Abstract
Background
Individual trajectories of drug use and drug-related problems are highly heterogeneous. There is no standard taxonomy of these trajectories, but one could be developed by defining natural categories based on changes in symptoms of substance-use disorders over time.
Methods
Our study was conducted in a community sample in Baltimore, Maryland. At baseline, all participants were using opioids and/or cocaine, but none were in treatment. Drug use and symptomatology were assessed again at 12 months (N=115).
Results
We defined Quitters as participants who had not used for at least 30 days at follow-up (17%). For the remaining participants, we performed longitudinal cluster analysis on DSM symptom-counts, identifying three trajectory clusters: newly or persistently Symptomatic (40%) participants, Chippers (21.5%) with few symptoms, and Converted Chippers (21.5%) with improved symptom counts.
Logistic regression showed that profiles of Quitters did not resemble Chippers, but instead resembled Symptomatic participants, having high probability of disorderly home neighborhood, nonwhite race, and negative mood. Quitters tended to have two protective factors: initiating opioid-agonist treatment during the study (reffect = 0.25, CL95 0.02–0.48) and lack of polydrug use (reffect = 0.25, CL95 0.004–.49). Converted Chippers tended to be white, with orderly home neighborhoods and less negative mood (reffects 0.24 to 0.31, CL95 0.01–0.54).
Conclusions
Changes in DSM symptomology provided a meaningful measure of individual trajectories. Quitters shared psychosocial characteristics with Symptomatic participants, but not with participants who continued to use with few symptoms. This suggests that Quitters abstained out of necessity, not because their problems were less severe.
Keywords: Cocaine, Opioid, Substance Use Disorder, DSM-5, Trajectory
Introduction
Most published research on trajectories of drug use—initiation, escalation, moderation, quitting—has focused on data from people who have substance-use disorders (SUDs) and seek treatment. However, even within that defined subset of use patterns, trajectories are highly heterogeneous (Genberg et al., 2011; Grant et al., 2016; Hasin et al., 2013; Hser, Huang, Brecht, Li, & Evans, 2008; Hser, Huang, Chou, & Anglin, 2007; Simpson, Joe, & Broome, 2002; Sobell, Ellingstad, & Sobell, 2000). People who seek treatment can differ substantially from people who have SUDs and do not seek treatment (Humphreys, 2015; Moos, 1994; Ray, Bujarski, Yardley, Roche, & Hartwell, 2017).
As for people who use drugs but do not have a SUD—their existence is documented chiefly through annual population surveys such as the National Survey on Drug Use and Health (Center for Behavioral Health Statistics and Quality, 2017), which does not follow them longitudinally. Ethnographic studies of non-addicted people who use drugs have achieved classic status (Waldorf, Reinarman, & Murphy, 1992; N.E. Zinberg, 1984) but are still comparatively rare (Duncan, White, & Nicholson, 2003).
A related issue is that there is no standard method to assess drug-related problem trajectories. Problem severity can follow its own trajectory, somewhat independent of absolute levels of use. For example, some people may use drugs regularly for years while accruing few or no drug-related problems; these people have traditionally been called chippers (Shiffman, 1989; N. E. Zinberg & Jacobson, 1976a). Others may transiently have enough problems to meet criteria for a SUD, but later become chippers; these people have been called converted chippers (Shiffman, Paty, Kassel, Gnys, & Zetterl-Segal, 1994). Others may use drugs only intermittently but accrue many problems when they do. Of the studies we have cited, some have attempted to describe these trajectories of problems, but none have had a way to quantify them over time. (A note on the term chippers: in the tobacco literature, chippers are operationally defined as people who smoke no more than five cigarettes per day, but our usage reflects the original definition, developed for people who use heroin; it refers only to the seeming absence of compulsion and use-related problems (N. E. Zinberg & Jacobson, 1976a), without regard to amount of use.)
One candidate method for assessing trajectories of drug-related problems is built into the diagnostic criteria for SUDs. In the US, the predominant system is the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) (American Psychiatric Association, 2013), which operationalizes SUDs in terms of 11 criteria or symptoms. Each symptom is a manifestation of the overarching idea that a SUD is a pattern of “problematic use” (in earlier editions, “maladaptive use”) that causes “clinically significant impairment or distress.” The presence of any two of the 11 symptoms is sufficient for a SUD diagnosis, leading to considerable heterogeneity among people who are diagnosed. However, for the purposes of assessing possible outcomes among all people who use drugs, heterogeneity is a strength.
We took this symptom-based approach to trajectory classification, using cohort data that we collected between 2012 and 2015 (ClinicalTrials.gov Identifier: NCT01571752). Our study recruitment was by advertising and word of mouth, not by probability sampling, but we enrolled all people who agreed to participate, regardless of whether they used drugs. Thus, we sampled from a broader base than has been typical in treatment studies or in many longitudinal studies. We assessed drug use and DSM symptomatology at two clinic visits 12 months apart. We called the study Health Outcomes by Neighborhood (HON) because we were especially interested in environmental predictors of transitions (e.g., from nonuse to use, or nonproblematic use to problematic use).
In the HON protocol, we specified an a priori method for categorizing drug-symptom trajectories, based on absolute increases or decreases in symptom count over 12 months. Our preliminary analyses (not shown) exposed a problem: our a priori method was insensitive to important aspects of the data, such as the number of symptoms at baseline. Therefore, we undertook the current analyses: (1) to develop a data-driven approach to categorizing trajectories of drug use and problems, and (2) to examine the criterion validity of the resultant categories, using a few of the main predictors specified in our HON protocol. These predictors included demographic variables, person-level measures of psychological health, and objective indices of neighborhood poverty and disorder. Our focus here is on methodology for defining trajectories of use and consequences among the enrollees who were already using opioids or cocaine. We focus here on opioid and cocaine use because primary use of these drug classes was the most commonly reported and most problematic kind of use in our sample. In a separate report, we will address the broader aims of the HON study—a full characterization of risk and protective factors in our whole sample, including people who use drugs other than opioids and cocaine, and also people who do not use drugs.
