Key Points
Question
Do multiple exposures to general anesthesia in children have a negative association with neurocognitive function and brain imaging?
Findings
In this cohort study of 212 eligible survivors of childhood acute lymphoblastic leukemia treated at a single institution between 2000 and 2010 who underwent 5699 exposures to general anesthesia, higher cumulative doses of fluranes and propofol and longer anesthesia duration significantly contributed to neurocognitive impairment and neuroimaging abnormalities at a median of 7.52 years since diagnosis, beyond the known results of neurotoxic chemotherapies.
Meaning
In pediatric populations with chronic health conditions who undergo multiple anesthesia exposures, limiting exposure to general anesthesia may be warranted.
Abstract
Importance
Limited studies have reported associations between anesthesia and neurocognitive and neuroimaging outcomes, particularly in pediatric patients who undergo multiple exposures to anesthesia as part of chronic disease management.
Objective
To investigate whether general anesthesia is associated with neurocognitive impairment and neuroimaging abnormalities in long-term survivors of childhood acute lymphoblastic leukemia.
Design, Setting, and Participants
A cohort study of 212 survivors of childhood acute lymphoblastic leukemia who received treatment between July 7, 2000, and November 3, 2010, and follow-up at a mean (SD) of 7.7 (1.7) years post diagnosis, was conducted at an academic medical center. Of 301 survivors who were alive and eligible for participation, 217 individuals (72.1%) agreed to participate in long-term follow-up. Data analysis was performed from August 23, 2017, to May 3, 2018.
Exposures
For 5699 anesthesia procedures, data on duration and cumulative doses of all anesthetics, sedatives, analgesics, anxiolytics, and neuromuscular blockers were abstracted, along with cumulative doses of high-dose intravenous methotrexate and number of triple intrathecal chemotherapy treatments.
Main Outcomes and Measures
Neurocognitive measures of attention, processing speed, executive function, and intelligence were examined. Brain volumes, cortical thickness, and diffusion tensor imaging of the whole brain, corpus callosum, frontal lobes, and parietal lobes were evaluated.
Results
Of the 217 study participants, 212 were included in both neurocognitive and brain imaging analysis. Of these, 105 were female (49.5%); mean (SD) age at diagnosis was 14.36 (4.79) years; time since diagnosis was 7.7 (1.7) years. Adjusting for chemotherapy doses and age at diagnosis, neurocognitive impairment was associated with higher propofol cumulative dose (relative risk [RR], 1.40 per 100 mg/kg; 95% CI, 1.11-1.75), flurane exposure (RR, 1.10 per exposure; 95% CI, 1.01-1.21), and longer anesthesia duration (RR, 1.03 per cumulative hour; 95% CI, 1.00-1.06). Slower processing speed was associated with higher propofol dose (estimate [est], −0.30; P = .04), greater number of exposures to fluranes (est, −0.14; P = .01), and longer anesthesia duration (est, −0.04; P = .003). Higher corpus callosum white matter diffusivity was associated with dose of propofol (est, 2.55; P = .01) and duration of anesthesia (est, 2.40; P = .02). Processing speed was significantly correlated with corpus callosum diffusivity (r = −0.26, P < .001).
Conclusions and Relevance
Higher cumulative anesthesia exposure and duration may be associated with neurocognitive impairment and neuroimaging abnormalities in long-term survivors of childhood acute lymphoblastic leukemia, beyond the known outcomes associated with neurotoxic chemotherapies. Anesthesia exposures should be limited in pediatric populations with chronic health conditions who undergo multiple medical procedures.
This cohort study examines the neurocognitive and neuroimaging outcomes associated with multiple use of general anesthesia in survivors of childhood acute lymphoblastic leukemia.
Introduction
Long-term survivors of childhood acute lymphoblastic leukemia (ALL) treated with chemotherapy are at risk for neurocognitive impairment, with frequency of impairment as high as 40%.1,2 However, impairment is not fully accounted for by chemotherapy agents, young age at diagnosis, or female sex, which are commonly identified risk factors.3 An additional factor for neurocognitive impairment in survivors is repeated exposure to general anesthesia, which has recently been identified by the US Food and Drug Administration as having a negative association with brain development in young children.4
In noncancer populations, findings on neurocognitive effects and general anesthesia are mixed, with some studies showing no difference after exposure5,6,7,8,9 and others demonstrating increased risk of long-term learning disabilities, academic problems, or neurocognitive problems.10,11,12,13 However, most of these studies have limitations, such as inclusion of children who had received only 1 exposure to anesthesia, examination of limited cognitive outcomes, lack of investigation of specific anesthetic agents, and limited information on anesthesia doses or duration. Addressing these limitations may help in understanding of general anesthesia and brain function.
To our knowledge, no published study has examined the association between anesthesia frequency and dose of specific agents on brain integrity in survivors of childhood cancer. Given the effects of neurotoxic chemotherapies on brain function in survivors of childhood cancer, the additional damage from general anesthesia may be particularly problematic.
