Abstract
Objectives:
Little is known about bone mineralization and osteocyte lacunae properties in chronic kidney disease mineral bone disorder (CKD-MBD).
Methods:
In this retrospective study, we measured the bone mineralization density distribution (BMDD) and osteocyte lacunar section (OLS) 2D-characteristics by quantitative backscatter electron imaging in Straumann drill biopsy samples from n=58 patients with CKD-MBD. Outcomes were studied in relation to serum parathyroid hormone (PTH), alkaline phosphatase (APH), histomorphometric bone turnover and treatment with cinacalcet or phosphate binders.
Results:
Lower calcium concentrations in bone from high turnover (average degree of bone mineralization -6.2%, p<0.001) versus low turnover patients were observed. OLS-characteristics were distinctly different (p<0.01 to p<0.05) in patients with highest compared to those with lowest turnover. Patients with cinacalcet had different OLS-characteristics (p<0.05) compared to those without cinacalcet. Furthermore, patients with phosphate binders had differences in BMDD and OLS-characteristics (p<0.05) compared to patients without phosphate binders.
Conclusions:
Our findings suggest that in patients with CKD-MBD secondary hyperparathyroidism and increased bone turnover decrease the average degree of bone matrix mineralization. Conversely, density and lacunar size of the osteocytes are increased compared to adynamic bone disease pointing at distinct patterns of bone mineralization and osteocyte lacunar properties in these two disease entities.
Keywords: Chronic Kidney Disease Mineral Bone Disorder (CKD-MBD), Bone Biopsy, Bone Mineralization, Osteocyte Lacunae, Histomorphometry
Introduction
Chronic kidney disease is commonly associated with skeletal abnormalities which might persist up to several years after successful kidney transplantation[1,2]. Patients suffer from fractures as well as from calcification of soft tissues and blood vessels described by the term chronic kidney disease - mineral bone disorder (CKD-MBD)[3-7],e.g..
Non-invasive imaging has become successful for studying the microstructure/microarchitecture of cancellous and cortical bone in patients[8-11]. However, the in vivo imaging techniques cannot provide information on mineralization defects in patients with CKD-MBD, thus a bone biopsy sample might be important for the diagnosis of bone abnormalities[12], which have traditionally been termed renal osteodystrophy (ROD)[13] and are usually defined by the standardized TMV (turnover, mineralization, volume) -system[14,15]. Histomorphometrically characterized bone turnover in CKD-MBD ranges from adynamic bone disease to osteitis fibrosa with dramatically increased bone turnover. Additionally, the biopsy sample offers information on bone volume and mineralization defects (based on histomorphometric osteoid-related parameters and/or mineralization lag time).
The aim of the present study was to use a large collection of bone biopsy samples for the investigation of material characteristics based on the analysis of the bone mineralization density distribution (BMDD) and the osteocyte lacunae section (OLS) characteristics. The BMDD is not only an important determinant of the mechanical performance of the bone material but might also help to understand pathophysiological mechanisms in diseased bone. The OLS characteristics were of special interest, because abnormalities in osteocyte number and function were observed recently in CKD-MBD[16]. The present measurements were related to previously obtained histomorphometric and biochemical characteristics as well as to treatment with cinacalcet or phosphate binders.
Materials & methods
Patients and biopsy samples
The measured iliac crest Straumann drill biopsy samples (4 mm diameter) were obtained from 58 patients with CKD-MBD for the purpose of diagnosis and treatment of renal bone disease. Samples from patients who received bisphosphonate treatment were excluded from this study. Inclusion criteria for this study were the presence of a high-quality bone biopsy sample with sufficient bone volume eligible for the BMDD and OLS analysis and a patients’ age >18 years. Patients were treated for renal disease in several clinics in Germany. Diagnosis of CKD-MBD was made according to Kidney Disease Outcomes Quality Initiative (KDOQI) and Kidney Disease Improving Global Outcomes (KDIGO) criteria[17]. Our study group comprised patients with hemodialysis (HD, n=38), peritoneal dialysis (PD, n=4), patients after kidney transplantation (KTX, n=15) and one patient without HD or KTX (CKD stage 3). In the 15 patients with KTX, bone biopsy samples were obtained between 2 and 198 months (median 39.0 months) after KTX. The patients’ estimated glomerular filtration rates (eGFR) ranged between 20 and 64 ml/min. Six of the patients had returned to hemodialysis after KTX. All but one of the KTX patients were treated with glucocorticoids (13 received 5mg prednisolone per day, one patient 10 mg methylprednisolone per day) at the time of biopsy. Additional immunosuppressive therapy included cyclosporin A and mycophenolate in 8 patients, and tacrolimus and mycophenolate in 3 patients. For 4 patients no information was available. Fourteen patients of the entire cohort experienced fractures. Vertebral fractures were reported for 10 patients, peripheral fractures (ribs, pelvis, femur) for 6 patients. Patients’ characteristics are summarized in Table 1. In this retrospective study, biopsy samples came from different medical centers in Germany and tetracycline labelling (i.e. TMV classification) was not available for the total cohort. All biopsy samples were studied for cancellous bone structure (BV/TV), mineralization defects (by OS/BS) and static histomorphometric parameters using a Merz ocular grid[18] and Osteoplan II image analysis system[19]. The cellular characteristics Ob.S/BS and Oc.S/BS were used for the functional classification to high (either Ob.S/BS or Oc.S/BS had to be higher than 3.2 % or 2.1 %, respectively), normal or low turnover (both Ob.S/BS and Oc.S/BS had to be lower than 1 %)[20]. Biochemical characteristics were measured as described previously[20].
Table 1.
