Key Teaching Points.
-
•
Painful left bundle branch block (LBBB) syndrome is a rare disorder in which a rate-related LBBB aberration causes debilitating exertional chest discomfort. The diagnosis is made clinically. The presence of tall T waves in normally conducted beats in the right precordial leads (quantified as an S wave–to–T wave ratio of ≤1.8) is supportive of this diagnosis.
-
•
The pathophysiology of this disorder is unknown. Proposed mechanisms include a disorder in the afferent neural network responsible for introception (heartbeat awareness) that may be abnormally activated during aberrant ventricular conduction in some patients, as well as disordered left ventricular activation causing dyssynchrony.
-
•
First-line treatment of painful LBBB is usually sinus node suppression using beta-blockers or ivabradine. Alternative therapies include cardiac resynchronization therapy or His-bundle pacing. However, in some cases, right ventricular pacing alone may be sufficient to control patient symptoms.
Introduction
Painful left bundle branch block (LBBB) syndrome is a disorder whereby rate-related ventricular conduction aberrancy results in exertional chest discomfort and/or dyspnea. Because this condition is uncommon in the general population and because it is frequently underrecognized by health care providers, its pathophysiology and treatment have not been studied in depth.
Since its earliest description in 19461 a variety of treatment strategies have been utilized, including pharmacologic sinus node suppression (designed to limit heart rate response to exercise, thus avoiding aberrant conduction altogether); in at least 2 instances,2, 3 His-bundle pacing has also been successfully employed to treat this disorder.
We report the case of a patient with painful LBBB syndrome who experienced marked symptom improvement with right ventricular (RV) pacing alone, as well as with left ventricular (LV) synchronous pacing.
Case report
A 46-year-old man with a past medical history notable for hypertension and hyperlipidemia presented to our outpatient clinic with 1 year of exertional chest pain. He described his symptoms as an anginal sensation in the mid sternum that would occur suddenly and predictably with moderate exertion and would always resolve after several minutes of rest. He also reported associated dyspnea and dizziness. Coronary angiography performed at an outside facility revealed no obstructive epicardial lesions. Transthoracic echocardiography and subsequent cardiac magnetic resonance imaging showed normal resting biventricular cavity sizes and systolic function along with mild-to-moderate mitral regurgitation; additionally, cardiac magnetic resonance imaging revealed no late gadolinium enhancement.
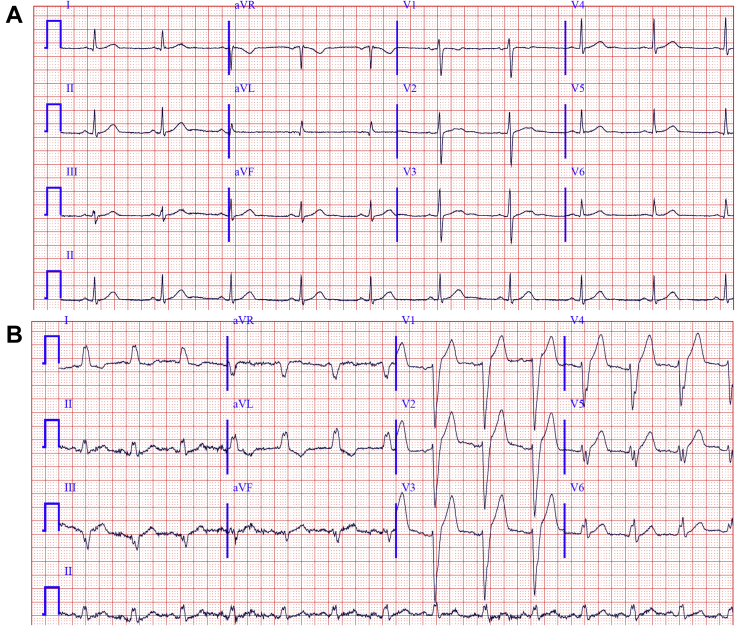
During an exercise treadmill stress test, the patient developed LBBB at a rate of 120 beats per minute (bpm); this was associated with an immediate reproduction of his typical symptoms. The LBBB and symptoms resolved abruptly during recovery at a rate of 80 bpm. Twelve-lead electrograms at rest and during exertion are shown in Figure 1; note that when LBBB was present, QRS width was 140 ms.
Figure 1.
Twelve-lead electrocardiogram (ECG) obtained at rest and with minor exertion. A: Resting 12-lead ECG showed sinus rhythm at 60 bpm; there were no abnormalities. B: During minor exertion, the patient developed left bundle branch block with QRS width of 140 ms; there was borderline leftward QRS axis and there were tall T waves in the right precordial leads (S/T ratio of 1.71). The patient complained of squeezing chest pain at that time.
