Abstract
Container-based sanitation (CBS) within a comprehensive service system value chain offers a low-cost sanitation option with potential for revenue generation but may increase microbial health risks to sanitation service workers. This study assessed occupational exposure to rotavirus and Shigella spp. during CBS urine collection and subsequent struvite fertilizer production in eThekwini, South Africa. Primary data included high resolution sequences of hand-object contacts from annotated video and measurement of fecal contamination from urine and surfaces likely to be contacted. A stochastic model incorporated chronological surface contacts, pathogen concentrations in urine, and literature data on transfer efficiencies of pathogens to model pathogen concentrations on hands and risk of infection from hand-to-mouth contacts. The probability of infection was highest from exposure to rotavirus during urine collection (∼10–1) and struvite production (∼10–2), though risks from Shigella spp. during urine collection (∼10–3) and struvite production (∼10–4) were non-negligible. Notably, risk of infection was higher during urine collection than during struvite production due to contact with contaminated urine transport containers. In the scale-up of CBS, disinfection of urine transport containers is expected to reduce pathogen transmission. Exposure data from this study can be used to evaluate the effectiveness of measures to protect sanitation service workers.
Introduction
Wastewater treatment is shifting toward a new paradigm that recognizes the value of resource recovery.1 Simultaneously, the Sustainable Development Goals call for universal access to safely managed sanitation by 2030, which will require providing new sanitation services to more than 4.5 billion people.2 Integrating these concepts to provide low-cost sanitation services that prioritize nutrient recovery can facilitate capture of nitrogen and phosphorus from human excreta otherwise lost to the environment.3
Container-based sanitation (CBS) is a low-cost sanitation service that can incorporate nutrient recovery. In a CBS system, human excreta is collected at latrines in sealable containers. The containers are removed by sanitation service workers and transported to centralized facilities for treatment before disposal or further processing for resource recovery. When combined with urine-diverting toilets, CBS allows urine and feces to be collected and managed separately. CBS does not require expensive or infeasible sewer infrastructure and thus is amenable to small scale service providers, community based programs, and entrepreneurs.4−9 Human excreta may be processed into a variety of marketable products such as fertilizer or animal feed, whose sale can contribute to system finance.10,11
One obstacle toward modernizing sanitation practices and integrating resource recovery is the need to ensure human health and safety throughout the sanitation value chain. In the absence of a sewer system, CBS relies on service workers to manage excreta. Such interactions with excreta increases risks of enteric disease transmission (i.e., diarrheagenic E. coli, Shigella spp., norovirus, rotavirus, and helminths).12−14 In the pursuit of economically attractive decentralized sanitation services like CBS, safe processes for collection, transport, and processing of excreta are critical to long-term success.
Risks to sanitation service workers along the value chain are likely driven by nondietary ingestion exposures to pathogens via accidental ingestion, inhalation, and hand-to-mouth contacts.15 Prior risk assessments on excreta reuse, conducted largely in the context of agricultural land application, assume risks are driven by accidental ingestion. For example, to assess risks to farmworkers from wastewater application to agricultural fields, estimates of accidental ingestion ranged from 1 to 10 mL of wastewater or 1–100 mg of wastewater-treated soil.16,17 Similarly, risk assessments of urine application to agriculture assumed 1 mL of urine is accidentally ingested per application.18,19 Accidental ingestion is classified as a direct ingestion exposure route, in which exposures to contaminated sources (feces and urine) are direct. Although accidental ingestion is possible, a substantially more likely route of ingestion for daily activities is indirect ingestion, in which hand-to-mouth or hand to peri-oral (around the mouth) contacts are the source of exposure following hand contacts with contaminated sources.20 Indirect ingestion occurs when pathogens contact skin (from a pathogen source like urine or feces or via an intermediate surface or liquid) and then the skin contacts the mouth.
Contributions from hand-to-mouth contacts are frequently neglected or aggregated into assumptions about accidental ingestion.17 Data on hand-to-mouth contact frequency rates are available for children in various settings,21−23 but fewer studies report adult hand-to-mouth contact frequency.20,24 Due to the lack of adult hand-to-mouth contact frequency, studies estimating microbial risks from indirect (i.e., hand to peri-oral) contacts rely on simplifying assumptions. Other studies integrate activity data with risk assessments but rely on activity data from different populations, assuming activities among the two populations were similar. For example, Beamer et al. assumed the behaviors of adults in an office setting were most similar to children 7–12 years old outdoors, citing a lack of adult data as motivation for this assumption.25 Zhao et al. assumed hand-to-mouth contact frequency for U.S. adults based on a study of sedentary adults videotaped while working at a desk.26 Additional studies used videography in exposure assessments but did not use the data to calculate infection risks from hand-to-mouth contacts.27−30 The present study uses videography and associated annotation directly in a microbial risk assessment of the study population, providing an opportunity to identify contact events most responsible for subsequent infection risks.
We apply this approach to evaluate occupational microbial health risks of container-based urine collection and subsequent struvite fertilizer production for nutrient recovery. These represent two primary components of a sanitation value chain within a decentralized network of container-based sanitation systems in the municipality of eThekwini, South Africa. Risks are evaluated within a quantitative microbial risk assessment (QMRA) framework, with an exposure assessment based on primary, site-specific data. The exposure assessment focuses on risks resulting from hand to peri-oral contacts, which are captured using time-lapse videographic data of sanitation workers during urine collection and struvite processing. Microbial and tracer sampling of both workers’ hands and frequently contacted surfaces was conducted in parallel. The video and sample data are integrated into a computer model to evaluate the sanitation workers’ infection risks from pathogens in urine over time. Rotavirus and Shigella spp. were selected as reference pathogens for the assessment due to their epidemiological importance in the study region and as contributors to diarrheal disease burden in developing countries more broadly.12,31 Globally, rotavirus and Shigella spp. were the two leading causes of diarrheal deaths in 2015, resulting in 165 000 to 241 000 and 85 000 to 278 700 deaths, respectively.32 Quantifying risks and identifying factors driving risks informs control strategies to ensure safe management of a promising, low-cost, sanitation service with resource recovery.
Methods
Overall Approach
Study Site
The eThekwini Water and Sanitation (EWS) unit manages a large network of urine diverting dry toilets (UDDTs) in rural townships within the municipality, which also includes the large coastal city of Durban. Primary data was collected in August 2014 within the scope of VUNA (Valorisation of Urine Nutrients in Africa), a research project to optimize urine collection and treatment for fertilizer production.33 UDDTs at households that participated in the study were equipped with 20-L plastic jerry cans to capture the urine outside of the dry toilet superstructure. The containers were collected by EWS staff approximately weekly.34 Staff transferred urine by pouring into receiving tanks in municipality vans or trucks and returned the urine containers to the UDDTs. The urine receiving tanks were then transported to a centralized research facility (Newlands Mashu) where urine was processed for nutrient recovery using struvite reactors35 or biological nitrification reactors.36,37 The struvite production protocol35 entailed filling 40 L reactors with urine using a submersion pump in the receiving tanks, dosing magnesium in the reactors, gently mixing the contents, and filtering the mixture through a cloth bag to capture the struvite. In accordance with EWS training, study participants generally wore facemasks and rubber or latex gloves during observation. Human subjects approval was obtained from the University of KwaZulu Natal Biomedical Research Ethics Committee (BREC, BE147/13) and informed consent was obtained from all study participants.
Modeling Approach
Health risks to sanitation service workers were assessed following the World Health Organization QMRA framework, which includes problem formulation, exposure assessment, health effects assessment, and risk characterization and management.38 The problem formulation identified urine exposure as the primary risk, given widespread presence of pathogens in urine. A previous hazard assessment of urine collected in this region identified several pathogens of concern.12 Rotavirus and Shigella spp. were selected as reference viral and bacterial pathogens, respectively, for the model because they were frequently detected, present significant health risks in developing countries, and dose–response curves are available. Because direct contact of urine with the mouth (i.e., via accidental ingestion during a spill) was assumed to be rare or nonexistent, the exposure assessment focused on indirect contact. Indirect contact was modeled as pathogens contacting skin either from urine or via a surface assumed to be contaminated with urine prior to skin contacting the mouth.
Exposure Assessment
Videography
First person videography was used to collect data on the frequency, duration, and chronology of hand–object (i.e., surfaces, opposite hand, face, and mouth) contacts. Portable cameras (GoPro Hero3 White Edition, GoPro, Inc., San Mateo, CA) with supplementary battery packs were worn by study participants on the head, such that the camera’s field of view captured the subject’s hand movements as well as their peri-oral region. Study participants (all trained municipality staff) were asked to conduct their typical urine collection and struvite production procedures. Over 3 days, 12.6 person-hours of video were collected during urine collection to capture a minimum of 30 household visits for urine collection by three study participants. Additionally, 11.6 person-hours of video were collected over 2 days during struvite production to capture the production of at least 10 batches of struvite by two study participants.
Video Translation
Video Translator for the Personal Computer (VTDPC, Version 1.0.0.0, 2014 Arizona Board of Regents on behalf of The University of Arizona) was provided by Robert A. Canales to extract microlevel activity time series (MLATS) data from the first-person video.39 MLATS are temporal sequences of every object the hands contact over the duration of a video-recorded event, with subsecond resolution. The VTDPC templates included 18 object classes for urine collection (Table S2) and 7 object classes for struvite production (Table S3). The object classes were decided based on preliminary video review, in which objects with similar surface types (e.g., metal, cloth, and skin) and expected contamination levels were categorized into object classes. Whether or not an object was visibly wet (urine-contaminated) on contact was also tracked for each object contacted. See the Supporting Information Methods for further details. Summary results of surface contact frequencies and durations are presented as mean (μ) ± standard deviation (σ) [range].
