Abstract
Objective:
This study aimed to examine whether WBV becomes a possible modality for the primary prevention of osteoporosis by exploring WBV frequency that has positive effects on bone properties in adult rats.
Methods:
Thirty-six 24-week-old rats were divided into one control and 5 experimental groups, which underwent WBV at various frequencies (15, 30, 45, 60 or 90 Hz), with a magnitude of 0.5 g, for 15 min/day, 5 days/week, for 8 weeks. Bone size, muscle weight and bone mechanical strength were measured at the end of experimental period. Bone mass, trabecular bone microarchitecture (TBMA) and cortical bone geometry were analyzed by micro-CT. Circulating bone formation/resorption markers were determined by ELISA.
Results:
Body weight-corrected soleus weight in all experimental groups and body weight-corrected extensor digitorum longus muscle weight in the 15 and 30 Hz groups were significantly higher than those of the control group, respectively. Femur trabecular thickness and width were significantly higher in the 15 Hz group than in the control group. However, there was no difference in bone mechanical strength or bone formation/resorption markers among all groups.
Conclusion:
These results suggest that WBV at low-frequencies may become a potent modality for the primary prevention of osteoporosis in adults.
Keywords: Whole Body Vibration, Frequency, Trabecular Bone Microarchitecture, Cortical Bone Geometry, Bone Mechanical Strength
Introduction
Osteoporosis is a major public health concern worldwide. Mechanical stress is required for bone to maintain its function[1], and to this end, physical activity is important for bone health[2]. Although whole body vibration (WBV) within the range of resonance frequencies of 1-10 Hz, which is formed by the car[3] or train[4], may cause diseases and health problems to the human, especially a low-back pain, WBV may represent a potent non-pharmacological approach to address osteoporosis. In fact, WBV was reported to prevent bone loss and the rate of falls[5]. However, the effects of WBV alone on bone mineral density (BMD) are controversial, with some studies reporting a positive[6-9] or negative[10] effect and others reporting no effect[11-14]. In addition, the effects of WBV on bone properties was reported in children and postmenopausal women, but not in young adults[15]. WBV action on bone growth in children may differ from that on bone deterioration in the elderly. Moreover, the effects of WBV on BMD were found to be stronger in the elderly and osteoporotic women, compared with non-osteoporotic men[16]. In animal studies, WBV at low-magnitude and high-frequency was shown to positively influence bone mass, BMD, bone mineral content (BMC), trabecular bone microarchitecture (TBMA), stiffness and mechanical strength in the femur[17,18], while a recent study has reported negative effects of WBV at low-magnitude and high-frequency on femoral bone properties[10]. Another study showed that WBV at high-frequency has beneficial effects on bone properties, whereas WBV at low-frequency has negative effects[19]. In addition to frequency, WBV magnitude had the impact on bone properties including trabecular bone volume[20,21] and femoral cancellous bone stiffness[14]. The sensitivity of trabecular bone to mechanical loading is also impacted by animal strain[22]. Collectively, these studies suggest that the effects of WBV on bone can differ greatly by WBV conditions and subjects, underscoring the need for osteoporosis prevention strategies tailored to each subject.
Using an osteopenia rat model, most studies examined whether WBV can improve bone mass or prevent the further development of osteoporosis[23-25], while only a few studies used normal adult rats for assessing the WBV effects on bone[19,21]. In adults, maintaining and increasing bone mass play an important role in the primary prevention of osteoporosis. For this end, it is also important to gain a clearer picture of how WBV, which is a passive exercise modality, influences adult bone properties. There were few reports on the effects of WBV frequencies on bone properties. In two studies, the higher frequency was reported to have positive effects on bone properties compared with the lower frequency (8 Hz vs 52 Hz vs 90 Hz, 45 Hz vs 90 Hz)[19,25]. So, we have speculated that the higher frequency of bone mechanical loading, which is induced by WBV at higher frequency, would exert more positive influence on bone properties. To examine this speculation, therefore, the present study has focused on the effects of WBV at five different frequencies (15 Hz - 90 Hz) on adult bone properties. On the other hand, Wang et al.[26] reported that age-related bone loss in normal male rats starts mostly from 9 months of age when bone growth is completed. In view of this backdrop, using normal male rats aged 6 months, the present study aimed to examine whether WBV for 2 months becomes a possible modality for the primary prevention of osteoporosis by exploring optimal WBV frequency that has beneficial effects on bone properties (bone mass, structure and strength). In clinic, WBV at optimal frequency may become a potent modality for the primary prevention of osteoporosis in adult subjects.
Materials and methods
This study was approved by the Committee of Research Facilities of Laboratory Animal Science of Kio University and was performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No.85-23, revised in 1996).