The initial therapeutic strategy was aimed at suppressing sinus node function; however, both metoprolol succinate and ivabradine failed to adequately attenuate sinus rates at low doses. At higher doses, the patient reported intractable side effects, including severe fatigue and dyspnea. Consequently, a decision was made to proceed with a cardiac resynchronization therapy (CRT) pacemaker device implant. This included the placement of a quadripolar lead in the posterolateral branch of the coronary sinus, a bipolar lead in the midseptal aspect of the right ventricle, and another bipolar lead in the right atrial (RA) appendage. Postimplant, the device was programmed to deliver RA-LV sequential pacing (DDD with LV-only ventricular output) with a rate-responsive baseline rate. The patient reported dramatic improvement in his symptoms immediately after device implant.
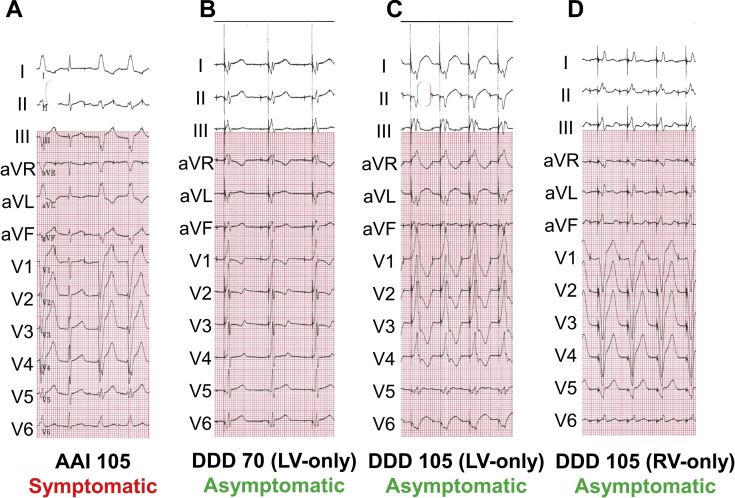
Approximately 1 month later, the patient attended a routine follow-up outpatient appointment, during which various pacemaker programming maneuvers were pursued (Figure 2). First, the device was programmed to AAI mode and the base rate was gradually increased from 60 bpm. As expected, there was normal ventricular conduction at baseline and LBBB aberrancy occurred at 105 bpm (Figure 2A); LBBB was associated with an immediate onset of symptoms. Next, the device was programmed to an RA-LV sequential pacing mode (DDD with LV-only ventricular output) and again the base rate was increased from 60 bpm. At low rates, we observed ventricular fusion (Figure 2B). At 105 bpm, the ventricular complexes were fully paced (Figure 2C); although the patient was aware of the elevated heart rates, he denied any chest pain. Finally, the device was programmed to an RA-RV sequential pacing mode (DDD with RV-only ventricular output) and the base rate was gradually increased (Figure 2D). At 105 bpm, the ventricular complexes were fully paced and were wider than during native LBBB conduction (160 ms); however, the patient again denied any chest pain.
Figure 2.
Pacing maneuvers done approximately 1 month after cardiac resynchronization therapy pacemaker device implant. A: The patient developed left bundle branch block and associated chest pain during atrial-only pacing at 105 beats per minute (bpm) (AAI mode). B: With right atrial (RA)-left ventricular (LV) synchronous pacing at 70 bpm (DDD mode with LV-only ventricular output) there were ventricular fusion complexes; the patient had no symptoms. C: As the pacemaker base rate was increased to 105 bpm, the ventricular complexes became purely paced; at this point, the patient was aware of the elevated heart rate but he denied any chest pain. D: The device was then programmed to RA–right ventricular (RV) synchronous pacing at 105 bpm (DDD mode with RV-only ventricular output), during which the patient remained chest pain free.
Discussion
Although rate-related LBBB aberrancy is a frequently observed phenomenon in clinical practice, it is nearly always asymptomatic. This makes the fact that an apparent minority of patients with rate-related LBBB aberrancy develop debilitating symptoms all the more intriguing.
We recently reported a case series of 50 patients with painful LBBB syndrome.4 We noted that most affected individuals exhibited normal LV function and their electrocardiograms were normal immediately before and immediately after the aberrancy was observed. When present, LBBB was associated with an inferior QRS axis and tall T waves in the precordial leads (quantified as maximum S/T ratio of ≤1.8). The patient reported in the present work fits this profile, albeit his QRS axis was pointed borderline leftward.
