Key Points
Question
How has use of anti–vascular endothelial growth factor agents changed over time?
Findings
In this cohort study of US Medicare Part B beneficiaries nationally, aflibercept injections increased by 69.4% from 2013 to 2015. By 2015, the 100 ophthalmologists performing the highest volume of ranibizumab injections, as gauged by number of injections administered, accounted for 31.0% of all ranibizumab injections performed nationally, while the 100 ophthalmologists performing the highest volume of aflibercept injections accounted for 17.6% of all aflibercept injections, and the 100 ophthalmologists performing the highest volume of bevacizumab injections accounted for 19.6% of all bevacizumab injections.
Meaning
The frequency of anti–vascular endothelial growth factor injection has been increasing from 2013 to 2015; throughout this time, a small portion of ophthalmologists accounted for a large share of the national use.
Abstract
Importance
The frequency of anti–vascular endothelial growth factor (VEGF) injections has grown exponentially with the introduction of bevacizumab, ranibizumab, and most recently aflibercept. The cost associated with these medications has garnered significant national attention, warranting a granular analysis of their use.
Objective
To analyze trends in anti-VEGF injections for US Medicare Part B beneficiaries from 2012 to 2015.
Design, Setting, and Participants
This observational cohort study used 2012-2015 data from the Centers for Medicare & Medicaid Services Medicare Part B Provider Utilization Files to analyze trends in intravitreal injections of anti-VEGF medications among Medicare Part B beneficiaries and their health care professionals.
Main Outcomes and Measures
The primary outcome measure was distribution of and change over time in the number of anti-VEGF injections performed for ranibizumab, aflibercept, and bevacizumab.
Results
A total of 2 574 124 intravitreal injections were performed by 3348 ophthalmologists in the outpatient setting for Medicare Part B beneficiaries during the 2015 calendar year; 100 ophthalmologists (3.0%) performed the highest volume of intravitreal injections. The total number of intravitreal injections administered in 2015 was 870 843 for aflibercept, 697 412 for ranibizumab, and 1 147 432 for bevacizumab. Ranibizumab injections decreased by 7.1% from 2012 to 2015 and bevacizumab injections decreased by 17.1%. From 2013 to 2015, aflibercept injections increased by 69.4%. The 100 ophthalmologists performing the highest volume of ranibizumab injections, as gauged by number of injections administered, accounted for 31.0% (95% CI, 30.994%-30.997%) of all ranibizumab injections nationally. The 100 ophthalmologists performing the highest volume of aflibercept injections accounted for 17.6% (95% CI, 17.638%-17.641%) of all aflibercept injections and the 100 ophthalmologists performing the highest volume of bevacizumab injections accounted for 19.6% (95% CI, 19.649%-19.653%) of all bevacizumab injections administered nationally to Medicare Part B beneficiaries. The highest number of injections per 1000 Medicare Part B beneficiaries occurred in Nebraska (aflibercept), Tennessee (ranibizumab), and South Dakota (bevacizumab).
Conclusions and Relevance
A total of 3.0% of ophthalmologists account for 17.6% to 31.0% of the total number of anti-VEGF injections administered nationally in the Medicare Part B population. Overall, bevacizumab and ranibizumab injections have decreased, coinciding with a 69.4% increase in aflibercept injections.
This cohort study analyzes trends in anti–vascular endothelial growth factor (anti-VEGF) injections for US Medicare Part B beneficiaries from 2012 to 2015.
