |
ADHD: attention deficit hyperactivity disorder CGI: Clincal Global Impressions DBD: Disruptive behavior disorder IQ: intelligence quotient RCT: randomised clinical trial TAU: treatment as usual VvGK: Dutch translation of the Disruptive Behaviour Disorders Rating Scale WFIRS: Weiss Functional Impairment Rating Scale
Differences between protocol and review
We were unable to use all of our methods as specified in our protocol, Storebø 2010, in this review update. We have archived these for use in future updates of this review in Table 2.
There are some changes in the number of authors between the protocol published in 2010 and the original review version published in 2012 as Mette Elmose Andersen, Signe Joost Hansen, Nadia Pedersen, Britta Tendal, Britta Tendal, and Erlend G Faltinsen now are new authors. Christian Gluud, Erik Simonsen, and Ole Jakob Storebø have been authors of both versions of the review and the protocol. Dorte Damm, and Per Hove Thomsen, who were both authors of the published protocol as well as the original version of the review, are not authors of this updated version of the review. The reason for this change is that Dorte Dam and Per Hove Thomsen did not have the time to participate in the work with this update and therefore the new authors were invited to help with the work.
We changed the databases that we planned to search in the protocol because of: lack of access [AMED] and because no unique relevant records were identified in the previous search [AMED].
Contributions of authors
Ole Jakob Storebø: development of protocol, study selection, data extraction, 'Risk of bias' assessment, data analysis, contact person, GRADE assessment, development of the final review ‐ original and update
Mette Elmose Andersen: study selection, data extraction, 'Risk of bias' assessment, development of the final review ‐ update
Maria Skoog: development of protocol, data extraction, 'Risk of bias' assessment, development of the final review ‐ original and update
Signe Joost Hansen: study selection , data extraction, 'Risk of bias' assessment, development of the final review ‐ update
Erik Simonsen: 'Risk of bias' assessment, development of the final review ‐ original and update
Nadia Pedersen: study selection, data extraction, 'Risk of bias' assessment, development of the final review ‐ update
Britta Tendal: study selection, data extraction, 'Risk of bias' assessment, development of the final review ‐ update
Henriette E Callesen: trial selection, data extraction, 'Risk of bias' assessment, development of the final review ‐ update
Erlend Faltinsen: trial selection, data extraction, 'Risk of bias' assessment, data analysis
Christian Gluud: development of protocol, advising on statistical methods and analysis, GRADE assessment, development of the final review ‐ original and update
All authors read and approved the final version of the review before submission.
Ole Jakob Storebø is the guarantor for the review.
Sources of support
Internal sources
None, Other.
External sources
-
CopenhagenTrial Unit, Denmark.
Support with TSA analyses
-
Research Library, Psychiatric Research Unit, Region Zealand, Roskilde, Denmark.
Support to develop search strategy and search databases
-
Department of Psychology, University of Southern Denmark, Denmark.
Financial support
Declarations of interest
Ole Jakob Storebø is an Associate Editor with the Cochrane Developmental, Psycosocial and Learning Problems Group. Mette Elmose Andersen ‐ none known Maria Skoog ‐ none known Signe Joost Hansen ‐ none known Erik Simonsen ‐ none known Nadia Pedersen ‐ none known Britta Tendal ‐ none known Henriette E Callesen ‐ none known Erlend G Faltinsen ‐ none known Christian Gluud ‐ none known
Ole Jakob Storebø, Maria Skoog, Erik Simonsen, and Christian Gluud were involved in the Storebø 2012 trial, which is included in this review. This trial was assessed by Nadia Pedersen, Mette Elmose, Signe Joost, and Mathilde Holmsov. These authors independently assessed the eligibility of this study, extracted data from it, assessed the risk of bias within it and assessed the quality of the evidence provided by it.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abikoff 2004 {published data only}
- Abikoff H, Hectman L, Klein RG, Gallagher R, Fleiss K, Etcovitch J, et al. Social functioning in children with ADHD treated with long‐term methylphenidate and multimodal psychosocial treatment. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(7):820‐9. [DOI: 10.1097/01.chi.0000128797.91601.1a; PUBMED: 15213583] [DOI] [PubMed] [Google Scholar]
- Abikoff H, Hectman L, Klein RG, Weiss G, Fleiss K, Etcovitch J, et al. Symptomatic improvement in children with ADHD treated with long‐term methylphenidate and multimodal psychosocial treatment. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(7):802‐11. [DOI: 10.1097/01.chi.0000128791.10014.ac; PUBMED: 15213581] [DOI] [PubMed] [Google Scholar]
- Hechtman L, Abikoff H, Klein RG, Weiss G, Respitz C, Kouri J, et al. Academic achievement and emotional status of children with ADHD treated with long‐term methylphenidate and multimodal psychosocial treatment. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(7):812‐9. [DOI: 10.1097/01.chi.0000128796.84202.eb; PUBMED: 15213582] [DOI] [PubMed] [Google Scholar]
- Klein RG, Abikoff H, Hechtman L, Weiss G. Design and rationale of controlled study of long‐term methylphenidate and multimodal psychosocial treatment in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(7):792‐801. [DOI: 10.1097/01.chi.0000128798.91601.fe; PUBMED: 15213580] [DOI] [PubMed] [Google Scholar]
Antshel 2003 {published data only}
- Antshel KM, Remer R. Social skills training in children with attention deficit hyperactivity disorder: a randomized‐controlled clinical trial. Journal of Clinical Child and Adolescent Psychology 2003;32(1):153‐65. [DOI: 10.1207/S15374424JCCP3201_14; PUBMED: 12611031] [DOI] [PubMed] [Google Scholar]
Azad 2014 {published data only}
- Azad MA, Faramarzi S, Arefi M, Farhadi T, Fakkar A. The effectiveness of meta‐cognitive knowledge on reduced hyperactivity and improved attention in children afflicted with attention deficit and hyperactivity disorder (ADHD) in the primary school. Journal of Advances in Cognitive Science 2014;16(1):49‐57. [icssjournal.ir/article‐1‐223‐en.html] [Google Scholar]
Bloomquist 1991 {published data only}
- Bloomquist ML, August GJ, Ostrander R. Effects of a school‐based cognitive‐behavioral intervention for ADHD children. Journal of Abnormal Child Psychology 1991;19(5):591‐605. [PUBMED: 1770187] [DOI] [PubMed] [Google Scholar]
- Bloomquist ML, Braswell L. Multicomponent cognitive‐behavior intervention for attention‐deficit hyperactivity disordered children. Data on file 1989.
