Abstract
Addiction is a disease in which, after a period of recreational use, a subset of individuals develops compulsive use that does not stop even in light of major negative consequences. Here, we review the evidence for underlying epigenetic remodeling in brain in two settings. First, excessive dopamine signaling during drug use may modulate gene expression, altering synaptic function and circuit activity and leading over time to maladaptive behaviors in vulnerable individuals. Second, on a longer timescale, life experience can shape the epigenetic landscape in brain and thereby may contribute to an individual’s vulnerability by amplifying drug-induced changes in gene expression that drive the transition to addiction. We conclude by exploring how epigenetic mechanisms might serve as therapeutic targets for addiction treatments.
Drug addiction is one of the leading causes of disability in the world today, with an estimated annual cost to the U.S. of more than $740 billion annually related to crime, lost work productivity, and health care (National Institute on Drug Abuse, 2018 [based on 2007–2013 statistics]). Similar European surveys, using somewhat different metrics, estimate the cost at ~65 billion Euros (Olesen et al., 2012). While the U.S. is currently facing an epidemic of opioid addiction, with over 40,000 opioid-related deaths reported in 2017 alone (Center for Disease Control and Prevention, 2017), the country has experienced repeated cycles of use of several abused substances over the past half century, with use of one drug class increasing just as use of another declines. Indeed, we are beginning to see another spike in use of psychostimulants (cocaine, methamphetamine) as today’s policy efforts focus more on opioids. Despite the devastating impact of addiction on humanity, available pharmacological and psychosocial treatments remain inadequately effective for most people.
The past two decades have witnessed compelling advances in our understanding of how addictive drugs affect the brain initially and, with repeated exposure, induce longer-lasting changes that drive the compulsive seeing and taking of drugs that defines addiction. The field has established the initial molecular targets of virtually all drugs of abuse and demonstrated that expression of those targets converges in defined brain regions, which comprise the brain’s reward circuitry. This circuitry controls responses to emotional stimuli (rewarding and aversive), motivation to prioritize and consume rewards (e.g., food, sex, social interaction), executive restraint over that consumption, and shaping future behavior through learning- and memory-related functions. All drugs of abuse, despite their initial effects on distinct molecular targets, exert a series of shared functional effects on the reward circuitry to corrupt these processes, which in the extreme cause an individual to lose control over drug consumption (Nestler, 2005).
An essential question in the field has been through what mechanism does repeated consumption of a drug of abuse cause the usurpation of brain reward circuits? One major effort in the field has focused on how repeated drug exposure alters the activity of individual neurons within these brain regions and the strength of their synaptic contacts, in essence, reordering the reward circuitry to drive the abnormal behavior that characterizes an addicted state. A largely separate major effort has focused on how repeated drug exposure alters the molecular constituents of individual brain cells to alter the functioning. A third general theme is why some individuals are vulnerable to the circuit and molecular plasticity induced by drug consumption, while other individuals remain resistant. Ultimately, an understanding of drug addiction will require a synthesis of all three experimental perspectives to delineate bidirectionally how cell-autonomous molecular adaptations in vulnerable individuals drive the altered functioning of the larger neural circuits within which those cells operate, and how circuit-level disruptions caused by drugs contribute to the cells’ molecular adaptations. We argue that these questions can best be solved through an iterative process, which requires the study of molecules, cells, and circuits.
The goal of this review is to set the stage for this integrated experimental effort, which is only beginning to be employed. We start with a brief overview of the brain’s reward circuitry focusing on the importance of dopaminergic transmission as a shared target for all drugs of abuse. We then introduce the concept of epigenetics, and associated regulation of gene transcription, which we view as a fundamental mechanism by which repeated drug exposure can change the brain for a lifetime and render an individual more susceptible to addiction later in life. This is followed by an overview of the synaptic and circuit changes induced by addictive drugs driving maladaptive behavior, as well as of the epigenetic and transcriptional regulation documented to date in addiction models. The review ends by outlining how a combined effort is poised to advance our grasp of addiction and eventually contribute to new treatments.
Dopaminergic Signaling and Addiction
Increased dopaminergic signaling from the midbrain ventral tegmental area (VTA) to the nucleus accumbens (NAc) in ventral striatum is hypothesized as the initial common action of all addictive drugs (Lüscher and Ungless, 2006). The effect is a strong reinforcement, which with chronic access may shape behavior in all subjects (here termed maladaptive behavior), but leads to compulsive consumption only in some individuals. The hypothesis is rooted in direct measures of extracellular dopamine (DA) levels in NAc (Di Chiara and Imperato, 1988) and in the observation that electrical activation of the medial forebrain bundle (which includes VTA→NAc axons) (Olds and Milner, 1954) or optogenetic excitation of VTAdopamine neurons in mice and rats (Witten et al., 2011; Ilango et al., 2014; Pascoli et al., 2015) drives reinforcement. In general, rats self-stimulate brain regions that receive dopamine inputs, presumably through axonal stimulation (Roberts et al., 1977). Conversely, dopamine receptor antagonists prevent reinforcement of psychostimulants in rats (Maldonado et al., 1993; McGregor and Roberts, 1993) and primates (Bergman et al., 1989). Within the population of VTA dopamine neurons, those projecting to the medial NAc shell appear to be the primary target of addictive drugs, demonstrated with micro-dialysis and fast scan cycle voltametry (Aragona et al., 2008; Di Chiara and Imperato, 1988; Stuber et al., 2005) or genetically encoded fluorescent probes (Wei et al., 2018). This is also the case for heroin, which causes a rapid dopamine transient in the NAc medial shell (Corre et al., 2018) driven by disinhibition of VTA dopamine neurons (Johnson and North, 1992).
One function of midbrain dopamine neurons may be the generation of a reward prediction error (RPE) signal that can drive associative learning (Schultz et al., 1997). The RPE hypothesis provides a theoretical framework that can link pharmacological dopamine enhancement to reward learning and ultimately addiction (Keiflin and Janak, 2015). Which population of midbrain dopamine neurons are stimulated turns out to be critical, as conditioned responding only occurs with VTA stimulation and not stimulation of the nearby substantia nigra (Saunders et al., 2018). The latter can drive motor invigoration, but animals fail to learn the significance of Pavlovian cues. Even within the VTA, dopamine neurons display considerable heterogeneity based on differences in their afferent and efferent connections as well as their responses to rewarding and aversive stimuli (Lammel et al., 2014). Additional work is needed to define the relative contribution of these various subpopulations to the addiction process.
Taken together, we consider addiction as a disease that is linked etiologically to a specific pharmacological substance in a vulnerable individual and characterized by maladaptive compulsive consumption of that substance. While dopamine projections from VTA to NAc trigger the development of this pathological behavior, additional neuromodulator systems and circuits also contribute, as will be seen below.
Epigenetic Regulation
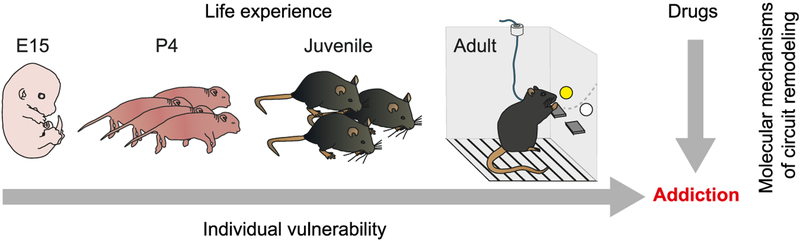
The term epigenetics, used most broadly, describes changes in chromatin structure that are associated with alterations in gene expression including those induced in the fully differentiated adult brain in response to a host of environmental stimuli such as addictive drugs. A drug alters gene expression and epigenetic mechanisms in two ways: as a direct effect of the drug activating its specific molecular target and downstream signaling cascades, or indirectly via increases in dopamine signaling and its downstream cascades. For the maladaptive changes shared across all classes of abused drugs, the latter seems of particular interest. Epigenetic mechanisms operate throughout life and play an important role in mediating the effect of lifetime exposures on the organism. Such mechanisms are likely involved in events during early development that contribute to variations across individuals that arise stochastically even in the face of constant genome sequence and similar environment (Honegger and de Bivort, 2018). By analogy with cell differentiation, where certain types of epigenetic modifications once they occur are permanent, it is possible that certain epigenetic changes resulting from behavioral experience or random developmental events underlie permanent changes in brain function, which could confer vulnerability to transition from recreational to compulsive use of drugs of abuse (Figure 1).