Introduction
Since the identification of vascular endothelial growth factor (VEGF) as a mediator of retinal and choroidal neovascularization,1,2 anti-VEGF agents have been universally adopted as the first-line treatment for several ocular conditions.3,4,5,6,7,8 Pegaptanib (an anti-VEGF RNA aptamer) was approved for neovascular age-related macular degeneration (AMD) in 2004.9,10 Approval of bevacizumab (human anti-VEGF) in combination with chemotherapy for colon cancer came in 2004,11,12,13 with evidence for the use of bevacizumab for neovascular AMD emerging soon after.14,15 This approval coincided with the development of ranibizumab, an anti-angiogenic monoclonal antibody fragment.16 Ranibizumab has been shown to be highly effective for neovascular AMD,17,18 macular edema after branch retinal vein occlusion19,20 and central retinal vein occlusion,21,22 and diabetic macular edema.23,24 Ranibizumab was approved by the US Food and Drug Administration (FDA) for multiple ocular conditions, while use of bevacizumab in ophthalmology remains off-label. Aflibercept, a “VEGF-trap” (an agent that blocks the action of VEGF)25 approved by the FDA in 2011, is likewise effective for AMD,26 macular edema after retinal vein occlusion,27 and diabetic macular edema.28
There is a growing body of high-quality evidence illustrating the modest differences in the efficacy of these 3 anti-VEGF therapies. The Comparison of Age-Related Macular Degeneration Treatments Trials (CATT), published in 2011, represented a seminal publication comparing anti-VEGF agents.29 The study group showed that visual gains with monthly bevacizumab for patients with neovascular AMD were noninferior to visual gains with monthly ranibizumab. For neovascular AMD, the data suggest that both agents, when given monthly, were similarly effective with regard to visual gains. This landmark trial paved the way for future comparisons of bevacizumab, ranibizumab, and aflibercept. For neovascular AMD, the data suggest that all 3 agents are equally effective with regard to visual gains.30,31 Recent evidence for treating diabetic macular edema shows that aflibercept is more effective than bevacizumab and ranibizumab in patients with poorer initial visual acuity after 1 year of treatment.32 An interdrug difference among patients with poorer visual acuity persisted with respect to mean visual acuity change from baseline, although no clinically relevant difference (eg, secondary outcomes of gaining ≥10 letters) persisted beyond 1 year. Furthermore, there were no differences in mean change in visual acuity at 1 or 2 years among the 3 drugs in patients with better baseline visual acuity.33 Vision gains are also similar between the 3 agents in the setting of retinal vein occlusion.34,35
The approximately 40-fold price differences between these anti-VEGF therapies in the context of comparable proven efficacy for AMD, diabetic macular edema, and retinal vein occlusion alongside the growing economic burden of health care costs warrant an exploration of feasible, cost-effective stewardship. In 2010, bevacizumab and ranibizumab accounted for approximately $2 billion of Medicare Part B expenditures,36 and by 2015 aflibercept was the leading Medicare Part B medication in overall spending, with aflibercept and ranibizumab accounting for 12% of the Medicare Part B budget annually.37 Considering the variability in the cost of anti-VEGF therapies (aflibercept, 2.0 mg, $1850; ranibizumab, 0.3 mg, $1170 and 0.5 mg, $1950; and bevacizumab repackaged, 1.25 mg, $60)38,39 and the increasing number of injections performed annually, there is much to be gained by a granular analysis of national use of anti-VEGF therapies. We hypothesize that there is a top-heavy distribution such that a small percentage of ophthalmologists account for a disproportionate amount of drug administration nationally. We set out to understand better the national use of the 3 main anti-VEGF agents by examining Medicare Part B claims data from 2012 to 2015.
Methods
Data Source
The Centers for Medicare & Medicaid Services (CMS) Medicare Part B Provider Utilization Files were accessed to gather the data for analysis in this observational cohort study. Specifically, we analyzed the 2012-2015 Medicare Part B Provider Utilization Files (the most recent data set made publicly available at study onset). The data files provide information about the spending on and use of Medicare Part B drugs (eg, drugs administered in physicians’ offices and outpatient settings). Vanderbilt University Medical Center Institutional Review Board exempted this study from review because the study did not meet the criteria for research as described in 45 CFR §46.102(d). Patient consent was not required, as no protected health information was used to analyze these publicly available records.
Data were refined to include all claims for intravitreal injection (Current Procedural Terminology code 67028) submitted nationally. These procedures were further delineated using Healthcare Common Procedure Coding System (HCPCS) code J2778 for ranibizumab services, code J0178 for aflibercept services, and codes J9035, J3490, and J3590 for bevacizumab services. Code J2778 represents 0.1 mg of ranibizumab injected, code J0178 represents 1 mg of aflibercept injected, and codes J9035, J3490, and J3590 all represent commonly submitted claims for off-label ophthalmologic use of bevacizumab. Ophthalmologists were not included in the data set if they did not submit claims for at least 11 services of a particular drug in the calendar year.
The total number of services billed for nationally was tabulated and then converted to injections using the following formula: 1 ranibizumab injection = 4 units (services) of ranibizumab (mean of 0.3-mg and 0.5-mg doses), 1 aflibercept injection = 2 units (services) of aflibercept, and 1 bevacizumab injection = 1 unit (service) of bevacizumab. Data were stratified across states and compared with the number of Medicare beneficiaries in each state. Further analysis was conducted to identify the ophthalmologists administering the highest volume of the 3 medications, as determined by total number of injections billed for.
