Key Points
Question
What is the neuroinhibitory potential of myelin-associated glycoprotein in comparison with vincristine, as measured via quantification of fluorescent intensity of the facial nerve after an axotomy injury?
Findings
In this laboratory experiment on 12 rats transgenic for the Thy-1 Gfp gene, myelin-associated glycoprotein significantly reduced fluorescent intensity in comparison with saline at weeks 3, 4, and 5 after an initial injury. Myelin-associated glycoprotein demonstrated similar intensity results as vincristine at weeks 4 and 5.
Meaning
These findings suggest that myelin-associated glycoprotein may have potential as a specific neuroinhibitor for patients with lower facial asymmetry after facial nerve injury.
This laboratory animal experiment assesses whether myelin-associated glycoprotein, a specific neuroinhibitor, can prevent neuroregeneration with efficacy comparable with that of vincristine, a well-established neurotoxin, and a saline control treatment.
Abstract
Importance
Aberrant synkinetic movement after facial nerve injury can lead to prominent facial asymmetry and resultant psychological distress. The current practices of neuroinhibition to promote greater facial symmetry are often temporary in nature and require repeated procedures.
Objective
To determine whether myelin-associated glycoprotein (MAG), a specific neuroinhibitor, can prevent neuroregeneration with efficacy comparable with that of vincristine, a well-established neurotoxin.
Design, Setting, and Participants
Rats transgenic for Thy-1 cell surface antigen–green fluorescent protein (Thy1-Gfp) were randomized into 3 groups. Each rat received bilateral crush axotomy injuries to the buccal and marginal mandibular branches of the facial nerves. The animals received intraneural injection of saline, MAG, or vincristine.
Main Outcomes and Measures
The animals were imaged via fluorescent microscopy at weeks 1, 3, 4, and 5 after surgery. Quantitative fluorescent data were generated as mean intensities of nerve segments proximal and distal to the axotomy site. Electrophysiological analysis, via measurement of compound muscle action potentials, was performed at weeks 0, 3, 4, and 5 after surgery.
Results
A total of 12 rats were included in the study. Administration of MAG significantly reduced fluorescent intensity of the distal nerve in comparison with the control group at week 3 (mean [SD], MAG group: 94 [11] intensity units vs control group: 130 [11] intensity units; P < .001), week 4 (MAG group: 81 [19] intensity units vs control group: 103 [9] intensity units; P = .004), and week 5 (MAG group: 76 [10] intensity units vs control group: 94 [10] intensity units; P < .001). In addition, rats treated with MAG had greater fluorescent intensity than those treated with vincristine at week 3 (mean [SD], MAG group: 94 [11] intensity units vs vincristine group: 76 [6] intensity units; P = .03), although there was no significant difference for weeks 4 and 5. At week 5, both MAG and vincristine demonstrated lower distal nerve to proximal nerve intensity ratios than the control group (control group, 0.94; vs MAG group, 0.82; P = .01; vs vincristine group; 0.77; P < .001). There was no significant difference in amplitude between the experimental groups at week 5 of electrophysiological testing.
Conclusions and Relevance
Lower facial asymmetry and synkinesis are common persistent concerns to patients after facial nerve injury. Using the Thy1-Gfp rat, this study demonstrates effective inhibition of neuroregeneration via intraneural application of MAG in a crush axotomy model, comparable with results with vincristine. By potentially avoiding systemic toxic effects of vincristine, MAG demonstrates potential as an inhibitor of neural regeneration for patients with synkinesis.
Level of Evidence
NA.
Introduction
The facial nerve (FN) is responsible for motor control of the ipsilateral mimetic facial muscles. Consequences of main trunk injury include oral incompetence, corneal irritation, and difficulty breathing.
The phenomenon of synkinesis typically emerges months after initial insult.1 Synkinesis involves involuntary movement of 1 facial muscle group with attempted activation of a distinct group. Lower facial static and dynamic asymmetries are problematic. Paralysis of the marginal mandibular branch results in an asymmetric smile owing to activation of unaffected muscles on the contralateral side, including actions of the depressor labii inferioris and depressor anguli oris.2
Contemporary medical treatments for facial asymmetry include temporary neurectomy with a local paralytic agent and chemodenervation with onabotulinum toxin A (Botox); these lend greater symmetry to the opposing sides.3,4,5 Challenges include variable results and the need for repeated injections every few months.
Surgical options include deanimation procedures, such as selective neurectomy or resection of the depressor labii inferioris muscle on the contralateral side.2,6 Even with targeted procedures, there is potential for regrowth, as well as the risk of inadvertent injury.7 Previous neural inhibition studies have demonstrated successful use of chemotherapeutic agents, including vincristine.8,9 The concerns with chemotherapeutic agents lie with their narrow therapeutic windows and potential for adverse systemic effects.10 Thus, there is motivation to identify specific neural inhibitors that would target individual components of the peripheral nerve.
Myelin-associated glycoprotein (MAG), a membrane protein of the immunoglobulin gene superfamily, has demonstrated potential as a specific inhibitor of axonal regrowth in murine models.11 The inhibitory efficacy of MAG has not been directly compared with an established neuroinhibitor, such as vincristine, in cranial or peripheral nerve models. Thus, we aimed to assess the inhibitory efficacy of MAG in comparison with vincristine in the transgenic Thy-1 cell surface antigen–green fluorescent protein (Thy1-Gfp) rat model with the goal of identifying a less toxic and more effective approach to treat synkinesis.
Methods
Animal Housing
All interventions were performed in accordance with the National Institutes of Health guidelines. The experimental protocol was approved by the University of Michigan institutional animal care and use committee. Adult Thy1-Gfp rats were quarantined and housed in a central facility. All animals were provided a 12-hour light-dark cycle and a temperature-controlled and humidity-controlled environment.