Figure 1. Impact of Epigenetic Remodeling on Addiction.
Life experience, such as early life stress, may shape the epigenetic landscape during development and eventually determine the individual vulnerability of addiction. In addition, addictive drugs may modulate gene expression via epigenetic mechanism. Molecular changes may occur through signaling of the drug-activated target or indirectly via increase of dopamine. The latter may constitute a molecular basis of neural circuit adaptation, such as drug-evoked synaptic plasticity common to all addictive drugs.
The last decade has witnessed important advances in understanding how the organization of chromatin controls the expression of specific genes (Berger, 2007; Maze et al., 2010). Many types of such epigenetic mechanisms affect the readability of the DNA double helix, which is wrapped around octamers of histones to form the unit of chromatin called a nucleosome (Box 1; Figure 2). In many tissues, including the brain, numerous types of histone modifications (e.g., acetylation, methylation, and phosphorylation, among others), along with the “writers,” “erasers,” and “readers” underlie these regulatory mechanisms. Also important are changes in nucleosome spacing and turnover, methylation of DNA itself, and several types of non-coding RNAs (ncRNAs), such as microRNAs (miRNAs) and long non-coding RNAs. Even the 3D structure of chromatin is subject to dynamic regulation. Through “looping,” distant regions of the genome can be brought into close proximity, affecting gene expression (Dekker and Mirny, 2016; Rowley and Corces, 2016). While many of these modifications are transient, some are long lived and may contribute to lasting cellular plasticity. However, no particular mode of epigenetic regulation is inherently stable, which means that the longer-lasting epigenetic changes can only be ascertained by empirical study. Moreover, numerous epigenetic mechanisms work in concert to control gene expression, which requires their exploration in a coordinated manner.
Box 1. Overview of Epigenetic Regulation.
DNA is condensed into the nucleus of the cell in a highly organized and compact manner referred to as chromatin. The functional unit of chromatin, the nucleosome, is composed of ~147 base pairs wrapped around core histone octamers consisting of 2 copies of each of the following proteins: histones H2A, H2B, H3, and H4 (Figure 2). Each histone protein can undergo numerous post-translational modifications (PTMs) in which different functional groups are covalently added to amino acid residues of their N-terminal tails—e.g., acetylation, methylation, phosphorylation, ubiquitination, SUMOylation, crotonylation, citrullination, and ADP-ribosylation, among others (Walker and Nestler, 2018). These modifications not only alter the structure of the nucleosome, but also change the interaction of DNA with the associated histones, thus increasing or decreasing the likelihood of transcription at a given locus. Histone modifications are diverse, and we are only beginning to understand how the diverse combinations of histone PTMs influence or indicate specific transcriptional states (Maze et al., 2014). Finally, histone modifications are added or removed by a large family of enzymes referred to as “writers” and “erasers,” respectively, making them reversible and dynamic epigenetic modifications. For example, histone acetylation is catalyzed by histone acetyltransferases, and reversed by histone deacetylases (HDACs). “Readers” refer to a host of proteins that bind to a specific PTM (or combination thereof) to effect changes in chromatin structure (e.g., nucleosome spacing) and the binding of the transcriptional machinery at a locus to ultimately control gene transcription.
An important epigenetic regulator in addition to histone modifications is DNA methylation, which occurs with the addition of a methyl group to cytosine-phospho-guanine (CpG) at the C5 position (5-mC). In development, it plays a pivotal role in tissue-specific gene expression, cellular differentiation, X chromosome inactivation, imprinting of parental alleles, and repetitive element silencing. DNA methylation at gene promoters is generally associated with repression, while methylation in gene bodies has been associated with active transcription (Cholewa-Waclaw et al., 2016; Kinde et al., 2015). A related cytosine modification, 5-hydroxy-mC (5-hmC), is concentrated in gene bodies and is enriched in brain and associated with active gene transcription. Recent genome-wide characterization of DNA methylation has revealed non-cytosine methylation, which also appears to regulate gene transcription. While DNA methylation has traditionally been characterized as a stable epigenetic mark, recent evidence shows that it is much more transient than previously thought. It is clear that DNA methylation and histone modifications participate in an “epigenetic conversation” to regulate transcription. For example, DNA methylation can recruit methylation-binding proteins, which in turn recruit HDACs, leading to a repressed chromatin state.
A suite of genome-wide sequencing tools has made it increasingly feasible to characterize this wide range of epigenetic modifications in discrete brain regions, populations of the same cell type within a region, and even all cells in a region at the single-cell level. RNA sequencing (RNA-seq) captures all RNAs expressed including diverse types of non-coding RNAs. Chromatin immunoprecipitation sequencing (ChIP-seq) enables genome-wide delineation of histone modifications and the binding of transcription factors, histone writers, erasers, and readers, chromatin regulatory proteins, etc. across the genome. An assay for transposase-accessible chromatin sequencing (ATAC-seq) maps nucleosomes in a genome-wide manner to identify regions that are accessible for transcription. Bisulfite sequencing provides a genome-wide measure of DNA methylation. Finally, Hi-C is a sequencing approach that provides a genome-wide map of the 3D structure of chromatin, which places widely separated genomic regions into close proximity. Most of these approaches are now being applied to the level of single cells (Luo et al., 2018). These and related methods are transforming the field’s ability to define epigenetic regulation in the brain.
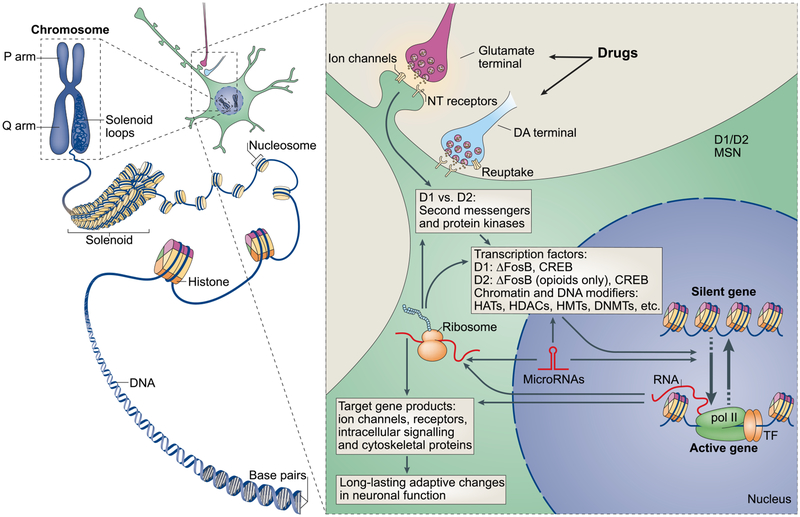
Figure 2. Integrated Scheme of Epigenetic and Synaptic Mechanisms of Addiction.
DNA is organized by wrapping around histone octomers to form nucleosomes, which are then further organized and condensed to form chromosomes (left). Only by temporarily unraveling compacted chromatin can the DNA of a specific gene be made accessible to the transcriptional machinery. Addictive drugs act through synaptic targets such as reuptake mechanisms, ion channels, and neurotransmitter (NT) receptors to alter intracellular signaling cascades (right). This can occur either directly or indirectly via increased dopaminergic transmission which, through its respective G-protein coupled receptors (GPCRs), affects downstream signaling cascades. This leads to the activation or inhibition of transcription factors (TFs) and of many other nuclear targets. These processes ultimately result in the induction or repression of particular genes, including those for non-coding RNAs such as microRNAs; altered expression of some of these genes can in turn further regulate gene transcription. It is proposed that some of these drug-induced changes at the chromatin level are extremely stable and thereby underlie the long-lasting behaviors that define addiction.
CREB, cyclic AMP-responsive element binding protein; DNMTs, DNA methyltransferases; HATs, histone acetyltransferases; HDACs, histone deacetylases; HDMs, histone demethylases; HMTs, histone methyltransferases; MEF2, myocyte-specific enhancer factor 2; NF-κB, nuclear factor-κB; pol II, RNA polymerase II. Modified from Robison and Nestler (2011).