Pathogen Contamination
The pathogen surface density (genome copies cm–2) for each object class i that was contacted by the study participants was calculated indirectly. Specifically, surface density was assumed to be a function of the equivalent volume of urine on a surface and the concentration of the pathogen in the urine as follows:
| 1 |
where ρAP,i (genome copies cm–2) is the surface density of pathogen P on object class i, V/Aurine,i (mL cm–2) is the volume of urine (mL) per unit surface area (cm2) on object class i, and CP,urine (genome copies mL–1) is the concentration of the pathogen in the urine.
The volume of urine per unit surface area (V/Aurine,i) was estimated using E. coli contamination as an indicator. This approach is based on the concept of human fecal equivalents,40−42 which uses relative levels of E.. coli in environmental samples and in feces as a proxy to estimate pathogen concentrations. Fecal-contaminated urine was assumed to be the main source of E. coli on the surfaces tested. Specifically, urine volume per unit surface area was estimated using the ratio of E. coli contamination on a surface (object class i) to the E. coli contamination in urine:
| 2 |
where ρAE.coli, i is the surface density of E. coli, or number of E. coli per unit surface area (colony forming units, CFU cm–2), and CE.coli,Urine is the concentration of E. coli in urine (CFU mL–1) as measured on the same day. E. coli contamination was measured in urine and on a range of surfaces using E. coli/total coliform petrifilm dryplates (3M, St. Paul, MN), according to manufacturer’s recommendations. A total 42 surfaces were sampled using polyester-tipped swabs prewetted with sterile 1/4-strength Ringer’s solution (see the Supporting Information, Methods section for further details).
The approach for measuring pathogen contamination on surfaces was modified for object types that were both observed as wet (i.e., visibly urine-contaminated) and on which E. coli or a tracer for urine contamination were measured for validation, as described in the following section. Specifically, the estimate of the surface density of a pathogen on the object (ρAP,i) was modified from eq 1 to assume pathogen contamination was a function of pathogen concentration in the urine and the amount of urine on the surface, or
| 3 |
where CP,urine is the concentration of the pathogen in the urine (genome copies mL−1, which is equivalent to genome copies cm−3) and h is the film thickness of urine on the surface (2.2 × 10–3 cm) based on the average of high and low values for water on hands and corrected for the density of urine.43
Validation of Urine Volume and Hand Contamination
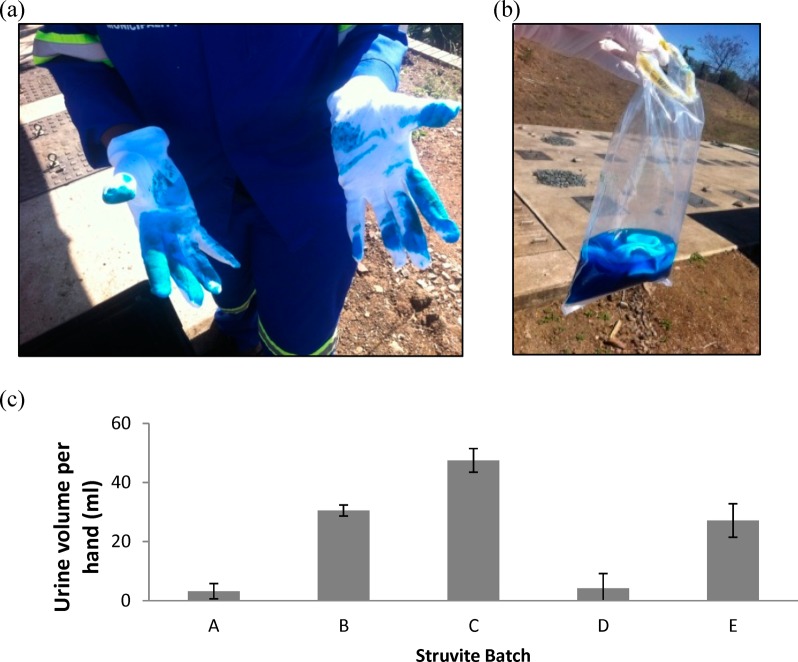
During video translation, surfaces were categorized as visibly wet (urine-contaminated) or dry based on observation. A tracer exposure study was conducted using a method modified from a previous study44 to validate visibly observed urine exposure. Use of tracers also allowed quantitative estimates of the volume of urine on workers’ hands and on the struvite reactor surfaces after struvite production. In brief, 250 L of urine was spiked with Brilliant Blue (BB) food coloring to achieve an approximate 10–3 M starting concentration. Contact with urine during struvite production resulted in transfer of dyed urine to the hands of study participants, who were provided with white cotton gloves over latex gloves, or to reactor surfaces. The amount of urine on the hands and the surfaces was then estimated by quantifying the BB spectrophotometrically (629 nm, Orberco SP 600 Spectrophotometer, Orbeco-Hellige Inc./Tintometer GmbH, Dortmund, Germany).
BB was quantified on hands by removing the cotton gloves after struvite production and extracting BB into distilled water (Figure 1). BB was quantified from surfaces by swabbing (approximately 100 cm2 per surface) with cotton-tipped swabs and extracting BB in 10 mL of 1/4-strength Ringer’s solution. The BB concentration measured in the urine and the extracts were then used to calculate the volume of urine that had come in contact with the hand or surface, as follows:
| 4 |
where VUrine,i is the volume (mL) of urine on object i, CBB,i is the concentration of BB measured in the object i sample extract (mol L–1), Vextract is the volume of distilled water used to extract BB from gloves or surface (mL), Vdiluent is the total volume of other liquids added to the extract (urine or other) during the period of use (mL), and CBB,urine is the concentration of BB measured in the urine (mol L–1). Spike tests of urine containing BB onto cotton gloves indicated sufficient recovery of BB. See the Methods section in the Supporting Information and Figures S4 and S5 and Table S4 for further details on method development for the validation of urine exposure.
Figure 1.
Urine volume measured on hands during struvite production from a tracer study. (a) Urine was augmented with dye and study participants wore cotton gloves over latex gloves during struvite production. (b) Cotton gloves were extracted in distilled water to measure the volume of urine exposure spectrophotometrically. (c) Five batches of struvite (A through E) were produced for the study. For each struvite batch produced, the average (±SD) volume of urine that contacted left and right hands for two study participants is presented.
Model
Dose
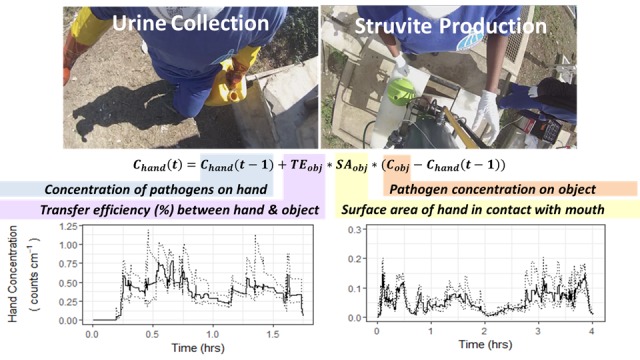
Calculations of ingested pathogen dose from indirect contacts with urine required modeling the pathogen concentration on the hand through time (e.g., Figure 2). Changes in concentration on the hand over time are influenced by the difference in concentration between the object and the hand during the contact event at a point in time, so transfer can occur in both directions. The concentration of pathogen P on hand H (right or left) was thus calculated as previously described, using a stochastic-mechanistic framework:30,45
| 5 |
where ρAP,H(t) is the surface density (genome copies cm−2) of the pathogen P on the hand H at time t, Δt is the time step between two subsequent contacts (which is nonuniform), TP,i→H is the transfer efficiency (%) of pathogen P from object class i to the hand H, fi→H is the fractional surface area, or the fraction of the hand’s total surface area, in contact with object class i during a contact event, and ρAP,i is the surface density of pathogen P on object i (genome copies cm−2). The parameter values in the model were described by probability distribution functions (PDFs) drawn from primary data collection on activity data and pathogen contamination determined in the present study. Transfer efficiencies and fractional surface areas of contact were drawn from literature (Methods section in the Supporting Information and Tables S5 and S6). In this generalized equation, the hand may be gloved or not-gloved, with model input parameters adjusted accordingly.
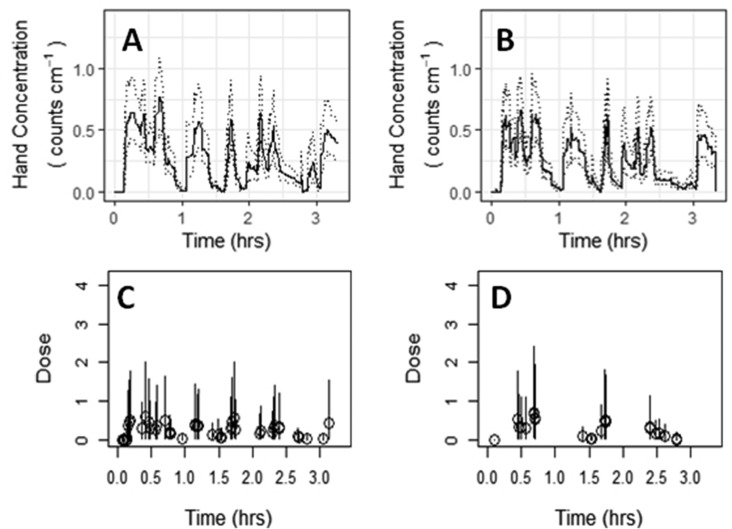
Figure 2.