Statistical Analysis
Medicare Part B use data were downloaded and organized into spreadsheets in Excel (Microsoft Corp). The prescription rates of aflibercept, bevacizumab, and ranibizumab were summarized by counts per 1000 beneficiaries. The prescription rates by the 100 ophthalmologists performing the highest volume of injections were reported for each medication with the 95% CIs constructed using bootstrap method. In particular, for each medication, we sampled the patient-level data set with replacement, found the 100 ophthalmologists performing the highest volume of injections, and then calculated the prescription rate by the 100 ophthalmologists performing the highest volume of injections. This procedure was repeated 1000 times, and 95% CIs were constructed using the 2.5% and 97.5% quantiles of the 1000 estimates calculated from bootstrap samples. The pairwise rate differences and their 95% CIs were constructed similarly. The maps of injections per 1000 Medicare beneficiaries for aflibercept, bevacizumab, and ranibizumab were plotted. Bonferroni correction was used to adjust for the pairwise comparisons among the 3 groups. A significance level of P = .0167 (P = .05/3) was used for statistical inference. All analyses were implemented using R, version 3.3.0 (R Foundation for Statistical Computing), with packages fiftystater and viridis for plotting.
Results
A total of 2 574 124 intravitreal injections (Current Procedural Terminology code 67028) were performed in the outpatient setting for Medicare Part B beneficiaries for the 2015 calendar year. These injections were administered by 3348 ophthalmologists (ophthalmologists performing ≤10 intravitreal injections in 2015 were excluded from the data file); 100 ophthalmologists (3.0%) performed the highest volume of intravitreal injections.
The total number of intravitreal injections administered in 2015 was 870 843 for aflibercept (HCPCS code J0178), 697 412 for ranibizumab (HCPCS code J2778), and 1 147 432 for bevacizumab (HCPCS codes J9035, J3490, and J3590) (Table). Ranibizumab claims in the Medicare population decreased by 7.1% from 2012 to 2015. Similarly, bevacizumab claims decreased by 17.1% during this period. Conversely, aflibercept claims increased by 69.4% from 2013 to 2015.
Table. Anti–Vascular Endothelial Growth Factor Injections Administered to Medicare Part B Population in 2015.
| Injections | Total No. of Intravitreal Injections | Aflibercept | Ranibizumab | Bevacizumab |
|---|---|---|---|---|
| Total No. of injections | 2 574 124a | 870 843 | 697 412 | 1 147 432 |
| Total No. (%) of injections administered by ophthalmologists with highest volume of intravitreal injections | 332 203 (12.9) | 153 467 (17.6) | 216 142 (31.0) | 225 083 (19.6) |
| Mean No. of injections administered by ophthalmologists with highest volume of intravitreal injections | 3322 | 1535 | 2161 | 2251 |
Total excludes clinicians administering 10 or fewer intravitreal injections in 2015.
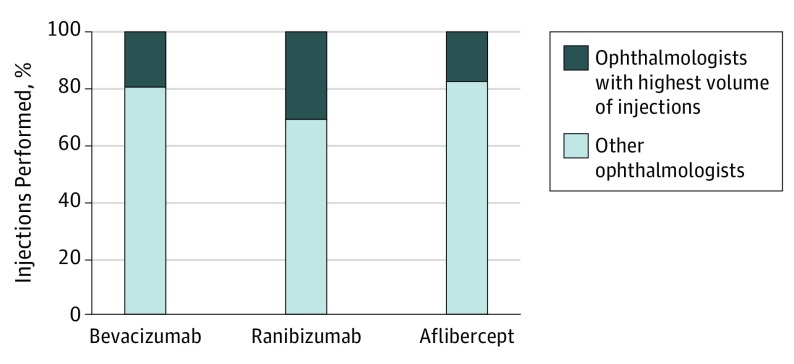
The 100 ophthalmologists with the highest volume of intravitreal injections performed 332 203 intravitreal injections in the Medicare Part B population in 2015, or 12.9% of the national total. The 100 ophthalmologists performing the highest volume of ranibizumab injections, as gauged by the number of injections administered, accounted for 31.0% (95% CI, 30.994%-30.997%) of all ranibizumab injections nationally in 2015 for Medicare Part B beneficiaries. Similarly, the 100 ophthalmologists performing the highest volume of aflibercept injections accounted for 17.6% (95% CI, 17.638%-17.641%) of all aflibercept injections and the 100 ophthalmologists performing the highest volume of bevacizumab injections accounted for 19.6% (95% CI, 19.649%-19.653%) of all bevacizumab injections in Medicare Part B beneficiaries (Figure 1). The percentage of use by the 100 ophthalmologists performing the highest volume of injections was statistically significantly greater for ranibizumab when compared with aflibercept (17.6%; 95% CI, 17.638%-17.641%; P < .001) and bevacizumab (19.6%; 95% CI, 19.649%-19.653%; P < .001).