Bul 2016 {published data only}
- Bul KC, Kato PM, Oord S, Danckaerts M, Vreeke LJ, Willems A, et al. Behavioral outcome effects of serious gaming as an adjunct to treatment for children with attention‐deficit/hyperactivity disorder: a randomized controlled trial. Journal of Medical Internet Research 2016;18(2):e26. [DOI: 10.2196/jmir.5173; PMC4773597; PUBMED: 26883052] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2015 {published data only}
- Choi ES, Lee WK. Comparative effects of emotion management training and social skills training in Korean children with ADHD. Journal of Attention Disorders 2015;19(2):138‐46. [DOI: 10.1177/1087054713496460; PUBMED: 23929521] [DOI] [PubMed] [Google Scholar]
Cohen 1981 {published data only}
- Cohen NJ, Sullivan J, Minde K, Novak C, Helwig C. Evaluation of the relative effectiveness of methylphenidate and cognitive behavior modification in the treatment of kindergarten‐aged hyperactive children. Journal of Abnormal Child Psychology 1981;9(1):43‐54. [PUBMED: 7217537] [DOI] [PubMed] [Google Scholar]
Evans 2016 {published data only}
- Evans SW, Langberg JM, Schultz BK, Vaughn A, Altaye M, Marshall SA, et al. Evaluation of a school‐based treatment program for young adolescents with ADHD. Journal of Consulting and Clinical Psychology 2016;84(1):15‐30. [DOI: 10.1037/ccp0000057; PUBMED: 26501496] [DOI] [PubMed] [Google Scholar]
- Langberg JM, Evans SW, Schultz BK, Becker SP, Altaye M, Girio‐Herrera E. Trajectories and predictors of response to the challenging horizons program for adolescents with ADHD. Behavior Therapy 2016;47(3):339‐54. [DOI: 10.1016/j.beth.2016.01.001; PUBMED: 27157028] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz BK, Evans SW, Langberg JM, Schoemann AM. Outcomes for adolescents who comply with long‐term psychosocial treatment for ADHD. Journal of Consulting and Clinical Psychology 2017;85(3):250‐61. [DOI: 10.1037/ccp0000172; PMC5321617; PUBMED: 28221060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hannesdottir 2017 {published data only}
- Hannesdottir DK, Ingvarsdottir E, Bjornsson A. The OutSMARTers program for children with ADHD. Journal of Attention Disorders 2017;21(4):353‐64. [DOI: 10.1177/1087054713520617; PUBMED: 24505061] [DOI] [PubMed] [Google Scholar]
Meftagh 2014a {published data only}
- Meftagh SD, Najimi A, Mohammadi N, Ghanizadeh A, Rahimi C, Amini MM. The most effective intervention for attention deficit‐hyperactivity disorder: using continuous performance test. Psychiatria Danubina 2014;26(2):165‐71. [PUBMED: 24909254] [PubMed] [Google Scholar]
MTA 1999 {published data only}
- Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott GR, Greenhill LL, et al. NIMH collaborative multimodal treatment study of children with ADHD (MTA): design methodology, and protocol evolution. Journal of Attention Disorders 1997;2(3):141‐58. [DOI: 10.1177/108705479700200301] [DOI] [Google Scholar]
- Arnold LE, Chuang S, Davies M, Abikoff HB, Conners CK, Elliot GR, et al. Nine months of multicomponent behavioral treatment for ADHD and effectiveness of MTA fading procedures. Journal of Abnormal Child Psychology 2004;32(1):39‐51. [PUBMED: 14998110] [DOI] [PubMed] [Google Scholar]
- Arnold LE, Elliot M, Sachs L, Bird H, Kraemer HC, Wells KC, et al. Effects of ethnicity on treatment attendance, stimulant response/dose, and 14‐month outcome in ADHD. Journal of Consulting and Clinical Psychology 2003;71(4):713‐27. [PUBMED: 12924677] [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, March JS, Abikoff H, Arnold LE, Cantwell DP, Conners CK, et al. Comprehensive assessment of childhood attention‐deficit hyperactivity disorder in the context of a multisite, multimodal clinical trial. Journal of Attention Disorder 1997;1(4):217‐34. [DOI: 10.1177/108705479700100403] [DOI] [Google Scholar]
- Jensen PS, Arnold LE, Swanson JM, Vitiello B, Abikoff HB, Greenhill LL, et al. 3‐year follow‐up of the NIMH MTA study. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(8):989‐1002. [DOI: 10.1097/CHI.0b013e3180686d48; PUBMED: 17667478] [DOI] [PubMed] [Google Scholar]
- Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, et al. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):147‐58. [DOI: 10.1097/00004583-200102000-00009; PUBMED: 11211363] [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. A 14‐month randomized clinical trial of treatment strategies for attention‐deficit/hyperactivity disorder. Multimodal treatment study of children with ADHD. Archives of General Psychiatry 1999;56(12):1073‐86. [PUBMED: 10591283] [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. Moderators and mediators of treatment response for children with attention‐deficit/hyperactivity disorder: the Multimodal Treatment study of children with Attention‐deficit/hyperactivity disorder. Archives of General Psychiatry 1999;56(12):1088‐96. [PUBMED: 10591284] [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow‐up: 24‐month outcomes of treatment strategies for attention‐deficit/hyperactivity disorder. Pediatrics 2004;113(4):754‐61. [PUBMED: 15060224] [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow‐up: changes in effectiveness and growth after the end of treatment. Pediatrics 2004;113(4):762‐9. [PUBMED: 15060225] [DOI] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: prospective follow‐up of children treated for combined‐type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(5):484‐500. [DOI: 10.1097/CHI.0b013e31819c23d0; PMC3063150; PUBMED: 19318991] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. The MTA follow‐up into adolescence: implications for personalized treatment. The International Society for Research in Child and Adolescent Psychopathology Conference; 2009 Jun 19; Seattle (WA). Seattle (WA): ISRCAP, 2009.
- Wells KC, Pelham WE, Kotkin RA, Hoza B, Abikoff HB, Abramowitz A, et al. Psychosocial treatment strategies in the MTA study: rationale, methods, and critical issues in design and implementation. Journal of Abnormal Child Psychology 2000;28(6):483‐505. [PUBMED: 11104313] [DOI] [PubMed] [Google Scholar]
Pfiffner 1997 {published data only}
- Pfiffner LJ, McBurnett K. Social skills training with parent generalization: treatment effects for children with attention deficit disorder. Journal of Consulting and Clinical Psychology 1997;65(5):749‐57. [PUBMED: 9337494] [DOI] [PubMed] [Google Scholar]
Pfiffner 2007 {published data only}
- Pfiffner LJ, Yee Mikami A, Huang‐Pollock C, Easterlin B, Zalecki C, McBurnett K. A randomized, controlled trial of integrated home‐school behavioral treatment for ADHD, predominantly inattentive type. Journal of American Academy of Child and Adolescent Psychiatry 2007;46(8):1041‐50. [DOI: 10.1097/chi.0b013e318064675f; PUBMED: 17667482] [DOI] [PubMed] [Google Scholar]
Pfiffner 2014 {published data only}
- Haack LM, Villodas M, McBurnett K, Hinshaw S, Pfiffner LJ. Parenting as a mechanism of change in psychosocial treatment for youth with ADHD, predominantly inattentive presentation. Journal of Abnormal Child Psychology 2017;45(5):841‐55. [DOI: 10.1007/s10802-016-0199-8; PMC5352559; PUBMED: 27628742] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfiffner LJ, Hinshaw SP, Owens E, Zalecki C, Kaiser NM, Villodas M, et al. A two‐site randomized clinical trial of integrated psychosocial treatment for ADHD‐inattentive type. Journal of Consulting and Clinical Psychology 2014;82(6):1115‐27. [DOI: 10.1037/a0036887; PMC4244306; PUBMED: 24865871] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfiffner 2016 {published data only}
- Pfiffner LJ, Rooney M, Haack L, Villodas M, Delucchi K, McBurnett K. A randomized controlled trial of a school‐implemented school‐home intervention for attention‐deficit/hyperactivity disorder symptoms and impairment. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(9):762‐70. [DOI: 10.1016/j.jaac.2016.05.023; PUBMED: 27566117] [DOI] [PubMed] [Google Scholar]
Qian 2017 {published data only}
- Qian Y, Chen M, Shuai L, Cao QJ, Yang L, Wang YF. Effect of an ecological executive skill training program for school‐aged children with attention deficit hyperactivity disorder: a randomized controlled clinical trial. Chinese Medical Journal 2017;130(13):1513‐20. [DOI: 10.4103/0366-6999.208236; PMC5494912; PUBMED: 28639564 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schramm 2016 {published data only}