It has also been suggested that sinus node suppression (either by pharmacologic means5 or via physical conditioning6) is frequently not effective, although this may have been a consequence of publication bias; that is to say, case reports of patients responding to relatively conservative treatment have selectively not been published in the literature. In our case series review,4 we proposed that device-based therapies could also be useful in some instances. Indeed, 2 recent studies reported that His-bundle pacing can been used to effectively treat this syndrome2, 3; in both of these reports, His-bundle pacing at high rates yielded narrow, pseudo-fused complexes, suggesting capture of predestined left bundle branch fibers beyond the level of functional block. The fact that His-bundle pacing can control symptoms makes intuitive sense, since this strategy strives to approximate normal ventricular depolarization. It also suggests that CRT could offer similar benefit; indeed, a single case of successful treatment of painful LBBB with CRT has been reported.7
In contrast, any form of RV pacing will result in highly abnormal ventricular activation and would not be expected to ameliorate symptoms related to aberrant ventricular conduction. Indeed, the patient described by Suryanarayana and colleagues3 experienced symptoms during RV septal pacing similar to those during rate-related LBBB conduction. Yet, in the present case report, our patient had no symptoms during RV septal pacing with QRS complexes wider (160 ms) and more inferiorly directed than those seen with native LBBB (140 ms, borderline left axis deviation). To our knowledge, this is only the second reported instance where RV-only pacing successfully treated painful LBBB syndrome4 (case 4 in this reference). This highlights the fact that symptom pathogenesis is not broadly explained by overall ventricular dyssynchrony (resulting from either LBBB or RV pacing). Instead, it is a subtler process whereby even relatively similar modes of ventricular activation (and/or repolarization) can have dramatically different consequences. We previously proposed that these subtleties may reflect variations in afferent neural networks responsible for introception (heartbeat awareness).8 However, this has not yet been proven and thus the exact pathophysiology of this syndrome remains unknown.
Regardless of the mechanism, the clinical implication of this case report is that RV-only pacing may be sufficient for the treatment of some patients with painful LBBB syndrome. Consequently, during device implant, patients who report no symptoms with intraprocedural RV-only pacing could conceivably avoid having to undergo more complex lead placement (such as His-bundle lead or coronary sinus lead placement). In addition, one should recognize that pacing from different locales within the right ventricle may produce differing clinical response. Therefore, it may be justifiable to test pace from multiple sites (eg, the RV apex, mid-RV septum, and the RV outflow tract) prior to permanent pacing lead delivery.
Though the implications raised by this and similar patient reports are intriguing, it is important to acknowledge the limitations inherent to single case reports. Most important, the pacing maneuvers discussed in the present work were not done in a double-blinded fashion: while the patient was unaware of what was being done, the treating physicians were in control of the effected therapy. The inherent bias may limit the generalizability of the observed result.
Conclusion
Painful LBBB is an uncommon and rarely recognized disorder usually treated with sinus node suppression or, occasionally, His-bundle pacing. However, in some cases, RV-only pacing may also be effective in controlling patients’ symptoms. This suggests a complex underlying pathophysiology. Electrophysiologic testing prior to device implantation has been proposed as a means to identify patients who will respond to RV-only pacing.
References
- 1.Eichert H. Transient bundle branch block associated with tachycardia. Am Heart J. 1946;31:511–518. doi: 10.1016/0002-8703(46)90436-x. [DOI] [PubMed] [Google Scholar]
- 2.Viles-Gonzalez J.F., Mahata I., Anter E., D’Avila A. Painful left bundle branch block syndrome treated with his bundle pacing. J Electrocardiol. 2018;51:1019–1022. doi: 10.1016/j.jelectrocard.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Suryanarayana P.G., Frankel D.S., Marchlinski F.E., Schaller R.D. Painful left bundle branch syndrome treated successfully with permanent His bundle pacing. HeartRhythm Case Rep. 2018;4:439–443. doi: 10.1016/j.hrcr.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shvilkin A., Ellis E.R., Gervino E.V., Litvak A.D., Buxton A.E., Josephson M.E. Painful left bundle branch block syndrome: clinical and electrocardiographic features and further directions for evaluation and treatment. Heart Rhythm. 2016;13:226–232. doi: 10.1016/j.hrthm.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Perin E., Petersen F., Massumi A. Rate-related left bundle branch block as a cause of non-ischemic chest pain. Cathet Cardiovasc Diagn. 1991;22:45–46. doi: 10.1002/ccd.1810220111. [DOI] [PubMed] [Google Scholar]
- 6.Heinsimer J.A., Skelton T.N., Califf R.M. Rate-related left bundle branch block with chest pain and normal coronary arteriograms treated by exercise training. Am J Med Sci. 1986;292:317–319. doi: 10.1097/00000441-198611000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Czuriga D., Lim P.O. Cardiac resynchronization therapy relieves intractable angina due to exercise-induced left bundle branch block without left ventricular systolic dysfunction: a detailed case study. J Cardiovasc Electrophysiol. 2016;27:609–612. doi: 10.1111/jce.12911. [DOI] [PubMed] [Google Scholar]
- 8.Gray M.A., Taggart P., Sutton P.M. A cortical potential reflecting cardiac function. Proc Natl Acad Sci U S A. 2007;104:6818–6823. doi: 10.1073/pnas.0609509104. [DOI] [PMC free article] [PubMed] [Google Scholar]