Abstract
The burden of pain is unequal across racial and ethnic groups. In addition to racial and ethnic differences in the experience of pain, there are racial and ethnic disparities in the assessment and treatment of pain. In this article, we provide a nonexhaustive review of the biopsychosocial mechanistic factors contributing to racial and ethnic differences in both the experience and treatment of pain. Using a modified version of the Socioecological Model, we focus on patient-, provider- and system-level factors including coping, perceived bias and discrimination, patient preferences, expectations, patient/provider communication, treatment outcomes and healthcare access. In conclusion, we provide psychosocial factors influencing racial and ethnic differences in pain and highlight future research targets and possible solutions to reduce these disparities.
Keywords: : disparities, ethnicity, mechanisms, pain, psychosocial, race
Practice points.
Racial and ethnic differences in pain coping, particularly for catastrophizing, praying and stoicism, may be driving differences in the experience of pain.
There is mixed evidence suggesting perceived bias and discrimination may lead to increased experimental and clinical pain among racial and ethnic minorities.
False beliefs and implicit bias may be driving racial and ethnic disparities in the assessment and treatment of pain.
Cultural differences among people of diverse racial and ethnic backgrounds lead to differences in pain treatment preferences.
Racial and ethnic minority patients have worse pain expectations which may influence pain treatment disparities. However, there is evidence that expectations are malleable and may serve as a good target for intervention.
Factors including language barriers, lack of access to interpreting services, cultural differences in communication styles and health literacy all impact patient/provider communication and can lead to poor treatment adherence and outcomes.
Future research should examine how the above-mentioned factors interact with each other as well as race and ethnicity. Further, research is limited among Asian–Americans, Native Americans, Alaskan Natives, Hawaiian and Pacific Islanders, and Middle-Eastern Americans. Thus, future research should aim to characterize pain among these groups.
Chronic pain affects nearly 100 million Americans and costs US$635 billion annually [1]. However, the burden of pain is unequal across racial and ethnic groups. Indeed, some studies have shown that compared with white individuals, black individuals are more sensitive to and less tolerant of experimental pain and report greater pain-related disability, suffering and psychological symptoms in response to clinical pain [2,3,4]. There is evidence that while Hispanic–Americans report fewer pain conditions and less pain interference than non-Hispanic white individuals (NHWs), they endorse more severe clinical pain and demonstrate greater pain sensitivity and less tolerance for experimental pain [5]. While much of the literature examining racial and ethnic differences in pain have focused on black, Hispanic and NHW groups, there is some evidence that pain also differs among Asian and Native Americans. Among individuals with painful knee osteoarthritis, Asian–Americans endorse higher levels of pain intensity [6]. Further, Asian–Americans demonstrate a lower pain tolerance and threshold and greater pain sensitivity compared with white individuals [7,8]. On the other hand, there is some evidence that Native Americans are less pain sensitive than white individuals [9].
In addition to racial and ethnic differences in the experience of pain, there are also racial and ethnic disparities in pain treatment. According to a review by Anderson and colleagues, such treatment disparities are present across treatment settings [10]. For example, minority patients are rated as having less severe pain, are less likely to receive comprehensive diagnostic and treatment approaches for pain, and receive less analgesics than white persons [11,12,13,14,15,16,17,18,19,20]. Minority patients are more likely to have their pain underestimated by providers, less likely to receive opioids as part of their pain management regimen and receive less aggressive pain treatment than white patients [21,22,23,24,25]. Further, non-white patients seen in the emergency department are 22–30% less likely to receive analgesic medications, 17–30% less likely to receive narcotics, have longer wait times and are less likely to be admitted compared with white patients [26].
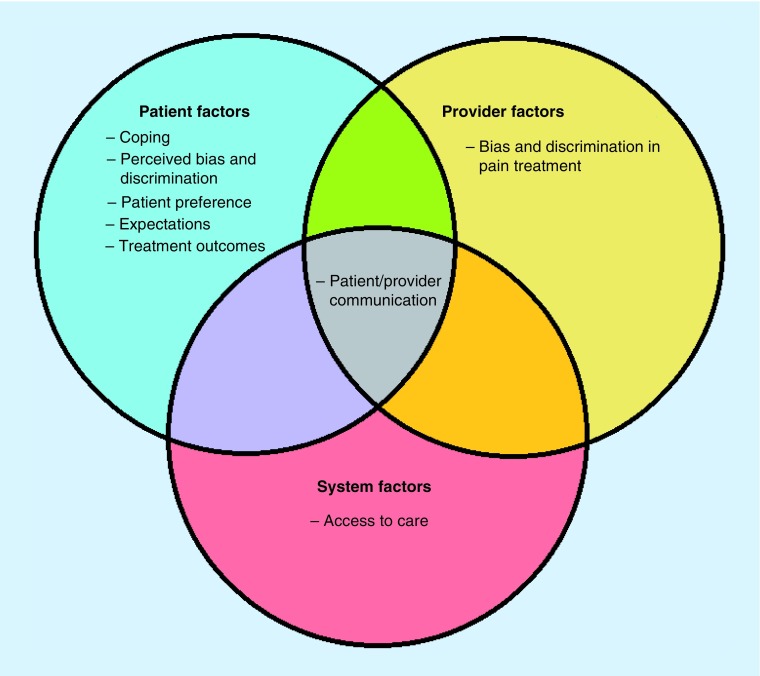
Taken together, there are considerable racial and ethnic differences in the experience and treatment of pain in the USA. Recent literature has aimed not only to quantify these differences but to determine possible mechanisms of and factors contributing to them. The aim of this review is to provide a non-exhaustive discussion of several possible patient-, provider-, and system-level psychosocial mechanistic factors contributing to the above described racial and ethnic differences in pain. We will use a modified version of the socioecological model as a framework for our discussion. The socioecological model considers the role of the individual, relationships, community and societal factors on healthcare (Figure 1) [27]. The first level, individual factors, includes such factors as expectations, beliefs and coping. The second level, relationship factors, includes the influence of providers and provider-level factors. The third level, community factors, includes the physical environment such as the treating institutions or neighborhood considerations. Finally, the fourth level, societal factors, includes social and cultural norms as well as health, economic and educational policies. It is important to note that these factors do not occur in isolation but instead interact across levels (e.g., bias and racism at the provider level impact patient expectations, an individual first level factor). For the purpose of this review, we have collapsed the third and fourth levels, community and environmental factors, as these factors are enmeshed within the pain disparities literature and are often times the most difficult to change. As Campbell and Edwards provided a comprehensive review of ethnic differences in both the experience and management of pain in 2012 [28], this review will focus primarily on more recent findings. Additionally, we will briefly touch on possible solutions and future research targets aimed at reducing racial and ethnic disparities.
Figure 1. . Modified socioecological model of patient, provider and system level factors influencing racial and ethnic disparities in pain.
Coping
One possible patient-related mechanism contributing to the observed race and ethnic differences in the experience of pain is pain-related coping. Catastrophizing, a cognitive and emotional response to pain characterized by rumination (“I cannot seem to keep it out of my mind”), magnification (“I am afraid the pain will get worse”) and helplessness (“I feel I cannot go on”), is a pain coping strategy associated with higher pain levels, poorer physical functioning and disability, and increased pain interference [29,30,31,32]. Prior studies have demonstrated that, compared with NHWs, black and Hispanic–Americans catastrophize more in response to pain [33,34,35]. Moreover, catastrophizing mediates race differences in experimental pain tolerance with black Americans catastrophizing more than white Americans which is associated with a poorer pain tolerance [36,37].
It is important to note that there is some debate whether catastrophizing is truly a coping strategy or whether, instead, it can be better characterized as a pain appraisal [38]. Indeed, evidence shows that catastrophizing is associated with other appraisal constructs such as coping efficacy [39]. Furthermore, coping typically refers to strategies used to minimize the impact of stress on overall wellbeing [40], and catastrophizing is consistently shown to be associated with poor pain-related outcomes [41,42]. However, it is important to consider that distress reduction may not be the primary goal of coping [43]. Catastrophizing, for example, may also serve to activate an individual’s social environment by soliciting assistance, support or empathy [38,44,45,46]. Because the nature and goal of catastrophizing may differ by racial and ethnic group, specifically as a result of social and cultural influences, more research is needed to determine the function of catastrophizing among persons with diverse racial and ethnic backgrounds. Indeed, an understanding of the function of catastrophizing (e.g., to gain social support, elicit empathy, legitimize pain) may assist in the development of culturally tailored pain treatments.
Beyond catastrophizing, there are also notable racial and ethnic differences in the use of praying and religious coping in response to pain. A recent meta-analysis found that compared with white Americans, black Americans endorse the use of praying more frequently in response to pain [33]. Several studies also demonstrate that Hispanic–Americans pray more in response to pain compared with NHWs [34,35,47,48,49]. However, Hispanic and black Americans report similar use of religious coping in response to pain [34,35,47,48,49]. These findings are consistent with the broader literature on race and religion which indicates that Hispanic and black Americans pray more, self-identify as more religious and endorse the use of more religion-based coping [50,51,52]. Praying in response to pain has been associated with greater pain severity, pain interference and disability [34,53,54,55] but also with greater psychological wellbeing [56]. It is possible that racial and ethnic differences in prayer and religious coping are driving racial and ethnic differences in the pain experience. Indeed, results of experimental pain studies of healthy, pain-free individuals demonstrate that prayer mediates race differences in experimental pain tolerance such that black participants are more likely to endorse the use of prayer to cope with pain and, as a result, demonstrate lower pain tolerance [36]. However, there are considerable limitations of this literature. First, prayer has only been examined of a mechanism of black/white race differences in experimental pain among healthy subjects. Thus, it remains unclear how prayer impacts pain among other racial and ethnic groups and how it functions in the context of clinical pain. Additionally, prayer is usually measured as a passive coping strategy. Because passive strategies are associated with poorer pain outcomes, it is unclear whether the act of praying or rather the passive nature of the prayer is contributing to these race differences. In a recent study of healthy, pain-free individuals, Meints and colleagues found that pain tolerance was poorer for those engaged in passive compared with active prayer and that was particularly true for black participants [57]. Moreover, religious coping and prayer may function differently based on social and cultural factors that cannot be disentangled from ethnicity and race. For example, preferred prayer type (e.g., meditative, intercessory and passive) may vary based on religious affiliation, which certainly differs by race. In a survey of religion in the USA, fewer white than black individuals endorsed being Protestant, while more white persons than black persons identified as Catholic [58]. While most of the literature has focused on racial and ethnic differences in catastrophizing and prayer, other differences in pain coping also exist. Among Hispanic–Americans, stoicism is a common coping strategy [59,60]. Remaining stoic in the face of pain may be used as an attempt to maintain physical functioning, which is a cultural value among Hispanic–Americans. Indeed, in Hispanic culture, shirking social duties is associated with perceptions of weakness [59,60]. Among Asian–Americans, the use of positive thinking and using exercise to cope with pain [61] are preferred coping strategies. These strategies are all active strategies which have been associated with less pain and improved function [62]. Given the cultural differences enmeshed within racial and ethnic differences, it is possible that different coping strategies are more effective at reducing pain and improving function for some racial and ethnic groups but not others. Understanding the differential function of coping between racial and ethnic groups may help to develop culturally sensitive, tailored pain treatments that aid in reducing disparities.
