Abstract
Objectives:
This project proposes a unique methodology utilizing electromyography and mechanomyography to determine the intensity of a desired movement which may be useful in further developing decomposition algorithms for prosthetic controls.
Methods:
Ten males performed isometric leg extension muscle actions corresponding to 20, 40, 60, 80, and 100% of their maximal voluntary isometric contraction force. The duration and amplitude of the gross lateral movement of the mechanomyographic signal as well as electromechanical delay were measured during each contraction.
Results:
The results indicated that the duration of the gross lateral movement decreased with increases in intensity (20<40=60<80<100% maximal voluntary isometric contraction) and that the amplitude of the gross lateral movement increased with increases in intensity (20<40=60<80<100% maximal voluntary isometric contraction). In addition, electromechanical delay decreased with each increase in intensity. These measurements occurred within 40 ms from the onset of the electromyographic signal.
Conclusions:
Thus, these measurements may be incorporated into existing prosthetic control algorithms to reduce grasp times and identify the intensity of a movement earlier. In addition, the gross lateral movement and electromechanical delay measurements may provide more intuitive controls for prosthetic users.
Keywords: Electromechanical Prosthetic, Myoelectric Control, Real-Time Control, GLM, EMD
Introduction
In 2005, a total of 1.6 million individuals in the United States were living with a loss of limb and it has been projected that by 2050 this number will rise to 3.6 million[1]. With an expected increase in amputees, the need for prosthetic development also increases. It has been reported[2-5] that 30 to 50% of amputees are unsatisfied with the comfort or functionality of their prosthetic. The majority of prosthetics are body powered or myoelectric. Body powered prosthetics rely on a series of cables and harnesses attached to non-affected joints to move the prosthesis through joint manipulation of the unaffected limb. It has been reported[6] that body powered prostheses require high levels of force to actuate which may result in greater fatigue or difficulty of use, especially for children[7]. In addition, many amputees reported[2,5] discontinuing the use of body powered prosthetics due to their lack of comfort, aesthetic appeal, and functionality.
Recently, the development of myoelectric prosthetics aimed to address the issues related to aesthetics, difficultly of use, comfort, and functionality posed by body powered prosthetics. Myoelectric prosthetics use the electromyographic (EMG) signal from muscles of the affected limb to control the movements of an externally powered prosthetic. The EMG signal consists of motor unit action potentials which reflect the neural signal sent from the spinal cord to the muscles[8]. Thus, through decomposition methods the EMG signal could be used to interpret the intensity and desired movement which can then be used to control myoelectric prosthetics. The decomposition of the EMG signal can be performed a number of ways including: wavelet analysis, auto-regression, short-term or fast Fourier transform, Fuzzy logic system, artificial intelligence, or higher order statistics[9]. Many prosthetics utilize only one decomposition method, however, it is likely that a combination of many methodologies will be required to address the current issues with myoelectric prosthetics. Specifically, in a recent study by Farina et al[8], they examined current myoelectric controlled upper-limb prostheses and identified specific issues that need to be further developed which included: more intuitive, closed-loop, adaptive, robust real-time controls (<200 ms), minimal number of recording electrodes, limited complexity, and low power consumption[8].
Based on the reports of amputees[2-5] and the findings of Farina et al[8], there is a need for decomposition algorithms that can create more intuitive and robust real-time myoelectric prosthetics (<200 ms). Previous studies[10-14] have reported myoelectric prosthetic grasp times from 400 to 5,000 ms, however, there are currently no standardized measurements for reporting these grasp times[15] (i.e. inclusion of signal onset, decomposition time, etc. in grasp time measurement). The grasp times ranging from 400 to 5,000 ms are well beyond what is considered a real-time control (<200 ms) defined by Farina et al[8]. Therefore, further examination and development of new signal acquisition techniques and decomposition algorithms are needed to create a more robust real-time myoelectric prosthetic.