Simulated rotavirus concentration on hands for left (A) and right (B) hands with corresponding dose events (bottom) for one person-day (person 3, day 2) monitored during urine collection. Dark lines (A and B) represent median simulations; dotted lines (A and B) represent fifth and 95th percentile simulations. Open circles (C and D) represent median dose, with the error bars representing the fifth and 95th percentile simulation values.
The model was implemented using a Monte Carlo framework to account for parameter uncertainty and variability as previously described.45,29 For each period of observation, pathogen contamination on hands and ingested dose were simulated 1000 times. For comparison, Julian et al. showed convergence of the median simulation value for nonpathogenic E. coli contamination on hands of Vietnamese farmers using a similar framework within 100 simulations.29 Within each simulation, activity data were fixed (using the exact chronology of each video segment) while pathogen contamination, transfer efficiencies, and fractional surface area varied randomly from within the descriptive PDFs. Several assumptions are implicit in the model construct that are consistent with previous implementations of similar models.29,30,46 First, the concentration distribution of the pathogen on each object is assumed constant in time, unless the object is the other hand of the study participant. Second, the transfer efficiency was assumed the same in both directions (hand-to-object or object-to-hand). Third, inactivation of pathogens on surfaces or the hand was not included in the model because prior modeling work on pathogen transfer between surfaces showed contributions of inactivation are negligible in the time frame (<1 h) monitored.30,45 This may result in some overestimation of risk.47 Finally, the duration of contact with an object did not change the estimate of pathogen transfer in the model.45,48,49
Dose of infectious pathogens ingested was assumed to occur due to hand-to-mouth contacts. To provide a representative sample of dose from single hand-to-mouth contacts, and due to the high proportion of facemask use among study participants, hand-to-facemask contacts were modeled as contributors to dose. While such hand-to-peri-oral contacts may contribute to overall pathogen exposure and ingestion, the cumulative risk estimates are expected to overestimate risks among the study population and provide an upper estimate for sanitation workers who do not use personal protective equipment.20 The instantaneous dose DP(tj) of pathogen P ingested following a single hand-to-mouth contact j occurring at time tj was modeled as
| 6 |
where rinf:gc is the ratio of infectious pathogens to genome copies, fH→M is the fractional surface area of the hand in contact with the mouth during a contact event, SH is the total surface area of the hand (cm2), and TP,H→M is the transfer efficiency of pathogen P between the hand and the mouth. To account for the application of polymerase chain reaction (PCR) assays in the determination of pathogen concentrations (as genome copies rather than infectious pathogens), an estimated 10% of genome copies of both rotavirus and Shigella spp. were assumed to be infectious (rinf:gc = 0.1).50,51 The total surface area of the hand (SH) was assumed to be 970 cm2 (the average of the median surface area of hands for men (1070 cm2) and women (870 cm2).43
The total dose of pathogen P ingested for all hand-to-mouth contacts was estimated by calculating the sum of doses from all hand-to-mouth contacts during the period of observation. For J total hand-to-mouth contact events the total dose is
| 7 |
The probability (or risk) of rotavirus or Shigella spp. infection (P(I)) for a given dose D was calculated using a beta-Poisson model:
| 8 |
which represents the dose–response relationship for the infection of humans with these pathogens. The preferred optimized parameters are α = 0.253, N50 = 6.17 for rotavirus and α = 0.265, N50 = 1.48 × 103 for Shigella flexneri.52 The probability of infection was calculated for both (1) the instantaneous dose, Dp(tj), resulting from each pathogen P and individual hand-to-peri-oral contact event j, and (2) the cumulative dose, Dp, resulting from the sum doses of all doses over the observation period, as shown in eq 7. The former is used to estimate average risk of infection per hand-to-peri-oral contact, and the latter is used to estimate the total risk over the observation period for each study participant workday. Five workdays were assessed for urine collection and four for struvite production. The model was implemented using the statistical software package R (version 3.4.3).
Sensitivity Analysis
A sensitivity analysis was conducted to evaluate the influence of each model parameter uncertainty on model outputs, using a method modified from Xue et al.53 The output chosen for the sensitivity analysis was the resulting time-averaged rotavirus concentration on hands to indicate influence of parameters on exposure and to remove the effect of hand-to-mouth contact timing from the evaluation. Parameter influence was indicated by holding all parameter values at their expected value based on estimated probability distribution functions and iteratively changing one parameter to either the low (25th percentile of probability distribution function) or high (75th percentile) value, respectively, for each parameter. When the low was not physiologically relevant (for example, a fractional surface area below zero), value was replaced with the relevant lower boundary of zero.
Results and Discussion
E. coli Detection on Surfaces Indicates Urine Contamination
The volume of urine on surfaces was estimated using the E. coli concentration in sampled urine relative to the surface density of E. coli on surfaces. Of the 6 urine samples collected from urine collection tanks (collected from different communities or on different days), 4 (66%) were positive for E. coli with concentrations ranging from 10 to 1.3 × 104 CFU mL–1 (Table S1). Each urine tank contained urine collected from tens to hundreds of households, so E. coli measurements may be considered representative of average fecal contamination levels in collected urine. In addition, 42 surface swab samples were collected for E. coli analysis, of which 14 (33%) were positive with surface densities ranging from 0.05 to 23 CFU cm–2. Based on measurements of E. coli on surfaces and in urine, the estimated urine volume (urine volume equivalents) calculated for each surface category ranged from 5 × 10–4 to 2.6 × 10–2 mL cm–2 (Table 1).
Table 1. Summary of E. coli Results for Surface Samples When Categorized for Urine Collection or Struvite Production As Well As Calculated Urine Volume Equivalents by Surface Category and Calculated Concentrations of Pathogens on Surfaces.
| study component | surface category | positive result/no. of samples (%) | E. coli μ ± σ (CFU/100 cm2)a | urine volume equivalent, μ ± σ (mL/100 cm2)a | rotavirus surface concentration, μ ± σ (counts/100 cm2) | Shigella spp. surface concentration, μ ± σ (counts/100 cm2) |
|---|---|---|---|---|---|---|
| urine collection | urine storage tank | 5/9 (56%) | 38 ± 95 | 0.05 ± 0.13 | 2.4 ± 6.5 | 5.1 ± 13.6 |
| metal (vehicle doors/handles) | 0/7 (0%) | <5 | 0 | 0 | 0 | |
| plastic urine transport container (jerry can) | 4/15 (27%) | 160 ± 590 | 0.6 ± 1.8 | 27 ± 91 | 57 ± 191 | |
| other plastic (seat cushion) | 0/1 (0%) | <5 | 0 | 0 | 0 | |
| textile (dry uniform) | 0/1 (0%) | <5 | 0 | 0 | 0 | |
| struvite production | urine storage tank | 8/21 (38%) | 129 ± 501 | 0.08 ± 0.23 | 3.8 ± 11.7 | 8.1 ± 24.4 |
| pump plastic tubing | 3/3 (100%) | 192 ± 162 | 0.26 ± 0.22 | 13 ± 14 | 27 ± 26 | |
| solid reactor components | 3/3 (100%) | 57 ± 60 | 0.08 ± 0.08 | 4.8 ± 7.9 | 7.9 ± 9.2 |
The mean (μ) and standard deviation (σ) were calculated assuming 0 CFU/100 cm2 for measurements below the detection limit.
The calculation of urine volume equivalents assumes that the primary source of E. coli on the sampled surfaces was from contamination with the urine and that the concentration distribution was constant throughout the activities assessed. There are trade-offs to applying field E. coli measurements in deriving viable pathogen concentration distributions in this way. E. coli and other Gram-negative bacteria are known to inactivate rapidly in stored urine.54,55 Therefore, E. coli measurements may improve the accuracy of viable concentration estimates for Gram-negative bacterial pathogens in urine such as Shigella spp. However, use of E. coli to calculate urine volume equivalents may underestimate the actual urine volume on surfaces and thus the concentrations of more persistent pathogens including rotavirus. Normalization of surface swab E. coli results with that of E. coli concentrations in urine samples collected on the same day was intended to mitigate this issue. Finally, the presence of E. coli on surfaces from sources other than urine (for example, animal feces) would lead to an overestimation of the urine volume equivalent. However, surface densities of E. coli on a variety of object classes with low likelihood of contact with urine were low or nondetectable (Table 1).
Microlevel Activities Indicate a Majority of Worker Time Was Spent Traveling by Foot during Urine Collection and near the Reactor during Struvite Production
Video was recorded during collection of urine transport containers from a total of 39 UDDTs. An additional 26 UDDTs were visited for which the urine transport containers were checked but not removed from the connection pipe for emptying. Video also captured the transfer of urine from the containers into the urine storage tanks in the municipality vehicle and the return of the transport containers. Of the total time recorded, 35 ± 7% of participant time was spent inside the municipality vehicle, 59 ± 7% of the time was spent outside the vehicle and away from the UDDT which typically indicated foot travel between UDDTs, 6 ± 1% of the time was at or within approximately 0.5 m of the UDDT, and 4 ± 3% of the time was spent pouring urine into the storage tank.
In the sanitation value chain, nutrient recovery from excreta follows collection. In this study, nutrient recovery from urine processing involved the production of struvite in manually operated field reactors. During the production of ten batches of struvite over 2 days, participants spent 53 ± 10% of the time next to the struvite reactor (within the drainage zone surrounding the reactor) and 12 ± 3% within view of the urine storage tank, from which urine was pumped into the reactor. The remaining time (37 ± 11%) was in the surrounding outdoor workspace (Table S8). The Results section in the Supporting Information contains further details on workers’ activities during urine collection and struvite production.