Perceived bias & discrimination
Perceived bias and discrimination may also be related to the experience of pain among racial and ethnic minorities. About half of Hispanic and 70% of black people in the USA report that they have experienced discrimination or have been treated unfairly because of their race or ethnicity [63]. Discrimination is associated with increased psychological distress, which may in turn be associated with the development of chronic pain [64]. However, studies directly linking perceived discrimination to pain have resulted in mixed findings for both experimental and clinical pain.
In experimental pain paradigms, perceived racial discrimination predicted lower heat pain tolerance among African–Americans with painful knee osteoarthritis and greater mechanical temporal summation of pain in African–Americans with sickle cell disease [65,66]. However, perceived discrimination was not associated with cold pain in a sample of African–American patients with knee osteoarthritis [67]. Due to the considerable inter- and intragroup differences in pain sensitivity as measured by quantitative sensory testing (which may be influenced by other patient-level factors) [68] as well as differences in methodology between studies (e.g., heat vs cold pain), variability in the relationships between perceived discrimination and pain sensitivity is to be expected.
While examinations of the relationship between discrimination and clinical pain have shown more consistent results, there is still some variability in findings. Perceived discrimination was found to be associated with more bodily pain among African– and Chinese–Americans [69,70]. Furthermore, perceived racial discrimination was associated with greater pain intensity among African–American women with osteoarthritis [71] and was shown to be the strongest predictor of reported back pain among African–Americans [72]. Greater discrimination among African–Americans with sickle cell disease was also associated with a stronger relationship between temporal summation of pain and clinical pain intensity, suggesting that perceived discrimination may be related to increased central sensitization among patients with sickle cell disease [66]. On the other hand, Carlisle and colleagues found that perceived discrimination was associated with chronic pain only for Hispanic–Americans but not African– Caribbean or Asian–Americans [73]. While the results from the Carlisle et al. study are incongruent with those of the other studies discussed here, this study examined perceived discrimination among an epidemiological sample of three racial and nine ethnic subgroups including both foreign- and native-born respondents. Thus, the relationships typically observed between perceived discrimination and pain may be differentially impacted by this wide range of ethnic identity [74], demonstrating how patient-level factors and societal factors interact to shape pain perception.
Taken together, these experimental and clinical findings suggest the need for a more nuanced understanding of the role of perceived bias and discrimination in the experience of pain. Racial and ethnic minorities consistently report greater perceived bias and discrimination which is associated with greater clinical pain among ethnic and racial minorities [75]. Moreover, the magnitude of the relationship between discrimination and pain seems to differ based on race and ethnicity. Indeed, Cuban Americans, for example, are often perceived as more closely resembling caucasians. Thus, they may be at lower risk for experiencing overt discrimination. African–Caribbeans, on the other hand, may be more akin in appearance to African–Americans, and thus face similar discrimination as African–Americans [73].
Bias & discrimination in pain treatment
In addition to high rates of perceived racial and ethnic discrimination across general contexts, many racial and ethnic minorities report bias and discrimination in relation to pain care, a provider-level influence. For example, African–Americans report discrimination related to their ability to receive quality pain care [76]. Indeed, higher rates of perceived discrimination among African–Americans are associated with greater hopelessness which is, in turn, associated with worse pain management outcomes [77]. Patients with sickle cell disease, a painful condition that occurs primarily in those of African descent, report even greater discrimination from healthcare providers than previously reported by African –Americans [77]. Further, race-based discrimination among this population is associated with difficulty persuading providers about pain. Thus, the perceived discrimination and bias among an already marginalized group is being compounded which may also impact the quality of pain care they are receiving. Hispanic–Americans, on the other hand, endorse the beliefs that health professionals do not understand their pain, do not believe them when they say they are in pain and do not care about their pain [59,76]. These beliefs may be influenced by the cultural value ‘personalismo’, a preference for developing personal, caring relationships with providers [59]. While the racial and ethnic disparities in pain treatment cannot be disputed, it remains unclear the extent to which various patient- (e.g., treatment preference), provider- (e.g., implicit bias) and system-level factors (e.g., access to care) drive these disparities. In an attempt to isolate the effects of race and ethnicity on pain judgements and treatment decisions, a number of researchers have used vignette-based studies with mixed results. For example, Druckman and colleagues found that medical staff rated black individuals as having a higher pain tolerance than white individuals [78]. However, another study found that healthcare professionals rated African–American virtual human patients as having greater pain and, as a result, were more likely to recommend opioid treatment [79]. These discrepancies in findings may be attributed to differences in methodology (e.g., traditional vignette vs virtual human patients), differences in provider type (division 1 sports medical staff vs nurses and physicians) as well as individual differences between types of vignette patients (student athlete with anterior cruciate ligament [ACL] injury vs patient with chronic low back pain). Because studies often examine racial disparities in pain assessment and care from a nomothetic perspective (i.e., group level differences), important individual level differences may be missed. By using both nomothetic and ideographic approaches, Hirsh and colleagues failed to find group based racial differences in the assessment and treatment of pain, but they did find that race influenced both the assessment of and treatment decisions for pain at the individual level. That is, when examined individually, race influenced single providers’ judgments and recommendations for pain [80,81]. Taking this a step further, Burgess et al. found that race differences in the likelihood to prescribe opioids varied as a factor of physician gender and cognitive load. Male physicians were less likely to prescribe opioids for black patients when under high cognitive load but more likely to prescribe opioids for black patients when under low cognitive load [82]. Thus, the variability among study results may be attributed to provider-level factors such as cognitive load and provider gender or type, or other clinically relevant patient-factors such as injury type.
Racial and ethnic disparities in pain care may also be driven by false beliefs about minority patients. In a primary care setting, black and Hispanic patients were seen as more likely to require scrutiny for potential drug abuse than white patients [83] suggesting that providers may believe these individuals are at greater risk for the misuse and abuse of opioid prescriptions. Hoffman and colleagues also found that nearly half of the medical students involved in their study endorsed false beliefs about biological differences between black and white individuals. For example, some of the medical students endorsed the belief that “black people’s skin is thicker than white people’s skin." Those who endorsed such beliefs then reported lower pain judgment ratings for black patients as compared with white patients [84]. Similar beliefs have been established among athletic trainers. Relative to injured white players, injured black National Football League players were deemed more likely to play in a subsequent game with the assumption that they felt less pain than white players [85]. Such false beliefs about minority patients may be driven by both provider- and system-level factors. For example, providers’ assessment and treatment decision may be a result of racial or ethnic bias. On the other hand, it may be a systematic problem resulting from educational deficiencies within medical schools and educational curricula for providers.
While healthcare providers may deny the influence of race on their pain-related judgements, race continues to influence these judgments [86]. This may be because these biases are occurring at the subconscious level rather than deliberately. Indeed, Mather and colleagues found that participants both perceived and responded to white pain more than black pain when shown faces below the conscious level of awareness but not when the faces were shown for a longer consciously discernable time [87]. A recent meta-analysis synthesized the findings of studies using the implicit association task to examine the effects of implicit racial bias on healthcare outcomes [88]. Researchers found that, of the 37 studies using the implicit association task to study health disparities, 31 studies found evidence for pro-white or antiminority bias across levels of training and disciplines. However, there were mixed results about the relationship between implicit bias and healthcare outcomes with six out of the 14 studies examining the association between implicit bias and healthcare outcomes finding that higher implicit bias was associated with disparities in treatment recommendations. These results lend support for the notion that at least some of the disparities in pain care are associated with provider-level bias.
It is clear that racial and ethnic bias and discrimination are influencing the racial and ethnic disparities in pain treatment; however, solutions to address racial and ethnic bias are needed to begin reducing disparities in pain care. A number of strategies have been suggested to reduce racial bias overall: using equal status contact including interacting with racial minorities in situations of shared power (e.g., engaging in recreational, occupational or classroom activities in which people of the racial/ethnic majority and minority share the same social status and are equals), providing exposure to counter-stereotypic minority exemplars (i.e., a member of a minority group that goes against a standardized stereotype of that minority), competing on teams with a diverse racial or ethnic makeup and practicing associating positive words with members of other racial or ethnic groups [89]. However, these strategies were developed outside of the clinical context primarily with undergraduate participants.
Patient preferences
Beyond provider factors, a variety of patient-related factors may be contributing to the racial and ethnic disparities in pain treatment. One such factor is patient preference. Not surprisingly, cultural differences result in differences in pain treatment preferences among people of diverse racial and ethnic backgrounds. In one study, Cherniack and colleagues found that compared with white and non-white Hispanic individuals, black individuals are the most likely to choose or desire prescription treatment for low back pain [90]. This preference seems to differ, however, based on the source of pain. For example, black individuals with cancer pain report that they prefer to not take pain medication, but instead feel that they ‘have to’ [91]. This preference stems from fears of losing control as well as concern of medication side effects and addiction [21,91]. White individuals, on the other hand, prioritize pain relief above the minimization of side effects [21].