The aims of the present study were to examine associations between cumulative dose and exposure duration of general anesthesia and neurocognitive and neuroimaging outcomes in long-term survivors of childhood ALL treated with chemotherapy only, adjusting for the known risk factors of chemotherapy dose, young age at diagnosis, and female sex. It was hypothesized that increased exposure to general anesthesia would have negative associations with neurocognitive and brain imaging outcomes, beyond the outcomes associated with chemotherapy and demographics.
Methods
Study Population
Eligible survivors were identified from the 408 children with ALL who were treated within the Total Therapy XV protocol, with inclusion and exclusion criteria previously reported,14,15 at St Jude Children’s Research Hospital between July 7, 2000, and November 3, 2010. Data analysis was performed from August 23, 2017, to May 3, 2018. Eligibility criteria for the present long-term follow-up study included receiving treatment at St Jude Children’s Research Hospital, being more than 5 years post diagnosis, and 8 years or older at the time of evaluation. Survivors were excluded from the study (n = 54) if they had a history of a noncancer-related neurodevelopmental or genetic disorder associated with neurocognitive impairment, had a subsequent brain injury unrelated to cancer, experienced a relapse or secondary cancer that required additional chemotherapy, received cranial radiotherapy for central nervous system relapse or bone marrow transplantation, or were not proficient in English.
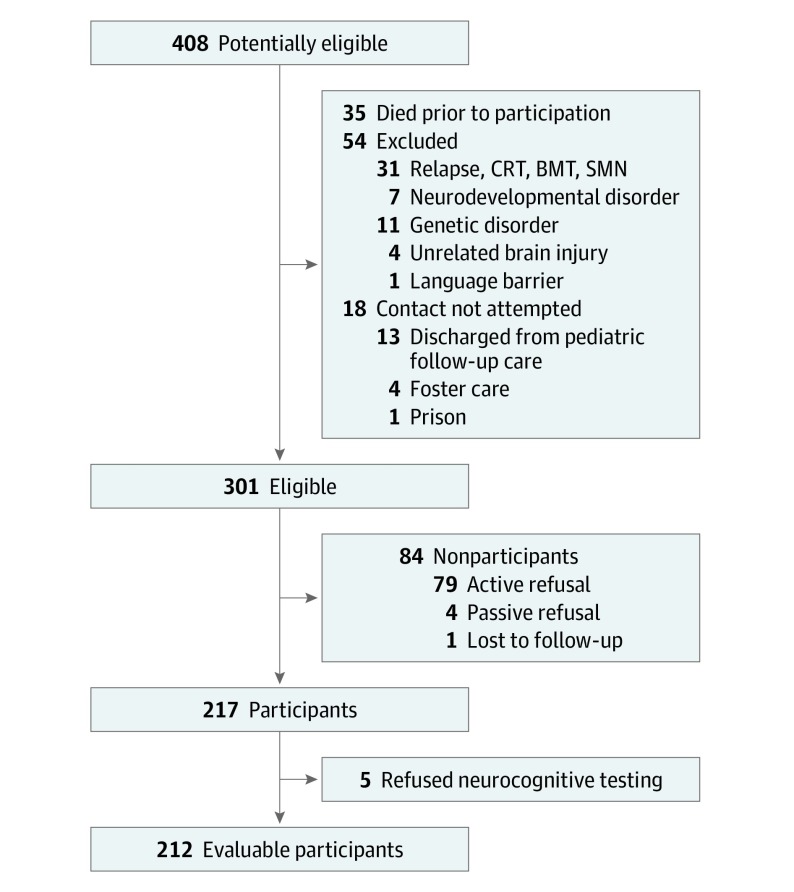
Survivors were actively recruited from the complete list of patients treated within the Total Therapy XV protocol through mailings, telephone calls, and clinical visits in an attempt to evaluate all eligible survivors. Recruitment for the study was not attempted for survivors (n = 18) who were discharged from pediatric follow-up care or were in foster care or prison. Of the 301 survivors who were alive and eligible for the study, 217 individuals (72.1%) participated in long-term follow-up; however, an additional 5 survivors declined participation in the neurocognitive assessment, resulting in 212 evaluable participants (70.4%) for the present analysis (Figure). Approval from the institutional review board at St Jude Children’s Research Hospital was obtained for the study protocol, and informed assent/consent was obtained from all participants and/or parents, as appropriate. Participants received financial compensation.
Figure. Participant Enrollment Flowchart.
BMT indicates bone marrow transplant; CRT, chemoradiotherapy; and SMT, smooth muscle tumors.