Recent years have brought numerous methodological advances (see Box 1), which have made the study of these multiple epigenetic endpoints feasible in both animal and human brain tissue examined postmortem. These approaches have successively enabled studies of gene expression and epigenetic regulation in discrete rodent and human brain regions, in populations of specific neural and non-neural cell types within those targeted brain regions, and most recently in individual cells in those regions. Such detailed analyses are essential given that each cell type is characterized by its unique epigenome consistent with cell-type-specific patterns of gene expression and regulation, which are only beginning to be elaborated in brain (Akbarian et al., 2015).
Synaptic Plasticity Underlying Drug-Induced Addiction-like Behavior
Consistent with shared increases in dopamine signaling across classes of drugs of abuse, animals share behavioral responses to acute and repeated drug exposure. Psychostimulants, opiates, and other abused substances increase locomotor activity in rodents upon initial exposure and cause a progressive increase in such locomotor activation with repeated drug doses, a phenomenon termed locomotor sensitization, which can last for weeks or months (Robinson and Berridge, 2001). Animals also learn to prefer an environment paired with drug exposure, referred to as conditioned place preference. Rodents self-administer drugs of abuse volitionally and over time will work hard (e.g., avidly press a lever) to obtain additional doses and are not deterred by a progressive ratio schedule (requirement to press the lever at accelerating rates) (Everitt and Robbins, 2000) or by receiving an aversive stimulus along with the drug (Vanderschuren and Everitt, 2005). The drive to self-administer a drug increases during periods of abstinence within the first month, called incubation of drug craving (Grimm et al., 2001), and relapse to self-administration, even after prolonged abstinence (weeks, months), is stimulated by exposure to the drug itself, to cues associated with the drug, or by stress (Bobadilla et al., 2017; Venniro et al., 2016).
A large literature has implicated specific, drug-evoked forms of synaptic plasticity in maladaptive behaviors. A comprehensive review can be found elsewhere (Lüscher, 2016); only a few examples are provided here. An initial dose of an addictive drug causes a potentiation of excitatory afferents onto VTA dopamine neurons (Ungless et al., 2001; Saal et al., 2003). Potentiation of excitatory glutamatergic afferents from medial prefrontal cortex (mPFC) and ventral hippocampus onto D1 receptor-expressing medium spiny neurons (MSNs) of the NAc has been causally linked to cue-associated drug-seeking behavior (Pascoli et al., 2014). While the induction of such plasticity typically requires dopamine, expression mechanisms vary and metabotropic glutamate receptors may limit the potentiation (McCutcheon et al., 2011). For excitatory transmission, insertion of AMPA glutamate receptors—and in some cases insertion of GluA2-lacking, calcium-permeable AMPA receptors—into the postsynaptic plasma membrane is a common feature (Bellone and Lüscher, 2006). Conversely, drug-evoked plasticity of GABA transmission is expressed predominantly by a presynaptic mechanism, typically involving a change in the release probability of GABA (Bocklisch et al., 2013). NAc neurons also express calcium-permeable AMPA receptors following drug exposure, particularly when cocaine is self-administered (Conrad et al., 2008; Wolf and Tseng, 2012). Cocaine and opiate exposure likewise regulates the total number of functional glutamatergic synapses on NAc MSNs, as silent synapses—those displaying NMDA receptor, but not AMPA receptor, responses—appear and recede over the course of drug self-administration, withdrawal, and relapse (Dong, 2016). Many forms of drug-evoked plasticity or the mechanisms by which the brain eventually restores baseline transmission depend on protein synthesis. For example, the GluA2-lacking α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) inserted after a first exposure to an addictive drugs are exchanged for with GluA2-containing receptors, which are synthesized de novo. In the NAc, the concomitant activation of D1R and N-methyl-D-aspartate receptors (NMDARs) triggers the MAP-kinase-ERK pathway (Bertran-Gonzalez et al., 2008) with an impact on transcription.
Circuit Remodeling Underlying Habits and Compulsion
The NAc and several upstream regions that innervate the NAc via glutamatergic neurons—prefrontal cortex (PFC), ventral hippocampus, basolateral amygdala, and thalamus, each of which receives dopamine projections from the VTA—emerge as the main loci of dopamine-mediated circuit remodeling. The region that has received the most attention to date is the medial PFC (mPFC), with the top-down glutamatergic projections from the mPFC to the NAc and several other subcortical regions having been linked to maladaptive behavior and individual vulnerability in addiction models (Figure 3). Distinct subregions of mPFC modulate cocaine taking with opposing effects. Stimulation of the more dorsal prelimbic (PL) subregion promotes drug consumption, while stimulation of the more ventral infralimbic (IL) subregion restrains relapse after extinction (Peters et al., 2008). This straightforward go and/or no-go model is rooted in the idea of segregated projections to NAc core versus shell, respectively. Recent observations, however, paint a more complex picture: both regions can drive and inhibit drug seeking as a function of the context and drug history (Moorman et al., 2015). A more refined model thus takes into account mPFC→NAc projections of individual neurons, which intermingle in PL and IL to reach NAc core and shell. Given the myriad of functions attributed to the mPFC, further refinement of the model seems warranted. In this context, the neural correlates of habitual actions are relevant. With repetition, IL activity exceeds PL activity, and IL inactivation restores goal-directed behavior. This model posits that habitual performance may be driven with a switch from PL to IL (Chen et al., 2013; Mihindou et al., 2013). While appealing, this hypothesis is also likely oversimplified and its relationship to compulsive action remains elusive. Other regions of the PFC have been implicated as well, in particular, the dysfunction of the orbitofrontal cortex (OFC), which may contribute to compulsive drug intake (Lucantonio et al., 2014; Pascoli et al., 2015).
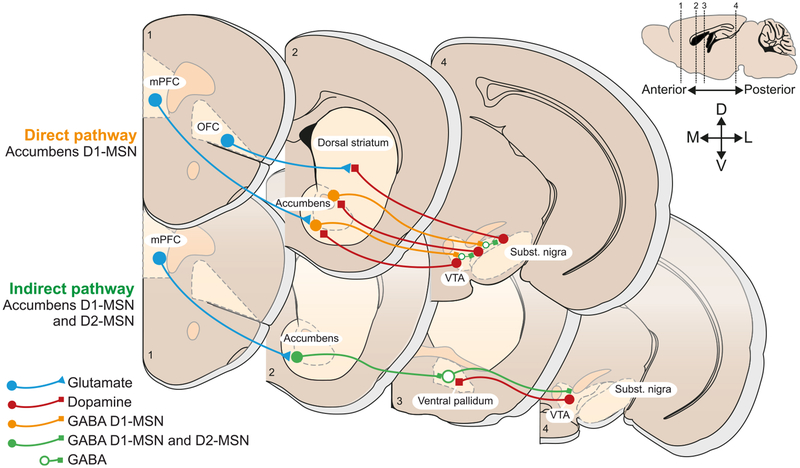
Figure 3. Brain Circuits Implicated in Maladaptive Behavior that May Undergo Epigenetic Remodeling.
The core projections targeted by addictive drugs include the glutamatergic cortical top down control of the midbrain to striatal loops. Two main streams can be distinguished: the direct pathway exclusively made of D1R-expressing medium spiny neurons and the indirect pathway that contains both D1 and D2R-MSNs and reaches the midbrain via a relay in the pallidum. With increasing duration of drug exposure and transition toward compulsive abuse, more and more dorsal loops may be recruited.