Animal care and experimental protocol
A total of thirty-six 24-week-old male Wistar rats (Japan SLC, Inc., Hamamatsu, Japan) were used in this study. Rats were housed in standard cages in an animal facility where the room temperature and lighting were controlled (temperature, 22-24°C; lighting, 12:12-h light-dark cycle), and were fed standard rodent chow (CE-2; CLEA Japan Inc., Tokyo, Japan) and water ad libitum during the experiment. Rats were divided into one control (CON) and five experimental groups (n=6 each). All experimental groups were treated with WBV (vertical direction vibration, magnitude 0.5±0.025 g, 15 min/day, 5 days/week) at 5 different frequencies (15, 30, 45, 60 and 90 Hz) using a vibration device system (Big Wave G-MasterPRO; Asahi Seisakusho Co. Ltd., Tokyo, Japan) for a period of 8 weeks.
After the WBV intervention, blood, muscles and bone samples were collected from all rats. Serum samples, obtained by centrifuging blood at 1000 g for 30 minutes, were stored at -80°C until biochemical analysis and enzyme-linked immunosorbent assay (ELISA). Bilateral soleus and extensor digitorum longus (EDL) muscles were harvested and weighed. Since there were two reports on the effects of WBV frequencies on femoral rat bone[19,25], we have also analyzed femoral bone properties in this study. Bilateral femurs’ wet weight and length were measured after the removal of soft tissue. Muscle weight and wet bone weight were corrected by body weight (BW). Right and left femurs were stored in saline and 70% ethanol, respectively, until analyzed. Right femurs were used for the measurements of bone mechanical strength, and left femurs were used not only for the analyses of bone mass, TBMA and cortical bone geometry (CBG) but also for the measurements of dry and ash bone weight.
Analyses of bone mass, TBMA and CBG
Analyses of bone mass and TBMA were performed as previously reported[27]. Using X-ray micro-computed tomography (Micro-CT; Hitachi Medical Corporation, Tokyo, Japan), the left distal femur was scanned in the high-definition mode (70 kV, 90 µA, voxel size 20.2 µm) for TBMA analysis. The region of interest (ROI) for TBMA of the distal femur was a 2-mm portion of the femur metaphysis, and the first slice was scanned 1 mm proximal from the physeal-metaphyseal demarcation. In addition, the diaphysis of the femur was simultaneously scanned with the Micro-CT device in the high-definition mode (70 kV, 90 µA, voxel size of 17.2 µm) for CBG analysis. The ROI for CBG was a 2-mm portion of the center of the femur diaphysis. Scanned data were transmitted to a personal computer, and TBMA and CBG of the ROI were analyzed using bone analysis software (TRI BON 3D; Ratoc System Engineering Co. Ltd., Tokyo, Japan).
Tissue volume (TV), bone volume (BV), bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), trabecular width (Tb.W), connectivity density (Conn.D) and structure model index (SMI) were assessed as TBMA parameters for the femur metaphysis. Cortical bone volume (CV), medullary volume (MV), cortical bone fraction (CV/(CV+MV)), cortical bone thickness (Ct.Th), cortical bone section area (Ct.Ar) and periosteal perimeter (Ps.Pm) were assessed as CBG parameters for the femur diaphysis. Moreover, a BMD phantom was simultaneously scanned under the same scanning conditions to obtain tissue mineral density (TMD), bone mineral content (BMC) and volume BMD (vBMD; BMC/TV).
Measurement of bone mechanical strength
The maximum load of the right femur was measured by a 3-point bending strength test using a Universal Testing Machine (Autograph AGS; Shimadzu Corp., Kyoto, Japan), and energy absorption was calculated. Bones were supported by 2 fulcrums (5 mm in diameter), and the distance between the fulcrums was half of the bone length. The bone center was then pressed downward at a speed of 1 mm/min.
Dry and ash bone weight measurements
After left femurs were used for assessing TBMA/CBG parameters, they were dehydrated in 100% ethanol for 48 hours and then heated at 100°C for 24 hours in a drying machine (Yamato Kagaku, Tokyo, Japan) to obtain dry bone weight. Bones were then burned to ash at 600°C for 24 hours with an electric furnace (Nitto Kagaku Co. Ltd., Nagoya, Japan) to obtain ash weight.
Biochemical analyses and ELISA
Serum samples were analyzed for calcium, inorganic phosphorus (IP), total protein, triglyceride and alkaline phosphatase (ALP). In addition, serum osteocalcin (OC) and tartrate-resistant acid phosphatase-5b (TRACP-5b) levels were determined with commercially available ELISA kits for OC (Immunodiagnostic Systems Ltd., Boldon, UK) and TRACP-5b (Immunodiagnostic Systems Ltd., Boldon, UK), respectively.