Beyond preferences for medications, there are also differences in preference in the type of pain care sought and perceived efficacy of pain care modalities. Compared with white patients, black and Asian–Americans are less likely to prefer seeing a pain care specialist, instead choosing to see their primary care provider [92]. Among patients with painful lower extremity osteoarthritis, black patients perceive over-the-counter medications, physical therapy and complementary care (e.g., massage, prayer and herbal medicine) as more efficacious while perceiving joint replacement as less efficacious compared with non-Hispanic white patients [93]. Black Americans are also less willing to undergo total joint replacement than white individuals [94,95]; one contributor to the lower reported willingness of black Americans to undergo total knee arthroplasty (TKA) is the perceived helpfulness of prayer as a nonsurgical alternative for managing joint pain (Ang et al. 2002). Consistent with the sociological model, such differences in surgical preference may also be related to social and economic factors. For example, black patients are less likely than white patients to have had family or friends who had a joint replacement or to have a good understanding of joint replacement [95,96,97], perhaps due to previous economic disadvantages. That is, black patients have historically experienced higher rates of being under- or uninsured, thereby having less access to healthcare [98]. Furthermore, they frequently exhibit less health literacy [99]. Taken together, these patient- and system-level factors may continue to influence treatment preferences. Similarly to black Americans, Hispanic–Americans express hesitance to take pain medications [59,76,100,101,102,103]. Instead, they prefer pain self-management and other noninvasive medical treatments including creams and ointments, electric blankets, folk remedies (i.e., teas or natural healers), practicing yoga and visiting a chiropractor [104]. Further, they are more likely than white and black patients to choose over the counter medications for pain [90]. It is important to note, however, that while Hispanic patients prefer to avoid taking pain medications, they still express a desire to have it available to them should they need it [104].
There are numerous factors that impact treatment preferences among Hispanic individuals. Their hesitance to take pain medication may stem from a belief that pain should be overcome without medication unless absolutely necessary, such as when it interferes with ones’ ability to work or perform in social roles [101]. Furthermore, such reluctance may be a result of fear. Indeed, Hispanic–Americans report considerable fear about adverse treatment outcomes [76,102]. In fact, they report more fear of negative outcomes than non-Hispanic white individuals [100]. Specifically, they are concerned about addiction as well as medication side effects [101,104]. Cultural beliefs about the meaning of pain may also impact treatment preferences. Specifically, Hispanic–Americans report that both admitting to being in pain as well as using pain medications are perceived as weaknesses [101,104,105]. Thus, remaining stoic and choosing to refrain from medication use may serve to save face within their community. In addition to impacting treatment preferences, Hispanic culture may impact decisions about seeking pain care. Hispanic–Americans feel a strong sense of responsibility to provide for their family which may, in turn, prohibit them from seeking pain care altogether [104].
Collectively, this body of work highlights cultural forces that shape patient preference for pain treatment. While preferences vary by race and ethnicity, there are also intraracial and intra-ethnic differences in preference based, in part, on the type of painful condition to be treated.
Expectations
The construct of patient expectations is a growing and increasingly more relevant topic in healthcare as systems shift toward greater patient-centered care. Patients’ expectations have not only been recognized as an influential component for treatment satisfaction [106,107,108,109,110] but have also been associated with pain treatment outcomes [111,112,113,114,115]. Several patient-level mechanisms have been proposed to explain these associations, including their role as triggers for physiologic responses, as motivators throughout treatment courses, as effectors alongside anxiety to either reduce or magnify symptoms, as directors of attentional resources that allow patients to focus or ignore certain symptoms and as forces affecting the understanding of the course of a disease or its treatment [116]. Understanding racial/ethnic differences in pain expectations has significant clinical implications. Not only can expectations serve as predictive tools for treatment outcomes, but given their modifiability [117,118] can also be targets for interventions prior to treatment.
Racial and ethnic differences in pain expectations have been documented in several studies, largely indicating that poorer pain expectations are experienced by minority patients. For example, several studies have demonstrated that, compared with white patients, black or African–American patients have worse pain expectations from joint replacement surgery [118,119,120,121] and that these differences in expectations account for the race differences in willingness to undergo these surgical procedures [119,121]. Although most of the literature examining race differences in pain expectations has been conducted for joint arthroplasty, an additional study has examined race differences in pain management expectations in the emergency department. In this study, authors found that though Hispanic–Americans reported greater expectations for pain relief than non-Hispanic white individuals, the difference was not significant [122].
Collectively, this provides evidence that not only do racial and ethnic differences in pain expectations exist, but that these differences may be influencing disparities in treatments. There is also preliminary evidence that these expectations may be malleable. Weng and colleagues tested the efficacy of an educational intervention in reducing disparities in expectations between groups and found that pain expectations for African–Americans improved dramatically, while those of caucasians remained mostly unchanged [118]. Thus, expectations may be considered as a viable intervention target in the mission to reduce racial and ethnic disparities in pain care, and education-oriented interventions that have been shown to have substantial behavioral effects on outcomes such as surgery rates among African–American patients [123].
Patient–provider communication
Effective communication between patients and providers is central to pain management, as patients share their concerns and providers solicit information from their patients to guide treatment recommendations [124,125,126]. Research indicates that better patient–provider communication (e.g., effective questioning, expression of empathy and concern, and shared decision making [127,128,129]) is beneficial for patients’ adherence to treatment recommendations [130], engagement in self-management for chronic pain [124,126] and satisfaction with care [131,132]. A number of factors may impact patient–provider communication, especially among racial and ethnic minorities, including language barriers, cultural differences in communication styles and health literacy.
According to the 2010 census, 21% of US population speaks a language other than English with 9% reporting limited English proficiency [133]. The language barrier that results from the patient and provider speaking different languages can result in inadequate pain control [134]. Indeed, language has been documented as a barrier to pain care especially among Hispanic–Americans. Less than 20% of health professionals treating Hispanic pain patients report proficiency in Spanish [135]. Furthermore, Hispanic–Americans believe that because they do not speak English, it is difficult to receive treatment for pain [76]. This is compounded by the fact that Spanish-speaking Hispanic–Americans are less likely to seek pain treatment than English-speaking Hispanic–Americans [76]. Spanish-speaking Hispanic–Americans report difficulty describing their pain experience to their provider as well as understanding the clinical recommendations of non-Spanish-speaking providers [59,136]. In emergency departments, Spanish-speaking patients are less likely to understand their diagnosis and treatment plan, are less likely to be satisfied with their care and the patient–provider relationship and are less likely to return when necessary than their English-speaking counterparts [137,138].
One way to improve pain outcomes among Spanish-speaking Americans is by using medical interpreters. Indeed, Spanish-speaking Americans who always received interpreters were more likely to report higher pain control, timely pain management and greater perceived provider helpfulness to treat their pain [139]. Despite the benefits of using interpreters, a number of concerns remain. First, interpreters are not always available [140], which needs to be addressed at the system-level. Further, while interpreters may share speech communities (i.e., they speak the same languages as the patient and provider), their interpretation of pain may differ culturally and thus nuances may be lost [141]. Further, medical competence among interpreters is vital. Without competent interpretation, adequate assessment of pain and teaching of pain management skills are nearly impossible [142,143].
Some may also argue that because pain rating scales have been translated and validated across many languages, language should not pose a barrier in the assessment of pain. However, the translation of pain rating scales may still convey different meanings across cultures [134,143]. Because languages may be the same despite cultural differences (e.g., Mexicans and Spaniards both speak Spanish), these tools may inadequately characterize the experience of pain [144,145]. Furthermore, different words may have different meanings across cultures. For example, researchers found that patients may deny having pain after a painful procedure but later admit to have an ‘almost intolerable ache’ [145]. In this case, the patient and provider understood the word ‘pain’ quite differently.
In addition to language barriers, cultural differences in communication styles may also impact disparities in pain care. In a study of Hispanic older adults with painful osteoarthritis, researchers found that while most stayed on topic, only half spoke clearly and explicitly, half indicated the location of their pain, one-third reported pain intensity and one-quarter used medical terminology [147]. Similarly, only a few older black adults with painful osteoarthritis used medical terminology and used explicit descriptions of their pain that provided a clear mental image to the provider [148]. Among Alaskan Natives and American Indians, there is a tendency to understate the severity of pain symptoms and use vague descriptors such as ‘ache’ to express severe pain and disability [149]. Furthermore, when asked to rate their pain on a numeric rating scale, some Native American patients choose a favorite or sacred number instead of the number that indicates their level of pain [150].
In order to address the cultural barriers to effective communication between providers and pain patients, intervention can occur at the patient-, provider- or system-level. First, providers may consider using the Explanatory Model Interview for Pain Assessment which accounts for cultural norms [151] and has been shown to improve the assessment and treatment of pain [152]. Furthermore, because providers report inadequate cultural competence training despite their high caseload of racial and ethnically diverse patients with chronic pain [135], it is vital to enhance cultural competency among providers [153] perhaps by including additional cultural competency training within medical school curricula. Finally, as evidenced in a study by McDonald and colleagues [154], coaching patients may be useful in helping racially and ethnically diverse patients describe their pain more accurately which may, in turn, lead to improved pain treatment outcomes.
Many studies have also shown that low health literacy affects communication between physicians and patients as well as health outcomes [155,156,157,158]. In fact, patients with low health literacy are more likely to have difficulties accessing and understanding health-related information, are less likely to ask questions and feel less confident about communicating with their providers [159,160]. This pattern of communication could have negative consequences on the assessment of pain, as patients limit the information they share with their providers. Briggs and colleagues found that patients with pain commonly have difficulty understanding medical instructions from their physicians and report lack of knowledge about treatment options for chronic pain management [161]. There is also some association between low health literacy and higher pain intensity [162].