It has been reported[16-18] that many myoelectric prosthetics require extensive training to become proficient enough to improve the performance of activities of daily living (ADL). In addition, the greater the number of hours of training required to become proficient enough to utilize a prosthetic are related to greater amputee drop-out rates. Therefore, as new signal acquisition techniques and decomposition algorithms are developed, consideration to the intuitive nature of the device should be given to increase amputee-prosthetic retention and usability of the prosthetic.
The primary method for controlling myoelectric prosthetics currently utilizes the EMG signal, however, some recent studies have proposed the use of the mechanomyographic (MMG) signal. The MMG signal measures the lateral oscillations of the activated muscle and occurs after the onset of the EMG signal[19,20]. The use of the MMG signal for prosthetic controls is relatively new compared to body powered or EMG based prosthetics and have primarily been proposed in research laboratories and not commercially available prosthetics. For example, Silva et al[21], reported using the root mean square (RMS) amplitude of the MMG signal to identify extension and flexion movements from the forearm, however, there were incorrect movements reported due to artifact or unconscious movements. In addition, Al-Mulla and Sepulveda[22] proposed a wavelet decomposition process for the MMG signal to control extension and flexion movements, but was only able reach 70% accuracy. Thus, the use of MMG signals to control prosthetics needs further development before they can be widely applied in clinical or commercial settings.
Few studies[23] have attempted to combine EMG and MMG signals for the control of prosthetics. For example, Xiloyannis et al[23], used Gaussian Process and Vector Auto-Regressive Moving Average Models to map muscle activity of the forearm, however, with low decomposition accuracy, this method could not be applied to prosthetics. In addition, Kimoto and Yamada[24] proposed a new EMG, MMG, and oxygen consumption sensor designed for the control of prosthetics by using EMG RMS, MMG RMS, as well as EMG mean power frequency (MPF), and MMG MPF. The design proposed by Kimoto and Yamada[24], however, reported that their sensor and algorithm was unable to discern between extension and flexion movements of the forearm. Thus, further development of an EMG and MMG based decomposition algorithm needs to be explored before this methodology can be used for the control of prosthetics.
The current study proposes unique signal acquisition techniques and decomposition theories which may lead to more intuitive controls and a reduction in decomposition algorithm time by examining the initiation of the EMG and MMG signals to provide earlier indications of the desired intensity and movement. Specifically, combining electromechanical delay measurements and simple decomposition of the gross lateral movement of the MMG signal may provide useful information for the control of myoelectric prosthetics. The electromechanical delay can be measured from the onset of the EMG signal to the onset of the MMG signal (EMDE-M). It has been suggested[20] that an increase in intensity results in a decrease in EMDE-M. It has also been suggested[19,20] that EMDE-M values are muscle-specific and may provide information about the desired movement. With contraction there is a gross lateral movement of the muscle which pulls in-line with the long axis of the bone causing large deflections in the MMG signal. Therefore, this projects proposes’ a unique methodology utilizing EMG and MMG to determine the intensity of the desired movement which may be useful in further developing decomposition algorithms for prosthetic controls.
Material & methods
Subjects
Ten men (mean ± SD age 23±2.7 yr; body mass 78.6±5.7 kg; height 173.2±6.3 cm) volunteered to participate in this study. The subjects were non-amputees and free from any musculoskeletal injuries or neuromuscular disorders. This study was approved by the Institutional Review Board, and all subjects signed a written informed consent and completed a health history questionnaire prior to participation. In addition, this study was performed in agreement with the ethical principles stated in the Declaration of Helsinki[25].