Clinical characteristics of the study cohort with CKD-MBD.
| Entire Study Cohort (n=58) | Subgroups by Turnover | Reference (peak bone mass) | |||
|---|---|---|---|---|---|
| High turnover (n=39) | Normal turnover (n=4)§ | Low turnover (n=15) | |||
| Αge (yrs) | 56 (45; 67) | 55 (40; 64) | 63 (52; 71) | 62 * (53; 75) | --- |
| Μale/female (nb.) | 27/31 | 17/22 | 0/4 | 10/5 | --- |
| HD or PD (nb.) | 42 | 31 | 2 | 9 | --- |
| duration of dialysis (months) | 56 (21; 81) | 55 (27; 83) | 70 (16; 123) | 57 (21; 78) | --- |
| Cinacalcet (nb.) | 25 | 23 | 1 | 1 | --- |
| Phosphatebinder (nb.) | 29 | 26 | 1 | 2 | --- |
| Patients with fx (nb.) | 14 | 4 | 4 | 6 | --- |
| Patients with PTX (nb.) | 9 | 5 | 0 | 4 | --- |
| Biochemical1 | |||||
| PTH (pg/ml) | 264 (75; 826) | 475 (156; 1047) | 64 (62; 345) | 90 ** (40; 189) | 10-65 |
| APH (µmol/l×s) | 1.9 (1.6; 2.8) | 2.1 (1.6; 2.9) | 1.2 (1.2; 2.6) | 1.9 (1.1; 2.9) | 0.6-2.20 |
| Ca (mmol/l) | 2.34 (2.24; 2.52) | 2.35 (2.26; 2.52) | 2.24 (1.84; 2.32) | 2.38 (2.23; 2.63) | 2.2-2.65 |
| 25OHD3 (nmol/l) | 64 (36; 90) | 63 (37; 90) | 119 (63; 175) | 65 (22; 83) | 75-375 |
| Phosphate (mmol/l) | 1.58 (1.12; 1.92) | 1.63 (1.21; 2.00) | 1.14 (1.06; 1.43) | 1.44 (0.97; 2.00) | 0.76-1.37 |
| Histomorphometric[2 | |||||
| BV/TV (%) | 16.6 (10.3; 21.8) | 17.4 (11.0; 21.7) | 14.5 (5.7; 21.1) | 16.10 (9.60; 22.70) | 20.43 (4.0) |
| OS/BS (%) | 3.7 (0.6; 11.9) | 6.1 (3.4; 17.5) | 1.0 (0.2; 6.4) | 0.00 (0.00; 0.40) | 14.3 (3.0) |
| Ob.S/BS (%) | 3.4 (0.3; 8.3) | 5.1 (3.3; 14.2) | 1.4 (0.8; 1.9) | 0.00 (0.00; 0.00) | 4.5 (3.2) |
| ES/BS (%) | 15.1 (10.0; 26.3) | 21.8 (13.3; 30.3) | 12.4 (10.6; 32.8) | 7.20 (4.20; 10.40) | 6.1 (1.9) |
| Oc.S/BS (%) | 3.3 (0.2; 9.2) | 6.8 (3.2; 13.3) | 1.4 (0.3; 2.0) | 0.0 (0.0; 0.0) | 2.5 (1.5) |
Data shown are median (25th; 75th percentiles) or mean (SD).
Abbreviations: nb.= number of patients; HD = hemodialysis; PD = peritoneal dialysis; PTX = parathyroidectomy; PTH = parathyroid hormone, APH = alkaline phosphatase, Ca = calcium, 25OHD3 = 25-hydroxyvitamin D3; BV/TV = bone volume per tissue volume, OS/BS = osteoid surface per bone surface, Ob.S/BS = osteoblast surface per bone surface; ES/BS = eroded surface per bone surface; Oc.S/BS osteoclast surface per bone surface; nomenclature according to Parfitt et al[49.
no statistical comparison due to small sample size
p<0.01,
p<0.05 low versus high turnover (adjusted for age if appropriate)
1 Biochemical information was not available from all patients;
2 No statistical comparison between bone turnover groups as histomorphometry was used to define bone turnover status.
3 Reference data are from a previous work by Delling[18.
Quantitative backscatter electron imaging (qBEI)
For the measurement of the BMDD, qBEI images were acquired using the following settings on the microscope (DSM 962, Zeiss, Oberkochen, Germany, equipped with a four-quadrant semi-conductor backscatter electron detector): accelerating voltage 20 kV, probe current 110±0.4 pA, working distance 15 mm, and a scan speed of 100 seconds per frame. Entire available cancellous tissue areas (up to 4 mm x 10 mm) of the biopsy samples were recorded in a series of images with each 50x nominal magnification (pixel resolution of 3.52 µm). After image calibration, the grey-levels in the images could be transferred to Ca weight % (wt %) values[21] (Figure 1). Thus, the grey level-histograms (denoted BMDD) provided information about the frequency distribution of pixel sized-bone areas with a certain calcium content (Figure 1). The histogram bin width was 0.17 wt % Ca. Five parameters were obtained from the BMDD and compared to adult reference data[21,22]: CaMean= the weighted mean Ca-concentration of the bone area; CaPeak= the histogram peak position, indicating the most frequently measured calcium concentration; CaWidth= the full width at half maximum of the distribution, describing the variation in mineralization density; CaLow= the percentage of low mineralized bone (<5th percentile of our reference BMDD); CaHigh= the percentage of highly mineralized bone areas (>95th percentile of our reference BMDD).
Figure 1.
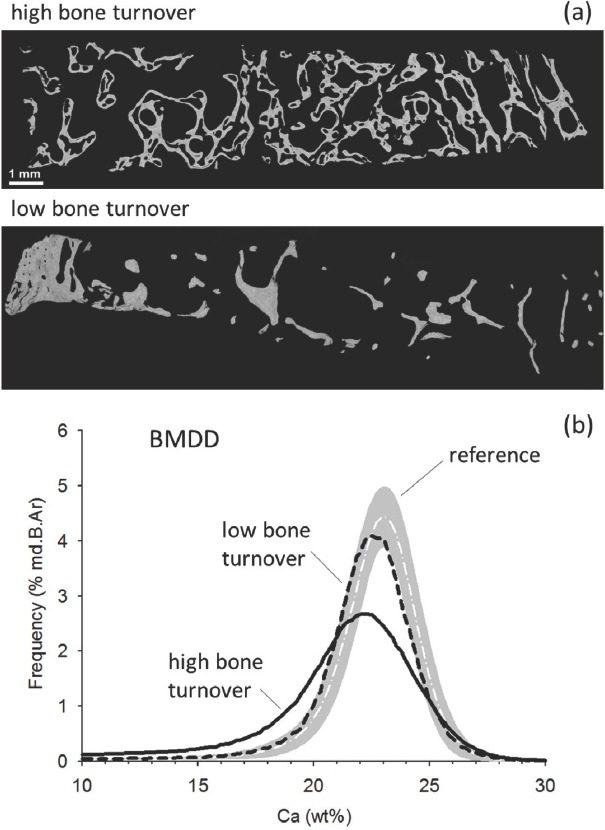
a) Backscatter electron overview image of the cross-sectional area of Straumann drill samples from patients with high and low turnover (bar indicates 1 mm). Brighter pixel grey levels in the image indicate higher Ca content. The high turnover sample is from a 49 years old male patient who suffered from secondary hyperparathyroidism. Before his bone biopsy was obtained parathyroidectomy was performed. At the time of bone biopsy, his PTH level was 318 pg/l, APH was 5.95 µmol/l×s. Cellular surfaces Ob.S/BS and Oc.S/BS were 11.60% and 7.40% respectively (for reference levels see Table 1). The biopsy sample shows trabecular tunneling, a typical feature in PTH excess. The low turnover sample is from a 53 years old female patient who had a PTH level of 36 pg/l, APH of 0.7 µmol/l×s and both Ob.S/BS and Oc.S/BS of 0.0%. b) The corresponding BMDD curves derived from qBEI images of higher magnification (see method section) covering the total cancellous bone area. The solid line represents the BMDD from the high turnover patient, the dashed line that of the low turnover patient. The white dashed line indicates the reference BMDD published previously[21], the gray band the corresponding ± 1SD values. The high turnover BMDD is indicating a higher portion of lower mineralized areas as well as a larger heterogeneity in mineralization (larger peak width) compared to low turnover BMDD. Both BMDDs are shifted towards lower mineral content compared to the reference BMDD.