2. Material and Methods
2.1. Participants
The current sample is drawn from an observational study (HON) that enrolled drug using and non-drug using men and women from 2012 to 2015. There were 290 participants who reported current use of cocaine, opioids, or both at the baseline visit. Of these 290 participants, 141 returned to provide data at the 12-month visit. This 48.6% follow-up rate matched our expectation (based on previous experience with similar populations and previous studies (Genberg et al., 2011)) that about 50% of participants making first visits would complete the study. Of these 141 who completed the study, six participants were not assigned a trajectory because their data were inconsistent: they denied any past use of opioids and/or cocaine at Visit 2 despite testing positive for drug use and/or endorsing past use at Visit 1. Because one of the environmental variables we focus on applies only within city limits, our main analysis only includes data from the 115 participants who lived in Baltimore City and completed both visits. To assess possible differences between participants who completed the study versus those who did not, we performed an additional analysis using between-groups t-tests and chi-square tests to compare the 115 participants who lived within city limits and completed both visits to the 94 participants who lived within city limits but did not return for Visit 2.
Participants included in these analyses (n=115) provided informed consent and were 18–75 years old, 85.2% African American and 73.9% male. All participants reported using cocaine and/or opioids at least 12 times in their lifetime and at least once in the past 30 days at the baseline visit. A subset (39.1%) reported that they initiated opioid agonist treatment at some time during the year-long study.
We maintained bimonthly contact with participants by telephone between the baseline and 12-month visits to maximize follow-up rates. Participants who did not return within 15 months of their baseline visit were not eligible to provide follow-up data. We compensated participants $100 at each of the two visits for answering questionnaires ($20 if the 12-month “visit” had to be conducted by phone), plus $10 for each bimonthly phone update.
2.2. Measures
Drug Use and DSM-5 SUD symptoms
At baseline, we administered the Patterns of Substance Use and Drug Use interview (modified from the PhenX Toolkit). This is a semistructured interview that assesses current and lifetime drug use and treatment. We categorized participants as having current use of opioids, cocaine, or both if they reported use at least 12 times in their lifetime, and at least once in the past 30 days (Hamilton et al., 2011) and verified drug use by urine analysis at baseline and follow-up. We also administered the HON DSM Questionnaire, a semistructured interview assessing 11 DSM-5 criteria (American Psychiatric Association, 1994, 2013) for SUDs during the past 30 days. These data, collected at baseline and the 12-month visit, were used to assess trajectories of symptoms. It is important to note that our use of the term trajectory is not meant to imply that we captured all changes in symptoms over 12-months—more or different timepoints could cause trajectories to take on different shapes. Nonetheless, the term is applicable in that we measured the course of symptoms over time.
Environmental risk and protective factors
We characterized participants’ neighborhoods of residence in two ways:
The Neighborhood Inventory for Environmental Typology (NIfETy), an onsite-observer-rated measure of physical and social disorder along specific blockfaces (Furr-Holden et al., 2010). Each of ten indicators of disorder (e.g., abandoned buildings, litter, drug paraphernalia) is scored as present vs. absent. More details of our methods for scoring the NIfETy have been published previously (Sarker et al. 2016).
Mean monetary (dollar) value of homes within a 500-meter radius of each participant’s place of residence was obtained from the Maryland Department of Assessments and Taxation. We log-transformed the values to reduce skew. Other investigations have used property value to predict health status, including obesity (Coffee et al., 2013; Drewnowski, Aggarwal, Tang, & Moudon, 2015).
Person-level risk and protective factors
In addition to demographic variables, we used baseline responses on the Profile of Mood States (POMS), a questionnaire assessing self-reported mood (anger, fatigue, vigor, sadness) (Cranford et al., 2006) during the 30 days prior to the baseline visit. Also, in the bimonthly phone updates, we asked about current treatment for SUDs, including treatment with medication, counseling, and support-group attendance.
2.3. Data Analyses
Before performing unsupervised cluster analyses on the DSM-5 symptom-count data, we removed the subset of participants who reported at least 30 days of abstinence from opioids and cocaine at their 12-month visit. We defined these participants as a “cluster” of Quitters. Our rationale was that a symptom count of zero would have a qualitatively different meaning for people with ongoing use compared to people who became abstinent.
For the remaining participants, we performed longitudinal cluster analysis —using K-Means for Longitudinal Data for participants who used a single drug, and with K-Means for Joint Longitudinal Data for participants who used cocaine and opioids— with the “kml” and “kml3d” software packages, respectively (Genolini & Falissard, 2011). Initially, we ran three separate cluster analyses, for participants who used cocaine, opioids, and both, respectively. The clusters obtained were highly similar across drug classes, and some of the clusters were also similar within drug classes (i.e., the trajectories could be categorized as increasing, decreasing, remaining low, or remaining high). Therefore, to avoid small cell sizes while maintaining clinical interpretability, subclusters that had similar trajectories were combined. The Chippers (N. E. Zinberg & Jacobson, 1976b) cluster consisted of participants who had low symptom counts (i.e., 3 symptoms or less) at both visits. The Converted Chippers (Shiffman et al., 1994) consisted of participants who improved from either a moderate or high symptom count at Visit 1 (4–11 symptoms) to a low count at Visit 2 (3 symptoms or less). One small cluster could not be combined with a larger cluster based on trajectory, per se, because only a few participants in the study had a marked increase in symptoms over the course of the year (going from 0–1 at the start of the study to 6–11 symptoms at the end). Therefore, these participants were combined with a larger cluster that shared the same final outcome: participants who had moderate or high symptom counts (i.e., 4–11 symptoms) at both visits. Thus, the Symptomatic cluster consisted of participants who either remained or became symptomatic by the end of the study. Note that participants who used both cocaine and opioids were included in the Symptomatic cluster if they had moderate or high symptom counts for either drug class at the 12-month visit, even if they had few or no symptoms related to the other class. Also note that cluster names were conferred for ease of reference and only apply to the 12-month time frame within which we conducted our assessments; they are not directly comparable to designations based on lifetime patterns of use.
