Abstract
Pulmonary metastases are a sign of advanced malignancy and an omen of poor prognosis. Once primary tumors metastasize, they become notoriously difficult to treat and interdisciplinary management often involves a combination of chemotherapy, radiotherapy, and surgery. Over the last 25 years, the emerging body of evidence has recognized the curative potential of pulmonary metastasectomy. Surgical resection of pulmonary metastases is now commonly considered for patients with controlled primary disease, absence of widely disseminated extrapulmonary disease, completely resectable lung metastases, sufficient cardiopulmonary reserve, and lack of a better alternative systemic therapy. Since the development of these selection criteria, other prognostic factors have been proposed to better predict survival and optimize the selection of surgical candidates. Disease-free interval (DFI), completeness of resection, surgical approach, number and laterality of lung metastases, and lymph node metastases all play a dynamic role in determining patient outcomes. There is a definite need to continue reviewing these prognosticators to identify patients who will benefit most from pulmonary metastasectomy and those who should avoid unnecessary loss of lung parenchyma. This literature review aims to explore and synthesize the last 25 years of evidence on the long-term survival, prognostic factors, and patient selection process for pulmonary metastasectomy.
Keywords: pulmonary metastasectomy, lung metastasectomy, metastases, lung resection, survival
Introduction
The lungs are the most common site of metastatic malignancy.1–7) Pulmonary metastases can arise from primary cancers within the lung or more frequently from extrapulmonary sites as a result of hematogenous, lymphatic, or transcoelomic spread. Colorectal cancer, osteogenic and soft tissue sarcoma, head and neck cancer, malignant melanoma, germ cell tumors, and renal cell carcinomas exhibit the greatest proclivity for pulmonary metastatic spread.1–7) Once primary tumors metastasize, they become notoriously difficult to treat and confer much higher morbidity and mortality. However, in select patients, pulmonary metastasectomy can be performed with curative intent. Complete surgical excision is often technically feasible with low perioperative morbidity and mortality.1,3,5,8–26)
Pulmonary resection is only considered suitable for patients with control of primary disease, no widely disseminated or uncontrolled extrapulmonary metastases, completely resectable lung metastases, and adequate cardiopulmonary reserve to tolerate surgery.1,2,9,17)
In the last 25 years, a substantial body of evidence has gradually recognized the curative potential of pulmonary metastasectomy.1) Resection of lung metastases in carefully selected patients is now widely accepted as a surgical intervention with significant therapeutic worth in the interdisciplinary management of metastatic malignancy. Current literature reports associated improvements in both overall and disease-free survival following pulmonary metastasectomy, with 5-year survival rates ranging between 20% and 40% compared to historical controls.1) Despite the growing evidence, it should be emphasized that only a proportion of patients diagnosed with lung metastases satisfy the typical criteria for surgical intervention. It is recognized that many dynamic factors, including the natural history of advanced malignancy, may prove to have the greatest influence on observed patient outcomes irrespective of surgical intervention.
This literature review aims to explore and synthesize the last 25 years of evidence on the long-term survival, prognostic factors, and patient selection process for surgical resection of pulmonary metastases.
Methods
Identification of studies
Eligibility criteria for inclusion in this literature review were predetermined. All relevant prospective and retrospective studies reporting prognostic outcomes of patients undergoing pulmonary metastasectomy with curative intent were identified. Specifically, this included any systematic reviews, cohort studies or case control series examining long-term survival, disease-free interval (DFI), and prognostic indicators of survival following pulmonary metastasectomy. To date, no randomized controlled trials (RCTs) investigating outcomes following pulmonary metastasectomy have been published. Studies considered eligible for inclusion reported on adult patients aged over 18 years, a diagnosis of metastatic cancer, primary focus on pulmonary metastasectomy regardless of type of resection or surgical approach, analysis of prognostic factors, and an outcome measure of long-term survival.
Search strategy
The literature search was conducted in accordance with the PRISMA 2009 guidelines.27) The electronic database search was performed by a single independent investigator via Medline (Ovid, 1946-present), EMBase (Ovid, 1946-present), and Cochrane Library. The search terms included but were not limited to: metastatic cancer, lung metastases, pulmonary metastases, lung metastasectomy, pulmonary metastasectomy, resection, and pulmonary surgical procedure. The search was restricted to publications in English or translated to English and no restrictions on publication date or publication status were imposed on the search. A supplemental manual search of journals and lists within the identified articles was undertaken for additional records.
Search results
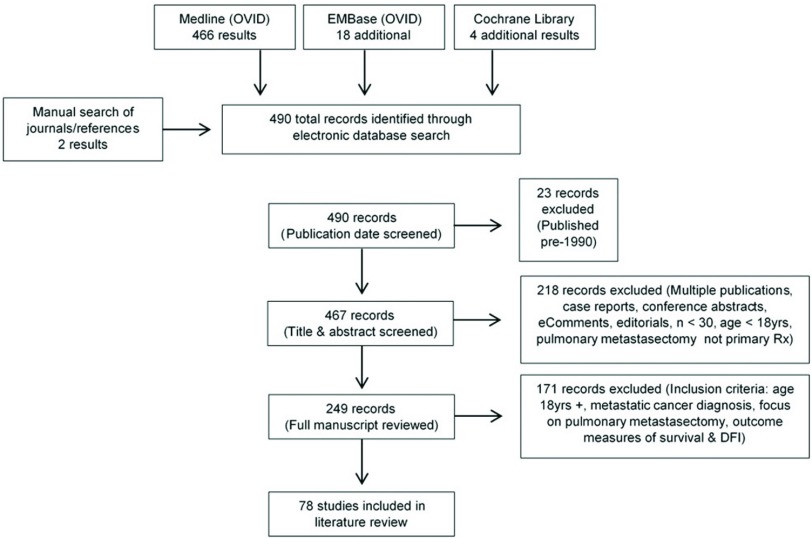
Electronic database searching retrieved a total of 490 records once duplications were removed. The final search date was 30th March 2016 and all identified articles were published between 1983 and 2016. In order for this literature review to reflect outcomes for patients undergoing modern surgical, anesthetic, and diagnostic imaging techniques only articles published in the last 25 years since 1990 were included. Following a title and abstract screen, a further 218 articles, which did not provide clinical research outcomes, were then excluded. These included duplicate or multiple publications, case commentaries, conference abstracts, eComments, and editorials. When multiple publications were identified, the most recent article was retained for review. In addition, any studies involving pediatric populations, containing a sample size of less than 30 patients or not primarily investigating pulmonary metastasectomy were also excluded. The remaining articles were then reviewed in full text and 171 articles were excluded in accordance with the aforementioned eligibility criteria. The final 78 full-text articles were comprehensively reviewed for inclusion in this review article (Fig. 1).
Fig. 1. Study selection process. DFI: disease-free interval.