Figure 1. Proportion of National Aflibercept, Ranibizumab, and Bevacizumab Injections Performed in Medicare Part B Population in 2015 by 100 Ophthalmologists With Highest Volume of Injections.
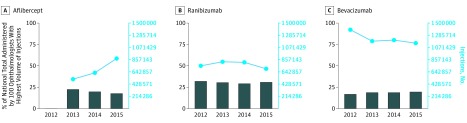
Similar data were analyzed for years 2012-2014 to identify any trends (eTable 1 in the Supplement). Use by the 100 ophthalmologists performing the highest volume of injections (vs the national total) was stable for ranibizumab over time (31.9% in 2012 and 31.0% in 2015). Bevacizumab’s share of the national total among the 100 ophthalmologists performing the highest volume of injections increased from 17.0% in 2012 to 19.6% in 2015. For aflibercept, the share of the national total among the 100 ophthalmologists performing the highest volume of injections decreased from 22.4% in 2013 to 17.6% in 2015.
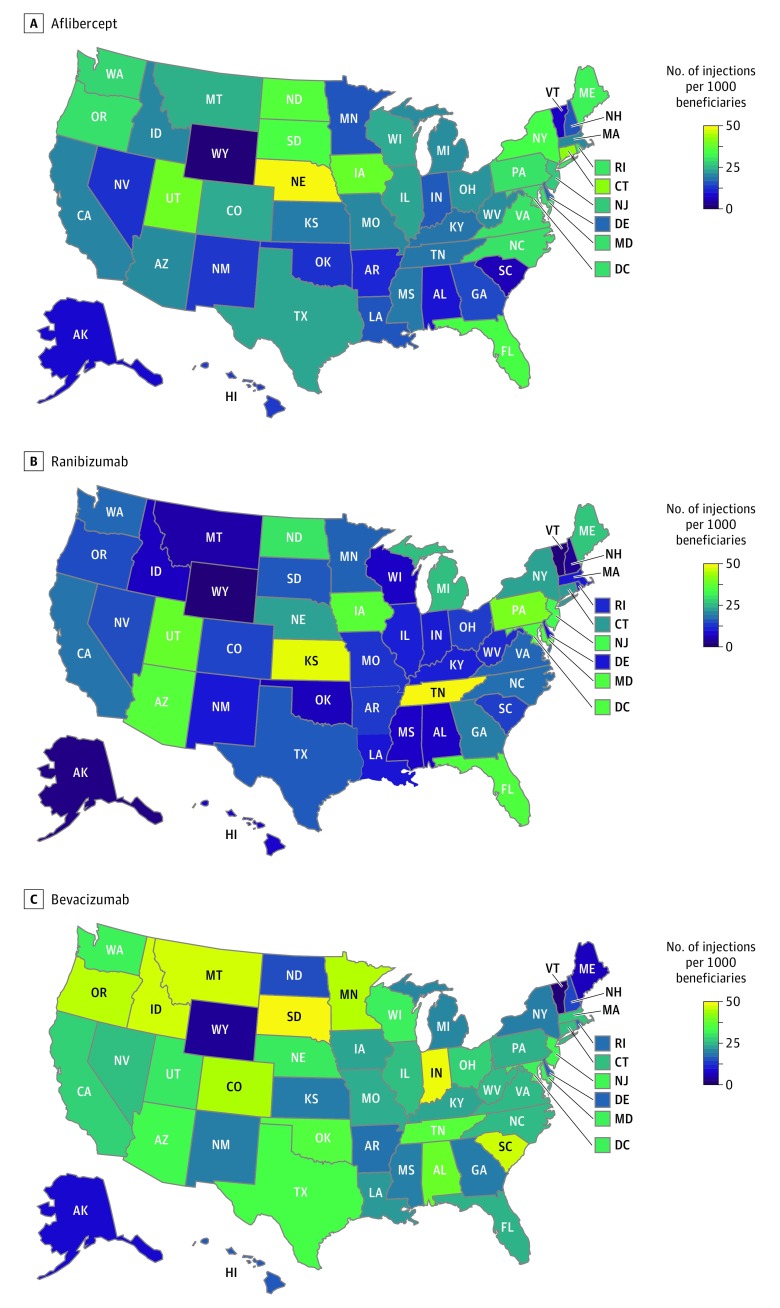
The highest number of injections per 1000 Medicare Part B beneficiaries occurred in Nebraska (aflibercept), Tennessee (ranibizumab), and South Dakota (bevacizumab) (eTable 2 in the Supplement). The geographical distribution is demonstrated in Figure 2.