Animal Treatment and Experimental Design
Twelve rats were randomized into 3 groups of 4 rats each, which made up groups receiving isotonic saline (the control group), MAG (0.30 μg/mL), and vincristine (0.1 mg/mL). In the entire cohort, bilateral crush injuries were performed. This consisted of 2 separate crush applications via smooth-surfaced jeweler’s forceps to the buccal and marginal mandibular branches for 30 seconds each.12,13 After this procedure, an intraneural injection of group-specific substrate was performed.
Surgical Techniques
Surgical procedures were performed in a dedicated room with sterile equipment. General anesthesia was induced via isoflurane and maintained throughout. An operating microscope was used (Wild M690 [Leica]). A 2-mm incision was made inferior to the animal’s posterior canthus. The buccal and marginal branches of the FN were identified, and the overlying fascia was dissected. Under × 25 magnification, each branch was isolated, and a crush injury was performed. A suture marker was placed adjacent to each axotomy site for reference.
Intraneural Injection
An intraneural injection of the group-specific substrate was performed in stereotaxic fashion immediately after the crush injury immediately proximal and distal to the injury site. A glass capillary needle was attached to a gastight syringe. The syringe was introduced to the surgical field, and the stereotaxic instrument was used to pierce the epineurium of the nerve infiltrate group–specific substrate for 30 seconds. Two total injections were performed per branch (1 proximal and 1 distal to the crush site). After completion of the injections, the wound was reapproximated via absorbable monocryl sutures. Tissue adhesive was then applied. An identical procedure was performed to the contralateral side.
Nerve Harvest and Fluorescent Microscopy
At their respective end points, rats were anesthetized. Under the microscope, the FN and its branches were identified. A similar procedure was performed for the right face. The overlying skin was excised to allow for exposure for fluorescent microscopy. While anesthetized, the rats were given a weight-appropriate infusion of intracardiac pentobarbital and were euthanized. An MZIII inverted fluorescent microscope (Leica) was used to image the FN and its branches in vivo. Multiple images were taken at × 1.5 and × 2.5 magnification.
Quantitative Assessment of Fluorescent Intensity
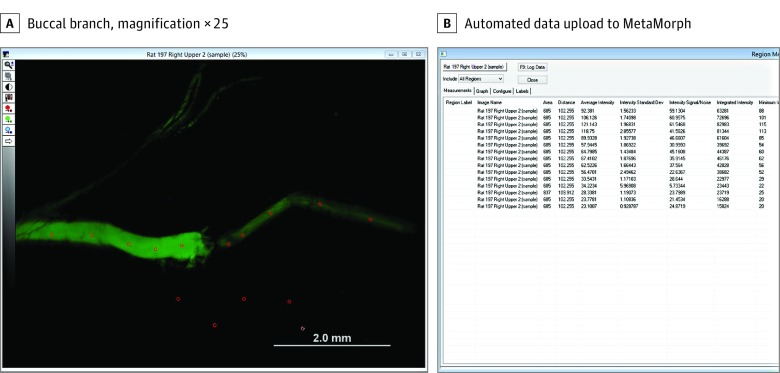
After imaging at 4080 × 3072 resolution, quantification of fluorescent intensity was performed via MetaMorph software (Molecular Devices) per a previously described protocol.14 Using high-resolution images at ×2.5 magnification, measurements of fluorescent intensity (mean [SD] intensity and minimum and maximum intensities) were obtained from 5 regions of interest each from nerve proximal to the crush axotomy site, distal to the axotomy site, and surrounding background. Standardized sampling of regions of interest was conducted by placing the regions of interest at equidistant points from the crush injury longitudinally at the midpoint of the nerve t by an observer blinded to the corresponding experimental group. The mean value of the 5 points from each segment was then determined. A representative analysis is shown in Figure 1. Mean (SD) values were generated from these measurements and pooled for quantitative analysis.
Figure 1. Screen Demonstrating Method for Uploading and Quantifying Quantitative Fluorescence Data.
A, Buccal branch imaged at × 2.5 magnification. The red circles in the fluorescent image represent regions of interest sampled to quantify fluorescence. A simplified systematic equidistant sampling along the length of each nerve site was performed to ensure representative sampling for quantitative analysis.
Electrophysiological Assessment
At 0 (baseline), 2, 3, 4, and 5 weeks after surgery, electrophysiological data were collected. Viking Quest Portable EMG System (CareFusion) software and hardware were used for collection and analysis. Compound muscle action potential (CMAP) values were obtained via repetitive electrophysiological testing.15 The animals were anesthetized for the procedure; 28-gauge electrodes were placed proximally to approximate the extracranial portion of the animal’s FN and distally in the vibrissal pad. Repetitive nerve stimulations were performed to obtain CMAP values. This procedure was performed bilaterally.
Statistical Analysis
Statistical analysis was performed via Prism version 7.0 (GraphPad) software. All results for quantitative analysis were reported with means (SDs). Means for quantitative fluorescent data were evaluated using repeated-measures 1-way analysis of variance and t tests. All statistical tests of significance were 2-sided with α of .05. Sample size analysis was based on data analysis from previous similar studies.16
Results
Animal Survival
All animals enrolled in the study survived to study conclusion. The rats gained weight appropriately throughout the study period. There were no untoward effects from the bilateral surgeries.