Activity Data and Microbial Sampling Highlighted Frequent Hand Contacts with Contaminated Surfaces
Videography identified the sanitation service worker collecting urine spends approximately 12 ± 4% [7–20%] of their total time handling the urine transport container via 54 ± 22 [33–94] contacts per hour with the urine transport container (Table S7). Similarly, 3 ± 4% [0–14%] of the time was spent contacting the urine storage tank, corresponding to 26 ± 32 [7–115] contacts per hour (Table S7). Contacts with visibly wet surfaces and contacts directly with urine were rare during urine collection, indicating that contact with urine-contaminated surfaces was infrequent (Table S8). However, concentrations of E. coli from swabs of the urine transport container were high (1.60 ± 5.90 CFU cm–2) relative to other surfaces (Table 1). Because the concentrations of E. coli on swabbed surfaces were used to estimate urine contamination, the E. coli concentration implies urine contamination even if the surface was not visibly wet. The frequent contacts with urine-contaminated surfaces thus suggest infection risks for service workers was elevated when interacting with urine transport containers and urine storage tanks. The finding is also reflected in the sensitivity analysis, which highlighted pathogen contamination on the urine transport containers as the most influential variable on estimated pathogen contamination of hands (Figure S1). The findings indicate that pathogen risks during urine collection can be substantially reduced by interventions to clean the transport containers in the field. Examples include immediate surface disinfection protocols or use of secondary containment systems during collection and transport.
Infection risks to sanitation service workers during struvite production were predominately from the visibly wet cloth reactor components (i.e., filters used to capture struvite; Figure S2). Service workers contacted such cloth reactor components 20 ± 9% [8–31%] of the participant time spent producing struvite, corresponding to 33 ± 14 [13–53] times per hour (Table S9). Surfaces that were visibly wet with urine, often including the cloth reactor components, were contacted an average of 25 ± 17 [2–25] times per hour (Table S10). Therefore, the potential for high-volume contamination of hands or gloves directly with urine, and exposure to pathogens in urine, was higher during struvite production than urine collection. The finding suggests struvite production risks may be driven more by liquid-to-skin transfers than surface-to-skin transfers.49 Pathogen concentration distributions estimated for the cloth reactor components based on the transfer of a liquid film were lower than pathogen surface densities determined for urine transport containers (Tables S5 and S6). As a result, pathogen concentrations modeled on service worker hands (which drive overall risk of infection from hand-mouth contacts) were typically higher during urine collection than struvite production.
Estimated Pathogen Contamination on Surfaces Was Low but Widespread
The application of urine volume equivalents allows estimation of pathogen concentrations on surfaces for any pathogen for which the concentration in urine is known. Pathogen concentrations in urine for rotavirus and Shigella spp. in the present study were inferred from detection frequency determined in a previous survey of diarrheal pathogens in source-separated urine in eThekwini.12 Specifically, the mean (μ̂) and standard deviation (σ̂) of rotavirus and Shigella spp. concentrations in urine were calculated using a maximum likelihood estimator applied to results from Bischel et al. as described in the Supporting Information (eqs S1–S3). With rotavirus (34% positive, n = 29) and Shigella spp. (61% positive, n = 18) prevalence data and a detection limit of 110 copies mL–1,12 the concentrations of pathogens in urine were calculated as 47 ± 33 copies mL–1 for rotavirus and 105 ± 49 copies mL–1 for Shigella spp. The previous hazard assessment survey was based on PCR assays and thus does not represent infectious particle concentrations. However, within freshly contaminated urine, a portion of the detected genome copies are assumed to be infectious, in part because inactivation of pathogens may be slow (e.g., for dsRNA viruses like rotavirus56) relative to the time scale of collection (weekly). Due to the small size of urine transport containers (20 L) and frequent collection of urine, the effect of months of urine storage on microbial health risks was not evaluated herein.
For objects that were observed as wet, the pathogen surface density was calculated to be 0.10 ± 0.07 copies cm–2 for rotavirus and 0.23 ± 0.11 copies cm–2 for Shigella spp. (Tables S4 and S5). For several surface categories, the average pathogen surface densities calculated from E. coli measurements were greater than the concentration calculated using the presumed film thickness. The values derived from the E. coli measurements were used as the model input concentration for both wet and dry surfaces of these categories (the plastic urine jerry can containers used during urine collection, the pump components and plastic tubing contacted during struvite). All other wet objects contacted were modeled using concentration distributions determined from the film thickness of urine that is expected to transfer from the wet object to the hand.
Pathogen Concentration on Hands Varied Rapidly
Second-by-second simulations of concentrations of pathogens were modeled on the left and right-hand of each study participant performing urine collection (Figures 2, S6, and S7) or struvite production activities (Figures S8 and S9). Rapidly changing concentrations on hands through time reflect the inherently complex processes of an individual’s constant movement through and interaction with their environment. Nevertheless, the range of hand concentrations was consistently between 0 and 2 Shigella spp. cm–2 or between 0 and 1 rotavirus cm–2 for all participants. Increasing the number of simulations beyond 1000 may further refine the 95% simulation values. Although the magnitude in pathogen concentrations on hands varied between simulations (Figures S6–S9), the temporal trends were consistent.
Tracer Validation of Urine Measurements on Hands and Surfaces Supports Use of E. coli As Indicator of Urine Contamination in This Study Setting
A tracer study (using BB) was conducted as an independent measure to evaluate the accuracy of urine volume equivalents determined from E. coli field measurements. Based on the tracer study, the volume of urine on a single hand during the production of one batch of struvite was 23 ± 18 mL per hand (n = 20) with range of <0.2 to 52 mL per hand (Figure 1). Assuming a hand surface area of 970 cm2, the urine volume per unit area is 2.4 × 10–2 ± 1.9 × 10–2 mL cm–2. These results represent an integrated volume of urine exposure for each struvite batch prepared because the dye was absorbed in the cotton gloves as opposed to transferred. Applying the calculated concentrations of rotavirus and Shigella spp. in urine, the average volumes correspond to 1 and 3 genome copies cm–2, respectively. As expected, these integrated pathogen surface density measurements are greater than the instantaneous pathogen surface density modeled on hands throughout several batches of struvite production, but they provide an upper bound to pathogen concentrations expected on participant hands. An integrated measure of hand contamination in this way may overestimate the risk of pathogen exposure.
The volume of urine on contaminated surfaces determined from swabs taken during the tracer study was 4 × 10–4 ± 3 × 10–4 mL cm–2 (n = 3). While the sample size is small, this volume is within range of the amount of urine estimated from E. coli measurements (urine volume equivalents) on solid reactor components collected on a different day during struvite production (Table 1). Taken together, the tracer validation study results support the application of E. coli measurements in modeling urine and pathogen exposure in this setting. Of note, the striking color of BB tracer may have influenced participant behaviors. However, no difference was observed in frequency of contacts during videography when BB tracer was not used (day 1) as compared when it was used (day 2).
Hand-to-Mouth Contacts Pose a Risk for Pathogen Ingestion
Ingestion of pathogens is likely following contacts of the hand to the mouth or to the area around the mouth. As demonstrated by videography, direct hand-to-mouth contacts were rare, occurring 0.3 ± 0.7 [0–2] times per hour during urine collection (Table S7). Of the rarely observed hand-to-mouth contacts, most occurred after study participants had removed their gloves. Bias resulting from video observation cannot be excluded and may have influenced observed rates. The hand-to-mouth contact rates observed among sanitation workers in the present study were substantially lower than those observed for U.S. office workers (6 times per hour)21 and more closely aligned with Vietnamese farmers using human feces for agriculture (0–1 times per hour).38 Cherrie et al. suggest that hand-to-mouth contact rates vary by idle time.20 Sanitation service workers and farmers are expected to be less idle than office workers. Low rates of direct hand-to-mouth contacts may also be due to the use of facemasks. Study participants wore facemasks throughout much of the urine collection and struvite production activities. During urine collection, participants contacted their facemask 13 ± 8 [0–22] times per hour (Table S7). Inappropriate removal of personal protective equipment (PPE) is an infection risk factor.57 Hand-to-facemask contact events were thus modeled as potential opportunities for pathogen exposure, resulting in 65 ± 39 [1–111] nonzero doses simulated during urine collection and 32 ± 23 [9–61] simulated during struvite production (Table 2).
Table 2. Summary of 1,000 Simulation Results for Dose and Risk Probabilities during Urine Collection and Struvite Productionba.