Although low health literacy affects many patients, it disproportionately impacts racial and ethnic minorities [99]. Thus, low health literacy may be a contributing factor in the racial and ethnic differences in the treatment of pain. Fortunately, health literacy adapted pain education has shown encouraging results for African–American patients with low literacy [163,164]. In a recent study, patients with significant socioeconomic risk factors (e.g., minority status, low-socioeconomic status) received information about the biopsychosocial model of pain and reported improvements in pain intensity and interference following the 10-week group treatment [164].
Racial & ethnic differences in pain treatment outcomes
Despite considerable evidence on racial and ethnic differences in the prevalence, experience and management of pain, few studies have addressed racial and ethnic differences in pain treatment outcomes. Understanding the potential impact of race and ethnicity on pain outcomes is critically important when developing effective targeted interventions.
Racial & ethnic differences in surgical treatment outcomes
In addition to the racial and ethnic disparities in the rates of orthopedic surgeries [165,166,167,168,169,170,171], there are also racial and ethnic differences in the outcomes of these procedures. In a retrospective review of data collected for the Spine Patient Outcomes Research Trial, Schoenfeld and colleagues discovered that white patients had significantly better pain outcomes than black patients and other non-white patients (i.e., Hispanic, Native American, Asian) [172]. Following hip and knee arthroplasty, black patients report greater pain intensity, pain-related disability, pain frequency and higher rates of postoperative pain compared with white patients [173,174,175,176]. Similarly, Barrack and colleagues found that black and Hispanic minority patients were more likely to report pain in the 30 days following total knee arthroplasty compared with white patients [177] while Khor et al. demonstrated that non-white patients undergoing lumbar fusion were less likely to have improved disability, back and leg pain [178]. However, while Goodman and colleagues found that worse pain outcomes were associated both with race and socioeconomic factors, the effect was more pronounced in black patients exemplifying a double disparity. Barrack and colleagues, on the other hand, found that their results were no longer significant when controlling for socioeconomic factors [177]. Collectively, these studies suggest that there are racial and ethnic differences in pain outcomes for orthopedic surgeries. However, it remains unclear how socioeconomic factors impact these results.
Racial & ethnic differences in nonsurgical outcomes for pain
Although there are consistent racial and ethnic differences in pain outcomes following orthopedic surgeries, the results are mixed for racial and ethnic differences in nonsurgical pain outcomes. In studies examining the efficacy of non-steroidal anti-inflammatory drugs for patients with painful osteoarthritis, researchers found no differences between white and non-white patients or between Asian and non-Asian populations [179,180]. That is, changes in pain scores were consistent across racial and ethnic groups. On the other hand, Dominick and colleagues found that among veterans with osteoarthritis, African–Americans rated medications as more helpful than white patients [181]. Beyond pharmaceutical treatments, several studies have examined racial and ethnic differences in multidisciplinary pain treatment program outcomes. In a large retrospective study performed at the Department of Veterans Affairs, Burgess and colleagues found no significant association between race and pain outcomes for a multidisciplinary pain treatment program [182]. However, in Merry and colleagues study examining race differences in pain-related treatment outcomes, both black and white participants experienced a reduction in pain-related interference, but only white participants reported reduced pain severity [183]. On the other hand, results of a study comparing outcomes of a multidisciplinary pain rehabilitation program between African–American and caucasian patients indicated that African–Americans experience less improvement in pain interference, affective distress, role physical function, social function and depression following treatment [184].
The conflicting results present across studies could be explained by several factors. There exists significant confusion and lack of a standard when operationalizing patients into racial and ethnic subgroups. This is not only problematic for conclusions drawn within studies but is also a significant limitation for aggregating results across studies. Studies are also inconsistent in both the reporting and the adjustment for potential confounders (i.e., education and income). This presents a challenge in the ability to discern the true cause for differing outcomes. Furthermore, the variety of pain outcome measures makes cross-study comparisons challenging. Finally, patient-level psychosocial variables, such as expectations and others mentioned above, may be driving these differences.
Healthcare access
At the system level, differential access to healthcare can account for some of the disparities in the racial and ethnic disparities in pain. It is important to note that while we will briefly touch upon the influence of healthcare access disparities in a broad sense, our focus will primarily be on the research that ties these factors directly to racial and ethnic disparities in pain. Among these factors, we include differential rates of health insurance coverage and quality, access to high quality pain treatment and access to appropriate treatment options.
There are 28.5 million uninsured people in the USA. Unfortunately, this burden is heavier among racial and ethnic minorities who are at greater risk of being uninsured or underinsured [185]. Indeed, in 2017, 94% of non-Hispanic white persons had health insurance coverage while only 90 and 84% of black and Hispanic–Americans, respectively, had coverage [185]. Furthermore, rates of government-issued health insurance (i.e., medicare and medicaid) is highest among black and Hispanic persons, compared with non-Hispanic white persons [185]. This inadequate access to health insurance may contribute to minorities’ difficulties receiving evaluation and treatment of painful conditions.
Even among insured individuals, there remain broad disparities in the pain treatments available to racial and ethnic minority patients. For example, racial and ethnic minorities are less likely to receive higher quality care. Quality of healthcare and resulting healthcare outcomes have been shown to be better within private, teaching and high-volume healthcare settings [186]. For total joint replacement surgeries, a common treatment for painful osteoarthritis, hospitals and surgeons with higher volumes of patients show better outcomes [187]. Unfortunately, racial and ethnic minority patients are disproportionately underserved by these high-volume, private and teaching hospitals. Instead, they are more likely to be treated at a safety net urban hospital, a hospital with higher medicaid utilization rates than other hospitals in the area. In fact, while urban hospitals have a minority patient population of approximately 19%, safety net urban hospitals have minority populations of over 40%. Due to racial and ethnic residential segregation [188] along with the geographic distribution of private, high-volume and teaching hospitals, minority patients are at greater risk for having poorer quality care.
Along with poorer quality of care, racial and ethnic minority patients are also less likely to receive appropriate and specialized pain treatment. Indeed, only 8% of non-Hispanic white patients report difficulty accessing specialist care, whereas 16% of black and 22% of Hispanic–American patients report the same difficulty [189]. Similarly, compared with black patients, white patients are more likely to endorse the belief that healthcare professionals who treat pain are easily accessible [76]. Perhaps, this is why African–Americans with hip and knee pain are more likely to obtain care in the emergency department than in a clinic [190]. Access to appropriate healthcare also stretches from the clinic to the pharmacy where access to opioid medications is limited in minority communities [191]. In fact, in one study only 25% of pharmacies in predominantly non-white neighborhoods had sufficient opioid supplies compared with 72% in predominantly white neighborhoods [192]. Together, these studies provide evidence for the influence of access to care on disparities in the experience and treatment of pain.
Conclusion
In this review, we used a modified version of the socioecological model (see Figure 1) framework to highlight patient-, provider- and system-level psychosocial mechanisms that may be underlying the racial and ethnic disparities present in the experience and treatment of pain. At the patient level, we see that coping, perceived bias and discrimination, patient preference, expectations, and differential outcomes all influence racial and ethnic disparities. Actual bias and discrimination in relation to the treatment of pain and differential access to pain care are provider- and system-level factors, respectively. As the socioecological model suggest [27], factors within the model are nested within one another demonstrating the complex nature of the relationship between variables at each level. Indeed, factors within each domain may interact (e.g., expectations about treatment outcomes may be related to having a lower health literacy, which may in turn be related to systemic factors such as socioeconomic status and historically poor access to care). Furthermore, some factors such as patient–provider communication, fit within each of the domains: patient, provider and system. Indeed, while language barriers may come at the intersection of the patient and provider, they present a system-level need for bilingual providers or interpreter services.
While there are well-documented racial and ethnic differences in the experience and treatment of pain, much of this literature has examined black versus white difference. Although the body of research conducted about pain among Hispanic–Americans has grown, this group represents 16% of the US population and is one of the fastest growing demographic groups in the USA [193]. Thus, more research is needed on this group to adequately represent and understand their pain experience. Future studies should specifically address the influence of acculturation on patients’ subscription to mainstream pain management options as opposed to perpetuating their country of origin’s practices. Furthermore, there is limited research focused on the pain experience among Asian–Americans, Native Americans, Alaskan Natives, Hawaiian and Pacific Islanders, and Middle-Eastern Americans. For example, while there are a small number of studies focused on the experience of pain in patients of Middle Eastern descent living abroad [194], we were unable to find a single US-based study. Moreover, there are little to no comparisons of pain among these understudied ethnic and cultural groups. Future research should aim to characterize pain among these racial and ethnic minorities. Such research could result in reduced racial and ethnic disparities in pain treatment and lead to more culturally competent care.
This manuscript is not without limitation. Much of the research we reviewed is cross-sectional, and thus causal pathways cannot be determined. Furthermore, we did not systematically search the literature, nor did we have stringent inclusion/exclusion criteria. Thus, we may have missed articles that help explain racial and ethnic differences in pain. That said, we have highlighted a number of possible mechanisms that have received attention in the disparities literature. Future research may seek to examine other possible mechanism of racial and ethnic differences in pain such as the influence of familial models of pain, perceived injustice, accessibility and proper training. Additional, use of experimental or longitudinal methodology will allow for the determination of causal pathways. Such research can aid in the development of culturally sensitive interventions for pain as well as enhancing medical training focused on culturally competent pain treatment.
Future perspective
Over the last decade, there has been a shift in attention toward addressing racial and ethnic pain disparities. While we certainly have a better understanding of the psychosocial mechanisms that may be driving racial and ethnic pain disparities, especially for black Americans, we are only beginning to see this knowledge translated into strategies for reducing the disparities. For example, Adam Hirsh is attempting to address disparities at the provider level by examining the effectiveness of a perspective-taking intervention aimed at reducing racial bias [80,195]. In the coming decade, we can expect to see additional efforts aimed at addressing provider- and system-level factors (e.g., addressing expectations by facilitating patient–provider communication or organizing community based support groups). Furthermore, we expect to see a continued shift toward personalized medicine, which will address patient-level factors. Indeed, Kelli Allen is examining the efficacy of culturally sensitive coping skills training for African–Americans with painful osteoarthritis [196,197]. We expect the development of other culturally tailored psychosocial treatments for pain aimed at improving pain outcomes among minority patients and thus reducing pain disparities.