Lastly, it is important to note that many participants endorsed the use of substances other than opioids and cocaine (primarily marijuana and alcohol, with a few participants endorsing the use of sedatives), but only 16 participants ever met SUD criteria for these substances over the course of the study (7 Symptomatic, 4 Converted Chippers, 3 Chippers and 2 Quitters).
To evaluate the criterion validity of the clusters, we conducted a multinomial logistic regression. The predictors were some of the main demographic, psychological, and environmental variables collected as part of the parent study; the outcome variable was cluster membership, with Symptomatic Use as the reference group. Variables tested but not retained were sex and age. Variables included in the final model were race, use of opioid agonist therapy during any of the 12 months of follow-up, drug-use class (opioids, cocaine, or both), POMS score as a measure of mental health, and mean residential value and NIfETy score as measures of the socioeconomic level and orderliness of the participant’s neighborhood. All variables were scaled to be between 0 and 1, as recommended by the authors of the multinomial software (Venables, 2002) and each variable was included in the final model as a main effect, with no interactions. Results are reported as odds ratios and 95% confidence intervals. Because odds ratios are affected by the units of measure of the predictor, we also report effect-size r values (reffect size) calculated from Z scores (Rosnow, Rosenthal, & Rubin, 2000). All analyses were performed with R version 3.5 (2015, The R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Participants who completed the study (n=115) did not differ from those who did not return for Visit 2 (n=94) in terms of sex, race, opioid symptoms, or stimulant symptoms: sex [χ2(1) = .39, p = .53 (Visit 2 completers: men, 73.9%, women, 26%; only Visit 1: men, 78.6%, women, 21.4%)]; race [χ2(1) = 1.99, p = .16 (Visit 2 completers: nonwhite, 85.2% white, 14.8%; only Visit 1: nonwhite, 78.6%, white, 21.4%), NIfETy score, t207 = −.59, p = .56 (Visit 2 completers: NIfETy, 5.71; only Visit 1: NIfETy, 5.52)];, opioid symptoms [t207 = −.32, p = .76 (Visit 2 completers: 3.96, only Visit 1, 3.81)]; stimulant symptoms (includes cocaine) [t207 = .87, p = .38 (Visit 2 completers: 2.28, only Visit 1, 2.67)]. However, participants who completed only Visit 1 had worse mood states as measured by POMS scores [t207 = −2.32, p = .02 (Visit 2 completers: POMS −2.91, only Visit 1,−4.04)], and were slightly younger [t207 = 2.2, p = .02 (Visit 2 completers: age, 45.58, only Visit 1, 42.55)].
The cluster we designated as Converted Chippers (n = 25; 21.5% of the total sample), included opioid Converted Chippers (Fig 1a) and cocaine Converted Chippers Fig 1e), as well as participants with dual opioid/cocaine use (Fig. 1i), who either showed reductions in symptom counts for both opioids and cocaine or reductions for one drug class coupled with consistently low symptom counts for the other drug class.
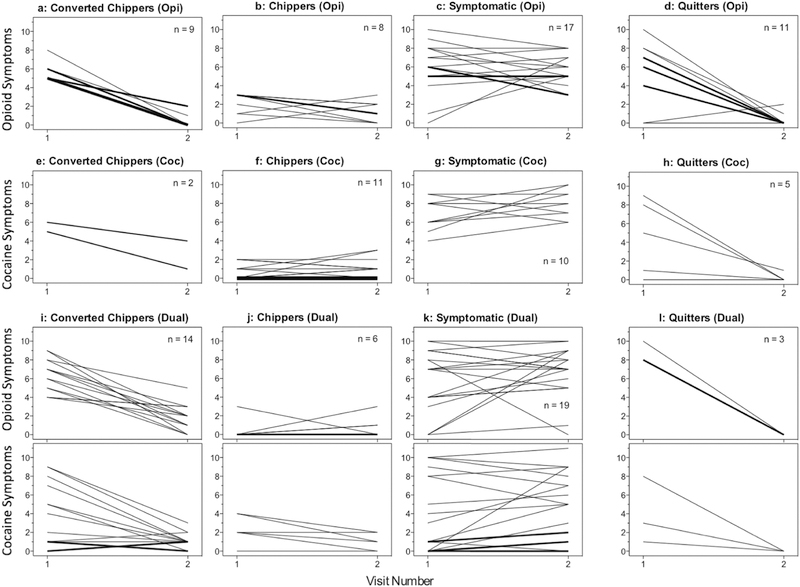
Figure 1.
Spaghetti plots showing symptom-count trajectories for the 115 individual participants assessed at two visits 12 months apart. Trajectories represent the number of DSM-5 SUD symptoms (out of a possible 11) endorsed at each visit. Each thin line represents one participant, thicker lines are in proportion to the number of participants showing the same trajectory. Note that: 1) participants who became abstinent over the course of the study were classified as Quitters; and 2) participants who had symptoms for both opioids and cocaine (dual use) were included in the Symptomatic Cluster if they had persistent or emergent symptoms for either drug, even if they had few or no symptoms related to the other.
Rows show baseline drug-use categories:
1: opioids only (n = 45, 39%).
2: cocaine only (n = 28, 24%).
3 and 4: dual use (n = 42, 37%).
Columns show clusters of 12-month trajectories:
1: Converted Chippers—high or moderate symptomatology that resolved by the 12-month visit (n = 25, 21.5%).
2: Chippers—low or no symptomatology at both visits (n = 25, 21.5%).
3: Symptomatic Use—high or moderate symptomatology at both visits; dual use was assigned to this group if their trajectory for either drug class met criteria (n = 46, 40%).
4: Quitters—defined outside of cluster analysis; the criterion was 30-day abstinence at the 12-month visit, regardless of symptom trajectory (n = 19, 17%).