| study component | pathogen | study participant/study day | total time evaluated (s) | number of facemask/mouth contacts observed | number of nonzero doses simulated | average of median single doses, DP(tj) [average of lower 95% CI, average of upper 95% CI] | average of median single risks [average lower 95% CI, average upper 95% CI] | cumulative risk [lower 95% CI, upper 95% CI] |
|---|---|---|---|---|---|---|---|---|
| urine collection | rotavirus | P1/D1 | 6290 | 70/0 | 67 | 4.0 × 10-1 [3.2 × 10-2, 1.4] | 1.5 × 10-1 [1.8 × 10-2, 3.0 × 10-1] | 6.5 × 10-1 [3.6 × 10-1, 7.4 × 10-1] |
| P2/D1 | 6429 | 2/0 | 1 | 2.7 × 10-1 [1.9 × 10-2, 1.1] | 1.2 × 10-1 [1.1 × 10-2, 2.8 × 10-1] | 1.2 × 10-1 [1.1 × 10-2, 2.8 × 10-1] | ||
| P1/D2 | 12012 | 126/3 | 111 | 2.8 × 10-1 [2.2 × 10-2, 9.9 × 10-1] | 1.1 × 10-1 [1.3 × 10-2, 2.4 × 10-1] | 6.6 × 10-1 [3.9 × 10-1, 7.6 × 10-1] | ||
| P3/D2 | 11956 | 95/8 | 86 | 2.4 × 10-1 [1.9 × 10-2, 8.6 × 10-1] | 9.5 × 10-2 [1.1 × 10-2, 2.0 × 10-1] | 6.3 × 10-1 [3.3 × 10-1, 7.3 × 10-1] | ||
| P2/D3 | 8885 | 60/0 | 60 | 4.3 × 10-1 [3.6 × 10-2, 1.5] | 1.6 × 10-1 [2.0 × 10-2, 3.1 × 10-1] | 6.5 × 10-1 [3.7 × 10-1, 7.4 × 10-1] | ||
| Shigella spp. | P1/D1 | 6290 | 70/0 | 67 | 7.8 × 10-1 [6.2 × 10-2, 2.7] | 1.8 × 10-3 [1.4 × 10-4, 6.0 × 10-3] | 9.4 × 10-2 [9.3 × 10-3, 2.2 × 10-1] | |
| P2/D1 | 6429 | 2/0 | 1 | 4.5 × 10-1 [4.0 × 10-2, 1.8] | 1.0 × 10-3 [9.1 × 10-5, 4.1 × 10-3] | 1.0 × 10-3 [9.1 × 10-5, 4.1 × 10-3] | ||
| P1/D2 | 12012 | 126/3 | 111 | 5.6 × 10-1 [4.5 × 10-2, 2] | 1.3 × 10-3 [1.0 × 10-4, 4.4 × 10-3] | 1.1 × 10-1 [1.1 × 10-2, 2.4 × 10-1] | ||
| P3/D2 | 11956 | 95/8 | 86 | 4.6 × 10-1 [3.7 × 10-2, 1.6] | 1.0 × 10-3 [8.5 × 10-5, 3.7 × 10-3] | 7.5 × 10-2 [7.2 × 10-3, 1.9 × 10-1] | ||
| P2/D3 | 8885 | 60/0 | 60 | 8.2 × 10-1 [6.5 × 10-2, 2.9] | 1.8 × 10-3 [1.5 × 10-4, 6.4 × 10-3] | 8.9 × 10-2 [8.6 × 10-3, 2.1 × 10-1] | ||
| struvite production | rotavirus | P1/D1 | 14537 | 64/NTb | 61 | 2.8 × 10-2 [2.3 × 10-3, 1.1 × 10-1] | 1.6 × 10-2 [1.3 × 10-3, 5.3 × 10-2] | 3.4 × 10-1 [6.9 × 10-2, 5.1 × 10-1] |
| P2/D1 | 14710 | 48/NTb | 42 | 6.2 × 10-2 [5.0 × 10-3, 2.4 × 10-1] | 3.3 × 10-2 [2.9 × 10-3, 1.0 × 10-1] | 3.9 × 10-1 [9.6 × 10-2, 5.5 × 10-1] | ||
| P1/D2 | 5776 | 14/NTb | 14 | 6.0 × 10-2 [4.6 × 10-3, 2.5 × 10-1] | 3.2 × 10-2 [2.7 × 10-3, 1.1 × 10-1] | 2.4 × 10-1 [3.5 × 10-2, 4.3 × 10-1] | ||
| P2/D2 | 7281 | 9/NTb | 9 | 7.7 × 10-2 [6.1 × 10-3, 3.1 × 10-1] | 4.1 × 10-2 [3.6 × 10-3, 1.3 × 10-1] | 2.2 × 10-1 [3.0 × 10-2, 4.0 × 10-1] | ||
| Shigella spp. | P1/D1 | 14537 | 64/NTb | 61 | 6.1 × 10-2 [4.5 × 10-3, 2.3 × 10-1] | 1.4 × 10-4 [1.0 × 10-5, 5.1 × 10-4] | 8.3 × 10-3 [6.2 × 10-4, 2.9 × 10-2] | |
| P2/D1 | 14710 | 48/NTb | 42 | 1.4 × 10-1 [1.1 × 10-2, 5.1 × 10-1] | 3.1 × 10-4 [2.5 × 10-5, 1.1 × 10-3] | 1.3 × 10-2 [1.0 × 10-3, 4.3 × 10-2] | ||
| P1/D2 | 5776 | 14/NTb | 14 | 1.4 × 10-1 [1.0 × 10-2, 5.4 × 10-1] | 3.1 × 10-4 [2.3 × 10-5, 1.2 × 10-3] | 4.3 × 10-3 [3.2 × 10-4, 1.6 × 10-2] | ||
| P2/D2 | 7281 | 9/NTb | 9 | 1.7 × 10-1 [1.4 × 10-2, 6.5 × 10-1] | 4.0 × 10-4 [3.1 × 10-5, 1.5 × 10-3] | 3.5 × 10-3 [2.8 × 10-4, 1.3 × 10-2] |
Single doses and risks refer to those encountered from a single hand-mouth contact. Cumulative risk is presented for each study participant workday.
Not Translated: for struvite production, facemask and mouth contacts were not distinguished during translation.
Risks of Infection from Exposure to Both Rotavirus and Shigella spp. Were High
The estimated median dose for a single hand-to-mouth contact from 1000 simulations varied from 2.8 × 10–2 to 4.3 × 10–1 infectious rotavirus and 6.1 × 10–2 to 8.2 × 10–1 infectious Shigella spp. (Figure 3, Table 2). The median risk per single hand-to-mouth contact from 1000 simulations, described as probability of infection (P(I)), ranged from 1.6 × 10–2 to 1.6 × 10–1 for rotavirus and 1.4 × 10–4 to 1.8 × 10–3 for Shigella spp. Of note, rotavirus presents a higher risk of infection despite a lower dose than Shigella spp. because rotavirus requires lower doses to initiate infection than Shigella spp. Cumulative risks, representing the risk of infection from the summed dose events occurring throughout the observation period, were similarly higher for rotavirus than Shigella spp. (Figure 3 and Table 2). The average risk of infection for a single hand-to-mouth contact reported here are substantially lower than the risks reported in other studies assuming accidental ingestion of urine. Specifically, Höglund et al. report an estimated an average ± standard deviation probability of infection of 0.56 ± 0.22 from a single accidental ingestion of unstored urine during removal of clogs from urine diversion pipes.18 Ahmed et al. report a probability of infection nearly equal to unity (1.0) for application of urine in gardening.58 While a realistic frequency of accidental ingestion events during urine collection and processing is difficult to accurately assess, such occurrences are likely rare in comparison to indirect ingestion of pathogens through hand-to-mouth contacts. Accidental ingestion of urine was not observed in the present study.
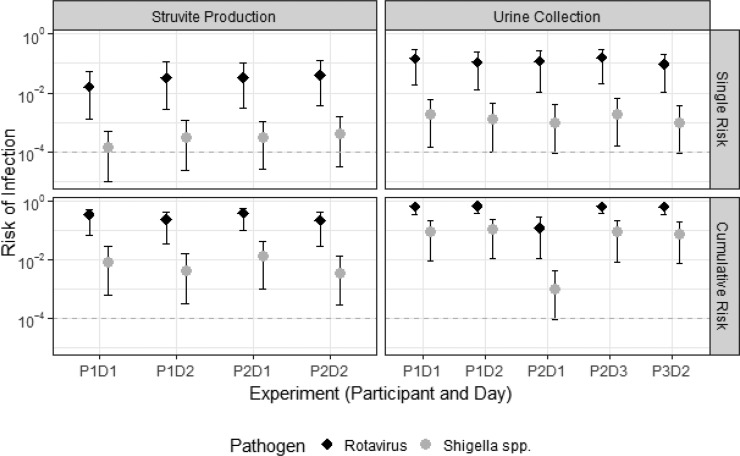
Figure 3.
Risk of infection from a single exposure (top) and from cumulative exposures throughout the period of time when video was captured (bottom) during struvite production (left) and urine collection (right). Dots represent median risk. Error bars represent the upper and lower 95th percentile simulations. Dashed gray line represents risk of 10–4 infections, which is the U.S. EPA recommended tolerable disease burden per person per year.
The U.S. EPA tolerable disease burden of 10–4 infections per person per year was exceeded for both rotavirus and Shigella spp. from a single hand-to-mouth contact event during both urine collection and struvite production (Figures 3 and 4 and Table 2). The elevated risk was due primarily to the pathogen concentration in urine, which was expected to be high in this study due to the frequent detection of pathogens in urine. The model also applies pathogen concentrations estimated from integrated community sampling, or pooled samples. A more likely scenario is that pathogen contamination varies substantially between households, as it is driven by the presence or absence of an active pathogen shedder. That is, urine from households without shedders likely pose low or no risk. Therefore, the modeled risks of infection may be considered a lower bound for risks during collection of urine from a household with active pathogen shedders. Of note, cumulative risks were modeled as a conservative scenario based on the summed dose occurring from all hand-to-facemask contacts observed. The frequency of hand-to-facemasks was substantially higher than hand-to-mouth contacts observed both here and elsewhere,29 so the cumulative risk provides an upper bound. Additional primary data quantifying the frequency of hand-to-mouth contacts for adults in a variety of settings and activities is needed and would provide greater context for observations in the present study.
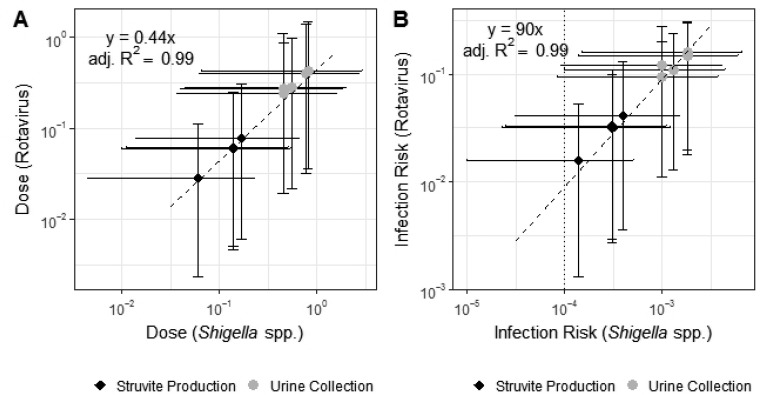
Figure 4.