Electromyographic and mechanomyographic signals
A bipolar surface electrode arrangement (Ag/AgCl, AccuSensor, Lynn Medical, Wixom, MI, USA) was placed on the vastus lateralis (VL) of the dominant leg (based on kicking preference) with an interelectrode distance of 30 mm. The skin was dry shaven, abraded, and cleaned with isopropyl alcohol prior to placing the electrodes. The bipolar electrode arrangement was then placed 66% of the distance between the anterior superior iliac spine and the lateral border of the patella and orientated at a 20° angle to approximate the pennation angle of the muscle fibers[26,27]. A reference electrode was placed over the anterior superior iliac spine. The EMG signals were zero-meaned and bandpass filtered (fourth-order Butterworth) at 10-500 Hz. The MMG signal was measured using uniaxial accelerometers (EGAS-S704-10, Measurement Specialties Inc., Hampton, VA) placed between the bipolar electrode arrangement on the VL. The MMG signals were zero-meaned and bandpass filtered (fourth-order Butterworth) at 5-100 Hz. All signals were simultaneously collected through a BioPac MP150 (BioPac System Inc., Goleta, CA) at a sampling frequency of 10,000 Hz. All signal processing was performed using custom programs written with LabVIEW software (Version 15.0, National Instruments, Austin TX).
Testing protocol
Subjects performed a warm-up consisting of 5 to 8 submaximal isometric leg extension contractions at approximately 50-80% of their perceived maximal voluntary isometric contraction (MVC) force. Then each subject performed 2, 6-s MVC muscle actions with 2-min of rest after each trial which was used to determine 100% MVC. Afterwards, subjects performed a series of randomly ordered submaximal isometric step muscle actions at 20, 40, 60, and 80% MVC which were determined from the highest MVC torque value of the 2 trials. Each isometric step muscle action was held for approximately 4-s with a digital display of their force production on a screen placed in front of the subjects. A minimum of 1-min of rest was given between each step isometric muscle action to avoid any effects of fatigue. All isometric muscle actions were performed at a knee joint angle of 120° (180° being full extension)[28].
Electromechanical delay measurements
The voluntary EMD measurements were determined from the onset of the EMG signal to the onset of the MMG signal (EMDE-M). The onset of EMG, MMG, and force were determined by the condition of three standard deviations (SDs) from the mean baseline noise observed for each signal[19,20] (Figure 1).
Figure 1.
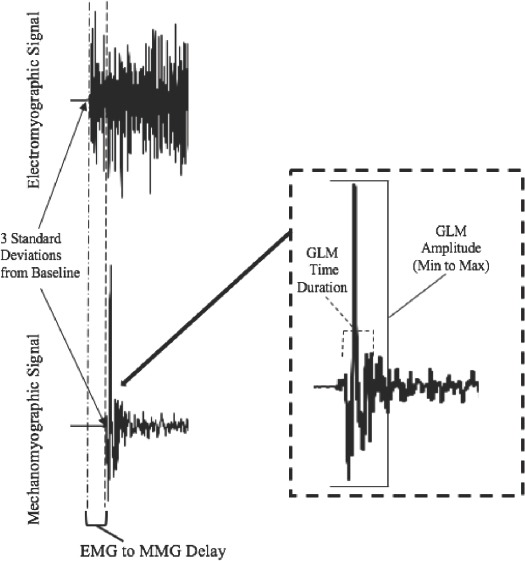
Depiction of the determination of electromechanical delay measurement from the electromyographic (EMG) and mechanomyographic (MMG) signals. The onset of the EMG and MMG signals were determined by a change greater than 3 standard deviations (SD) from a baseline/resting value. In addition, the location of the gross lateral movement (GLM) amplitude and duration as they relate to the mechanomyographic signal. The GLM Amplitude was determined from the absolute minimum to maximal value while the duration was determined from the onset of the mechanomyographic signal to the onset of the small lateral oscillations of the muscle.
Gross lateral movement measurements
The gross lateral movement was measured from the beginning of the MMG signal, determined by 3 SD from the baseline noise, until the beginning of the small oscillations of the muscle associated with the resonant frequency of the muscle[29] (Figure 1). The gross lateral movement amplitude (GLMAMP) was determined from the minimum to maximum peak values measured during the gross lateral movement (Figure 1). The gross lateral movement time-duration (GLMTime) was measured from the onset of the MMG signal to the beginning of the small lateral oscillations associated with the resonant frequency of the muscle recorded from the MMG signal (Figure 1).
Statistical analysis
Electromechanical delay
A repeated measures ANOVA was used to determine mean differences in EMDE-M by Intensity [1 (EMDE-M) x 5 (Intensity: 20, 40, 60, 80, and 100% MVC)] with post-hoc, pair-wise comparisons with Tukey’s LSD correction.