The cluster we designated as Chippers (n = 25; 21.5% of the total sample) had low symptom counts at both visits for both drug classes (Fig 1b, 1f, and 1j).
Within each of the three clusters of ongoing use (first three columns in Fig. 1), symptom trajectories were similar regardless of whether the drug class was cocaine or opioids. Only a small subcluster of participants had large numbers of emergent symptoms (i.e., sharp upward slopes in Figs. 1c, 1g and 1k); to maintain adequate cell sizes, we combined them with the subcluster of participants who had high symptom counts at both visits, forming the Symptomatic cluster (n = 46; 40% of total sample). Participants with dual opioid/cocaine use were included in the Symptomatic cluster if they had persistently high or emergent symptom counts for opioids or cocaine (Fig. 1k).
In our predefined Quitters cluster (n = 19; 17% of total sample), some participants continued to report SUD symptoms such as craving at 12 months (rightmost column in Fig. 1). Had the Quitters been classified according to the k-means clustering, 15 out of 19 (Figs. 1d, 1h and 1l) would have been in the Converted Chippers cluster, and 4 would have been in the Chippers cluster.
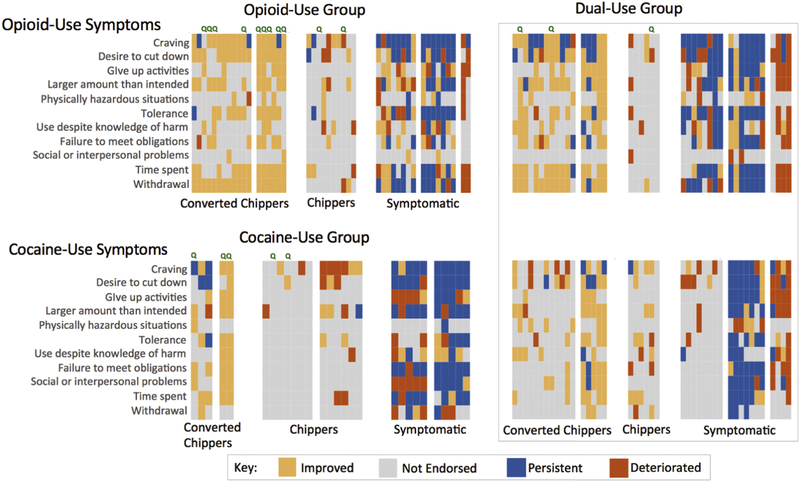
Figure 2 shows the symptoms more granularly, with each of the 11 possible symptoms color-coded for each participant in terms of its level and its direction of change (if any) between the baseline and 12-month visits. Some symptoms tended to not be endorsed at either time point (gray cells in Fig. 2); across the sample, there was little endorsement of “continued substance use despite having persistent or recurrent social or interpersonal problems,” or “recurrent substance use in situations when it is physically hazardous.” Remission of withdrawal-related symptoms was heavily endorsed among Converted Chippers, but most Chippers never endorsed withdrawal symptoms, either at baseline or 12 months.
Figure 2.
Heatmap showing changes in specific DSM-5 symptoms related to opioid use and/or cocaine use for each participant. Each column of cells shows data for one participant. Cell color indicates the trajectory for a single symptom, based on its presence vs. absence at the baseline visit and 12-month visit: (1) improved if endorsed only at baseline; (2) not endorsed if not endorsed at either visit; (3) persistent if endorsed at both visits; or (4) emergent if endorsed only at the 12-month visit. Green “Q” indicates a participant who reported being abstinent at Visit 2 and was therefore treated as a member of the Quitters “cluster” in regression analysis. Blocks of participants separated by white space within the Converted Chippers, Chippers and Symptomatic clusters represent subclusters.
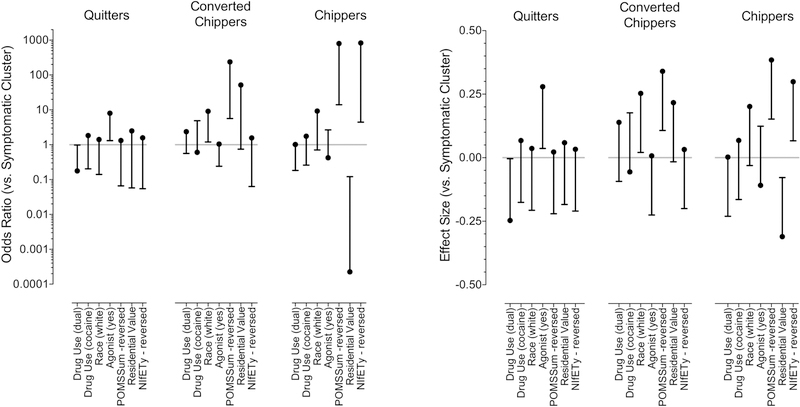
Figures 3 and 4 show inputs to, and results from, the multinomial logistic regression with the Symptomatic group as the reference category (see Supplementary File 1 for logistic regression coefficients). The overall model had a McFadden pseudo-R2 of 0.43, indicating excellent fit. Quitters were distinct from the two clusters of ongoing use (Chippers and Converted Chippers); they tended to resemble the Symptomatic group in having disorderly or impoverished home neighborhood (Figs. 3h and 4), nonwhite race (Figs. 3b and 4), and more negative mood reported on the POMS (Figs. 3g and 4). The only ostensible protective factors that were more likely among the Quitters (reffect = 0.25, 95% CI 0.02–0.48) than the Symptomatic group were opioid-agonist treatment (Figs. 3c and 4) and use of opioids alone as opposed to dual use of opioids and cocaine (Figs. 3d, 3f and 4); the estimated effect sizes for these two factors were similar, but the confidence interval for opioid-only use came much closer to including zero (reffect = 0.25, CL95 0.004–.49), suggesting the agonist treatment effect might be more reliable. Six participants, all within the Symptomatic cluster, reported attending 12-step support and/or counseling during the study. However, no participant reported 12-step support or counseling more than once at bimonthly updates through Visit 2.