If mPFC and OFC play a role in appropriately updating the affective value of stimuli and action outcome during goal-directed behavior, they may dysfunction in pathological states where compulsion is a key symptom. Analogies between obsessive-compulsive disorder (OCD) and addiction come to mind. OCD is characterized by upsetting, persistent thoughts, and repetitive behavioral responses to these thoughts (Alonso et al., 2015), while addiction is defined by compulsive drug taking despite negative consequences (Wise and Koob, 2014). A leading theory posits that compulsivity emerges from abnormal transition between habitual and goal-directed behaviors (Gillan et al., 2016; Voon et al., 2015), which is why the OFC has been implicated in both OCD and addiction in pre-clinical and human studies (Beucke et al., 2013; Fettes et al., 2017; Lucantonio et al., 2014). Lack of restraint for punished reward administration may acutely be coded by mPFC neurons projecting to the NAc (Kim et al., 2017) but is likely to extend to larger cortical areas in a chronic situation. Similarly, hypoactivity of the mPFC contributes to compulsive cocaine self-administration (Chen et al., 2013). Given the role of mPFC in pain perception (Ong et al., 2018), the decreased ability of electric shock to dampen drug consumption may also reflect decreased sensitivity to the shock (Pascoli et al., 2015). Moreover, the cellular and molecular mechanisms underlying mPFC hypoactivity remain poorly understood. The “progression to addiction” begins with the first exposure, and gradually consolidates during repeated, yet still controlled drug use. As use escalates, drug administration becomes compulsive in vulnerable individuals, resulting in loss of control over drug use (Belin-Rauscent et al., 2016; Everitt and Robbins, 2016). This progression may depend on habit formation discussed above, which slowly becomes more and more pronounced, eventually qualifying as compulsion. Alternatively, compulsion can be understood as the development of extreme goal-directed actions with a narrow focus (Vandaele and Janak, 2017). In an addiction model based on optogenetic self-stimulation of VTA dopamine neurons, compulsion was associated with a selective strengthening of the mPFC→dorsal striatum projection (Pascoli et al., 2018). The synaptic potentiation was observed in afferents of both D1 and D2 MSN projection neurons and mediated by a postsynaptic expression mechanism. Bidirectional in vivo manipulations of this projection exert direct effects on the compulsion: a long term depression (LTD) protocol renders compulsive mice more sensitive to the punishment, while artificial potentiation favored self-stimulation despite punishment. The induction mechanisms of these synaptic changes remain largely unknown, calling for molecular studies of the mPFC and OFC, as well as of the other major glutamatergic afferent regions to the NAc including ventral hippocampus, amygdala, and thalamus, among others, in the context of addiction and other psychiatric disorders. An appealing hypothesis is that with chronic drug exposure more and more dorsal loops may be recruited. In fact, anatomical constraints (Haber et al., 2000), lesion experiments (Belin and Everitt, 2008), and fast cycle voltammetry measurements of dopamine (Willuhn et al., 2012) at different stages of the disease progression support such a model. Taken together, emerging circuit models will guide the search for the underlying molecular mechanisms involved.
Genetics of Individual Vulnerability
Clinical studies have estimated that only 10%–20% of people who recreationally use psychostimulants or opiates will eventually become addicts, with other drugs showing lower transition rates (Egervari et al., 2017; Vsevolozhskaya and Anthony, 2016, 2017). These numbers are generally reproduced in rats (Deroche-Gamonet et al., 2004) and mice (Pascoli et al., 2015). Both genetic and environmental factors contribute to making an individual vulnerable to this progression to addiction while others remain unscathed.
Evidence for heritability of addiction is based on observations in twins (Bevilacqua and Goldman, 2009). The monozygotic to dizygotic twin concordance ratios for drug addictions are approximately 2:1 (Goldman et al., 2005). Moreover, when quantified using h2, heritabilities average ~50% for all classes of abused substances studied to date, which is surprising for a disease where choice and psychosocial factors are so strongly involved. Since differences in h2 between drugs may reflect the pharmacological efficacy of activating the mesolimbic dopamine system, it has been argued that this variation resides in the intrinsic addictiveness of a drug, which may be reflected in the strength of common drug-induced circuit adaptations (Goldman et al., 2005).
The underlying genetic mechanisms of addiction are highly complex. It is likely that sequence variations at many hundreds of genetic loci comprise the 50% heritability of addictions, with each individual locus contributing a minute fraction to individual differences in addiction vulnerability. Only a small subset of contributing genetic variations has been established to date. Several environmental factors have been associated with addiction vulnerability in humans, including early life trauma, disrupted family structure, and peer pressure, among many others, with certain forms of stress likewise increasing addiction vulnerability in laboratory rodents. For example, prepubertal deprivation of interactions with conspecifics may increase the risk for the transition to addiction (Baarendse et al., 2014). The rats were socially isolated between postnatal days 21 and 42, a time period roughly comparable to childhood and early adolescence in humans (McCutcheon et al., 2009; Wong et al., 2013), and their motivation for cocaine under a progressive ratio schedule was enhanced.
However, strong individual differences in vulnerability to addictive drugs are still observed in genetically homogeneous populations of animals (inbred rat and mouse lines) while maintaining constant environment to the extent possible (Pascoli et al., 2015). The biological basis of this non-genetic, non-environmental cause of individual variability is unknown but could involve stochastic events during development.
Individuality in Genetically Identical Conspecifics
“Stochastic individuality” (Honegger and de Bivort, 2018) is defined by behavioral variance despite identical genotypes exposed to identical environments. Examples range from startle response in clonal daughters from pea aphids (clover lice) to variance in identical twins, who grow up in the same household. Given that a multitude of factors shape behavior through complex interactions, even small environmental perturbations may promote random variation. In the case of addiction, positive feedback mechanisms may amplify the stochastic individuality such that a behavioral “bistability” would emerge (i.e., addicted versus recreational use). To parse the contribution of the different components, it may thus help to control for one and test the others. In this context, congenic mice on a C57BL/6 background have often been used in experimental models, thereby minimizing the contribution of genetic factors for behavioral traits. These mice are quasi clonal, confirmed by sequencing of the C57BL/6J strain at 6.5× coverage (Keane et al., 2011; Waterston et al., 2002), which revealed little (< 1%) intra-strain genetic variation. By contrast, significant inter-strain variability between widely used strains of laboratory mice is well known, for example, reflecting contributions of the two subspecies Mus musculus domesticus and Mus musculus musculus (Wade and Daly, 2005). Not surprisingly, several studies report behavioral differences between C57BL/6 sub-strains, including addiction-relevant traits: e.g., C57BL/6J display a stronger preference for alcohol than C57BL/6N (Blum et al., 1982; Hwa et al., 2011). However, there is no evidence for polymorphic variance within the C57BL/6J strain underlying drug-adaptive behavior. Regardless, substantial behavioral individuality emerges even among identical C57BL/6N or C57BL/6J mice (Freund et al., 2013), which could be explained by one of the following mechanisms: (1) remaining minimal residual segregation, as perfect inbreeding is impossible, (2) transcriptional and epigenetic drift caused by small differences in the environment, and (3) stochastic changes in gene expression and circuit function over a lifetime. The last two mechanisms may be linked, as epigenetic remodeling is posited to play an important role in stochastic developmental events. While this has yet to be demonstrated empirically, epigenetic regulation represents an appealing mechanism for controlling individual vulnerability to addictive drugs.