Gross lateral movement measurements
Two, separate, 1 x 5 (Intensity: 20, 40, 60, 80, and 100% MVC) repeated measures ANOVAs were used to examine GLMAMP and GLMTime with post-hoc pair-wise comparisons with Tukey’s LSD correction. If the assumption of sphericity was violated during any analysis, the Huynh-Feldt correction was used. An alpha of p≤0.05 was considered statistically significant for all statistical analyses (SPSS Version 22.0, Armonk, NY).
Results
Electromechanical delay
There was a significant 1-way repeated measures ANOVA (p<0.01, η2p =0.86) for EMDE-M by Intensity (20, 40, 60, 80, and 100% MVC). Post-hoc analyses indicated that 20>40 (p<0.01)>60 (p=0.02)>80 (p<0.01)>100% MVC (p<0.01) (Figure 2). In addition, Figure 3 depicts the individual responses for each of the subjects. All subjects exhibited decreases in EMDE-M with each increase in intensity (Figure 3).
Figure 2.
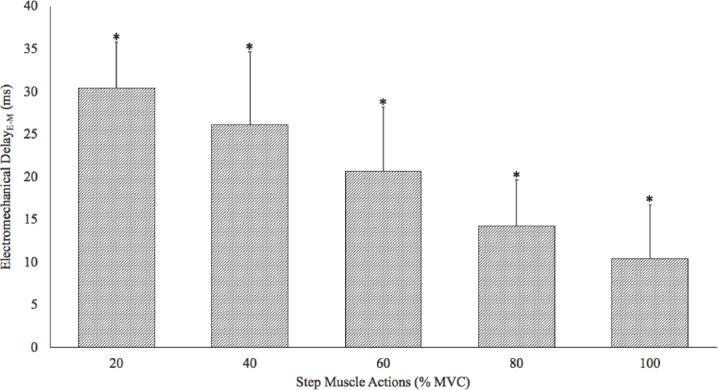
Composite (mean ± SD) electromechanical delay measurements determined from the onset of the electromyographic signal to the onset of the mechanomyographic signal. There was a significant decrease in the duration for the electromechanical delay with each increase in intensity (*= 20>40>60>80>100% maximal voluntary isometric contraction [MVC]).
Figure 3.
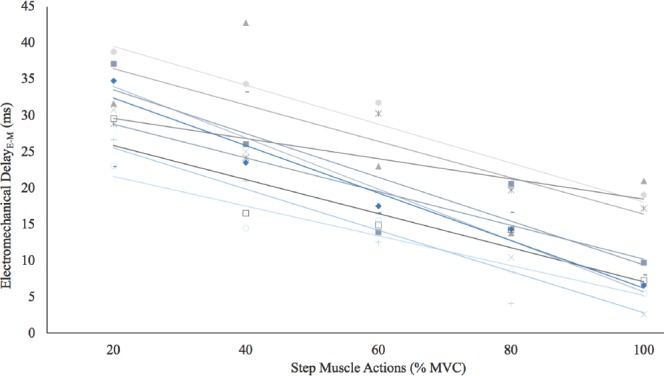
Individual responses for the electromechanical delay from the onset of the electromyographic signal to the onset of the mechanomyographic signal. There were decreases (r2=0.61 to 0.96; p<0.01) in the duration for the electromechanical delay with each increase in intensity (20>40>60>80>100% maximal voluntary isometric contraction [MVC]) for all subjects.
Gross lateral movement measurements
There were significant 1-way repeated measures ANOVAs by Intensity (20, 40, 60, 80, and 100% MVC) for both the GLMAMP (p<0.01, η2p =0.99) and GLMTime (p<0.01, η2p =0.97). Post-hoc analyses indicated that 20<40 (p<0.01)=60 (p=0.08)<80 (p<0.01)<100% MVC (p<0.01) for GLMTime (Figure 4). In addition, post-hoc analyses indicated that 20>40 (p<0.01)=60 (p=0.06)>80 (p<0.01)>100% MVC (p<0.01) for GLMAMP. Figure 5 and 6 depict the individual GLMAMP and GLMTime responses, respectively, for each subject. For GLMAMP, all subjects exhibited increases with each increase in intensity (Figure 5). For GLMTime, all subjects exhibited decreases with each increase in intensity (Figure 6).