- Schramm SA, Hennig T, Linderkamp F. Training problem solving and organizational skills in adolescents with attention‐deficit/hyperactivity disorder: a randomized controlled trial. Journal of Cognitive Education and Psychology 2016;15(3):391‐411. [DOI: 10.1891/1945-8959.15.3.391] [DOI] [Google Scholar]
Storebø 2012 {published data only}
- Storebø OJ, Gluud C, Winkel P, Simonsen E. Social‐skills and parental training plus standard treatment versus standard treatment for children with ADHD ‐ the randomised SOSTRA trial. PLOS One 2012;7(6):e37280. [DOI: 10.1371/journal.pone.0037280; PMC3380035 ; PUBMED: 22745657] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storebø OJ, Pedersen J, Skoog M, Thomsen PH, Winkel P, Gluud C, et al. Randomised social‐skills training and parental training plus standard treatment versus standard treatment of children with attention deficit hyperactivity disorder ‐ the SOSTRA trial protocol. Trials 2011;12:18. [DOI: 10.1186/1745-6215-12-18; PMC3038941; PUBMED: 21255399] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storebø OJ, Skoog M, Rasmussen PD, Winkel P, Gluud C, Pedersen J, et al. Attachment competences in children with ADHD during the social‐skills training and attachment (SOSTRA) randomized clinical trial. Journal of Attention Disorders 2015;19(10):865‐71. [DOI: 10.1177/1087054713520220; PUBMED: 24532801] [DOI] [PubMed] [Google Scholar]
Tabaeian 2010 {published data only}
- Tabaeian SR, Kalantari M, Amiri S, Neshatdoost HT, Molavi H. Effectiveness of social skills training on social competence on primary school children with ADHD. Journal of Psychology 2010;13(4):362‐76. [Google Scholar]
Tutty 2003 {published data only}
- Tutty S, Gephart H, Wurzbacher K. Enhancing behavioral and social skill functioning in children newly diagnosed with attention‐deficit hyperactivity disorder in a pediatric setting. Journal of Developmental and Behavioral Pediatrics 2003;24(1):51‐7. [PUBMED: 12584485] [DOI] [PubMed] [Google Scholar]
Van der Oord 2007 {published data only}
- Oord S, Prins PJM, Oosterlaan J, Emmelkamp PMG. Does brief, clinically based, intensive multimodal behaviour therapy enhance the effects of methylphenidate in children with ADHD?. European Child and Adolescent Psychiatry 2007;16(1):48‐57. [DOI: 10.1007/s00787-006-0574-z; PUBMED: 16972117] [DOI] [PubMed] [Google Scholar]
Waxmonsky 2010 {published data only}
- Waxmonsky JG, Waschbusch DA, Pelham WE, Draganac‐Cardona L, Rotella B, Ryan L. Effects of atomoxetine with and without behavior therapy on the school and home functioning of children with attention‐deficit/hyperactivity disorder. Journal of Clinical Psychiatry 2010;71(11):1535‐51. [DOI: 10.4088/JCP.09m05496pur; PUBMED: 20673557 ] [DOI] [PubMed] [Google Scholar]
Waxmonsky 2016 {published data only}
- Waxmonsky JG, Waschbusch DA, Belin P, Li T, Babocsai L, Humphery H, et al. A randomized clinical trial of an integrative group therapy for children with severe mood dysregulation. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(3):196‐207. [DOI: 10.1016/j.jaac.2015.12.011; PMC4764804; PUBMED: 26903253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkes Gillan 2016 {published data only}
- Wilkes‐Gillan S, Bundy A, Cordier R, Lincoln M, Chen YW. A randomised controlled trial of a play‐based intervention to improve the social play skills of children with attention deficit hyperactivity disorder (ADHD). PLOS One 2016;11(8):e0160558. [DOI: 10.1371/journal.pone.0160558; PMC4987013; PUBMED: 27529693] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuk‐chi 2005 {published data only}
- Yuk‐chi S. Effectiveness of Methylphenidate and Combined Treatment (Methylphenidate and Psychosocial Treatment) for Chinese Children with Attention‐Deficit/Hyperactivity Disorder in a Community Mental Health Center [PhD thesis]. Hong Kong (CN): The Chinese University of Hong Kong, 2005. [Google Scholar]
References to studies excluded from this review
Abikoff 2013 {published data only}
- Abikoff H, Gallagher R, Wells KC, Murray DW, Huang L, Lu F, et al. Remediating organizational functioning in children with ADHD: Immediate and long‐term effects from a randomized controlled trial. Journal of Consulting and Clinical Psychology 2013;81(1):113‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bussing 2016 {published data only}
- Bussing R, Nelson MM, Kurtz S. Parent‐child interaction therapy: treatment components and evidence‐base. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(10 Suppl. 1):S351. [Google Scholar]
Cionek Szpak 2012 {published data only}
- Cionek‐Szpak E, Holtmann M, Stadler C, Poustka F. EEG‐Neurobiofeedback in ADHD ‐ a treatment supplement or alternative‐rapport about the results of a controlled experimental trial. Neuropsychiatrie de l'Enfance et de l'Adolescence 2012;60(5 Suppl. 1):s267. [Google Scholar]
Cipolla 2013 {published data only}
- Cipolla DS. An investigation of the effects of a computer‐based intervention on the social skills of children with autism. Dissertation Abstracts International Section A: Humanities and Social Sciences 2013;73(11‐A(E)):No pagination specified. [Google Scholar]
Corkum 2005 {published data only}
- Corkum PV, McKinnon MM, Mullane JC. The effect of involving classroom teachers in a parent training program for families of children with ADHD. Child & Family Behavior Therapy 2005;27(4):29‐49. [Google Scholar]
Dodge 2011 {published data only}
- Dodge A. The effects of the fast track preventive intervention on the development of conduct disorder across childhood. Child Development 2011;82(1):331‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Döpfner 2004 {published data only}
- Döpfner M, Breuer D, Schürmann S, Metternich TW, Rademacher C, Lehmkuhl G. Effectiveness of an adaptive multimodal treatment in children with attention‐deficit/hyperactivity disorder ‐ global outcome. European Child and Adolescent Psychiatry 2004;13(Suppl. 1):117‐29. [DOI] [PubMed] [Google Scholar]
ESCAP 2013 {published data only}
- No authorship indicated. 15th International Congress of ESCAP ‐ European Society for Child and Adolescent Psychiatry, 2013 July 6‐10; Dublin (IRELAND). European Child & Adolescent Psychiatry 2013;22(Suppl. 2):S81‐S323. [DOI] [PubMed] [Google Scholar]
Feinfield 2004 {published data only}
- Feinfield KA, Baker BL. Empirical support for a treatment program for families of young children with externalizing problems. Journal of Clinical Child and Adolescent Psychology 2004;33(1):182‐95. [DOI] [PubMed] [Google Scholar]
Gol 2005 {published data only}
- Gol D, Jarsus T. Effect of a social skills training group on everyday activities of children with attention deficit‐hyperactivity disorder. Developmental Medicine and Child Neurology 2005;47(8):539‐45. [DOI] [PubMed] [Google Scholar]
Gonzalez 2002 {published data only}
- Gonzalez LO, Sellers EW. The effects of a stress‐management program on self‐concept, locus of control, and the acquisition of coping skills in school‐age children diagnosed with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychiatric Nursing 2002;15(1):5‐15. [DOI] [PubMed] [Google Scholar]
Grasmann 2011 {published data only}
- Grasmann D, Stadler C. VIA ‐ an intensive therapeutic treatment program for conduct disorders [VIA ‐ intensivtherapeutischer behandlungsansatz bei storungen des sozialverhaltens]. Zeitschrift fur Kinder und Jugendpsychiatrie und Psychotherapie 2011;39(1):23‐31. [DOI] [PubMed] [Google Scholar]
Horn 1990 {published data only}
- Horn WF, Ialongo N, Greenberg G, Packard T, Smith‐Winberry C. Additive effects of behavioral parent training and self‐control therapy with attention deficit/ hyperactivity disordered children. Journal of Clinical Child Psychology 1990;19(2):98‐110. [Google Scholar]
ICBM 2012 Meeting {published data only}
- Anonymous. ICBM 2012 Meeting. International Journal of Behavioral Medicine 2012;19(Suppl. 1):1‐342. [DOI] [PubMed] [Google Scholar]
Jans 2012 {published data only}