Procedure
Medical record abstraction was performed to capture data from all procedures requiring anesthesia between diagnosis and the follow-up neurocognitive assessment to account for all potential associations between anesthesia exposure and neurocognitive performance, regardless of whether the exposure occurred during or after cancer therapy. For each procedure in each participant, cumulative doses of all agents administered by an anesthetist/anesthesiologist during the procedure, type of procedure, anesthesia duration, participant’s weight, and respiratory or cardiac complications from anesthesia were recorded. The examined agents included anesthetics, sedatives, analgesics, anxiolytics, and neuromuscular blockers, hereafter referred to as anesthetic agents. Cumulative doses of neurotoxic chemotherapies as defined by the Children’s Oncology Group Long-term Follow-up Guidelines16 were also abstracted: high-dose intravenous methotrexate (with dose adjusted for body surface area) and number of triple intrathecal treatments (methotrexate, hydrocortisone, and cytarabine). Doses of anesthetic agents that were administered were adjusted for actual body weight.
Neurocognitive testing was administered by certified examiners under the supervision of a board-certified clinical neuropsychologist (K.R.K.). Testing followed standard clinical guidelines in a dedicated evaluation room. Order of testing was standardized, and participants’ schedules were controlled to reduce fatigue and other extraneous factors. Assessed neurocognitive domains included attention,17,18,19,20 processing speed,18,19,21,22 executive function,17,21,23 and intelligence.24
Structural magnetic resonance imaging (MRI) was conducted on a 3T platform (Siemens Trio; Siemens) and included T1-weighted imaging with a sagittal 3-dimension, magnetization-prepared, rapid gradient echo sequence (repetition time [TR], 1560 milliseconds; echo time [TE], 2.75; TI, 900 milliseconds; imaging resolution, 0.98 × 0.98 × 1.1 mm). FreeSurfer software was used to process the T1-weighted MRI images to assess brain volumes and cortical thickness.25 The MRI examination included diffusion tensor imaging using a double-spin, echoplanar imaging pulse sequence (TR, 10 000 milliseconds; TE, 100 milliseconds; b, 700 seconds/mm2; 3 × 1.8 × 1.8 mm; acquisition time, approximately 1.5 minutes each) with 4 acquisitions and 12 gradient directions to examine white matter microstructure in the whole brain, corpus callosum, frontal lobes, and parietal lobes. The MRI examination was performed within 24 hours of the neurocognitive testing. No sedation was used during the MRI examination.
Statistical Analysis
Descriptive measures were obtained for demographic and clinical variables. For the anesthesia variables, total number of procedures requiring anesthesia, cumulative weight-adjusted doses of each anesthetic agent, and cumulative anesthesia duration were calculated for each participant.
Neurocognitive performance for each measure was scored using age-based, national normative data, and then transformed into age-adjusted Z scores. These scores were then compared with the normative population (μ = 0, σ = 1). Survivors were classified as having global neurocognitive impairment if they performed 2 or more SDs below the age-adjusted national normative data on 3 or more neurocognitive measures. In the present neurocognitive battery containing 42 measures, there is an approximate 5% likelihood of being classified as having global neurocognitive impairment in the general population using this criterion.26 Multivariable log-binomial models were constructed to test the association of cumulative dose of each anesthetic agent with global neurocognitive impairment, with a modified Poisson approach27 used to generate quasi-likelihood estimators for the relative risk (RR) and 95% CIs by using robust error variances (GENMOD procedure, with Poisson distribution and log link), with adjustment for age at diagnosis, sex, total intrathecal count, and cumulative dose of high-dose intravenous methotrexate (model details in eMethods 1 in the Supplement). Only anesthetic agents that were administered to at least 30% of the survivors were included in the analysis.
One-sample t tests were applied to compare mean survivor performance on each test-specific neurocognitive measure with population norms, with P value correction for multiple comparisons using false discovery rate.28 Measures that were worse than population norms based on a significance level of .05 were examined in models with test-specific neurocognitive performance. All participants who completed either brain imaging or neurocognitive testing at long-term follow-up were included in the respective analyses. For the anesthetic agents that were significantly associated with global neurocognitive impairment, multivariable general linear models were applied to examine the association between cumulative dose of each anesthetic agent with test-specific neurocognitive performance and neuroimaging outcomes, with adjustment for age at diagnosis, age at evaluation for neuroimaging outcomes, sex, total intrathecal chemotherapy count, and cumulative high-dose intravenous methotrexate dose (eMethods 2 in the Supplement). Pearson product moment coefficients were calculated between the significant test-specific neurocognitive variables and significant neuroimaging variables. All analyses were conducted in SAS, version 9.4 (SAS Institute Inc).
Results
The demographic, clinical, and treatment characteristics for the survivors are presented in Table 1. Mean (SD) age at diagnosis was 14.4 (4.8) years and time since diagnosis was 7.7 (1.7) years, with a relatively equal proportion of males and females. For the 212 survivors, data were abstracted for 5699 exposures to anesthesia, occurring from diagnosis to date of long-term follow-up, of which 5395 exposures (94.7%) occurred during therapy. The mean (SD) number of anesthesia exposures was 26.9 (6.3) (range, 12-48), and mean (SD) cumulative duration of anesthesia exposure was 15.6 (5.7) hours (range, 4.3-40.9). Table 2 presents the frequency of participants receiving each anesthetic agent and the cumulative doses of each anesthetic agent. Fluranes (sevoflurane, isoflurane, desflurane, and enflurane) were reported in number of exposures rather than cumulative dose because the cumulative dose could not be accurately captured for these inhaled anesthetic agents. Propofol and fentanyl were the most commonly administered agents, with every survivor receiving them at least once. However, cumulative doses of propofol and fentanyl were not highly correlated (ρ = 0.19). Survivors completed multiple medical procedures that required anesthesia, with lumbar punctures and bone marrow aspirations being the most common (eFigure in the Supplement).