Qualitative Fluorescent Imaging
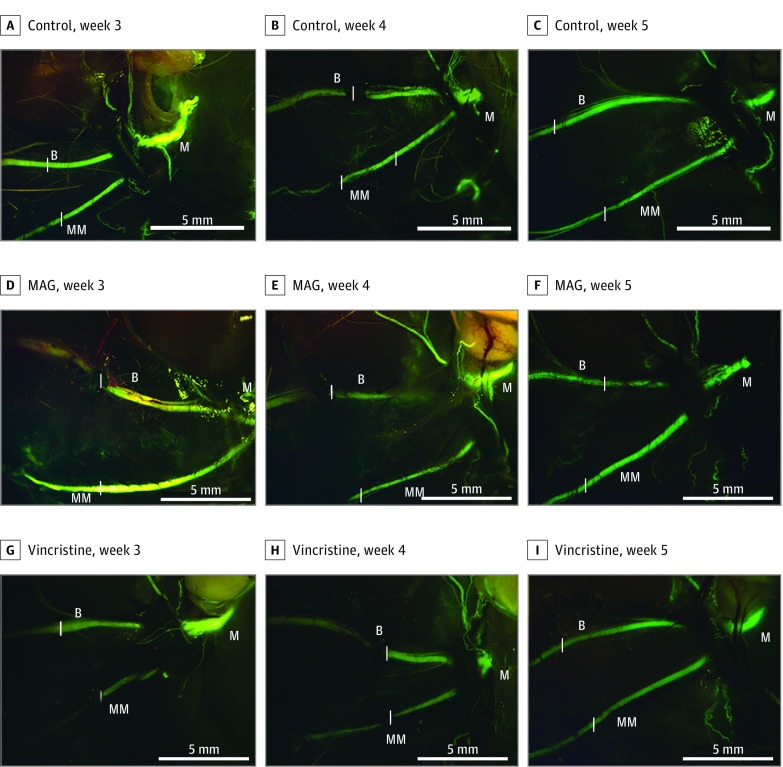
Transgenic Gfp rats allow for direct visualization and assessment of nerve regeneration longitudinally via fluorescent microscopy. There was no reduction in fluorescent intensity immediately after the crush injury. At week 1, there was uniform loss of fluorescence across all 3 groups distal to the site of the crush injury. Imaging at 1 week after surgery was difficult owing to inflammation and scar formation. Imaging was performed at weeks 3, 4, and 5 to grossly assess for neural regeneration. Results are shown in Figure 2.
Figure 2. Representative Images of Facial Nerve With Buccal and Marginal Branches Visible.
Nerve regeneration is demonstrated for the saline control group at weeks 3 (A), 4 (B), and 5 (C); the myelin-associated glycoprotein (MAG) group at weeks 3 (D), 4 (E), and 5 (F); and the vincristine group at weeks 3 (G), 4 (H), and 5 (I). Images were taken at × 1.25 magnification (scale bars represent 5 mm). The branches of the nerve are identified as main trunk (labeled M), buccal (labeled B), and marginal mandibular (labeled MM). Sites of injury are denoted by a white bar placed alongside the appropriate segment of each proximal nerve branch.
Quantitative Assessment of Fluorescent Intensity
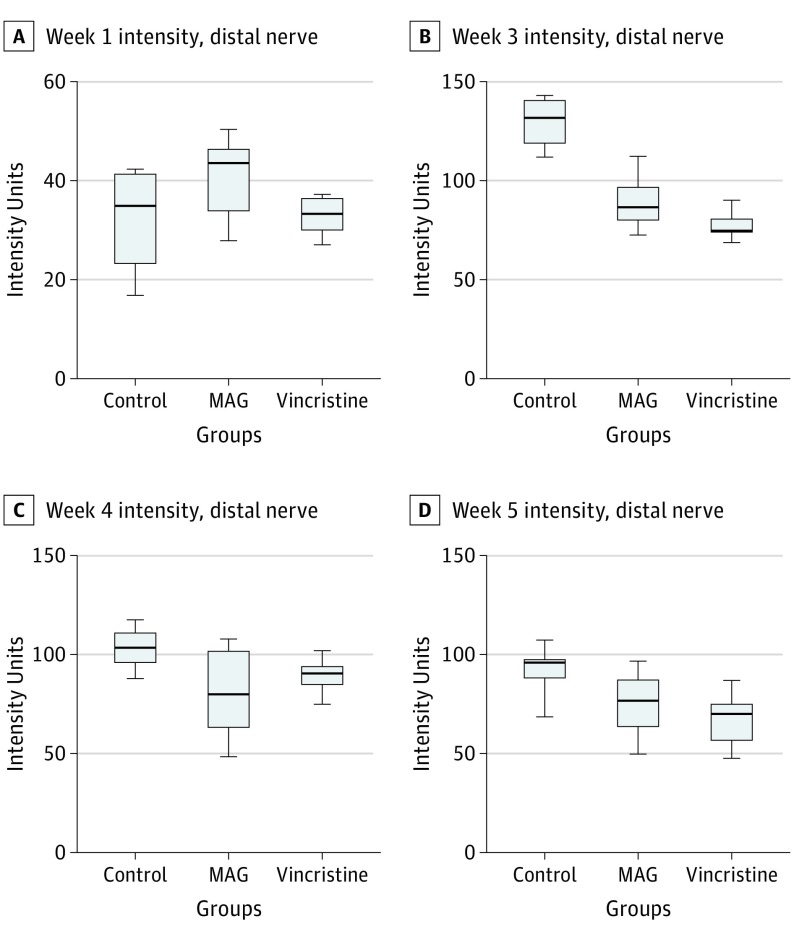
Fluorescent intensity was assessed for each peripheral branch proximal and distal to the injury site for each point. The intensity of the distal nerve for each group is modeled in Figure 3. Using repeated measures 1-way analysis of variance, we found a significant difference between the groups at all points (intensity units: control group: week 1, 34; week 3, 130; week 4, 103; and week 5, 94; MAG group: week 1, 41; week 3, 94; week 4, 81; and week 5, 76; vincristine group: week 1, 33; week 3, 76; week 4, 89; and week 5, 68; P = .003 overall). We performed further analysis between groups for each individual point. There were no significant differences among intensity measurements at 1 week after surgery. At week 3, the control group demonstrated greater intensity than the MAG group (mean [SD], MAG group: 130 [11] intensity units vs control group: 94 [11] intensity units; P < .001) and the vincristine group (mean [SD], vincristine group: 130 [11] intensity units vs control group: 76 [6] intensity units; P < .001). The MAG group (mean [SD], 94 [11] intensity units) demonstrated greater intensity than the vincristine group (76 [6] intensity units; P = .03) at this point. There was no significant difference in intensity between the MAG group (mean [SD], 81 [19] intensity units) and the vincristine groups (89 [7] intensity units; P = .50) at week 4 or across all 5 points (mean [SD], MAG group: 76 [10] intensity units vs vincristine group: 68 [11] intensity units; P = .33). When compared with the saline group at week 4 (mean [SD], 103 [9] intensity units), both the MAG group (mean [SD], 81 [19] intensity units; P = .004) and vincristine (mean [SD], 89 [7] intensity units; P = .004) demonstrated less intensity. These differences were observed 1 week later, when the MAG group (mean [SD], 76 [10] intensity units) and the vincristine group (mean [SD], 68 [11] intensity units) demonstrated significantly less intensity than saline control group at week 5 (mean [SD], 94 [10] intensity units; P < .001 for both comparisons).
