Key Points
Question
Is retinopathy observed in patients with yellow fever?
Findings
In this cross-sectional study of 64 patients who had received a confirmed diagnosis of yellow fever during 2 recent outbreaks in Southeastern Brazil, 13 patients (20%) had retinopathy, with the most common retinal findings being retinal nerve fiber layer infarct, superficial hemorrhage, and grayish outer retinal lesions. Elevated serum aspartate aminotransferase, total bilirubin, arterial lactate, and serum creatinine levels as well as disease severity and low platelet counts were associated with retinopathy in these patients.
Meaning
Retinopathy can be observed among patients with yellow fever but may be overlooked, especially if more severe disease precludes careful ophthalmoscopic examinations.
Abstract
Importance
Yellow fever still threatens people in endemic areas, and besides conjunctival icterus, little is known about the ocular changes that occur in these patients.
Objective
To characterize retinal changes in patients with confirmed yellow fever during 2 recent outbreaks of the disease in Minas Gerais, Southeastern Brazil.
Design, Setting, and Participants
This cross-sectional, observational study conducted at a single referral center for infectious diseases in Southeastern Brazil collected data between January 2017 and February 2018 from 94 consecutive patients with suspicion of yellow fever who were eligible for the study.
Main Outcomes and Measures
Patients underwent ophthalmic examination. Clinical findings, laboratory results, and occurrence of retinopathy and death during hospitalization were reported, including age, sex, comorbidities, disease severity, serum aspartate aminotransferase level, total bilirubin level, serum creatinine level, arterial lactate level, international normalized ratio, and platelet count at hospital admission.
Results
In total, 64 patients were included who had received a confirmed diagnosis of yellow fever, with a median (interquartile range) age of 47 (38-56) years, and 12 patients (19%) were women. Twenty eyes (16%) of 13 patients (20%) had retinopathy at the same time as yellow fever. The most common fundus changes among the 20 eyes were retinal nerve fiber layer infarcts (11 [55%]), superficial hemorrhages (7 [35%]) and grayish deep lesions (6 [30%]), possibly at the level of the outer retina or choroid. Aspartate aminotransferase levels higher than 3000 U/L (odds ratio [OR], 14.2; 95% CI, 3.5-77.8; P < .001), total bilirubin levels higher than 2.3 mg/dL (OR, 20.0; 95% CI, 4.4-159.7; P < .001), serum creatinine levels higher than 2.0 mg/dL (OR, 8.2; 95% CI, 2.1-36.0; P = .003), arterial lactate levels higher than 17.1/mg/dL (OR, 4.6; 95% CI, 1.1-19.0; P = .03), platelet count lower than 94 × 103/μL (OR, 7.8; 95% CI, 1.8-59.9; P = .004), and classification of disease as severe (OR, 11.7; 95% CI, 2.0-301.0; P = .003) were associated with retinopathy. Arterial hypertension, diabetes, international normalized ratio, and death were not associated with retinopathy.
Conclusions and Relevance
Retinopathy was present in 20% of patients with yellow fever and appeared to be associated with more severe systemic disease. Retinal nerve fiber layer infarcts and superficial hemorrhages, but not the grayish deep lesions, resembled those associated with other flavivirus (eg, dengue virus) infections. The clinical relevance of these findings may warrant further investigation.
This cross-sectional single-center study conducted in Southeastern Brazil evaluates clinical findings and biomarkers associated with retinopathy and characterizes retinal changes among patients with confirmed yellow fever during 2 recent outbreaks of the disease.
Introduction
Yellow fever (YF) is caused by the YF virus, the prototype of the Flaviviridae family, comprising roughly 70 positive-sense, single-stranded RNA viruses that replicate in the cytoplasm of infected cells.1,2 Yellow fever is a zoonotic infection that is endemic in tropical areas of South America and Africa and is transmitted in a sylvatic cycle involving wild nonhuman primates and the mosquitoes of the genus Haemagogus in Americas and the genus Aedes in Africa.1,2 “Jungle yellow fever” infection occurs when humans are sporadically bitten by infected sylvatic mosquitoes.1 “Urban yellow fever” infection is the result of the transmission of the YF virus between humans, mainly by Aedes aegypti.1
Yellow fever is the original viral hemorrhagic fever, a viral sepsis with high lethality characterized by fever, prostration, hemorrhage, shock, and hepatic, renal, and myocardial injury.1 Ocular involvement reported as associated with YF has been limited to conjunctival icterus or conjunctival hyperemia as well as to vaccine-induced intraocular inflammation.3,4,5,6
Despite the availability for decades of an effective vaccine, the disease remains threatening to travelers to and residents of endemic areas.2 Two consecutive outbreaks of YF recently occurred in Brazil, in the 2016 to 2017 and the 2017 to 2018 seasons, with a lethality rate of more than 30%.7,8,9,10 The number of human cases that were confirmed and reported surpassed 750 from July 2016 through June 2017 and surpassed 1100 from July 2017 through May 2018, with the epicenter of these outbreaks being the state of Minas Gerais in Southeastern Brazil.8 The purpose of the present study is to describe the retinal findings among patients with YF who were hospitalized at a referral center during these recent outbreaks in Brazil.