- Jans T, Jacob C, Hennighausen K, Roesler M, Haenig S, Sobanski E, et al. Treatment outcome of behavioral parent‐child training in childhood ADHD as a function of the treatment of maternal ADHD. Neuropsychiatrie de l'Enfance et de l'Adolescence 2012;60(Suppl. 5):S51. [Google Scholar]
Jans 2015 {published data only}
- Jans T, Jacob C, Warnke A, Zwanzger U, Groß‐Lesch S, Matthies S, et al. Does intensive multimodal treatment for maternal ADHD improve the efficacy of parent training for children with ADHD? A randomized controlled multicenter trial. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2015;56(12):1298‐313. [DOI] [PubMed] [Google Scholar]
Klein 1997 {published data only}
- Klein RG, Abikoff H. Behavior therapy and methylphenidate in the treatment of children with ADHD. Journal of Attention Disorders 1997;2(2):89‐114. [Google Scholar]
Kolko 1990 {published data only}
- Kolko DJ, Loar LL, Sturnick D. Inpatient social‐cognitive skills training groups with conduct disordered and attention deficit disordered children. Journal of Child Psychology and Psychiatry 1990;31(5):737‐48. [DOI] [PubMed] [Google Scholar]
Kolko 1999 {published data only}
- Kolko DJ, Bukstein OG, Barron J. Methylphenidate and behavior modification in children with ADHD and comorbid ODD or CD: main and incremental effects across settings. Journal of American Academic Child and Adolescent Psychiatry 1999;38(5):578‐86. [DOI] [PubMed] [Google Scholar]
Langberg 2008 {published data only}
- Langberg JM, Epstein JN. Efficacy of an organization skills intervention to Improve the academic functioning of students with attention‐deficit/hyperactivity disorder. School Psychology Quarterly 2008;23(3):407‐17. [Google Scholar]
Langberg 2012 {published data only}
- Langberg JM, Epstein JN, Becker SP, Girio‐Herrera E, Vaughn AJ. Evaluation of the Homework, Organization, and Planning Skills (HOPS) intervention for middle school students with ADHD as implemented by school mental health providers. School Psychology Review 2012;41(3):342‐64. [PMC free article] [PubMed] [Google Scholar]
Lessard 2011 {published data only}
- Lessard L, Coutu S. Efficacy of a social skills program for children presenting with heterogeneous psychiatric diagnoses. Canadian Journal of Behavioural Science/Revue Canadienne des Sciences du Comportement 2011;43(1):30‐9. [Google Scholar]
Leung 2017 {published data only}
- Leung C, Tsang S, Ng GSH, Choi SY. Efficacy of parent‐child interaction therapy with Chinese ADHD children: randomized controlled trial. Research on Social Work Practice 2017;27(1):36‐47. [Google Scholar]
Lim‐Ashworth 2013a {published data only}
- Lim‐Ashworth N, Ooi YP, Weng SJ, Lim CG, Fung DSS, Glenn A, et al. Preliminary findings on the effects of nutritional and social skills intervention among children with Attention Deficit Hyperactivity Disorder. Annals of the Academy of Medicine Singapore 2013;42(9 Suppl. 1):S162. [Google Scholar]
Malti 2012 {published data only}
- Malti T, Ribeaud D, Eisner M. Effectiveness of a universal school‐based social competence program: the role of child characteristics and economic factors. International Journal of Conflict and Violence 2012;6(2):249‐59. [Google Scholar]
Miranda 2002 {published data only}
- Miranda A, Presentación J, Soriano M. Effectiveness of a school‐based multicomponent program for the treatment of children with ADHD. Journal of Learning Disabilities 2002;35(6):547‐63. [DOI] [PubMed] [Google Scholar]
Molina 2008 {published data only}
- Molina BSG, Flory K, Bukstein OG, Greiner AR, Baker JL, Krug V, et al. Feasibility and preliminary efficacy of an after‐school program for middle schoolers with ADHD. Journal of Attention Disorders 2008;12(3):207‐17. [DOI] [PubMed] [Google Scholar]
NCT02574273 {published data only}
- NCT02574273. Pilot trial of a social skills group treatment (Secret Agent Society Program). clinicaltrials.gov/ct2/show/NCT02574273 (first received 12 October 2015).
NCT03176108 {published data only}
- NCT03176108. Evaluation of a parent/child cognitive‐behaviorial therapy program in ADHD children with emotional dysregulation profile. clinicaltrials.gov/ct2/show/NCT03176108 (first received 5 June 2017).
Ostberg 2012 {published data only}
- Ostberg M, Rydell AM. An efficacy study of a combined parent and teacher management training programme for children with ADHD. Nordic Journal of Psychiatry 2012;66(2):123‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfiffner 2018 {published data only}
- Pfiffner LJ, Rooney ME, Jiang Y, Haack LM, Beaulieu A, McBurnett K. Sustained effects of collaborative school‐home intervention for Attention‐Deficit/Hyperactivity Disorder symptoms and impairment. Journal of the American Academy of Child and Adolescent Psychiatry 2018; Vol. 57, issue 4:245‐51. [DOI] [PubMed]
Power 2012a {published data only}
- Power TJ, Mautone JA, Soffer SL, Clarke AT, Marshall SA, Sharman J, et al. A family‐school intervention for children with ADHD: results of a randomized clinical trial. Journal of Consulting and Clinical Psychology 2012;80(4):611‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pumpuang 2012 {published data only}
- Pumpuang W, Phuphaibul R, Orathai P, Putdivarnichapong W. Effectiveness of a collaborative home‐school behavior management program for parents and teachers of children with Attention Deficit Hyperactivity Disorder. Pacific RIM International Journal of Nursing Research 2012;16(2):138‐53. [Google Scholar]
Rosén 1984 {published data only}
- Rosén LA, O´Leary SG, Joyce AS, Conway G, Pfiffner L. The importance of prudent negative consequences for maintaining the appropriate behavior of hyperactive students. Journal of Abnormal Child Psychology 1984;12(4):581‐604. [DOI] [PubMed] [Google Scholar]
Sibley 2013 {published data only}
- Sibley MH, Pelham WE Jr, Derefinko KJ, Kuriyan AB, Sanchez F, Graziano PA. A pilot trial of Supporting Teens' Academic Needs Daily (STAND): a parent‐adolescent collaborative intervention for ADHD. Journal of Psychopathology and Behavioral Assessment 2013;35(4):436‐49. [Google Scholar]
Sibley 2013a {published data only}
- Sibley MH. Supporting Teens' Academic Needs Daily (STAND): a parent‐adolescent collaborative intervention for ADHD. Dissertation Abstracts International: Section B: The Sciences and Engineering 2013;74(3‐B(E)):No pagination specified. [Google Scholar]
Sibley 2016 {published data only}
- Sibley MH, Graziano PA, Kuriyan AB, Coxe S, Pelham WE, Rodriguez L, et al. Parent‐teen behavior therapy + motivational interviewing for adolescents with ADHD. Journal of Consulting and Clinical Psychology 2016;84(8):699‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vidal 2015 {published data only}
- Vidal R, Castells J, Richarte V, Palomar G, Garcia M, Nicolau R, et al. Group therapy for adolescents with attention‐deficit/hyperactivity disorder: a randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry 2015;54(4):275‐82. [DOI] [PubMed] [Google Scholar]
Wolraich 1978 {published data only}
- Wolraich M, Drummond T, Salomon MK, O´Brian ML, Sivage C. Effects of methylphenidate alone and in combination with behavior modification procedures on the behavior and academic performance of hyperactive children. Journal of Abnormal Child Psychology 1978;6(1):149‐61. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01019252 {published data only}
- NCT01019252. Skills training for adolescents with ADHD [Compensatory executive functioning skills training in adolescents with ADHD]. clinicaltrials.gov/ct2/show/NCT01019252 (first received 20 November 2009).
References to ongoing studies
IRCT201609186834N11 {published data only}
- IRCT201609186834N11. The effect of psycho‐educational group therapy of emotional intelligence on enhancing of emotional intelligence of adolescents with ADHD. www.irct.ir (first received 30 October 2016).
NCT01330849 {published data only}
- NCT01330849. Toolkit for school behavior modification in children with attention‐deficit/hyperactivity disorder (ADHD) [A randomized controlled trial of a behavior modification toolkit in primary school children with ADHD behaviors]. clinicaltrials.gov/ct2/show/NCT01330849 (first received 5 April 2011).