Footnotes
Author contributions
SM Meints, A Cortes and CA Morais: conception and design of work, drafting manuscript, final approval and accountability for integrity of work. RR Edwards: conception and design of work, revision of manuscript for intellectual content, final approval, accountability for integrity of work.
Financial & competing interests disclosure
This work is supported by the NIH under award (grant number: T32 AR055885). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Institute of Medicine. Relieving pain in America. The National Academies Press; Washington, DC, USA: (2011). [Google Scholar]
- 2.Green C, Baker T, Sato Y, Washington T. Race and chronic pain: a comparative study of young black and white Americans presenting for management. J. Pain 4(4), 176–183 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Green CR, Anderson KO, Baker TA. et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 4, 277–294 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Kim HJ, Yang GS, Greenspan JD. et al. Racial and ethnic differences in experimental pain sensitivity: systematic review and meta-analysis. Pain 158(2), 194–211 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Hollingshead NA, Meints SM, Miller MM, Robinson ME, Hirsh AT. A comparison of race-related pain stereotypes held by white and black individuals. J. Appl. Soc. Psychol. 46(12), 718–723 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn H, Weaver M, Lyon DE. et al. Differences in clinical pain and experimental pain sensitivity between Asian Americans and whites with knee osteoarthritis. Clin. J. Pain 33(2), 174–180 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrom C, Bair E, Maixner W. et al. Demographic predictors of pain sensitivity: results from the OPPERA study. J. Pain 18(3), 295–307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowell LN, Mechlinl B, Jil E, Addamol M, Girdlerl SS. Asians differ from non-Hispanic whites in experimental pain sensitivity. Eur. J. Pain 15(7), 764–771 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palit S, Kerr KL, Kuhn BL. et al. Exploring pain processing differences in Native Americans. Health Psychol. 32(11), 1127 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J. Pain 10(12), 1187–1204 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Carey TS, Mills Garrett J. The relation of race to outcomes and the use of health care services for acute low back pain. Spine 28, 390–394 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Taylor BA, Casas-Ganem J, Vaccaro AR, Hilibrand AS, Hanscom BS, Albert TJ. Differences in the work-up and treatment of conditions associated with low back pain by patient gender and ethnic background. Spine 30(3), 359–364 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Todd KH, Deaton C, D'Adamo AP, Goe L. Ethnicity and analgesic practice. Ann. Emerg. Med. 35(1), 11–16 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA 269(12), 1537–1539 (1993). [PubMed] [Google Scholar]
- 15.Heins JK, Heins A, Grammas M, Costello M, Huang K, Mishra S. Disparities in analgesia and opioid prescribing practices for patients with musculoskeletal pain in the emergency department. J. Emerg. Nurs. 32(3), 219–224 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Hostetler MA, Auinger P, Szilagyi PG. Parenteral analgesic and sedative use among ED patients in the United States: combined results from the National Hospital Ambulatory Medical Care Survey (NHAMCS) 1992–1997. Am. J. Emerg. Med. 20(3), 139–143 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Tamayo-Sarver JH, Hinze SW, Cydulka RK, Baker DW. Racial and ethnic disparities in emergency department analgesic prescription. Am. J. Public Health 93(12), 2067–2073 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald DD. Gender and ethnic stereotyping and narcotic analgesic administration. Res. Nurs. Health 17(1), 45–49 (1994). [DOI] [PubMed] [Google Scholar]
- 19.Ng B, Dimsdale JE, Rollnik JD, Shapiro H. The effect of ethnicity on prescriptions for patient-controlled analgesia for post-operative pain. Pain 66(1), 9–12 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Ng B, Dimsdale JE, Shragg GP, Deutsch R. Ethnic differences in analgesic consumption for postoperative pain. Psychosom. Med. 58(2), 125–129 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Meghani SH, Byun E, Gallagher RM. Time to take stock: a meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med. 13(2), 150–174 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Singhal A, Tien Y-Y, Hsia RY. Racial-ethnic disparities in opioid prescriptions at emergency department visits for conditions commonly associated with prescription drug abuse. PLoS ONE 11(8), e0159224 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drwecki BB, Moore CF, Ward SE, Prkachin KM. Reducing racial disparities in pain treatment: the role of empathy and perspective-taking. Pain 152(5), 1001–1006 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Kaseweter KA, Drwecki BB, Prkachin KM. Racial differences in pain treatment and empathy in a Canadian sample. Pain Res. Manag. 17(6), 381–384 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staton LJ, Panda M, Chen I. et al. When race matters: disagreement in pain perception between patients and their physicians in primary care. J. Natl. Med. Assoc. 99(5), 532–538 (2007). [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AA, Zogg CK, Zafar SN. et al. Analgesic access for acute abdominal pain in the emergency department among racial/ethnic minority patients: a nationwide examination. Med. Care 53(12), 1000–1009 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Bronfenbrenner U. Ecological models of human development. International Encyclopedia of Education 3(2), 37–43 (1994). [Google Scholar]
- 28.Campbell C, Edwards R. Ethnic differences in pain and pain management. Pain Management. 2(3), 219–230 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards R, Cahalan C, Mensing G, Smith M. Pain, catastrophizing, and depression in the rheumatic diseases. Nat. Rev. Rheumatol. 7(4), 216–225 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. J. Pain 17(9 Suppl.), T70–T92 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quartana P, Campbell C. Pain catastrophizing: a critical review. Expert Rev. Neurother. 9(5), 745–758 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wertli MM, Burgstaller JM, Weiser S, Steurer J, Kofmehl R, Held U. Influence of catastrophizing on treatment outcome in patients with nonspecific low back pain: a systematic review. Spine 39(3), 263–273 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Meints SM, Miller MM, Hirsh AT. Differences in pain coping between black and white Americans: a meta-analysis. J. Pain 17(6), 642–653 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards RR, Moric M, Husfeldt B, Buvanendran A, Ivankovich O. Ethnic similarities and differences in the chronic pain experience: a comparison of African American, Hispanic, and white patients. Pain Med. 6, 88–98 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Gagnon CM, Matsuura JT, Smith CC, Stanos SP. Ethnicity and interdisciplinary pain treatment. Pain Pract. 14(6), 532–540 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Meints SM, Hirsh AT. In vivo praying and catastrophizing mediate the race differences in experimental pain sensitivity. J. Pain 16(5), 491–497 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Meints SM, Stout M, Abplanalp S, Hirsh AT. Pain-related rumination, but not magnification or helplessness, mediates race and sex differences in experimental pain. J. Pain 18(3), 332–339 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Sullivan MJ, Thorn B, Haythornthwaite JA. et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin. J. Pain 17, 52–64 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Geisser M, Robinson ME, Riley JL. Pain beliefs, coping, and adjustment to chronic pain. Let’s focus more on the negative. Pain Forum 8, 161–168 (1999). [Google Scholar]
- 40.Lazarus RS, Folkman S. Stress, Appraisal and Copin. Springer Publishing Company, NY, USA: (1984). [Google Scholar]
- 41.Severeijns R, Van Den Hout MA, Vlaeyen JW, Picavet HS. Pain catastrophizing and general health status in a large Dutch community sample. Pain 99(1–2), 367–376 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Severeijns R, Vlaeyen JW, Van Den Hout MA, Weber WE. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin. J. Pain 17(2), 165–172 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Coyne JC, Racioppo MW. Never the Twain shall meet? Closing the gap between coping research and clinical intervention research. Am. Psychol. 55(6), 655 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Lackner JM, Gurtman MB. Pain catastrophizing and interpersonal problems: a circumplex analysis of the communal coping model. Pain 110(3), 597–604 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Gauthier LR, Rodin G, Zimmermann C. et al. The communal coping model and cancer pain: the roles of catastrophizing and attachment style. J. Pain 13(12), 1258–1268 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Thorn BE, Ward LC, Sullivan MJ, Boothby JL. Communal coping model of catastrophizing: conceptual model building. Pain 106, 1–2 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Cohen LA, Bonito AJ, Akin DR. et al. Toothache pain: behavioral impact and self-care strategies. Spec. Care Dentist 29(2), 85–95 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Hastie BA, Riley JL, Fillingim RB. Ethnic differences and responses to pain in healthy young adults. Pain Med. 6(1), 61–71 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Townley S, Papaleontiou M, Amanfo L. et al. Preparing to implement a self-management program for back pain in New York City senior centers: what do prospective consumers think? Pain Med. 11(3), 405–415 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Culver JL, Arena PL, Wimberly SR, Antoni MH, Carver CS. Coping among African–American, Hispanic, and non-Hispanic white women recently treated for early stage breast cancer. Psychol. Health 19(2), 157–166 (2004). [Google Scholar]
- 51.Chatters L, Taylor R, Jackson J. Religious coping among African Americans, Caribbean Blacks and Non-Hispanic whites. J. Community Psychol. 36(3), 371–386 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chatters LM, Taylor RJ, Bullard KM, Jackson JS. Race and ethnic differences in religious involvement: African Americans, Caribbean blacks and non-Hispanic whites. Ethn. Racial Stud. 32(7), 1143–1163 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geisser ME, Robinson ME, Henson CD. The coping strategies questionnaire and chronic pain adjustment: a conceptual and empirical reanalysis. Clin. J. Pain 10, 98–106 (1994). [DOI] [PubMed] [Google Scholar]
- 54.Robinson ME, Riley JL, Myers CD. et al. The Coping Strategies Questionnaire: a large sample, item level factor analysis. Clin. J. Pain 13, 43–49 (1997). [DOI] [PubMed] [Google Scholar]
- 55.Novy DM, Nelson DV, Hetzel RD, Squitieri P, Kennington M. Coping with chronic pain: sources of intrinsic and contextual variability. J. Behav. Med. 21, 19–34 (1998). [DOI] [PubMed] [Google Scholar]
- 56.Abraído-Lanza AF, Vásquez E, Echeverría SE. En las manos de Dios [in God's hands]: religious and other forms of coping among Latinos with arthritis. J. Consult. Clin. Psychol. 72(1), 91 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meints SM, Mosher C, Rand KL, Ashburn-Nardo L, Hirsh AT. An experimental investigation of the relationships among race, prayer, and pain. Scand. J. Pain. 18(3), 545–553 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pew Forum U.S. religious landscape survey: religious affiliation. www.pewtrusts.org/en/research-and-analysis/reports/2008/02/25/us-religious-landscape-survey-religious-affiliation (2008).