Osteocyte lacunae sections (OLS) characteristics
For OLS-analysis, qBEI images of cancellous bone with a nominal magnification 130x (0.88 µm/pixel) were acquired (Figure 2A), which were transformed to binary images using a threshold based on a fixed grey level (5.2 wt% Ca) (Figure 2B). For details see previous work[23]. The 2D-analyses of OLS were performed based on a custom-made macro in ImageJ software (version 1.50f; NIH, Bethesda, MD, USA)[24]. Subsequently, the OLS were extracted using a minimum and maximum size threshold of 5 µm2 and 80 µm2, respectively. A total bone area of 2.66 (2.01; 3.36) mm2 and a total number of 716 (488; 956) OLS (median, 25th; 75th percentiles) were analyzed per each sample. Five parameters were obtained (Figure 2C-F):
Figure 2.
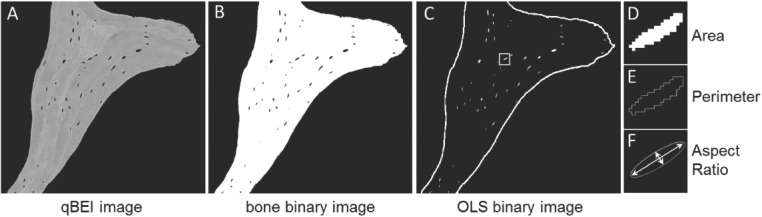
Measurement of the OLS characteristics. qBEI images of a magnification of 130x were acquired per sample (one of such images is shown in A). These images were then transferred to binary images showing mineralized bone in white and non-mineralized area in black (B). Finally, the contrast was converted and size thresholds (between 5 µm2 and 80 µm2) applied, thus showing the OLS to be analyzed in white (perimeter of mineralized bone area indicated by white solid line) (C). From such images, OLS-porosity and OLS-density were deduced. Additionally, OLS-area (D), OLS-perimeter (E) and OLS-aspect ratio were calculated (F).
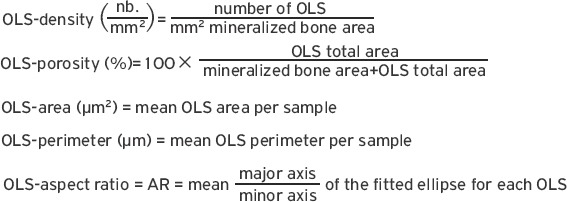
OLS-AR= 1 indicates a circle and increasing values indicate increasingly elongated shape of the OLS. OLS-AR values >10 were excluded. The viability of the cells within the OLS cannot be evaluated by this method.
For the analysis of potential differences in OLS characteristics due to bone turnover, we compared the fourth of the cohort with the most diverging bone turnover indices (7 samples with highest turnover versus 7 samples with lowest turnover). Turnover was defined primarily by Ob.S/BS and additionally by Oc.S/BS and PTH. Those 7 patients classified to highest turnover had Ob.S/BS from 14.9% to 29.4%, Oc.S/BS from 6.6% to 24.2%, and PTH levels from 502 pg/l to 1874 pg/l. Those 7 classified to lowest turnover had Ob.S/BS=0% and Oc.S/BS=0%, and PTH levels from 33.5 pg/l to 127 pg/l.
Statistical analysis
SigmaStat Version 4.0 (Systat Software Inc., San Jose, CA, USA) was used for statistical analysis. For the comparison of two groups t-tests (for normally distributed data) or Mann-Whitney rank sum tests (for non-normally distributed data) were used, if there was no evidence that a covariate affected the values of the dependent variable. Additionally, analysis of covariance (i.e. age or bone turnover) was performed if there was evidence that one of these parameters might have a significant effect on the comparison of the subgroups (in these cases p-values for adjusted means are presented). The sample size of the group with normal turnover (n=4) was too small for statistical comparisons, but the data were included in the correlation analysis. Spearman rank order correlation was used to test the association of BMDD or OLS- characteristics with clinical or histomorphometric outcomes within the total study cohort (n=58 patients).
Results
Comparison high vs. low bone turnover patients
Clinical Characteristics and biochemical outcomes (Table 1): The patients with high turnover were significantly younger than those with low turnover (p=0.011). No significant differences between biochemical characteristics were observed (APH p=0.348, Ca p=0.657, 25OHD3 p=0.370, phosphate p=0.461), except for PTH, which was significantly higher in the high turnover group (5.3-fold, p=0.001; after adjustment for age p=0.006). Duration of hemodialysis was not significantly different between high versus low turnover patients (p=0.846).
Histomorphometric parameters were used to define the bone turnover groups and therefore not statistically compared between the high versus low turnover patients.