Figure 4.
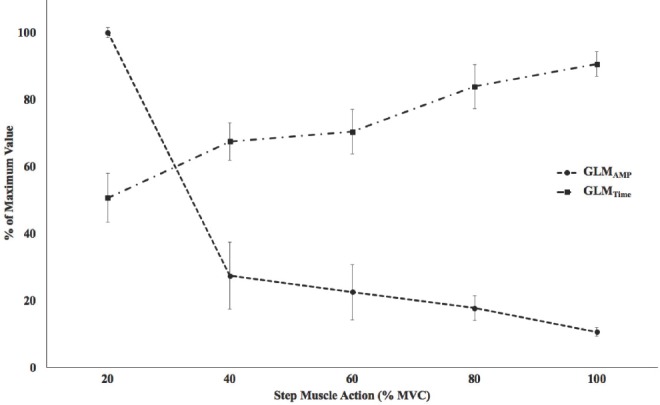
Composite (mean ± SD) gross lateral movement (GLM) amplitude (GLMAMP) and duration (GLMTime) as a % of maximal value during 20, 40, 60, 80, and 100% maximal voluntary isometric contraction (MVC). There were significant increases in GLMAMP with increases in intensity (20<40=60<80<100% MVC). There were significant decreases in GLMTime with increases in intensity (20>40= 60>80>100% MVC).
Figure 5.
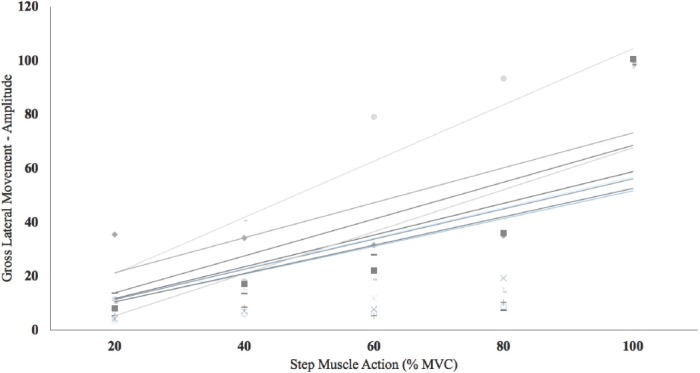
Individual responses for the gross lateral movement amplitude measurements during 20, 40, 60, 80, and 100% maximal voluntary isometric contractions (MVC). All subjects exhibited increases (r2=0.39 to 0.85; p<0.01 to 0.02) in gross lateral movement amplitude with an increase in intensity.
Figure 6.
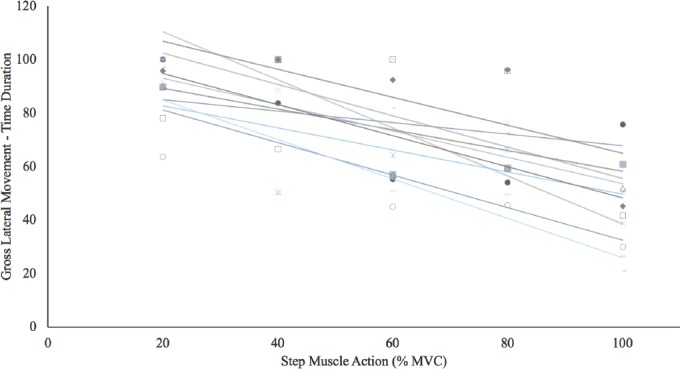
Individual responses for the gross lateral movement duration measurements during 20, 40, 60, 80, and 100% maximal voluntary isometric contractions (MVC). All subjects exhibited decrease (r2=0.29 to 0.79; p<0.01 to 0.04) in gross lateral movement duration with an increase in intensity.