- 59.Sherwood GD, Mcneill JA, Palos G, Starck P. Perspectives on pain: a qualitative analysis of the Hispanic pain experience. NT Res. 8(5), 364–377 (2003). [Google Scholar]
- 60.Villarruel AM. Mexican–American cultural meanings, expressions, self-care and dependent-care actions associated with experiences of pain. Res. Nurs. Health 18(5), 427–436 (1995). [DOI] [PubMed] [Google Scholar]
- 61.Liao KY-H, Henceroth M, Lu Q, Leroy A. Cultural differences in pain experience among four ethnic groups: a qualitative pilot study. J. Behav. Health 5, 75–81 (2016). [Google Scholar]
- 62.Brown GK, Nicassio PM. Development of a questionnaire for the assessment of active and passive coping strategies in chronic pain patients. Pain 31, 53–64 (1987). [DOI] [PubMed] [Google Scholar]
- 63.Pew Research Center. On views of race and inequality, blacks and whites are worlds apart. (2016). www.pewsocialtrends.org/2016/06/27/on-views-of-race-and-inequality-blacks-and-whites-are-worlds-apart/
- 64.Brown TT, Partanen J, Chuong L, Villaverde V, Griffin AC, Mendelson A. Discrimination hurts: the effect of discrimination on the development of chronic pain. Soc. Sci. Med. 204, 1–8 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Goodin BR, Pham QT, Glover TL. et al. Perceived racial discrimination, but not mistrust of medical researchers, predicts the heat pain tolerance of African Americans with symptomatic knee osteoarthritis. Health Psychol. 32(11), 1117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mathur VA, Kiley KB, Haywood C Jr. et al. Multiple levels of suffering: discrimination in health-care settings is associated with enhanced laboratory pain sensitivity in sickle cell disease. Clin. J. Pain 32(12), 1076–1085 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herbert MS, Goodin BR, Bulls HW. et al. Ethnicity, cortisol, and experimental pain responses among persons with symptomatic knee osteoarthritis. Clin. J. Pain 33(9), 820–826 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maier C, Baron R, Tölle T. et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 150(3), 439–450 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Dugan SA, Lewis TT, Everson-Rose SA, Jacobs EA, Harlow SD, Janssen I. Chronic discrimination and bodily pain in a multiethnic cohort of midlife women in the study of women's health across the nation. Pain 158(9), 1656–1665 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burgess DJ, Grill J, Noorbaloochi S. et al. The effect of perceived racial discrimination on bodily pain among older African American men. Pain Med. 10(8), 1341–1352 (2009). [DOI] [PubMed] [Google Scholar]
- 71.Taylor JLW, Campbell CM, Thorpe RJ, Whitfield KE, Nkimbeng M, Szanton SL. Pain, racial discrimination, and depressive symptoms among African American women. Pain Manag. Nurs. 19(1), 79–87 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edwards R. The association of perceived discrimination with low back pain. J. Behav. Med. 31(5), 379–389 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Carlisle SK. Perceived discrimination and chronic health in adults from nine ethnic subgroups in the USA. Ethn. Health 20(3), 309–326 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yip T, Gee GC, Takeuchi DT. Racial discrimination and psychological distress: the impact of ethnic identity and age among immigrant and United States-born Asian adults. Dev. Psychol. 44(3), 787 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pascoe EA, Smart Richman L. Perceived discrimination and health: a meta-analytic review. Psychol. Bull. 135(4), 531 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen M, Ugarte C, Fuller I, Haas G, Portenoy RK. Access to care for chronic pain: racial and ethnic differences. J. Pain 6(5), 301–314 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Ezenwa M, Fleming M. Racial disparities in pain management in primary care. J. Pain 13(4), S20 (2012). [PMC free article] [PubMed] [Google Scholar]
- 78.Druckman JN, Trawalter S, Montes I, Fredendall A, Kanter N, Rubenstein AP. Racial bias in sport medical staff's perceptions of others' pain. J. Soc. Psychol. 158(6), 721–729 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Wandner LD, Heft MW, Lok BC. et al. The impact of patients' gender, race, and age on health care professionals' pain management decisions: an online survey using virtual human technology. Int. J. Nurs. Stud. 51(5), 726–733 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirsh AT, Hollingshead NA, Ashburn-Nardo L, Kroenke K. The interaction of patient race, provider bias, and clinical ambiguity on pain management decisions. J. Pain 16(6), 558–568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hirsh A, George S, Robinson M. Pain assessment and treatment disparities: a virtual human technology investigation. Pain 143, 106–113 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burgess DJ, Phelan S, Workman M. et al. The effect of cognitive load and patient race on physicians' decisions to prescribe opioids for chronic low back pain: a randomized trial: table 1. Pain Med. 15, 965–974 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Becker WC, Starrels JL, Heo M, Li X, Weiner MG, Turner BJ. Racial differences in primary care opioid risk reduction strategies. Ann. Fam. Med. 9(3), 219–225 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc. Natl. Acad. Sci. USA 113(16), 4296–4301 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trawalter S, Hoffman KM, Waytz A. Racial bias in perceptions of others' pain. PLoS ONE 7(11), e48546 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hirsh A, Jensen M, Robinson M. Evaluation of nurses' self-insight into their pain assessment and treatment decisions. J. Pain 11(5), 454–461 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mathur VA, Richeson JA, Paice JA, Muzyka M, Chiao JY. Racial bias in pain perception and response: experimental examination of automatic and deliberate processes. J. Pain 15(5), 476–484 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maina IW, Belton TD, Ginzberg S, Singh A, Johnson TJ. A decade of studying implicit racial/ethnic bias in healthcare providers using the implicit association test. Soc. Sci. Med. 199, 219–222 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Drwecki BB. Education to identify and combat racial bias in pain treatment. AMA J. Ethics 17(3), 221 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Cherniack EP, Ceron-Fuentes J, Florez H, Sandals L, Rodriguez O, Palacios JC. Influence of race and ethnicity on alternative medicine as a self-treatment preference for common medical conditions in a population of multi-ethnic urban elderly. Complement. Ther. Clin. Pract. 14(2), 116–123 (2008). [DOI] [PubMed] [Google Scholar]
- 91.Meghani SH, Keane A. Preference for analgesic treatment for cancer pain among African Americans. J. Pain Symptom Manage. 34(2), 136–147 (2007). [DOI] [PubMed] [Google Scholar]
- 92.Wong MD, Asch SM, Andersen RM, Hays RD, Shapiro MF. Racial and ethnic differences in patients' preferences for initial care by specialists. Am. J. Med. 116(9), 613–620 (2004). [DOI] [PubMed] [Google Scholar]
- 93.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Variation in perceptions of treatment and self-care practices in elderly with osteoarthritis: a comparison between African American and white patients. Arthritis Care Res. (Hoboken) 45(4), 340–345 (2001). [DOI] [PubMed] [Google Scholar]
- 94.Allen KD, Golightly YM, Callahan LF. et al. Race and sex differences in willingness to undergo total joint replacement: the Johnston County Osteoarthritis Project. Arthritis Care Res. (Hoboken) 66(8), 1193–1202 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suarez-Almazor ME, Souchek J, Kelly PA. et al. Ethnic variation in knee replacement: patient preferences or uninformed disparity? Arch. Intern. Med. 165(10), 1117–1124 (2005). [DOI] [PubMed] [Google Scholar]
- 96.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Understanding ethnic differences in the utilization of joint replacement for osteoarthritis: the role of patient-level factors. Med. Care 40(Suppl. 1), I44–I51 (2002). [DOI] [PubMed] [Google Scholar]
- 97.Hausmann LRM, Mor M, Hanusa BH. et al. The effect of patient race on total joint replacement recommendations and utilization in the orthopedic setting. J. Gen. Intern. Med. 25(9), 982–988 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wehby GL, Lyu W. The impact of the ACA Medicaid expansions on health insurance coverage through 2015 and coverage disparities by age, race/ethnicity, and gender. Health Serv. Res. 53(2), 1248–1271 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kutner M, Greenburg E, Jin Y, Paulsen C. The health literacy of America's adults: results from the 2003 National Assessment of Adult Literacy. NCES 2006–483. National Center for Education Statistics (2006). https://nces.ed.gov/pubs2006/2006483.pdf [Google Scholar]
- 100.Katz JN, Lyons N, Wolff LS. et al. Medical decision-making among Hispanics and non-Hispanic whites with chronic back and knee pain: a qualitative study. BMC Musculoskelet. Disord. 12(1), 78 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Monsivais DB, Engebretson JC. “I'm just not that sick” pain medication and identity in Mexican American women with chronic pain. J. Holist. Nurs. 30(3), 188–194 (2012). [DOI] [PubMed] [Google Scholar]
- 102.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. J. Pain 5(6), 317–328 (2004). [DOI] [PubMed] [Google Scholar]
- 103.Rutledge DN, Cantero PJ, Ruiz JE. Chronic pain management strategies used by low-income overweight Latinos. Chronic Illn. 9(2), 133–144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Torres CA, Thorn BE, Kapoor S, Demonte C. An examination of cultural values and pain management in foreign-born Spanish-speaking Hispanics seeking care at a federally qualified health center. Pain Med. 18(11), 2058–2069 (2017). [DOI] [PubMed] [Google Scholar]