Discussion
Historical context
The first published report of pulmonary metastasectomy in 1882 described the resection of two incidental pulmonary metastases during the surgical excision of a chest wall sarcoma.28) In 1927, Divis then outlined the first planned metastasectomy29) while Alexander and Haight reported the earliest series of lung resection for solitary metastases in 1947, demonstrating significant 3-year survival in 24 patients.30) The largest case series during this period was conducted at the Mayo Clinic by Thomford et al. in 1965. This observational study demonstrated a 5-year survival of 31% among 205 patients undergoing 221 lung resections for metastatic cancer and proposed principles for patient selection.31) During the 1960s to 1970s, reports of pulmonary metastasectomy for specific primary tumor types emerged and in 1979 McCormack and Martini demonstrated the potential survival benefits following multiple lung resections for patients with bilateral or multiple metastases.32) Since then the momentum of evidence supporting the utility of pulmonary metastasectomy has been building gradually until Pastorino et al. published a landmark large-scale cohort study in 1997. The International Registry of Lung Metastases was established in 1991 aiming to assess the long-term outcomes of pulmonary metastasectomy. From the 5206 cases examined in the registry, Pastorino et al. reported a 5-year survival of 13% to 36% and concluded that surgical resection of lung metastases is a safe and potentially curative procedure.1) Since this time extensive literature has emerged supporting pulmonary metastasectomy as a surgical intervention of significant therapeutic value in the interdisciplinary management of metastatic cancer.
Current evidence base
Historically, metastatic spread has always been an omen of poor prognosis. While it is still widely acknowledged as a sign of advanced malignant disease, in recent decades the greater efficacy of systemic therapy and advancements in diagnostic imaging have significantly improved the prognosis of patients diagnosed with metastatic cancer. As survival outcomes continue to improve, the role of pulmonary metastasectomy with curative intent is increasingly germane in the modern age.
Selection criteria
In the last 25 years, a surge in published observational studies has established the current evidence base. Case series, cohort studies, and review articles published since the 1990s outline an abundance of retrospective data that supports the potential survival benefits of resecting lung metastases in select patients.1,2,9,33,34) This broad foundation of evidence has heralded the almost ubiquitous acceptance of pulmonary metastasectomy as a safe and potentially curative surgical intervention. Careful selection of appropriate surgical candidates is paramount to ensure lung resection can be performed with curative intent and minimal perioperative morbidity or mortality. Reported perioperative mortality and morbidity ranges from 0% to 2.5% and 1.0% to 16.0%, respectively.14,16,35,36) Often the process of selection is guided primarily by clinical evaluation and imaging. Since the majority of pulmonary metastases are asymptomatic, patient symptoms rarely feature in the decision-making process. Symptomatic metastases are uncommon but in approximately 15% of patients symptoms can include cough, hemoptysis, and chest discomfort.37) Most commonly lung metastases are identified as incidental findings on radiographic imaging. The criteria for selecting patients for surgery have been gradually expanding since first proposed by Thomford et al. in 1965.31) Pulmonary metastasectomy is now considered suitable for patients with:
Controlled or complete eradication of primary disease,
No widely disseminated or uncontrollable extrapulmonary disease,
Completely resectable lung metastases,
Sufficient cardiopulmonary reserve to tolerate surgery,
Lack of better alternative systemic therapy.1,2,9,17,31,33,34,37–39)
These general selection criteria are now widely recognized with more than 400 publications confirming its utility.40) However, there remains a need to develop specific and robust guidelines to optimize the process of selecting surgical candidates for pulmonary resection. There is also a need to ascertain the true proportion of patients with secondary lung metastases that are amenable to curative surgery, but the true clinical denominator is notoriously difficult to establish accurately.1,24,33)
Survival
The primary malignancies that exhibit the greatest proclivity for metastatic spread to the lungs include colorectal cancer, osteogenic and soft tissue sarcoma, malignant melanoma, head and neck cancer, germ cell tumors, breast cancer, and renal cell carcinoma.1–7) Each primary cancer exhibits a different natural history and pattern of spread and therefore confers markedly different survival rates following pulmonary resection. Overall, current literature reports 5-year and 10-year survival rates of 20.0% to 48.0% and 15.8% to 37.7%, respectively, for all primary cancers following pulmonary metastasectomy.1,25,40–44)
The 78 articles included in this review reported a wide range of survival rates specific to primary tumor types. Colorectal cancer exhibited overall 5-year survival of 24.4% to 82.0% and 10-year survival of 11.0% to 48.4%, with a median survival time of between 33.9 months to 98.0 months.2,4,8–20,36,39,45–48) Sarcoma including both osteosarcoma and soft tissue sarcoma is reported to have post-pulmonary metastasectomy survival of 15.0% to 54.6% 5-year survival and 11.0% to 44.9% 10-year survival, with a median survival of 16.0 months to 60.2 months.3,5,7,21,23,24,26,35,38,49–53) Malignant melanoma holds a notoriously dismal prognosis once metastatic spread occurs. Following resection of metastatic melanoma to the lung, long-term survival ranges from 14.0% to 41.0% 3-year survival and 21.0% to 34.0% 5-year survival, with a median survival ranging between 19.0 months to 35 months.1,2,22,44,54) In contrast, survival following the resection of germ cell tumor metastases is markedly more optimistic with 5-year and 10-year survival ranging between 42.0% to 82.0% and 63.0% to 86.0%, respectively.6,55–57) Head and neck cancers are reported to have 5-year survival of 29.1% to 57.9% and 10-year survival of up to 35.0%, with a median survival 26 months.34,58) Similarly, breast cancer has reported 5-year survival of 38.0% to 59.6% and 10-year survival of 22.0% to 43.0% following pulmonary metastasectomy, exhibiting a median survival between 35.0 and 82.4 months.59–61) Renal cell carcinoma has also been demonstrated to exhibit 3-year and 5-year survival rates of 49.0% and 31.0%, respectively.62) Overall, the wide variability in survival rates published in current literature most likely the reflects a myriad of influential factors including patient selection bias, natural history of metastatic disease, efficacy of available systemic therapy, and disparities in patient follow-up periods.