BMDD outcomes: Two examples of biopsy sample sections with corresponding BMDD from high and low turnover patients are shown in [Figure 1]. As both patient groups comprised patients with HD or PD and patients after KTX, we first compared these subgroups and found no statistically significant differences for BMDD after adjustment for bone turnover (i.e. Ob.S/BS) (all p>0.05). Consequently, these subgroups were not distinguished for further analysis. Comparison of BMDD-parameters between high and low turnover patients revealed significant differences (Table 2): High turnover patients had lower CaMean (-6.2 %, p<0.001), lower CaPeak (-4.8 %, p<0.05), higher CaWidth (+13.6 %, p<0.001), higher CaLow (+56 %, p<0.001) and lower CaHigh (-71 %, p=0.003) compared to those with low turnover. As CaMean and CaHigh showed a significant dependency on age (Spearman rank order correlation R=0.28 and R=0.29, respectively, both p<0.05), comparison for these two parameters was adjusted for age: CaMean remained significantly different (p<0.001) while CaHigh was not significantly different between high and low turnover patients after adjustment for age.
Table 2.
BMDD obtained from cancellous bone.
| High turnover (n=39) | Normal turnover(n=4)§ | Low turnover (n=15) | Adult Reference | |
|---|---|---|---|---|
| CaMean (wt%) | 19.82°°° (19.09; 20.70) | 21.04 (20.67; 21.64) | 21.32 *** °°° (20.66; 21.80) | 22.24 (21.84; 22.50) |
| CaPeak (wt%) | 21.14°°° (20.45; 22.01) | 22.18 (21.92; 22.57) | 22.36 *** °°° (21.66; 22.88) | 22.96 (22.70; 23.14) |
| CaWidth (Δwt%) | 4.33°°° (4.16; 4.51) | 3.81 (3.64; 4.51) | 3.81 *** °° (3.29; 3.99) | 3.29 (3.12; 3.47) |
| CaLow (%md.B.Ar) | 14.11°°° (10.24; 19.86) | 9.28 (7.80; 10.51) | 9.04 *** °°° (5.96; 9.85) | 4.52 (3.87; 5.79) |
| CaHigh (%md.B.Ar) | 0.96°°° (0.35; 2.94) | 4.61 (2.94; 7.74) | 3.34 (1.97; 6.41) | 4.62 (3.52; 6.48) |
The comparison to reference BMDD data revealed in both high and low turnover groups a shift to lower calcium concentrations: CaMean was lower (-10.9 % and -4.1 %, both p<0.001), CaPeak was lower (-7.9 % and -3.1 %, both p<0.001), CaWidth was higher (+32 %, p<0.001 and +16 %, p<0.004), and CaLow was higher (+212 % and +100 %, both p<0.001) in high and low turnover patients, respectively, compared to reference. CaHigh was lower (-79 %, p<0.001) in the high turnover patients compared to reference (for low turnover patients p=0.220). Furthermore, strong correlations between BMDD-parameters with serum PTH, APH as well as with histomorphometric indices were observed (Table 3). No significant relationship of the BMDD outcomes with other serum parameters including phosphate, OHVitD3 or calcium were observed.
Table 3.
Spearman correlation analysis of cancellous BMDD versus serum parameters or versus static histomorphometric parameters.
| PTH n=48 | APH n=49 | Phosphate (n=47) | OS/BS n=58 | Ob.S/BS n=58 | ES/BS n=58 | Oc.S/BS n=58 | |
|---|---|---|---|---|---|---|---|
| CaMean | -0.46*** | -0.46*** | ns | -0.57*** | -0.61*** | -0.41*** | -0.49*** |
| CaPeak | -0.44** | -0.34* | -0.29* | -0.49*** | -0.54*** | -0.40** | -0.47*** |
| CaWidth | 0.35* | 0.44** | ns | 0.54*** | 0.54*** | 0.31* | 0.37*** |
| CaLow | 0.46*** | 0.55*** | ns | 0.64*** | 0.67*** | 0.44*** | 0.52*** |
| CaHigh | -0.37** | -0.31* | -0.30* | -0.42*** | -0.49*** | -0.30* | -0.42** |
Data show Spearman correlation coefficients.
p≤0.001,
p<0.01
p<0.05. Positive correlation coefficients indicate that one parameter is increasing with the increasing other, while negative correlation coefficients indicate that one parameter is decreasing with the other parameter increasing.
OLS characteristics: Among the 14 patients studied for OLS-characteristics, OLS-area and OLS-AR were significantly related to patients’ age (therefore comparison for these parameters was adjusted for age). Bone from the patients with the highest turnover had significantly higher OLS-porosity (+38%, p=0.009), OLS-density (+21 %, p=0.031), and OLS-perimeter (+7 %, p=0.013) compared to bone from the patients with lowest turnover. OLS-area and OLS-AR were not significantly different between these patient groups after adjustment for age (Figure 3).
Figure 3.
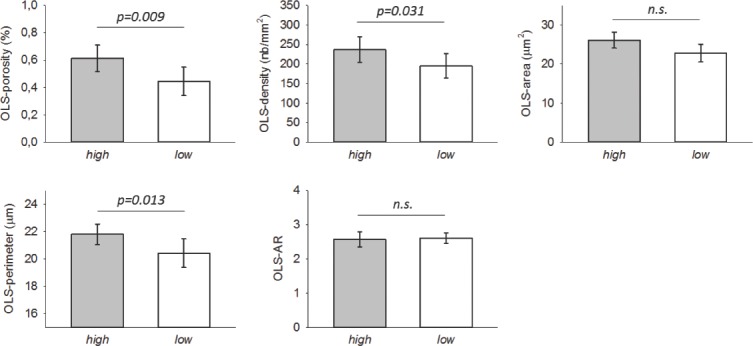
Comparison of OLS outcomes for n=7 with highest bone turnover versus those from the n=7 with lowest bone turnover. Bars indicate mean and SD (p-values after adjustment for age, n.s. not significant).
OLS outcomes were significantly correlated with PTH and APH (Figure 4, Table 4). Correlations of OLS-characteristics with histomorphometric indices of bone turnover were not performed as bone turnover indices (Ob.S/BS, Oc.S/BS, OS/BS) were zero for all patients in the lowest turnover group.
Figure 4.