Methods
Participants
This is a cross-sectional, observational, single-center study. All patients with suspicion of YF admitted to a regional referral center for the 2 outbreaks between January 2017 and February 2018 were eligible for the study. An additional patient confirmed with YF and admitted to the intensive care unit (ICU) of our university hospital (Hospital das Clínicas da Universidade Federal de Minas Gerais) was also included. Only patients who had received a diagnosis of YF confirmed by real-time polymerase chain reaction analyses or by a positive specific IgM antibody–capture enzyme-linked immunosorbent assay result were included. Other infectious causes were appropriately excluded. Specifically, serological tests for the presence of dengue virus, chikungunya virus, leptospirosis, rickettsiosis, and viral hepatitis were performed, with negative results. Patients admitted to the ICU with clinical instability or with serum aspartate aminotransferase (AST) levels higher than 2000 U/L (to convert to microkatals per liter, multiply by 0.0167), serum creatinine levels higher than 2.0 mg/dL (to convert to micromoles per liter, multiply by 88.4), or an international normalized ratio (INR) greater than 1.5 were considered to have severe systemic disease.11 All patients underwent dilated indirect ophthalmoscopy by at least 2 uveitis specialists (L.C., S.O., or D.V.-S.), and retinopathy was considered in the setting of acute fundus changes consistent with YF. Whenever possible, retinal lesions were documented by a portable 45° fundus camera (Horus digital fundus camera; Medimaging Integrated Solution), a conventional digital fundus camera (Canon CR2; Canon Inc), or even indirect ophthalmoscopy with a smartphone and a 20-diopter lens. Two patients with stable clinical conditions also underwent multimodal imaging (reflectance, fluorescein angiography, and spectral-domain optical coherence tomography) with Heidelberg Spectralis HRA-OCT (Heidelberg Engineering), at the ophthalmic imaging unit of our university hospital. Patients admitted during the 2017 to 2018 outbreak had ocular symptoms investigated and were also tested with an Amsler grid. We assessed age, sex, comorbidities (including arterial hypertension and diabetes), AST levels, total bilirubin levels, serum creatinine levels, arterial lactate levels, INR, and platelet count levels at hospital admission as well as the occurrence of retinopathy, necessity of ICU admission, and death during hospitalization. For patients with nonsevere systemic disease who had only venous lactate levels measured, we estimated arterial lactate levels using the formula: arterial lactate = −0.259 + (venous lactate) × 0.996.12 The present study was approved by the institutional review board of the Universidade Federal de Minas Gerais and was conducted in accordance with the tenets of the Declaration of Helsinki.13 Owing to an acute epidemic and public health emergency, the institutional review board granted a waiver of informed patient consent so that data could be retrospectively analyzed.
Statistical Analysis
The Mann-Whitney test and the Fisher exact test with a mid–P value adjustment were used to compare continuous and categorical variables between subgroups, respectively. To estimate odds ratio (OR) confidence intervals, we used the Fisher exact test with a mid–P value adjustment. Multivariate analysis was conducted using logistic regression.
Classification and regression trees, a predictive model, were used to determine thresholds of each variable to predict retinopathy.14 The thresholds of the variables were obtained minimizing the sum of the Gini impurity within subgroups. Gini impurity is a measure of statistical dispersion that is zero when all cases in a group have the same measured outcome and that increases as the group becomes more heterogeneous, with a maximum value of 1.14 For example, in the present study, a subgroup in which none or all patients had retinopathy would have a Gini impurity value of zero. Thresholds were obtained requiring a minimum reduction of 0.01 in the sum within subgroups of the Gini impurity and a minimum size of 10 patients in each subgroup, to avoid overfitting. Only 1 split was permitted for each variable. All calculations were conducted using R, version 3.5.1 (The R Foundation).15 Decision trees were built using the library rpart.16
Results
We assessed 94 patients admitted with suspicion of YF between January 2017 and February 2018. Sixty-four patients who had received a confirmed diagnosis were included in the study. The median (interquartile range) age was 47 (38-56) years, and 12 patients (19%) were women. The median (interquartile range) values of the laboratory findings were as follows: AST levels, 978 (313-4713) U/L; total bilirubin levels, 1.0 (0.8-3.1) mg/dL (to convert to micromoles per liter, multiply by 17.104); serum creatinine levels, 1.1 (0.8-1.4) mg/dL; arterial lactate levels, 9.0 (7.2-16.2) mg/dL (to convert to millimoles per liter, multiply by 0.111); INR, 1.1 (1.0-1.5); and platelet count 94 (67-131) × 103/μL (to convert to ×109 per liter, multiply by 1). Thirty-six patients (56%) had severe disease, 25 patients (39%) needed ICU admission, and 11 patients (17%) died during hospitalization.