Discussion
Electromechanical delay
In the current study, there were mean decreases in EMDE-M with each increase in intensity level (20>40>60>80>100% MVC) (Figure 2). This was also true for each individual subject (Figure 3). These findings were similar to those of Smith et al[19], who reported greater EMDE-M during submaximal compared to maximal isometric leg extension muscle actions from the VL. In addition, these findings were similar to those of Yavuz et al[30], who reported decreases in EMD, determined from the onset of the EMG signal to the onset of force production, during 10 to 50% MVC step isometric muscle actions from the triceps surea. The EMDE-M values represent the duration for motor unit action potentials to propagate and stimulate movement from the muscle, which has been termed excitation-contraction coupling[19,20,31]. Specifically, EMDE-M occurs prior to the muscles movement and is not associated with taking up the extra tendon slack of muscle[19,20,31]. Since EMDE-M reflects the time duration before the muscle moves, it does not include the series elastic component, which is typically referred to as EMDM-F, and is the counterpart of the overall electromechanical delay measurement from the onset of the EMG signal to the initiation of force production (EMDE-F). The EMDM-F and EMDE-F measures were purposefully excluded from this study as amputees’ prosthetic devices take the place of the missing limb where force production is traditionally measured. Amputees, however, are often still able to contract their muscles of the affected limb which provides the EMDE-M measure. Thus, EMDE-M reflects excitation-contraction coupling which occurs prior to force production and, therefore, EMDE-M may be an intuitive early indicator of the desired movement and intensity level in amputees. Specifically, any muscle that can be voluntarily contracted by an amputee may be done at different perceived intensities which will exhibit unique EMDE-M values that can potentially be used to control a prosthetic.
The initial grasp time of a prosthetic may, theoretically, be reduced by using EMDE-M as a complimentary measurement to existing algorithms. That is, myoelectric controlled prosthetics which utilize wavelet, EMG RMS, or auto-regression algorithms obtain the required biosignals following the onset of the EMG signal and gross lateral movement. The EMDE-M measurement, however, can be obtained in approximately 10 to 30 ms from the first peripherally detectable indicator of a muscle activation (onset of EMG signal). Therefore, utilizing EMDE-M may provide an indicator of the desired movement and intensity level in amputees earlier than previously developed methods. Thus, EMDE-M may have potential implications for decreasing grasp times and increasing the intuitive control mechanisms in existing myoelectric prosthetic control algorithms by providing earlier muscle activation information about the desired movement and intensity.
Gross lateral movement of the mechanomyographic signal
The GLMAMP increased and GLMTime decreased with increases in force production from 20 to 40%, 60 to 80%, and 80 to 100%, but not from 40 to 60% MVC during isometric leg extension muscle actions (Figure 4). In addition, all subjects exhibited increases in GLMAMP and decreases in GLMTime with increases in intensity (Figure 5 and 6). To our knowledge, no previous studies have examined the minimum to maximum value (GLMAMP) or duration (GLMTime) from the gross lateral movement during step muscle actions (Figure 1). These findings indicated that the intensity of pull on the muscle during the gross lateral movement was generally related to GLMAMP. That is, as the intensity of a movement increases there is a general increase in GLMAMP. In addition, these data suggested intensity-related changes in the duration for the muscle to pull in-line with the long axis of the bone (GLMTime). Specifically, low intensities resulted in greater durations to pull the muscle in-line with the bone compared to higher intensities. These relationships were observed with each increase in intensity, except from 40 to 60% MVC. Therefore, both GLMAMP and GLMTime provided similar information and may be useful for determining an intensity range (i.e. low, moderate, and high intensity) of a desired movement.