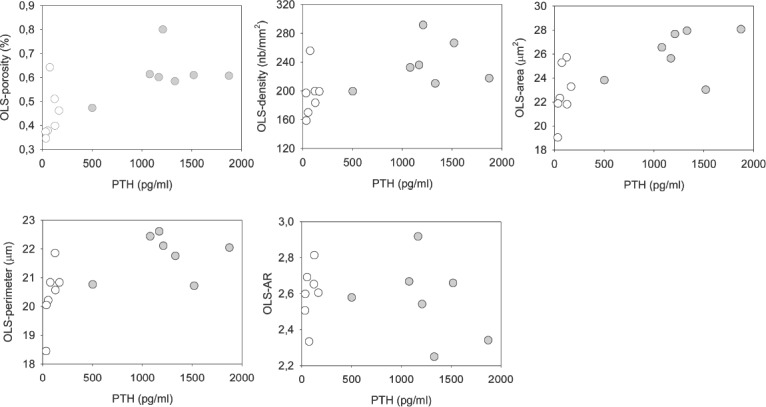
Relationships between OLS parameters and PTH. Grey indicates high turnover, white low turnover patients (for correlation coefficient and p-values see Table 4).
Table 4.
Spearman correlation analysis of OLS-characteristics versus serum parameters.
| PTH n=14 | APH n=14 | |
|---|---|---|
| OLS-porosity | 0.66** | n.s. (p=0.195) |
| OLS-density | 0.68** | n.s. (p=0.225) |
| OLS-area | 0.71** | 0.58* |
| OLS-perimeter | 0.64* | n.s. (0.087) |
| OLS-aspect ratio | n.s. (p=0.750) | n.s. (p=0.510) |
Data show Spearman correlation coefficients.
p<0.01,
p<0.05, n.s. not significant
Positive correlation coefficients indicate that one parameter is increasing with the increasing other parameter.
BMDD and OLS-characteristics subcohort analysis by treatment
We studied our biopsy cohort for cinacalcet effects. Cinacalcet acts as a calcimimetic by allosteric activation of the calcium-sensing receptor. Previously we observed lowered bone matrix mineralization in patients with an activating mutation of the calcium sensing receptor (causing a primary form of hypoparathyroidism) independent of bone turnover[25,26], therefore cinacalcet effects were of specific interest. From the total cohort n=25 patients received cinacalcet and n=31 never received cinacalcet (for 2 patients no information was available). The majority of patients who received cinacalcet had high bone turnover (n=23 had high, n=1 had normal, and n=1 had low turnover). After adjustment for bone turnover (i.e. Ob.S/BS), no differences in BMDD were observed (all p>0.05). Among the subgroup studied for OLS-characteristics after adjustment for Ob.S/BS no differences in OLS-porosity, OLS-density or OLS-AR were observed (all p>0.05), while OLS-area and OLS-perimeter were both significantly higher (+4.6% and +1.5%, p<0.05) in those (n=7) with cinacalcet compared to those without (n=6).
From the total cohort n=29 patients received phosphate binders (of different types) and n=22 never received phosphate binders (for the remaining patients no information was available). After adjustment for bone turnover (Ob.S/BS), CaPeak (-3.2 %, p<0.05) was significantly lower in those with phosphate-binders, while all other BMDD parameters were not different compared to those without phosphate binders (all p>0.05). Furthermore, OLS-characteristics were not significantly different, except OLS-density which was 25% higher (p<0.05) in patients with phosphate binders (n=5) compared to those without phosphate binders (n=8) (all comparisons for OLS-characteristics were adjusted for Ob.S/BS).
Discussion
In this retrospective study, we assigned patients to high, normal or low bone turnover based on histomorphometric bone formation and resorption and found significant differences for biochemical characteristics, BMDD and OLS parameters.
Among the biochemical factors only PTH was significantly different in the high versus low turnover patients. Although its measurement and interpretation in patients with CKD-MBD might have some limitations[3,4,27,28], lower PTH levels were found in our patients with reduced bone turnover. The PTH of such low turnover patients was clearly different from those with high turnover renal bone disease also in other works[20,29,30]. The duration of hemodialysis was not found to be a predicting factor for the high and low turnover status of the patients. However, our patients with low bone turnover were older. Older age is one of the known factors associated with adynamic bone disease[31,32].
The analysis of BMDD revealed significant differences between high and low turnover patients. The BMDD measured by qBEI has to be distinguished from the analysis of mineralization by histology. While the latter shows the presence of abnormally thick osteoid and/or unmineralized areas within the bone matrix (mineralization defects), the BMDD represents the distribution of calcium concentrations in the mineralized bone matrix. The BMDD has only minor variation with age and other biological factors in healthy adult individuals[21,33]. Abnormal BMDD is contributing to altered mechanical properties of the bone material and is frequently observed in pathological conditions with increased fragility[34,35]. Deviation from normal BMDD usually indicates abnormal bone turnover and/or mineralization processes[22]. While bone turnover determines the average tissue age (younger in high turnover, older tissue age in low turnover condition), the mineralization processes describe the time course of mineral accumulation in the newly formed osteoid and the final level of mineralization within each bone structural unit (BSU or bone packet). If the latter mineralization processes are delayed or the final level of mineralization is decreased this will lead to an overall lower bone matrix mineralization[22].
The observed differences in the BMDD, i.e. the lower degree of bone matrix mineralization and the higher percentage of low mineralized bone areas in high versus low turnover patients were not surprising. The higher the bone turnover, the higher is the percentage of newly formed, lower mineralized bone matrix leading to an overall lower degree and higher heterogeneity of mineralization. The noticed differences in BMDD due to the level of bone turnover are well known for other patient groups without CKD-MBD[22] and were also observed between renal osteodystrophy (ROD) III versus ROD II patients of another cohort[36] and between patients with adynamic bone disease and those with osteitis fibrosa[37]. Furthermore, our BMDD findings are in line with previously observed differences by Fourier transform infrared spectroscopy and nanoindentation measurements between high and low turnover CKD-MBD patients[29].
A surprising result was that the low turnover patients had a decreased degree of mineralization compared to reference BMDD obtained from healthy individuals. As described above deviations from normal BMDD in pathologic cases are mainly due to abnormalities in bone turnover and/or the mineralization processes. Typically, in abnormally low bone turnover situations (with in average much older tissue age than that in normal bone turnover condition) bone matrix mineralization is increased[22]. Thus, in this context our findings of abnormally low degree of bone matrix mineralization in the low turnover patients was surprising. However, our findings are in line with the results for another cohort of adult patients with CKD-MBD which has previously been published[38]. The latter and the data of the present study suggest abnormal mineralization processes (i.e. slower mineral accrual and lower final level of mineralization within each BSU) in patients with CKD-MBD. However, no indication for such abnormalities in mineralization processes were observed in children with CKD-MBD[39].