Twenty-nine patients (45%) were admitted between January 2017 and June 2017, during the 2016 to 2017 outbreak, and 35 patients (55%) were admitted between July 2017 and February 2018, during the 2017 to 2018 outbreak. Fundus changes were observed in 20 eyes (16%) of 13 patients (20%) with YF, 3 of 29 patients (10%) from the 2016 to 2017 outbreak and 10 of 35 patients (29%) from the 2017 to 2018 outbreak. Among patients with retinopathy, 7 of 13 (54%) had bilateral involvement (Table 1). The most common retinal findings were nerve fiber layer infarct (11 of 20 eyes; 55%) (Figure 1), superficial hemorrhage (7 of 20 eyes; 35%) (Figure 1 and Figure 2), including a Roth spot in 1 eye (Figure 2), and grayish lesions (6 of 20 eyes; 30%), possibly at the level of the outer retina or choroid (Figure 3). Other fundus changes that were observed among the 64 patients with confirmed YF infection but were considered unlikely to be associated with YF were optic disc pallor (2 patients [3%]), disc-cup asymmetry (1 patient [2%]), unspecific retinochoroidal scars (2 patients [3%]), focal retinal pigment epithelial hyperplasia (2 patients [3%]), and focal retinal pigment epithelial atrophy (1 patient [2%]). Among the 30 patients who were admitted with suspicion of YF but had this infection ruled out, the observed fundus changes were superficial hemorrhages (1 patient [3%]), solitary peridiscal retinal nerve fiber layer (RNFL) infarct (1 patient [3%]), choroidal nevus (1 patient [3%]), retinochoroidal scars (1 patient [3%]), retinal drusen (2 patients [7%]), synchysis scintillans (1 patient [3%]), myelinated RNFL (1 patient [3%]), and myopic degeneration (1 patient [3%]).
Table 1. Characterization of 13 Patients With Retinopathy Associated With Yellow Fever.
| Patient No. | AST, U/L | Total Bilirubin, mg/dL | Creatinine, mg/dL | Arterial Lactate, mg/dL | INR | Platelet Count, ×103/μL | Severe Disease | Retinal Finding |
|---|---|---|---|---|---|---|---|---|
| 1 | 14 | 5.9 | 4.2 | 20.7 | 1.9 | 93 | Yes | OD: RNFL infarcts; OS: RNFL infarcts and superficial hemorrhages |
| 2 | 12 872 | 7.9 | 4.3 | 18.0 | 2.5 | 42 | Yesa | OS: RNFL infarcts |
| 3 | 4560 | 9.8 | 2.2 | 8.1 | 1.0 | 73 | Yes | OD: RNFL infarcts and superficial hemorrhages; OS: RNFL infarcts |
| 4 | 9325 | 2.4 | 0.9 | 9.9 | 1.0 | 115 | Yes | OD: superficial hemorrhages |
| 5 | 10 613 | 3.0 | 1.1 | 9.9 | 1.0 | 139 | Yesa | OS: RNFL infarcts |
| 6 | 58 | 4.1 | 2.3 | 5.4 | 1.0 | 82 | Yes | OS: superficial hemorrhages |
| 7 | 1274 | 0.6 | 0.8 | 10.8 | 1.0 | 74 | No | OU: RNFL infarcts |
| 8 | 11 333 | 7.1 | 2.2 | 24.3 | 2.3 | 45 | Yes | OD: superficial hemorrhages; OS: Superficial hemorrhages and Roth spot |
| 9 | 16 220 | 6.7 | 8.9 | 149.5 | 5.0 | 88 | Yesa | OS: RNFL infarcts |
| 10 | 50 | 2.4 | 1.3 | 21.6 | 1.3 | 73 | Yes | OU: Grayish deep lesions |
| 11 | 12 196 | 2.8 | 0.9 | 72.1 | 1.6 | 59 | Yes | OS: RNFL infarcts |
| 12 | 5172 | 6.3 | 2.2 | 10.8 | 1.2 | 61 | Yes | OD: grayish deep lesions; OS: grayish deep lesions, superficial hemorrhages, and RNFL infarcts |
| 13 | 3437 | 1.3 | 0.5 | 12.6 | 1.1 | 19 | Yes | OU: grayish deep lesions |
Abbreviations: AST, aspartate aminotransferase; INR, international normalized ratio; RNFL, retinal nerve fiber layer.