NCT02937142 {published data only}
- NCT02937142. Cognitive behavior group therapy in adolescents with attention deficit hyperactivity disorder [Cognitive behavior group therapy in adolescents (14‐18 years) with attention deficit hyperactivity disorder (ADHD)]. clinicaltrials.gov/ct2/show/NCT02937142 (first received 14 October 2016).
Yang 2015 {published data only}
- Yang L, Shuai L, Chen M, Qian Y, Wang Y. Efficacy of an integrated behavioural therapy for children with Attention‐Deficit/Hyperactivity Disorder: a randomized controlled trial. ADHD: Attention Deficit and Hyperactivity Disorders 2015;7(Suppl. 1):S94. [DOI: 10.1007/s12402-015-0169-y; P‐25‐0008] [DOI] [Google Scholar]
Additional references
Abikoff 2011 [pers comm]
- Abikoff H. Cochrane review: social skills training [personal communication]. Email to: OJ Storebø 28 January 2011.
Achenbach 1991
- Achenbach TM. Integrative Guide for the 1991 CBCL/4‐18, YSR, and TRF Profiles. Burlington (VT): University of Vermont, Department of Psychology, 1991. [Google Scholar]
Almerie 2015
- Almerie MQ, Okba Al, Marhi M, Jawoosh M, Alsabbagh M, Matar HE, et al. Social skills programmes for schizophrenia. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD009006.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrews 2013a
- Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck‐Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719‐25. [DOI: 10.1016/j.jclinepi.2012.03.013; PUBMED: 23312392] [DOI] [PubMed] [Google Scholar]
Andrews 2013b
- Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation ‐ determinants of a recommendation's direction and strength. Journal of Clinical Epidemiology 2013;66(7):726‐35. [DOI: 10.1016/j.jclinepi.2013.02.003; PUBMED: 23570745] [DOI] [PubMed] [Google Scholar]
Antshel 2010 [pers comm]
- Antshel K. [personal communication]. Email to: OJ Storebø 16 December 2010.
Antshel 2011 [pers comm]
- Antshel K. [personal communication]. Email to: OJ Storebø 13 July 2011.
Attkisson 1982
- Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Evaluation and Program Planning 1982;5(3):233‐7. [PUBMED: 10259963] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI: 10.1016/j.jclinepi.2010.07.015; PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Barkley 1987
- Barkley RA. Defiant Children: A Clinician's Manual for Parent Training. 1st Edition. The Guilford Press, 1987. [Google Scholar]
Barkley 2002
- Barkely RA, Fischer M, Smallish L, Fletcher K. The persistence of attention‐deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. Journal of Abnormal Psychology 2002;111(2):279‐89. [PUBMED: 12003449] [PubMed] [Google Scholar]
Berkley 1996
- Berkey CS, Mosteller F, Lau J, Antman EM. Uncertainty of the time of first significance in random effects cumulative meta‐analysis. Controlled Clinical Trials 1996;17(5):357‐71. [DOI: 10.1016/S0197-2456(96)00014-1] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI: 10.1016/j.jclinepi.2007.10.007; PUBMED: 18411040] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI: 10.1093/ije/dyn188; PUBMED: 18824466] [DOI] [PubMed] [Google Scholar]
Brunetti 2013
- Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. Journal of Clinical Epidemiology 2013;66(2):140‐50. [DOI: 10.1016/j.jclinepi.2012.04.012; PUBMED: 22863410] [DOI] [PubMed] [Google Scholar]
Castellanos 2006
- Castellanos FX, Sonuga‐Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends in Cognitive Sciences 2006;10(3):117‐23. [DOI: 10.1016/j.tics.2006.01.011; PUBMED: 16460990] [DOI] [PubMed] [Google Scholar]
Castellini 2018
- Castellini G, Bruschettini M, Gianola S, Gluud C, Moja L. Assessing imprecision in Cochrane systematic reviews: a comparison of GRADE and Trial Sequential Analysis. Systematic Reviews 2018;7:110. [DOI: 10.1186/s13643-018-0770-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013
- Chan A‐W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI: 10.7326/0003-4819-158-3-201302050-00583; PMC5114123; PUBMED: 23295957] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2012
- Chang Z, Lichtenstein P, Larsson H. The effects of childhood ADHD symptoms on early‐onset substance use: a Swedish twin study. Journal of Abnormal Child Psychology 2012;40(3):425‐35. [DOI: 10.1007/s10802-011-9575-6; PUBMED: 21947618] [DOI] [PubMed] [Google Scholar]
Cochrane Hepato‐Biliary Group 2019
- Cochrane Hepato‐Biliary Group. Information for authors. hbg.cochrane.org/information‐authors (accessed prior to 5 June 2019).
Conners 1998
- Conners KC, Sitarenios G, Parker JDA, Epstein JN. The Revised Conners´ Parent Rating Scale (CPRS‐R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology 1998;26(4):257‐68. [PUBMED: 9700518] [DOI] [PubMed] [Google Scholar]
Conners 2008a
- Conners KC. Conners Comprehensive Behavior Rating Scales (Conners CBRS). Toronto (ON): Multi‐Health Systems Inc, 2008. [Google Scholar]
Conners 2008b
- Conners KC. Conners 3. Toronto (ON): Multi‐Health Systems Inc, 2008. [Google Scholar]
Cortese 2016
- Cortese S, Moreira‐Maia CR, Fleur D, Morcillo‐Peñalver C, Rohde LA, Faraone SV. Association between ADHD and obesity: a systematic review and meta‐analysis. American Journal of Psychiatry 2016;173(1):34‐43. [DOI: 10.1176/appi.ajp.2015.15020266; PUBMED: 26315982] [DOI] [PubMed] [Google Scholar]
Czamara 2013
- Czamara D, Tiesler CM, Kohlböck G, Berdel D, Hoffmann B, Bauer CP, et al. Children with ADHD symptoms have a higher risk for reading, spelling and math difficulties in the GINIplus and LISAplus cohort studies. PLOS ONE 2013;8(5):e63859. [DOI: 10.1371/journal.pone.0063859; PMC3664565; PUBMED: 23724008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daley 2014b
- Daley D, Oord S, Ferrin M, Danckaerts M, Doepfner M, Cortese S, et al. Behavioral interventions in attention‐deficit/hyperactivity disorder: a meta‐analysis of randomized controlled trials across multiple outcome domains. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(8):835‐47. [DOI: 10.1016/j.jaac.2014.05.013; PUBMED: 25062591] [DOI] [PubMed] [Google Scholar]
Dalsgaard 2002
- Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH. Conduct problems, gender and adult psychiatric outcome of children with attention‐deficit hyperactivity disorder. British Journal of Psychiatry 2002;181(5):416‐21. [PUBMED: 12411268] [DOI] [PubMed] [Google Scholar]
Dalsgaard 2015
- Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB, Pedersen MG. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet 2015;385(9983):2190‐6. [DOI: 10.1016/S0140-6736(14)61684-6] [DOI] [PubMed] [Google Scholar]
Dawson 2010
- Dawson P, Guare R. Executive Skills in Children and Adolescents: A Practical Guide to Assessment and Intervention. 2nd Edition. New York: The Guilford Press, 2010. [Google Scholar]
De Boo 2007
- Boo GM, Prins PJM. Social incompetence in children with ADHD: possible moderators and mediators in social‐skills training. Clinical Psychology Review 2007;27(1):78‐97. [DOI: 10.1016/j.cpr.2006.03.006; PUBMED: 16814435] [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomised trials. Statistics in Medicine 2002;21(19):2971–80. [DOI: 10.1002/sim.1301; PUBMED: 12325113] [DOI] [PubMed] [Google Scholar]
DSM‐5
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). 5th Edition. Washington (DC): American Psychiatric Publishing, 2013. [Google Scholar]
DSM‐III
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐III). 3rd Edition. Washington (DC): American Psychiatric Association, 1980. [Google Scholar]
DSM‐IV
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington (DC): American Psychiatric Association, 1994. [Google Scholar]
DSM‐IV‐R
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐III‐R). 3rd Edition. Washington (DC): American Psychiatric Association, 1987. [Google Scholar]
DSM‐IV‐TR
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR). 4th Edition. Washington (DC): American Psychiatric Association, 2000. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI: 10.1136/bmj.315.7109.629; PMC2127453; PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliot 1991
- Elliot SN, Gresham FM. Social Skills Intervention Guide: Practical Strategies for Social Skills Training. Circle Pines, MN: American Guidance Service, 1991. [Google Scholar]
Erskine 2016
- Erskine HE, Norman RE, Ferrari AJ, Chan GC, Copeland WE, Whiteford HA, et al. Long‐term outcomes of attention‐deficit/hyperactivity disorder and conduct disorder: a systematic review and meta‐analysis. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(10):841–50. [doi: 10.1016/j.jaac.2016.06.016; PUBMED: 27663939] [DOI] [PubMed] [Google Scholar]
Eunethydis 2010
- Eunethydis. Eunethydis 1st international ADHD conference: from data to best clinical practice. European Child & Adolescent Psychiatry 2010;19(Suppl. 1):5‐91. [DOI: 10.1007/s00787-010-0117-5] [DOI] [PubMed] [Google Scholar]
Evans 2017 [pers comm]
- Evans S. Questions regarding your study [personal communication]. Email to: J Signe 29 December 2017.