Figure 2. Geographical Distribution of Mean Number of Anti–Vascular Endothelial Growth Factor Injections per 1000 Medicare Part B Beneficiaries in 2015 for Aflibercept, Ranibizumab, and Bevacizumab.
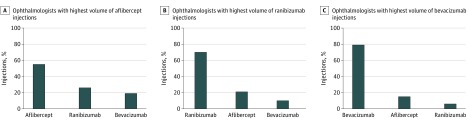
Thirteen ophthalmologists are among the 100 ophthalmologists performing the highest volume of aflibercept injections and the 100 ophthalmologists performing the highest volume of ranibizumab injections. Nine ophthalmologists are among the 100 ophthalmologists performing the highest volume of aflibercept injections and the 100 ophthalmologists performing the highest volume of bevacizumab injections. Conversely, there were no ophthalmologists overlapping between the 100 ophthalmologists performing the highest volume of ranibizumab injections and the 100 ophthalmologists performing the highest volume of bevacizumab injections.
Discussion
In 2015, more than 2.5 million intravitreal injections were administered by 3 348 ophthalmologists across the country. Using CMS Medicare Part B claims data to identify the number of injections administered for aflibercept, ranibizumab, and bevacizumab, we were able to identify several important injection trends.
Confirming our hypothesis, there is a top-heavy distribution when examining the ophthalmologists performing the highest volume of injections. For 2015, the 100 ophthalmologists performing the highest volume of injections (as identified by number of injections billed for in the calendar year) for each medication represent 3% of qualifying anti-VEGF injecting providers. This cohort, however, accounted for 31.0% of all ranibizumab administered nationally in the Medicare Part B population, compared with 17.6% for aflibercept (P < .001) and 19.6% for bevacizumab (P < .001) administered in the Medicare Part B population.
There are many points to consider when interpreting these data. The data set included all practitioners performing more than 10 intravitreal injections annually (the CMS data set excludes data from those performing 10 or fewer services). It is not surprising that the vitreoretinal specialists performing the highest volume of intravitreal injections would perform a large share of national injections compared with colleagues performing a lower volume of intravitreal injections or general ophthalmologists who may only perform a few intravitreal injections annually. The question remains whether these 3 groups represent distinct cohorts. Specifically, are there certain clinicians performing a high volume of injections who use all 3 agents with high frequency, or do the above groups preferentially choose a certain medication?
Exploration of the data provides further insight on this matter. Nine of the top injectors of aflibercept also inject bevacizumab in high numbers. Conversely, there was no overlap between the 100 ophthalmologists performing the highest volume of ranibizumab injections and the 100 ophthalmologists performing the highest volume of bevacizumab injections. The data indicate that certain clinicians who perform a high volume of intravitreal injections are injecting a large amount of aflibercept and bevacizumab or a large amount of aflibercept and ranibizumab. However, the ophthalmologists performing the highest volume of ranibizumab injections do not, according to the above data, incorporate bevacizumab with the same frequency. The injection distribution of the ophthalmologists performing the highest volume of intravitreal injections is illustrated in Figure 3.
Figure 3. Distribution of Anti–Vascular Endothelial Growth Factor Drug Choice by 100 Ophthalmologists With Highest Volume of Injections for Aflibercept, Ranibizumab, and Bevacizumab.
The CATT results, published in 2011, demonstrated that bevacizumab and ranibizumab, when given monthly, were similarly efficacious with regard to visual gains in the treatment of neovascular AMD.29 In 2012, shortly after CATT, the most common practice for physicians was the use of all 3 agents, followed by the use of bevacizumab alone.40 Our study found that in the 4 years after the release of the CATT results, ranibizumab claims in the Medicare population initially rose but subsequently decreased by 7% overall and that the 100 ophthalmologists performing the highest volume of ranibizumab injections consistently accounted for 29% to 32% of the national total annually. Meanwhile, bevacizumab claims decreased during the study period by 17%. Despite this reduction, the ophthalmologists performing the highest volume of injections have accounted for an increasing percentage of national use. This cohort was consistently using bevacizumab at the time the CATT results were released and subsequently continued to see the value of incorporating this low-cost alternative in their practice. At the same time, the reductions in ranibizumab and bevacizumab claims coincided with an increase in aflibercept usage from 2013 to 2015. As more aflibercept was administered nationally, the distribution became less top-heavy (Figure 4). These trends warrant discussion of the financial and geographic context for selection of anti-VEGF agents.