Epigenetic Regulation in the Addiction Process
An increasing number of studies have investigated epigenetic alterations elicited by cocaine or other addictive drugs (Nestler, 2014). They report that drug-induced changes in gene expression are associated with many types of epigenetic events, including several forms of histone modifications, DNA methylation, and miRNAs. Most of this work has focused on NAc and has examined such epigenetic changes at individual candidate genes of interest and increasingly genome-wide by use of chromatin immunoprecipitation followed by deep sequencing (chromatin immunoprecipitation sequencing [ChIP-seq]) and related methods (see Box 1). For example, cocaine or morphine regulation of histone acetylation and methylation, and of DNA methylation, have been mapped genome-wide in NAc and correlated with changes in gene expression in addiction models (e.g., Damez-Werno et al., 2016; Feng et al., 2014; Massart et al., 2015; Maze et al., 2010; Renthal et al., 2009; Sadakierska-Chudy et al., 2017; Sun et al., 2017). Moreover, manipulation of the writers and erasers of these individual histone and DNA marks specifically in NAc of adult animals has been shown to control drug-elicited behaviors, including self-administration. For example, large numbers of gene promoters are hyper-methylated in NAc after extended withdrawal from cocaine but become hypo-methylated during cue-induced reinstatement of self-administration (Massart et al., 2015). Injections of a DNA methyltransferase inhibitor into the NAc abolished reinstatement. Further molecular and pharmacological studies in which writers and erasers of DNA methylation are suppressed or activated in NAc specifically, or systemically, support a complex role of DNA methylation in cocaine-related behaviors (LaPlant et al., 2010; Sadri-Vakili, 2015; Vaillancourt et al., 2017). Manipulations of histone deacetylases (HDACs) and histone methyltransferases likewise change behavioral responses to cocaine, but the effects appear highly dependent on the timing of manipulations (e.g., Kennedy et al., 2013; Sadri-Vakili, 2015). Similarly, several miRNAs have been shown to be upregulated after cocaine or other drug exposure, while others are downregulated (Doura and Unterwald, 2016). Modulation of expression of these miRNAs in the striatum has been shown to alter a variety of drug-related behaviors (Chandrasekar and Dreyer, 2011; Hollander et al., 2010; Quinn et al., 2015). Regulation of nucleosome spacing and 3D chromatin structure in NAc has also been implicated in the actions of drugs of abuse (Engmann et al., 2017; Sun et al., 2017). A major weakness of the extant literature is that most studies to date have examined whole extracts of NAc; it is essential moving forward to capture drug-induced changes in this range of epigenetic endpoints at the level of individual neurons and other cell types that comprise this brain region. Preliminary reports of this cell-type-specific approach are beginning to appear (Mews et al., 2018; Zhang et al., 2018). Likewise, parallel studies are needed of individual cell types within other critical brain reward regions.
A criticism of the epigenetics field in general is that chromatin modifications simply reflect changes in gene expression but do not in and of themselves represent the key drivers of such changes, which according to this traditional viewpoint rely mostly on transcription factors. Drug regulation of transcription factors is no doubt crucial for transcriptional regulation, but recent evidence directly establishes the causal involvement of epigenetic mechanisms as well. Using new tools that allow the direct targeting of a histone-modifying enzyme to a single gene within NAc neurons, even within a single type of NAc neuron, has been shown to bidirectionally control expression levels of the targeted gene, with increased histone acetylation promoting that gene’s expression and increased repressive histone methylation exerting the opposite effect (Hamilton et al., 2018; Heller et al., 2014; 2016). These studies are important milestones in the neuroepigenomics field because they demonstrate that drug-induced epigenetic modifications are not simply passive bystanders of changes in gene expression but contribute causally to such transcriptional regulation. More recently, CRISPR tools have been used to execute several types of such locus-specific epigenome editing within the brain (Gallegos et al., 2018; Hamilton et al., 2018; Liu et al., 2018; Lorsch et al., 2018). This new approach of “causal epigenomics” facilitates the ability to establish the role of epigenetic modes of regulation in neural phenomena and even raises the advent of eventually utilizing such approaches as novel therapeutics for addiction and other CNS diseases.
Epigenetic Basis of Individual Variability
Only a few studies to date have reported epigenetic screening of vulnerability endophenotypes. One measured responding to a natural reward, progressive ratio breakpoint, and cue-induced reinstatement of drug seeking in rats to determine their vulnerability to compulsive drug taking. In the “vulnerable” rats, several miRNAs were found at elevated levels in the striatum (Quinn et al., 2015). Other studies have used the link between stress and propensity to addiction to hypothesize about epigenetic markers that contribute to addiction vulnerability (Cadet, 2016). RNA-seq studies have begun to map genome-wide cocaine-induced changes in gene expression in NAc and several other reward-related regions as a consequence of exposure to stress earlier in life (Walker and Nestler, 2018); however, further work is needed to investigate the epigenetic mechanisms that underlie this priming effect of stress. Because stress increases the likelihood of developing compulsive drug taking, and because stress and addictive drugs drive some similar epigenetic changes (Covington et al., 2011), it has been proposed that epigenetic modifications induced by stress might underlie lifelong vulnerability to addiction. However, this hypothesis has yet to be tested directly.
Another line of research examines the heritability of epigenetic changes that occur in sperm or egg cells (Morrow and Flagel, 2016; Szutorisz and Hurd, 2016; Vassoler et al., 2013). Animals that are exposed to any of several drugs of abuse have offspring with different rates of drug vulnerability compared with control animals. There is correlative evidence that part of this trans-generational transmission of changes in drug responses might be mediated by drug-induced epigenetic modifications in sperm cells; however, these reports lack causal evidence. Moreover the mechanism of induction remains elusive. Are epigenetic changes in sperm or egg cells a direct effect of the drug in the gonads or the results of altered brain function? And how does an epigenetic modification in sperm or egg cells program highly specific changes in a given cell type and circuit in the brain? Until it is feasible to selectively replicate and block a drug-induced epigenetic modification (e.g., DNA methylation event, miRNA) in sperm or egg, and demonstrate consequent effects and understand underlying mechanisms on brain and behavior through multiple generations, the question of trans-generational epigenetic inheritance of addiction vulnerability must be viewed with caution.
Taken together, there are heuristic reasons to expect that epigenetic mechanisms mediate the ability of behavioral experience during a lifetime to program an individual for greater or lesser vulnerability to compulsive drug use later in life, but limited experimental evidence. Such studies are now required and should be integrated with the substantial and still growing literature of the epigenetic remodeling that underlies drug-induced maladaptive behavior in adults. This combined effort will define the ways in which stress and other forms of life experience leave epigenetic marks—or “chromatin scars”—at specific genomic loci, which may, when paired with pharmacologically evoked, strong dopamine transients in adolescence or adulthood favor the transition to addiction.
Bridging Epigenetic Regulation with Synaptic and Circuit Plasticity
The well-described changes in synaptic plasticity that are induced in mesocorticolimbic circuits by repeated exposure to addictive drugs may mediate a state of addiction rooted in cell-autonomous changes in gene expression and epigenetic modifications in constituent cell types of these circuits. However, while diverse types of epigenetic changes and associated altered expression of specific target genes have been strongly implicated in behavioral aspects of addiction as discussed earlier, there remains a paucity of studies that have sought to directly link epigenetic and transcriptional regulation in a given cell type to addiction-associated synaptic and circuit plasticity. We propose that bridging these levels of analysis is a fundamental need in addiction research.
Studies of two transcription factors implicated in addiction through epigenetic mechanism illustrate this research direction. Several drugs of abuse have been shown to activate CREB (cAMP response element binding protein) and ΔFosB (a truncated product of the FosB gene) in NAc (Robison and Nestler, 2011). CREB activation occurs in both the D1 and D2 subtypes of NAc MSNs defined by the dopamine receptor they predominantly express, whereas ΔFosB activation occurs selectively within D1-type MSNs in response to all drugs of abuse except for opiates, which interestingly induce the protein in both cell types (Lobo et al., 2013). CREB activation in NAc MSNs serves a negative feedback, homeostatic role, as it opposes the behavioral effects of cocaine and opiates (Robison and Nestler, 2011). This action increases the intrinsic excitability of these neurons (Dong et al., 2006). CREB activation also contributes to drug-induced synaptic plasticity in NAc by mediating cocaine induction of the GluN2B subunit of NMDA glutamate receptors and associated changes in dendritic morphology (Bellone and Lüscher, 2012; Dong and Nestler, 2014). These actions appear to be the same in D1- and D2-type NAc MSNs. ΔFosB, in contrast, exerts opposing effects on these two MSN subtypes in NAc, with increased AMPA receptor function induced in D1-type MSNs and decreased AMPA receptor function induced in D2-type MSNs (Grueter et al., 2013). Likewise, ΔFosB increases the formation of silent synapses on D1 MSNs, consistent with a ΔFosB-induced increase in thin dendritic spines on this cell type but reduces silent synapses on D2 MSNs. Genome-wide studies have examined the range of target genes controlled by CREB and ΔFosB in NAc (Renthal et al., 2009) and, not surprisingly, genes involved in cell excitability and synaptic function are prominent among their targets. However, these analyses have not yet been performed on a cell-type-specific basis, which is a high priority particularly for ΔFosB given different actions in the two NAc cell types. CREB and ΔFosB action have also been related to several modes of epigenetic modifications, including histone acetylation and methylation on a genome-wide and candidate gene basis (Renthal et al., 2009), but this too must now be repeated in a cell-type-specific manner. Ultimately, it may also prove useful to carry out transcriptomic and epigenomic mapping at the level of single cells, given the considerable heterogeneity that has been observed to date even among the same cell type (Gokce et al., 2016). However, technical advances in these methods will be needed to dramatically increase the coverage of the genome that they provide.