Statistical analyses
All values are expressed as mean ± standard deviation. Differences in effects of WBV frequency on measured parameters between the CON group and experimental groups were examined using Dunnett’s test. Overall differences among experimental groups were determined by one-way ANOVA, and differences between individual groups were examined using the Bonferroni post hoc test. Effect sizes were presented as η2 in one-way ANOVA of all groups. All statistical analyses were performed using Excel Statistics software (BellCurve for Excel version 2.11 for Windows; Social Survey Research Information Co., Ltd., Tokyo, Japan). P<0.05 was considered statistically significant.
Results
BW, food intake, muscle weight and bone size
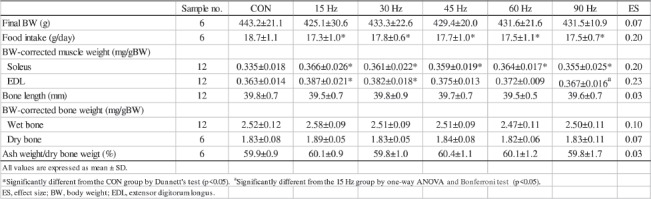
Table 1 summarizes the results for BW, food intake, BW-corrected muscle weight and bone size (bone length, BW-corrected wet and dry bone weight, and ash weight/dry bone weight) of the femur in all groups. Final BW did not significantly differ among groups, although food intake was significantly greater in the CON group than in all experimental groups. BW-corrected soleus weight was heavier in all experimental groups than in the CON group. BW-corrected EDL weight was heavier in the 15 and 30 Hz groups than in the CON group, and heavier in the 15 Hz group than in the 90 Hz group. Bone size did not significantly differ among groups.
Table 1.
Body weight, food intake, muscle weight and femoral bone size.
Bone mass parameters
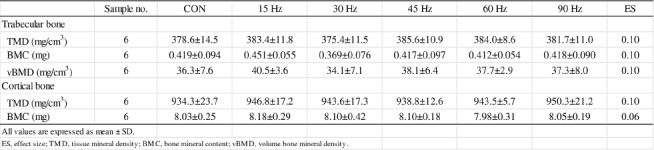
There were no differences in bone mass parameters of trabecular and cortical bones among all groups (Table 2).
Table 2.
Bone mass parameters of trabecular and cortical bones in femur.
TBMA and CBG parameters
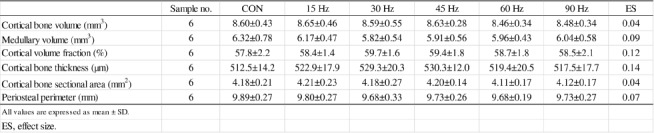
Figure 1 and Table 3 show TBMA and CBG parameters of the femur in all groups, respectively. Tb.Th and Tb.W of the femur were significantly higher in the 15 Hz group than in the CON group (Figure 1). Like bone mass parameters, there were no differences in CBG parameters among all groups (Table 3).
Figure 1.
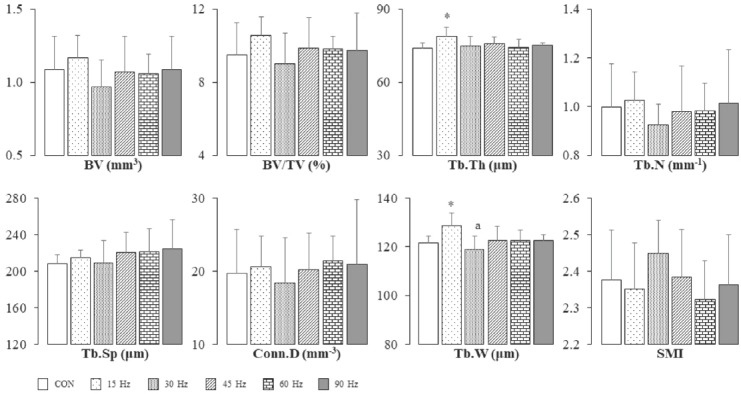
Trabecular bone microarchitecture parameters of femoral metaphysis. *Significantly different from the CON group by Dunnet test (p<0.05). aSignificantly different from the 15 Hz group by one-way ANOVA and Bonferroni test (p<0.05). Bar=SD. Sample no.=6. Effect sizes; BV, 0.09; BV/TV, 0.10; Tb.Th, 0.26; Tb.N, 0.05; Tb.Sp, 0.08; Conn.D, 0.03; Tb.W, 0.33; SMI, 0.11. BV, bone volume; BV/TV, bone volume ratio; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation; Conn.D, connectivity density; Tb.W, trabecular width; SMI, structure model index.
Table 3.
Cortical bone geometry of femur in all groups.
Bone mechanical strength
There were no differences in maximum load and energy absorption of the femur between the CON group and all experimental groups (Figure 2).
Figure 2.