Faraone 2000
- Faraone SV, Biederman J, Spencer T, Wilens T, Seidman LJ, Mick E, et al. Attention‐deficit/hyperactivity disorder in adults: an overview. Biological Psychiatry 2000;48(1):9‐20. [PUBMED: 10913503] [DOI] [PubMed] [Google Scholar]
Fohlmann 2009a
- Fohlmann AH. Social skills training [Social færdighedstræning]. In: Nordentoft M, Melau M, Iversen T, Kjær S editor(s). Psychosis in Adolescents. Symptoms, Treatment and the Future [Psykose hos Unge. Symptomer, Behandling og Fremtid]. Copenhagen (DK): Psykiatrifondens Forlag, 2009:161‐89. [Google Scholar]
Fohlmann 2009b [pers comm]
- Storebø OJ. Social Skills Training [personal communication]. Email to: AH Fohlmann 10 April 2009.
Ford 2003
- Ford T, Goodman R, Meltzer H. The British Child and Adolescent Mental Health Survey 1999: the prevalence of DSM‐IV disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42(10):1203–11. [DOI: 10.1097/00004583-200310000-00011; PUBMED: 14560170] [DOI] [PubMed] [Google Scholar]
Franke 2012
- Franke B, Faraone SV, Asherson P, Buitelaar J, Bau CHD, Ramos‐Quiroga JA, et al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Molecular Psychiatry 2012;17(10):960‐87. [DOI: 10.1038/mp.2011.138; PMC3449233; PUBMED: 22105624] [DOI] [PMC free article] [PubMed] [Google Scholar]
Froehlich 2011
- Froehlich TE, Anixt JS, Loe IM, Chirdkiatgumchai V, Kuan L, Gilman RC. Update on environmental risk factors for attention‐deficit/hyperactivity disorder. Current Psychiatry Reports 2011;13(5):33‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE Working Group
- GRADE Working Group. GRADE. www.gradeworkinggroup.org (accessed 11 December 2018).
GRADEpro GDT 2015 [Computer program]
- McMaster University. GRADEpro GDT. Version accessed prior to 12 March 2018. Hamilton (ON): McMaster University, 2015.
Gresham 1990
- Gresham FM, Elliot SN. Social Skills Rating System. Circle Pines (MN): Assistance Service, 1990. [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383–94. [DOI: 10.1016/j.jclinepi.2010.04.026; PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395–400. [DOI: 10.1016/j.jclinepi.2010.09.012; PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407–15. [DOI: 10.1016/j.jclinepi.2010.07.017; PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283–93. [DOI: 10.1016/j.jclinepi.2011.01.012; PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294–302. [DOI: 10.1016/j.jclinepi.2011.03.017; PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303–10. [DOI: 10.1016/j.jclinepi.2011.04.014; PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311–6. [DOI: 10.1016/j.jclinepi.2011.06.004; PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151–7. [DOI: 10.1016/j.jclinepi.2012.01.006; PUBMED: 22542023] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158–72. [DOI: 10.1016/j.jclinepi.2012.01.012; PUBMED: 22609141] [DOI] [PubMed] [Google Scholar]
Guyatt 2013c
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles ‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173–83. [DOI: 10.1016/j.jclinepi.2012.08.001; PUBMED: 23116689] [DOI] [PubMed] [Google Scholar]
Hannesdottir 2018 [pers comm]
- Hannesdottir D. [personal communication]. Email to: S Joost 18 January 2018.
Hannesdottir 2018b [pers comm]
- Hannesdottir D. [personal communication]. Email to: S Joost 9 February 2018.
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ICD‐10
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders. Clinical Descriptions and Diagnostic Guidelines. Geneva (CH): World Health Organization, 1992. [Google Scholar]
ICD‐9
- World Health Organization. International Classification of Diseases. 9th Edition. Vol. 1, Geneva (CH): World Health Organization, 1977. [Google Scholar]
ICH 1996
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice E6(R1). www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (accessed 6 May 2011).
International Congress on ADHD 2009
- International Congress on ADHD. 2nd International Congress on ADHD: from childhood to adult disease. www.adhd‐federation.org/fileadmin/user_upload/PDFs/ADHD_Final_Program.pdf (accessed prior to 29 May 2019).
Jakobsen 2014
- Jakobsen JC, Gluud C, Winkel P, Lange T, Wetterslev J. The thresholds for statistical and clinical significance – a five‐step procedure for evaluation of intervention effects in randomised clinical trials. BMC Medical Research Methodology. 2014;14:34. [DOI: 10.1186/1471-2288-14-34; PMC4015863; PUBMED: 24588900] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kadesjö 2002
- Kadesjö B. ADHD in Children and Adults [ADHD hos Barn och Vuxna]. Stockholm (SE): Socialstyrelsen, 2002. [Google Scholar]
Kavale 1997
- Kavale KA, Mathur SR, Forness SR, Rutherford Jr RB, Quinn MM. Effectiveness of social skills training for students with behaviour disorders: a meta‐analysis. Advances in Learning and Behavioral Disabilities 1997;11:1‐26. [Google Scholar]
Kendal 1982
- Kendall PC, Braswell L. Cognitive‐behavioral self‐control therapy for children: a components analysis. Journal of Consulting and Clinical Psychology 1982;50:672‐90. [DOI] [PubMed] [Google Scholar]
Keshavan 2003
- Keshavan MS, Sujata M, Mehra A, Montrose DM, Sweeney JA. Psychosis proneness and ADHD in young relatives of schizophrenia patients. Schizophrenia Research 2003;59(1):85‐92. [12413647] [DOI] [PubMed] [Google Scholar]
Keus 2010
- Keus F, Wetterslev J, Gluud C, Laarhoven CJHM. Evidence at a glance: error matrix approach for overviewing available evidence. BMC Medical Research Methodology 2010;10:90. [DOI: 10.1186/1471-2288-10-90] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2002
- Kjaergard LL, Gluud C. Citation bias of hepato‐biliary randomized clinical trials. Journal of Clinical Epidemiology 2002;55(4):407‐10. [PUBMED: 11927210] [DOI] [PubMed] [Google Scholar]
Koisaari 2015
- Koisaari T, Michelsson K, Holopainen JM, Maksimainen R, Päivänsalo J, Rantala K, et al. Traffic and criminal behavior of adults with attention deficit‐hyperactivity with a prospective follow‐up from birth to the age of 40 years. Traffic Injury Prevention 2015;16(8):824‐30. [DOI: 10.1080/15389588.2015.1029068; PUBMED: 25837647] [DOI] [PubMed] [Google Scholar]
Kwak 2002
- Kwak K, Park H, Kim C. The Manual for the Korean WISC‐III. Seoul: Special Education Publishing Co, 2002. [Google Scholar]
Landau 1991
- Landau S, Moore LA. Social skill deficits in children with attention‐deficit hyperactivity disorder. School Psychology Review 1991;20(2):235‐51. [Google Scholar]
Lau 1995
- Lau J, Schmid CH, Chalmers TC. Cumulative meta‐analysis of clinical trials builds evidence for exemplary medical care. Journal of Clinical Epidemiology 1995;48(1):45‐57. [DOI: 10.1016/0895-4356(94)00106-Z; PUBMED: 7853047] [DOI] [PubMed] [Google Scholar]
Lauth 2004
- Lauth GW, Fellner C. Course of therapy and long‐term effects of a multimodal therapy program in ADHD. Single case studies [Therapieverlauf und langzeiteffekt eines multimodalen trainingsprogramms bei Aufmerksamkeitsdefizit‐/Hyperaktivitätsstörungen. Einzelfallstudien]. Kindheit und Entwicklung 2004;13(3):167‐79. [DOI: 10.1026/0942-5403.13.3.167] [DOI] [Google Scholar]
Liberman 1988
- Liberman RP. Social skills training. In: Liberman RP editor(s). Psychiatric Rehabilitation of Chronic Mental Patients. Washington (DC): American Psychiatric Press, 1988. [Google Scholar]
Mager 2005
- Mager W, Milich R, Harris MJ, Howard A. Intervention groups for adolescents with conduct problems: is aggression harmful or helpful?. Journal of Abnormal Child Psychology 2005;33(3):349‐62. [DOI: 10.1007/s10802-005-3572-6] [DOI] [PubMed] [Google Scholar]