SI conversion factors: To convert AST to microkatals per liter, multiply by 0.0167; total bilirubin and serum creatinine to micromoles per liter, multiply by 17.104 and 88.4, respectively; lactate to millimoles per liter, multiply by 0.111; platelet count to ×109 per liter, multiply by 1.
Deceased.
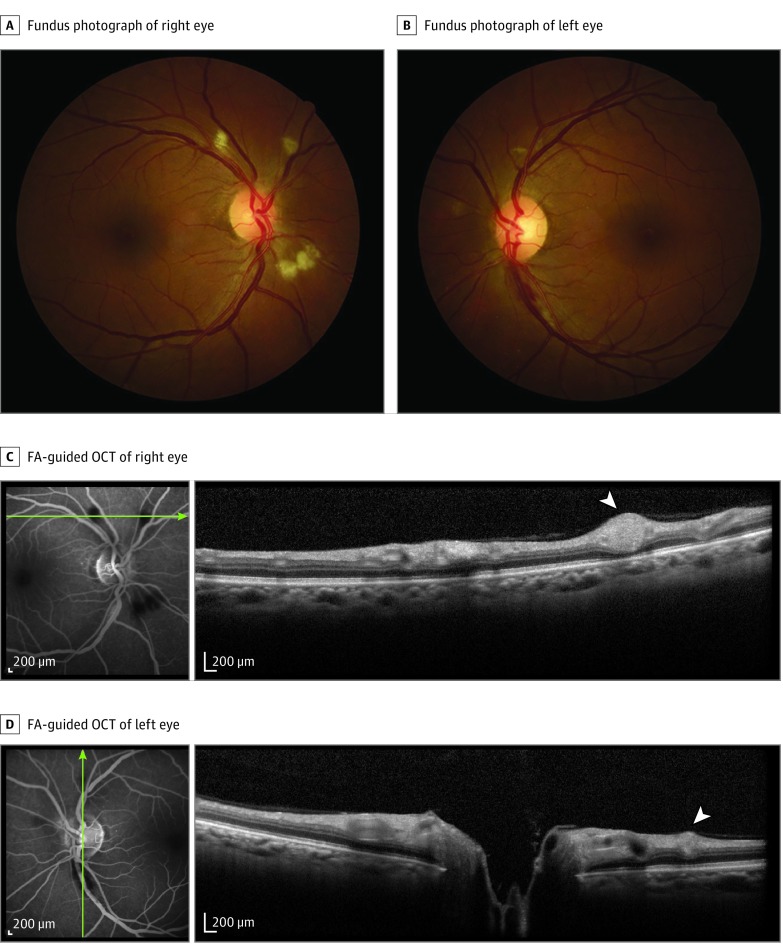
Figure 1. Images From Patient With Bilateral Retinopathy and Confirmed Yellow Fever Admitted to Intensive Care Unit Because of Systemic Decompensation With Hepatic and Renal Failure, Encephalopathy, and Profuse Gastrointestinal Bleeding.
Fundus photographs show retinal nerve fiber layer (RNFL) infarcts (A and B); a flame-shaped hemorrhage is present in the inferotemporal arcade of left eye (B). Fluorescein angiography (FA) discloses hypofluorescent blockage at the level of RNFL infarcts, with no evidence of hyperfluorescence of the optic disc, macula, or retinal blood vessels (C and D). Fluorescein angiography–guided optical coherence tomography (OCT) (C and D) shows hyperreflective nodules at the RNFL (white arrowheads) corresponding to the RNFL infarcts. Green lines indicate retinal topography of OCT section.
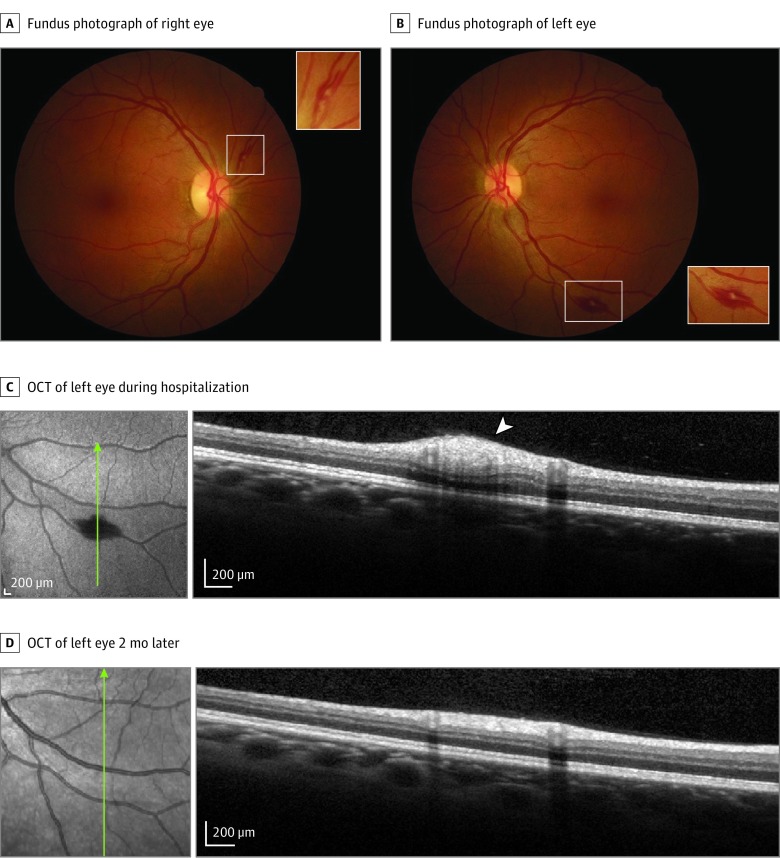
Figure 2. Images From Patient With Bilateral Retinopathy Admitted to Intensive Care Unit Because of Systemic Decompensation With Renal Failure, Thrombocytopenia, and Coagulopathy.