Prognostic factors
A number of prognostic indicators have been proposed in an attempt to predict better survival outcomes for patients undergoing pulmonary metastasectomy. Primary tumor type and histology, DFI, completeness of resection, surgical approach, number and laterality of lung metastases, and lymph node metastases all play a dynamic role in determining patient outcomes. There is a definite need to continue reviewing these prognosticators to optimize the selection process for surgical candidates. Judicious preoperative screening will identify patients who will benefit most from pulmonary metastasectomy and those who should avoid unnecessary loss of lung parenchyma.26)
Primary tumor
Primary tumor type and histology remain important considerations in the interdisciplinary approach to metastatic disease. Germ cell cancers are consistently reported as demonstrating the best survival post-pulmonary metastasectomy of all primary tumor types.1,6,55–57) Survival following the development of germ cell tumor metastases has improved dramatically since the advent of cisplatin-based chemotherapy, but residual pulmonary nodules remain common requiring surgical intervention. In 1997, Pastorino et al. demonstrated that patients with germ cell tumors had by far the best survival, with 68% 5-year and 63% 10-year survival.1) In contrast, metastatic melanoma has limited systemic treatment options and proves to hold the worst prognosis.1,22,54) Peterson et al. conducted the largest cohort study of 1720 patients in 2007 reporting a dismal median survival of 7.3 months after the development of pulmonary metastases without intervention. However, significant survival advantage was found for patients undergoing complete resection of lung metastases when compared with patients not undergoing surgical resection, with 5-year survival rates of 21% compared with 3%.54)
Primary sarcomas exhibit the largest range of histological subtypes. Suzuki et al. in 2006 compared the histology of 105 sarcoma patients, finding no prognostic influence on the survival of patients with lung metastases from osteosarcoma or soft tissue sarcoma subtypes.7) Similarly, Kim et al. ascertained no survival difference between the four most common sarcoma histological subtypes of osteosarcoma, malignant fibrous histiocytoma, leiomyosarcoma, and chondrosarcoma.24) It is noted that these findings may be limited by relatively small comparison groups (n <20). In contrast, Younes et al. in 2012 outlined the significant difference in survival between histological subtypes of sarcoma patients. By conducting a large cohort study involving 440 patients with larger comparison groups (n >60), osteosarcoma and soft tissue sarcoma patients demonstrated statistically significant differences in median survival of 27.0 months and 41.8 months, respectively.44)
Advanced stage colorectal cancer remains one of the most common causes of pulmonary metastases and currently no studies directly comparing surgical intervention with supportive care have been reported.63,64) Stage IV colorectal cancer without chemotherapy or surgical intervention notably carries a dismal prognosis, with a 5-year survival rate of less than 10%63,64) and a median survival time of 5.0 months even with optimal supportive care.65,66) Similarly, inoperable metastatic head and neck cancers exhibit an equally poor median survival of between 5.7 and 6.1 months despite systemic therapy, with no reported studies comparing chemotherapy or surgery with supportive care alone.67,68) Historically, metastatic breast cancer holds an ominous prognosis with a reported median survival of 24 months69) and 5-year survival of 5% to 10% following traditional chemotherapy regimens.70–72) However, breast cancer survival rates have improved dramatically over the last two decades. The introduction of national breast cancer screening programs, improved diagnostic and biopsy techniques, coupled with advancements in targeted chemotherapy, hormonal therapy, and aggressive surgical management has seen 5-year survival rates rise from 52% to 85.1% since the 1970s.72) Tumor histology is undoubtedly an important prognostic factor following pulmonary metastasectomy as it guides the consideration of adjuvant therapy and ultimately, the selection of patients with favorable natural history may prove to have the greatest impact on overall survival outcomes.
Disease-free interval
DFI is consistently shown in recent literature to have a significant influence on long-term survival of pulmonary metastasectomy patients. This measure can provide a clinical impression of the either indolent or aggressive nature of a primary malignancy. Of the 56 studies examining DFI included in this review, 42 studies determined DFI to be a significant prognostic indicator of survival. Blackmon et al. in 2009 published a series examining 234 sarcoma patients. Results from evaluating DFI as a continuous variable found that for every month DFI increased there was a significant improvement in survival, and they concluded that a DFI > 24 months appears to be one of the most important prognostic factors.49) Furthermore, Rena et al. in 2006 compared DFI periods of 0–36 months and >36 months in 202 patients diagnosed with a variety of primary epithelial tumors, ascribing to the International Registry of Lung Metastases classification.1) A DFI of >36 months was demonstrated to hold significantly improved prognosis, with 5-year and 10-year actuarial survivals of 52% and 17%, respectively.42) These results corroborated the findings from Pastorino et al. in the International Registry of Lung Metastases, which first outlined that a DFI of >36 months is associated with improved 5-year and 10-year survival rates of 45% and 29%, respectively, when compared with a DFI of 12–35 months (31% 5-year and 22% 10-year survival) or <12 months (33% 5-year and 27% 10-year survival).1)
Conflicting evidence does exist surrounding the utility of DFI as a prognostic indicator. In this review, 14 studies determined no statistically significant link between DFI and long-term survival. McCormack et al. in 1992 compared 144 patients undergoing lung resection for colorectal metastases. No significant survival difference was found when comparing patients presenting with synchronous metastases, a DFI of <12 months and a DFI of >12 months.47) Since then a number of studies have demonstrated similar non-significant results in metastatic sarcoma, melanoma, breast, and renal cancer populations.7,22,60,62)
The definition of DFI also remains heterogeneous.11) Traditionally, DFI is defined as the period of time between the primary treatment of a malignancy and the first sign of disease recurrence or metastatic spread. It is generally accepted as an indicator of the rate of disease progression and thereby correlated with prognosis. However, DFI appears frequently in current literature defined as the period of time between primary cancer treatment and the first sign of lung metastases or date of pulmonary metastasectomy.3,5,15,21,23,24,35,45,50,51,58) This definition, unless explicitly stated, fails to acknowledge if extrapulmonary metastases have developed prior to lung metastases. Perhaps even more problematic is when DFI is calculated as the time from primary treatment until the date of pulmonary metastasectomy,5,23) as clearly the date of metastasectomy does not equate to the date of relapse. Commonly, a suspicious nodule discovered during follow-up is monitored for sinister signs of growth or evolution and consequently may not be resected until months or years after first being detected. A significant overestimation of DFI can therefore occur and this must be considered when interpreting results. Ultimately, while the traditional definition of DFI may arguably be a more accurate reflection of disease progression, it is still difficult to ascertain and can be inaccurate. It is dependent on the symptoms of relapse and the degree of comprehensive follow-up imaging. But with the current practice of regular radiological surveillance to detect disseminated disease, it can allow for a much more reliable estimation of true DFI.