Majewicz‐Hefley 2007
- Majewicz‐Hefley A, Carlson JS. A meta‐analysis of combined treatments for children diagnosed with ADHD. Journal of Attention Disorders 2007;10(3):239‐50. [DOI: 10.1177/1087054706289934; PUBMED: 17242418] [DOI] [PubMed] [Google Scholar]
Meichenbaum 1978
- Meichenbaum D. Cognitive‐Behavior Modification: An Integrative Approach. New York (NY): Plenum Press, 1978. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42. [DOI: 10.1016/j.jclinepi.2013.02.004; PUBMED: 23623694] [DOI] [PubMed] [Google Scholar]
Nakao 1994
- Nakao K, Treas J. Updating occupational prestige and socioeconomic scores: how the new measures measure up. Sociological Methodology 1994;24:1‐72. [DOI: 10.2307/270978] [DOI] [Google Scholar]
Neale 2010
- Neale BM, Medland SE, Ripke S, Asherson P, Franke B, Lesch KP, et al. Meta‐analysis of genome‐wide association studies of attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2010;49(9):884‐97. [DOI: 10.1016/j.jaac.2010.06.008; PMC2928252; PUBMED: 20732625] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newcorn 2008
- Newcorn JH. Co‐morbidity in adults with ADHD. CNS Spectrums 2008;13(Suppl. 12):12‐5. [DOI: 10.1017/S1092852900003199] [DOI] [PubMed] [Google Scholar]
NICE 2009
- National Collaborating Centre for Mental Health, commissioned by the National Institute for Health and Clinical Excellence. Attention Deficit Hyperactivity Disorder: Diagnosis and Management of ADHD in Children, Young People and Adults. National Clinical Practice Guideline Number 72. Leicester and London: The British Psychological Society and The Royal College of Psychiatrists, 2009. [PubMed] [Google Scholar]
NICE 2018
- NICE. Attention deficit hyperactivity disorder: diagnosis and management. www.nice.org.uk/guidance/ng87/resources/attention‐deficit‐hyperactivity‐disorder‐diagnosis‐and‐management‐pdf‐1837699732933 (accessed 12 July 2018).
Nigg 2005
- Nigg JT, Casey BJ. An integrative theory of attention‐deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology 2005;17(3):785‐806. [DOI: 10.1017/S0954579405050376; PUBMED: 16262992] [DOI] [PubMed] [Google Scholar]
Nordic ADHD konference 2010
- Nordic ADHD konference 2010. Nordic ADHD conference: lifelong perspectives of special needs [Nordic ADHD konference: livslange perspektiver of specielle behov]. www.bupnet.dk/media/ADHD‐konference_maj_2010‐_Program%5B1%5D.pdf (accessed prior to 29 May 2019).
Norman 2003
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health‐related quality of life: the remarkable universality of half a standard deviation. Medical Care 2003;41(5):582‐92. [DOI: 10.1097/01.MLR.0000062554.74615.4C; PUBMED: 12719681] [DOI] [PubMed] [Google Scholar]
Pasini 2007
- Pasini A, Paloscia C, Alessandrelli R, Porfirio MC, Curatolo P. Attention and executive functions profile in drug naive ADHD subtypes. Brain and Development 2007;29(7):400‐8. [DOI: 10.1016/j.braindev.2006.11.010; PUBMED: 17207595] [DOI] [PubMed] [Google Scholar]
Pelham 1992
- Pelham WE Jr, Gnagny EM, Greenslade KE, Milich R. Teacher ratings of DSM‐III‐R symptoms for disruptive behaviour disorder.. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31(2):210‐8. [DOI: 10.1097/00004583-199203000-00006; PUBMED: 1564021] [DOI] [PubMed] [Google Scholar]
Perroud 2014
- Perroud N, Cordera P, Zimmermann J, Michalopoulos G, Bancila V, Prada P, et al. Comorbidity between attention deficit hyperactivity disorder (ADHD) and bipolar disorder in a specialized mood disorders outpatient clinic. Journal of Affective Disorders 2014;168:161‐6. [DOI: 10.1016/j.jad.2014.06.053; PUBMED: 25051093] [DOI] [PubMed] [Google Scholar]
Pfiffner 2011 [pers comm]
- Pfiffner L. Cochrane review: Social skills training [personal communication]. Email to: OJ Storebø 26 May 2011.
Pfiffner 2011b [pers comm]
- Pfiffner L. Cochrane review: Social skills training [personal communication]. Email to: OJ Storebø 25 May 2011.
Pineda 2003
- Pineda DA, Lopera F, Palacio JD, Ramirez D, Henao GC. Prevalence estimations of attention‐deficit/hyperactivity disorder: differential diagnoses and comorbidities in a Columbian sample. International Journal of Neuroscience 2003;113(1):49‐71. [PUBMED: 12691001] [DOI] [PubMed] [Google Scholar]
Polanczyk 2007
- Polanczyk G, Rohde LA. Epidemiology of attention‐deficit/hyperactivity disorder across the lifespan. Current Opinion in Psychiatry 2007;20(4):386‐92. [DOI: 10.1097/YCO.0b013e3281568d7a; PUBMED: 17551354] [DOI] [PubMed] [Google Scholar]
Punja 2016
- Punja S, Shamseer L, Hartling L, Urichuk L, Vandermeer B, Nikles J, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD009996.pub2; PUBMED: 26844979] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Savovic 2018
- Savović J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JPT, et al. Association between risk‐of‐bias assessments and results of randomized trials in Cochrane Reviews: the ROBES Meta‐Epidemiologic Study. American Journal of Epidemiology 2018;187(5):1113‐22. [DOI: 10.1093/aje/kwx344; PMC5928453; PUBMED: 29126260] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savovíc 2012
- Savović S, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429–38. [DOI: 10.7326/0003-4819-157-6-201209180-00537; PUBMED: 22945832] [DOI] [PubMed] [Google Scholar]
Schachar 1997
- Schachar RJ, Tannock R, Cunningham C, Corkum PV. Behavioral, situational, and temporal effects of treatment of ADHD with methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(6):754‐63. [DOI: 10.1097/00004583-199706000-00011; PUBMED: 9183129] [DOI] [PubMed] [Google Scholar]
Schmidt 2009
- Schmidt S, Peterman F. Developmental psychopathology: attention deficit hyperactivity disorder (ADHD). BMC Psychiatry 2009;9:58. [DOI: 10.1186/1471-244X-9-58; PMC2751746 ; PUBMED: 19761584] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schultz 1995
- Schultz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [PUBMED: 7823387] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI: 10.1136/bmj.c332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sergeant 2003
- Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: a neuropsychological perspective. Neuroscience and Biobehavioral Reviews 2003;27(7):583‐92. [PUBMED: 14624803] [DOI] [PubMed] [Google Scholar]
Storebø 2014
- Storebø OJ, Simonsen E. Is ADHD an early stage in the development of borderline personality disorder?. Nord Journal of Psychiatry 2014;68(5):289–95. [DOI: 10.3109/08039488.2013.841992; 24117059] [DOI] [PubMed] [Google Scholar]
Storebø 2015
- Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD009885.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Storebø 2018
- Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non‐randomised studies.. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD012069.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swanson 2007
- Swanson JM, Hinshaw SP, Arnold LE, Gibbons RD, Marcus S, Hur K, et al. Secondary evaluations of MTA 36‐month outcomes: propensity score and growth mixture model analyses. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(8):1003‐14. [DOI: 10.1097/CHI.0b013e3180686d63; PUBMED: 17667479] [DOI] [PubMed] [Google Scholar]
Thalheimer 2002
- Thalheimer W, Cook S. How to calculate effect sizes from published research articles: a simplified methodology. www.bwgriffin.com/gsu/courses/edur9131/content/Effect_Sizes_pdf5.pdf (accessed 28 May 2011).