Linear correlation between (Y-axis) rotavirus and (X-axis) Shigella spp. for simulated hand-to-mouth contacts for (A) dose and (B) infection risk during (black diamond) struvite production and (gray dot) urine collection. Each point represents the median instantaneous dose modeled for each study participant. Horizontal and vertical error bars represent the 95% confidence intervals of the modeled doses. Infection risk is higher than (dotted line) U.S. EPA tolerable disease burden of 10–4 infections per person per year for both rotavirus and Shigella spp.
Rotavirus dose and risks were linearly correlated with Shigella spp. dose and risks, respectively (Figure 4). Although a day’s activities consist of contact with wide ranging surfaces, the transfer efficiencies, concentrations on surfaces, and surface areas of contact applied for each of these surfaces are linearly combined to calculate the concentration on hands through time. For the activities monitored, the range, frequency and timing of surfaces contacted by different individuals on different days (in the collection of nearly 40 urine transport containers and in the production of 10 batches of struvite) was similar enough to maintain a linear relationship between the pathogen dose. The linear relationship between rotavirus and Shigella spp. doses (rotavirus dose ≈0.44 Shigella spp. dose) reflects the ratio of the concentration of rotavirus to the concentration of Shigella spp. in urine (47 genome copies rotavirus to 105 genome copies Shigella spp.). The finding suggests the predictability of doses to other pathogens based on estimated concentrations in urine. The uncertainty bounds captured by the model provides important information when extrapolating results to other pathogens.
The linear relationship between rotavirus and Shigella spp. risks is more complicated (rotavirus risk ∼90 Shigella spp. risk), as it is influenced by both dose and dose–response curves. The dose–response curves for rotavirus and Shigella spp. are coincidentally both modeled as beta-Poisson with similar shape parameters (αrotavirus = 0.265, αShigella spp.= 0.253). Therefore, the observed linear relationship for risks is not extensible to other pathogens with dose–response relationships described by other distributions (i.e., exponential) or substantially different beta-Poisson shape parameters.
Modeling Highlights Effective Risk Management Strategies during Container-Based Resource Recovery
Both dose and risk were higher during urine collection than during struvite production. Struvite production was expected to result in higher risks for sanitation service workers due to more frequent contacts with urine and contaminated surfaces. However, urine collection requires frequent contact with highly contaminated transport containers. Therefore, in this setting, urine transport containers are important drivers of pathogen surface densities on hands and of potential ingested dose. By comparison, the primary driver of model variability for struvite production was the pathogen concentration on the cloth reactor components (Figure S2), which were saturated with urine. To reduce risks, controlling pathogen contamination in urine is a promising intervention. Urine collection occurs when contamination of urine with feces is recent and pathogen concentrations in the urine are greatest. Both storage and treatment, for example with the addition of lime, reduce pathogen contamination.59 With storage, pathogen concentrations decline in urine due to the natural production of the disinfectant ammonia during urea hydrolysis and increased pH.60 Storage alone can reduce infection risks: Ahmed et al. (2017) estimate infection risks are sufficiently reduced when urine is stored for four months 15 °C (approximately the average winter temperature in Durban) or 10 days at 25 °C (approximately the average summer temperature).61 However, storage is not feasible in CBS due to logistic and/or space constraints. Similar to solid waste collection from households, CBS urine collection is needed at regular intervals to facilitate proper toilet usage at the household (to avoid discharge of urine to the environment) and to minimize costs of urine collection for nutrient recovery.34 Centralized storage, though potentially more feasible, would not reduce risks for sanitation workers collecting urine from households and transporting to centralized storage facilities. Lime is an effective treatment because it markedly increases the pH (>11), which increases pathogen inactivation and prevents the loss of ammonia due to evaporation in storage. However, the highly alkaline pH also poses safety risks to urine collectors. This study suggests the most practical approaches may be interrupting exposures to contaminated urine transport containers, which may include cleaning and/or secondary containment procedures, promotion of personal protective equipment, and raising awareness among sanitation service workers to promote hand and environmental hygiene. Specific hygiene interventions upon participant entry of urine collectors into the vans and trucks used during collection would be a logical control point.
Study Limitations
The inherently complex nature of the field study led to several limitations of this research. First, the presence of background E. coli may have increased the calculated concentrations of pathogens on surfaces, which relied on E. coli measurements to determine the volume of urine contamination. However, E. coli levels were below the detection limit in two-thirds of surface swab samples (Table 1), indicating low background concentrations on a diversity of objects throughout the study area. Regardless, future risk assessments would benefit from quantitative estimates of pathogen contamination based on direct measurements. Second, the model construct relied on a number of simplifying assumptions as previously discussed. The development of rapid on-site microbial field sampling tools would allow assumptions regarding time-dependent pathogen concentration distributions to be evaluated and refined in the model. With such data available, differences in transfer efficiencies from wet to dry surfaces or dry to wet surfaces may also be incorporated in future applications of this model.49 Finally, the videographic approach for quantifying human-environment interactions relies on high quality first-person videos and requires extensive data processing time. Due to the cost associated with collection and annotation of videos, sample size of study participants was limited. Videography provides the benefit of tracking human movements through space and time to generate thousands of data points for each subject and activity evaluated. Such high resolution data allows assessment of within-participant heterogeneity. However, between-participant heterogeneity is limited by small sample sizes and the potential for activity bias from participants who are aware their actions are being recorded. Study of the microbial health risk potential (e.g., number of hand-to-mouth contacts) during a variety of container-based and other waste collection and transport techniques for nonsewered sanitation systems in different cities and countries would provide valuable context to the human-environment interaction results obtained in this study. Improved computer-vision algorithms to aid processing could dramatically improve data collection.62
Significance
CBS is a promising and appropriate alternative to expensive sewer distribution systems, with potential for rapid and widespread expansion in developing countries. Yet, in particular for CBS urine collection, potential microbial health risks for sanitation service workers have been overlooked. This study was unique in its combination of tools and techniques to evaluate microbial health risks due to indirect exposure during container-based sanitation and resource recovery through manual struvite production. An important objective of this study was to describe and model the complexity of realistic scenarios of pathogen transmission during urine collection and handling. Overwhelmingly, exposure assessments model risks using assumptions of direct ingestion of low volumes of urine.61,63 In the present study, risks were driven by indirect exposure via pathogen transfer from urine to surfaces, surfaces to hands, and hands to the mouth, while no accidental ingestion was observed. By modeling these processes, we observed that the risk of infection via indirect exposure depends on a combination of factors including the timing, extent and frequency of contacts with contaminated surfaces, the level of surface contamination, the frequency and timing of hand-to-mouth contacts, and the dose–response relationship for the pathogen of interest. Risks reliant on assumptions of accidental ingestion neglect the dynamics of exposures from hand-to-mouth contacts, which are more typical of daily activities. Understanding the extent to which pathogens in urine may be transmitted, and routes of transmission, can help in communicating risks to those involved in manual urine collection or processing and to identify appropriate intervention strategies. The results reaffirm the need to follow standard practices related to proper training and implementation of safety protocols in the expansion of CBS, urine collection and waste resource recovery. The methods applied in this study can also be used to evaluate impacts of such interventions. With the changing paradigm of wastewater to that of resource recovery, the health of sanitation service workers should not be placed in jeopardy as services are expanded.
Acknowledgments
This study was undertaken in the framework of the Valorisation of Urine Nutrients in Africa (VUNA) project funded by the Bill & Melinda Gates Foundation (www.vuna.ch, Grant No. OPP1011603). Additional funding was provided by the U.S. National Science Foundation International Research Fellowship Program (Grant No. 1159225), the Swiss National Science Foundation (Grant No. 200021_146829/1), and discretionary funding provided by Eawag and EPFL. Thanks to the study volunteers, Robert Canales for providing the Video Translator for the Personal Computer software, Loic Decrey for processing samples, Nicola Rodda for use of laboratory facilities, EWS staff and Chris Buckley for facilitating the research, and Ana Karina Pitol for helpful discussions.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.9b01092.