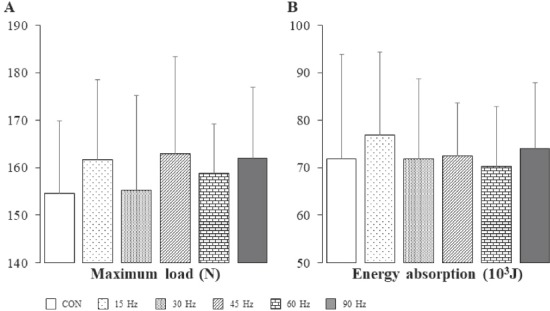
Maximum load (A) and energy absorption (B) of femur in all groups. Bar=SD. Sample no.=6. Effect sizes; Maximum load, 0.04; Energy absorption, 0.02.
Biochemical analyses
Serum concentration of inorganic phosphorus was significantly higher in the 90 Hz group than in the CON, 15 Hz and 30 Hz groups (Table 4). On the other hand, there were no significant differences in serum levels of OC and TRACP-5b among all groups (Figure 3).
Table 4.
Serum biochemical analyses.
Figure 3.
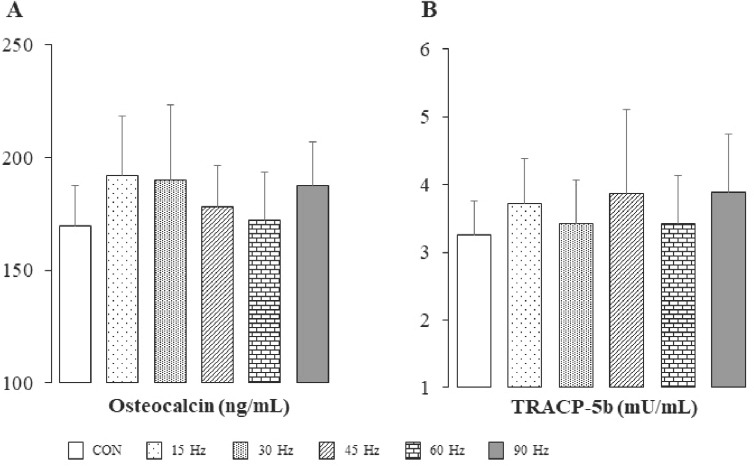
Serum levels of osteocalcin (A) and tartrate-resistant acid phosphatase-5b (B) in all groups. Bar=SD. Sample no.=6. Effect sizes; OC, 0.15; TRACP-5b, 0.10. TRACP-5b, tartrate-resistant acid phosphatase-5b.
Discussion
In the present study, WBV at 15 Hz/0.5 g for 8 weeks increased Tb.Th and Tb.W of the femur trabecular bone in adult male rats. Rubin et al. previously reported a >30% increase in trabecular density and BV/TV of the proximal femur in female sheep treated with vertical vibration at a frequency of 30 Hz and a magnitude of 0.3 g for 20 min/day, 5 days/week, for 1 year, compared with control sheep[18]. Several investigators, as well as Rubin et al.[18], used 0.3 g as the magnitude to examine the effects of WBV on bone in animal studies[17,20-22,24], where its positive effects were observed. On the other hand, other investigators used 1 g or more magnitude in animal bone studies using WBV[14,20]. A vibration stimulus of <1 g is generally considered to be low-magnitude, whereas a stimulus of ≥1 g is considered to be high-magnitude[28-30]. Therefore, we have used the half of 1 g, i.e., 0.5 g as low-magnitude in all WBV experiments. In another study, 3-month-old Sprague Dawley rats, which were treated with WBV (90 Hz, 0.5 mm amplitude, for 15 min/day, twice a day, 7 days/week, for 35 days), showed an increase in both the maximum load and stiffness of the femur[23]. Thus, even if the strain on bone is small, i.e., low-magnitude, WBV at relatively high-frequencies could augment bone properties. Two previous studies reported the effects of WBV frequencies on bone properties. Pasqualini et al.[19] investigated the effects of different vibration frequencies (8, 52 and 90 Hz, magnitude of 0.7 g, for 10 min/day, 5 days/week, for 4 weeks) in mature Wistar rats, and found that, compared with the control, WBV at 90 Hz significantly increases BV/TV of the femur, Tb.Th of the tibia and Conn.D, BV/TV and trabecular BMD of L2 vertebra, whereas WBV at 8 Hz significantly decreases trabecular BMD of the tibia and femur. Judex et al.[25] reported that WBV at higher frequency has more positive effects on TBMA parameters of distal femur in ovariectomized rats (45 Hz and 90 Hz, magnitude of 0.15 g, for 10 min/day, 5 days/week, for 4 weeks). These results suggest that higher frequency brings forth positive effects on bone properties compared with lower frequencies. On the contrary, in this study, Tb.Th and Tb.W of TBMA parameters were significantly increased in the 15 Hz group, but not in the 90 Hz group.