Thomas 2015
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention‐deficit/hyperactivity disorder: a systematic review and meta‐analysis. Pediatrics 2015;135(4):e994‐e1001. [DOI: 10.1542/peds.2014-3482; PUBMED: 25733754] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [DOI: 10.1093/ije/dyn179; PUBMED: 18824467] [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Walter SD, Johnston BC, Furukawa TA, Guyatt GH. Pooling health‐related quality of life outcomes in meta‐analysis ‐ a tutorial and review of methods for enhancing interpretability. Research Synthesis Methods 2011;2:188‐203. [DOI] [PubMed] [Google Scholar]
US FDA 2011
- US Food, Drug Administration. Communication about an ongoing safety review of stimulant medications used in children with attention‐deficit/hyperactivity disorder (ADHD). www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm165858.htm (accessed 6 May 2011).
Van der Oord 2008
- Oord S, Prins PJM, Oosterlaan J, Emmelkamp PMG. Efficacy of methylphenidate, psychosocial treatments and their combination in school‐aged children with ADHD: a meta‐analysis. Clinical Psychology Review 2008;28(5):783‐800. [DOI: 10.1016/j.cpr.2007.10.007; PUBMED: 18068284] [DOI] [PubMed] [Google Scholar]
Van Eck 2010
- Eck K, Finney JS, Evans S. Parent report of ADHD symptoms of early adolescents: a confirmatory factor analysis of the Disruptive Behavior Disorders scale. Educational and Psychological Measurement 2010;70:1042‐59. [Google Scholar]
Walcott 2004
- Walcott CM, Landau S. The relation between disinhibition and emotion regulation in boys with attention deficit hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology 2004;33(4):772‐82. [DOI: 10.1207/s15374424jccp3304_12; PUBMED: 15498744] [DOI] [PubMed] [Google Scholar]
Waschbusch 2011 [pers comm]
- Waschbusch D. Cochrane review: Social skills training [personal communication]. Email to: OJ Storebø 22 June 2011.
Wechsler 2003
- Wechsler D. Wechsler Intelligence Scale for Children. 4th Edition. London (UK): Pearson, 2003. [Google Scholar]
Weller 2010
- Weller EB, Weller RA, Fristad MA, Rooney MT, Schecter BA. Children's Interview for Psychiatric Syndromes (ChIPS). Journal of the American Academy of Child & Adolescent Psychiatry 2000;39(1):76‐84. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI: 10.1016/j.jclinepi.2007.03.013; PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI: 10.1186/1471-2288-9-86; PMC2809074; PUBMED: 20042080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17:39. [DOI: 10.1186/s12874-017-0315-7; PMC5397700; PUBMED: 28264661] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whalen 1985
- Whalen CK, Henker B. The social worlds of hyperactive (ADHD) children. Clinical Psychology Review 1985;5(5):447‐78. [DOI: 10.1016/0272-7358(85)90004-2] [DOI] [Google Scholar]
Willcut 2012
- Willcutt EG. The prevalence of DSM‐IV attention‐deficit/hyperactivity disorder: a meta‐analytic review. Neurotherapeutics 2012;9(3):490‐9. [DOI: 10.1007/s13311-012-0135-8; PMC3441936; PUBMED: 22976615] [DOI] [PMC free article] [PubMed] [Google Scholar]
Willis 2019
Wolraich 2007
- Wolraich ML, McGuinn L, Doffing M. Treatment of attention deficit hyperactivity disorder in children and adolescents: safety considerations. Drug Safety 2007;30(1):17‐26. [DOI: 10.2165/00002018-200730010-00003; PUBMED: 17194168] [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schultz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI: 10.1136/bmj.39465.451748.AD; PMC2267990; PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
World Congress on ADHD 2011
- World Congress on ADHD. 3rd World Congress on ADHD: from childhood to adult disease. ADHD Attention Deficit and Hyperactivity Disorders 2011;3(2):77‐234. [DOI: 10.1007/s12402-011-0058-y] [DOI] [Google Scholar]
World Congress on ADHD 2013
- World Congress on ADHD. 4th World Congress on ADHD: from childhood to adult disease. ADHD Attention Deficit and Hyperactivity Disorders 2013;5(2):111‐247. [DOI: 10.1007/s12402-013-0109-7] [DOI] [Google Scholar]
World Congress on ADHD 2015
- World Congress on ADHD. 5th World Congress on ADHD: from child to adult disorder. ADHD Attention Deficit and Hyperactivity Disorders 2015;7(Suppl. 1):S1‐S119. [DOI: 10.1007/s12402-015-0169-y] [DOI] [PubMed] [Google Scholar]
World Congress on ADHD 2017
- World Congress on ADHD. 6th World Congress on ADHD: from child to adult disorder. ADHD Attention Deficit and Hyperactivity Disorders 2017;9(Suppl. 1):S1‐S55. [10.1007/s12402‐017‐0224‐y] [DOI] [PubMed] [Google Scholar]
Xu 2018
- Xu G, Strathearn L, Liu B, Yang B, Bao W. Twenty‐year trends in diagnosed Attention‐Deficit/Hyperactivity Disorder among US children and adolescents, 1997‐2016. JAMA Network Open 2018;1(4):e181471. [DOI: 10.1001/jamanetworkopen.2018.1471] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoshimasu 2012
- Yoshimasu K, Barbaresi WJ, Colligan RC, Voigt RG, Killian JM, Weaver AL, et al. Childhood ADHD is strongly associated with a broad range of psychiatric disorders during adolescence: a population‐based birth cohort study. Journal of Child Psychology and Psychiatry and Allied Disciplines 2012;53(10):1036‐43. [DOI: 10.1111/j.1469-7610.2012.02567.x; PMC3608464; PUBMED: 22647074] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zametkin 1987
- Zametkin AJ, Rapoport JL. Neurobiology of attention deficit disorder with hyperactivity: where have we come in 50 years?. Journal of the American Academy of Child and Adolescent Psychiatry 1987;26(5):676‐86. [DOI: 10.1097/00004583-198709000-00011; PUBMED: 2889717] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Storebø 2010
- Storebø OJ, Skoog M, Damm D, Thomsen PH, Simonsen E, Gluud C. Social skills training for children with attention deficit hyperactivity disorder (ADHD). Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD008223] [DOI] [PubMed] [Google Scholar]
Storebø 2011
- Storebø OJ, Skoog M, Damm D, Thomsen PH, Simonsen E, Gluud C. Social skills training for attention deficit hyperactivity disorder (ADHD) in children aged 5 to 18 years. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008223.pub2; PUBMED: 22161422] [DOI] [PubMed] [Google Scholar]