In the current work, we compared osteocyte lacunae section (OLS) characteristics between highest and lowest bone turnover patients based on a recently established method[23]. Strikingly, we found significantly higher OLS-porosity in the high turnover group. This higher porosity was due to both higher OLS-density and OLS-area. The latter is suggesting a morphologic change of the osteocyte lacunae in high turnover CKD-MBD. Recently, Yajima et al. described morphologic differences in osteocyte lacunae depending on patients’ PTH levels[16]. Moreover, parathyroidectomy decreased osteocyte lacunar porosity and number in CKD-MBD patients with secondary hyperparathyroidism[40]. It might be assumed that under conditions of high PTH and consequently high bone formation/turnover osteolysis (i.e. the release of mineral) by osteocytes provides a contributing mechanism for the disposal of calcium. In fact, there is evidence for osteocytic osteolysis associated with high PTH levels[41-43].
The association of serum measures such as PTH or APH e.g. with histomorphometric bone turnover indices have already been reported[44], however, the present work shows for the first time the strong negative correlation of PTH and APH with bone matrix mineralization in adult patients with CKD-MBD. Noteworthy, if we exclude the patients with PTH at the extreme ends (which are greater than 600 ng/L and lower than 100 ng/L) for the correlation analysis, the significant relationship with bone matrix mineralization disappears (all Spearman rank correlations p>0.05, data not shown). This is in line with the lack of correlations of histomorphometric bone formation rate with PTH within these limits as reported by others[27].
Regarding the cinacalcet effects, it has to be mentioned that we found lowered bone matrix mineralization densities in a previous analysis of patients with hypoparathyroidism due to an activating mutation of the calcium sensing receptor[25]. This previous unexpected finding led to the hypothesis of a direct and bone turnover independent action of the calcium sensing receptor on bone mineralization and motivated our interest in potential cinacalcet treatment effects on the BMDD. As cinacalcet is primarily given to patients with high turnover and known to reduce histomorphometric indices of bone turnover in CKD-MBD[45,46], we performed the BMDD comparison after adjustment for Ob.S/BS and found no significant differences between the patients with compared to those without cinacalcet. This might suggest that activation of the calcium sensing receptor did not affect bone matrix mineralization in our study cohort, however definite conclusions are not possible as we have no information on the bone mineralization from paired biopsy samples. It is also noteworthy that bone from patients treated with cinacalcet had larger OLS area and perimeter compared to those without. This was not expected when considering the results from a recent case report[47]. However, as already mentioned above, we have no information on the bone tissue and material before treatment in patients who received cinacalcet, thus our analysis cannot contribute to the clarification of open questions about the clinical usefulness of treatment with calcimimetics in view of potential safety aspects and costs.
Furthermore, BMDD comparison after adjustment for Ob.S/BS, revealed lower CaPeak and higher OLS-density in the patients with phosphate binders compared to those without. Whether, this has any clinical relevance is unknown. The phosphate binders used in this retrospective study were of different types (aluminum-, calcium carbonate- or lanthanum based). Apart from bone histology findings in patients and animals treated with lanthanum based phosphate binders[48,49], no information on effects of phosphate binders on bone matrix mineralization is available so far.
Limitations of our study are its retrospective design and the heterogeneity of the study population, which is primarily due to the nature of the CKD course. The biopsy samples were obtained from a cohort with CKD-MBD of different etiology at different clinical centers at a time when FGF23 measurements were not routinely provided. We have also no information on dual energy X-ray absorptiometry scans of the patients. This might be considered as a limitation. However, it has to be noted that in patients with end-stage renal disease osteoporosis definition according to the WHO (by bone mineral density and presence of fractures) fails[5]. An additional limitation of our study is, that no information on vascular calcification or tetracycline labelling (thus no TMV-classification) was available. This did not allow for full clinical and histomorphometric analysis for correlation with bone matrix mineralization. On the other hand, the strength of the study is the large number of biopsy samples fully characterized by static bone formation and resorption, which could be measured for BMDD and for OLS characteristics by a novel tool first applied here in CKD-MBD.
We conclude that especially PTH and histomorphometric bone turnover/formation parameters were predictors of the relative level of bone matrix mineralization among our adult patients with CKD-MBD. However, the absolute level of bone matrix mineralization was generally decreased compared to normal suggesting additionally altered mineralization processes. Moreover, our OLS data indicate that in conditions of secondary hyperparathyroidism and increased bone turnover, the density and lacunar size of the osteocytes is distinctly higher than in adynamic bone disease in this cohort with CKD-MBD.
Acknowledgements
The authors thank Petra Keplinger, Sonja Lueger and Phaedra Messmer for sample preparation and qBEI measurements at the Bone Material Laboratory of the Ludwig Boltzmann Institute of Osteology, Vienna, Austria. This work was supported by the AUVA (Austrian Social Insurance for Occupational Risk) and the WGKK (Social Health Insurance Vienna).