Figure 3. Box-and-Whisker Plots for Fluorescent Intensity of Nerve Distal to the Axotomy Site for Experimental Groups by Week.
There was significantly greater intensity for the saline control group in comparison with the MAG group for week 3 (lower distal nerve to proximal nerve intensity ratios, 0.82 vs 0.76; P = .05) (B), week 4 (intensity ratios, 0.90 vs 0.70; P < .001) (C), and week 5 (intensity ratios, 0.94 vs 0.82; P < .001) (D). Similarly, there was greater intensity for the saline control group in comparison with the vincristine group for week 3 (intensity ratios, 0.82 vs 0.62; P < .001) (B), week 4 (intensity ratios, 0.90 vs 0.70; P < .001) (C), and week 5 (intensity ratios, 0.94 vs 0.77; P < .001) (D). The MAG-treated animals had significantly greater intensity than the vincristine-treated animals in week 3 (intensity ratios, 0.76 vs 0.62; P < .001) (B).
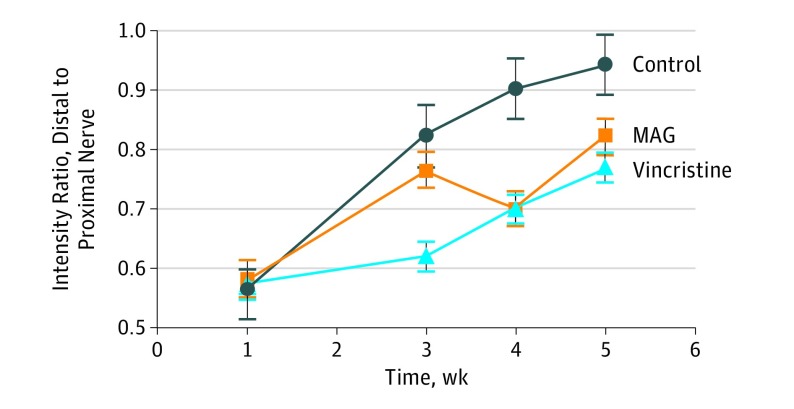
The ratio of distal to proximal nerve fluorescent intensity was measured between groups and is modeled in Figure 4. There was no significant difference between groups 1 week postsurgery. At the final point, there was a lower ratio for MAG (0.82; P = .01) and vincristine (0.77; P < .001) compared with the saline control group (0.94).
Figure 4. Comparison of Ratio of Distal Nerve Intensity to Proximal Nerve Intensity by Week.
At week 5, the saline control group demonstrated a significantly greater ratio in comparison to both the myelin-associated glycoprotein (MAG) group (lower distal nerve to proximal nerve intensity ratios, 0.94 vs 0.82; P = .01) and the vincristine group (intensity ratios, 0.94 vs 0.77; P < .001).
Electrophysiological Analysis
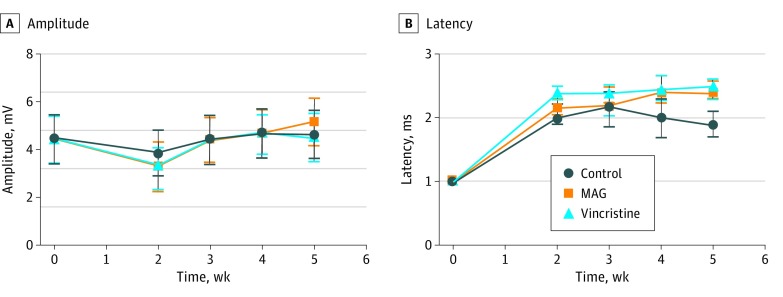
Electrophysiological data (CMAP and latency values) were compiled at weeks 0, 2, 3, 4, and 5 (Figure 5). Baseline values among groups were nonsignificant. For CMAP values, each of the groups experienced a decline in amplitude at week 2, with a return to baseline levels by week 3. The dips in CMAP values for the MAG group at week 2 (3.85 mV) and the vincristine group (3.92 mV; P = .14) in comparison with the saline control group at week 2 (3.32 mV; P = .18) were not significant. With regard to latency values, the MAG group (2.4 ms; P = .02) and vincristine group (2.5 ms; P = .002) demonstrated significantly delayed latency in comparison with the control group at week 5 (1.9 ms). There was no difference in latency values between MAG and vincristine for any of the analyzed weeks.