WBV-induced mechanical stress to bone is attributable to both vibration loading and muscle contraction. The action of vibration loading may be explainable by the daily stress stimulus theory, which identifies both strain magnitude and the number of loading cycles as an appropriate maintenance loading signal[31]. The resultant strain magnitude required for maintaining the bone mass was approximately 340 µɛ, and the stress exponent value m was calculated to be approximately 2.24 and 1.04 in our 15 Hz and 90 Hz groups, respectively[31]. The present rats in the 15 Hz group, which underwent 13,500 cycles of vibration per day, seem to have been given the loading to maintain/increase bone mass, because the stress exponent value m for maintaining the bone mass was reported to be approximately 2.5 in the range of 10,000-36,000 cycles per day[31]. On the other hand, the 90 Hz group with 1.04 of the stress exponent value m underwent 81,000 cycles per day. Since more than 100,000 cycles per day were reported to increase bone mass in case of approximately 1.0 of the stress exponent value m[31], it seems likely that the TBMA parameters remain unchanged in our 90 Hz group. In addition, WBV at 17 Hz with a magnitude of 0.5 g, as well as WBV at 45 Hz with a magnitude of 3.0 g, increased tibia bending stress and femur compression stress in ovariectomized rats, and these two vibration frequencies were able to prevent the reduction of bone mechanical strength, which was caused by ovariectomy[24]. Therefore, the combination of frequencies and magnitude appear to influence the TBMA parameters.
Muscle contraction during WBV results from a tonic vibration reflex, which involves the contraction/relaxation cycle of muscles during WBV[29]. Larger muscle mass is generally considered to generate stronger muscle strength. In our adult rats, BW-corrected muscle weight of soleus and EDL was significantly heavier in the 15 Hz group than in the CON group. Such an increase in relative muscle mass appears to have been induced by the exercise effects of WBV. Likewise, in tail-suspended rats[32] and sarcopenia model mice[33], muscle mass, fiber cross-sectional area or muscle strength of lower limb muscles could be improved by WBV. Moreover, in normal mice, not only soleus cross-sectional area and type I and II fiber area but also trabecular BV and CV of tibia were increased by WBV[34]. WBV was also reported to increase or improve muscle strength, power and flexibility[35]. Furthermore, muscle strength and size were found to correlate with BMD and BMC[36]. Bone can continuously bear both gravitational loading and loading induced by muscle contraction, and consequently, in the bone–muscle unit, these mechanical loading could play a pivotal role in determining not only muscle mass/structure but also bone mass/structure[36]. In addition, oscillatory stimulation of muscles regulates both mechanical bone strain and fluid dynamics. For instance, in tail-suspended model rats, stimulation at 10 Hz and 20 Hz could generate the maximum matrix strain and the maximum intramedullary pressure, respectively[37]. In view of these observations, it seems likely that TBMA parameters are influenced by mechanical loading accompanied with WBV-induced muscle contraction.
Although mechanical loading, as mentioned above, may have positive effects on bone properties, the reason why only15 Hz-WBV increased Tb.Th and Tb.W still remains to be resolved in this study. In addition to loading cycles and muscle contraction, it should be noted that transmissibility can play a role in contributing to the strength of stress applied to bone. In human, transmissibility of lower limb joints (e.g., hip, knee and ankle) and spine was shown to differ by frequencies[38], suggesting that in rats, too, transmissibility of joints may depend upon frequency. Likewise, transmissibility may differ among bones. In rats, the femur transmissibility may be different from that of the tibia, which was reported to be less than 5 µɛ[25]. In a previous study of Wehrle et al. (35 Hz and 45 Hz, magnitude of 0.3 g, for 20 min/day, 5 days/week, for 10 and 21 days)[39], WBV at lower frequency of 35 Hz, like our 15 Hz-WBV, showed positive effects on bone properties as compared with WBV at higher frequency of 45 Hz. WBV at 35 Hz increased BV/TV and Tb.N of femur, but not CBG parameters[39]. Wehrle et al.[39] reported that the signal transition of WBV to bone matrix depends on body mass, individual anatomy, a posture during WBV, species, age, gender and genetic predisposition. In addition, 45 Hz-WBV with a magnitude of 0.1 g or 1.0 g showed positive effects on BV/TV, Tb.Th and Tb.N of tibia, whereas 45 Hz-WBV with a magnitude of 0.3 g showed no effects on tibia bone parameters[20]. Thus, the complex mechanism comprised of multiple factors appears to exist in bone-response to WBV.