Figure 4. Trend in Number of Injections Administered Annually in Medicare Part B Population vs Percentage of Use by 100 Ophthalmologists With Highest Volume of Injections for Aflibercept, Ranibizumab, and Bevacizumab.
Decision making regarding choice of anti-VEGF agent is complex. Despite relatively equivocal clinical outcomes and safety data in many circumstances, the differences in some clinical situations or the medicolegal climate in specific regions may bias physicians toward FDA-approved therapies, possibly reflective of general associations between malpractice risk and defensive medicine.41 Patient preferences are likewise complex and may be associated with individual outcome preferences relative to treatment burden.42,43,44 In addition, concerns about and access to reliable compounding pharmacies may limit the availability of bevacizumab.45 Furthermore, the frequency of injections may contribute to the choice of anti-VEGF agent. A large amount of the data on this topic has been published since 2015, meaning that the trends we are seeing may change in the upcoming years to reflect these new findings.
More important, individual clinicians may be incentivized toward one treatment option vs another. In 2013-2014, Open Payments and Provider Utilization and Payment data from CMS showed that 7% of ophthalmologists received 90% of the $4.5 million payments, for consultations and other services from the pharmaceutical manufacturers of ranibizumab and aflibercept.46 A 2017 study by Singh et al reported that those who received these payments were more likely to use ranibizumab or aflibercept compared with off-label bevacizumab.46
The average sales price of a medication is reimbursed by Medicare plus an additional 6% (4.3% owing to federal budget sequestration). Bevacizumab is not priced for ophthalmic use using the average sales price; thus, the reimbursement is a fraction of that received for ranibizumab and aflibercept, which are both priced using the average sales price. Fluctuations in this pricing, even during the course of a year, could affect margin and influence decision making regarding choice of drug. In addition, rarely discussed manufacturer rebates in the form of volume discounts to certain practices further complicate decision making regarding choice of anti-VEGF agent. These financial motivations affecting choice of anti-VEGF agent must not be overlooked.
Examining the distribution of claims across the country, the highest number of injections per 1000 Medicare beneficiaries occurred in Nebraska for aflibercept, Tennessee for ranibizumab, and South Dakota for bevacizumab. There are data on regional differences in anti-VEGF use. Ophthalmologists practicing in states with higher injection rates are more likely to use higher-cost anti-VEGF agents.47 In addition, Medicare beneficiaries have increased odds of receiving ranibizumab vs bevacizumab for neovascular AMD if they independently: were nonblack, lived in urban areas or in zip codes with higher median incomes, and lived in New England (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont) or East South Central regions (Alabama, Kentucky, Mississippi, and Tennessee).48 Similarly, for diabetic macular edema, the highest frequencies of bevacizumab use (vs ranibizumab or aflibercept) occur in the Mountain, West South Central, and East South Central divisions compared with the highest frequencies of ranibizumab use occurring in the mid-Atlantic, West North Central, and New England divisions.49
Limitations
Despite the findings noted above, it is important to acknowledge the limitations of this analysis. The data here are restricted to Medicare Part B beneficiaries, excluding patients with private insurance plans and Medicaid, although there is no evidence to suggest that ophthalmologists’ selection of agent is different depending on the patient’s insurance. Units of drug billed for are used as a proxy for intravitreal injections; the data here were converted to injections. Ophthalmologists billing for 10 or fewer services in a calendar year are not included in the CMS data set, which could affect distribution statistics. We cannot account for the number of injections that were left out of the CMS data set completely and accept that all sources of data may not be included. Although this large data set analysis can provide associations, we cannot draw conclusions about individual physician practice patterns.
Conclusions
In this analysis, we use Medicare Part B claims data to isolate important use and geographic trends in choice of anti-VEGF medication. Use of anti-VEGF agents has increased substantially and continues to increase annually.37 We identify the presence of a top-heavy distribution, with 3% of ophthalmologists performing intravitreal injections accounting for 17% to 31% of a specific agent’s national use. More important, the results are consistent from 2012 to 2015; the data suggest that publication of the CATT results29 had limited influence on anti-VEGF selection patterns. Approval of aflibercept by the FDA, while predictably affecting use of the other 2 available agents, had a more significant association with bevacizumab use than ranibizumab use. Physicians performing the largest number of injections more commonly use aflibercept and ranibizumab or aflibercept and bevacizumab. None of the ophthalmologists performing the highest volume of ranibizumab injections incorporate bevacizumab with the same frequency. Our analysis provides valuable information and critical insights into the distribution of anti-VEGF agent use across the country at clinician and state levels.