Figure 5. Comparison of Electrophysiological Data Between the Experimental Groups With Amplitude, Measured in Millivolts, and Latency, Measured in Milliseconds.
There was no statistical significance between the differences in the values of the 3 groups at any of the points for compound muscle action potential values. The animals returned to baseline amplitude values by week 3 of testing. In regard to latency testing, there was a significant difference between the myelin-associated glycoprotein (MAG) group (2.4 ms) and the control group at week 5 (1.9 ms; P = .02) and between the vincristine group (2.5 ms) and the control group for week 4 (2.0 ms; P = .03) and week 5 (vincristine, 2.5 ms vs control, 1.9 ms; P = .002).
Discussion
In this study, MAG inhibited neural regeneration as effectively as vincristine when administered via intraneural injection in a crush axotomy model in the rat facial nerve. Myelin-associated glycoprotein works via action on the small guanosine triphosphatases (GTPases) Ras homolog gene family, member A (RhoA) and Ras-related C3 botulinum toxin substrate 1 (Rac1); specifically, it activates RhoA and inhibits Rac1, leading to a nonpermissive environment for nerve fiber elongation.17 There has also been a clearly established link between oligodendrocyte myelin glycoprotein expression and facial nerve transection.18 Manipulation of the Rho family of GTPases has been extensively studied in relation with central nervous system injuries and neurodegenerative disorders.19,20 The primary focus of these previous studies involves RhoA knockdown or dysregulation to improve axonal regeneration and inhibit retrograde apoptosis signaling.21,22 Previous studies have also assessed the specific neuroinhibitory effect of MAG via the counting of distinct axon profiles via immunohistochemistry-based methods and assessment of neurite outgrowth via quantification of neurite fluorescence.11,23,24,25
Data regarding the efficacy of neuroinhibition in comparison with established neurotoxins in peripheral nerve are lacking. Vincristine has demonstrated efficacy in improving laryngeal adductor function by inhibiting reinnervation to the canine posterior cricoarytenoid muscle after recurrent laryngeal nerve injury.26 It has also effectively inhibited neural regeneration after a crush injury to the posterior tibial nerve in a rodent model.8 To our knowledge, this is the first study to attempt to establish efficacy of a RhoA-activating membrane protein with that of a potent neurotoxin, an important consideration when deciding on the translational potential of MAG as a neuroinhibitor.
Traditional measures of neuroinhibition involve quantitative histomorphometry for assessment of axonal regeneration (eg, total axon count, axon density, axon diameter).27,28,29,30 Functional outcomes include analyses of eye and vibrissal pad movement, assessment of innervation of motor end plates, and electrophysiological analyses.29,31,32 The timing of neuroinhibitory, or nerve regeneration, experiments in the mouse or rat model must be carefully planned because of the superlative regenerative potential of these animals; if the animals recover from their injury prior to the established end point of the experiment, then detection of neuroenhancement or neuroinhibition may be lost via type 2 error.33
The Thy1-Gfp rat model is uniquely equipped to assess effects on nerve regeneration or prolonged inhibition, because the animal can be serially imaged to document a return of the fluorescent protein under microscopy. The construct of the Thy1-XFP mouse lines is a transgenic Sprague Dawley rat founder line created in collaboration with genOway using pronuclear injection.34 The initial study by Magill et al35 demonstrated the definitive return of fluorescence in peripheral branches after 4 weeks following crush injury to the main trunk. The validity of using the Thy1-Gfp rat as a model for nerve regeneration was established via comparative analysis of histomorphometric and functional parameters with wild-type Sprague Dawley rats after sciatic nerve injury.36 Subsequently, these transgenic animals have primarily been used to assess timing of neural recovery after various injury patterns.37,38 Recovery is measured qualitatively, because scientists assess timing of nerve regeneration as concordant with the return of gross fluorescence.
In this study, MAG demonstrated greater intensity (less inhibition) than the positive control group that received vincristine at week 3, but this difference did not persist at weeks 4 and 5 after surgery. This may be associated with either a delayed onset of action for MAG in comparison with vincristine or a more sustained neuroinhibitory effect. The 3-week and 4-week points are of particular interest because they correspond to the period during which the animal would begin to demonstrate a plateau of recovery from a crush injury.12 We hypothesize that vincristine, which inhibits microtubule formation, causes a more acute cytotoxic response, with resultant early decline in intensity values. This pattern may differ from inhibition by MAG, which specifically activates RhoA and is able to suppress axonal elongation during the critical regenerative period. These findings are consistent with MAG’s purported inhibitory mechanism of action.17 The differential outcomes across the experimental timeline may therefore reflect the substances’ variable mechanisms of action.