WBV was reported to increase plasma concentrations of growth hormone and testosterone[40]. Furthermore, we previously reported that WBV can prevent a decrease in bone formation marker OC in rats with spinal cord injury[41]. On the other hand, the present WBV at various frequencies did not induce any change in bone formation/resorption markers. In view of these results, TBMA changes found in our 15 Hz-WBV group may have resulted from the complex interaction among multiple factors such as mechanical stress, muscle contraction, transmissibility, hormonal/physiological conditions, etc. in bone-response to WBV.
In this study, serum IP concentration was significantly higher in the 90 Hz group than in the CON, 15 Hz and 30 Hz groups. Phosphate metabolism is regulated mainly by fibroblast growth factor 23 (FGF23), which is produced by osteocytes and osteoblasts[42,43], as well as dietary phosphate, calcitriol and parathyroid hormone (PTH). Moreover, the rate of bone remodeling is thought to be another important factor for determining the plasma IP concentrations[40]. In our study, there was no significant difference in bone formation/resorption markers, i.e., OC and TRACP-5b, among all groups, suggesting similar bone remodeling rates in all groups. Therefore, the increased serum IP found in the 90 Hz group may have been caused by the suppression in the production and/or action of bone-derived FGF23. To explore the possible mechanism of serum IP increase by 90 Hz WBV, the determination of circulating levels of FGF23, calcitriol and PTH is required.
This study has some limitations. First, the sample sizes were small and thereby most of data were statistically underpowered. Therefore, it should be noted that the data, presented herein, may lead to potential over-interpretation. Studies using more animals are indispensable for further confirming the present results. Second, although WBV was performed at 5 different frequencies, only one magnitude (0.5 g) was tested. Several studies used approximately 0.3 g of magnitude in WBV[17,20-22,24]. Moreover, the previous studies showed that the effects of WBV on bone differ when various magnitudes are used at the same frequency[20,21,44]. Therefore, further studies will be needed to determine optimal magnitude of WBV at 15 Hz of frequency. Third, we have not measured dynamic strain in the femur. The measurements of this strain magnitude are needed to further confirm the beneficial effects of muscle contraction on bone quality. Fourth, we have determined circulating bone formation/resorption markers in response to WBV, but have not measured hormones and cytokines. Further studies, that assess both the effects of WBV on hormones/cytokines and how they influence bone parameters, are required. Finally, further studies are needed to explore optimal WBV conditions (frequency, magnitude, duration, times, period and these combinations) for augmenting bone mass and TBMA in adult rats.
Conclusions
In adult rats, WBV at 15 Hz of frequency was found to have beneficial effects on bone and the potency to increase TBMA, in particular, Tb.Th and Tb.W, in the femur. Thus, WBV at low-frequency may become a potent modality for the primary prevention of osteoporosis in adults.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research C (15K01445).
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Frost HM. The Utah Paradigm of Skeletal Physiology (Volume I) Nafplio: International Society of Musculoskeletal and Neuronal Interactions; 2004. [Google Scholar]
- 2.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American College of Sports Medicine. American College of Sports Medicine Position Stand:Physical activity and bone health. Med Sci Sports Exerc. 2004;36(11):1985–96. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 3.Funakoshi M, Taoda K, Tsujimura H, Nishiyama K. Measurement of whole-body vibration in taxi drivers. J Occup Health. 2004;46:119–24. doi: 10.1539/joh.46.119. [DOI] [PubMed] [Google Scholar]
- 4.Ismail AR, Nuawi MZ, How CW, Kamaruddin NF, Nor MJM, Makhtar NK. Whole body vibration exposure to train passenger. Am J Applied Sci. 2010;7(3):352–9. [Google Scholar]