Table 1. Survivor Demographics and Treatment Characteristics.
| Characteristic | Value |
|---|---|
| Sex, No. (%) | |
| Male | 107 (50.5) |
| Female | 105 (49.5) |
| Age at evaluation, y | |
| Mean (SD) | 14.36 (4.79) |
| Median (range) | 13.23 (8.05-26.49) |
| Age at diagnosis, y | |
| Mean (SD) | 6.66 (4.54) |
| Median (range) | 5.04 (1.02-18.45) |
| Time since diagnosis, y | |
| Mean (SD) | 7.70 (1.70) |
| Median (range) | 7.52 (5.06-12.54) |
| Race/ethnicity, No. (%) | |
| Non-Hispanic white | 156 (73.58) |
| Other | 56 (26.42) |
| Highest education, y | |
| Mean (SD) | 7.50 (3.96) |
| Median (range) | 7.00 (2.00-17.00) |
| Highest parental education, y | |
| Mean (SD) | 14.58 (2.80) |
| Median (range) | 14.00 (6.00-22.00) |
| Treatment risk stratum, No (%)a | |
| Low | 121 (57.08) |
| Standard/high | 91 (42.92) |
| CNS involvement, No. (%) | |
| No blasts in CSF | 172 (81.13) |
| <5% blasts in CSF | 31 (14.62) |
| ≥5% blasts in CSF | 1 (0.47) |
| Traumatic LP with blasts | 8 (3.77) |
| High-dose IV methotrexate, g/m2 b | |
| Mean (SD) | 15.33 (5.00) |
| Median (range) | 14.30 (6.07-38.97) |
| TIT chemotherapy, mL | |
| Mean (SD) | 203.42 (85.84) |
| Median (range) | 190.48 (17.19-492.86) |
| TIT chemotherapy (No. of counts) | |
| Mean (SD) | 14.61 (4.05) |
| Median (range) | 13.00 (9.00-24.00) |
Abbreviations: CNS, central nervous system; CSF, cerebrospinal fluid; IV, intravenous; LP, lumbar puncture; TIT, triple intrathecal.
Criteria for low-risk acute lymphocytic leukemia (ALL): B-cell precursor ALL with DNA index 1.16 or greater, TEL-AML1 fusion, or age 1 to 9.9 years and white blood cell count of less than 50 × 109 cells per liter (to convert to cell count per microliter, multiply by 0.001). Must not have (1) CNS 3 status (≥5 white blood cells per liter of CSF with morphologically identifiable blasts or cranial nerve palsy), (2) overt testicular leukemia, (3) adverse genetic features (t(9;22) or BCR-ABL fusion, t(1;19) with E2A-PBX1 fusion, rearranged MLL, or hypodiploidy [<45 chromosomes]), or (4) poor early response (≥1% lymphoblasts on day 19 or 26 of remission induction, 0.01% or more lymphoblasts by immunologic or molecular methods on remission date). Criteria for standard-risk ALL: All cases of T-cell ALL and those of B-cell precursor ALL that do not meet the criteria for low-risk or high-risk ALL. Criteria for high-risk ALL: t(9;22) or BCR-ABL fusion; induction failure or more than 1% leukemic lymphoblasts in the bone marrow on remission date, more than 0.1% leukemic lymphoblasts in the bone marrow in week 7 of continuation treatment (ie, before reinduction, approximately 14 weeks post remission induction), re-emergence of leukemic lymphoblasts by minimal residual disease (MRD) (at any level) in patients previously MRD negative, and persistently detectable MRD at lower levels.
Cumulative doses are listed for IV methotrexate and TIT (methotrexate, hydrocortisone, and cytarabine). High-dose IV methotrexate was calculated separately. High-dose IV methotrexate was defined as daily dose of 1 gram per square meter of body surface area of IV methotrexate.