eTable 1. Trend in Anti-VEGF Injections Administered in Medicare Part B Population (2012-2015)
eTable 2. Geographic Disparities in Anti-VEGF Injections by State
References
- 1.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306-1309. doi: 10.1126/science.2479986 [DOI] [PubMed] [Google Scholar]
- 2.Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246(4935):1309-1312. doi: 10.1126/science.2479987 [DOI] [PubMed] [Google Scholar]
- 3.Adamis AP, Shima DT, Tolentino MJ, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia–associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996;114(1):66-71. doi: 10.1001/archopht.1996.01100130062010 [DOI] [PubMed] [Google Scholar]
- 4.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92(23):10457-10461. doi: 10.1073/pnas.92.23.10457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci U S A. 1996;93(10):4851-4856. doi: 10.1073/pnas.93.10.4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozaki H, Seo MS, Ozaki K, et al. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000;156(2):697-707. doi: 10.1016/S0002-9440(10)64773-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim LA, D’Amore PA. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol. 2012;181(2):376-379. doi: 10.1016/j.ajpath.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sternberg P Jr, Durrani AK. Evolving concepts in the management of retinopathy of prematurity. Am J Ophthalmol. 2018;186:xxiii-xxxii. doi: 10.1016/j.ajo.2017.10.027 [DOI] [PubMed] [Google Scholar]
- 9.Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR; VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group . Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805-2816. doi: 10.1056/NEJMoa042760 [DOI] [PubMed] [Google Scholar]
- 10.Ng EW, Shima DT, Calias P, Cunningham ET Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5(2):123-132. doi: 10.1038/nrd1955 [DOI] [PubMed] [Google Scholar]
- 11.Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti–vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19(3):843-850. doi: 10.1200/JCO.2001.19.3.843 [DOI] [PubMed] [Google Scholar]
- 12.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21(1):60-65. doi: 10.1200/JCO.2003.10.066 [DOI] [PubMed] [Google Scholar]
- 13.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335-2342. doi: 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 14.Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112(6):1035-1047. doi: 10.1016/j.ophtha.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36(4):331-335. doi: 10.3928/1542-8877-20050701-14 [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Wiesmann C, Fuh G, et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293(4):865-881. doi: 10.1006/jmbi.1999.3192 [DOI] [PubMed] [Google Scholar]
- 17.Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 18.Brown DM, Kaiser PK, Michels M, et al. ; ANCHOR Study Group . Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444. doi: 10.1056/NEJMoa062655 [DOI] [PubMed] [Google Scholar]
- 19.Campochiaro PA, Heier JS, Feiner L, et al. ; BRAVO Investigators . Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102-1112.e1. doi: 10.1016/j.ophtha.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 20.Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118(8):1594-1602. doi: 10.1016/j.ophtha.2011.02.022 [DOI] [PubMed] [Google Scholar]
- 21.Brown DM, Campochiaro PA, Singh RP, et al. ; CRUISE Investigators . Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124-1133.e1. doi: 10.1016/j.ophtha.2010.02.022 [DOI] [PubMed] [Google Scholar]
- 22.Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118(10):2041-2049. doi: 10.1016/j.ophtha.2011.02.038 [DOI] [PubMed] [Google Scholar]
- 23.Nguyen QD, Brown DM, Marcus DM, et al. ; RISE and RIDE Research Group . Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801. doi: 10.1016/j.ophtha.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 24.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399-2405. doi: 10.2337/dc10-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holash J, Davis S, Papadopoulos N, et al. VEGF-trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393-11398. doi: 10.1073/pnas.172398299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heier JS, Brown DM, Chong V, et al. ; VIEW 1 and VIEW 2 Study Groups . Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548. doi: 10.1016/j.ophtha.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 27.Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology. 2015;122(3):538-544. doi: 10.1016/j.ophtha.2014.08.031 [DOI] [PubMed] [Google Scholar]
- 28.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247-2254. doi: 10.1016/j.ophtha.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 29.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ; CATT Research Group . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908. doi: 10.1056/NEJMoa1102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014;(8):CD005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarwar S, Clearfield E, Soliman MK, et al. Aflibercept for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2016;2:CD011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203. doi: 10.1056/NEJMoa1414264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359. doi: 10.1016/j.ophtha.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott IU, VanVeldhuisen PC, Ip MS, et al. ; SCORE2 Investigator Group . Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion: the SCORE2 randomized clinical trial. JAMA. 2017;317(20):2072-2087. doi: 10.1001/jama.2017.4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayanan R, Panchal B, Das T, Chhablani J, Jalali S, Ali MH; MARVEL study group . A randomised, double-masked, controlled study of the efficacy and safety of intravitreal bevacizumab versus ranibizumab in the treatment of macular oedema due to branch retinal vein occlusion: MARVEL report No. 1. Br J Ophthalmol. 2015;99(7):954-959. doi: 10.1136/bjophthalmol-2014-306543 [DOI] [PubMed] [Google Scholar]
- 36.Department of Health and Human Services, Office of the Inspector General. Medicare payments for drugs used to treat wet age-related macular degeneration. https://oig.hhs.gov/oei/reports/oei-03-10-00360.pdf. Published April 2012. Accessed May 3, 2018. [Google Scholar]
- 37.Patel S. Medicare spending on anti–vascular endothelial growth factor medications. Ophthalmol Retina. 2018;2(8):785-791. doi: 10.1016/j.oret.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 38.Ross EL, Hutton DW, Stein JD, Bressler NM, Jampol LM, Glassman AR; Diabetic Retinopathy Clinical Research Network . Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: analysis from the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial. JAMA Ophthalmol. 2016;134(8):888-896. doi: 10.1001/jamaophthalmol.2016.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Red Book Online [database]. Greenwood Village, CO: Truven Health Analytics Inc; 2016.