- 5.Ma C, Liu A, Sun M, Zhu H, Wu H. Effect of whole-body vibration on reduction of bone loss and fall prevention in postmenopausal women:a meta-analysis and systematic review. J Orthop Surg Res. 2016;11:24. doi: 10.1186/s13018-016-0357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai CL, Tseng SY, Chen CN, Liao WC, Wang CH, Lee MC, Hsu PS. Effect of 6 months of whole body vibration on lumbar spine bone density in postmenopausal women:a randomized controlled trial. Clin Interv Aging. 2013;8:1603–9. doi: 10.2147/CIA.S53591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck BR, Norling TL. The effect of 8 mos of twice-weekly low- or higher intensity whole body vibration on risk factors for postmenopausal hip fracture. Am J Phys Med Rehabil. 2010;89(12):997–1009. doi: 10.1097/PHM.0b013e3181f71063. [DOI] [PubMed] [Google Scholar]
- 8.Humphries B, Fenning A, Dugan E, Guinane J, MacRae K. Whole-body vibration effects on bone mineral density in women with or without resistance training. Avizt Space Environ Med. 2009;80(12):1025–31. doi: 10.3357/asem.2573.2009. [DOI] [PubMed] [Google Scholar]
- 9.Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women:a randomized controlled pilot study. J Bone Miner Res. 2004;19(3):352–9. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 10.Zhang T, Gao J, Fang J, Gong H. Multiscale investigation on the effects of additional weight bearing in combination with low-magnitude high-frequency vibration on bone quality of growing female rats. J Bone Miner Metab. 2018;36:157–69. doi: 10.1007/s00774-017-0827-6. [DOI] [PubMed] [Google Scholar]
- 11.Kiel DP, Hannan MT, Barton BA, Bouxsein ML, Sisson E, Lang T, Allaire B, Dewkett D, Carroll D, Maqaziner J, Shane E, Leary ET, Zimmerman S, Rubin CT. Low-magnitude mechanical stimulation to improve bone density in persons of advanced age:A randomized, placebo-controlled trial. J Bone Miner Res. 2015;30(7):1319–28. doi: 10.1002/jbmr.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liphardt AM, Schipilow J, Hanley DA, Boyd SK. Bone quality in osteopenic postmenopausal women is not improved after 12 months of whole-body vibration training. Osteoporos Int. 2015;26(3):911–20. doi: 10.1007/s00198-014-2995-8. [DOI] [PubMed] [Google Scholar]
- 13.Gómez-Cabello A, González-Agüero A, Morales S, Ara I, Casajús JA, Vincente-Rodriquez G. Effects of a short-term whole body vibration intervention on bone mass and structure in elderly people. J Sci Med Sport. 2014;17(2):160–4. doi: 10.1016/j.jsams.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Runge WO, Ruppert DS, Mercellin-Little DJ, Dahners LE, Harrysson OLA, Weinhold PS. Bone changes after short-term whole body vibration are confined to cancellous bone. J Musculoskelet Neuronal Interact. 2018;18(4):485–92. [PMC free article] [PubMed] [Google Scholar]
- 15.Slatkovska L, Alibhai SMH, Beyene J, Cheung AM. Effect of whole-body vibration on BMD:a systematic review and meta-analysis. Osteoporos Int. 2010;21(12):1969–80. doi: 10.1007/s00198-010-1228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zha DS, Zhu QA, Oei WW, Zheng JC, Wu SH, Xu ZX, Li T, Chen JT. Does whole-body vibration with alternative tilting increase bone mineral density and change bone metabolism in senior people. Aging Clin Exp Res. 2012;24(1):28–36. doi: 10.3275/7517. [DOI] [PubMed] [Google Scholar]
- 17.Rubin C, Turner AS, Muller R, Mittra E, McLeod K, Lin W, Qin YX. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17(2):349–57. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 18.Rubin C, Turner AS, Mallomckrodt C, Jerome C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002;30(3):445–52. doi: 10.1016/s8756-3282(01)00689-5. [DOI] [PubMed] [Google Scholar]
- 19.Pasqualini M, Lavet C, Elbadaoui M, Vanden-Bossche A, Laroche N, Gnyubkin V, Vico L. Skeletal site-specific effects of whole body vibration in mature rats:from deleterious to beneficial frequency-dependent effects. Bone. 2013;55(1):69–77. doi: 10.1016/j.bone.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Christiansen BA, Silva MJ. The effect of varying magnitudes of whole-body vibration on several skeletal sites in mice. Ann Biomed Eng. 2006;34(7):1149–56. doi: 10.1007/s10439-006-9133-5. [DOI] [PubMed] [Google Scholar]
- 21.Lynch MA, Brodt MD, Silva MJ. Skeletal effects of whole-body vibration in adult and aged mice. J Orthop Res. 2010;28(2):241–7. doi: 10.1002/jor.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Judex S, Donahue LR, Rubin C. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J. 2002;16(10):1280–2. doi: 10.1096/fj.01-0913fje. [DOI] [PubMed] [Google Scholar]
- 23.Tezval M, Biblis M, Sehmish S, Schmelz U, Kolios L, Rack T, Stuermer KM, Stuermer EK. Improvement of femoral bone quality after low-magnitude, high-frequency mechanical stimulation in the ovariectomized rat as an osteopenia model. Calcif Tissue Int. 2011;88(1):33–40. doi: 10.1007/s00223-010-9423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oxlund BS, Ørtoft G, Andreassen TT, Oxlund H. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone. 2003;32(1):69–77. doi: 10.1016/s8756-3282(02)00916-x. [DOI] [PubMed] [Google Scholar]