Table 2. Characteristics of Anesthetic Agents.
| Anesthetic Agent | Participants Receiving Agent, No. (%) | Cumulative Dose per Participant | |
|---|---|---|---|
| Mean (SD)a | Median (range) | ||
| Propofol, mg/kg | 212 (100) | 148.39 (70.27) | 132.35 (40.48-372.98) |
| Fentanyl, μg/kg | 212 (100) | 17.46 (7.67) | 16.41 (4.81-54.39) |
| Fluranes (exposures)b | 203 (95.8) | 2.37 (1.48) | 2.00 (0-10.00) |
| Glycopyrrolate, μg/kg | 147 (69.3) | 19.58 (23.07) | 5.84 (0-166.15) |
| Pentobarbital sodium, mg/kg | 132 (62.3) | 20.05 (12.77) | 5.89 (0-65.30) |
| Meperidine hydrochloride, mg/kg | 125 (59.0) | 4.15 (2.96) | 1.08 (0-11.69) |
| Midazolam hydrochloride, mg/kg | 122 (57.6) | 0.18 (0.19) | 0.04 (0-0.89) |
| Morphine sulfate, mg/kg | 66 (31.1) | 0.22 (0.29) | 0 (0-1.61) |
| Rocuronium bromide, mg/kg | 47 (22.2) | 1.07 (1.08) | 0 (0-5.00) |
| Diazepam, mg/kg | 33 (15.6) | 0.50 (0.50) | 0 (0-2.47) |
| Neostigmine methylsulfate, mg/kg | 29 (13.7) | 0.06 (0.03) | 0 (0-0.14) |
| Ketamine hydrochloride, mg/kg | 11 (5.2) | 2.92 (2.82) | 0 (0-8.54) |
| Vecuronium bromide, mg/kg | 8 (3.8) | 0.14 (0.09) | 0 (0-0.31) |
| Atropine, mg/kg | 8 (3.8) | 0.01 (0.00) | 0 (0-0.02) |
| Cisatracurium besylate, mg/kg | 7 (3.3) | 0.19 (0.09) | 0 (0-0.33) |
| Ephedrine hydrochloride, mg/kg | 5 (2.4) | 0.39 (0.12) | 0 (0-0.49) |
| Succinylcholine chloride, mg/kg | 3 (1.4) | 1.22 (0.13) | 0 (0-1.37) |
Mean (SD) cumulative dose is based only on the individuals who received the agent.
Number of exposures was identified for fluranes because cumulative dose cannot be accurately captured for inhaled anesthetic agents.
Of the 212 survivors, 91 individuals (42.9%) were classified as having general neurocognitive impairment. In multivariable models examining the associations of anesthesia doses and duration with global neurocognitive impairment (Table 3), survivors had an increased risk of global neurocognitive impairment after exposure to propofol (RR, 1.40 per 100 mg/kg; 95% CI, 1.11-1.75; P = .004; 64% increased risk for mean exposure of 148.39 mg/kg) and fluranes (RR, 1.10 per exposure; 95% CI, 1.01-1.21; P = .03; 26% increased risk for mean of 2.37 exposures), and with longer cumulative anesthesia duration (RR, 1.03 per hour; 95% CI; 1.00-1.06; P = .04; 55% increased risk for mean duration of 15.6 hours). Univariate analyses can be found in eTable 1 in the Supplement.
Table 3. Multivariable Regression Models Predicting Global Neurocognitive Impairmenta.
| Parameters | Global Neurocognitive Impairmentb | |
|---|---|---|
| RR (95% CI) | P Value | |
| Propofol, per 100 mg/kg | 1.40 (1.11-1.75) | .004 |
| Fentanyl, per 10 μg/kg | 1.20 (0.99-1.45) | .07 |
| Midazolam hydrochloride, per 1 mg/kg | 1.07 (0.42-2.73) | .89 |
| Meperidine hydrochloride, per 1 mg/kg | 0.98 (0.93-1.04) | .47 |
| Morphine sulfate, per 1 mg/kg | 0.70 (0.28-1.70) | .43 |
| Pentobarbital sodium, per 10 mg/kg | 1.02 (0.90-1.15) | .74 |
| Glycopyrrolate, per 1 μg/kg | 1.00 (1.00-1.01) | .14 |
| Fluranes (per exposure)c | 1.10 (1.01-1.21) | .03 |
| Duration (per h) | 1.03 (1.00-1.06) | .04 |
Abbreviation: RR, relative risk.
Models are adjusted for age at diagnosis, sex, total intrathecal chemotherapy count, and cumulative dose of high-dose intravenous methotrexate.
Global neurocognitive impairment is defined as 3 or more tests falling more than 2 standard deviations below the age-adjusted population normative means.
Number of exposures was identified for fluranes because cumulative dose cannot be accurately captured for inhaled anesthetic agents.
Associations between specific neurocognitive tests and cumulative dose of propofol, number of exposures to fluranes, and cumulative anesthesia duration were examined with multivariable general linear models, adjusting for age at diagnosis, sex, use of high-dose intravenous methotrexate, and intrathecal count. Only the 19 neurocognitive measures for which survivors differed from population norms after false-discovery rate correction were examined; data on comparison with population norms have been published.2 Slower processing speed was noted with higher propofol dose (letter sequencing: estimate [est], −0.30; P = .04), higher number of exposures to fluranes (color naming: est, −0.09; P = .03; digit symbol: est, −0.08; P = .04; letter sequencing: est, −0.14; P = .01), and longer anesthesia duration (digit symbol: est, −0.03; P = .03; letter sequencing: est, −0.04; P = .003) (eTable 2 in the Supplement). Univariate analyses can be found in eTable 3 in the Supplement. Higher propofol dose was associated with worse attention (omissions: est, −0.46; P = .02; spatial span: est, −0.24; P = .04). A greater number of exposures to fluranes was associated with worse executive function (Rey complex figure: est, −0.25; P = .03).