The interpretation of electrophysiological data are interesting with regard to the fluorescent intensity data. Previous studies have demonstrated that a crush injury does not induce GFP degradation or extensive Wallerian degeneration.37 We found that while all groups had a modest decrease in mean amplitude values at week 2, CMAP amplitude returned to baseline by week 3. This is consistent with prior studies by Hadlock et al12 in which rats demonstrate functional signs of recovery by day 11 and a plateau of recovery by days 19 to 28. Given that regenerating nerve does not have to traverse a suture line or gap with a crush injury, there is likely preservation of Schwann cell tubule continuity and earlier restoration of neurocircuity.39 Crush injury therefore allows for earlier generation and transmission of action potentials with subsequent contraction of the end-organ muscle. The latency data are similar, in that all groups demonstrated an initially delayed latency value; while the control group did experience some recovery, the MAG and vincristine groups remained delayed. Demonstrating significant differences between groups on all electrophysiological outcome measures may require use of transection or nerve-gap injury models.40,41,42 In this study, a crush injury was chosen to allow for translational application because it mimics the injury pattern seen in patients with Bell palsy.
Given the challenges that patients with FN injury experience with aberrant synkinetic activation of previously distinct facial muscle groups, a specific neural inhibitor such as MAG holds potential as an adjunctive treatment to prevent nerve regrowth and reestablish lower facial symmetry. Future studies could consider assessing the neuroinhibitory potential of MAG in transection or nerve gap models; this would allow for a prolonged period of nerve regrowth and the potential to correlate intensity outcomes with electrophysiological outcomes.12 In addition, studies with the Thy1-Gfp rat may correlate intensity outcomes with histomorphometric and/or functional parameters.
Limitations
Given that MAG has not been previously used in a rat facial nerve model, concentrations were determined based on prior literature. In a prior study, Tomita et al11 used a lower concentration of MAG for topical application to sciatic nerves of adult mice. Our group therefore increased the relative concentration of MAG for more site-specific intraneural application. The concentration of vincristine was determined from prior animal studies.8,9 An additional limitation is about any potential injury from the injections themselves. While this concept was not studied in this article, previous studies have demonstrated only intraneural edema and no evidence of demyelination or Wallerian degeneration with intrafascicular saline injections.43
One unexpected pattern in the data is the varying absolute florescence intensity for the control groups, which decreases from weeks 3 to 5 and appears to be a result contrary to the expected pattern. This paradoxical result highlights the importance of normalizing data when using this method. In the present study, each animal serves as its own internal control, and these normalized data demonstrated the expected trend for the control group. The method for fluorescent quantification of facial nerve regeneration, while promising, will benefit from subsequent refinement in standardization of nerve exposure, imaging environment, and more reliable comparison of absolute values across points.
An important consideration not addressed by the present study is whether MAG or vincristine might suppress axonal fluorescence even in the absence of direct neural injury. Prior work including use of sham controls suggests that an intact nerve sheath serves as a barrier that can insulate the nerve against neuroinhibition arising from topical application, but the effects of isolated topical application of vincristine and MAG in a Thy-1 Gfp facial nerve rat model await future study.44,45
Another limitation of these data is about the limited sensitivity of electrophysiological measures for detecting differences in reinnervation outcomes. Despite neuroinhibition by MAG or vincristine after a crush injury, the preservation of Schwann cell continuity would potentially continue to allow the propagation of electrical stimuli.12,39 Ideally, testing would have been performed 1 week after injury; however, collecting data at this point was discouraged by the institutional animal care and use committee overseeing this study, given the fresh wound and concern for stress to the animals associated with anesthetic for electrode placement at this early point. Finally, additional future studies would be particularly valuable for determining which histomorphometric parameters on cross-sectional staining of nerve fibers correlate most closely with fluorescent intensity, because density, fiber density, fiber count, nerve caliber, myelination, and other parameters may all influence the fluorescent intensity observed in this experimental model.
Conclusions
We used the transgenic Thy1-Gfp rat model to demonstrate efficacy of MAG as a specific inhibitor to nerve recovery after a crush injury, as measured via quantitative fluorescent intensity. In comparison with vincristine, a known neurotoxin and chemotherapeutic agent, MAG demonstrates its specific inhibitory action in delaying nerve recovery during the animal’s greatest regenerative phase (in weeks 3-4) with results comparable with vincristine after 4 weeks. By avoiding the systemic effects of a cytotoxic agent such as vincristine, MAG demonstrates potential as an inhibitor of neural regeneration for patients with lower facial asymmetry or synkinetic movements. Future studies may better elucidate its inhibitory effect after complete nerve injury.