Detailed methods on quantifying pathogen contamination of surfaces; use of Brilliant Blue dye for validation of urine volume and hand contamination; parameter values used in the model; detailed results on microlevel activity time series; sensitivity analyses for rotavirus on hands during urine collection and struvite production; intertranslator comparison of microlevel activity time series data; extraction efficiency and calibration curves of exposure estimates using Brilliant Blue dye; exposure profiles for Shigella spp. on left and right hands during urine collection; surface categories and category components used during videography of both urine collection and struvite production; summary of microlevel activity time series data for both urine collection and struvite production; and personal protective equipment use, location, urine splashing, and videography quality for both urine collection and struvite production (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Guest J. S.; Skerlos S. J.; Barnard J. L.; Beck M. B.; Daigger G. T.; Hilger H.; Jackson S. J.; Karvazy K.; Kelly L.; Macpherson L.; et al. A New Planning and Design Paradigm to Achieve Sustainable Resource Recovery from Wastewater. Environ. Sci. Technol. 2009, 43 (16), 6126–6130. 10.1021/es9010515. [DOI] [PubMed] [Google Scholar]
- World Health Organization; UNICEF Progress on Drinking Water; Sanitation and Hygiene; 2017. [Google Scholar]
- Fuhrmeister E. R.; Schwab K. J.; Julian T. R. Estimates of Nitrogen, Phosphorus, Biochemical Oxygen Demand, and Fecal Coliforms Entering the Environment Due to Inadequate Sanitation Treatment Technologies in 108 Low and Middle Income Countries. Environ. Sci. Technol. 2015, 49 (19), 11604–11611. 10.1021/acs.est.5b02919. [DOI] [PubMed] [Google Scholar]
- Bohnert K.; Chard A. N.; Mwaki A.; Kirby A. E.; Muga R.; Nagel C. L.; Thomas E. A.; Freeman M. C. Comparing Sanitation Delivery Modalities in Urban Informal Settlement Schools: A Randomized Trial in Nairobi, Kenya. Int. J. Environ. Res. Public Health 2016, 13 (12), 1189. 10.3390/ijerph13121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel K.; Tilmans S.; Kramer S.; Sklar R.; Tillias D.; Davis J. User Perceptions of and Willingness to Pay for Household Container-Based Sanitation Services: Experience from Cap Haitien, Haiti. Environ. Urban. 2015, 27 (2), 525–540. 10.1177/0956247815596522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilmans S.; Russel K.; Sklar R.; Page L.; Kramer S.; Davis J. Container-Based Sanitation: Assessing Costs and Effectiveness of Excreta Management in Cap Haitien, Haiti. Environ. Urban. 2015, 27 (1), 89–104. 10.1177/0956247815572746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M.; Bufardi A.; Tilley E.; Zurbrügg C.; Truffer B. A Compatibility-Based Procedure Designed to Generate Potential Sanitation System Alternatives. J. Environ. Manage. 2012, 104, 51–61. 10.1016/j.jenvman.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Tilley E. Cost-Effectiveness and Community Impacts of Two Urine-Collection Programs in Rural South Africa. Environ. Sci. Water Res. Technol. 2016, 2 (2), 320–335. 10.1039/C5EW00237K. [DOI] [Google Scholar]
- Ernst & Young LLP; Water & Sanitation for the Urban Poor. The World Can’t Wait for Sewers: Advancing Container-Based Sanitation Businesses as a Viable Answer to the Global Sanitation Crisis, 2017.
- Diener S.; Semiyaga S.; Niwagaba C. B.; Murray A.; Birane J.; Mbéguéré M.; Effah J.; Zurbrugg C.; Strande L. A Value Proposition : Resource Recovery from Faecal Sludge — Can It Be the Driver for Improved Sanitation ?. Resources, Conserv. Recycl. 2014, 88, 32–38. 10.1016/j.resconrec.2014.04.005. [DOI] [Google Scholar]
- Tilley E.; Gantenbein R.; Khadka R.; Zurbrügg C.; Udert K. M. Social and Economic Feasibility of Struvite Recovery from Urine at the Community Level in Nepal. Int. Conf. Nutr. Recover. from Wastewater Streams 2009, 169–178. [Google Scholar]
- Bischel H. N.; Özel Duygan B. D.; Strande L.; McArdell C. S.; Udert K. M.; Kohn T. Pathogens and Pharmaceuticals in Source-Separated Urine in eThekwini, South Africa. Water Res. 2015, 85, 57–65. 10.1016/j.watres.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Stenström T. A.; Seidu R.; Nelson E.; Christian Z.. Microbial Exposure and Health Assessments in Sanitation Technologies and Systems; Stockholm Environment Institute: Stockholm, Sweden, 2011. [Google Scholar]
- Katukiza A. Y.; Ronteltap M.; van der Steen P.; Foppen J. W. A.; Lens P. N. L. Quantification of Microbial Risks to Human Health Caused by Waterborne Viruses and Bacteria in an Urban Slum. J. Appl. Microbiol. 2014, 116 (2), 447–463. 10.1111/jam.12368. [DOI] [PubMed] [Google Scholar]
- Fracchia L.; Pietronave S.; Rinaldi M.; Giovanna Martinotti M. Site-Related Airborne Biological Hazard and Seasonal Variations in Two Wastewater Treatment Plants. Water Res. 2006, 40 (10), 1985–1994. 10.1016/j.watres.2006.03.016. [DOI] [PubMed] [Google Scholar]
- WHO WHO Guidelines for the Safe Use of Wastewater, Excreta and Greywater; WHO: Geneva, Switzerland, 2006; Vol. II, p 204. [Google Scholar]
- Schoen M. E.; Ashbolt N. J.; Jahne M. A.; Garland J. Risk-Based Enteric Pathogen Reduction Targets for Non-Potable and Direct Potable Use of Roof Runoff, Stormwater, and Greywater. Microb. Risk Anal. 2017, 5, 32–43. 10.1016/j.mran.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund C.; Stenström T. A.; Ashbolt N. Microbial Risk Assessment of Source-Separated Urine Used in Agriculture. Waste Manage. Res. 2002, 20 (2), 150–161. 10.1177/0734242X0202000207. [DOI] [PubMed] [Google Scholar]
- Ahmed W.; Hamilton K. A.; Vieritz A.; Powell D.; Goonetilleke A.; Hamilton M. T.; Gardner T. Microbial Risk from Source-Separated Urine Used as Liquid Fertilizer in Sub-Tropical Australia. Microb. Risk Anal. 2017, 5, 53–64. 10.1016/j.mran.2016.11.005. [DOI] [Google Scholar]
- Cherrie J. W.; Semple S.; Christopher Y.; Saleem A.; Hughson G. W.; Philips A. How Important Is Inadvertent Ingestion of Hazardous Substances at Work?. Ann. Occup. Hyg. 2006, 50 (7), 693–704. [DOI] [PubMed] [Google Scholar]
- Kwong L. H.; Ercumen A.; Pickering A. J.; Unicomb L.; Davis J.; Luby S. P. Hand- and Object-Mouthing of Rural Bangladeshi Children 3–18 Months Old. Int. J. Environ. Res. Public Health 2016, 13 (6), 563. 10.3390/ijerph13060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou M.-C.; Özkaynak H.; Beamer P.; Dang W.; Hsi H.-C.; Jiang C.-B.; Chien L.-C. Mouthing Activity Data for Children Aged 7 to 35 Months Old in Taiwan Ming-Chien. J. Exposure Sci. Environ. Epidemiol. 2015, 25 (4), 388–398. 10.1038/jes.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US-EPA Child-Specific Exposure Factors Handbook, 2008.
- Nicas M.; Best D. A Study Quantifying the Hand-to-Face Contact Rate and Its Potential Application to Predicting Respiratory Tract Infection. J. Occup. Environ. Hyg. 2008, 5 (6), 347–352. 10.1080/15459620802003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer P.; Plotkin K. R.; Gerba C. P.; Sifuentes L. Y.; Koenig D. W.; Reynolds K. A. Modeling of Human Viruses on Hands and Risk of Infection in an Office Workplace Using Micro-Activity Data. J. Occup. Environ. Hyg. 2015, 12 (4), 266–275. 10.1080/15459624.2014.974808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.; Eisenberg J. E.; Spicknall I. H.; Li S.; Koopman J. S. Model Analysis of Fomite Mediated Influenza Transmission. PLoS One 2012, 7 (12), e51984. 10.1371/journal.pone.0051984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci A.; Donzelli G.; Cioni L.; Federigi I.; Lombardi R.; Verani M. Quantitative Microbial Risk Assessment for Workers Exposed to Bioaerosol in Wastewater Treatment Plants Aimed at the Choice and Setup of Safety Measures. Int. J. Environ. Res. Public Health 2018, 15, 1490. 10.3390/ijerph15071490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunger N.; Teske S. S.; Nappier S.; Haas C. N. Recreational Use Assessment of Water-Based Activities, Using Time-Lapse Construction Cameras. J. Exposure Sci. Environ. Epidemiol. 2012, 22 (3), 281–290. 10.1038/jes.2012.4. [DOI] [PubMed] [Google Scholar]
- Julian T. R.; Vithanage H. S. K.; Li M.; Kuroda M.; Pitol A. K.; Hong P.; Nguyen L.; Canales R. A.; Fujii S.; Harada H. High Time-Resolution Simulation of E. Coli on Hands Reveals Large Variation in Microbial Exposures amongst Vietnamese Farmers Using Human Excreta for Agriculture. Sci. Total Environ. 2018, 635 (April), 120–131. 10.1016/j.scitotenv.2018.04.100. [DOI] [PubMed] [Google Scholar]
- Julian T. R.; Pickering A. J. A Pilot Study on Integrating Videography and Environmental Microbial Sampling to Model Fecal Bacterial Exposures in Peri-Urban Tanzania. PLoS One 2015, 10 (8), e0136158 10.1371/journal.pone.0136158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff K. L.; Nataro J. P.; Blackwelder W. C.; Nasrin D.; Farag T. H.; Panchalingam S.; Wu Y.; Sow S. O.; Sur D.; Breiman R. F.; et al. Burden and Aetiology of Diarrhoeal Disease in Infants and Young Children in Developing Countries (the Global Enteric Multicenter Study, GEMS): A Prospective, Case-Control Study. Lancet 2013, 382 (9888), 209–222. 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Troeger C.; Forouzanfar M.; Rao P. C.; Khalil I.; Brown A.; Reiner R. C.; Fullman N.; Thompson R. L.; Abajobir A.; Ahmed M.; et al. Estimates of Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoeal Diseases: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17 (9), 909–948. 10.1016/S1473-3099(17)30276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udert K. M.; Buckley C. A.; Wächter M.; McArdell C. S.; Kohn T.; Strande L.; Zöllig H.; Hug A.; Oberson A.; Etter B. Technologies for the Treatment of Source-Separated Urine in the eThekwini Municipality. Water SA 2015, 41, 212–221. 10.4314/wsa.v41i2.06. [DOI] [Google Scholar]
- Tilley E. Cost-Effectiveness and Community Impacts of Two Urine-Collection Programs in Rural South Africa. Environ. Sci. Water Res. Technol. 2016, 2, 320–335. 10.1039/C5EW00237K. [DOI] [Google Scholar]
- Rhoton S.; Grau M.; Brouckaert C. J.; Gounden G.; Buckley C. A.. Field Operation of a Simple Struvite Reactor to Produce Phosphorus Fertiliser from Source-Separated Urine in eThekwini. In WISA Biennal Conference May 25–28, 2014; Mbombela, Mpumalanga, South Africa, 2014; pp 1–5.