For the neuroimaging outcomes, higher white matter mean diffusivity in the corpus callosum (mean [SD], 0.91 [0.05]) was associated with the dose of propofol (est, 2.55; P = .01) and duration of anesthesia (est, 2.40; P = .02) (eTable 4 in the Supplement). No other significant associations were found between the anesthesia and neuroimaging variables. Correlations between the significant test-specific neurocognitive variables and significant neuroimaging variables demonstrated associations between the corpus callosum mean diffusivity and Omissions (r = −0.19; P = .02), Letter Sequencing (r = −0.22; P = .005), and Digit Symbol (r = −0.26; P < .001).
Discussion
This study provides novel data on associations between general anesthesia and neurocognitive and neuroimaging outcomes in long-term survivors of childhood ALL treated within a chemotherapy-only protocol. The frequency and dose-specific outcomes of anesthesia exposure on brain integrity have not, to our knowledge, been previously examined in long-term survivors of childhood cancer. Overall, our study revealed several findings: (1) long-term survivors of childhood ALL receive significant, repeated exposure to general anesthesia during and after therapy; (2) after adjusting for chemotherapy exposure and demographics, greater exposure to propofol and fluranes and longer cumulative anesthesia duration were associated with increased risk of general neurocognitive impairment and dysfunction on tests of processing speed and attention; and (3) greater exposure to propofol and longer cumulative anesthesia duration were associated with worse white matter integrity in the corpus callosum, which was associated with the observed neurocognitive impairment.
Long-term survivors of childhood ALL experienced considerable anesthesia exposure during treatment for leukemia, with the survivors in our sample undergoing a mean of 15.6 hours of general anesthesia across a mean of 26.9 different exposures. The RR of general neurocognitive impairment for the average survivor resulted in a 64% increased risk following propofol exposure (mean cumulative dose, 148.39 mg/kg), 26% increased risk following exposures to fluranes (mean number, 2.37), and 55% increased risk associated with cumulative anesthesia duration (mean, 15.6 hours). For survivors receiving the maximum amount of each key exposure in our study, risk was increased by 247% with propofol, 164% with fluranes, and 214% with anesthesia duration. For our current leukemia protocol (ie, Total Therapy XVII; ClinicalTrials.gov identifier NCT03117751), we have eliminated purely research procedures that require anesthesia and have reduced duration of medically necessary events that require anesthesia. Based on the planned protocol procedures, these changes should result in a reduction of 25% in the propofol dose and 47% in the number of flurane exposures and total anesthesia duration. Assuming equivalent effect sizes, these reductions should virtually eliminate excess risk for neurocognitive impairment after adjusting for neurotoxic chemotherapy.
Higher propofol doses and longer anesthesia duration were associated with worse white matter integrity in the corpus callosum, as well as worse performance on measures of attention and processing speed. Corpus callosum mean (SD) diffusivity in survivors (0.91 [0.05]) was more than 1 SD above that of the corpus callosum in typically developing adolescents (0.75 [0.15]).29 This difference suggests that anesthetic agents such as propofol may affect interhemispheric white matter connectivity and disrupt efficient communication between neurons, resulting in processing speed and attention impairments.
Studies in nonhuman primates suggest that exposure to propofol and fluranes increases apoptosis by inducing caspase-3 activation. Propofol30 and fluranes31 were found to induce apoptosis of both white and gray matter, with the most vulnerable cells being oligodendrocytes engaged in myelinogenesis. In our study, the observed processing speed impairments and associated higher white matter mean diffusivity in the corpus callosum suggest a pattern of myelin dysfunction in survivors who received higher doses and underwent longer durations of anesthesia. In addition, caspase-3 activation was associated with increases in amyloid-β protein and hyperphosphorylated tau levels in the brain, which have been observed in neurodegenerative disorders such as Alzheimer disease.32 An association between elevated total tau levels in the cerebrospinal fluid during chemotherapy and difficulties with attention, processing speed, and white matter integrity in ALL survivors.33
A second potential mechanism underlying the processing speed and attention impairments in long-term survivors is neuroinflammation, as repeated exposure to propofol34 and fluranes35 has been reported to increase the expression of the proinflammatory cytokines tumor necrosis factor, IL-1, and IL-6 in animal models. Oligodendrocytes, the cellular component of myelin, have been shown to be particularly sensitive to neurotoxic effects of increased inflammation and stress responses.36 In this survivor sample, an association between elevated homocysteine during therapy and cognitive dysfunction at long-term follow-up has previously been identified.2 Administration of the chemotherapeutic agent methotrexate is associated with elevated homocysteine levels,37 a biomarker of acute inflammation with demonstrated vascular toxicity and neurotoxicity. Survivors are therefore already at risk for elevated levels of inflammation and resulting cognitive dysfunction during therapy due to methotrexate exposure. High exposure to propofol and fluranes combined with elevated inflammation and neurotoxic effects from methotrexate administration may compound the negative association with survivors’ neurocognition.