References
- 1.Fujiwara K, Furuta Y, Nakamaru Y, Fukuda S. Comparison of facial synkinesis at 6 and 12 months after the onset of peripheral facial nerve palsy. Auris Nasus Larynx. 2015;42(4):271-274. doi: 10.1016/j.anl.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 2.Hussain G, Manktelow RT, Tomat LR. Depressor labii inferioris resection: an effective treatment for marginal mandibular nerve paralysis. Br J Plast Surg. 2004;57(6):502-510. doi: 10.1016/j.bjps.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 3.Wei LA, Diels J, Lucarelli MJ. Treating buccinator with botulinum toxin in patients with facial synkinesis: a previously overlooked target. Ophthalmic Plast Reconstr Surg. 2016;32(2):138-141. doi: 10.1097/IOP.0000000000000449 [DOI] [PubMed] [Google Scholar]
- 4.Cooper L, Lui M, Nduka C. Botulinum toxin treatment for facial palsy: a systematic review. J Plast Reconstr Aesthet Surg. 2017;70(6):833-841. doi: 10.1016/j.bjps.2017.01.009 [DOI] [PubMed] [Google Scholar]
- 5.Choi KH, Rho SH, Lee JM, Jeon JH, Park SY, Kim J. Botulinum toxin injection of both sides of the face to treat post-paralytic facial synkinesis. J Plast Reconstr Aesthet Surg. 2013;66(8):1058-1063. doi: 10.1016/j.bjps.2013.04.012 [DOI] [PubMed] [Google Scholar]
- 6.Breslow GD, Cabiling D, Kanchwala S, Bartlett SP. Selective marginal mandibular neurectomy for treatment of the marginal mandibular lip deformity in patients with chronic unilateral facial palsies. Plast Reconstr Surg. 2005;116(5):1223-1232. doi: 10.1097/01.prs.0000182219.40800.56 [DOI] [PubMed] [Google Scholar]
- 7.van Veen MM, Dusseldorp JR, Hadlock TA. Long-term outcome of selective neurectomy for refractory periocular synkinesis. Laryngoscope. 2018;128(10):2291-2295. doi: 10.1002/lary.27225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paydarfar JA, Paniello RC. Functional study of four neurotoxins as inhibitors of post-traumatic nerve regeneration. Laryngoscope. 2001;111(5):844-850. doi: 10.1097/00005537-200105000-00016 [DOI] [PubMed] [Google Scholar]
- 9.Yian CH, Paniello RC, Gershon Spector J. Inhibition of motor nerve regeneration in a rabbit facial nerve model. Laryngoscope. 2001;111(5):786-791. doi: 10.1097/00005537-200105000-00006 [DOI] [PubMed] [Google Scholar]
- 10.Aghajan Y, Yoon JM, Crawford JR. Severe vincristine-induced polyneuropathy in a teenager with anaplastic medulloblastoma and undiagnosed Charcot-Marie-Tooth disease. BMJ Case Rep. 2017;2017:bcr-2016-218981. doi: 10.1136/bcr-2016-218981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomita K, Kubo T, Matsuda K, Yano K, Tohyama M, Hosokawa K. Myelin-associated glycoprotein reduces axonal branching and enhances functional recovery after sciatic nerve transection in rats. Glia. 2007;55(14):1498-1507. doi: 10.1002/glia.20566 [DOI] [PubMed] [Google Scholar]
- 12.Hadlock TA, Kowaleski J, Lo D, Mackinnon SE, Heaton JT. Rodent facial nerve recovery after selected lesions and repair techniques. Plast Reconstr Surg. 2010;125(1):99-109. doi: 10.1097/PRS.0b013e3181c2a5ea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barghash Z, Larsen JO, Al-Bishri A, Kahnberg KE. Degeneration and regeneration of motor and sensory nerves: a stereological study of crush lesions in rat facial and mental nerves. Int J Oral Maxillofac Surg. 2013;42(12):1566-1574. doi: 10.1016/j.ijom.2013.04.017 [DOI] [PubMed] [Google Scholar]
- 14.Arechavala-Gomeza V, Kinali M, Feng L, et al. Immunohistological intensity measurements as a tool to assess sarcolemma-associated protein expression. Neuropathol Appl Neurobiol. 2010;36(4):265-274. doi: 10.1111/j.1365-2990.2009.01056.x [DOI] [PubMed] [Google Scholar]
- 15.Schulz A, Walther C, Morrison H, Bauer R. In vivo electrophysiological measurements on mouse sciatic nerves. J Vis Exp. 2014;51181(86). doi: 10.3791/51181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichols CM, Brenner MJ, Fox IK, et al. Effects of motor versus sensory nerve grafts on peripheral nerve regeneration. Exp Neurol. 2004;190(2):347-355. doi: 10.1016/j.expneurol.2004.08.003 [DOI] [PubMed] [Google Scholar]
- 17.Niederöst B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22(23):10368-10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama Y, Fujiwara T, Kubo T, et al. Reduction of oligodendrocyte myelin glycoprotein expression following facial nerve transection. J Chem Neuroanat. 2008;36(3-4):209-215. doi: 10.1016/j.jchemneu.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 19.Stankiewicz TR, Linseman DA. Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration. Front Cell Neurosci. 2014;8(314). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutekunst CA, Tung JK, McDougal ME, Gross RE. C3 transferase gene therapy for continuous conditional RhoA inhibition. Neuroscience. 2016;339:308-318. doi: 10.1016/j.neuroscience.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Zhang G, Rodemer W, Jin LQ, Shifman M, Selzer ME. The role of RhoA in retrograde neuronal death and axon regeneration after spinal cord injury. Neurobiol Dis. 2017;98:25-35. doi: 10.1016/j.nbd.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boghdadi AG, Teo L, Bourne JA. The involvement of the myelin-associated inhibitors and their receptors in CNS plasticity and injury. Mol Neurobiol. 2018;55(3):1831-1846. doi: 10.1007/s12035-017-0433-6 [DOI] [PubMed] [Google Scholar]