Conclusion
These findings indicated that the combination of EMG and MMG allowed for an early indication of the intensity of a desired movement through the analysis of EMDE-M, GLMAMP, and GLMTime. These measurements typically occurred during the first 40 ms after the onset the EMG signal. In addition, EMDE-M, GLMAMP, and GLMTime reflect unique physiological mechanisms which occur prior to the portion of the signal that most algorithms utilize to control a prosthetic. Therefore, incorporating these measurements into existing algorithms may help in reducing grasp times by identifying the intensity of a movement earlier. It is also plausible that these measurements are more intuitive for users due to these measurements being directly related to the intensity of a contraction. It is important to note, however, that this study utilized a healthy population and that these methods should be further investigated in amputees and with different muscles. In conclusion, EMDE-M, GLMAMP, and GLMTime may be useful for earlier detection of the intensity of a movement in myoelectric prosthetic control algorithms.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States:2005 to 2050. Arch Phys Med Rehab. 2008;89(3):422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Cupo M, Sheredos S. Clinical evaluation of a new, above-elbow, body-powered prosthetic arm:a final report. J Rehabil Res Dev. 1998;35(4):431–446. [PubMed] [Google Scholar]
- 3.Doeringer J, Hogan N. Performance of above elbow body-powered prostheses in visually guided unconstrained motion tasks. IEEE Tran Biomed Eng. 1995;42(6):621–631. doi: 10.1109/10.387202. [DOI] [PubMed] [Google Scholar]
- 4.Pezzin L, Dillingham T, MacKenzie E, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services 1. Arch Phys Med Rehab. 2004;85(5):723–729. doi: 10.1016/j.apmr.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Carey S, Lura D, Highsmith M. Differences in myoelectric and body-powered upper-limb prostheses:systematic literature review. J Rehabil Res Dev. 2015;52(3):247–262. doi: 10.1682/JRRD.2014.08.0192. [DOI] [PubMed] [Google Scholar]
- 6.Carrozza M, Suppo C, Sebastiani F, Massa B, Vecchi F, Lazzarini R, Cutkosky M, Dario P. The SPRING hand:development of a self-adaptive prosthesis for restoring natural grasping. Auton Robot. 2004;16(2):125–141. [Google Scholar]
- 7.Kruit J, Cool J. Body-powered hand prosthesis with low operating power for children. J Med Eng Tech. 1989;13(1-2):129–133. doi: 10.3109/03091908909030212. [DOI] [PubMed] [Google Scholar]
- 8.Farina D, Jiang N, Rehbaum H, Holobar A, Graimann B, Dietl H, Aszmann OC. The extraction of neural information from the surface EMG for the control of upper-limb prostheses:emerging avenues and challenges. IEEE Trans Neural Syst Rehab Eng. 2014;22(4):797–809. doi: 10.1109/TNSRE.2014.2305111. [DOI] [PubMed] [Google Scholar]
- 9.Reaz M, Hussain M, Mohd-Yasin F. Techniques of EMG signal analysis:detection, processing, classification and applications. Bio Proc Onl. 2006;8(1):11–35. doi: 10.1251/bpo115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dechev N, Cleghorn W, Naumann S. Multiple finger, passive adaptive grasp prosthetic hand. Mechanism and machine theory. 2001;36(10):1157–1173. [Google Scholar]
- 11.Light C, Chappell P. Development of a lightweight and adaptable multiple-axis hand prosthesis. Med Engin Phys. 2000;22(10):679–684. doi: 10.1016/s1350-4533(01)00017-0. [DOI] [PubMed] [Google Scholar]
- 12.Dalley S, Wiste T, Withrow T, Goldfarb M. Design of a multifunctional anthropomorphic prosthetic hand with extrinsic actuation. IEEE Transactions Mechat. 2009;14(6):699–706. [Google Scholar]
- 13.Cipriani C, Controzzi M, Carrozza M. Mechanical design of a transradial cybernetic hand. Intel Robot Syst. 2008;1(2):22–26. [Google Scholar]