Complete vs incomplete resection
Standard oncological principles have always mandated the complete resection lung metastases, wherever possible, to reduce the risk of local recurrence. In the last few decades, completeness of resection has been consistently shown to be a predictor of improved survival following pulmonary metastasectomy.1,14,20,54,62) Actuarial survival after complete metastasectomy is 36% at 5 years and 26% at 10 years compared with survival of 13% at 5 years and 7% at 10 years following incomplete resection.1) Murthy et al. in 2005 reported completeness of resection as critical to survival. By examining 92 patients with metastatic renal cell cancer, they compared 63 patients who had complete resection with 29 patients who underwent incomplete resection. Results demonstrated 3-year and 5-year survival rates following complete resection of 59% and 42%, respectively, compared with 22% and 8%, respectively, following incomplete resection. They concluded that incomplete resection is the strongest predictor of poor survival.62) More recently, in 2013 Younes et al. retrospectively compared 120 patients with colorectal pulmonary metastases. They demonstrated a highly significant difference in median survival following complete and incomplete resection of 34.9 months and 12.2 months, respectively.20) Countless publications have since elicited similar results and surgical approaches that ensure clear margins with no residual macroscopic or microscopic disease are now standard practice.40,59,60)
Surgical approach
While it is unanimous that complete resection is vital for survival benefit, the surgical approach used to achieve complete resection not universal. Open thoracotomy and minimally invasive video-assisted thoracoscopic surgery (VATS) are currently the most common surgical approaches, with median sternotomy and clamshell thoracotomy less commonly discussed. Historically, posterolateral thoracotomy was standard practice. Proponents today maintain that an open approach allows for the bimanual palpation of an entire lung to detect small nodules not seen on imaging.11,25,42,57) In mid-1990s, the advent of VATS for lung metastases raised concerns over its apparent limited ability to palpate the lung for small lesions through minimally invasive incisions.73) In 1996, McCormack et al. prospectively evaluated the role of VATS by first performing thorascopic resection of all radiographically detected disease followed by a thoracotomy and bimanual palpation, revealing that 56% of patients had residual metastatic lesions after VATS resection.74) Thus, fears emerged that residual disease may remain without comprehensive bimanual palpation, leading to higher recurrence rates and poorer survival outcomes.25,73) However, since the 1990s significant advancements in high-resolution helical computed tomography (CT) technology, combined positron emission tomography (PET)-CT scanning57) and considerable refinements in minimally invasive surgical techniques have occurred. These improvements in the modern era have heralded the increasing the ability to detect and resect even small sub-centimeter lung nodules which were previously unseen. The clear division in opinions was surveyed by the European Society of Thoracic Surgeons (ESTS). In 2008, they reported a large-scale survey of current pulmonary metastasectomy practices, with Internullo et al. revealing that 65.1% consider lung palpation to be mandatory, 34.9% consider it not always necessary, and 40% use a thorascopic approach with curative intent.75)
Despite the ongoing debate surrounding bimanual palpation and the ability to resect all nodules, it remains most salient that there is no definitive difference in long-term outcomes between the two main surgical approaches. Current evidence has consistently reported similar recurrence rates and no significant difference in survival outcomes between open and thorascopic surgery.12,14,23,38,40,42,44,59) Pulmonary recurrence rates postmetastasectomy range from 30.6% to 69.0% regardless of the principal surgical approach.14,42,48) While neither operative approach translates directly to improved long-term survival over the other, there are undeniable advantages to a VATS approach. Decreased postoperative pain, preservation of short-term lung function, shorter period of chest tube drainage, and reduced hospital length of stay are significant advantages of minimally invasive surgery.16,53) A number of patients also present with bilateral disease or eventually develop recurrent disease and a minimally invasive approach can make staged bilateral or repeat lung resections less difficult.53) Rodriguez et al. in 2014 examined the rates of postoperative morbidity and mortality in a large prospective multicenter study of 532 patients with metastatic colorectal cancer.16) The morbidity and mortality rates were 15.6% and 0.4%, respectively. In addition, they suggested a significant protective effect of VATS resection over open thoracotomy in preventing postoperative morbidity, most commonly including ongoing air leaks, atelectasis, pneumonia, arrhythmias, and acute respiratory distress syndrome.16) Postoperative pain scores have also shown to be significantly lower after the cessation of epidural analgesia in minimally invasive thorascopic surgery by Paiva and Wright in 2004. By prospectively randomizing 52 patients to a minimally invasive thorascopic approach or limited thoracotomy, they demonstrated lower visual analogue scale (VAS) pain scores following a thorascopic approach (VAS score of 3.8) compared with thoracotomy (VAS score of 5.2).76) Ultimately, there are currently no definitive guidelines in current literature that govern the widespread implementation of an open thoracotomy or VATS surgical approach, but there appears to be an increasing movement toward minimally invasive surgery for its undeniable benefits.
Other prognostic factors
Conflicting reports continue to emerge regarding the impact of other prognostic indicators including number and laterality of pulmonary metastases, lymph node status, and repeat metastasectomy. The presence of solitary pulmonary metastases has been found to confer better prognosis in a number of cohort studies;1,17,37,39) however, many studies also report the presence of multiple metastases has no significant influence on survival.12,14,41,43) Any reported survival benefit associated with single metastases may be related to the rate of disease progression, resectability, decreased loss of lung parenchyma, and subsequent functional volume. On the other hand, multiple metastases are often a sign of more widely disseminated disease and have been shown to be a risk factor for incomplete resection and can therefore be associated with poor survival.62) The impact of unilateral compared with bilateral distribution of lung metastases is equally as contentious. In this review, 15 articles reported laterality as a non-significant prognosticator3,8,12–14,22,23,41–44,50,59) while 11 articles described unilateral disease as a significant factor in improved survival.4,9,17,20,21,24,39,45,51) Similarly, there are broadly inconsistent results published concerning the influence of lymph node status and the impact of repeat metastasectomy.11,35,38,41) Clearly, there is a need to continue refining these prognostic criteria to optimize the selection process of pulmonary metastasectomy candidates.
Limitations of current evidence
A cogent evidence base recognizing the curative potential of pulmonary metastasectomy has now been established. However, it must be acknowledged that the widespread acceptance of such surgery is founded on retrospective case series of highly selected patients.52,77) The longitudinal design of case series and cohort studies provides valuable prognostic data by deriving relative risk of death and long-term survival, but the utility of observational studies is limited. Case series and cohort studies are unable to establish direct patterns of causation between exposure and outcome. In addition, these surgical series often include patients with many different primary cancers, receiving a variety of neoadjuvant or adjuvant therapies or have small cohort sizes with limited follow-up. It is therefore difficult to determine the true therapeutic efficacy of pulmonary metastasectomy in prolonging survival based on retrospective data analysis. Fiorentino et al. expressed concerns over the current quality of evidence in 2010, concluding that “the quality [of evidence] is not sufficient to draw inferences concerning the effectiveness of this surgery.”46) There is indeed a lack of prospective studies and to date no randomized controlled data has been published. Aberg et al. in 1997 wrote “it is a scientific fallacy to assume pulmonary metastasectomy patients have the same prognosis as an untreated group with the same disease, a control group with the same characteristics as those operated on is lacking.”77) It must therefore be acknowledged that long-term survival without pulmonary metastasectomy may not necessarily approach zero and the inferred survival benefits from such surgery may be influenced by other factors.
The inherent selection bias of a surgical cohort chosen for their more positive prognostic features is one major factor. Selecting patients with (1) controlled primary disease; (2) no uncontrollable extrapulmonary disease; (3) resectable lung metastases; (4) sufficient cardiopulmonary reserve to tolerate surgery; (5) lack of a better alternative systemic therapy is now standard practice.1,2,9,17) Surgical resection of lung metastases is often not an option for patients with rapidly progressive or widely disseminated disease. Therefore, any associated survival benefit following pulmonary metastasectomy may be in fact strongly influenced by the selection of surgical candidates who are more likely to survive.52) The natural history of tumor progression in these highly selected patients may be the ultimate determinant of long-term outcomes. It is possible any survival advantage may indeed be a reflection of the indolent nature of metastatic disease in surgical cohorts.36) Without adequate non-operative control data, it will remain difficult to quantitatively analyze the direct survival benefit from surgical resection. Obtaining such control data however is challenging, if not impossible. Clinical reason dictates that allowing even low-volume disease to remain without treatment is not conducive to cure. And thus it is ethically difficult to justify not offering surgical intervention to observe survival outcomes for non-operative patients. Comparing systemic medical therapy with surgical intervention is perhaps the next best option. Case matched series by Salah et al. in 2013 compared sarcoma patients with resectable lung metastases. Superior progression free and overall survival was demonstrated in patients undergoing lung resection when compared with those receiving systemic therapy alone. Overall median survival in the surgical cohort was 34.0 months compared with 12.4 months in patients receiving systemic treatment.50) Similar associated survival benefits were published by Yhim et al. in 2010. They demonstrated significantly longer survival in patients undergoing neoadjuvant systemic therapy and pulmonary metastasectomy (4-year overall survival of 82.1%) for breast cancer than those undergoing systemic therapy alone (4-year overall survival of 31.6%).61) It is noted, however, that these case matched series are limited by small comparison group numbers, selection bias, and heterogeneity between groups without a randomization process. Nevertheless, despite the inherent selection bias within the current evidence base, offering surgical intervention as an option within an interdisciplinary treatment approach still represents the best chance of cure for patients with low-volume metastatic disease.