Fundus photographs show superficial hemorrhages (white rectangles; A and B), including a Roth spot in left eye (B). Insets are magnifications of the areas shown in the white rectangles. Optical coherence tomography (OCT) of the left eye over the Roth spot (white arrowhead) is shown during hospitalization (C) and 2 months later, after recovery (D). Green lines indicate retinal topography of OCT section.
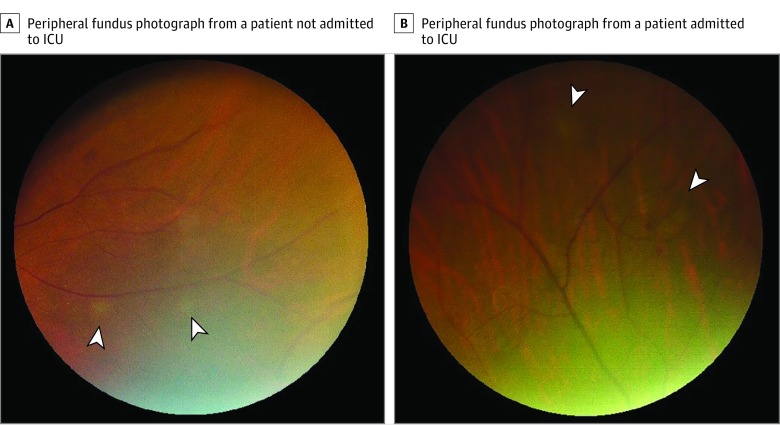
Figure 3. Fundus Photographs Showing Peripheral, Grayish, Deep Lesions, Possibly at the Level of the Outer Retina or Choroid.
A, Right eye of a patient with severe systemic disease but who did not need intensive care unit (ICU) admission. B, Left eye of a patient with severe systemic disease who was admitted to the ICU because of hemodynamic instability. Both patients presented with bilateral retinopathy. The white arrowheads indicate deep lesions.
In the 2017 to 2018 outbreak, none of the patients with retinopathy reported visual problems and 2 patients without retinopathy (4%) reported eye pain. All patients, except those unconscious at the ICU, who therefore could not be tested, had normal Amsler grid test results.
Clinical findings and laboratory biomarkers of retinopathy (vs no retinopathy) are provided in Table 2. Median (range; interquartile range) AST levels (9325 [688-16 220; 3437-12 196] U/L vs 652 [85-24 820; 265-2196] U/L; P = .007), total bilirubin levels (4.1 [0.6-9.8; 2.4-6.7] mg/dL vs 0.9 [0.31–14.5; 0.7-2.1] mg/dL; P < .001), serum creatinine levels (2.2 [0.5-9.9; 0.9-2.3] mg/dL vs 1.0 [0.4–5.6; 0.8-1.3] mg/dL; P = .049), frequency of severe systemic disease (12 of 13 [92%] vs 24 of 51 [47%]; P = .003), and rate of ICU admission (9 of 13 [69%] vs 16 of 51 [31%]; P = .02) were higher in patients with retinopathy vs those without retinopathy. In addition, the median (range; interquartile range) platelet count (73 [19-139; 59-88] × 103/μL vs 103 [32-362; 69-139] × 103/μL; P = .02) was lower in patients with retinopathy vs those without retinopathy. Classification of disease as severe11 was associated with retinopathy, with 12 of 39 patients (31%) with severe disease presenting fundus changes consistent with YF retinopathy vs only 1 of 25 patients (4%) with nonsevere disease displaying retinopathy (OR, 9.3; 95% CI, 1.6-238.9; P = .009). Admission to the ICU was also associated with retinopathy (OR, 4.7; 95% CI, 1.3-20.3; P = .02), whereas arterial hypertension and diabetes were not associated.