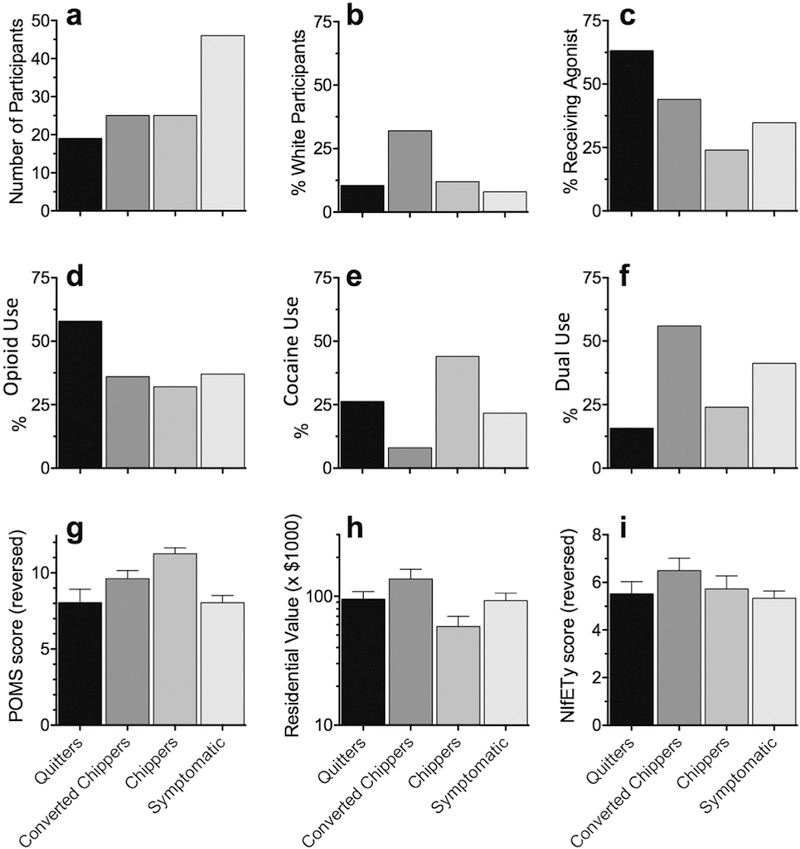
Figure 3.
Summary values of variables included in the multinomial logistic regression model, showing percentage in each cluster (including the “cluster” of Quitters, defined prior to clustering). (a) number of participants in each cluster; (b) percentage of participants self-described as being white; (c) percentage of participants who initiated opioid agonist therapy between the baseline and 12-month visits;* (d, e, f) percentage of participants who enrolled in the study with use of opioids, cocaine, or both, respectively; (g) summary rating on the Profile of Mood States (POMS) questionnaire, referring to the 30-day period prior to joining the study, with higher values indicating a “better” state; (h) average value of residences in the participant’s home neighborhood; and (i) Neighborhood Inventory for Environmental Typology (NIfETy) ratings, with higher values indicating a more orderly neighborhood environment.
*77.3% of the overall sample expressed interest in treatment at Visit 1, 87% of Symptomatic, 78.9% of Quitters, 92% of Converted Chippers and 44% of Chippers.
Figure 4.
Results of multinomial logistic regression on external criterion variables (the same ones described for Fig. 4) as predictors of membership in each cluster, with Symptomatic Use as the reference group. Each variable is plotted so that its ostensibly protective levels (more stable moods, more orderly neighborhood, etc.) are on the top half of the y axis.
The left panel shows odds ratios (points) and 95% confidence intervals (lines). Because the magnitude of the odds ratios is affected by the units of measure of the predictor, the right panel shows effect sizes (reffect) and 95% confidence intervals for the same analysis. Confidence intervals are shown in only one direction to emphasize inclusion or exclusion of the null: predictors whose confidence intervals do not cross the central gray line had statistically significant associations with cluster membership. All tests were two-tailed.
In general, Quitters were similar to ongoing Symptomatic Use group (the reference category) except for having used agonist treatment between the baseline and 12-month visits. In contrast, Chippers and Converted Chippers had significantly higher likelihoods of several person-level and environmental protective factors than ongoing Symptomatic Use.
In contrast to the other clusters, those who had few problems (Chippers) or whose problems ameliorated during the study (Converted Chippers) had high probabilities of residence in orderly neighborhoods, white race, and less negative mood on the POMS (reffects 0.24 to 0.31, 95% CIs 0.01–0.54).
There was an unexpected divergence between the two neighborhood-related variables (Figs. 4h, 4i and 5) that predicted membership in the Chippers cluster: Chippers tended to live in neighborhoods with NIfETy scores indicating a high degree of orderliness, but with relatively low residential values.
4. Discussion
Use of DSM symptom counts as trajectory indices
It has long been recognized that the individual criteria described in the DSM for SUDs (and, in previous editions, for Substance Dependence) are not interchangeable as indicators of severity. Nonetheless, the DSM-5 incorporates the idea that a higher symptom count is likely to suggest higher severity and gives cutoffs for levels of severity (mild, 2–3; moderate, 4–5; severe, 6–11). We did not base our analyses on the DSM-5 severity categories because their use has received only mixed support in the literature to date. However, the categories do appear consistent with our clusters: our Symptomatic cluster consisted of people who progressed into or maintained symptom counts that would be categorized as moderate or severe, whereas our Chippers and Converted Chippers progressed into or maintained symptom counts that would be categorized as mild or as not meeting diagnostic criteria.
With or without labeled degrees of severity, our results indicate that changes in DSM-5 symptomology can be a useful index of drug-related problems over time in people who use opioids, cocaine, or both. Even though we used a nonprobability sample with a sample size not on an epidemiological scale and a follow-up period of only 12 months, we found a wide range of baseline severities and longitudinal changes—and showed that they cohered into interpretable categories. These categories would not have been detectable if we had measured only frequency of use.