The relative contribution of pulmonary metastasectomy to overall survival is also influenced by the administration of systemic chemotherapy and radiotherapy. Over the last few decades, neoadjuvant or adjuvant chemotherapeutic regimes have evolved substantially. These regimes are increasingly individualized based on specific patient characteristics, tumor biology, and drug efficacy.45) Radical improvements in systemic therapy, as evidenced in the management of germ cell tumors and breast cancer, have also changed the role of surgical resection. It is therefore challenging for retrospective cohort studies to provide clear unbiased results on the potential benefits of pulmonary metastasectomy in patients receiving neoadjuvant or adjuvant systemic therapy. Currently, the majority of solid primary tumors that metastasize to the lung remain relatively unresponsive to available systemic chemotherapy regimens37) and it has been reported that the extent of neoadjuvant or adjuvant chemotherapy does not have a statistically significant impact on survival outcomes after pulmonary metastasectomy.38,40,48) However, these reports stem from basic binary analyses comparing those who did or did not receive systemic therapy, rather than in depth analysis of specific regimes taking into account tumor biology and drug efficacy. Further prospective evidence examining similar chemotherapy or radiotherapy protocols is required to accurately describe its true impact on survival post-pulmonary metastasectomy.
Future directions
The future indications and optimal timing for pulmonary metastasectomy within the interdisciplinary management of metastatic disease remain dynamic. Advances in chemotherapy regimens and the evolution of immunotherapy have the potential treat low-volume metastatic disease without the need for surgical intervention. In the setting of metastatic colorectal cancer, the modern age of oxaliplatin- and irinotecan-based chemotherapy alongside targeted immunotherapy agents including bevacizumab, cetuximab, and panitumumab has seen significant improvements in disease-free and overall survival.78–81) It is unlikely, however, that this will render metastasectomy surgery obsolete. These advances in systemic therapy may enable a subset of patients with advanced metastatic cancer who exhibit incomplete tumor response and develop residual drug-resistant clones to be considered for curative surgery.82,83) The timing of pulmonary metastasectomy in the future, therefore, may evolve to follow after induction or definitive chemotherapy when low-volume drug-resistant disease remains.
Image-guided ablative therapies including radiofrequency ablation (RFA), cryoablation, microwave ablation (MWA), and laser-induced thermal therapy (LITT) have also emerged as potential minimally invasive alternatives to surgical resection.84) RFA and cryoablation are more commonly offered and have been shown to have similar 5-year survival rates to surgical resection.84–86) De Baere et al., in a large retrospective analysis of 562 patients, reported overall 5-year survival of 51.5% and median survival of 62 months following RFA for pulmonary metastases, however 67% of procedures were complicated by a pneumothorax, with 58% of those patients requiring intercostal catheter insertion.87) RFA like pulmonary metastasectomy is founded predominantly upon retrospective data and therefore liable to the same bias.85) Currently, no prospective randomized data have been published comparing surgery to RFA.84,85) In 2014, Schlijper et al. conducted a systematic review comparing pulmonary metastasectomy to RFA concluding that although current evidence largely supports surgery as the most effective option, no firm conclusions can made without more robust data.85,86) The safety and efficacy of cryoablative therapy for pulmonary metastases is currently being examined in the ECLIPSE trial, a prospective single arm study.88) With final outcomes yet to be published, interim analysis of the initial 35 patients has demonstrated overall tumor control in 91.4% of patients, however a complete tumor response was seen in 21.2% of patients and metastatic recurrence occurred in 40% of patients at 12 months post-cryoablation.88)
Stereotactic ablative radiation (SABR), is also known as stereotactic body radiation therapy (SBRT), offers a non-invasive option in the interdisciplinary management of pulmonary metastases. A treatment option traditionally reserved for peripherally located oligometastatic disease in those individuals who are considered poor surgical candidates.89,90) While encouraging outcomes have been reported in single modality studies, comparisons between SABR and metastasectomy are limited to a small number of retrospective studies.89,90) In 2018, Lee et al. conducted a retrospective study comparing outcomes of SABR with metastasectomy finding no significant difference in local tumor control rates and overall survival.90) However, a significant improvement in progression-free survival following metastasectomy was described with 41.6% remaining disease free compared with 11.9% in the SABR group at 2 years.90) Jingu et al. reported a systematic review of SABR for the control of pulmonary metastases, revealing variable local tumor control rates of between 25.0% and 97.5%, and also identified that SABR is significantly less effective at local control of oligometastatic disease from colorectal cancer than from other primary malignancies.89)
In current practice, image-guided ablation and SABR offer the opportunity to treat non-surgical candidates with limited tumor burden, but the undoubted benefits of preserving lung parenchyma in patients with compromised respiratory function or those requiring multiple treatments may lead to these modalities becoming increasingly adopted in a future interdisciplinary approach.87,88) While these ablative therapies do offer a minimally invasive option, they do not enable the same histopathological scrutiny as surgical resection, which can guide adjuvant systemic treatment.84) Ablation destroys malignant tissues and tumor samples are not obtained.84,91) Compounding the issue is when no tissue diagnosis is available prior to ablative therapy, and often a new or enlarging lung mass on CT with PET avidity is considered diagnostic of metastatic disease.87) Without more robust data, it is not known whether they lead to equivalent or better survival outcomes and quality of life when compared with definitive surgical resection. What is known is that surgical resection offers tissue samples for histopathology and clearly defined treatment margins, which image-guided ablative modalities are unable to.84)
The future need for comprehensive prospective data and RCTs is undeniable. In 2008, the ESTS published the pulmonary metastasectomy project, concluding that “the level of evidence to support current practice is too low to set firm recommendations to the members of the ESTS … In the absence of a randomised controlled trial looking at the effectiveness of pulmonary metastasectomy on survival and quality of life, it is unlikely that current practice will ever be influenced.”75) However, the feasibility of conducting such a trial remains uncertain. Valerie Rusch has noted the dynamic nature of pulmonary metastasectomy surgery explaining that “In the era of both rapidly evolving molecular medicine and technology, it can be increasingly difficult to perform randomized controlled trials that attempt to determine the benefit of surgical resection. Such trials require many years to develop and perform … Major technological improvements in radiotherapy and ablative therapies also have the potential to alter the indications for surgery or render surgical intervention obsolete.”92)
Shonio et al. in 2009 stated that a prospective randomized trial deriving the benefit of surgical resection over supportive care alone would be ethically difficult and impractical.58) Multiple authors have since echoed these views, with opinions on the feasibility of conducting such a study ranging from “unlikely” to “impossible.”14,26,53) How do you concretely define the cohort of patients to be randomized? Is it ethically justifiable to consent patients to potentially receive non-active treatment? Can such a study reliably deduce the exact number needed to treat (NNT) to find those patients who will truly achieve survival benefit from pulmonary metastasectomy, and at what cost to those patients randomized to the conservative or non-treatment arm? These are just some of the questions surrounding the development of such prospective research. While comparing surgical resection to active monitoring is not ethically acceptable, a comparison with systemic medical therapies is currently underway. Treasure et al. in 2009 established the Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) trial to discover the patterns of survival when comparing pulmonary metastasectomy and best medical therapy. This ambitious phase III RCT is aiming to address the need for prospective data for metastatic colorectal cancer patients. It is indeed hoped that this study will be able to answer the feasibility questions raised and optimize the patient selection process. The PulMiCC trial is currently ongoing and it is anticipated the results will be of keen interest in the thoracic oncology field.