Of course, CREB and ΔFosB are just two of numerous transcription factors implicated in drug action. Recent transcriptome-wide mapping of gene expression changes induced in NAc and several other mesocorticolimbic brain regions by cocaine self-administration has revealed prominent lasting effects of drug exposure—genes whose expression is primed or desensitized in concert with incubation of drug craving and relapse behavior, with several transcription factors in addition to CREB and ΔFosB deduced to play an important role (Walker et al., 2018). Future work is needed to causally implicate these other factors in drug action as well as define the cell specificity of their actions and the accompanying epigenetic modifications that occur to drive long-lasting changes in gene expression.
This literature provides an ideal foundation upon which to now delineate the precise steps through which individual or combinations of transcription factors in a given cell type—and the associated epigenetic modifications at specific genomic loci—mediate cell and circuit plasticity associated with addiction. For example, tools exist to control levels of endogenous CREB or ΔFosB in specific cell types, even within specific subsets of those cells that form microcircuits with other brain regions, as noted above. Tools also exist to target CREB or ΔFosB to a single gene of interest in a neuronal cell type in vivo (Lorsch et al., 2018). These various tools can now be used to construct a stepwise understanding of how a drug of abuse, like cocaine or heroin, modifies a single transcription factor and associated epigenetic parameters to alter expression of selected genes, and how that relates to altered excitability of the cell, the strength of its synaptic inputs, and together the strength and pattern of its outputs.
As this type of synthetic analysis moves forward, it will be possible to then take a developmental perspective and analyze how events early in life—as well as stochastic changes during development—prime changes in gene expression via epigenetic mechanisms for a lifetime and how that renders cells and circuits more vulnerable to addiction. Recent work for example has shown that early life stress causes lifelong changes in steady-state expression levels of numerous genes in mesocorticolimbic brain regions as well as primes or desensitizes many other genes for altered expression in response to subsequent stress in adulthood (Peña et al., 2017). These types of analyses are now underway in addiction models and will provide novel insight into how life experience controls addiction vulnerability.
Outlook for Therapeutic Advances
Virtually all available treatments for drug addiction act on the initial protein targets for drugs of abuse, such as opioid receptors (methadone, buprenorphine) and nicotinic cholinergic receptors (varenicline). It has been much harder to target core addiction mechanisms in the brain. One limitation of drug discovery efforts to date is that they have concentrated on candidate protein targets of interest. Our expectation is that the unbiased, genome-wide efforts described above, made possible by advanced genomic and epigenetic approaches, will better focus therapeutic discovery on the genes and biochemical pathways, and their synaptic and circuit consequences, that are most prominently involved in drug craving and relapse. A related possibility is whether targeting the lasting epigenetic mechanisms that drive lifelong changes in addiction vulnerability represent an additional path for medication development. As noted earlier, there are encouraging findings based on the use of inhibitors of HDACs, histone or DNA methyltransferases, or chromatin remodeling proteins in animal models (e.g., Egervari et al., 2017; LaPlant et al., 2010; Massart et al., 2015; Walker and Nestler, 2018). However, a major challenge of this approach is that such molecules, now in development for cancer therapy (e.g., HDAC inhibitors, bromodomain inhibitors), target ubiquitously expressed proteins and likely have too many off-target effects for treatment of drug addiction. Whether it will be feasible to generate small molecules that exert more selective effects on the brain is an empirical question that will be answered with future investigations. The advent of locus-specific epigenome editing broadens still further the range of approaches that could potentially target such mechanisms more selectively, but such studies are in very early stages of investigation.
ACKNOWLEDGMENTS
We thank Michaël Loureiro for help with the graphics. E.J.N. is supported by grants from the National Institute on Drug Abuse. C.L. is a European Research Council (ERC) advanced grant awardee and also receives funds from the Swiss National Science Foundation. We thank Shannon Wolfman for the help on an earlier version of the manuscript.
Footnotes
DECLARATION OF INTERESTS
C.L. is a member of the following scientific advisory boards: Stalicla SA, Geneva, Phénix, and IRP Foundations.
REFERENCES
- Akbarian S, Liu C, Knowles JA, Vaccarino FM, Farnham PJ, Crawford GE, Jaffe AE, Pinto D, Dracheva S, Geschwind DH, et al. ; PsychENCODE Consortium (2015). The PsychENCODE project. Nat. Neurosci 18, 1707–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso P, López-Solà C, Real E, Segalàs C, and Menchón JM (2015). Animal models of obsessive-compulsive disorder: Utility and limitations. Neuropsychiatr. Dis. Treat 11, 1939–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, and Wightman RM (2008). Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J. Neurosci 28, 8821–8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJJ, Limpens JHW, and Vanderschuren LJMJ (2014). Disrupted social development enhances the motivation for cocaine in rats. Psychopharmacology (Berl.) 231, 1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, and Everitt BJ (2008). Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57, 432–441. [DOI] [PubMed] [Google Scholar]
- Belin-Rauscent A, Daniel M-L, Puaud M, Jupp B, Sawiak S, Howett D, McKenzie C, Caprioli D, Besson M, Robbins TW, et al. (2016). From impulses to maladaptive actions: The insula is a neurobiological gate for the development of compulsive behavior. Mol. Psychiatry 21, 491–499. [DOI] [PubMed] [Google Scholar]
- Bellone C, and Lüscher C (2006). Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat. Neurosci 9, 636–641. [DOI] [PubMed] [Google Scholar]
- Bellone C, and Lüscher C (2012). Drug-evoked plasticity: Do addictive drugs reopen a critical period of postnatal synaptic development? Front. Mol. Neurosci 5, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SL (2007). The complex language of chromatin regulation during transcription. Nature 447, 407–412. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, and Spealman RD (1989). Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J. Pharmacol. Exp. Ther 251, 150–155. [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, and Girault J-A (2008). Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci 28, 5671–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, Kaufmann C, and Kathmann N (2013). Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry 70, 619–629. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, and Goldman D (2009). Genes and addictions. Clin. Pharmacol. Ther 85, 359–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Briggs AH, DeLallo L, Elston SF, and Ochoa R (1982). Whole brain methionine-enkephalin of ethanol-avoiding and ethanol-preferring c57BL mice. Experientia 38, 1469–1470. [DOI] [PubMed] [Google Scholar]
- Bobadilla A-C, Heinsbroek JA, Gipson CD, Griffin WC, Fowler CD, Kenny PJ, and Kalivas PW (2017). Corticostriatal plasticity, neuronal ensembles, and regulation of drug-seeking behavior. Prog. Brain Res 235, 93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocklisch C, Pascoli V, Wong JCY, House DRC, Yvon C, de Roo M, Tan KR, and Lüscher C (2013). Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science 341, 1521–1525. [DOI] [PubMed] [Google Scholar]
- Cadet JL (2016). Epigenetics of stress, addiction, and resilience: Therapeutic implications. Mol. Neurobiol 53, 545–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (2017). https://wonder.cdc.gov/.