- 40.Baisiwala S, Bundorf MK, Pershing S. Physician utilization patterns for VEGF-inhibitor drugs in the 2012 United States Medicare population: bevacizumab, ranibizumab, and aflibercept. Ophthalmic Surg Lasers Imaging Retina. 2016;47(6):555-562. doi: 10.3928/23258160-20160601-07 [DOI] [PubMed] [Google Scholar]
- 41.Bilimoria KY, Chung JW, Minami CA, et al. Relationship between state malpractice environment and quality of health care in the United States. Jt Comm J Qual Patient Saf. 2017;43(5):241-250. doi: 10.1016/j.jcjq.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 42.Baxter JM, Fotheringham AJ, Foss AJ. Determining patient preferences in the management of neovascular age-related macular degeneration: a conjoint analysis. Eye (Lond). 2016;30(5):698-704. doi: 10.1038/eye.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller S, Agostini H, Ehlken C, Bauer-Steinhusen U, Hasanbasic Z, Wilke T. Patient preferences in the treatment of neovascular age-related macular degeneration: a discrete choice experiment. Ophthalmology. 2016;123(4):876-883. doi: 10.1016/j.ophtha.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 44.Droege KM, Caramoy A, Kersten A, et al. Patient preference of ranibizumab treatment regimen for neovascular age-related macular degeneration—monthly injections versus pro re nata. Graefes Arch Clin Exp Ophthalmol. 2014;252(1):31-34. doi: 10.1007/s00417-013-2412-6 [DOI] [PubMed] [Google Scholar]
- 45.Yannuzzi NA, Klufas MA, Quach L, et al. Evaluation of compounded bevacizumab prepared for intravitreal injection. JAMA Ophthalmol. 2015;133(1):32-39. doi: 10.1001/jamaophthalmol.2014.3591 [DOI] [PubMed] [Google Scholar]
- 46.Singh N, Chang JS, Rachitskaya AV. Open payments database: anti–vascular endothelial growth factor agent payments to ophthalmologists. Am J Ophthalmol. 2017;173:91-97. doi: 10.1016/j.ajo.2016.09.026 [DOI] [PubMed] [Google Scholar]
- 47.Erie JC, Barkmeier AJ, Hodge DO, Mahr MA. High variation of intravitreal injection rates and Medicare anti–vascular endothelial growth factor payments per injection in the United States. Ophthalmology. 2016;123(6):1257-1262. doi: 10.1016/j.ophtha.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 48.Gower EW, Stein JD, Shekhawat NS, Mikkilineni S, Blachley TS, Pajewski NM. Geographic and demographic variation in use of ranibizumab versus bevacizumab for neovascular age-related macular degeneration in the United States. Am J Ophthalmol. 2017;184:157-166. doi: 10.1016/j.ajo.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 49.Wu CM, Wu AM, Greenberg PB, Yu F, Lum F, Coleman AL. Frequency of bevacizumab and ranibizumab injections for diabetic macular edema in Medicare beneficiaries. Ophthalmic Surg Lasers Imaging Retina. 2018;49(4):241-244. doi: 10.3928/23258160-20180329-05 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Trend in Anti-VEGF Injections Administered in Medicare Part B Population (2012-2015)
eTable 2. Geographic Disparities in Anti-VEGF Injections by State