Strengths and Limitations
Strengths of this study include the use of a population with repeated exposures to anesthesia, examination of several cognitive domains using a comprehensive test battery, examination of the results occurring with commonly used agents administered during general anesthesia, and examination of the dose-specific effects of each agent. The findings of our study should be interpreted in the context of its limitations as well. Survivors’ neurocognitive function was compared with nationally standardized, age-adjusted population norms rather than a control group; however, adjustment for chemotherapy exposure would not have been possible with a control group and it was not feasible to recruit a control group that underwent similarly extensive exposure to anesthesia. No neurocognitive data were obtained at baseline before anesthesia exposure owing to treatment beginning immediately after diagnosis and because limited cognitive tests are available for very young children at the peak age of diagnosis for ALL. Also, because white matter develops differentially throughout the brain, the findings in the present study may differ from investigations of other populations in which anesthesia exposure occurred at a different age than that of our sample. Nonetheless, this study provides novel data on the frequency and dose-specific associations between anesthesia exposure and cognitive function and brain integrity in long-term survivors of childhood cancer.
Conclusions
The findings from our study suggest that long-term survivors of childhood ALL experience significant neurocognitive impairment and abnormal white matter integrity related to exposure to propofol, fluranes, and longer anesthesia duration. Propofol and fluranes are common anesthetic agents used across populations, and the results of the present study are applicable to other pediatric populations with chronic health conditions that require multiple procedures conducted under general anesthesia. Clinically, efforts should be made to limit the use of general anesthesia, particularly propofol and fluranes, when feasible alternatives exist for providing sufficient pain control and sedation. Options include limiting use of general anesthesia in this population unless clinically necessary and using psychological techniques, such as cognitive-behavioral intervention38 and distraction,39,40 to reduce the need for general anesthesia during diagnostic procedures such as lumbar punctures and MRI scans. Such efforts may improve long-term outcomes in children with chronic health conditions. Future studies should use a prospective research design and additional pediatric samples with chronic health conditions to further evaluate neurocognitive and neuroimaging outcomes of anesthesia exposure.
eMethods 1. Modified Poisson Approach
eMethods 2. Multivariable General Limited Model Approach
eFigure. Mean Frequency and Mean Cumulative Duration of Procedures Requiring Anesthesia per Participant
eTable 1. Univariate Models Predicting Global Neurocognitive Impairment
eTable 2. Multivariable Models Predicting Test-Specific Neurocognitive Performance
eTable 3. Univariate Regression Models Predicting Test-Specific Neurocognitive Performance
eTable 4. Multivariable Regression Models Predicting Neuroimaging Outcomes
References
- 1.Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude Lifetime Cohort study. J Clin Oncol. 2013;31(35):1456-1463. doi: 10.1200/JCO.2012.48.2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krull KR, Cheung YT, Liu W, et al. Chemotherapy pharmacodynamics and neuroimaging and neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2016;34(22):2644-2653. doi: 10.1200/JCO.2015.65.4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan PC, Patel SK, Dilley K, et al. ; Children’s Oncology Group Long-term Follow-up Guidelines Task Force on Neurocognitive/Behavioral Complications After Childhood Cancer . Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: a report from the Children’s Oncology Group. Arch Pediatr Adolesc Med. 2007;161(8):798-806. doi: 10.1001/archpedi.161.8.798 [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-review-results-new-warnings-about-using-general-anesthetics-and. Published April 27, 2017. Accessed January 10, 2019.
- 5.Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315(21):2312-2320. doi: 10.1001/jama.2016.6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson AJ, Disma N, de Graaff JC, et al. ; GAS consortium . Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387(10015):239-250. doi: 10.1016/S0140-6736(15)00608-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko WR, Liaw YP, Huang JY, et al. Exposure to general anesthesia in early life and the risk of attention deficit/hyperactivity disorder development: a nationwide, retrospective matched-cohort study. Paediatr Anaesth. 2014;24(7):741-748. doi: 10.1111/pan.12371 [DOI] [PubMed] [Google Scholar]
- 8.Hansen TG, Pedersen JK, Henneberg SW, et al. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114(5):1076-1085. doi: 10.1097/ALN.0b013e31820e77a0 [DOI] [PubMed] [Google Scholar]
- 9.Fan Q, Cai Y, Chen K, Li W. Prognostic study of sevoflurane-based general anesthesia on cognitive function in children. J Anesth. 2013;27(4):493-499. doi: 10.1007/s00540-013-1566-z [DOI] [PubMed] [Google Scholar]
- 10.Flick RP, Katusic SK, Colligan RC, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128(5):e1053-e1061. doi: 10.1542/peds.2011-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology. 2012;117(3):494-503. doi: 10.1097/ALN.0b013e3182644684 [DOI] [PubMed] [Google Scholar]
- 12.Ing CH, DiMaggio CJ, Malacova E, et al. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014;120(6):1319-1332. doi: 10.1097/ALN.0000000000000248 [DOI] [PubMed] [Google Scholar]
- 13.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110(4):796-804. doi: 10.1097/01.anes.0000344728.34332.5d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pui C-H, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730-2741. doi: 10.1056/NEJMoa0900386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ClinicalTrials.gov Therapy for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia. NCT00137111. https://clinicaltrials.gov/ct2/show/NCT00137111. Accessed March 1, 2009.