Table 2. Biomarkers of Retinopathy in Patients With Yellow Fevera.
| Demographic | Patients, No. (%) | |
|---|---|---|
| No Retinopathy (n = 51) | Retinopathy (n = 13) | |
| Age, median (IQR), y | 48 (36-56) | 46 (40-51) |
| Female sex | 10 (20) | 2 (15) |
| Comorbidityb | ||
| Arterial hypertension | 14 (27) | 4 (33) |
| Diabetes | 5 (10) | 2 (17) |
| Laboratory findings | ||
| AST, U/L | ||
| Median (IQR) | 652 (265-2196) | 9325 (3437-12 196) |
| >3000c | 9 (18) | 10 (77) |
| Total bilirubin, mg/dL | ||
| Median (IQR)c | 0.9 (0.7-2.1) | 4.1 (2.4-6.7) |
| >2.3c | 10 (20) | 11 (85) |
| Creatinine, mg/dL | ||
| Median (IQR)c | 1.0 (0.8-1.3) | 2.2 (0.9-2.3) |
| >2.0c | 6 (12) | 7 (54) |
| Arterial lactate, mg/dLd | ||
| Median (IQR) | 9 (7.2-11.7) | 12.3 (8.1-20.7) |
| >17.1c | 7 (15) | 6 (46) |
| INR | ||
| Median (IQR) | 1.1 (1.0-1.3) | 1.3 (1.0-1.9) |
| >1.5 | 8 (16) | 5 (38) |
| Platelet count, ×103/μL | ||
| Median (IQR)c | 103 (69-139) | 73 (59-88) |
| <94c | 20 (39) | 11 (85) |
| Disease severity | ||
| Severec | 24 (47) | 12 (92) |
| ICU admissionc | 16 (31) | 9 (69) |
| Deceased | 8 (16) | 3 (23) |
Abbreviations: AST, aspartate aminotransferase; ICU, intensive care unit; INR, international normalized ratio; IQR, interquartile range.
SI conversion factors: To convert AST to microkatals per liter, multiply by 0.0167; total bilirubin and serum creatinine to micromoles per liter, multiply by 17.104 and 88.4, respectively; lactate to millimoles per liter, multiply by 0.111; platelet count to ×109 per liter, multiply by 1.
Continuous variables are expressed as median (IQR) and categorical variables as absolute number (percentage).
One missing value in the group with retinopathy.
Statistically significant at α < .05.
Five missing values in the group without retinopathy.
Using classification and regression trees as a prediction model for retinopathy, we found thresholds for AST, total bilirubin, serum creatinine, and arterial lactate levels as well as for INR and platelet count, respecting the described criteria. We then sought to evaluate whether an association existed between retinopathy and those laboratory biomarkers (Table 2). AST levels higher than 3000 U/L (OR, 14.2; 95% CI, 3.5-77.8; P < .001), total bilirubin levels higher than 2.3 mg/dL (OR, 20.0; 95% CI, 4.4-159.7; P < .001), serum creatinine levels higher than 2.0 mg/dL (OR, 8.2; 95% CI, 2.1-36.0; P = .003), arterial lactate levels higher than 17.1 mg/dL (OR, 4.6; 95% CI, 1.1-19.0; P = .03), platelet count lower than 94 × 103/μL (OR, 7.8; 95% CI, 1.8-59.9; P = .004), severe disease (OR, 11.7; 95% CI, 2.0-301.0; P = .003), and ICU admission (OR, 4.7; 95% CI, 1.3-20.3; P = .02) were associated with retinopathy. By contrast, an INR greater than 1.5 (OR, 3.3; 95% CI, 0.8-13.1; P = .10) and death (OR, 1.6; 95% CI, 0.3-7.1; P = .54) were not associated with retinopathy. In a multivariate analysis using as independent variables those laboratory values with the greatest ORs in univariate analyses (AST levels >3000 U/L, total bilirubin levels >2.3 mg/dL, serum creatinine levels >2.0 mg/dL, and platelet count <94 × 103/μL), only total bilirubin levels higher than 2.3 mg/dL were associated with retinopathy (OR, 8.3; 95% CI, 1.2-77.9; P = .04).
Discussion
We report retinal changes among 13 patients with confirmed YF who were examined at a referral center in Southeastern Brazil. The country had recently experienced the largest outbreak of YF in its history, associated with periurban sylvatic transmission, but raising awareness of the risk of urban dissemination through Aedes aegypti mosquitoes, which were also implicated in recent outbreaks of Zika virus, chikungunya, and dengue in Brazil and in other parts of the world. According to the Brazilian Ministry of Health, more than 80% of the confirmed cases of YF in these outbreaks occurred in males.8 This presumably results from greater exposure of male rural workers in high risk areas. The emergence of these mosquito-borne viral diseases has been of global concern, unveiling novel yet unrecognized ocular manifestations, as we describe herein for YF.