Conclusion
The diagnosis of lung metastases is widely acknowledged as a sign of advanced malignant disease with poor prognosis. Modern interdisciplinary management of metastatic cancer mandates the consideration of all treatment options from chemotherapy or radiotherapy to surgical intervention. Over the last few decades, the potential survival benefits following pulmonary metastasectomy has been increasingly recognized in medical literature. The foundation of evidence has seen the widespread acceptance of lung resection for metastatic cancer as a safe and potentially curative procedure.1,2,9,33,34) However, controversy does exist with concerns over the quality of such evidence.52,77) Future prospective data will support the development of specific and robust guidelines to optimize the process of selecting surgical candidates for pulmonary metastasectomy.
Disclosure Statement
No conflicts of interest to declare.
References
- 1).Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997; 113: 37-49. [DOI] [PubMed] [Google Scholar]
- 2).Pfannschmidt J, Egerer G, Bischof M, et al. Surgical intervention for pulmonary metastases. Dtsch Arztebl Int 2012; 109: 645-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Dear RF, Kelly PJ, Wright GM, et al. Pulmonary metastasectomy for bone and soft tissue sarcoma in Australia: 114 patients from 1978 to 2008. Asia Pac J Clin Oncol 2012; 8: 292-302. [DOI] [PubMed] [Google Scholar]
- 4).Inoue M, Ohta M, Iuchi K, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2004; 78: 238-44. [DOI] [PubMed] [Google Scholar]
- 5).Lin AY, Kotova S, Yanagawa J, et al. Risk stratification of patients undergoing pulmonary metastasectomy for soft tissue and bone sarcomas. J Thorac Cardiovasc Surg 2015; 149: 85-92. [DOI] [PubMed] [Google Scholar]
- 6).Liu D, Abolhoda A, Burt ME, et al. Pulmonary metastasectomy for testicular germ cell tumors: a 28-year experience. Ann Thorac Surg 1998; 66: 1709-14. [DOI] [PubMed] [Google Scholar]
- 7).Suzuki M, Iwata T, Ando S, et al. Predictors of long-term survival with pulmonary metastasectomy for osteosarcomas and soft tissue sarcomas. J Cardiovasc Surg 2006; 47: 603-8. [PubMed] [Google Scholar]
- 8).Cho JH, Kim S, Namgung M, et al. The prognostic importance of the number of metastases in pulmonary metastasectomy of colorectal cancer. World J Surg Oncol 2015; 13: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013; 20: 572-9. [DOI] [PubMed] [Google Scholar]
- 10).Hattori N, Kanemitsu Y, Komori K, et al. Outcomes after hepatic and pulmonary metastasectomies compared with pulmonary metastasectomy alone in patients with colorectal cancer metastasis to liver and lungs. World J Surg 2013; 37: 1315-21. [DOI] [PubMed] [Google Scholar]
- 11).Jarabo JR, Fernández E, Calatayud J, et al. More than one pulmonary resections or combined lung-liver resection in 79 patients with metastatic colorectal carcinoma. J Surg Oncol 2011; 104: 781-6. [DOI] [PubMed] [Google Scholar]
- 12).Park JS, Kim HK, Choi YS, et al. Outcomes after repeated resection for recurrent pulmonary metastases from colorectal cancer. Ann Oncol 2010; 21: 1285-9. [DOI] [PubMed] [Google Scholar]
- 13).Perera NK, Knight SR. Outcomes after pulmonary metastasectomy for colorectal cancer. ANZ J Surg 2014; 84: 556-9. [DOI] [PubMed] [Google Scholar]
- 14).Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg 2007; 84: 324-38. [DOI] [PubMed] [Google Scholar]
- 15).Rama N, Monteiro A, Bernardo JE, et al. Lung metastases from colorectal cancer: surgical resection and prognostic factors. Eur J Cardiothorac Surg 2009; 35: 444-9. [DOI] [PubMed] [Google Scholar]
- 16).Rodríguez-Fuster A, Belda-Sanchis J, Aguiló R, et al. Morbidity and mortality in a large series of surgical patients with pulmonary metastases of colorectal carcinoma: a prospective multicentre Spanish study (GECMP-CCR-SEPAR). Eur J Cardiothorac Surg 2014; 45: 671-6. [DOI] [PubMed] [Google Scholar]
- 17).Saito Y, Omiya H, Kohno K, et al. Pulmonary metastasectomy for 165 patients with colorectal carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg 2002; 124: 1007-13. [DOI] [PubMed] [Google Scholar]
- 18).Salah S, Watanabe K, Park JS, et al. Repeated resection of colorectal cancer pulmonary oligometastases: pooled analysis and prognostic assessment. Ann Surg Oncol 2013; 20: 1955-61. [DOI] [PubMed] [Google Scholar]
- 19).Salah S, Watanabe K, Welter S, et al. Colorectal cancer pulmonary oligometastases: pooled analysis and construction of a clinical lung metastasectomy prognostic model. Ann Oncol 2012; 23: 2649-55. [DOI] [PubMed] [Google Scholar]
- 20).Younes RN, Abrao F, Gross J. Pulmonary metastasectomy for colorectal cancer: long-term survival and prognostic factors. Int J Surg 2013; 11: 244-8. [DOI] [PubMed] [Google Scholar]
- 21).Casson AG, Putnam JB, Natarajan G, et al. Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 1992; 69: 662-8. [DOI] [PubMed] [Google Scholar]
- 22).Chua TC, Scolyer RA, Kennedy CW, et al. Surgical management of melanoma lung metastasis: an analysis of survival outcomes in 292 consecutive patients. Ann Surg Oncol 2012; 19: 1774-81. [DOI] [PubMed] [Google Scholar]
- 23).García Franco CE, Torre W, Tamura A, et al. Long-term results after resection for bone sarcoma pulmonary metastases. Eur J Cardiothorac Surg 2010; 37: 1205-8. [DOI] [PubMed] [Google Scholar]
- 24).Kim S, Ott HC, Wright CD, et al. Pulmonary resection of metastatic sarcoma: prognostic factors associated with improved outcomes. Ann Thorac Surg 2011; 92: 1780-6. [DOI] [PubMed] [Google Scholar]
- 25).Monteiro A, Arce N, Bernardo J, et al. Surgical resection of lung metastases from epithelial tumors. Ann Thorac Surg 2004; 77: 431-7. [DOI] [PubMed] [Google Scholar]
- 26).Sardenberg RA, Figueiredo LP, Haddad FJ, et al. Pulmonary metastasectomy from soft tissue sarcomas. Clinics (Sao Paulo) 2010; 65: 871-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Ann Intern Med 2009; 151: W-65-W-94. [DOI] [PubMed] [Google Scholar]
- 28).Weinlechner JW. Tumoren an der brustwand und deren behnadlung resection der rippeneroffnung der brusthohle und partielle entfernung der lunge. Wien Med Wochenschr 1882; 32: 589-91. [Google Scholar]