- 25.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40(6):1333–9. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Banu J, McMahan CA, Kalu DN. Male rodent model of age-related bone loss in man. Bone. 2001;29(2):141–8. doi: 10.1016/s8756-3282(01)00483-5. [DOI] [PubMed] [Google Scholar]
- 27.Minematsu A, Nishii Y, Imagita H, Takeshita D, Sakata S. Long-term intake of green tea extract causes mal-conformation of trabecular bone microarchitecture in growing rats. Calcif Tissue Int. 2018;102(3):358–67. doi: 10.1007/s00223-017-0358-0. [DOI] [PubMed] [Google Scholar]
- 28.Lau E, Al-Dujaili S, Guenther A, Liu D, Wang L, You L. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46:1508–15. doi: 10.1016/j.bone.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasturi GC, Adler RA. Osteoporosis:nonpharmacologic management. Physical Med Rehab. 2011;3(6):562–72. doi: 10.1016/j.pmrj.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Beck BR. Vibration therapy to prevent bone loss and falls:mechanisms and efficacy. Curr Osteoporos Rep. 2015;13:381–9. doi: 10.1007/s11914-015-0294-8. [DOI] [PubMed] [Google Scholar]
- 31.Qin YX, Rubin CT, McLeod KJ. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J Orthop Res. 1998;16(4):482–9. doi: 10.1002/jor.1100160414. [DOI] [PubMed] [Google Scholar]
- 32.Sun KT, Leung KS, Siu PM, Qin L, Cheung WH. Differential effects of low-magnitude high-frequency vibration on reloading hind-limb soleus and gastrocnemius medialis muscles in 28-day tail-suspended rats. J Musculoskelet Neuronal Interact. 2015;15(4):316–24. [PMC free article] [PubMed] [Google Scholar]
- 33.Guo AY, Leung KS, Qin JH, Chow SK, Cheung WH. Effect of Low-Magnitude, High-Frequency Vibration Treatment on Retardation of Sarcopenia:Senescence-Accelerated Mouse-P8 Model. Rejuvenation Res. 2016;19(4):293–302. doi: 10.1089/rej.2015.1759. [DOI] [PubMed] [Google Scholar]
- 34.Xie L, Rubin C, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol. 2008;104(4):1056–62. doi: 10.1152/japplphysiol.00764.2007. [DOI] [PubMed] [Google Scholar]
- 35.Alam MM, Khan AA, Farooq M. Effect of whole-body vibration on neuromuscular performance:a literature review. Work. 2018;59(4):571–83. doi: 10.3233/WOR-182699. [DOI] [PubMed] [Google Scholar]
- 36.Tagliaferri C, Wittrant Y, Davicco MJ, Walrand S, Coxam V. Muscle and bone, two interconnected tissues. Ageing Res Rev. 2015;21:55–70. doi: 10.1016/j.arr.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Qin YX, Lam H. Intramedullary pressure and matrix strain induced by oscillatory skeletal muscle stimulation and its potential in adaptation. J Biomech. 2009;42(2):140–5. doi: 10.1016/j.jbiomech.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiiski J, Heinonen A, Järvinen TL, Kannus P, Sievänen H. Transmission of vertical whole body vibration to the human body. J Bone Miner Res. 2008;23(8):1318–25. doi: 10.1359/jbmr.080315. [DOI] [PubMed] [Google Scholar]
- 39.Wehrle E, Wehner T, Heilmann A, Bindl R, Claes L, Jakob F, Amling M, Ignatis A. Distinct frequency dependent effects of whole-body vibration on non-fracture bone and fracture healing in mice. J Orthop Res. 2014;32(8):1006–13. doi: 10.1002/jor.22629. [DOI] [PubMed] [Google Scholar]
- 40.Bosco C, Iacovelli M, Tsarpela O, Cardinale M, Bonifazi M, Tihanyi J, Viru M, De Lorenzo A, Viru A. Hormonal responses to whole-body vibration in men. Eur J Appl Physiol. 2000;81(6):449–54. doi: 10.1007/s004210050067. [DOI] [PubMed] [Google Scholar]
- 41.Minematsu A, Nishii Y, Imagita H, Takeshita D, Sakata S. Whole-body vibration can attenuate the deterioration of bone mass and trabecular bone microstructure in rats with spinal cord injury. Spinal Cord. 2016;54:597–603. doi: 10.1038/sc.2015.220. [DOI] [PubMed] [Google Scholar]
- 42.Pendio MGMG, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2014;27(11):2039–48. doi: 10.1007/s00467-012-2175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi Z, Liu W, Lu J. The mechanism underlying the beneficial effects of exercise on bone remodeling:Role of bone-derived cytokines and microRNAs. Prog Biophys Mol Biol. 2016;122(2):131–9. doi: 10.1016/j.pbiomolbio.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Rubin CT, Sommerfeldt DW, Judex S, Qin Y. Inhibition of osteopenia by low magnitude, high-frequency mechanical stimuli. Drug Discov Today. 2001;6(16):848–58. doi: 10.1016/s1359-6446(01)01872-4. [DOI] [PubMed] [Google Scholar]