- Chandrasekar V, and Dreyer J-L (2011). Regulation of MiR-124, Let-7d, and MiR-181a in the accumbens affects the expression, extinction, and reinstatement of cocaine-induced conditioned place preference. Neuropsychopharmacology 36, 1149–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau H-J, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, and Bonci A (2013). Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496, 359–362. [DOI] [PubMed] [Google Scholar]
- Cholewa-Waclaw J, Bird A, von Schimmelmann M, Schaefer A, Yu H, Song H, Madabhushi R, and Tsai LH (2016). The role of epigenetic mechanisms in the regulation of gene expression in the nervous system. J. Neurosci 36, 11427–11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L-J, Shaham Y, Marinelli M, and Wolf ME (2008). Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454, 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre J, van Zessen R, Loureiro M, Patriarchi T, Tian L, Pascoli V, and Lüscher C (2018). Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement. eLife 7, 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE 3rd, Maze I, Sun H, Bomze HM, DeMaio KD, Wu EY, Dietz DM, Lobo MK, Ghose S, Mouzon E, et al. (2011). A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron 71, 656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damez-Werno DM, Sun H, Scobie KN, Shao N, Rabkin J, Dias C, Calipari ES, Maze I, Peña CJ, Walker DM, et al. (2016). Histone arginine methylation in cocaine action in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 113, 9623–9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, and Mirny L (2016). The 3D genome as moderator of chromosomal communication. Cell 164, 1110–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, and Piazza PV (2004). Evidence for addiction-like behavior in the rat. Science 305, 1014–1017. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, and Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 85, 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y (2016). Silent synapse-based circuitry remodeling in drug addiction. Int. J. Neuropsychopharmacol 19, pyv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, and Nestler EJ (2014). The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol. Sci 35, 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, and Malenka RC (2006). CREB modulates excitability of nucleus accumbens neurons. Nat. Neurosci 9, 475–477. [DOI] [PubMed] [Google Scholar]
- Doura MB, and Unterwald EM (2016). MicroRNAs modulate interactions between stress and risk for cocaine addiction. Front. Cell. Neurosci 10, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egervari G, Ciccocioppo R, Jentsch JD, and Hurd YL (2017). Shaping vulnerability to addiction - the contribution of behavior, neural circuits and molecular mechanisms. Neurosci. Biobehav. Rev 85, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engmann O, Labonté B, Mitchell A, Bashtrykov P, Calipari ES, Rosenbluh C, Loh YE, Walker DM, Burek D, Hamilton PJ, et al. (2017). Cocaine-induced chromatin modifications associate with increased expression and three-dimensional looping of Auts2. Biol. Psychiatry 82, 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, and Robbins TW (2000). Second-order schedules of drug reinforcement in rats and monkeys: Measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl.) 153, 17–30. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, and Robbins TW (2016). Drug addiction: Updating actions to habits to compulsions ten years on. Annu. Rev. Psychol 67, 23–50. [DOI] [PubMed] [Google Scholar]
- Feng J, Wilkinson M, Liu X, Purushothaman I, Ferguson D, Vialou V, Maze I, Shao N, Kennedy P, Koo J, et al. (2014). Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens. Genome Biol 15, R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettes P, Schulze L, and Downar J (2017). Cortico-striatal-thalamic loop circuits of the orbitofrontal cortex: Promising therapeutic targets in psychiatric illness. Front. Syst. Neurosci 11, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzler M, Krüger A, Sachser N, Lindenberger U, and Kempermann G (2013). Emergence of individuality in genetically identical mice. Science 340, 756–759. [DOI] [PubMed] [Google Scholar]
- Gallegos DA, Chan U, Chen L-F, and West AE (2018). Chromatin regulation of neuronal maturation and plasticity. Trends Neurosci 41, 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Robbins TW, Sahakian BJ, van den Heuvel OA, and van Wingen G (2016). The role of habit in compulsivity. Eur. Neuropsychopharmacol 26, 828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokce O, Stanley GM, Treutlein B, Neff NF, Camp JG, Malenka RC, Rothwell PE, Fuccillo MV, Südhof TC, and Quake SR (2016). Cellular taxonomy of the mouse striatum as revealed by single-cell RNA-seq. Cell Rep 16, 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, and Ducci F (2005). The genetics of addictions: Uncovering the genes. Nat. Rev. Genet 6, 521–532. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, and Shaham Y (2001). Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412, 141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, and Malenka RC (2013). ΔFosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc. Natl. Acad. Sci. USA 110, 1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, and McFarland NR (2000). Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci 20, 2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PJ, Burek DJ, Lombroso SI, Neve RL, Robison AJ, Nestler EJ, and Heller EA (2018). Cell-type-specific epigenetic editing at the Fosb gene controls susceptibility to social defeat stress. Neuropsychopharmacology 43, 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller EA, Cates HM, Peña CJ, Sun H, Shao N, Feng J, Golden SA, Herman JP, Walsh JJ, Mazei-Robison M, et al. (2014). Locus-specific epigenetic remodeling controls addiction- and depression-related behaviors. Nat. Neurosci 17, 1720–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller EA, Hamilton PJ, Burek DD, Lombroso SI, Peña CJ, Neve RL, and Nestler EJ (2016). Targeted epigenetic remodeling of the Cdk5 gene in nucleus accumbens regulates cocaine- and stress-evoked behavior.J. Neurosci 36, 4690–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Im H-I, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, and Kenny PJ (2010). Striatal microRNA controls cocaine intake through CREB signalling. Nature 466, 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger K, and de Bivort B (2018). Stochasticity, individuality and behavior. Curr. Biol 28, R8–R12. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, and Miczek KA (2011). Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol. Clin. Exp. Res 35, 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Broker CJ, Wang DV, and Ikemoto S (2014). Phasic excitation of ventral tegmental dopamine neurons potentiates the initiation of conditioned approach behavior: Parametric and reinforcement-schedule analyses. Front. Behav. Neurosci 8, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, and North RA (1992). Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci 12, 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, et al. (2011). Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiflin R, and Janak PH (2015). Dopamine prediction errors in reward learning and addiction: From theory to neural circuitry. Neuron 88, 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Feng J, Robison AJ, Maze I, Badimon A, Mouzon E, Chaudhury D, Damez-Werno DM, Haggarty SJ, Han MH, et al. (2013). Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat. Neurosci 16, 434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Ye L, Jennings JH, Pichamoorthy N, Tang DD, Yoo AW, Ramakrishnan C, and Deisseroth K (2017). Molecular and circuit-dynamical identification of top-down neural mechanisms for restraint of reward seeking. Cell 170, 1013–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinde B, Gabel HW, Gilbert CS, Griffith EC, and Greenberg ME (2015). Reading the unique DNA methylation landscape of the brain: Non-CpG methylation, hydroxymethylation, and MeCP2. Proc. Natl. Acad. Sci. USA 112, 6800–6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, and Malenka RC (2014). Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76 (Pt B), 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iñiguez SD, et al. (2010). Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat. Neurosci 13, 1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Wu H, Krzisch M, Wu X, Graef J, Muffat J, Hnisz D, Li CH, Yuan B, Xu C, et al. (2018). Rescue of fragile X syndrome neurons by DNA methylation editing of the FMR1 gene. Cell 172, 979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, et al. (2013). ΔFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J. Neurosci 33, 18381–18395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsch ZS, Hamilton PJ, Ramakrishnan A, Parise EM, Wright WJ, Salery M, Lepack A, Mews P, Issler O, McKenzie A, et al. (2018). Zfp189 mediates stress resilience through a CREB-regulated transcriptional network in prefrontal cortex. bioRxiv. 10.1101/403733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Takahashi YK, Hoffman AF, Chang CY, Bali-Chaudhary S, Shaham Y, Lupica CR, and Schoenbaum G (2014). Orbitofrontal activation restores insight lost after cocaine use. Nat. Neurosci 17, 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Rivkin A, Zhou J, Sandoval JP, Kurihara L, Lucero J, Castanon R, Nery JR, Pinto-Duarte A, Bui B, et al. (2018). Robust single-cell DNA methylome profiling with snmC-seq2. Nat. Commun 9, 3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C (2016). The emergence of a circuit model for addiction. Annu. Rev. Neurosci 39, 257–276. [DOI] [PubMed] [Google Scholar]
- Lüscher C, and Ungless MA (2006). The mechanistic classification of addictive drugs. PLoS Med. 3, e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Robledo P, Chover AJ, Caine SB, and Koob GF (1993). D1 dopamine receptors in the nucleus accumbens modulate cocaine self-administration in the rat. Pharmacol. Biochem. Behav 45, 239–242. [DOI] [PubMed] [Google Scholar]
- Massart R, Barnea R, Dikshtein Y, Suderman M, Meir O, Hallett M, Kennedy P, Nestler EJ, Szyf M, and Yadid G (2015). Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J. Neurosci 35, 8042–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Covington HE 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, et al. (2010). Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science 327, 213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Shen L, Zhang B, Garcia BA, Shao N, Mitchell A, Sun H, Akbarian S, Allis CD, and Nestler EJ (2014). Analytical tools and current challenges in the modern era of neuroepigenomics. Nat. Neurosci 17, 1476–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, White FJ, and Marinelli M (2009). Individual differences in dopamine cell neuroadaptations following cocaine self-administration. Biol. Psychiatry 66, 801–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, and Tseng KY (2011). Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J. Neurosci 31, 14536–14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, and Roberts DC (1993). Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res 624, 245–252. [DOI] [PubMed] [Google Scholar]
- Mews P, Kronman H, Ramakrishnan A, Sidoli S, Peck EG, Garcia B, and Nestler EJ (2018). Mechanisms of epigenetic priming in cocaine addiction. Program No. 161.04, Neuroscience Meeting Planner (San Diego, CA: Society for Neuroscience). [Google Scholar]
- Mihindou C, Guillem K, Navailles S, Vouillac C, and Ahmed SH (2013). Discriminative inhibitory control of cocaine seeking involves the prelimbic pre-frontal cortex. Biol. Psychiatry 73, 271–279. [DOI] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, and Aston-Jones G (2015). Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 1628 (Pt A), 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, and Flagel SB (2016). Neuroscience of resilience and vulnerability for addiction medicine: From genes to behavior. Prog. Brain Res. 223, 3–18. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. (2018). https://www.drugabuse.gov/related-topics/trends-statistics.