- 23.Tang S, Qiu J, Nikulina E, Filbin MT. Soluble myelin-associated glycoprotein released from damaged white matter inhibits axonal regeneration. Mol Cell Neurosci. 2001;18(3):259-269. doi: 10.1006/mcne.2001.1020 [DOI] [PubMed] [Google Scholar]
- 24.Torigoe K, Lundborg G. Selective inhibition of early axonal regeneration by myelin-associated glycoprotein. Exp Neurol. 1998;150(2):254-262. doi: 10.1006/exnr.1997.6775 [DOI] [PubMed] [Google Scholar]
- 25.Wong VSC, Picci C, Swift M, Levinson M, Willis D, Langley B. α-Tubulin acetyltransferase is a novel target mediating neurite growth inhibitory effects of chondroitin sulfate proteoglycans and myelin-associated glycoprotein. eNeuro. 2018;5(1):ENEURO.0240-17.2018. doi: 10.1523/ENEURO.0240-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paniello RC, Park A. Effect on laryngeal adductor function of vincristine block of posterior cricoarytenoid muscle 3 to 5 months after recurrent laryngeal nerve injury. Ann Otol Rhinol Laryngol. 2015;124(6):484-489. doi: 10.1177/0003489414566182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner MJ, Mackinnon SE, Rickman SR, et al. FK506 and anti-CD40 ligand in peripheral nerve allotransplantation. Restor Neurol Neurosci. 2005;23(3-4):237-249. [PubMed] [Google Scholar]
- 28.Kawamura DH, Hadlock TA, Fox IK, Brenner MJ, Hunter DA, Mackinnon SE. Regeneration through nerve isografts is independent of nerve geometry. J Reconstr Microsurg. 2005;21(4):243-249. doi: 10.1055/s-2005-871751 [DOI] [PubMed] [Google Scholar]
- 29.Brenner MJ, Dvali L, Hunter DA, Myckatyn TM, Mackinnon SE. Motor neuron regeneration through end-to-side repairs is a function of donor nerve axotomy. Plast Reconstr Surg. 2007;120(1):215-223. doi: 10.1097/01.prs.0000264094.06272.67 [DOI] [PubMed] [Google Scholar]
- 30.Brenner MJ, Hess JR, Myckatyn TM, Hayashi A, Hunter DA, Mackinnon SE. Repair of motor nerve gaps with sensory nerve inhibits regeneration in rats. Laryngoscope. 2006;116(9):1685-1692. doi: 10.1097/01.mlg.0000229469.31749.91 [DOI] [PubMed] [Google Scholar]
- 31.Hadlock T, Lindsay R, Edwards C, et al. The effect of electrical and mechanical stimulation on the regenerating rodent facial nerve. Laryngoscope. 2010;120(6):1094-1102. [DOI] [PubMed] [Google Scholar]
- 32.Hadlock T, Kowaleski J, Lo D, et al. Functional assessments of the rodent facial nerve: a synkinesis model. Laryngoscope. 2008;118(10):1744-1749. doi: 10.1097/MLG.0b013e31817f5255 [DOI] [PubMed] [Google Scholar]
- 33.Brenner MJ, Moradzadeh A, Myckatyn TM, et al. Role of timing in assessment of nerve regeneration. Microsurgery. 2008;28(4):265-272. doi: 10.1002/micr.20483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng G, Mellor RH, Bernstein M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41-51. doi: 10.1016/S0896-6273(00)00084-2 [DOI] [PubMed] [Google Scholar]
- 35.Magill CK, Moore AM, Borschel GH, Mackinnon SE. A new model for facial nerve research: the novel transgenic Thy1-GFP rat. Arch Facial Plast Surg. 2010;12(5):315-320. doi: 10.1001/archfacial.2010.71 [DOI] [PubMed] [Google Scholar]
- 36.Kemp SWP, Phua PD, Stanoulis KN, et al. Functional recovery following peripheral nerve injury in the transgenic Thy1-GFP rat. J Peripher Nerv Syst. 2013;18(3):220-231. doi: 10.1111/jns5.12035 [DOI] [PubMed] [Google Scholar]
- 37.Placheta E, Wood MD, Lafontaine C, Frey M, Gordon T, Borschel GH. Macroscopic in vivo imaging of facial nerve regeneration in Thy1-GFP rats. JAMA Facial Plast Surg. 2015;17(1):8-15. doi: 10.1001/jamafacial.2014.617 [DOI] [PubMed] [Google Scholar]
- 38.Moore AM, Borschel GH, Santosa KB, et al. A transgenic rat expressing green fluorescent protein (GFP) in peripheral nerves provides a new hindlimb model for the study of nerve injury and regeneration. J Neurosci Methods. 2012;204(1):19-27. doi: 10.1016/j.jneumeth.2011.10.011 [DOI] [PubMed] [Google Scholar]
- 39.Tomatsuri M, Okajima S, Ide C. Sprout formation at nodes of Ranvier of crush-injured peripheral nerves. Restor Neurol Neurosci. 1993;5(4):275-282. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki K, Suzuki Y, Tanihara M, et al. Reconstruction of rat peripheral nerve gap without sutures using freeze-dried alginate gel. J Biomed Mater Res. 2000;49(4):528-533. doi: [DOI] [PubMed] [Google Scholar]
- 41.Cho HH, Jang S, Lee SC, et al. Effect of neural-induced mesenchymal stem cells and platelet-rich plasma on facial nerve regeneration in an acute nerve injury model. Laryngoscope. 2010;120(5):907-913. [DOI] [PubMed] [Google Scholar]
- 42.Farrag TY, Lehar M, Verhaegen P, Carson KA, Byrne PJ. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. 2007;117(1):157-165. doi: 10.1097/01.mlg.0000249726.98801.77 [DOI] [PubMed] [Google Scholar]
- 43.Whitlock EL, Brenner MJ, Fox IK, Moradzadeh A, Hunter DA, Mackinnon SE. Ropivacaine-induced peripheral nerve injection injury in the rodent model. Anesth Analg. 2010;111(1):214-220. doi: 10.1213/ANE.0b013e3181de574e [DOI] [PubMed] [Google Scholar]
- 44.Fink BR, Byers MR, Middaugh ME. Dynamics of colchicine effects on rapid axonal transport and axonal morphology. Brain Res. 1973;56(C):299-311. doi: 10.1016/0006-8993(73)90343-0 [DOI] [PubMed] [Google Scholar]
- 45.Sotgiu ML, Biella G, Firmi L, Pasqualucci V. Topical axonal transport blocker vincristine prevents nerve injury-induced spinal neuron sensitization in rats. J Neurotrauma. 1998;15(12):1077-1082. doi: 10.1089/neu.1998.15.1077 [DOI] [PubMed] [Google Scholar]