- 16.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group Long-term Follow-up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979-4990. doi: 10.1200/JCO.2004.11.032 [DOI] [PubMed] [Google Scholar]
- 17.Conners CK, Connelly V, Campbell S, MacLean M, Barnes J. Conners’ Continuous Performance Test. 2nd ed New York: North Tonawanda; 2001. [Google Scholar]
- 18.Wechsler D. Wechsler Intelligence Scale for Children. 4th ed San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 19.Wechsler D. Wechsler Adult Intelligence Scale. 4th ed San Antonio, TX: Psychological Corporation; 2008. [Google Scholar]
- 20.Wechsler D. Wechsler Memory Scale. 4th ed San Antonio, TX: Psychological Corporation; 2009. [Google Scholar]
- 21.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- 22.Strauss E, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed London: Oxford University Press; 2006. [Google Scholar]
- 23.Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial. Lutz, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 24.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 25.FreeSurfer https://surfer.nmr.mgh.harvard.edu. Accessed December 1, 2016.
- 26.Ingraham LJ, Aiken CB. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology. 1996;10(1):120. doi: 10.1037/0894-4105.10.1.120 [DOI] [Google Scholar]
- 27.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 29.Sener RN. Diffusion MRI: apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput Med Imaging Graph. 2001;25(4):299-326. doi: 10.1016/S0895-6111(00)00083-5 [DOI] [PubMed] [Google Scholar]
- 30.Creeley C, Dikranian K, Dissen G, Martin L, Olney J, Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth. 2013;110(suppl 1):i29-i38. doi: 10.1093/bja/aet173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brambrink AM, Back SA, Riddle A, et al. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72(4):525-535. doi: 10.1002/ana.23652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505-508. doi: 10.1001/jamaneurol.2013.5847 [DOI] [PubMed] [Google Scholar]
- 33.Cheung YT, Khan RB, Liu W, et al. Association of cerebrospinal fluid biomarkers of central nervous system injury with neurocognitive and brain imaging outcomes in children receiving chemotherapy for acute lymphoblastic leukemia. JAMA Oncol. 2018;4(7):e180089. doi: 10.1001/jamaoncol.2018.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han D, Jin J, Fang H, Xu G. Long-term action of propofol on cognitive function and hippocampal neuroapoptosis in neonatal rats. Int J Clin Exp Med. 2015;8(7):10696-10704. [PMC free article] [PubMed] [Google Scholar]
- 35.Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17(11):705-717. doi: 10.1038/nrn.2016.128 [DOI] [PubMed] [Google Scholar]
- 36.Garver DL, Holcomb JA, Christensen JD. Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. Int J Neuropsychopharmacol. 2008;11(1):49-61. doi: 10.1017/S1461145707007730 [DOI] [PubMed] [Google Scholar]
- 37.Kishi S, Griener J, Cheng C, et al. Homocysteine, pharmacogenetics, and neurotoxicity in children with leukemia. J Clin Oncol. 2003;21(16):3084-3091. doi: 10.1200/JCO.2003.07.056 [DOI] [PubMed] [Google Scholar]
- 38.Ellis JA, Spanos NP. Cognitive-behavioral interventions for children’s distress during bone marrow aspirations and lumbar punctures: a critical review. J Pain Symptom Manage. 1994;9(2):96-108. doi: 10.1016/0885-3924(94)90162-7 [DOI] [PubMed] [Google Scholar]
- 39.Törnqvist E, Månsson Å, Hallström I. Children having magnetic resonance imaging: a preparatory storybook and audio/visual media are preferable to anesthesia or deep sedation. J Child Health Care. 2015;19(3):359-369. doi: 10.1177/1367493513518374 [DOI] [PubMed] [Google Scholar]
- 40.Koller D, Goldman RD. Distraction techniques for children undergoing procedures: a critical review of pediatric research. J Pediatr Nurs. 2012;27(6):652-681. doi: 10.1016/j.pedn.2011.08.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Modified Poisson Approach
eMethods 2. Multivariable General Limited Model Approach
eFigure. Mean Frequency and Mean Cumulative Duration of Procedures Requiring Anesthesia per Participant
eTable 1. Univariate Models Predicting Global Neurocognitive Impairment
eTable 2. Multivariable Models Predicting Test-Specific Neurocognitive Performance
eTable 3. Univariate Regression Models Predicting Test-Specific Neurocognitive Performance
eTable 4. Multivariable Regression Models Predicting Neuroimaging Outcomes