- Nestler EJ (2005). Is there a common molecular pathway for addiction? Nat. Neurosci 8, 1445–1449. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2014). Epigenetic mechanisms of drug addiction. Neuropharmacology 76 (Pt B), 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, and Milner P (1954). Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol 47, 419–427. [DOI] [PubMed] [Google Scholar]
- Olesen J, Gustavsson A, Svensson M, Wittchen HU, and Jönsson B; CDBE2010 study group; European Brain Council (2012). The economic cost of brain disorders in Europe. Eur. J. Neurol 19, 155–162. [DOI] [PubMed] [Google Scholar]
- Ong W-Y, Stohler CS, and Herr DR (2018). Role of the prefrontal cortex in pain processing. Mol. Neurobiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, and Lüscher C (2014). Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509, 459–464. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Hiver A, and Lüscher C (2015). Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron 88, 1054–1066. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Hiver A, Van Zessen R, Loureiro M, Achargui R, Harada M, Flakowski J, and Lüscher C (2018). Stochastic synaptic plasticity underlying compulsion in a model of addiction. Nature 564, 366–371. [DOI] [PubMed] [Google Scholar]
- Peña CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, Issler O, Loh YE, Leong T, Kiraly DD, et al. (2017). Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 356, 1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, and Kalivas PW (2008). Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats.J. Neurosci 28, 6046–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn RK, Brown AL, Goldie BJ, Levi EM, Dickson PW, Smith DW, Cairns MJ, and Dayas CV (2015). Distinct miRNA expression in dorsal striatal subregions is associated with risk for addiction in rats. Transl. Psychiatry 5, e503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE 3rd, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, et al. (2009). Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron 62, 335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, and Fibiger HC (1977). On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol. Biochem. Behav 6, 615–620. [DOI] [PubMed] [Google Scholar]
- Robinson TE, and Berridge KC (2001). Incentive-sensitization and addiction. Addiction 96, 103–114. [DOI] [PubMed] [Google Scholar]
- Robison AJ, and Nestler EJ (2011). Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci 12, 623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley MJ, and Corces VG (2016). The three-dimensional genome: Principles and roles of long-distance interactions. Curr. Opin. Cell Biol 40, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, and Malenka RC (2003). Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37, 577–582. [DOI] [PubMed] [Google Scholar]
- Sadakierska-Chudy A, Frankowska M, Jastrzębska J, Wydra K, Miszkiel J, Sanak M, and Filip M (2017). Cocaine administration and its withdrawal enhance the expression of genes encoding histone-modifying enzymes and histone acetylation in the rat prefrontal cortex. Neurotox. Res 32, 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri-Vakili G (2015). Cocaine triggers epigenetic alterations in the corticostriatal circuit. Brain Res 1628 (Pt A), 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Richard JM, Margolis EB, and Janak PH (2018). Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci 21, 1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, and Montague PR (1997). A neural substrate of prediction and reward. Science 275, 1593–1599. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PEM, Carelli RM, and Wightman RM (2005). Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology 30, 853–863. [DOI] [PubMed] [Google Scholar]
- Sun H, Damez-Werno DM, Scobie KN, Shao N-Y, Dias C, Rabkin J, Wright KN, Mouzon E, Kabbaj M, Neve R, et al. (2017). Regulation of BAZ1A and nucleosome positioning in the nucleus accumbens in response to cocaine. Neuroscience 353, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, and Hurd YL (2016). Epigenetic effects of cannabis exposure. Biol. Psychiatry 79, 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, and Bonci A (2001). Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411, 583–587. [DOI] [PubMed] [Google Scholar]
- Vaillancourt K, Ernst C, Mash D, and Turecki G (2017). DNA methylation dynamics and cocaine in the brain: Progress and prospects. Genes (Basel) 8, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandaele Y, and Janak PH (2017). Defining the place of habit in substance use disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 87, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, and Everitt BJ (2005). Behavioral and neural mechanisms of compulsive drug seeking. Eur. J. Pharmacol 526, 77–88. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, and Pierce RC (2013). Epigenetic inheritance of a cocaine-resistance phenotype. Nat. Neurosci 16, 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, and Shaham Y (2016). Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog. Brain Res 224, 25–52. [DOI] [PubMed] [Google Scholar]
- Voon V, Derbyshire K, Rück C, Irvine MA, Worbe Y, Enander J, Schreiber LRN, Gillan C, Fineberg NA, Sahakian BJ, et al. (2015). Disorders of compulsivity: A common bias towards learning habits. Mol. Psychiatry 20, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vsevolozhskaya OA, and Anthony JC (2016). Transitioning from first drug use to dependence onset: Illustration of a multiparametric approach for comparative epidemiology. Neuropsychopharmacology 41, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vsevolozhskaya OA, and Anthony JC (2017). Estimated probability of becoming a case of drug dependence in relation to duration of drug-taking experience: A functional analysis approach. Int. J. Methods Psychiatr. Res 26, e1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CM, and Daly MJ (2005). Genetic variation in laboratory mice. Nat. Genet 37, 1175–1180. [DOI] [PubMed] [Google Scholar]
- Walker DM, and Nestler EJ (2018). Neuroepigenetics and addiction. Handb. Clin. Neurol 148, 747–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Cates HM, Loh YE, Purushothaman I, Ramakrishnan A, Cahill KM, Lardner CK, Godino A, Kronman HG, Rabkin J, et al. (2018). Cocaine self-administration alters transcriptome-wide responses in the brain’s reward circuitry. Biol. Psychiatry 84, 867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, et al. ; Mouse Genome Sequencing Consortium (2002). Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562. [DOI] [PubMed] [Google Scholar]
- Wei C, Han X, Weng D, Feng Q, Qi X, Li J, and Luo M (2018). Response dynamics of midbrain dopamine neurons and serotonin neurons to heroin, nicotine, cocaine, and MDMA. Cell Discov 4, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Everitt BJ, and Phillips PE (2012). Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc. Natl. Acad. Sci. USA 109, 20703–20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, and Koob GF (2014). The development and maintenance of drug addiction. Neuropsychopharmacology 39, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, et al. (2011). Recombinase-driver rat lines: Tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, and Tseng KY (2012). Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: When, how, and why? Front. Mol. Neurosci 5, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WC, Ford KA, Pagels NE, McCutcheon JE, and Marinelli M (2013). Adolescents are more vulnerable to cocaine addiction: Behavioral and electrophysiological evidence. J. Neurosci 33, 4913–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhou J, Pang Y, Rivkin A, Assakura Miyazaki P, Rashid M, Bartlett A, Sandoval J, Nery JR, Jacobs M, Williams E, et al. (2018). Linking cortical projection cell types to epigenetic profiles Program No. 519.12, Neuroscience Meeting Planner (San Diego, CA: Society for Neuroscience; ). [Google Scholar]