Ocular changes previously described in YF have been limited to conjunctival icterus or hyperemia and vaccine complications.3,4,5,6 Searching MEDLINE and PubMed for “ocular” and “yellow fever” on November 26, 2018, we found only 13 articles, of which only 3 cited any ocular finding associated with the disease or its vaccine (a live-attenuated YF virus).3,4,5 We hypothesize that retinopathy associated with YF has probably been overlooked because of the absence of ocular symptoms and also because of the severity of systemic involvement, frequently demanding admission to the ICU and limiting ophthalmic assessment in this context.
Overall, 13 of 64 patients (20%) with confirmed YF had evidence of retinopathy, 3 of 29 (10%) from the 2016 to 2017 outbreak and 10 of 35 (29%) from the 2017 to 2018 outbreak. Our results showed an association between abnormal laboratory biomarkers and occurrence of retinopathy. Elevated AST levels, elevated total bilirubin levels, and renal failure have already been associated with higher mortality in patients with YF.1,17 Disease classified as severe11 was associated with retinopathy in the present study, with 12 of 39 patients (31%) with severe disease presenting fundus changes consistent with YF retinopathy vs only 1 of 25 patients (4%) with nonsevere disease displaying retinopathy. Admission to the ICU was also associated with retinopathy, whereas arterial hypertension and diabetes were not associated with retinopathy.
In multivariate analysis, only total bilirubin levels higher than 2.3 mg/dL were associated with retinopathy (P = .04), confirming that an elevated total bilirubin level may be the most important biomarker of retinopathy because it also had the greatest OR in the univariate analysis. However, owing to the limited sample size and consequent restricted statistical power of the study, we could not assert that other variables were not associated with retinopathy.
The most common fundus changes among the 20 eyes were RNFL infarcts (11 [55%]), superficial hemorrhages (7 [35%]), including Roth spots, and grayish deep lesions that appeared to be at the level of the outer retina or choroid (6 [30%]). The observed RNFL infarcts and superficial hemorrhages were nonspecific and similar to those described among individuals infected by other flaviviruses, such as dengue virus.3,18,19,20 These acute microangiopathic changes are probably associated with occlusion of RNFL precapillary arterioles (Figure 1 and Figure 2) owing to immune-mediated endothelial damage and coagulopathy, peaking at the nadir of thrombocytopenia.19,20,21 In the present study, retinopathy was found to be associated with thrombocytopenia, supporting this hypothesis. The pathophysiology of the grayish deep lesions is unknown, and we were unable to document them using multimodal imaging. However, we hypothesize that these lesions might also result from ischemic changes, but at the level of the choriocapillaris/retinal pigment epithelium/deep retina. Lesions with a pale-gray appearance at the level of photoreceptors have been described after Ebola virus disease,22,23 sharing some resemblance to the peripheral grayish lesions described among the patients with YF in the present study. However, these lesions were disclosed during acute illness in YF, in contrast to those observed during convalescence among Ebola virus survivors. In addition, the characteristic sharp angulated margins of retinal lesions associated with Ebola virus were not observed among the patients with YF in the present study.
Limitations
Our study has some limitations. Only patients who had received a confirmed diagnosis of YF and had been admitted to a referral center were included, and they may not represent all infected patients. At the other end of the spectrum, patients with fulminant disease rapidly progressing to death may not have been examined. Subtle retinal changes, such as the grayish deep peripheral lesions, might have been overlooked in some patients. Widefield imaging and a consistent retinal imaging protocol would have been useful to better document, quantify, and follow up retinal changes in these patients. Unfortunately, this was not possible in the setting of these 2 outbreaks with acutely ill patients, many of whom had been admitted to the ICU. Finally, as a cross-sectional study, causality of retinal changes cannot be definitely inferred.
Conclusions
Up to 20% of patients with YF displayed retinal changes, even in the absence of reports by patients of ocular problems. The results of the present study indicated that these changes were associated with laboratory biomarkers of more severe systemic disease. These findings suggest that ophthalmic screening of patients with YF should be encouraged so that further studies may assess and validate retinopathy as a possible prognostic marker in patients with YF.