- 14.Kamikawa Y, Maeno T. Underactuated five-finger prosthetic hand inspired by grasping force distribution of humans. Intel Robot Syst. 2008;1(4):717–722. [Google Scholar]
- 15.Belter J, Segil J, SM B. Mechanical design and performance specifications of anthropomorphic prosthetic hands:a review. J Rehab Res Dev. 2013;50(5):599. doi: 10.1682/jrrd.2011.10.0188. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard S, Galway H, Milner M. Myoelectric training methods for the preschool child with congenital below-elbow amputation. A comparison of two training programmes. Bone Joint J. 1985;67(2):273–277. doi: 10.1302/0301-620X.67B2.3980540. [DOI] [PubMed] [Google Scholar]
- 17.Millstein S, Heger H, Hunter G. Prosthetic use in adult upper limb amputees:a comparison of the body powered and electrically powered prostheses. Prost Ortho Int. 1986;10(1):27–34. doi: 10.3109/03093648609103076. [DOI] [PubMed] [Google Scholar]
- 18.Vidovic M, Paredes L, Hwang H, Amsu S, Pahl J, Hahne J, Graimann B, Farina D, Müller M. Covariate shift adaptation in EMG pattern recognition for prosthetic device control. Med Biol Soc. 2014;36(1):4370–4373. doi: 10.1109/EMBC.2014.6944592. [DOI] [PubMed] [Google Scholar]
- 19.Smith C, Housh T, Hill E, Johnson G, Schmidt R. Changes in Electromechanical Delay During Fatiguing Dynamic Muscle Actions. Muscle Nerve. 2016;56(2):315–320. doi: 10.1002/mus.25502. [DOI] [PubMed] [Google Scholar]
- 20.Smith C, Housh T, Hill E, Johnson G, Schmidt R. Dynamic versus Isometric Electromechanical Delay in Non-Fatigued and Fatigued Muscle:A Combined Electromyographic, Mechanomyographic, and Force Approach. J Electromyog Kine. 2017;33(1):34–38. doi: 10.1016/j.jelekin.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Silva J, Chau T, Goldenberg A. MMG-based multisensor data fusion for prosthesis control. Eng Med Biol Soc. 2003;3(1):2909–2912. [Google Scholar]
- 22.Al-Mulla M, Sepulveda F. Novel Pseudo-Wavelet function for MMG signal extraction during dynamic fatiguing contractions. Sensors. 2014;14(6):9489–9504. doi: 10.3390/s140609489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiloyannis M, Gavriel C, Thomik A, Faisal A. Dynamic forward prediction for prosthetic hand control by integration of EMG, MMG and kinematic signals. Neur Eng. 2015;1(2):611–614. [Google Scholar]
- 24.Kimoto A, Yamada Y. A new layered sensor for simultaneous measurement of EMG, MMG and oxygen consumption at the same position. Med Biol Eng Comp. 2015;53(1):15–22. doi: 10.1007/s11517-014-1208-0. [DOI] [PubMed] [Google Scholar]
- 25.World Medical Association Declaration of Helsinki:ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 26.Abe T, Kumagai K, Brechue WF. Fascicle length of leg muscles is greater in sprinters than distance runners. Med Sci Sports Exerc. 2000;32(6):1125–1129. doi: 10.1097/00005768-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hägg G. European recommendations for surface electromyography. Roessingh Research and Development. 1999;8(2):13–54. [Google Scholar]
- 28.Kulig K, Andrews J, Hay J. Human strength curves. Exer Sport Sci Rev. 1984;12(1):417–466. [PubMed] [Google Scholar]
- 29.Orizio C. Muscle sound:bases for the introduction of a mechanomyographic signal in muscle studies. Crit Rev Biomed Eng. 1993;21(3):201–243. [PubMed] [Google Scholar]
- 30.Yavuz Ş, Şendemir-Ürkmez A, Türker K. Effect of gender, age, fatigue and contraction level on electromechanical delay. Clin Neurophysiol. 2010;121(10):1700–1706. doi: 10.1016/j.clinph.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 31.Cè E, Rampichini S, Agnello L, Limonta E, Veicsteinas A, Esposito F. Effects of temperature and fatigue on the electromechanical delay components. Muscle Nerve. 2013;47(4):566–576. doi: 10.1002/mus.23627. [DOI] [PubMed] [Google Scholar]