- 29).Divis G. Einbertrag zur operativen, Behandlung der lungengeschuuliste. Acta Chir Scand 1927; 62: 329-34. [Google Scholar]
- 30).Alexander J, Haight C. Pulmonary resection for solitary metastatic sarcomas and carcinomas. Surg Gynecol Obstet 1947; 85: 129-46. [PubMed] [Google Scholar]
- 31).Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg 1965; 49: 357-63. [PubMed] [Google Scholar]
- 32).McCormack PM, Martini N. The changing role of surgery for pulmonary metastases. Ann Thorac Surg 1979; 28: 139-45. [DOI] [PubMed] [Google Scholar]
- 33).Pastorino U. Lung metastasectomy: why, when, how. Crit Rev Oncol Hematol 1997; 26: 137-45. [DOI] [PubMed] [Google Scholar]
- 34).Young ER, Diakos E, Khalid-Raja M, et al. Resection of subsequent pulmonary metastases from treated head and neck squamous cell carcinoma: systematic review and meta-analysis. Clin Otolaryngol 2015; 40: 208-18. [DOI] [PubMed] [Google Scholar]
- 35).Anraku M, Yokoi K, Nakagawa K, et al. Pulmonary metastases from uterine malignancies: results of surgical resection in 133 patients. J Thorac Cardiovasc Surg 2004; 127: 1107-12. [DOI] [PubMed] [Google Scholar]
- 36).Landreneau RJ, De Giacomo T, Mack MJ, et al. Therapeutic video-assisted thoracoscopic surgical resection of colorectal pulmonary metastases. Eur J Cardiothorac Surg 2000; 18: 671-6. [DOI] [PubMed] [Google Scholar]
- 37).Rusch VW. Pulmonary metastasectomy. Current indications. Chest 1995; 107: 322S-31S. [DOI] [PubMed] [Google Scholar]
- 38).Pfannschmidt J, Klode J, Muley T, et al. Pulmonary metastasectomy in patients with soft tissue sarcomas: experiences in 50 patients. Thorac Cardiovasc Surg 2006; 54: 489-92. [DOI] [PubMed] [Google Scholar]
- 39).Iizasa T, Suzuki M, Yoshida S, et al. Prediction of prognosis and surgical indications for pulmonary metastasectomy from colorectal cancer. Ann Thorac Surg 2006; 82: 254-60. [DOI] [PubMed] [Google Scholar]
- 40).Casiraghi M, De Pas T, Maisonneuve P, et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the “international registry of lung metastases”. J Thorac Oncol 2011; 6: 1373-8. [DOI] [PubMed] [Google Scholar]
- 41).Kandioler D, Krömer E, Tüchler H, et al. Long-term results after repeated surgical removal of pulmonary metastases. Ann Thorac Surg 1998; 65: 909-12. [DOI] [PubMed] [Google Scholar]
- 42).Rena O, Papalia E, Oliaro A, et al. Pulmonary metastases from epithelial tumours: late results of surgical treatment. Eur J Cardiothorac Surg 2006; 30: 217-22. [DOI] [PubMed] [Google Scholar]
- 43).Robert JH, Ambrogi V, Mermillod B, et al. Factors influencing long-term survival after lung metastasectomy. Ann Thorac Surg 1997; 63: 777-84. [DOI] [PubMed] [Google Scholar]
- 44).Younes RN, Fares AL, Gross JL. Pulmonary metastasectomy: a multivariate analysis of 440 patients undergoing complete resection. Interact Cardiovasc Thorac Surg 2012; 14: 156-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Chen F, Hanaoka N, Sato K, et al. Prognostic factors of pulmonary metastasectomy for colorectal carcinomas. World J Surg 2009; 33: 505-11. [DOI] [PubMed] [Google Scholar]
- 46).Fiorentino F, Hunt I, Teoh K, et al. Pulmonary metastasectomy in colorectal cancer: a systematic review and quantitative synthesis. J R Soc Med 2010; 103: 60-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).McCormack PM, Burt ME, Bains MS, et al. Lung resection for colorectal metastases. 10-year results. Arch Surg 1992; 127: 1403-6. [DOI] [PubMed] [Google Scholar]
- 48).Suzuki H, Kiyoshima M, Kitahara M, et al. Long-term outcomes after surgical resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 2015; 99: 435-40. [DOI] [PubMed] [Google Scholar]
- 49).Blackmon SH, Shah N, Roth JA, et al. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long-term survival. Ann Thorac Surg 2009; 88: 877-84. [DOI] [PubMed] [Google Scholar]
- 50).Salah S, Fayoumi S, Alibraheem A, et al. The influence of pulmonary metastasectomy on survival in osteosarcoma and soft-tissue sarcomas: a retrospective analysis of survival outcomes, hospitalizations and requirements of home oxygen therapy. Interact Cardiovasc Thorac Surg 2013; 17: 296-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Smith R, Pak Y, Kraybill W, et al. Factors associated with actual long-term survival following soft tissue sarcoma pulmonary metastasectomy. European J Surg Oncol 2009; 35: 356-61. [DOI] [PubMed] [Google Scholar]
- 52).Treasure T, Fiorentino F, Scarci M, et al. Pulmonary metastasectomy for sarcoma: a systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012; 2: pii: e001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Dossett LA, Toloza EM, Fontaine J, et al. Outcomes and clinical predictors of improved survival in a patients undergoing pulmonary metastasectomy for sarcoma. J Surg Oncol 2015; 112: 103-6. [DOI] [PubMed] [Google Scholar]
- 54).Petersen RP, Hanish SI, Haney JC, et al. Improved survival with pulmonary metastasectomy: an analysis of 1720 patients with pulmonary metastatic melanoma. J Thorac Cardiovasc Surg 2007; 133: 104-10. [DOI] [PubMed] [Google Scholar]
- 55).Pfannschmidt J, Hoffmann H, Dienemann H. Thoracic metastasectomy for nonseminomatous germ cell tumors. J Thorac Oncol 2010; 5: S182-S6. [DOI] [PubMed] [Google Scholar]
- 56).Pfannschmidt J, Zabeck H, Muley T, et al. Pulmonary metastasectomy following chemotherapy in patients with testicular tumors: experience in 52 patients. Thorac Cardiovasc Surg 2006; 54: 484-8. [DOI] [PubMed] [Google Scholar]
- 57).Ripley RT, Downey RJ. Pulmonary metastasectomy. J Surg Oncol 2014; 109: 42-6. [DOI] [PubMed] [Google Scholar]
- 58).Shiono S, Kawamura M, Sato T, et al. Pulmonary metastasectomy for pulmonary metastases of head and neck squamous cell carcinomas. Ann Thorac Surg 2009; 88: 856-60. [DOI] [PubMed] [Google Scholar]
- 59).Friedel G, Pastorino U, Ginsberg RJ, et al. Results of lung metastasectomy from breast cancer: prognostic criteria on the basis of 467 cases of the International Registry of Lung Metastases. Eur J Cardiothorac Surg 2002; 22: 335-44. [DOI] [PubMed] [Google Scholar]