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Lehmann G, Ott U, Stein G, Steiner T, Wolf G. Renal osteodystrophy after successful renal transplantation:a histomorphometric analysis in 57 patients. Transplant Proc. 2007;39:3153–8. doi: 10.1016/j.transproceed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Fratzl-Zelman N, Valta H, Pereira RC, Misof BM, Roschger P, Jalanko H, Wesseling-Perry K, Klaushofer K, Mäkitie O. Abnormally High and Heterogeneous Bone Matrix Mineralization After Childhood Solid Organ Transplantation:A Complex Pathology of Low Bone Turnover and Local Defects in Mineralization. J Bone Miner Res. 2017;32:1116–1125. doi: 10.1002/jbmr.3087. [DOI] [PubMed] [Google Scholar]
- 3.Martin KJ, Olgaard K, Coburn JW, Coen GM, Fukagawa M, Langman C, Malluche HH, McCarthy JT, Massry SG, Mehls O, Salusky IB, Silver JM, Smogorzewski MT, Slatopolsky EM, McCann L Bone Turnover Work Group. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis. 2004;43:558–65. doi: 10.1053/j.ajkd.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Cejka D. Renal bone disease. Wien Med Wochenschr. 2013;163:403–8. doi: 10.1007/s10354-013-0195-3. [DOI] [PubMed] [Google Scholar]
- 5.Miller PD. Chronic kidney disease and osteoporosis:evaluation and management. Bonekey Rep eCollection. 2014;25(3):542. doi: 10.1038/bonekey.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pimentel A, Ureña-Torres P, Zillikens MC, Bover J, Cohen-Solal M. Fractures in patients with CKD-diagnosis, treatment, and prevention:a review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int. 2017;92:1343–1355. doi: 10.1016/j.kint.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 7.McNerny EMB, Nickolas TL. Bone Quality in Chronic Kidney Disease:Definitions and Diagnostics. Curr Osteoporos Rep. 2017;15:207–213. doi: 10.1007/s11914-017-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonard MB. A structural approach to skeletal fragility in chronic kidney disease. Semin Nephrol. 2009;29:133–43. doi: 10.1016/j.semnephrol.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cejka D, Patsch JM, Weber M, Diarra D, Riegersperger M, Kikic Z, Krestan C, Schueller-Weidekamm C, Kainberger F, Haas M. Bone microarchitecture in hemodialysis patients assessed by HR-pQCT. Clin J Am Soc Nephrol. 2011;6:2264–71. doi: 10.2215/CJN.09711010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babayev R, Nickolas TL. Bone Disorders in Chronic Kidney Disease:An Update in Diagnosis and Management. Semin Dial. 2015;28:645–53. doi: 10.1111/sdi.12423. [DOI] [PubMed] [Google Scholar]
- 11.Bielesz B, Patsch JM, Fischer L, Bojic M, Winnicki W, Weber M, Cejka D. Cortical porosity not superior to conventional densitometry in identifying hemodialysis patients with fragility fracture. PLoS One. 2015;12(2):e0171873. doi: 10.1371/journal.pone.0171873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evenepoel P, Behets GJS, Laurent MR, D'Haese PC. Update on the role of bone biopsy in the management of patients with CKD-MBD. J Nephrol. 2017;30:645–652. doi: 10.1007/s40620-017-0424-8. [DOI] [PubMed] [Google Scholar]
- 13.Amling M, Grote HJ, Vogel M, Hahn M, Delling G. Three-dimensional analysis of the spine in autopsy cases with renal osteodystrophy. Kidney Int. 1994;46(3):733–43. doi: 10.1038/ki.1994.328. [DOI] [PubMed] [Google Scholar]
- 14.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G. Definition evaluation, and classification of renal osteodystrophy:A position statement from Kidney Disease:Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 15.Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium:analysis of 630 bone biopsies in black and white patients. J Bone Miner Res. 2011;26:1368–76. doi: 10.1002/jbmr.309. Erratum in:J Bone Miner Res. 2011;26:2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yajima A, Tsuchiya K, Burr DB, Minner DE, Condon KW, Miller CA, Satoh S, Inaba M, Nakayama T, Tanizawa T, Ito A, Nitta K. Osteocytic perilacunar/canalicular turnover in hemodialysis patients with high and low serum PTH levels. Bone. 2018;113:68–76. doi: 10.1016/j.bone.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 17.NKF:K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease Guideline 8B, in K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease Guidelines. New York: National Kidney Foundation; 2003. [PubMed] [Google Scholar]
- 18.Merz WA, Schenk RK. Quantitative structural analysis of human cancellous bone. Anat (Basel) 1970;75:54–66. doi: 10.1159/000143440. [DOI] [PubMed] [Google Scholar]
- 19.Delling G. Endokrine Osteopathien: Morphologie, Histomorphometrie und Differentialdiagnose (Endocrine bone diseases:morphology, histomorphometry and differential diagnosis) In: Büngeler W, Eder M, Lennert K, Peters G, Sandritter W, Seifert G, editors. Veröffentlichungen aus der Pathologie. Vol. 98. Gustav-Fischer-Verlag Stuttgart (article in German); 1975. [PubMed] [Google Scholar]
- 20.Lehmann G, Stein G, Hüller M, Schemer R, Ramakrishnan K, Goodman WG. Specific measurement of PTH (1-84) in various forms of renal osteodystrophy (ROD) as assessed by bone histomorphometry. Kidney Int. 2005;68:1206–14. doi: 10.1111/j.1523-1755.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- 21.Roschger P, Gupta HS, Berzlanovich A, Ittner G, Dempster DW, Fratzl P, Cosman F, Parisien M, Lindsay R, Nieves JW, Klaushofer K. Constant mineralization density distribution in cancellous human bone. Bone. 2003;32:316–323. doi: 10.1016/s8756-3282(02)00973-0. [DOI] [PubMed] [Google Scholar]
- 22.Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456–466. doi: 10.1016/j.bone.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Blouin S, Fratzl-Zelman N, Glorieux FH, Roschger P, Klaushofer K, Marini JC, Rauch F. Hypermineralization and High Osteocyte Lacunar Density in Osteogenesis Imperfecta Type V Bone Indicate Exuberant Primary Bone Formation. J Bone Miner Res. 2017;32:1884–1892. doi: 10.1002/jbmr.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ:25 years of image analysis. Nat Methods. 2012;9(7):671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theman TA, Collins MT, Dempster DW, Zhou H, Reynolds JC, Brahim JS, Roschger P, Klaushofer K, Winer KK. PTH(1-34) replacement therapy in a child with hypoparathyroidism caused by a sporadic calcium receptor mutation. J Bone Miner Res. 2009;24:964–73. doi: 10.1359/JBMR.081233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ovejero D, Misof BM, Gafni RI, Dempster D, Zhou H, Klaushofer K, Collins MT, Roschger P. Bone Matrix Mineralization in Patients With Gain-of-Function Calcium-Sensing Receptor Mutations Is Distinctly Different From that in Postsurgical Hypoparathyroidism. J Bone Miner Res. 2018 doi: 10.1002/jbmr.3638. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Garrett G, Sardiwal S, Lamb EJ, Goldsmith DJ. PTH--a particularly tricky hormone:why measure it at all in kidney patients? Clin J Am Soc Nephrol. 2013;8:299–312. doi: 10.2215/CJN.09580911. [DOI] [PubMed] [Google Scholar]