Symptom counts might be part of the solution to a longstanding debate in research on addiction treatment: what constitutes a meaningful outcome measure (Carroll et al., 2014; Donovan et al., 2012). In one of our clinical trials, we showed that contingency management caused reductions in past-30-day symptom counts for DSM-IV opioid and cocaine dependence (Epstein et al., 2009), and, more recently, another group has shown that Cognitive-Behavioral Therapy causes reductions in DSM-5 symptom count for Alcohol-Use Disorder (AUD) (Kiluk, Frankforter, Cusumano, Nich, & Carroll, 2018). Sustained drug abstinence is likely to continue to be an important metric of success in many contexts; however, symptom amelioration could be an important supplementary or, in some cases, alternative metric for treatment effectiveness.
Use of clustering to reveal natural categories of trajectories
Our analytic approach, k-means longitudinal clustering (Genolini & Falissard, 2011), does not seem to have been used previously in studies of symptom trajectories for SUDs. We turned to cluster analysis because the categorization method we had specified a priori was an unsatisfactory match to the patterns of symptom change we actually saw. Like most data-driven analyses, clustering raises issues of contamination by “researcher degrees of freedom” (Simmons, Nelson, & Simonsohn, 2011), because it requires investigator decisions about where to lump and where to split: diagnostic output from cluster-analysis software can offer guidance, but cannot replace expert knowledge of the subject area (Mooi & Starstedt, 2011). An a priori system may give a greater appearance of rigor but will also incorporate preexisting investigator biases (or oversights) about the likely distribution of the data. This problem might be resolved after publication of more cluster analyses like ours. Eventually, a data bank in the literature will enable investigators to specify better categorization methods a priori.
Implications of the current results for natural categories of trajectories
We did not undertake these analyses to provide a full picture of risk and protective factors for drug-related problems. Rather, we used demographic, psychological, and environmental variables to probe the criterion validity of our clusters. It should be emphasized that all of the predictive measures, including mood and neighborhood conditions, were obtained at the first visit and were framed in reference to the 30-day period before the first visit. Some noteworthy patterns emerged.
Most striking was that the participants who became abstinent in our 12-month time frame (Quitters, 17% of the sample) were similar to those who continued to use problematically (Symptomatic group, 40% of our sample). Both groups had high probabilities of what looked like risk factors for poor outcomes, both personally and environmentally: more negative mood reported on the POMS (Rausch, Nichinson, Lamke, & Matloff, 1990), nonwhite race (Carter et al., 2010; Hser et al., 2008; Molina, Alegria, & Chen, 2012; Zapolski, Pedersen, McCarthy, & Smith, 2014), and residence in disorderly or impoverished neighborhoods (Siahpush, Yong, Borland, Reid, & Hammond, 2009; Storr, Chen, & Anthony, 2004). In contrast, the participants who continued to use at 12 months, but nonproblematically (Chippers and Converted Chippers, 43% of our sample) had high probabilities of what looked like protective factors on the same measures.
This pattern of findings suggests that it would be an oversimplification to rank-order “success” with the Symptomatic group at one end, Chippers in the middle, and Quitters at the other end. Rather, our sample seemed to bifurcate into those whose use resulted in problems and those whose use did not, at least during a 12-month time frame. The Quitters in our sample had baseline symptomology similar to that of the Symptomatic group, and Quitters may have been motivated to quit, at least in part, by their experience of high(er) levels of negative consequences. This kind of motivation has sometimes been invoked in the phrase “hitting bottom”—an old-fashioned term that is not necessarily outmoded (Kirouac, Frohe, & Witkiewitz, 2015). It is important to note that, in using the term “hitting bottom,” we do not endorse the lore that it is a necessary precursor for change in people with drug-related problems (see Kirouac et al. 2015 for a more thorough discussion of the term and an attempt to operationalize it). Rather, we want to point out important overlaps between experiencing drug-use-related symptoms and quitting. Consistent with this, a combined analysis of data from three clinical trials showed that higher baseline AUD severity predicted both heavier drinking and more abstinence (i.e., either remaining symptomatic or quitting), whereas lower baseline AUD severity predicted greater likelihood of transition to low-risk drinking (i.e., becoming a converted chipper) (Witkiewitz et al., 2017). Interestingly, in our sample two Quitters reported drug craving as a symptom after they became abstinent, whereas several Chippers never reported drug craving.
There were two distinctions between our Quitters and our Symptomatic group. First, Quitters were less likely to dually use opioids and cocaine (Weiss, Martinez-Raga, & Hufford, 1996). Second, and more reliably, Quitters tended to enroll in opioid-agonist treatment between baseline and month 12. Baseline interest in treatment had been expressed by high proportions of each cluster (79% of Quitters, CL95 = 77–96%, 87% of Symptomatic Use, CL95 = 54–93%), so perhaps the difference lay partly in practical barriers to access, which we did not systematically assess.
One finding we had not expected was that the two neighborhood-related variables predicted cluster membership in divergent directions. Relative to the Symptomatic group, Chippers lived in neighborhoods that looked more orderly to trained observers—but that also had lower average property values. Examining individual cases, we found that Chippers tended to live in orderly neighborhoods that were on the periphery of disorderly neighborhoods, either in outer urban or near-suburban areas, though still within city limits. The Symptomatic group tended to live in disordered inner-urban neighborhoods where property values were low by regional standards but were still modestly higher than those of Chippers’ neighborhoods. The higher property values might reflect closeness to more sought-after areas. Visible aspects of the neighborhood—presence or absence of litter, abandoned buildings, broken windows, discarded drug paraphernalia, and so on—may have been more salient than the locational considerations that drove property values slightly down or up. The orderliness of Chippers’ neighborhoods may have both reflected and driven a social cohesion and collective efficacy that helped protect them against drug-related problems (although these protective effects may be filtered through individual residents’ perceptions (Brunton-Smith, Sturgis, & Leckie, 2018)). The disorderliness of the Symptomatic group’s neighborhoods may have conferred risks that were both psychological and practical: for example, in prior research, people who used drugs were more likely to be approached by someone selling drugs if they lived in more disadvantaged neighborhoods (Storr et al., 2004).