- 105.Carrion IV, Cagle JG, Van Dussen DJ, Culler KL, Hong S. Knowledge about hospice care and beliefs about pain management: exploring differences between Hispanics and non-Hispanics. Am. J. Hosp. Palliat. Care. 32(6), 647–653 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Bell RA, Kravitz RL, Thom D, Krupat E, Azari R. Unmet expectations for care and the patient–physician relationship. J. Gen. Intern. Med. 17(11), 817–824 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin. Orthop. Relat. Res. 468(1), 57–63 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eisler T, Svensson O, Tengstrom A, Elmstedt E. Patient expectation and satisfaction in revision total hip arthroplasty. J. Arthroplasty 17(4), 457–462 (2002). [DOI] [PubMed] [Google Scholar]
- 109.Noble PC, Conditt MA, Cook KF, Mathis KB. The John Insall Award: patient expectations affect satisfaction with total knee arthroplasty. Clin. Orthop. Relat. Res. 452, 35–43 (2006). [DOI] [PubMed] [Google Scholar]
- 110.Scott CE, Howie CR, MacDonald D, Biant LC. Predicting dissatisfaction following total knee replacement: a prospective study of 1217 patients. J. Bone Joint Surg. Br. 92(9), 1253–1258 (2010). [DOI] [PubMed] [Google Scholar]
- 111.Iles RA, Davidson M, Taylor NF. Psychosocial predictors of failure to return to work in non-chronic non-specific low back pain: a systematic review. Occup. Environ. Med. 65(8), 507–517 (2008). [DOI] [PubMed] [Google Scholar]
- 112.Mahomed NN, Liang MH, Cook EF. et al. The importance of patient expectations in predicting functional outcomes after total joint arthroplasty. J. Rheumatol. 29(6), 1273–1279 (2002). [PubMed] [Google Scholar]
- 113.Myers SS, Phillips RS, Davis RB. et al. Patient expectations as predictors of outcome in patients with acute low back pain. J. Gen. Intern. Med. 23(2), 148–153 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O'Malley KJ, Roddey TS, Gartsman GM, Cook KF. Outcome expectancies, functional outcomes, and expectancy fulfillment for patients with shoulder problems. Med. Care 42(2), 139–146 (2004). [DOI] [PubMed] [Google Scholar]
- 115.Gandhi R, Davey J, Mahomed N. Patient expectations predict greater pain relief with joint arthroplasty. J. Arthroplasty 24(5), 716–721 (2009). [DOI] [PubMed] [Google Scholar]
- 116.Flood AB, Lorence DP, Ding J, McPherson K, Black NA. The role of expectations in patients' reports of post-operative outcomes and improvement following therapy. Med. Care 31(11), 1043–1056 (1993). [DOI] [PubMed] [Google Scholar]
- 117.Mancuso CA, Graziano S, Briskie LM. et al. Randomized trials to modify patients' preoperative expectations of hip and knee arthroplasties. Clin. Orthop. Relat. Res. 466(2), 424–431 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weng HH, Kaplan RM, Boscardin WJ. et al. Development of a decision aid to address racial disparities in utilization of knee replacement surgery. Arthritis Rheum. 57(4), 568–575 (2007). [DOI] [PubMed] [Google Scholar]
- 119.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Differences in expectations of outcome mediate African American/white patient differences in “willingness” to consider joint replacement. Arthritis Rheum. 46(9), 2429–2435 (2002). [DOI] [PubMed] [Google Scholar]
- 120.Allen K, Oddone E, Coffman C, Keefe F. Racial differences in osteoarthritis pain and function: potential explanatory factors. Osteoarthritis Cartilage 18(2), 160–167 (2010). [DOI] [PubMed] [Google Scholar]
- 121.Riddle DL, Golladay GJ, Hayes A, Ghomrawi HM. Poor expectations of knee replacement benefit are associated with modifiable psychological factors and influence the decision to have surgery: a cross-sectional and longitudinal study of a community-based sample. Knee 24(2), 354–361 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee WW, Burelbach AE, Fosnocht D. Hispanic and non-Hispanic white patient pain management expectations. Am. J. Emerg. Med. 19(7), 549–550 (2001). [DOI] [PubMed] [Google Scholar]
- 123.Ibrahim SA, Blum M, Lee G-C. et al. Effect of a decision aid on access to total knee replacement for black patients with osteoarthritis of the knee: a randomized clinical trial. JAMA Surg. 152(1), e164225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dorflinger L, Kerns RD, Auerbach SM. Providers' roles in enhancing patients' adherence to pain self management. Transl. Behav. Med. 3(1), 39–46 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Farin E, Gramm L, Schmidt E. The patient–physician relationship in patients with chronic low back pain as a predictor of outcomes after rehabilitation. J. Behav. Med. 36(3), 246–258 (2013). [DOI] [PubMed] [Google Scholar]
- 126.Henry SG, Matthias MS. Patient–clinician communication about pain: a conceptual model and narrative review. Pain Med. 19(11), 2154–2165 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bensing JM, Dronkers J. Instrumental and affective aspects of physician behavior. Med. Care 30(4), 283–298 (1992). [DOI] [PubMed] [Google Scholar]
- 128.Roter DL, Frankel RM, Hall JA, Sluyter D. The expression of emotion through nonverbal behavior in medical visits. Mechanisms and outcomes. J. Gen. Intern. Med. 21(Suppl. 1), S28–S34 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roter DL, Stewart M, Putnam SM, Lipkin M Jr, Stiles W, Inui TS. Communication patterns of primary care physicians. JAMA 277(4), 350–356 (1997). [PubMed] [Google Scholar]
- 130.Zolnierek KBH, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med. Care 47(8), 826 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haskard KB, Williams SL, Dimatteo MR, Rosenthal R, White MK, Goldstein MG. Physician and patient communication training in primary care: effects on participation and satisfaction. Health Psychol. 27(5), 513 (2008). [DOI] [PubMed] [Google Scholar]
- 132.Ruberton PM, Huynh HP, Miller TA, Kruse E, Chancellor J, Lyubomirsky S. The relationship between physician humility, physician–patient communication, and patient health. Patient Educ. Couns. 99(7), 1138–1145 (2016). [DOI] [PubMed] [Google Scholar]
- 133.United States Census Bureau. Detailed languages spoken at home and ability to speak English for the population 5 years and over: 2009–2013. (2015). https://census.gov/data/tables/2013/demo/2009-2013-lang-tables.html
- 134.Davidhizar R, Giger J. A review of the literature on care of clients in pain who are culturally diverse. Int. Nurs. Rev. 51(1), 47–55 (2004). [DOI] [PubMed] [Google Scholar]
- 135.Chiauzzi E, Black RA, Frayjo K. et al. Health care provider perceptions of pain treatment in Hispanic patients. Pain Pract. 11(3), 267–277 (2011). [DOI] [PubMed] [Google Scholar]
- 136.Cohen LA, Harris SL, Bonito AJ. et al. Coping with toothache pain: a qualitative study of low-income persons and minorities. J. Public Health Dent. 67(1), 28–35 (2007). [DOI] [PubMed] [Google Scholar]
- 137.Crane JA. Patient comprehension of doctor–patient communication on discharge from the emergency department. J. Emerg. Med. 15(1), 1–7 (1997). [DOI] [PubMed] [Google Scholar]
- 138.Carrasquillo O, Orav EJ, Brennan TA, Burstin HR. Impact of language barriers on patient satisfaction in an emergency department. J. Gen. Intern. Med. 14(2), 82–87 (1999). [DOI] [PubMed] [Google Scholar]
- 139.Jimenez N, Moreno G, Leng M, Buchwald D, Morales LS. Patient-reported quality of pain treatment and use of interpreters in Spanish-speaking patients hospitalized for obstetric and gynecological care. J. Gen. Intern. Med. 27(12), 1602–1608 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ku L, Flores G. Pay now or pay later: providing interpreter services in health care. Health Aff. 24(2), 435–444 (2005). [DOI] [PubMed] [Google Scholar]
- 141.Angelelli C. Challenges in interpreters' coordination of the construction of pain. : Coordinating Participation in Dialogue Interpreting. Baraldi C, Gavioli L (Eds). John Benjamins Publishing Company, PA, USA, 251–268 (2012). [Google Scholar]
- 142.Cooper LA, Roter DL. Patient–provider communication: the effect of race and ethnicity on process and outcomes of healthcare. : Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR (). National Academies Press (US), Washinton DC, USA: (2003). [PubMed] [Google Scholar]
- 143.Lasch K, Greenhill A, Wilkes G, Carr D, Lee M, Blanchard R. Why study pain? A qualitative analysis of medical and nursing faculty and students' knowledge of and attitudes to cancer pain management. J. Palliat. Med. 5(1), 57–71 (2002). [DOI] [PubMed] [Google Scholar]
- 144.Campbell TS, Hughes JW, Girdler SS, Maixner W, Sherwood A. Relationship of ethnicity, gender, and ambulatory blood pressure to pain sensitivity: effects of individualized pain rating scales. J. Pain 5(3), 183–191 (2004). [DOI] [PubMed] [Google Scholar]
- 145.Gélinas C, Loiselle CG, Lemay S, Ranger M, Bouchard E, McCormack D. Theoretical, psychometric, and pragmatic issues in pain measurement. Pain Manag. Nurs. 9(3), 120–130 (2008). [DOI] [PubMed] [Google Scholar]
- 147.Jorge J, McDonald DD. Hispanic older adults' osteoarthritis pain communication. Pain Manag. Nurs. 12(3), 173–179 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Puia D, McDonald DD. Older black adult osteoarthritis pain communication. Pain Manag. Nurs. 15(1), 229–235 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jimenez N, Garroutte E, Kundu A, Morales L, Buchwald D. A review of the experience, epidemiology, and management of pain among American Indian, Alaska Native, and Aboriginal Canadian peoples. J. Pain 12(5), 511–522 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Burhansstipanov L, Hollow W. Native American cultural aspects of oncology nursing care. Semin. Oncol. Nurs. 17(3), 206–219 (2001). [DOI] [PubMed] [Google Scholar]
- 151.Kleinman A, Benson P. Anthropology in the clinic: the problem of cultural competency and how to fix it. PLoS Med. 3(10), e294 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Narayan MC. Culture's effects on pain assessment and management. Am. J. Nurs. 110(4), 38–47 (2010). [DOI] [PubMed] [Google Scholar]