- Bischel H. N.; Schertenleib A.; Fumasoli A.; Udert K. M.; Kohn T. Inactivation Kinetics and Mechanisms of Viral and Bacterial Pathogen Surrogates during Urine Nitrification. Environ. Sci. Water Res. Technol. 2015, 1, 65–76. 10.1039/C4EW00065J. [DOI] [Google Scholar]
- Fumasoli A.; Etter B.; Sterkele B.; Morgenroth E.; Udert K. M. Operating a Pilot-Scale Nitrification/distillation Plant for Complete Nutrient Recovery from Urine. Water Sci. Technol. 2016, 73 (1), 215–222. 10.2166/wst.2015.485. [DOI] [PubMed] [Google Scholar]
- Quantitative Microbial Risk Assessment: Application for Water Safety Management; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Zartarian V. G.; Ferguson A. C.; Leckie J. O. Quantified Mouthing Activity Data from a Four-Child Pilot Field Study. J. Expo. Anal. Environ. Epidemiol. 1998, 8 (4), 543–553. [PubMed] [Google Scholar]
- Mattioli M. C. M.; Davis J.; Boehm A. B. Hand-to-Mouth Contacts Result in Greater Ingestion of Feces than Dietary Water Consumption in Tanzania: A Quantitative Fecal Exposure Assessment Model. Environ. Sci. Technol. 2015, 49 (3), 1912–1920. 10.1021/es505555f. [DOI] [PubMed] [Google Scholar]
- Ottoson J.; Stenström T. A. Faecal Contamination of Greywater and Associated Microbial Risks. Water Res. 2003, 37 (3), 645–655. 10.1016/S0043-1354(02)00352-4. [DOI] [PubMed] [Google Scholar]
- Julian T. R. Environmental Transmission of Diarrheal Pathogens in Low and Middle Income Countries. Environ. Sci. Process. Impacts 2016, 18 (8), 944–955. 10.1039/C6EM00222F. [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency Exposure Factors Handbook; U.S. Environ. Prot. Agency: Washington, DC, 2011; pp 1–1466. [Google Scholar]
- Flores A. P.; Berenstein G. A.; Hughes E. A.; Zalts A.; Montserrat J. M. Pesticide Risk Assessment in Flower Greenhouses in Argentina: The Importance of Manipulating Concentrated Products. J. Hazard. Mater. 2011, 189 (1–2), 222–228. 10.1016/j.jhazmat.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Julian T. R.; Canales R. A.; Leckie J. O.; Boehm A. B. A Model of Exposure to Rotavirus from Nondietary Ingestion Iterated by Simulated Intermittent Contacts. Risk Anal 2009, 29 (5), 617. 10.1111/j.1539-6924.2008.01193.x. [DOI] [PubMed] [Google Scholar]
- De Man H.; Bouwknegt M.; van Heijnsbergen E.; Leenen E. J. T. M.; van Knapen F.; de Roda Husman A. M. Health Risk Assessment for Splash Parks That Use Rainwater as Source Water. Water Res. 2014, 54, 254–261. 10.1016/j.watres.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Pinfold J. V. Faecal Contamination of Water and Fingertip-Rinses as a Method for Evaluating the Effect of Low-Cost Water Supply and Sanitation Activities on Faeco-Oral Disease Transmission. I. A Case Study in Rural North-East Thailand. Epidemiol. Infect. 1990, 105 (2), 363–375. 10.1017/S0950268800047956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Hubal E. A.; Nishioka M. G.; Ivancic W. A.; Morara M.; Egeghy P. P. Comparing Surface Residue Transfer Efficiencies to Hands Using Polar and Nonpolar Fluorescent Tracers. Environ. Sci. Technol. 2008, 42 (3), 934–939. 10.1021/es071668h. [DOI] [PubMed] [Google Scholar]
- Pitol A. K.; Bischel H. N.; Kohn T.; Julian T. R. Virus Transfer at the Skin-Liquid Interface. Environ. Sci. Technol. 2017, 51 (24), 14417–14425. 10.1021/acs.est.7b04949. [DOI] [PubMed] [Google Scholar]
- Rigotto C.; Victoria M.; Moresco V.; Kolesnikovas C. K.; Corrêa A.; Souza D. S. M.; Miagostovich M. P.; Simões C. M. O.; Barardi C. R. M. Assessment of Adenovirus, Hepatitis A Virus and Rotavirus Presence in Environmental Samples in Florianopolis, South Brazil. J. Appl. Microbiol. 2010, 109 (6), 1979–1987. 10.1111/j.1365-2672.2010.04827.x. [DOI] [PubMed] [Google Scholar]
- Prez V. E.; Gil P. I.; Temprana C. F.; Cuadrado P. R.; Martínez L. C.; Giordano M. O.; Masachessi G.; Isa M. B.; Ré V. E.; Paván J. V.; et al. Quantification of Human Infection Risk Caused by Rotavirus in Surface Waters from Córdoba, Argentina. Sci. Total Environ. 2015, 538, 220–229. 10.1016/j.scitotenv.2015.08.041. [DOI] [PubMed] [Google Scholar]
- Center for Advancing Microbial Risk Assessment (CAMRA) . Quantitative Microbial Risk Assessment (QMRA) Wiki http://qmrawiki.canr.msu.edu/index.php/Dose_Response (accessed Jun 25, 2018).
- Xue J.; Zartarian V.; Moya J.; Freeman N.; Beamer P.; Black K.; Tulve N.; Shalat S. A Meta-Analysis of Children’s Hand-to-Mouth Frequency Data for Estimating Nondietary Ingestion Exposure. Risk Anal 2007, 27 (2), 411–420. 10.1111/j.1539-6924.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- Nordin A.; Niwagaba C.; Jönsson H.; Vinnerås B. Pathogen and Indicator Inactivation in Source-Separated Human Urine Heated by the Sun. J. Water, Sanit. Hyg. Dev. 2013, 3 (2), 181–188. 10.2166/washdev.2013.174. [DOI] [Google Scholar]
- Vinnerås B.; Nordin A.; Niwagaba C.; Nyberg K. Inactivation of Bacteria and Viruses in Human Urine Depending on Temperature and Dilution Rate. Water Res. 2008, 42 (15), 4067–4074. 10.1016/j.watres.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Decrey L.; Kohn T. Virus Inactivation in Stored Human Urine, Sludge and Animal Manure under Typical Conditions of Storage or Mesophilic Anaerobic Digestion. Environ. Sci. Water Res. Technol. 2017, 3 (3), 492–501. 10.1039/C6EW00311G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L.; Alfano-Sobsey E.; Rutala W. A.; Weber D. J.; Sobsey M. Virus Transfer from Personal Protective Equipment to Healthcare Employees’ Skin and Clothing. Emerging Infect. Dis. 2008, 14 (8), 1291–1293. 10.3201/eid1408.080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W.; Hamilton K. A.; Vieritz A.; Powell D.; Goonetilleke A.; Hamilton M. T.; Gardner T. Microbial Risk from Source-Separated Urine Used as Liquid Fertilizer in Sub-Tropical Australia. Microb. Risk Anal. 2017, 5, 53–64. 10.1016/j.mran.2016.11.005. [DOI] [Google Scholar]
- Randall D. G.; Krähenbühl M.; Köpping I.; Larsen T. A.; Udert K. M. A Novel Approach for Stabilizing Fresh Urine by Calcium Hydroxide Addition. Water Res. 2016, 95, 361–369. 10.1016/j.watres.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decrey L.; Kazama S.; Udert K. M.; Kohn T. Ammonia as an in-Situ Sanitizer: Inactivation Kinetics and Mechanisms of the ssRNA Virus MS2 by NH3. Environ. Sci. Technol. 2015, 49, 1060–1067. 10.1021/es5044529. [DOI] [PubMed] [Google Scholar]
- Ahmed W.; Hamilton K. A.; Vieritz A.; Powell D.; Goonetilleke A.; Hamilton M. T.; Gardner T. Microbial Risk from Source-Separated Urine Used as Liquid Fertilizer in Sub-Tropical Australia. Microb. Risk Anal. 2017, 5, 53–64. 10.1016/j.mran.2016.11.005. [DOI] [Google Scholar]
- Julian T. R.; Bustos C.; Kwong L. H.; Badilla A. D.; Lee J.; Bische H. N.; Canales R. A. Quantifying Human-Environment Interactions Using Videography in the Context of Infectious Disease Transmission. Geospat. Health 2018, 13 (1), 195–197. 10.4081/gh.2018.631. [DOI] [PubMed] [Google Scholar]
- Höglund C.; Stenström T. A.; Ashbolt N. Microbial Risk Assessment of Source-Separated Urine Used in Agriculture. Waste Manage. Res. 2002, 20 (2), 150–161. 10.1177/0734242X0202000207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.