- 60).Meimarakis G, Rüttinger D, Stemmler J, et al. Prolonged overall survival after pulmonary metastasectomy in patients with breast cancer. Ann Thorac Surg 2013; 95: 1170-80. [DOI] [PubMed] [Google Scholar]
- 61).Yhim HY, Han SW, Oh DY, et al. Prognostic factors for recurrent breast cancer patients with an isolated, limited number of lung metastases and implications for pulmonary metastasectomy. Cancer 2010; 116: 2890-901. [DOI] [PubMed] [Google Scholar]
- 62).Murthy SC, Kim K, Rice TW, et al. Can we predict long-term survival after pulmonary metastasectomy for renal cell carcinoma? Ann Thorac Surg 2005; 79: 996-1003. [DOI] [PubMed] [Google Scholar]
- 63).Field K, Lipton L. Metastatic colorectal cancer-past, progress and future. World J Gastroenterol 2007; 13: 3806-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Goldberg RM, Rothenberg ML, Van Cutsem E, et al. The continuum of care: a paradigm for the management of metastatic colorectal cancer. The Oncol 2007; 12: 38-50. [DOI] [PubMed] [Google Scholar]
- 65).Zacharakis M, Xynos ID, Lazaris A, et al. Predictors of survival in stage IV metastatic colorectal cancer. Anticancer Res 2010; 30: 653-60. [PubMed] [Google Scholar]
- 66).Scheithauer W, Rosen H, Kornek GV, et al. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 1993; 306: 752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Clavel M, Vermorken JB, Cognetti F, et al. Randomized comparison of cisplatin, methotrexate, bleomycin and vincristine (CABO) versus cisplatin and 5-fluorouracil (CF) versus cisplatin (C) in recurrent or metastatic squamous cell carcinoma of the head and neckA phase III study of the EORTC Head and Neck Cancer Cooperative Group. Ann Oncol 1994; 5: 521-6. [DOI] [PubMed] [Google Scholar]
- 68).Hong WK, Schaefer S, Issell B, et al. A prospective randomized trial of methotrexate versus cisplatin in the treatment of recurrent squamous cell carcinoma of the head and neck. Cancer 1983; 52: 206-10. [DOI] [PubMed] [Google Scholar]
- 69).Cheng YC, Ueno NT. Improvement of survival and prospect of cure in patients with metastatic breast cancer. Breast Cancer 2012; 19: 191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Falkson G, Gelman RS, Leone L, et al. Survival of premenopausal women with metastatic breast cancer. Long-term follow-up of Eastern Cooperative Group and Cancer and Leukemia Group B studies. Cancer 1990; 66: 1621-9. [DOI] [PubMed] [Google Scholar]
- 71).Greenberg PA, Hortobagyi GN, Smith TL, et al. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol 1996; 14: 2197-205. [DOI] [PubMed] [Google Scholar]
- 72).Parks RM, Cheung KL. Patient pathway for breast cancer: turning points and future aspirations. Future Oncol 2015; 11: 1059-70. [DOI] [PubMed] [Google Scholar]
- 73).Downey RJ, Bains MS. Open surgical approaches for pulmonary metastasectomy. Thorac Surg Clin 2016; 26: 13-8. [DOI] [PubMed] [Google Scholar]
- 74).McCormack PM, Bains MS, Begg CB, et al. Role of video-assisted thoracic surgery in the treatment of pulmonary metastases: results of a prospective trial. Ann Thorac Surg 1996; 62: 213-6. [DOI] [PubMed] [Google Scholar]
- 75).Internullo E, Cassivi SD, Van Raemdonck D, et al. Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 2008; 3: 1257-66. [DOI] [PubMed] [Google Scholar]
- 76).Paiva JM, Wright GM. Hand-assisted thoracoscopic surgery causes less postoperative pain than limited thoracotomy after cessation of epidural analgesia. Heart Lung Circ 2004; 13: 374-8. [DOI] [PubMed] [Google Scholar]
- 77).Aberg T. Selection mechanisms as major determinants of survival after pulmonary metastasectomy. Ann Thorac Surg 1997; 63: 611-2. [DOI] [PubMed] [Google Scholar]
- 78).André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343-51. [DOI] [PubMed] [Google Scholar]
- 79).Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335-42. [DOI] [PubMed] [Google Scholar]
- 80).Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007; 357: 2040-8. [DOI] [PubMed] [Google Scholar]
- 81).Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010; 28: 4697-705. [DOI] [PubMed] [Google Scholar]
- 82).Van der Jeught K, Xu HC, Li YJ, et al. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol 2018; 24: 3834-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000; 355: 1041-7. [DOI] [PubMed] [Google Scholar]
- 84).Treasure T. Surgery and ablative techniques for lung metastases in the Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) trial: is there equivalence? J Thorac Dis 2016; 8: S649-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).Lyons NJ, Pathak S, Daniels IR, et al. Percutaneous management of pulmonary metastases arising from colorectal cancer; a systematic review. Eur J Surg Oncol 2015; 41: 1447-55. [DOI] [PubMed] [Google Scholar]
- 86).Schlijper RC, Grutters JP, Houben R, et al. What to choose as radical local treatment for lung metastases from colo-rectal cancer: surgery or radiofrequency ablation? Cancer Treat Rev 2014; 40: 60-7. [DOI] [PubMed] [Google Scholar]
- 87).de Baère T, Aupérin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015; 26: 987-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).de Baere T, Tselikas L, Woodrum D, et al. Evaluating cryoablation of metastatic lung tumors in patients–safety and efficacy: the ECLIPSE trial–interim analysis at 1 year. J Thorac Oncol 2015; 10: 1468-74. [DOI] [PubMed] [Google Scholar]
- 89).Jingu K, Matsushita H, Yamamoto T, et al. Stereotactic radiotherapy for pulmonary oligometastases from colorectal cancer: a systematic review and meta-analysis. Technol Cancer Res Treat 2018; 17: 1533033818794936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90).Lee YH, Kang KM, Choi HS, et al. Comparison of stereotactic body radiotherapy versus metastasectomy outcomes in patients with pulmonary metastases. Thorac Cancer 2018; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91).Downey RJ. Surgery for colorectal and sarcomatous pulmonary metastases: history, current management, and future directions. Thorac Surg Clin 2006; 16: 133-7, v-vi. [DOI] [PubMed] [Google Scholar]
- 92).Rusch VW. Pulmonary metastasectomy: a moving target. J Thorac Oncol 2010; 5: S130-1. [DOI] [PubMed] [Google Scholar]