- 28.Delanaye P, Souberbielle JC, Lafage-Proust MH, Jean G, Cavalier E. Can we use circulating biomarkers to monitor bone turnover in CKD haemodialysis patients?Hypotheses and facts. Nephrol Dial Transplant. 2014;29:997–1004. doi: 10.1093/ndt/gft275. [DOI] [PubMed] [Google Scholar]
- 29.Malluche HH, Porter DS, Monier-Faugere MC, Mawad H, Pienkowski D. Differences in bone quality in low- and high-turnover renal osteodystrophy. J Am Soc Nephrol. 2012;23:525–32. doi: 10.1681/ASN.2010121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graciolli FG, Neves KR, Barreto F, Barreto DV, Dos Reis LM, Canziani ME, Sabbagh Y, Carvalho AB, Jorgetti V, Elias RM, Schiavi S, Moysés RMA. The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int. 2017;91:1436–1446. doi: 10.1016/j.kint.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 31.Malluche HH, Monier-Faugere MC. Risk of adynamic bone disease in dialyzed patients. Kidney Int Suppl. 1992;38:S62–7. [PubMed] [Google Scholar]
- 32.Drüeke TB, Massy ZA. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016;89:289–302. doi: 10.1016/j.kint.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Boivin G, Meunier PJ. The degree of mineralization of bone tissue measured by computerized quantitative contact microradiography. Calcif Tissue Int. 2002;70:503–511. doi: 10.1007/s00223-001-2048-0. [DOI] [PubMed] [Google Scholar]
- 34.Bala Y, Farlay D, Boivin G. Bone mineralization:from tissue to crystal in normal and pathological contexts. Osteoporos Int. 2013;24:2153–66. doi: 10.1007/s00198-012-2228-y. [DOI] [PubMed] [Google Scholar]
- 35.Fonseca H, Moreira-Gonçalves D, Coriolano HJ, Duarte JA. Bone quality:the determinants of bone strength and fragility. Sports Med. 2014;44:37–53. doi: 10.1007/s40279-013-0100-7. [DOI] [PubMed] [Google Scholar]
- 36.Haas M, Leko-Mohr Z, Roschger P, Kletzmayr J, Schwarz C, Domenig C, Zsontsich T, Klaushofer K, Delling G, Oberbauer R. Osteoprotegerin and parathyroid hormone as markers of high-turnover osteodystrophy and decreased bone mineralization in hemodialysis patients. Am J Kidney Dis. 2002;39:580–6. doi: 10.1053/ajkd.2002.31409. [DOI] [PubMed] [Google Scholar]
- 37.Ng AH, Hercz G, Kandel R, Grynpas MD. Association between fluoride, magnesium, aluminum and bone quality in renal osteodystrophy. Bone. 2004;34:216–24. doi: 10.1016/j.bone.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Cejka D, Benesch T, Krestan C, Roschger P, Klaushofer K, Pietschmann P, Haas M. Effect of teriparatide on early bone loss after kidney transplantation. Am J Transplant. 2008;8:1864–70. doi: 10.1111/j.1600-6143.2008.02327.x. [DOI] [PubMed] [Google Scholar]
- 39.Nawrot-Wawrzyniak K, Misof BM, Roschger P, Pańczyk-Tomaszewska M, Ziółkowska H, Klaushofer K, Fratzl-Zelman N. Changes in bone matrix mineralization after growth hormone treatment in children and adolescents with chronic kidney failure treated by dialysis:a paired biopsy study. Am J Kidney Dis. 2013;61:767–77. doi: 10.1053/j.ajkd.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Yajima A, Inaba M, Tominaga Y, Nishizawa Y, Ikeda K, Ito A. Increased osteocyte death and mineralization inside bone after parathyroidectomy in patients with secondary hyperparathyroidism. J Bone Miner Res. 2010;25:2374–81. doi: 10.1002/jbmr.126. [DOI] [PubMed] [Google Scholar]
- 41.Tazawa K, Hoshi K, Kawamoto S, Tanaka M, Ejiri S, Ozawa H. Osteocytic osteolysis observed in rats to which parathyroid hormone was continuously administered. J Bone Miner Metab. 2004;22:524–9. doi: 10.1007/s00774-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 42.Nango N, Kubota S, Hasegawa T, Yashiro W, Momose A, Matsuo K. Osteocyte-directed bone demineralization along canaliculi. Bone. 2016;84:279–288. doi: 10.1016/j.bone.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Tsourdi E, Jähn K, Rauner M, Busse B, Bonewald LF. Physiological and pathological osteocytic osteolysis. J Musculoskelet Neuronal Interact. 2018;18:292–303. [PMC free article] [PubMed] [Google Scholar]
- 44.Lehmann G, Ott U, Kaemmerer D, Schuetze J, Wolf G. Bone histomorphometry and biochemical markers of bone turnover in patients with chronic kidney disease Stages 3 - 5. Clin Nephrol. 2008;70:296–305. doi: 10.5414/cnp70296. [DOI] [PubMed] [Google Scholar]
- 45.Malluche HH, Monier-Faugere MC, Wang G, Frazã O JM, Charytan C, Coburn JW, Coyne DW, Kaplan MR, Baker N, McCary LC, Turner SA, Goodman WG. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol. 2008;69:269–78. doi: 10.5414/cnp69269. [DOI] [PubMed] [Google Scholar]
- 46.Behets GJ, Spasovski G, Sterling LR, Goodman WG, Spiegel DM, De Broe ME, D'Haese PC. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int. 2015;87:846–56. doi: 10.1038/ki.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yajima A, Tsuchiya K, Bonewald LF, Inaba M, Tominaga Y, Tanizawa T, Ito A, Nitta K. Case report:Electron microscopic evaluation of bone from a patient treated with cinacalcet hydrochloride, maxacalcitol, and alfacalcidol for hyperparathyroid bone disease with secondary hyperparathyroidism. Osteoporos Int. 2018;29:1203–1209. doi: 10.1007/s00198-018-4402-3. [DOI] [PubMed] [Google Scholar]
- 48.Yajima A, Inaba M, Tominaga Y, Tanaka M, Otsubo S, Nitta K, Ito A, Satoh S. Impact of lanthanum carbonate on cortical bone in dialysis patients with adynamic bone disease. Ther Apher Dial. 2013;17(Suppl.1):41–8. doi: 10.1111/1744-9987.12038. [DOI] [PubMed] [Google Scholar]
- 49.Damment S, Secker R, Shen V, Lorenzo V, Rodriguez M. Long-term treatment with lanthanum carbonate reduces mineral and bone abnormalities in rats with chronic renal failure. Nephrol Dial Transplant. 2011;26:1803–12. doi: 10.1093/ndt/gfq682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry:standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]