- 153.Tait RC, Chibnall JT. Racial/ethnic disparities in the assessment and treatment of pain: psychosocial perspectives. Am. Psychol. 69(2), 131 (2014). [DOI] [PubMed] [Google Scholar]
- 154.McDonald DD, Gifford T, Walsh S. Effect of a virtual pain coach on older adults' pain communication: a pilot study. Pain Manag. Nurs. 12(1), 50–56 (2011). [DOI] [PubMed] [Google Scholar]
- 155.Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann. Intern. Med. 155(2), 97–107 (2011). [DOI] [PubMed] [Google Scholar]
- 156.Nouri SS, Rudd RE. Health literacy in the ‘oral exchange’: an important element of patient–provider communication. Patient Educ. Couns. 98(5), 565–571 (2015). [DOI] [PubMed] [Google Scholar]
- 157.Parker R. Health literacy: a challenge for American patients and their health care providers. Health Promot. Int. 15(4), 277–283 (2000). [Google Scholar]
- 158.Williams MV, Davis T, Parker RM, Weiss BD. The role of health literacy in patient-physician communication. Fam. Med. 34(5), 383–389 (2002). [PubMed] [Google Scholar]
- 159.Aboumatar HJ, Carson KA, Beach MC, Roter DL, Cooper LA. The impact of health literacy on desire for participation in healthcare, medical visit communication, and patient reported outcomes among patients with hypertension. J. Gen. Intern. Med. 28(11), 1469–1476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Menendez ME, Van Hoorn BT, Mackert M, Donovan EE, Chen NC, Ring D. Patients with limited health literacy ask fewer questions during office visits with hand surgeons. Clin. Orthop. Relat. Res. 475(5), 1291–1297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Briggs AM, Jordan JE, Buchbinder R. et al. Health literacy and beliefs among a community cohort with and without chronic low back pain. Pain 150(2), 275–283 (2010). [DOI] [PubMed] [Google Scholar]
- 162.Köppen PJ, Dorner TE, Stein KV, Simon J, Crevenna R. Health literacy, pain intensity and pain perception in patients with chronic pain. Wien. Klin. Wochenschr. 130(1–2), 23–30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thorn BE, Day MA, Burns J. et al. Randomized trial of group cognitive behavioral therapy compared with a pain education control for low-literacy rural people with chronic pain. Pain 152(12), 2710–2720 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thorn BE, Eyer JC, Van Dyke BP. et al. Literacy-adapted cognitive behavioral therapy versus education for chronic pain at low-income clinics. Ann. Intern. Med. 168(7), 471 (2018). [DOI] [PubMed] [Google Scholar]
- 165.Collins JE, Deshpande BR, Katz JN, Losina E. Race-and sex-specific incidence rates and predictors of total knee arthroplasty: seven-year data from the Osteoarthritis Initiative. Arthritis Care Res. (Hoboken) 68(7), 965–973 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schoenfeld AJ, Sturgeon DJ, Dimick JB. et al. Disparities in rates of surgical intervention among racial and ethnic minorities in medicare accountable care organizations. Ann. Surg. 269 (3) 459–464 (2019). [DOI] [PubMed] [Google Scholar]
- 167.Shahid H, Singh JA. Racial/ethnic disparity in rates and outcomes of total joint arthroplasty. Curr. Rheumatol. Rep. 18(4), 20 (2016). [DOI] [PubMed] [Google Scholar]
- 168.Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national Medicare data. Ann. Rheum. Dis. 73(12), 2107–2115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Singh JA, Ramachandran R. Racial disparities in total ankle arthroplasty utilization and outcomes. Arthritis Res. Ther. 17(1), 70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Singh JA, Ramachandran R. Persisting racial disparities in total shoulder arthroplasty utilization and outcomes. J. Racial Ethn. Health Disparities 3(2), 259–266 (2016). [DOI] [PubMed] [Google Scholar]
- 171.Zhang W, Lyman S, Boutin-Foster C. et al. Racial and ethnic disparities in utilization rate, hospital volume, and perioperative outcomes after total knee arthroplasty. J. Bone Joint. Surg. Am 98(15), 1243–1252 (2016). [DOI] [PubMed] [Google Scholar]
- 172.Schoenfeld AJ, Lurie JD, Zhao W, Bono CM. The effect of race on outcomes of surgical or nonsurgical treatment of patients in the Spine Patient Outcomes Research Trial (SPORT). Spine 37(17), 1505–1515 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Goodman SM, Mandl LA, Parks ML. et al. Disparities in TKA outcomes: census tract data show interactions between race and poverty. Clin. Orthop. Relat. Res. 474(9), 1986–1995 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lavernia CJ, Alcerro JC, Contreras JS, Rossi MD. Ethnic and racial factors influencing well-being, perceived pain, and physical function after primary total joint arthroplasty. Clin. Orthop. Relat. Res. 469, 1838–1845 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lavernia CJ, Villa JM. Does race affect outcomes in total joint arthroplasty? Clin. Orthop. Relat. Res. 473(11), 3535–3541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Allen Butler R, Rosenzweig S, Myers L, Barrack RL. The Frank Stinchfield Award: the impact of socioeconomic factors on outcome after THA: a prospective, randomized study. Clin. Orthop. Relat. Res. 469(2), 339–347 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Barrack RL, Ruh EL, Chen J. et al. Impact of socioeconomic factors on outcome of total knee arthroplasty. Clin. Orthop. Relat. Res. 472(1), 86–97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Khor S, Lavallee D, Cizik AM. et al. Development and validation of a prediction model for pain and functional outcomes after lumbar spine surgery. JAMA Surg. (153 (7), 634–642 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Detora LM, Krupa D, Bolognese J, Sperling RS, Ehrich EW. Rofecoxib shows consistent efficacy in osteoarthritis clinical trials, regardless of specific patient demographic and disease factors. J. Rheumatol. 28(11), 2494–2503 (2001). [PubMed] [Google Scholar]
- 180.Lubis A, Wang W, Lima G, Fayyad R, Walker C. Comparing the safety and efficacy of celecoxib for the treatment of osteoarthritis in Asian and non-Asian populations: an analysis of data from two randomized, double-blind, placebo-controlled, active-comparator trials. Pain Ther. 6(2), 235–242 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dominick KL, Bosworth HB, Hsieh JB, Moser BK. Racial differences in analgesic/anti-inflammatory medication use and perceptions of efficacy. J. Natl Med. Assoc. 96(7), 928–932 (2004). [PMC free article] [PubMed] [Google Scholar]
- 182.Burgess DJ, Gravely AA, Nelson DB. et al. Association between pain outcomes and race and opioid treatment: retrospective cohort study of Veterans. J. Rehabil. Res. Dev. 53(1), 13–24 (2016). [DOI] [PubMed] [Google Scholar]
- 183.Merry B, Campbell CM, Buenaver LF. et al. Ethnic group differences in the outcomes of multidisciplinary pain treatment. J. Musculoskelet. Pain 19(1), 24–30 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hooten WM, Knight-Brown M, Townsend CO, Laures HJ. Clinical outcomes of multidisciplinary pain rehabilitation among African American compared with Caucasian patients with chronic pain. Pain Med. 13(11), 1499–1508 (2012). [DOI] [PubMed] [Google Scholar]
- 185.Berchick ER, Hood E, Barnett JC. Health insurance coverage in the United States: 2017. Curr. Popul. Rep. 60–264 (2018). [Google Scholar]
- 186.Institute of Medicine. Understanding and eliminating racial and ethnic disparities in healthcare. : Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR (). National Academies Press (US), DC, USA: (2003). [PubMed] [Google Scholar]
- 187.Meredith DS, Katz JN. Procedure volume as a quality measure for total joint replacement. Clin. Exp. Rheumatol. 25(6 Suppl. 47), 37–43 (2007). [PubMed] [Google Scholar]
- 188.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 116(5), 404 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Collins KS, Hall AG, Neuhaus C. US Minority Health: a Chartbook. The Commonwealth Fund, NY, USA: (1999). [Google Scholar]
- 190.Blake VA, Allegrante JP, Robbins L. et al. Racial differences in social network experience and perceptions of benefit of arthritis treatments among New York City Medicare beneficiaries with self-reported hip and knee pain. Arthritis Rheum. 47(4), 366–371 (2002). [DOI] [PubMed] [Google Scholar]
- 191.Green CR, Ndao-Brumblay SK, West B, Washington T. Differences in prescription opioid analgesic availability: comparing minority and white pharmacies across Michigan. J. Pain 6(10), 689–699 (2005). [DOI] [PubMed] [Google Scholar]
- 192.Morrison RS, Wallenstein S, Natale DK, Senzel RS, Huang LL. “We don't carry that” – failure of pharmacies in predominantly nonwhite neighborhoods to stock opioid analgesics. N. Engl. J. Med. 342(14), 1023–1026 (2000). [DOI] [PubMed] [Google Scholar]
- 193.Ennis S, Rios-Vargas M, Albert N. The Hispanic population: 2010 (2010 Census Briefs). US Census Bureau DC, USA: (2011). [Google Scholar]
- 194.Zander V, Eriksson H, Christensson K, Müllersdorf M. Development of an interview guide identifying the rehabilitation needs of women from the Middle East living with chronic pain. Int. J. Environ. Res. Public Health 12(10), 12043–12056 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hirsh A. Virtual perspective-taking to reduce race and SES disparities in pain care (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Allen KD, Arbeeva L, Cene CW. et al. Pain coping skills training for African Americans with osteoarthritis study: baseline participant characteristics and comparison to prior studies. BMC Musculoskelet. Disord. 19(1), 337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schrubbe LA, Ravyts SG, Benas BC. et al. Pain coping skills training for African Americans with osteoarthritis (STAART): study protocol of a randomized controlled trial. BMC Musculoskelet. Disord. 17, 359 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]