Limitations and Future Directions
Our study had several limitations. As we expected, based on previous studies with similar study designs in similar populations (Genberg et al., 2011), about half of the participants did not return for the second visit. Baseline levels of opioid and stimulant symptoms did not differ between completers and noncompleters, but noncompleters were slightly younger and reported worse mood at Visit 1 compared to completers. Based on our analysis of completers, mood scores suggest that many noncompleters would have been classified among the Quitter or Symptomatic clusters if they had returned at Visit 2; to the extent that this is true, our Visit 2 results might underestimate the proportions of people who become abstinent or who continue to use with many symptoms.
Self-reports of abstinence were verified by urinalysis on the two visit days, but this verification applies only to the few days before the test. The sample consisted mostly of African Americans with low incomes, which may limit the generalizability of our findings. However, the sample accurately represented the demographics of Baltimore, which is a potential strength of the study. Replication in other cities, and extension to suburban and rural areas, would increase confidence in the findings. In addition, our observer-rated indices of neighborhood disorder were based on site visits made in 2008, prior to participant enrollment in the study. Mitigating this limitation is the relative slowness of change in most Baltimore neighborhoods, especially lower-SES residential neighborhoods. Economic stagnation has kept them, in many instances, persistently disordered.
As we noted, the 12-month time frame of our study means that our cluster names are not directly comparable to designations based on lifetime patterns of use. For example, some of our Chippers might have been designated Converted Chippers if we had taken their full histories into account. Longer and more closely spaced follow-up visits would provide more information about the shapes of symptom trajectories, and perhaps provide insight into relapse after long periods of abstinence or low symptomology, especially if the follow-up visits included biological verification of drug use or abstinence. With those data, we could examine questions such as whether someone with persistently Symptomatic use could become a Chipper after a lengthy abstinence (Rounsaville, Kosten, & Kleber, 1987).
We treated all DSM-5 criteria with equal weight, using total symptom counts to assess change. There is a large body of research on the items that make up DSM criteria for SUDs, but most of it has focused on whether the criteria measure two underlying constructs (abuse and dependence) or just one. More research is needed to inform differential weighting of symptoms into severity scores specific to opioid and cocaine use disorder, like has been investigated for AUD (Lane & Sher, 2015; Steinley, Lane, & Sher, 2016).
At a minimum, though, our findings support the use of DSM-5 SUD symptom counts as a measure of drug-related problems (or use without problems) over time. Because transient or occasional drug use is normative at a population level (Chen & Kandel, 1995), the symptom-trajectory approach may help establish population norms for drug-related problems. It may also inform treatment-outcome measures, which could be used both for evaluation of program effectiveness and for approval of new treatments. Future studies should investigate DSM-5 symptomology as it relates to other person-level and environmental predictors and as it applies to other classes of substances.
Supplementary Material
Highlights.
Changes in DSM-5 symptoms over time can define and categorize courses of drug-related problems
Psychological health and home neighborhood can have protective or hazardous effects on trajectory
Over the year-long study, some people were able to “get away with” continuing to use with few symptoms
People who stopped using shared risk profiles with highly-symptomatic people who continued to use
The difference between cessation and ongoing problematic use may be access to treatment
Acknowledgements
This study was supported by the Intramural Research Program of NIH, NIDA.
Ethical approval for this study was granted by The NIH Addictions Institutional Review Board.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
The data presented here are not publicly available because other components of the study protocol are still ongoing: https://clinicaltrials.gov/ct2/show/NCT01571752
References
- American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Washington, DC: Anerican Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association. [Google Scholar]
- Brunton-Smith I, Sturgis P, & Leckie G (2018). How collective is collective efficacy? The importance of consensus in judgments about community cohesion and willingness to intervene. Criminology, 56(3), 608–637. [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, DeVito EE, Decker S, LaPaglia D, … Ball SA (2014). Toward empirical identification of a clinically meaningful indicator of treatment outcome: Features of candidate indicators and evaluation of sensitivity to treatment effects and relationship to one year follow up cocaine use outcomes. Drug and Alcohol Dependence, 137, 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Paris MM, Lam CY, Robinson JD, Traylor AC, Waters AJ, … Cinciripini PM (2010). Real-time craving differences between black and white smokers. Am J Addict, 19(2), 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2017). Results from the 2016 National Survey on Drug Use and Health: Detailed Tables . Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Chen K, & Kandel DB (1995). The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health, 85(1), 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee NT, Lockwood T, Hugo G, Paquet C, Howard NJ, & Daniel M (2013). Relative residential property value as a socio-economic status indicator for health research. Int J Health Geogr, 12, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, & Bolger N (2006). A procedure for evaluating sensitivity to within-person change: can mood measures in diary studies detect change reliably? Pers Soc Psychol Bull, 32(7), 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Bigelow GE, Brigham GS, Carroll KM, Cohen AJ, Gardin JG, … Wells EA (2012). Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction, 107(4), 694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Aggarwal A, Tang W, & Moudon AV (2015). Residential property values predict prevalent obesity but do not predict 1-year weight change. Obesity (Silver Spring), 23(3), 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan DF, White JB, & Nicholson T (2003). Using Internet-based surveys to reach hidden populations: case of nonabusive illicit drug users. Am J Health Behav, 27(3), 208–218. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Schmittner J, Umbricht A, Schroeder JR, Moolchan ET, & Preston KL (2009). Promoting abstinence from cocaine and heroin with a methadone dose increase and a novel contingency. Drug Alcohol Depend, 101(1–2), 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr-Holden CD, Campbell KD, Milam AJ, Smart MJ, Ialongo NA, & Leaf PJ (2010). Metric properties of the Neighborhood Inventory for Environmental Typology (NIfETy): an environmental assessment tool for measuring indicators of violence, alcohol, tobacco, and other drug exposures. Eval Rev, 34(3), 159–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genberg BL, Gange SJ, Go VF, Celentano DD, Kirk GD, & Mehta SH (2011).Trajectories of injection drug use over 20 years (1988–2008) in Baltimore, Maryland. Am J Epidemiol, 173(7), 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genolini C, & Falissard B (2011). KmL: a package to cluster longitudinal data. Comput Methods Programs Biomed, 104(3), e112–121. [DOI] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, … Hasin DS (2016). Epidemiology of DSM-5 Drug Use Disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry, 73(1), 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, … Haines J (2011). The PhenX Toolkit: get the most from your measures. Am J Epidemiol, 174(3), 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, … Grant BF (2013). DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry, 170(8), 834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Huang D, Brecht ML, Li L, & Evans E (2008). Contrasting trajectories of heroin, cocaine, and methamphetamine use. J Addict Dis, 27(3), 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Huang D, Chou CP, & Anglin MD (2007). Trajectories of heroin addiction: growth mixture modeling results based on a 33-year follow-up study. Eval Rev, 31(6), 548–563. [DOI] [PubMed] [Google Scholar]
- Humphreys K (2015). Addiction treatment professionals are not the gatekeepers of recovery. Subst Use Misuse, 50(8–9), 1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk BD, Frankforter TL, Cusumano M, Nich C, & Carroll KM (2018). Change in DSM-5 Alcohol Use Disorder criteria count and severity level as a treatment outcome indicator: Results from a randomized trial. Alcohol Clin Exp Res [DOI] [PMC free article] [PubMed]
- Kirouac M, Frohe T, & Witkiewitz K (2015). Toward the operationalization and examination of “hitting bottom” for problematic alcohol use: A literature review. Alcoholism Treatment Quarterly
- Lane SP, & Sher KJ (2015). Limits of Current Approaches to Diagnosis Severity Based on Criterion Counts: An Example with DSM-5 Alcohol Use Disorder. Clin Psychol Sci, 3(6), 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina KM, Alegria M, & Chen CN (2012). Neighborhood context and substance use disorders: a comparative analysis of racial and ethnic groups in the United States. Drug Alcohol Depend, 125 Suppl 1, S35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi E, & Starstedt A (2011). Cluster analysis. In Mooi E & Starstedt A (Eds.), A Concise Guide to Market Research (pp. 237–284). Berlin: Springer-Verlag [Google Scholar]
- Moos RH (1994). Treated or untreated, an addiction is not an island unto itself. Addiction, 89(5), 507–509. [DOI] [PubMed] [Google Scholar]
- Rausch JL, Nichinson B, Lamke C, & Matloff J (1990). Influence of negative affect on smoking cessation treatment outcome: A pilot study. Br J Addict, 85(7), 929–933. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Yardley MM, Roche DJO, & Hartwell EE (2017). Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Abuse, 43(6), 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosnow RL, Rosenthal R, & Rubin DB (2000). Contrasts and correlations in effect-size estimation. Psychol Sci, 11(6), 446–453. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Kosten TR, & Kleber HD (1987). The antecedents and benefits of achieving abstinence in opioid addicts: a 2.5-year follow-up study. Am J Drug Alcohol Abuse, 13(3), 213–229. [DOI] [PubMed] [Google Scholar]
- Shiffman S (1989). Tobacco “chippers”--individual differences in tobacco dependence. Psychopharmacology (Berl), 97(4), 539–547. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Kassel JD, Gnys M, & Zetterl-Segal M (1994). Smoking behavior and smoking history of tobacco chippers. Exp Clin Psychopharmacol, 2(2), 126–142. [Google Scholar]
- Siahpush M, Yong HH, Borland R, Reid JL, & Hammond D (2009). Smokers with financial stress are more likely to want to quit but less likely to try or succeed: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction, 104(8), 1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, & Simonsohn U (2011). False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol Sci, 22(11), 1359–1366. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, & Broome KM (2002). A national 5-year follow-up of treatment outcomes for cocaine dependence. Arch Gen Psychiatry, 59(6), 538–544. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Ellingstad TP, & Sobell MB (2000). Natural recovery from alcohol and drug problems: Methodological review of the research with suggestions for future directions. Addiction, 95(5), 749–764. [DOI] [PubMed] [Google Scholar]
- Steinley D, Lane SP, & Sher KJ (2016). Determining optimal diagnostic criteria through chronicity and comorbidity. In Silico Pharmacol, 4(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storr CL, Chen CY, & Anthony JC (2004). “Unequal opportunity”: Neighbourhood disadvantage and the chance to buy illegal drugs. J Epidemiol Community Health, 58(3), 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables WNR, B.D. (2002). Modern Applied Statistics with S Fourth Edition. New York Springer. [Google Scholar]
- Waldorf D, Reinarman C, & Murphy S (1992). Cocaine Changes: The Experience of Using and Quitting Philadelphia, PA: Temple University Press. [Google Scholar]
- Weiss RD, Martinez-Raga J, & Hufford C (1996). The significance of a coexisting opioid use disorder in cocaine dependence: An empirical study. Am J Drug Alcohol Abuse, 22(2), 173–184. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Pearson MR, Hallgren KA, Maisto SA, Roos CR, Kirouac M, … Heather N (2017). Who achieves low risk drinking during alcohol treatment? An analysis of patients in three alcohol clinical trials. Addiction, 112(12), 2112–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski TC, Pedersen SL, McCarthy DM, & Smith GT (2014). Less drinking, yet more problems: Understanding African American drinking and related problems. Psychol Bull, 140(1), 188–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinberg NE (1984). Drug, Set, and Setting: The Basis for Controlled Intoxicant Use New Haven, CT: Yale University Press. [Google Scholar]
- Zinberg NE, & Jacobson RC (1976a). The natural history of “chipping”. Am J Psychiatry, 133(1), 37–40. [DOI] [PubMed] [Google Scholar]
- Zinberg NE, & Jacobson RC (1976b). The natural history of “chipping”. Am J Psychiatry, 133(1), 37–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.