References
- 1.Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001;1(1):11-20. doi: 10.1016/S1473-3099(01)00016-0 [DOI] [PubMed] [Google Scholar]
- 2.Monath TP, Vasconcelos PF. Yellow fever. J Clin Virol. 2015;64:160-173. doi: 10.1016/j.jcv.2014.08.030 [DOI] [PubMed] [Google Scholar]
- 3.Merle H, Donnio A, Jean-Charles A, et al. Ocular manifestations of emerging arboviruses: dengue fever, chikungunya, Zika virus, West Nile virus, and yellow fever. J Fr Ophtalmol. 2018;41(6):e235-e243. doi: 10.1016/j.jfo.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 4.Biancardi AL, Moraes HV. Anterior and intermediate uveitis following yellow fever vaccination with fractional dose: case reports. Ocul Immunol Inflamm. 2018. doi: 10.1080/09273948.2018.1510529 [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Kumar A. Ocular manifestations of emerging flaviviruses and the blood-retinal barrier. Viruses. 2018;10(10):E530. doi: 10.3390/v10100530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moysidis SN, Koulisis N, Patel VR, et al. The second blind spot: small retinal vessel vasculopathy after vaccination against Neisseria meningitidis and yellow fever. Retin Cases Brief Rep. 2017;11(suppl 1):S18-S23. doi: 10.1097/ICB.0000000000000391 [DOI] [PubMed] [Google Scholar]
- 7.The Lancet Yellow fever: a major threat to public health. Lancet. 2018;391(10119):402. doi: 10.1016/S0140-6736(18)30152-1 [DOI] [PubMed] [Google Scholar]
- 8.Ministerio da Saude do Brasil. Monitoramento do período sazonal da febre amarela Brasil–2017/2018 [in Portuguese]. http://portalarquivos2.saude.gov.br/images/pdf/2018/maio/09/Informe-FA.pdf. Accessed May 4, 2019.
- 9.Paules CI, Fauci AS. Yellow fever–once again on the radar screen in the Americas. N Engl J Med. 2017;376(15):1397-1399. doi: 10.1056/NEJMp1702172 [DOI] [PubMed] [Google Scholar]
- 10.Dyer O. Yellow fever stalks Brazil in Zika’s wake. BMJ. 2017;356:j707. doi: 10.1136/bmj.j707 [DOI] [PubMed] [Google Scholar]
- 11.Governo do Estado de Minas Gerais—Secretaria de Estado de Saúde de Minas Gerais. Manejo clínico da febre amarela [in Portuguese]. http://www.saude.mg.gov.br/images/documentos/Manejo%20Clinico%20Febre%20Amarela%20SES-MG_03-02-2017.pdf. Published 2017. Accessed May 9, 2019.
- 12.Mikami A, Ohde S, Deshpande GA, Mochizuki T, Otani N, Ishimatsu S. Can we predict arterial lactate from venous lactate in the ED? Am J Emerg Med. 2013;31(7):1118-1120. doi: 10.1016/j.ajem.2013.03.034 [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 14.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984. [Google Scholar]
- 15.R Core Team. The R project for statistical computing. https://www.r-project.org. Accessed May 4, 2019.
- 16.Therneau TM, Atkinson EJ Mayo Foundation. An introduction to recursive partitioning using the RPART routines. https://cran.r-project.org/web/packages/rpart/vignettes/longintro.pdf. Published April 11, 2019. Accessed May 4, 2019.
- 17.Tuboi SH, Costa ZG, da Costa Vasconcelos PF, Hatch D. Clinical and epidemiological characteristics of yellow fever in Brazil: analysis of reported cases 1998-2002. Trans R Soc Trop Med Hyg. 2007;101(2):169-175. doi: 10.1016/j.trstmh.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 18.Kamath SJ, Nayak MK, Gour R, Singh N. Dengue stings the eye! J Clin Diagn Res. 2017;11(9):ND03-ND05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng AW, Teoh SC. Dengue eye disease. Surv Ophthalmol. 2015;60(2):106-114. doi: 10.1016/j.survophthal.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 20.Su DH, Bacsal K, Chee SP, et al. ; Dengue Maculopathy Study Group . Prevalence of dengue maculopathy in patients hospitalized for dengue fever. Ophthalmology. 2007;114(9):1743-1747. doi: 10.1016/j.ophtha.2007.03.054 [DOI] [PubMed] [Google Scholar]
- 21.Messaoudi I, Basler CF. Immunological features underlying viral hemorrhagic fevers. Curr Opin Immunol. 2015;36:38-46. doi: 10.1016/j.coi.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steptoe PJ, Scott JT, Baxter JM, et al. Novel retinal lesion in Ebola survivors, Sierra Leone, 2016. Emerg Infect Dis. 2017;23(7):1102-1109. doi: 10.3201/eid2307.161608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steptoe PJ, Momorie F, Fornah AD, et al. Multimodal imaging and spatial analysis of Ebola retinal lesions in 14 survivors of Ebola virus disease. JAMA Ophthalmol. 2018;136(6):689-693. doi: 10.1001/jamaophthalmol.2018.1248 [DOI] [PMC free article] [PubMed] [Google Scholar]